Abstract
Total sleep deprivation (TSD) induces a broad spectrum of cognitive, behavioral and cellular changes. We previously reported that long term (5–11 days) TSD in the rat, by the disk-over-water method, decreases the activity of the antioxidant enzyme superoxide dismutase (SOD) in the brainstem and hippocampus. To gain insight into the mechanisms causing cognitive impairment, here we explore the early associations between metabolic activity, antioxidant responses and working memory (one form of cognitive impairment). Specifically we investigated the impact of short term (6 h) TSD, by gentle handling, on the levels of the endogenous antioxidant, total glutathione (GSHt), and the activities of the antioxidative enzymes, SOD and glutathione peroxidase (GPx). Short term TSD had no significant impact on SOD activity, but increased GSHt levels in the rat cortex, brainstem and basal forebrain, and GPx activity in the rat hippocampus and cerebellum. We also observed increased activity of hexokinase, (HK), the rate limiting enzyme of glucose metabolism, in the rat cortex and hypothalamus. We further showed that 6h of TSD leads to increased exploratory behavior to a new environment, without impairing spontaneous alternation behavior (SAB) in the Y maze. We conclude that acute (6h) sleep loss may trigger compensatory mechanisms (like increased antioxidant responses) that prevent initial deterioration in working memory.
Keywords: total sleep deprivation, Y maze, metabolic activity, antioxidant responses, rat
Introduction
Sleep deprivation leads to impaired performance, characterized by cognitive slowing, memory impairment, decreased vigilance and the inability to sustain attention. Several investigators showed that spatial learning and memory, as assessed by performance in the Morris water maze, was impaired after total sleep deprivation (TSD), paradoxical sleep deprivation (PSD) or sleep fragmentation [11, 27,31].
It has been hypothesized that free radicals accumulate during prolonged waking as a result of enhanced metabolic activity, and may be responsible for some of the effects of sleep deprivation [21]. Free radicals are difficult to detect and quantify directly due to their extreme reactivity. The production of free radicals can be inferred from measurement of antioxidant responses and/or oxidative stress-induced products. Antioxidant responses include changes in the activities of antioxidative enzymes, superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and glutathione reductase (GR) and in the levels of the endogenous antioxidant, glutathione (GSH). If antioxidant responses are unable to successfully scavenge the free radicals, this will lead to oxidative stress resulting in damage to lipids, proteins and/or nucleic acids [8].
We previously reported that long term (5–11 days) TSD by the disk-over-water method decreased SOD activity in the rat hippocampus and brainstem [20]). Everson et al [7] reported that 5 or 10 days of TSD decreased total glutathione (GSHt) levels and CAT activity in the rat liver and increased GPx activity in the rat heart. D’Almeida et al [4] reported that 96h of PSD, by the platform technique, significantly decreased GSHt levels in the rat hypothalamus, while Silva et al [23] showed that 72h of PSD increased the ratio of oxidized/reduced glutathione levels and lipid peroxidation levels in the mouse hippocampus. A differential alteration in SOD activity, GSHt and lipid peroxidation levels across multiple brain regions in old (24 month) compared to young adult (8 month) rats, both subjected to 96h of PSD by the classical platform (flower pot) procedure, was observed by Singh et al [24].
We hypothesize that short-term (6h) total sleep deprivation increases glucose metabolism resulting in elevated free radical production and altered antioxidant responses. Glucose metabolism was analyzed by changes in the activity of its rate limiting enzyme, hexokinase (HK), while antioxidant responses were assessed by changes in the activities of the antioxidative enzymes, SOD and GPx and levels of the endogenous antioxidant, GSHt. Furthermore, we tried to correlate these biochemical changes with behavioral changes associated with performance in the Y maze.
Materials and Methods
Adult male Sprague Dawley rats (400–500g) were used for all experiments. Procedures were carried out in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals. Animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the Veterans Administration Greater Los Angeles Health Care System. The rats were kept on 12h:12h light-dark cycle (lights on at 8:00 AM and off at 8:00 PM), ambient temperature 24 ± 1°C, with food and water available ad libitum.
Rats were housed in individual cages and handled for 1 hour each day for 1 week. This gentle handling procedure included brushing their fur with a cotton tip applicator, introducing objects into their cages, disturbing their cage bedding, tapping on their cages and rotating their cages. After 1 week of habituation to the handling procedure the animals were divided into two groups: the sleep deprived (SD) rats were handled each time they showed physical signs of sleepiness (no motor activity), while the unhandled controls (UC) were left undisturbed in their cages. The animals were subjected to 6h of TSD (8AM-2PM) starting at lights-on. Sleep deprivation was carried out in the first part of the light period when sleep and slow wave activity are at their maximum [30].
In Experiment 1, after 6 hours of TSD, the sleep deprived rats were placed in the Y maze for behavioral testing (at 2PM). Control rats, which were left undisturbed, were tested on the Y maze one day prior, also at 2 PM. The symmetrical Y maze is made of acrylic and consists of three arms of equal size [10cm (width) x 30.5cm (inner length) x 40.5cm (outer length) x 20cm (height)] at an angle of 120°. The Y maze was placed in a room without any environmental cues. The rat was placed in the center of the Y maze and allowed to freely explore the maze for 8 min. An alternation was defined as the entry into all three arms on consecutive choices. An arm was considered to be entered when the rat’s hind paws were within the walls of the arm. The sequence and total number of arms entered was recorded manually as well as on a video tracking system, HVS Image Ltd. (Buckingham, U.K.). The spontaneous alternation behavior (SAB) is the correct number of sequential triads containing entries into all the three arms divided by the maximum possible number of alternations (which is the number of arm entries -2) x 100. The apparatus was cleaned using 70% ethanol, and the alcohol was allowed to evaporate for 5 minutes between trials, in order to remove any olfactory cues.
In Experiment 2, following 6 hours of TSD, the rats were sacrificed by decapitation after halothane (1 min.) anesthesia. Control rats (which were left undisturbed) were similarly sacrificed one day prior, also at 2 PM. The brain was quickly removed (2 min.), rinsed in pre-chilled saline and rapidly dissected on ice (5 min.). The cortex (2mm x lateral 6mm area, somatosensory cortex, AP 1.70 to −4.3; weight= 0.20g), hippocampus (1mm x lateral 3–6mm area, AP −2.30 to −6.30, weight= 0.11g), basal forebrain (2mm x lateral 2mm area, medial basal forebrain, AP 0.48 to −1.30, weight= 0.035g), hypothalamus (2mm x lateral 2mm area, AP −1.30 to −4.80, weight= 0.05g), brainstem (5mm x lateral 4mm area, AP −8.72 to −15.20, weight= 0.16g) and cerebellum (7mm x lateral 6mm area, AP −9.30 to −15.20, weight= 0.15g) were collected in pre-chilled (in dry ice) microcentrifuge tubes and immediately transferred to −80°C until analyzed. The total time taken from sacrifice to collection of the tissues was approximately 7 mins. Cooling the tissue in crushed ice has been shown to prevent autolysis and breakdown of cofactors and rapidly freezing the tissue helps to prevent enzymatic changes (Morton, 1955).
Biochemical Analysis
Each brain region was homogenized in a hand held homogenizer (Fisher, cat # 08-414-16C) with 20 strokes, in pre-chilled homogenizing buffer composed of 50 mM Tris HCl, pH 7.5, 50mM MgCl2 and 5mM EDTA, containing protease inhibitors (Roche Diagnostics, cat # 11836153001) to make a 10% homogenate (w/v). This homogenate was centrifuged in an Eppendorf micro-centrifuge (5415C) at 2,000 rpm (320xg) for 10 min. at 4°C. The pellet was discarded and the supernatant was re-centrifuged at 13,500 rpm (14,000xg) for 30 min. at 4°C. This supernatant was used for determining SOD, GPx and HK activities and GSHt levels.
The protein content of the samples was determined with the DC protein assay kit (Bio-Rad, cat # 500-0111). The amount of protein in the standards and samples was determined on a microtitre plate reader (Molecular Devices Emax precision microplate reader) at a wavelength of 750nm.
Superoxide Dismutase (SOD) activity
Superoxide dismutase (SOD) activity was measured according to the method of Misra and Fridovich [17]. Tissue extract was added to 1 ml of carbonate buffer (50mM, pH 10.2 containing 0.1mM EDTA) and the reaction initiated with 30ul epinephrine (30mM in 0.05% acetic acid). The rate of autoxidation of epinephrine was measured at 480nm for 180s. on a Hitachi U2000 spectrophotometer. Superoxide dismutase activity was expressed as units (U) of SOD/mg of protein, where one unit of SOD is defined as the amount of enzyme present that inhibits the autoxidation of epinephrine by 50%.
Glutathione Peroxidase (GPx) activity
Glutathione peroxidase (GPx) activity was determined according to the method of Somani and Husain [26]. The reaction mixture consisted of 100ul of GSH (0.01M), 100ul of NADPH (1.5mM) and 100ul of GR (0.24 units) in phosphate buffer (50mM). Tissue extract was added to 1ml of the reaction mixture and incubated at 37°C for 10 min. Then, 100ul of t-butyl hydroperoxide (12mM) was added to 900ul of the reaction mixture and the rate of oxidation of NADPH was measured at 340nm for 180s. The molar extinction coefficient of 6.22 × 103 (Mcm)−1 was used to determine GPx activity. Glutathione peroxidase activity was expressed as mM NADPH oxidized/min/mg protein.
Total Glutathione (GSHt) levels
Total glutathione (GSHt) was measured by the enzymatic recycling procedure in which reduced glutathione (GSH) is sequentially oxidized by 5, 5′-dithiobis-(2-nitrobenzoic acid) (DTNB) to oxidized glutathione (GSSG) which is then reduced by NADPH in the presence of GR back to GSH [10]. To 800ul of NADPH (0.3mM) and 100ul of DTNB (6mM), 100ul of either tissue extract or known amounts of GSH standard were added. The reaction was initiated with 10ul of GR (50 units/ml). All solutions were made up in stock buffer (pH 7.5) containing sodium phosphate (125mM) and sodium-EDTA (6.3mM). The rate of DTNB reduction was measured at 412nm continuously for 120s. Glutathione (GSH) was used as an external standard, and the level of total glutathione in the samples was expressed as nmoles GSH/g tissue.
Hexokinase (HK) activity
Hexokinase activity was measured according to the procedure of Knull et al [15]. Tissue extract was added to the reaction mixture consisting of 100ul each of glucose (33mM), ATP (67mM), MgCl2 (67mM), potassium HEPES (400mM, pH 7.5), 1-thioglycerol (100mM), NADP+ (6.4mM) and 10ul of glucose 6-phosphate dehydrogenase (1 unit) in a total volume of 1.0 ml. NADPH formation was followed at 340nm for 3 min. Hexokinase activity was expressed as μmole of NADPH formed/min/g tissue.
Statistical Analysis
Six sleep deprived (SD) and 6 unhandled control (UC) rats were tested in the Y maze. The total number of arm entries and the percentage of alternation were analyzed by the Student’s t-test. For the biochemical experiment, values from duplicate samples for each biochemical measure (SOD, GPx, GSHt, HK) were averaged to obtain one value point per animal. Six to twelve animals per biochemical measure were used. Normalized values for each biochemical measure for each individual brain region was calculated from the average of each data set. The Student’s t-test was used to determine significance differences between SD and UC rats for each brain region for each biochemical measure. Statistical significance was determined at the level of p<0.05.
RESULTS
In this study we investigated the effects of acute (6h) TSD, by gentle handling, on changes in (1) behavioral performance in the Y maze, and (2) antioxidant responses (SOD and GPx activity, GSHt levels), and metabolic activity (HK activity) by biochemical analysis.
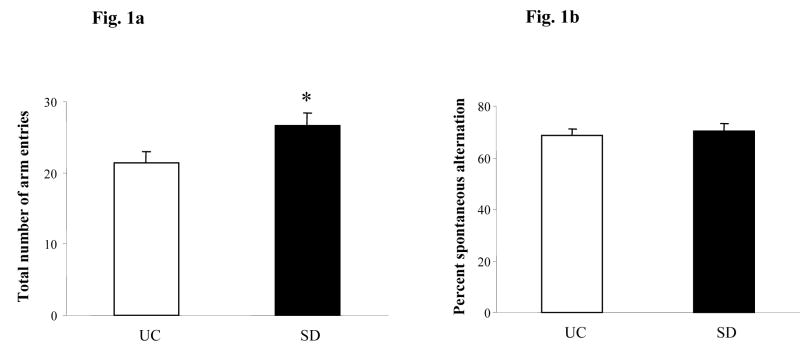
Sleep deprived (SD) rats had significantly more number of arm entries compared to UC rats (n=6, p=0.03, 28.6%, Fig. 1a). Both groups of rats performed well above the random choice (50%), however, there was no significant difference in the SAB between SD and UC rats (68.6% SD vs 70.5% UC, p>0.05, Fig, 1b),
Fig. 1.
Spontaneous alternation behavior (SAB) in the Y maze task. (a) Total number of arm entries, (b) Percent spontaneous alternation in SD (sleep deprived) and UC (unhandled control) rats. Data are expressed as mean ± S.E.M. * p<0.05 Student’s t-test.
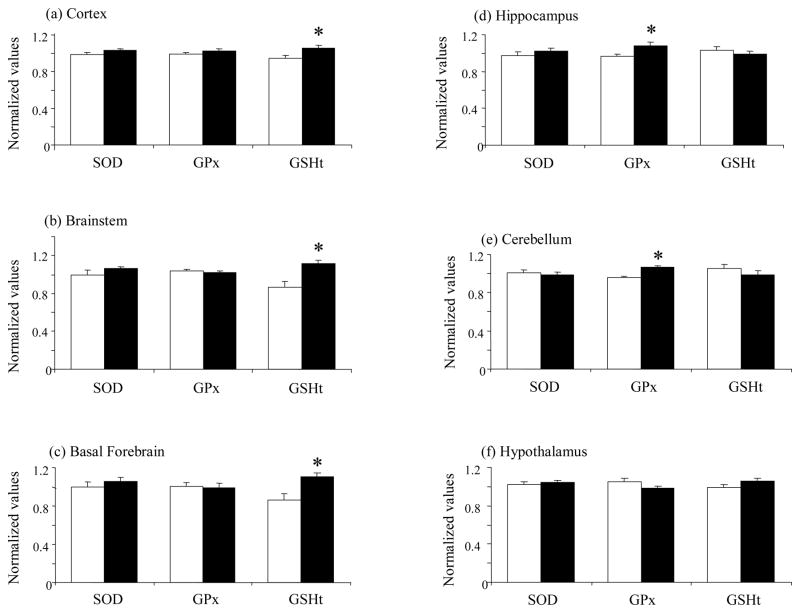
Rats subjected to 6h of TSD had higher GSHt levels in the cortex compared to unhandled controls (n=11, p=0.008, 12%, Fig. 2a). However no significant changes in either SOD or GPx (n=12 for each marker, p>0.05, Fig. 2a) activities were observed in this brain region.
Fig. 2.
Changes in antioxidant response markers in the rat cortex (a), brainstem (b), basal forebrain (c), hippocampus (d), cerebellum (e) and hypothalamus (f) after 6h total sleep deprivation. Superoxide dismutase (SOD) activity, glutathione peroxidase (GPx) activity and total glutathione (GSHt) levels. Data are expressed as mean normalized values ± S.E.M. UC= unhandled controls (white bar), SD= sleep deprived (black bar). * p<0.05 Student’s t-test.
Increased GSHt levels were also observed in the brainstem (n=10, p=0.006, 28%, Fig. 2b) and the basal forebrain (n=10, p=0.007, 28%, Fig. 2c) of rats subjected to acute sleep deprivation. There were no significant changes in either SOD or GPx activities in the brainstem (n=12 for each marker, p>0.05) or basal forebrain (n=12 for each marker, p>0.05) of sleep deprived compared to control rats.
Sleep deprived rats showed greater GPx activity in the hippocampus compared to control rats (n=12, p=0.03, 12%, Fig. 2d). There were no significant changes in the activity of SOD (n=12, p>0.05) or in the level of GSHt (n=11, p>0.05) in sleep deprived compared to unhandled rats.
Rats subjected to 6h of TSD also showed higher GPx activity in the cerebellum compared to unhandled controls (n=12, p=0.0002, 11%, Fig. 2e). Neither SOD activity (n=12) nor GSHt level (n=11) in the cerebellum were significantly altered by 6h of TSD. The hypothalamus did not show significant changes in any of the three antioxidant response markers (SOD, GPx, GSHt) studied here (n=12 for each marker) with 6h of TSD (Fig. 2f).
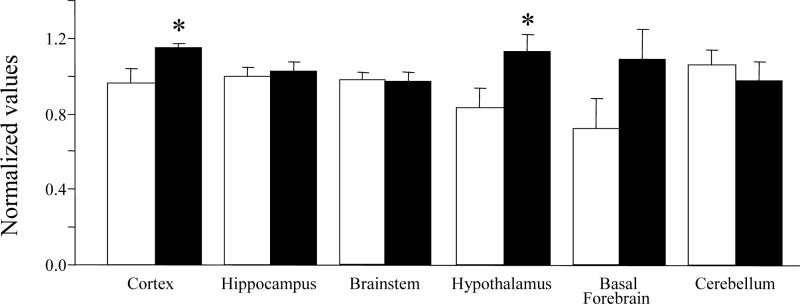
Hexokinase activity was significantly increased in the hypothalamus (n=6, p=0.05, 35%, Fig. 3) and cortex (n=6, p=0.04, 20%, Fig. 3) and insignificantly increased in the basal forebrain (n=6, p>0.05, 51%) of rats subjected to acute (6h) TSD compared to unhandled controls (UC).
Fig. 3.
Changes in hexokinase activity across different brain regions. Data are expressed as mean normalized values ± S.E.M. UC= unhandled controls (white bar), SD= sleep deprived (black bar). * p<0.05 Student’s t-test.
DISCUSSION
This study investigated the effects of 6h of TSD on metabolic activity, antioxidant responses and working memory. We chose to study 6h of TSD because this time period of sleep deprivation can be easily achieved by gentle handling, while longer periods of sleep deprivation would require more stressful procedures. Also, 6h of TSD has been shown to induce a significant increase in NREM sleep and slow wave activity, while 3h of TSD causes only a minor increase in total sleep time [30].
In this study we showed that 6h of TSD did not affect spontaneous alternation behavior (SAB) in the Y maze. Pierard et al [19] similarly reported that 3h of TSD in mice had no effect on SAB, in contrast 24h of TSD caused major disruptions in the ability of mice to run the alternation task. The mice chose to sleep rather than to explore the maze. Ten hours of TSD, on the other hand, decreased the SAB in deprived mice compared to non deprived mice. This suggests that the amount of sleep loss is an important factor affecting performance in the Y-maze. Guan et al [11] reported that 6h of TSD, by gentle handling, impaired spatial memory, but not spatial learning, and did not influence nonspatial learning or memory in rats, as assessed by performance in the Morris water maze. Smith and Rose [25] similarly reported that REM sleep is involved in spatial but not in non spatial learning in rats in the Morris water maze. The Y maze is a useful index of responsiveness to novelty, reflected by increased locomotor and exploratory behavior, as well as SAB, which is a measure of working memory [13]. SAB is the innate tendency of rodents to remember the position of the arm selected in the preceding choice and therefore serves as a measure of cognitive impairment. The Morris water maze, on the other hand, is used to assess spatial and non spatial learning and memory (acquisition, retention).
Our study also showed that 6h of TSD increased exploratory behavior in a new environment. This is consistent with a previous study by Albert et al [1] who reported that REM sleep deprived rats showed increased locomotor and exploratory activity and greater sensitivity to environmental stimuli compared to non deprived rats. Similarly, Tartar et al [28] recently reported that 24h of treadmill-induced TSD or sleep fragmentation in rats, increased exploratory behavior in an open field test. Sleep deprived rats showed increased number of entries into and time spent in the open field.
This is the first study showing that acute (6h) TSD, by gentle handling, increases antioxidant responses (SOD, GPx and GSHt) in multiple rat brain regions. Free radicals have been shown to regulate the activities of antioxidative enzymes, (SOD and GPx) and endogenous antioxidant (GSHt) [12]. Superoxide anions are free radicals produced in the mitochondria and endoplasmic reticulum as a by product of ATP synthesis. SOD converts superoxide anions into hydrogen peroxide and oxygen, while GPx, using glutathione as a cofactor, converts hydrogen peroxide and lipid hydroperoxides into oxygen and water or ROH respectively. Hydrogen peroxide and lipid hydroperoxides if not removed can produce the more reactive hydroxyl free radical, which can lead to oxidative stress. Superoxide anions can also react with nitric oxide to form peroxynitrite which can produce both hydroxyl radicals as well as nitrotyrosine, resulting in both oxidative and nitrosative stress.
The increase in total glutathione levels was the most pronounced antioxidant response after 6h of TSD, and may account for the ability of rats to compensate for any deficit in working memory and/or increased exploratory behavior. Cruz-Aguado et al [3] reported that diethylmaleate (DEM)-treated rats, who had reduced total glutathione levels, exhibited a motivational or sensorimotor deficit, leading to a reduction in their exploratory or locomotor behavior. These authors [3] further showed that glutathione depletion did not influence performance of animals in the passive avoidance test, although it resulted in impaired spatial acquisition but not retention in the Morris water maze. Dean et al [5] similarly reported that 2-cyclohexene-1-one (CHX)-treated rats and mice, who had reduced total glutathione levels, showed disruption of short term spatial memory.. These findings support our hypothesis that increased total glutathione levels may prevent deficits in working memory in rats subjected to 6h of TSD.
Here we show increased antioxidant responses in rats subjected to short term (6h) TSD, however, we previously reported decreased antioxidant responses in rats exposed to long term (5–11 days) TSD [20]. We propose that acute (short term) sleep loss increases the production of free radicals which then induces the antioxidant responses. Chronic (long term) sleep loss further increases the levels of free radicals, and the elevated antioxidant responses would be incapable of successfully scavenging these enhanced free radicals, resulting in damage to the antioxidative enzymes, thus leading to decreased antioxidant responses. This differential effect of acute and chronic sleep deprivation varies across brain regions. Increased antioxidant responses were observed in the rat cortex, hippocampus, basal forebrain, brainstem and cerebellum with 6h of TSD, while decreased responses were observed in the rat hippocampus and brainstem with 5–11 days of TSD [20]. The brain is not uniformly vulnerable to the effects of sleep deprivation. We speculate that this could be due to the fact that certain neuronal populations may be more susceptible to free radical production as a result of increased activity of these neurons due to prolonged waking. Cirelli [2] showed that transcriptional changes, in the rat cerebral cortex, associated with prolonged sleep loss differed significantly from short term sleep deprivation. She suggested that sleep loss may trigger an oxidative stress response in some brain regions, but the brain is capable of responding to this acute stress effectively and thus prevents oxidative damage, while chronic stress may result in irreversible changes [2].
Changes in antioxidant responses have also been observed with paradoxical sleep deprivation (PSD). D’Almeida et al [4] reported that 96h of PSD significantly decreased GSHt levels in the rat hypothalamus. Decreased GSHt levels and SOD activity as well as higher lipid peroxidation (thiobarbituric acid reactive substances) were also observed in the hippocampus, thalamus and hypothalamus of rats subjected to 96h of PSD [24]. These changes were greater in old (24 months) compared to adult (8 months) rats. On the other hand, increased SOD activity and lower lipid peroxidation was noted in the cortex and brainstem of PSD deprived rats, but these changes were greater in adult compared to old rats [24]. Silva et al [23] showed that 72h of PSD increased the ratio of oxidized/reduced glutathione and increased lipid peroxidation in the mouse hippocampus.
We hypothesize that elevated glucose metabolism, arising from increased energy demands during wakefulness, a period of high neuronal activity, may be a potential source of elevated free radicals. Ikeda et al. [14] proposed that free radicals are produced during wakefulness, a period of high neuronal activity, while Scharf et al., [22] proposed that free radicals may be activated by the depletion of cellular energy occurring during extended wakefulness. We previously reported that chronic TSD (>45h) causes degenerative changes in the rat supraoptic nucleus (SON) of the hypothalamus, a region of high metabolic activity [6]. Gip et al [9] reported that 6h of TSD decreased glycogen levels in the rat cerebellum and hippocampus, while increasing glucose levels in the cortex. They proposed that the regional effects of TSD on brain glycogen and glucose levels may be correlated with differing energy demands.
Hexokinase (HK), the enzyme that catalyzes the initial step in glucose metabolism, is the major factor governing the rate of glucose metabolism. In this study we showed that 6h of TSD increased HK activity in several rat brain regions. Thakkar and Mallick [29] similarly reported that 4 days of PSD, by the flower pot technique, increased HK activity in the rat brainstem, cerebellum and cerebrum. Knull et al [15] reported that intraperitoneal injection of glucose to galactose fed chicks increased HK activity in the cerebellum, while Mayer et al [16] reported that the HK activity of the small intestine of starved rats increased significantly within the first 15 min. of perfusion with 50mM glucose.
We show for the first time that acute (6h) TSD in the rat increases antioxidant responses in multiple brain regions. This may reflect an enhanced production of free radicals, arising in part from elevated glucose metabolism (in the cortex). The absence of antioxidant responses in the hypothalamus, despite an increase in HK activity would suggest that endogenous antioxidants in the rat hypothalamus were sufficient to scavenge the free radicals produced by increased glucose metabolism, resulting from 6h of TSD. Conversely antioxidants, other than SOD, GPx or GSHt, may be involved. Also increased antioxidant responses in the rat brainstem, basal forebrain, hippocampus and cerebellum could be due to free radicals produced from sources other than increased glucose metabolism. Furthermore 6h of TSD increased locomotor and exploratory behavior in a new environment without affecting SAB in the Y maze. Thus acute (6h) sleep loss may trigger mechanisms (like increased antioxidant responses) that prevent initial deterioration in working memory and also lead to increased exploratory behavior.
Acknowledgments
This research was supported by grants HL-060296, HL-41370 and MH-64109 and by the Medical Research Services of the Department of Veteran Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albert I, Cicala GA, Siegel J. The behavioral effects of REM sleep deprivation in rats. Psychophysiology. 1970;6:550–560. doi: 10.1111/j.1469-8986.1970.tb02244.x. [DOI] [PubMed] [Google Scholar]
- 2.Cirelli C. Cellular consequences of sleep deprivation in the brain. Sleep Med Rev. 2006;10:307–321. doi: 10.1016/j.smrv.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Cruz-Aguado R, Almaguer-Melian W, Diaz CM, Lorigados L, Bergado J. Behavioral and biochemical effects of glutathione depletion in the rat brain. Brain Res Bull. 2001;55:327–333. doi: 10.1016/s0361-9230(01)00484-1. [DOI] [PubMed] [Google Scholar]
- 4.D’Almeida V, Lobo LL, Hipólide DC, de Oliveira AC, Nobrega JN, Tufik S. Sleep deprivation induces brain region-specific decreases in glutathione levels. Neuroreport. 1998;9:2853–2856. doi: 10.1097/00001756-199808240-00031. [DOI] [PubMed] [Google Scholar]
- 5.Dean O, Bush AI, Berk M, Copolov DL, van den Buuse M. Glutathione depletion in the brain disrupts short-term spatial memory in the Y maze in rats and mice. Behav Brain Res. 2009;198:258–262. doi: 10.1016/j.bbr.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Eiland MM, Ramanathan L, Gulyani S, Gilliland M, Bergmann BM, Rechtschaffen A, Siegel JM. Increases in amino-cupric-silver staining of the supraoptic nucleus after sleep deprivation. Brain Res. 2002;945:1–8. doi: 10.1016/s0006-8993(02)02448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everson CA, Laatsch CD, Hogg N. Antioxidant defense responses to sleep loss and sleep recovery. Am J Physiol Regul Integr Comp Physiol. 2005;288:R374–R383. doi: 10.1152/ajpregu.00565.2004. [DOI] [PubMed] [Google Scholar]
- 8.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;15:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gip P, Hagiwara G, Sapolsky RM, Cao VH, Heller HC, Ruby NF. Glucocorticoids influence brain glycogen levels during sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1057–R1062. doi: 10.1152/ajpregu.00528.2003. [DOI] [PubMed] [Google Scholar]
- 10.Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 11.Guan Z, Peng X, Fang J. Sleep deprivation impairs spatial memory and decreases extracellular signal-regulated kinase phosphorylation in the hippocampus. Brain Res. 2004;1018:38–47. doi: 10.1016/j.brainres.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 12.Harris ED. Regulation of antioxidant enzymes. FASEB J. 1992;6:2675–2683. doi: 10.1096/fasebj.6.9.1612291. [DOI] [PubMed] [Google Scholar]
- 13.Hughes RN. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci Behav Rev. 2004;28:497–505. doi: 10.1016/j.neubiorev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Ikeda M, Ikeda-Sagara M, Okada T, Clement P, Urade Y, Nagai T, Sugiyama T, Yoshioka T, Honda K, Inoué S. Brain oxidation is an initial process in sleep induction. Neuroscience. 2005;130:1029–1040. doi: 10.1016/j.neuroscience.2004.09.057. [DOI] [PubMed] [Google Scholar]
- 15.Knull HR, Taylor WF, Wells WW. Effects of energy metabolism on in vivo distribution of hexokinase in brain. J Biol Chem. 1973;248:5414–5417. [PubMed] [Google Scholar]
- 16.Mayer RJ, Shakespeare P, Hübscher G. Glucose metabolism in the mucosa of the small intestine. Changes of hexokinase activity during perfusion of the proximal half of rat small intestine. Biochem J. 1970;116:43–48. doi: 10.1042/bj1160043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 18.Morton RK. Methods of extraction of enzymes from animal tissues. Methods Enzymol. 1955;1:25–51. [Google Scholar]
- 19.Pierard C, Liscia P, Philippin JN, Mons N, Lafon T, Chauveau F, Van Beers P, Drouet I, Serra A, Jouanin JC, Beracochea D. Modafinil restores memory performance and neural activity impaired by sleep deprivation. Pharmacol Biochem Behav. 2007;88:55–63. doi: 10.1016/j.pbb.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Ramanathan L, Gulyani S, Nienhuis R, Siegel JM. Sleep deprivation decreases superoxide dismutase activity in rat hippocampus and brainstem. Neuroreport. 2002;13:1387–1390. doi: 10.1097/00001756-200208070-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reimund E. The free radical flux theory of sleep. Med Hypotheses. 1994;43:231–233. doi: 10.1016/0306-9877(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 22.Scharf MT, Naidoo N, Zimmerman JE. Pack AI The energy hypothesis of sleep revisited. Prog Neurobiol. 2008;86:264–280. doi: 10.1016/j.pneurobio.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silva RH, Abílio VC, Takatsu AL, Kameda SR, Grassl C, Chehin AB, Medrano WA, Calzavara MB, Registro S, Andersen ML, Machado RB, Carvalho RC, Ribeiro Rde A, Tufik S, Frussa-Filho R. Role of hippocampal oxidative stress in memory deficits induced by sleep deprivation in mice. Neuropharmacology. 2004;46:895–903. doi: 10.1016/j.neuropharm.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 24.Singh R, Kiloung J, Singh S, Sharma D. Effect of paradoxical sleep deprivation on oxidative stress parameters in brain regions of adult and old rats. Biogerontology. 2008;9:53–62. doi: 10.1007/s10522-008-9124-z. [DOI] [PubMed] [Google Scholar]
- 25.Smith C, Rose GM. Posttraining paradoxical sleep in rats is increased after spatial learning in the Morris water maze. Behav Neurosci. 1997;111:1197–1204. doi: 10.1037//0735-7044.111.6.1197. [DOI] [PubMed] [Google Scholar]
- 26.Somani SM, Husain K. Interaction of exercise training and chronic ethanol ingestion on antioxidant system of rat brain regions. J Appl Toxicol. 1997;17:329–336. doi: 10.1002/(sici)1099-1263(199709)17:5<329::aid-jat452>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 27.Tartar JL, Ward CP, McKenna JT, Thakkar M, Arrigoni E, McCarley RW, Brown RE, Strecker R. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur J Neurosci. 2006;23:2739–2748. doi: 10.1111/j.1460-9568.2006.04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tartar JL, Ward CR, Cordeira JW, Legare SL, Blanchette AJ, McCarley RW, Strecker RE. Experimental sleep fragmentation and sleep deprivation in rats increases exploration in an open field test of anxiety while increasing plasma corticosterone levels. Behav Brain Res. 2009;197:450–453. doi: 10.1016/j.bbr.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thakkar M, Mallick BN. Rapid eye movement sleep-deprivation-induced changes in glucose metabolic enzymes in rat brain. Sleep. 1993;16:691–694. [PubMed] [Google Scholar]
- 30.Tobler I, Borbely AA. The effect of 3-h and 6-h sleep deprivation on sleep and EEG spectra of the rat. Behav Brain Res. 1990;36:73–78. doi: 10.1016/0166-4328(90)90161-7. [DOI] [PubMed] [Google Scholar]
- 31.Yang RH, Hu SJ, Wang Y, Zhang WB, Luo WJ, Chen JY. Paradoxical sleep deprivation impairs spatial learning and affects membrane excitability and mitochondrial protein in the hippocampus. Brain Res. 2008;1230:224–232. doi: 10.1016/j.brainres.2008.07.033. [DOI] [PubMed] [Google Scholar]