Abstract
Aims
Identifying early changes in hemostatic clot function as a result of tissue injury and hypoperfusion may provide important information regarding the mechanisms of traumatic coagulopathy. A combat-relevant swine model was used to investigate the development of coagulopathy during trauma by monitoring hemostatic function during increasing severity of shock.
Methods
Swine were injured (soft tissue + femur fracture) and hemorrhaged while continuously monitoring Oxygen Debt (OD) by indirect calorimetry at the airway. Hemostatic function was assessed by Thrombelastography (TEG), prothrombin time (PT), partial Thromboplastin time (PTT), and fibrinogen concentration and compared before hemorrhage (D0) and during shock when OD= 40 and 80 ml/kg. An instrumented sham group was used for comparison.
Results
N=23 swine (n=18 hemorrhage, n=5 sham) weighing 45+/−6 Kg were studied after removing an average of 34+/−14% of blood volume during hemorrhage. Hgb, Hct, platelet counts, PT and PTT did not change with increasing OD (p>0.05). Fibrinogen was reduced significantly by OD=40 ml/kg (mean diff =−59.9 mg/dl, 95% CI diff [−95.1, −24.6]). TEG parameters representing clot initiation (R) and polymerization (K and Alpha Angle) did not change with increasing OD during shock (p>0.053). Clot strength (MA) was reduced in the hemorrhage group by OD=80 ml/kg (mean diff = −4.1 mm, 95% CI diff [−7.4, −0.8]).
Conclusion
In this swine model of traumatic shock, fibrinogen was significantly reduced and an isolated reduction in clot strength (MA) was found with increasing OD. Fibrinogen consumption and altered platelet function may account for the earliest changes in hemostatic function during traumatic shock.
INTRODUCTION
Hemorrhage is a major contributor to mortality and morbidity in trauma patients and is complicated by the presence of coagulopathy.1 Both post-injury coagulopathy and base deficit are associated with increased morbidity and mortality in trauma patients, and tissue hypoperfusion is an important contributor to the early coagulopathy of trauma. 2,3,4,5,6,7 Anticoagulation with enhanced fibrinolysis mediated by the Protein C pathway has been proposed as an important biochemical mechanism mediating this relationship.8
However, when focusing on hemostatic clot formation, other potential contributors to the anticoagulant and fibrinolytic state described by Brohi et al. become possible.8 Altered platelet count or function, changes in fibrin fiber geometry, or changes in clot structure due to altered fibrin networks each play important roles during clot formation, and impairments of any of these processes have been shown experimentally to change the quality of the formed clot and strongly impact fibrinolysis.9,10,11 Observational data has revealed such early changes in clot quality during trauma when measured by means of Thrombelastography (TEG).12 Therefore, formation of dysfunctional hemostatic clot structure and function may not be just an end-product of altered biochemical processes associated with traumatic coagulopathy, but may also play an active role in the progression of coagulopathy, thus contributing to the vicious cycle of trauma-induced anticoagulation and fibrinolysis. Further investigation into the specific changes in clot quality and function taking place during the early period of traumatic shock are needed in order to define the potentially important contributions of early changes in clot structure and to develop potential countermeasures. This is especially important in settings such as combat where deliberate hypotensive resuscitation is contemplated as a means to reduce hemorrhage. Optimizing clot strength during this period might be considered a potential therapeutic target.
A primary limitation to experimental examination of traumatic coagulopathy has been the lack ofa standard and precise tool to measure severity of hypoperfusion and shock. Base deficit is related to tissue hypoperfusion and clinical studies have used base excess to define the relationship between hypoperfusion and coagulopathy.4,7 However, other comprehensive experimental measurements of oxygen metabolism including total body oxygen consumption (VO2) and Oxygen Debt (OD), achieved via indirect calorimetry, have demonstrated increased accuracy when quantifying injury severity and predicting mortality in experimental models.13 VO2 and/or OD measurements have been applied clinically to trauma patients, and high-risk surgical patients in intensive care units, however direct measures of oxygen metabolism via indirect calorimetry have not been measured alongside hemostatic functional assays in the clinical setting mostly due to technical difficulty obtaining the oxygen transport measurements.14,15
Much mystery still surrounds changes in clotting function taking place in the period after injury and prior to fluid resuscitation. Thus, characterizing changes in clotting function in a model of traumatic hemorrhagic shock at standardized levels of injury severity is a much needed first step towards identifying specific targets for further research.
We characterize the changes in clotting function taking place during the critical period after injury and prior to fluid resuscitation using TEG and an experimental model that allows for robust standardization of injury severity via continuous measurement of oxygen transport. We hypothesize that changes in hemostatic function will be present shortly after injury and will change as shock severity increases.
METHODS
Subjects and Instrumentation
This study was approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee. Immature domestic swine weighing 40–50kg were initially sedated with intramuscular ketamine/xylazine (20mg/kg, 2mg/kg) and anesthesia was induced with intravenous sodium pentathol (10–20 mg/kg). General anesthesia was then maintained using intravenous alpha-chloralose bolus (40–50 mg/kg) followed by continuous infusion (10 mg/kg/hour). Ventilatory muscle paralysis was maintained by pancuronium bromide 0.1mg/kg every 60 minutes. Animals were intubated and mechanically ventilated (iVent201, VersaMed Medical Systems, Inc., Pearl River, NY) at FiO2 21% to maintain an end-tidal CO2 level of 35–40 mmHg during a baseline equilibration period. Room air ventilation was used as means to mimic the combat setting where supplemental oxygen is not readily available. The left carotid artery was cannulated to allow for continuous recording of mean arterial pressure (MAP), and blood gas laboratory sampling. The right internal jugular vein was cannulated with a central venous oximetric catheter (Edwards Life Sciences, Irvine, CA) to allow for continuous monitoring of central venous hemoglobin oxygen saturation (ScvO2), blood gas sampling from the central venous circulation, and coagulation sampling. The left femoral artery was surgically exposed and cannulated to allow rapid arterial hemorrhage.
Indirect calorimetry was used to continuously measure oxygen consumption (VO2) breath-by-breath at the airway. Oxygen Debt (OD) was calculated at a frequency of 200 measurements per minute and expressed in ml/kg as the cummulative difference between VO2 during shock and VO2 at baseline integrated over time before and after active hemorrhage. OD increased or decreased in proportion to the magnitude of the difference between ongoing VO2 measurements vs. the baseline VO2 measurement, and the period of time spent at the current measurement and was displayed as a continuous measurement using integrated software (BIOPAC Systems Inc., Goleta, CA). Systemic blood gases and lacate measurements were made using the Stat Profile Critical Care Xpress bedside analyzer (Nova Biomedical Corp., Waltham, MA). Hematocrit (Hct), hemoglobin (Hgb), white blood cell (WBC) and platelet counts (PLT) were obtained using the VetScan HM2 Hematology System, bedside analyzer (Abaxid, Union City, CA). The Prothrombin time PT, Partial Thromboplastin Time (aPTT), and fibrinogen concentration were analyzed in platelet poor plasma by routine laboratory techniques using the Start-4 coagulation analyzer (Diagnostica Stago, Asnières, France). Hemostatic function was determined by Thrombelastography (TEG) (Haemoscope Corporation, Niles, IL). An aliquot of 340 μL of citrated whole blood was recalcified with 20 μL of 0.2M calcium chloride to initiate coagulation. The TEG parameters measured included the R-time (reaction time), which is a measure of the time it takes for clotting to start; the K-time (kinetics time), which is an indicator of how fast the strength of the clot increases once clotting has begun; and the MA (maximum amplitude), which is an indicator of the maximum structural integrity obtained by the clot.16 All coagulation measurements were performed at 37°C constant temperature. All devices were calibrated as directed by the manufacturers.
Traumatic Hemorrhage Protocol
Following instrumentation and baseline measurements, a captive bolt pistol was used to induce hind limb soft tissue injury and right hind limb mid-shaft femur fracture to simulate multisystem trauma. Mid-shaft femur fracture was confirmed in each animal by direct palpation and at necropsy. Simultaneously, subjects were hemorrhaged rapidly via the femoral artery catheter to an initial goal mean arterial pressure (MAP) of 30±5 mmHg. Hemorrhage was halted at goal MAP and subjects were maintained in shock, thus allowing OD to increase, throughout the remainder of the experiment. Blood samples for perfusion and coagulation analysis were obtained simultaneously from the central venous circulation at 3 time points; baseline prior to hemorrhage (D0), when OD= 40cc/kg (D40), and when OD = 80 cc/kg (D80) in order to standardize severity of injury for each group of samples. Subjects were not resuscitated with intravenous fluids, and were euthanized using intravenous potassium chloride (dose mg/kg) under anesthesia upon completion of the protocol. Body temperature was maintained in the normal porcine range, 38±1°C, by warming blankets and continuously monitored via rectal probe. A sham group instrumented as above but not hemorrhaged was used as controls.
Statistical Analysis
Continuous measurements were normally distributed and described using mean and standard error. Mean values were grouped by predetermined levels of OD (or time in sham controls) and compared for significant differences over time within groups and between experimental and sham groups using two-way repeated measures ANOVA. Tukey Kramer significance adjustment was used when comparing specific individual differences. Significant differences were defined as having a p value < 0.05 or a 95% confidence interval of the paired difference that did not include zero for individual comparisons.
RESULTS
Of 21 total subjects in the hemorrhage group, 18 swine weighing 45±6 Kg survived the initial hemorrhage and were included in analysis after having removed an average of 34±14% of total blood volume during the protocol. Another 5 subjects made up the instrumented sham group in which all steps were identical except traumatic injury and hemorrhage were withheld. Physiologic parameters measured in hemorrhage and sham groups are summarized in Table 1. Direct global measures of oxygen transport and metabolic correlates including OD, lactate, and venous pH changed significantly during traumatic hemorrhage confirming worsening severity of metabolic dysfunction with acidosis during shock (all p < 0.001). None of the same parameters changed significantly over time in the Sham group (ANOVA p > 0.05 for all parameters). Hgb, Hct, and platelet counts did not change over time during traumatic shock, but Hgb was significantly less in the hemorrhage group at OD = 80 ml/kg compared to the sham group (p=0.04). (Table 2.)
Table 1.
Physiologic parameters measured before (Oxygen Debt = 0 ml/kg) and during traumatic hemorrhagic shock (Oxygen Debt 40 and 80 ml/kg).
| Oxygen Debt (ml/kg) | |||
|---|---|---|---|
| Variable, Mean(SE) | 0 | 40 | 80 |
| Oxygen Metabolism/Perfusion | |||
| MAP (mmHg) | |||
| Hem | 109.8(1.8) | 35.5(1.8)*† | 29.6(1.8)*† |
| Sham | 113.8(3.4) | 100.1(3.4) | 102.5(3.4) |
| BE ecf (mol/l) | |||
| Hem | 2.2(0.7) | −0.1(0.7) | −2.1(0.7)* |
| Sham | 2.9(1.4) | 3.6(1.4) | 1.9(1.4) |
| pH | |||
| Hem | 7.4(0.01) | 7.32(0.01)*† | 7.29 (0.01)*† |
| Sham | 7.4(0.01) | 7.41(0.01) | 7.41(0.01) |
| Lactate (mmol/l) | |||
| Hem | 1.2(0.2) | 4.8(0.2)*† | 6.4(0.2)*† |
| Sham | 1.1(0.4) | 1.2(0.4) | 1.2(0.4) |
= different than OD= 0 cc/kg, p value < 0.05,
=different than corresponding Sham sample, p value < 0.05.
Table 2.
Blood cell counts and hematocrit measured before (Oxygen Debt = 0 ml/kg) and during severe traumatic hemorrhagic shock (Oxygen Debt = 80 ml/kg).
| Oxygen Debt (cc/kg) | ||
|---|---|---|
| Variable, Mean(SE) | 0 | 80 |
| Cell Counts | ||
| WBC (10^9/l) | ||
| Hem | 13.2(1.0) | 13.2(1.0) |
| Sham | 13.7(1.9) | 13.8(1.9) |
| Hgb (g/dl) | ||
| Hem | 9.7(0.3) | 8.9(0.3)† |
| Sham | 10.2(0.6) | 10.3(0.6) |
| Hct (%) | ||
| Hem | 29.5(1.0) | 26.9(1.1) |
| Sham | 29.7(2.0) | 26.3(2.0) |
| Platelet (10^9/l) | ||
| Hem | 299.0(25.5) | 261.9(27.3) |
| Sham | 265.8(51.1) | 360.8(51.1) |
WBC = white blood cell count, Hgb = hemoglobin concentration, Hct = hematocrit,
=different than corresponding Sham sample, p value < 0.05.
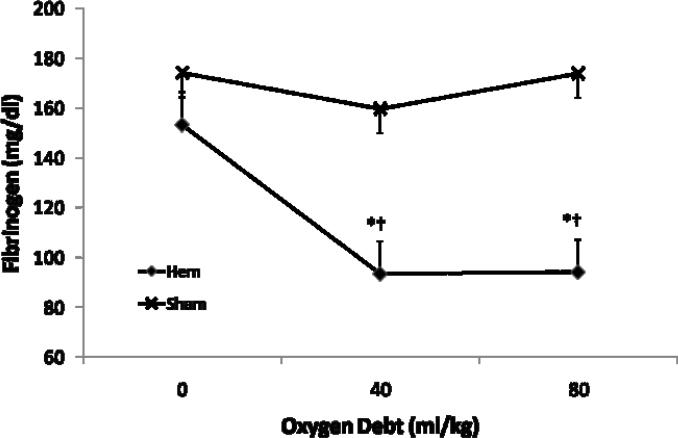
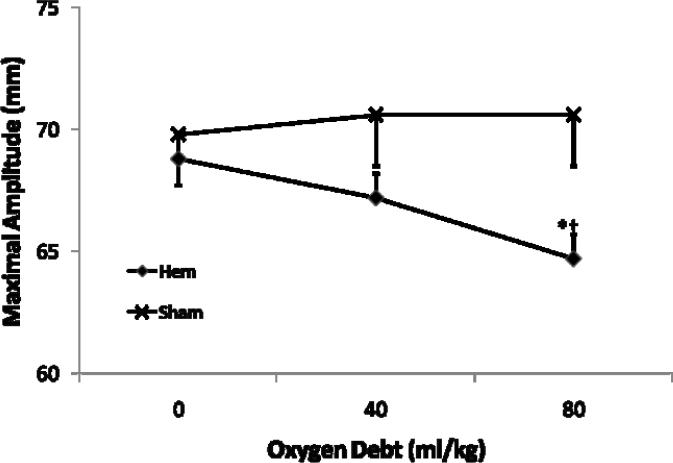
Coagulation changes during traumatic shock are summarized in Table 3. PT and PTT did not change significantly during shock and were not different between groups (p > 0.21). Fibrinogen concentration fell rapidly during early shock in the hemorrhage group becoming significantly reduced by D40 (mean diff D0 vs D40 = −59.9 mg/dl, 95% CI diff [−95.1, −24.6]) and remained so until completion of the protocol. (Fig. 1) There was no significant further reduction in fibrinogen levels between D40 and D80. Of the TEG parameters measured; clot initiation (R time), clot polymerization (K time), and clotting angle (Ang) did not vary significantly over time in the hemorrhage or sham groups (p > 0.053). However, clot strength (MA) became significantly decreased in the hemorrhage group by D80 (mean diff D0 vs. D80 = −4.1 mm, 95% CI diff [−7.4, −0.8]). (Fig. 2)
Table 3.
Coagulation parameters measured before (Oxygen Debt = 0 ml/kg) and during traumatic hemorrhagic shock (Oxygen Debt 40 and 80 ml/kg).
| Oxygen Debt (ml/kg) | |||
|---|---|---|---|
| Variable, Mean(SE) | 0 | 40 | 80 |
| Coagulation | |||
| PT (sec) | |||
| Hem | 13.0(0.5) | 13.8(0.6) | 13.3(0.5) |
| Sham | 13.8(0.6) | 13.0(0.6) | 12.6(0.6) |
| PTT (sec) | |||
| Hem | 26.2(1.7) | 25.4(2.0) | 23.7(1.9) |
| Sham | 25.5(2.2) | 22.5(2.0) | 22.3(2.0) |
| Fibrinogen (mg/dl) | |||
| Hem | 153.3(8.3) | 93.4(9.9)*† | 94.1(8.8)*† |
| Sham | 174.1(11.6) | 159.6(13.0) | 173.8(13.0) |
| Thrombelastrography | |||
|---|---|---|---|
| R Time (min) | |||
| Hem | 5.3(0.4) | 4.4(0.5) | 5.2(0.4) |
| Sham | 4.5(1.0) | 4.9(0.7) | 4.6(0.7) |
| K Time (min) | |||
| Hem | 1.5(0.1) | 1.3(0.1) | 1.5(0.1) |
| Sham | 1.2(0.2) | 1.2(0.2) | 1.3(0.2) |
| Ang (deg) | |||
| Hem | 70.3(1.3) | 73.0(1.5) | 69.5(1.4) |
| Sham | 75.1(3.0) | 74.2(2.4) | 73.8(2.3) |
| MA (mm) | |||
| Hem | 68.8(0.9) | 67.2(1.0) | 64.7(0.9)*† |
| Sham | 69.8(2.1) | 70.6(1.6) | 70.6(1.6) |
PT= Prothrombin Time, PTT= Partial Thromboplastin Time, Thrombelastography parameters; R= Clot initiation time, K = fibrin polymerization time, Ang = rate of fibrin polymerization/cross linking, MA = Maximal Amplitude of deflection representing clot strength as sum of fibrin network strength and platelet contraction.
= different than OD= 0 cc/kg, p value < 0.05,
=different than corresponding Sham sample, p value < 0.05
Figure 1.
Effect of severe hemorrhagic shock on blood Fibrinogen concentration (Mean +/− SE) before hemorrhage (Oxygen Debt = 0 cc/kg) and after induction of severe hemorrhagic shock (Oxygen Debt = 40 and 80 cc/kg). Hemorrhage group N= 18 subjects, Sham N= 5 subjects. *= different than OD= 0 cc/kg, p value < 0.05, †=different than corresponding Sham sample, p value < 0.05
Figure 2.
Effect of severe hemorrhagic shock on clot strength by Thrombelastography (TEG) Maximal Amplitude (Mean +/− SE) before hemorrhage (Oxygen Debt = 0 cc/kg) and after induction of severe hemorrhagic shock (Oxygen Debt = 40 and 80 cc/kg). Hemorrhage group N= 18 subjects, Sham N= 5 subjects. *=different than OD= 0 cc/kg, p value < 0.05. †=different than corresponding Sham sample, p value < 0.05
DISCUSSION
Our results suggest that primary changes in hemostatic function begin shortly after onset of traumatic hemorrhagic shock and continue to change according to the severity of shock. The earliest change in hemostatic function found was a reduction of clot strength. Consumption of fibrinogen combined with possible alterations in platelet function may contribute to the noted reduction in clot strength.
As expected, individual measures of systemic oxygen metabolism, perfusion, and anaerobic metabolism were highly correlated with increasing OD, indicating that OD did successfully describe a state of supply-depend hemorrhagic shock in our model. Oxygen debt (OD) is a complex measurement intended to quantify the cumulative degree of metabolic derangement having occurred during a period of supply-dependent shock. OD is affected by both the duration of shock and the severity of shock and, therefore, provided a precise estimate of shock severity as reflected by the lack of significant variability in corresponding metabolic indicators such as lactate and base excess at each preselected level of OD. Similar standardization of injury severity is not possible in traditional volume-or pressure-controlled models of hemorrhage as thehemorrhage volumes and blood pressures necessary to produce a standard OD among individuals is too variable reflecting individual compensatory responses to volume loss.17,13,18
The reason for such an early and dramatic fall in fibrinogen concentration in our study remains unclear. Fibrinogen may become lost in the initial hemorrhage volume, consumed primarily at local sites of injury by thrombin-induced coagulation, or sequestered by binding to activated platelets and deposited in microcirculatory tissue beds.19,20 However, we did not appreciate the simultaneous reduction in platelet count that has been typically seen during hemorrhagic shock by other investigators, making increased sequestration by platelets less likely.21 Acidosis has also been shown to increase fibrinogen consumption in a swine model and should be considered as a potentially important contributor to the witnessed fibrinogen consumption in our model.22 The behavior of fibrinogen and blood pH were quite similar in that the greatest change in both parameters took place between the D0 and D40 measurements, with a relative leveling-off thereafter. This effect may be specific to fibrinogen since no other estimates of hemostasis by PT, PTT, or TEG `R' time behaved similarly. The cause of the rapid consumption of fibrinogen is likely multifactorial and has been attributed to a “coagulation process” in experimental studies which have used heparin to successfully inhibit fibrinogen consumption during hemorrhagic shock.23
The significant loss of fibrinogen during shock may be directly related to the observed reduction in clot strength during severe shock. Clot strength (MA), as measured by TEG, is determined by multiple factors. During the process of clot formation, fibrin clot structure is first determined by the amount of thrombin generated, the amount of available fibrinogen, the rate of fibrin polymerization, pH, and degree of cross-linking.24,25,26,27 The clot then acquires its final functional properties after contraction by platelets.28,29 The amount of available fibrinogen alone can directly affect clot strength in the trauma population as demonstrated by Rugeri et. al. using rotational thromboelastography with platelet inhibition (FIBTEM).30 This functional test, in which platelet contraction is inhibited using cytochalasin D, leaves only the developing fibrin network to influence overall clot strength. This test was used to find a strong correlation between a reduction in clot strength by FIBTEM and falling fibrinogen concentration in a small sample of trauma patients.30 Similar early abnormalities affecting only TEG clot polymerization (K time) and clot strength (MA) were noted in a small retrospective review of penetrating injuries in the combat setting.31 In addition, experimental results similar to ours were also found in the normothermic arm of a swine hemorrhage study by Martini et al. that demonstrated a primary effect of hemorrhagic shock on clot strength (MA) and fibrinogen concentration prior to onset of other clotting abnormalities.32 Martini et al. has also found that the reduction in fibrinogen concentration during hemorrhage is a result of increased metabolic breakdown without an increase in synthesis and that increased fibrinogen breakdown corresponds with an isolated loss of clot strength by TEG.21 Furthermore, qualitative changes in clot fibrin structure can also directly impact clot function. Clots that are made up of thinner fibrin fibers with more cross-links are reported to have increased elastic modulus and are more resistant to fibrinolysis, while clots made up of larger fibers with fewer cross-links, which are characteristic of a reduced fibrinogen concentration, demonstrate lower elastic modulus and faster lysis.33,34 Changes in fibrinogen concentration and its effect on clot elastic modulus have also been detected in a mixed population of critically ill and injured patients and elastic modulus appears to vary linearly over a range of fibrinogen concentrations independent of platelet count.35,36 The changes in fibrinogen concentration noted in our study are likely to have contributed to the reduction in clot strength by TEG. Therefore, softer clots formed during shock may predispose them to enhanced rates of dissolution when exposed to lytic conditions. Importantly, the early reductions in clot strength that we noted occurred prior to any changes in clotting times, suggesting that early changes in clot quality and function may actually precede and possibly speed the onset of the hyperfibrinolytic state described by Brohi et al.7 These early changes in clot quality taking place prior to fluid resuscitation may be important to consider when developing further theories of traumatic coagulopathy. This is potentially critical considering the practice and debate of fluid-restricted resuscitations.
Changes in platelet-induced clot retraction may have also contributed our observed reduction of clot strength during shock. When examining the period between OD40 and OD80, it is obvious that MA continued to decline while fibrinogen concentration remained stable. This suggests that additional factors related to the development of MA may be affected during severe shock. Clot initiation time (R), as a reflection of thrombin generation, platelet counts, and hematocrit did not change significantly during this time period. Therefore, reduced platelet contraction causing impaired clot retraction or changes in fibrin network cross-linking remain as potential mediators of the noted reduction in clot strength between D40 and D80. However, TEG alone has limited ability to detect isolated changes in platelet activity and poorly discriminates between fibrinolysis and clot retraction without incorporation of control samples utilizing platelet inhibitors.37 The lack of simultaneous TEG data incorporating platelet inhibitors leaves us unable to definitively include a primary platelet effect on clot strength during hemorrhage.
Any conclusions drawn from our study should be tempered by several inherent limitations. First, the coagulation system of swine is known to be more reactive than that of humans and may have impacted our results.38 The model also does not allow for evaluation of clotting function in response to isolated hemorrhage without tissue trauma. The addition of severe tissue trauma and long-bone fracture in combination with severe hemorrhage likely exaggerated the coagulopathy produced due to increased factor consumption at the various sites of injury. However, we believe this to be a more clinically relevant model over hemorrhage alone. Overall, the design of the study is comparative in nature and should only be hypothesis generating. In keeping with such design, we are unable to draw mechanistic or causative conclusions from the data.
CONCLUSION
In this experimental model, traumatic hemorrhagic shock induced an early and isolated reduction in functional clot strength as measured by TEG. Changes in fibrinogen concentration and platelet function may play significant roles in the noted loss of clot strength. Further study of the early coagulopathy of trauma should include the contributions of fibrinogen metabolism and platelet function as important possible mediators of pathologic clotting function.
ACKNOWLEDGEMENTS
The study was sponsored in part by Prolong Pharmaceuticals (Monmouth, NJ) however; the sponsor was not involved in study design, data collection or analysis, or writing of the manuscript. N. White is supported in part by NIH training grant GM008695-09. The authors would like to thank the staff of the VCURES shock laboratory and VCU Coagulation Advancement laboratory for their efforts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST STATEMENT: The authors including NJW, EJM, DFB, and KRW have no potential conflicts of interest to disclose regarding personal or financial relationships related to this study.
REFERENCES
- 1.Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–193. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Macleod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early Coagulopathy Predicts Mortality in Trauma. J Trauma. 2003;55:39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 3.Siegel JH, Rivkind AI, Dalal S, Goodarzi S. Early physiologic predictors of injury severity and death in blunt multiple trauma. Arch Surg. 1990;125:498–508. doi: 10.1001/archsurg.1990.01410160084019. [DOI] [PubMed] [Google Scholar]
- 4.Rutherford EJ, Morris JA, Reed GW, Hall KS. Base deficit stratifies mortality and determines therapy. J Trauma. 1992;33:417–423. doi: 10.1097/00005373-199209000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Davis JW, Parks SN, Kaups KL, Gladen HE, O'Donnell-Nicol S. Admission base deficit predicts transfusion requirements and risk of complications. J Trauma. 1996;41:769–774. doi: 10.1097/00005373-199611000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Eberhard LW, Morabito DJ, Matthay MA, et al. Initial severity of metabolic acidosis predicts the development of acute lung injury in severely traumatized patients. Crit Care Med. 2000;28:125–131. doi: 10.1097/00003246-200001000-00021. [DOI] [PubMed] [Google Scholar]
- 7.Brohi K, Cohen MJ, Ganter MT, et al. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2008;64:1211–7. doi: 10.1097/TA.0b013e318169cd3c. discussion 1217. [DOI] [PubMed] [Google Scholar]
- 8.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF. Traumatic Coagulopathy: Initiated by hypoperfusion. Modulated through the protein C pathway? Annals of Surgery. 2007;245(5):812–18. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carr ME, Alving BM. Effect of fibrin structure on plasmin-mediated dissolution of plasma clots. Blood Coagulation and Fibrinolysis. 1995;6:567–573. doi: 10.1097/00001721-199509000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Katori N, Tanaka KA, Szlam F, Levy JH. The effects of platelet count on clot retraction and tissue plasminogen activator-induced fibrinolysis on thrombelastography. Anesth Analg. 2005;100:1781–5. doi: 10.1213/01.ANE.0000149902.73689.64. [DOI] [PubMed] [Google Scholar]
- 11.Collet JP, Park D, Lesty C, Soria J, Montalescot G, Weisel JW. Influence of fibrin network conformationand fibrin fiber diameter on fibrinolysis speed. Arterioscler Thromb Vasc Biol. 2000;20:1354–1361. doi: 10.1161/01.atv.20.5.1354. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann CR, Dwyer KM, Crews JD, Dols SJ, Trask AL. Usefulness of thrombelastography in assessment of trauma patient coagulation. The Journal of trauma. 1997;42(4):716–20. doi: 10.1097/00005373-199704000-00023. discussion 720. [DOI] [PubMed] [Google Scholar]
- 13.Roesner JP, Koch A, Bateman R, et al. Accurate and continuous measurement of oxygen deficit during haemorrhage in pigs. Resuscitation. 2009;80(2):259–63. doi: 10.1016/j.resuscitation.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Peerless JR, Epstein CD, Martin JE, Pinchak AC, Malangoni MA. Oxygen consumption in the early postinjury period: use of continuous, on-line indirect calorimetry. Crit Care Med. 2000;28:395–401. doi: 10.1097/00003246-200002000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Shoemaker WC, Appel PL, Kram HB. Role of oxygen debt in the development of organ failure sepsis, and death in high-risk surgical patients. Chest. 1992;102:208–215. doi: 10.1378/chest.102.1.208. [DOI] [PubMed] [Google Scholar]
- 16.Hartert H, Schaeder JA. The physical and biologic constants of thromboelastography. Biorheology. 1962;1:31–9. [Google Scholar]
- 17.Rixen D, Raum M, Holzgraefe B, Sauerland S, Nagelschmidt M, Neugebauer EA. A pig hemorrhagic shock model: oxygen debt and metabolic acidemia as indicators of severity. Shock. 2001;16(3):239–44. doi: 10.1097/00024382-200116030-00012. [DOI] [PubMed] [Google Scholar]
- 18.Dunham CM, Fabian M, Siegel JH, Gettings L. Relationship of plasma amino acids to oxygen debt during hemorrhagic shock. Circulatory shock. 1991;35(2):87–95. [PubMed] [Google Scholar]
- 19.Eichhorn ME, Ney L, Massberg S, Goetz AE. Platelet kinetics in the pulmonary microcirculation in vivo assessed by intravital microscopy. Journal of Vascular Research. 2002;39(4):330–9. doi: 10.1159/000065545. [DOI] [PubMed] [Google Scholar]
- 20.Blomquist S, Thorne J, Elmr O. Different effects of bleeding and soft-tissue trauma on pulmonary platelet trapping in pigs. The Journal of trauma. 1989;29(6):866–72. doi: 10.1097/00005373-198906000-00027. [DOI] [PubMed] [Google Scholar]
- 21.Martini WZ, Chinkes DI, Pusateri AE, Holcomb JB, Yu YM, Zhang XJ, Wolfe RR. Acute changes in fibrinogen metabolism and coagulation after hemorrhage in pigs. Am J Physiol Endocrinol Metab. 2005;289:E930–E934. doi: 10.1152/ajpendo.00137.2005. [DOI] [PubMed] [Google Scholar]
- 22.Martini WZ, Holcomb JB. Acidosis and coagulopathy: the differential effects on fibrinogen synthesis and breakdown in pigs. Annal Surg. 2007;246(5):831–835. doi: 10.1097/SLA.0b013e3180cc2e94. [DOI] [PubMed] [Google Scholar]
- 23.Leandoer L, Applegren L, Bergentz SE. Fibrinogen turnover after massive haemorrhage in heparinized dogs. Europ. Surg. Res. 1969;1:115–129. doi: 10.1159/000127467. [DOI] [PubMed] [Google Scholar]
- 24.Wolberg AS, Monroe DM, Roberts HR, Hoffman M. Elevated prothrombin results in clots with altered fiber structure: as a possible mechanism of the increased thrombotic risk. Hemostasis, Blood. 2003;101:3008–3013. doi: 10.1182/blood-2002-08-2527. [DOI] [PubMed] [Google Scholar]
- 25.Carr ME, Martin EJ, Carr SL. Delayed, reduced or inhibited thrombin production reduces platelet contractile force and results in weaker clot formation. Blood Coagulation and Fibrinolysis. 2002;13:193–197. doi: 10.1097/00001721-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Blomback B, Masahisa O. Fibrin gel structure and clotting time. Thromb. Res. 1982;25:51–70. doi: 10.1016/0049-3848(82)90214-6. [DOI] [PubMed] [Google Scholar]
- 27.Lorand L. Factor., XIII Structure, activation, and interactions with fibrinogen and fibrin. Annals N.Y. Academy of Sciences. 2000:291–311. doi: 10.1111/j.1749-6632.2001.tb03516.x. [DOI] [PubMed] [Google Scholar]
- 28.Jen CJ, McIntire LV. The Structural Properties and Contractile Force of a Clot. Cell Motility. 1982;2:445–455. doi: 10.1002/cm.970020504. [DOI] [PubMed] [Google Scholar]
- 29.Carr ME. Development of Platelet Contractile Force as a Research and Clinical Measure of Platelet Function. Cell Biochemistry and Biophysics. 2003;38:55–78. doi: 10.1385/CBB:38:1:55. [DOI] [PubMed] [Google Scholar]
- 30.Rugeri L, Levrat A, David JS, et al. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. J. Thrombosis Hemostasis. 2006;5:289–295. doi: 10.1111/j.1538-7836.2007.02319.x. [DOI] [PubMed] [Google Scholar]
- 31.Plotkin AJ, Wade CE, Jenkins DH, et al. A reduction in clot formation rate and strength assessed by thrombelastography is indicative of transfusion requirements in patients with penetrating injuries. The Journal of trauma. 2008;64(2 Suppl):S64–8. doi: 10.1097/TA.0b013e318160772d. [DOI] [PubMed] [Google Scholar]
- 32.Martini WZ, Cortez DS, Dubick MA, Park MS, Holcomb JB. Thrombelastography is better than PT, aPTT, and activated clotting time in detecting clinically relevant clotting abnormalities after hypothermia, hemorrhagic shock and resuscitation in pigs. The Journal of trauma. 2008;65(3):535–43. doi: 10.1097/TA.0b013e31818379a6. [DOI] [PubMed] [Google Scholar]
- 33.Carr ME, Carr SL. Fibrin structure and concentration alter clot elastic modulus but do not alter platelet mediated force development. Blood Coagulation and Fibrinolysis. 1994;6:79–86. doi: 10.1097/00001721-199502000-00013. [DOI] [PubMed] [Google Scholar]
- 34.Carr ME, Alving BM. Effect of fibrin structure on plasmin-mediated dissolution of plasma clots. Blood coagulation & fibrinolysis. 1995;6(6):567–73. doi: 10.1097/00001721-199509000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Dempfle C, Klsch T, Elmas E, et al. Impact of fibrinogen concentration in severely ill patients on mechanical properties of whole blood clots. Blood coagulation & fibrinolysis. 2008;19(8):765–70. doi: 10.1097/MBC.0b013e32830f1b68. [DOI] [PubMed] [Google Scholar]
- 36.Lang T, Johanning K, Metzler H, et al. The effects of fibrinogen levels on thromboelastometric variables in the presence of thrombocytopenia. Anesthesia & analgesia. 2009;108(3):751–8. doi: 10.1213/ane.0b013e3181966675. [DOI] [PubMed] [Google Scholar]
- 37.Bowbrick VA, Mikhailidis DP, Stansby G. Value of thromboelastography in the assessment of platelet function. Clin/Appl Thrombosis and Hemostasis. 2003;9(2):137–142. doi: 10.1177/107602960300900208. [DOI] [PubMed] [Google Scholar]
- 38.Velik-Salchner C, Schnrer C, Fries D, et al. Normal values for thrombelastography (ROTEM) and selected coagulation parameters in porcine blood. Thrombosis Research. 2006;117(5):597–602. doi: 10.1016/j.thromres.2005.05.015. [DOI] [PubMed] [Google Scholar]