Abstract
Holoprosencephaly (HPE), the most common developmental disorder of the human forebrain, is occasionally associated with the spectrum of agnathia, or virtual absence of the mandible. This condition results in a constellation of structural cerebral and craniofacial abnormalities. Here we present two new patients and review 30 patients from the literature with HPE and variants of agnathia. The majority of these patients are female and have the most severe forms of HPE, with cyclopia present more frequently than is usually observed in cohorts of patients with HPE. Also, many patients have additional clinical findings not typical in patients with classic HPE, particularly situs abnormalities. Recent animal studies suggest that the association of HPE and agnathia may relate to alterations in signaling from forebrain and foregut endoderm organizing centers and subsequent first pharyngeal arch development, although present models are inadequate to explain all of the clinical findings of this enigmatic human syndrome. Further research is required to better elucidate the causal and pathogenic basis of this association.
Keywords: holoprosencephaly, HPE, agnathia, dysgnathia, otocephaly, first pharyngeal arch, first branchial arch, situs inversus
INTRODUCTION
Holoprosencephaly (HPE) is the most common developmental disorder of the developing forebrain in humans. In addition to differences in cerebral structure, a select number of patients also present with craniofacial differences in the spectrum of malformations, ranging from isolated agnathia to otocephaly. The prevalence of agnathia spectrum in cohorts of patients with HPE ranges from 0.8% (1/121) [Croen et al., 1996] to 10% (3/30) [Blaas et al., 2002].
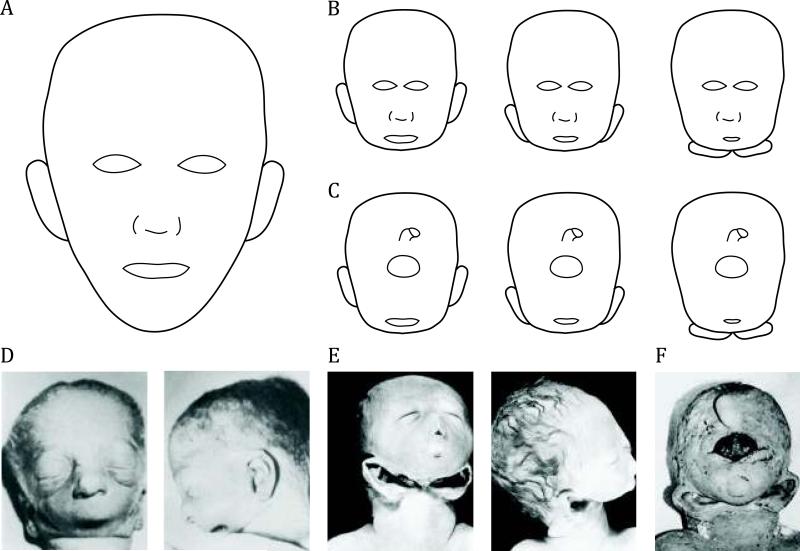
The spectrum of agnathia ranges from isolated agnathia, or virtual absence of the mandible, to otocephaly, which refers to a broader malformation of mandibular hypoplasia or agnathia, downward displacement of the ears and/or synotia (approximation of the ears in the midline), with or without aglossia (no tongue), and microstomia (small mouth) [Petrikovsky, 1999]. These can be isolated findings or they can be associated with HPE-associated manifestations, ranging from mild phenotypes to the relatively severe form of alobar HPE with cyclopia. (See Cohen review for more information on classic HPE, this issue.) “Dysgnathia complex” may also be used to describe findings similar to otocephaly, which may present with variable degrees of severity. Dysgnathia complex often results in fatal respiratory failure due to airway obstruction [Baker et al., 2004]. (See Figure 1 for illustration and photographs of agnathia spectrum.) The mildest end of the spectrum of agnathia, micrognathia or a small mandible, may occur with HPE [Cohen and Sulik, 1992], however patients with simply micrognathia are not included in this review.
Fig. 1.
Schematic representations (A-C) and photographic depictions (D-F) of patients with holoprosencephaly (HPE) and features of agnathia spectrum. Agnathia (virtual absence of the mandible) can be an isolated finding or associated with a broader syndrome of otocephaly, which includes mandibular hypoplasia or agnathia, downward displacement of the ears and/or synotia (approximation of the ears in the midline), with or without aglossia (no tongue) and microglossia (small mouth) [Petrikovsky, 1999]. A: Schematic normal craniofacial structure. B: Agnathia spectrum and mild HPE facial findings, shown here with hypotelorism. Progression, from left to right, shows isolated agnathia followed by otocephaly with low-set ears followed by ears fused in the midline. One or two nares may be present, nasal and oral orifices may be absent or not patent, and ocular orbits may be spaced normally (not shown). C: Agnathia and severe HPE with cyclopia (single ocular orbit, depicted as an oval here) and a proboscis (nose-like appendage, depicted as cylinder-like structure here) shown above the single orbit. A similar progression from agnathia to otocephaly is shown. A proboscis is commonly seen with cyclopia, and it may be seen below the eyes or be absent (not shown). Synophthalmia (two fused ocular globes in one orbit) may also occur. D: Photographs of Patient 10 with normal orbital placement, downslanting palpebral fissures, two patent nares, microstomia, a hypoplastic tongue, and agnathia. Photograph courtesy of Pauli et al. [1983], reprinted with permission of Wiley-Liss, Inc. a subsidiary of John Wiley & Sons, Inc. E: Photographs of Patient 13 with downslanting palpebral fissures, single incompletely patent nasal passage, midfacial hypoplasia, low set and posteriorly rotated ears, rudimentary mouth, absent mandible, and hypoplastic maxilla, palate, and tongue. Photograph courtesy of Leech et al. [1988], reprinted with permission of Wiley-Liss, Inc. a subsidiary of John Wiley & Sons, Inc. F: Photograph of Patient 15 with synophthalmia, a midline frontal proboscis, a chin-like structure with a blind ending cavity, agnathia, and ears simple and fused in the midline. Photograph adapted from Meinecke et al. [1990], reprinted with permission of Wiley-Liss, Inc. a subsidiary of John Wiley & Sons, Inc.
A subset of patients with HPE and agnathia presents with situs abnormalities, which are disturbances of left-right embryonic patterns, showing unusual spatial arrangements of thoracic and/or abdominal organs in relationship to one another. Given that situs terminology may not be used consistently, we will use the following clarification of terms, based on Ware and Belmont [2004]: situs solitus refers to normal left-right anatomical arrangement of organs in the thorax and abdomen. Situs inversus refers to complete reversal or a mirror-image of structures in the thorax and abdomen, maintaining typical asymmetry. Situs ambiguus refers to discordance of right and left patterns which are ordinarily asymmetric. Other terms that may be used to describe situs ambiguus include heterotaxia, partial situs inversus, and laterality sequence [Ware and Belmont, 2004]. To our knowledge, the first reported child with agnathia, HPE, and situs inversus was described by Leech et al. [1988]. At least five other patients with situs abnormalities have since been reported in the literature.
Historically, patients with agnathia were classified into two basic groups: those with cyclopia and those without cyclopia [Pauli et al., 1981]. Later, four subclassifications were given: agnathia alone, agnathia with holoprosencephaly, agnathia with situs inversus and visceral anomalies, and agnathia with holoprosencephaly and situs inversus with visceral anomalies [Leech et al., 1988]. Agnathia malformation spectrum was then proposed to encompass isolated agnathia in addition to agnathia in conjunction with other anomalies [Persutte et al., 1990].
While no definitive etiology has been elucidated for the association of HPE and agnathia, both hereditary factors, as demonstrated by the occurrence of HPE and agnathia in inbred guinea pigs [Wright, 1933; Wright and Wagner, 1934], and environmental factors have been proposed. HPE-agnathia has further been observed in sheep, mice, and rabbits [reviewed in Hersh et al., 1989]. The underlying defect appears to be related to the prechordal plate and tissues under its direct and indirect control. This includes the prosencephalic portion of the neural tube and related neural crest tissue, which is involved in forming the first pharyngeal or branchial arch [Persutte et al., 1990; Schiffer et al., 2002]. The first pharyngeal arch (the term used when referring to a human embryo) is made up of two processes: the maxillary process, which contributes to formation of the maxilla, premaxilla, zygomatic bones, and portions of the temporal bones, and the mandibular process, which contains Meckel's cartilage. Meckel's cartilage forms the incus and malleus, and then serves as a template for the mandible to form through membranous ossification of surrounding mesenchymal tissue [Sadler, 2000]. Such development may involve signals from an organizing center in the foregut endoderm, as discussed by Andersson et al. [2006]. (See Sulik review for further information on embryology, this issue.) More recently, studies in mice have demonstrated that the Sonic hedghog (Shh) signaling pathway and bone morphogenetic protein (BMP) pathway are involved in forebrain and craniofacial development. Mice deficient in Shh [Chiang et al., 1996] or modifiers of BMPs, such as Chordin, Noggin, and Twisted gastrulation homolog 1 (Twsg1) [Anderson et al., 2002; Petryk et al., 2004], have features that resemble HPE and agnathia in humans.
Here we present 32 patients with both HPE and features of agnathia, which include two new patients and 30 previously reported patients. We then review animal studies to discuss potential molecular etiologies. In addition, there are over 60 other reported patients who may fit into the spectrum; however the data are too incomplete or unobtainable to include in this series [Matsunaga and Shiota, 1977: 10 patients with HPE and “branchial arch anomalies”; 17 non-illustrated patients with cyclopia and agnathia, Keith [1909]; two patients with “agnathia-HPE complex” Okuno et al., [1996]; and a literature review of 37 patients, four of whom met criteria for inclusion here using original sources, Pauli et al. [1981].
CLINICAL FINDINGS
Newly Reported Patients
Patient 31 was a female, born at 24 weeks gestation to a 17-year-old primigravida mother, with a birth weight of 525 grams (~10th centile, based on Fenton, 2003), head circumference of 19.5 cm (3rd-10th centile), and length of 31 cm (10th-50th centile). The patient died perinatally. The pregnancy was uncomplicated, and no medications were used. Physical findings included extreme micrognathia, microstomia, two nares, normal position of the ears with overfolded helices, downslanting palpebral fissures, and camptodactyly of the right fourth and fifth digits. A postmortem total body X-ray showed “extreme micrognathia” and 13 ribs bilaterally. A head and brain MRI showed no mandible, probable choanal atresia, and an abnormal cerebrum with no central sulcus, dorsally fused lateral ventricles, and absent ventral falx cerebri, consistent with semilobar HPE. A full-body MRI showed no evidence of situs inversus. No permission was given for autopsy. Genetic studies included a normal female pattern on karyotype and 105K Agilent array-CGH. No other genetic test results are currently available. The patient's mother and father have no signs of midline defects, with the referring clinicians (NJ, AJvE) reporting no hypotelorism, midface hypoplasia, absent labial frenulum, single central incisor or anosmia, and no micrognathia, retrognathia, or developmental delay.
Patient 32 was a female, born at nearly 35 weeks gestation to a 17-year-old primigravida mother, with a birth weight of 1,141 grams (<3rd centile), head circumference of 24.5 cm (<3rd centile), and length of 37 cm (<3rd centile). The patient died within 12 hours after birth. The pregnancy was significant for polyhydramnios, premature rupture of membranes, and eclampsia necessitating delivery via cesarean section. Physical findings included no palpable fontanelles, a normal hair whorl, hypoplastic supraorbital ridges, downslanting palpebral fissures, proptotic eyes due to absent zygomatic arches and maxilla, agnathia, a single pointed nare with a dimple indicating the site of the mouth, and ears fused in the midline with bilateral preauricular pits. Limbs were notable for right-sided radial absence with an absent thumb, camptodactyly of the remaining right four fingers, and abnormal right palmar creases. The anus was perforate, external genitalia were female, and the back was somewhat hirsute without a normal gluteal crease. A prenatal ultrasound showed a monoventricle, and a sample from an amniocentesis was reported to reveal no cytogenetic abnormalities. XK aprosencephaly was diagnosed after birth. Autopsy revealed a large hydrocephalus that appeared to end in an open third ventricle, a “slightly small” cerebellum, and an absent corpus callosum, in addition to complete situs inversus with the thorax and abdominal contents reversed and additional genitourinary abnormalities. Consanguinity was reported, however the details are not known. (See Tables I and II for further clinical details and Figure 2 for photographs.)
Table I.
Patients with Holoprosencephaly and Features of Agnathia Spectrum
| Pt | Sex | HPE | Agnathia Spectrum | Facial Findings | Other Findings | GA, Death◇ | Genetic Testing | Reference, Pt # |
|---|---|---|---|---|---|---|---|---|
| 1 | M | NS | “Hypognathus” | “Two eyes situated in a single orbit,” proboscides above and below orbit, no mouth, ears horizontal in neck | Anterior fontanelle almost closed | NS | NS | Ballantyne, 1902, p.432 |
| 2 | NS | NS | Agnathia | Cyclopia, proboscis, ears inferiorly placed | NS | NS | NS | Keith, 1909, Fig 14* |
| 3 | NS | NS | Agnathia | Cyclopia, no nose or mouth, low-set ears | NS | NS | NS | Gartner, 1947, #2 |
| 4 | NS | NS | “Hypognathus” | Cyclopia, no nose, rudimentary buccal orifice, ears low-set | NS | NS | NS | Potter and Craig, 1961, Fig 24-43 |
| 5 | F | NS | “Hypognathus” | Cyclopia, no proboscis, astomia, ears almost fused | No other external abnormalities | ~30 wks, fetal demise | NS | Sarma, 1963, #1 |
| 6 | F | NS | Agnathia | Cyclopia, proboscis, ears partially fused, no mouth | Normal internal organs | 35 wks, neonatal | 46,XX | Mollica et al., 1979 |
| 7 | M | Alobar | Agnathia | Cyclopia, proboscis | NS | 40 wks, fetal demise | NS | Jones et al., 1980, #2 |
| 8 | F | Alobar | Agnathia, mandible “absent” | Cyclopia, proboscis, blind-ending ostium, anterior meningocele, ears fused, hypoplastic tongue | TEF, cardiopulmonary anomalies, adrenal hypoplasia, placental villitis | 35 wks, NS | 46,XX | Gaba et al., 1982 |
| 9 | F | Alobar | “Extremely small mandible” | Hypotelorism, microphthalmia, cebocephaly, microstomia, low-set ears | Cardiac, GI, GU defects, small adrenals, pituitary and some salivary glands absent | 26-27 wks, fetal demise | Not tested, see Pt 10 for Sib results | Pauli et al., 1983, #1; Sib of Pt 10 |
| 10 | F | “Agenesis of both olfactory bulbs” | “Extremely hypoplastic [mandible]” | Normal orbits, microstomia, hypoplastic anterior two-thirds of tongue | All salivary glands absent, normal heart and GI, 11 ribs bilaterally | 27 wks, fetal demise | 46,XX,der(18)t(6;18) (p24.1;p11.2)pat | Pauli et al., 1983, #2; Krassikoff and Sekhon, 1989; Overhauser et al., 1995, 1057P; Sib of Pt 9 |
| 11 | F | NS, no olfactory bulbs | Agnathia | Low-set eyes, synotia, astomia, proboscis, no maxilla | No salivary glands, twin-twin transfusion; monochorionic, diamniotic; mild HPE in Twin A | 15 wks, fetal demise | NS | Machin et al., 1985; Twin B |
| 12 | NS | Alobar | Agnathia | Cyclopia | NS | NS | NS | Carles et al., 1987 |
| 13 | M | Alobar | Agnathia | Downslanting palpebral fissures, single nare, low-set ears, hypoplastic maxilla | Complete situs inversus‡, visceral organs normal, 13 ribs bilaterally | 28-29 wks, neonatal | 46,XY,9qh+ | Leech et al., 1988 |
| 14 | F | Hydranencephaly | Agnathia | Cyclopia, astomia, proboscis, large cyst emanating from below proboscis to level of mid-epigastrium | Situs inversus‡, adrenal hypoplasia, aplastic pituitary | 21 wks, fetal demise | 46,XX | Robinson and Lenke, 1989; Persutte et al., 1990 |
| 15 | M | Alobar | Agnathia | Synophthalmia, proboscis, ears low-set and fused, hypoplastic oral cavity with no external opening | Situs inversus‡, cryptorchidism, hypoplastic testes, horseshoe-kidneys | 41 wks, neonatal | 46, XY | Meinecke et al., 1990 |
| 16 | F | Alobar | Agnathia | Severe hypotelorism, prominent ocular globes without lids, proboscis, astomia, ears almost fused in midline | Unilateral post-axial polydactyly, no visceral abnormalities | 25 wks, fetal demise | 46,XX | Rolland et al., 1991 |
| 17 | F | NS | “Severe mandibular hypoplasia” | Hypotelorism, microstomia, synotia, partial cebocephaly, absent nasal bones | GI, GU, cardiopulmonary and limb abnormalities; maternal sodium valproate use until 6-7 wks GA | 28 wks, fetal demise | 46,XX | Ades and Sillence, 1992 |
| 18 | F | Hydranencephaly | Agnathia | Hypertelorism, cleft lip and palate, poorly formed low-set ears, aplasia cutis, abnormal eyelids | GI, GU, cardiopulmonary abnormalities | 18 wks, fetal demise | Not tested, parental karyotypes normal | Porteous et al., 1993, #1; Sib of Pt 19 |
| 19 | NS | NS | Agnathia | Absent eyelids, midline cleft palate, simple low-set ears | Imperforate anus | NS, fetal demise | Not tested, parental karyotypes normal | Porteous et al., 1993, #2; Sib of Pt 18 |
| 20 | NS | Semilobar | Agnathia | NS | NS | NS | NS | Croen et al., 1996 |
| 21 | M | Alobar | Agnathia | Synophthalmia, proboscis, low-set ears, aglossia, microstomia (blind-end) | Situs inversus totalis ‡ | 26 wks, neonatal | 46,XY | Ozden et al., 2000 |
| 22 | M | Alobar | Agnathia | Eyes “not identified,” otocephaly, “tumor-like proboscis” | None | 30+ wks, fetal demise | 46,XY | Blaas et al., 2002, #15 |
| 23 | F | Alobar | Agnathia | Hypotelorism, otocephaly, proboscis, cystic enlargement of pharynx/larynx | Defects related to being dicephalus dipus tribrachius conjoined twin; twin's craniofacial structure normal | 20+ wks, fetal demise | 46,XX | Blaas et al., 2002, #16 |
| 24 | M | Anencephaly | Agnathia | Cyclopia, “two proboscides,” otocephaly | None | 13+ wks, fetal demise | 46,XY | Blaas et al., 2002, #30 |
| 25 | M | Alobar | Agnathia | Cyclopia, ears fused at midline, no external opening to oral cavity, no nasal cavity | Situs inversus partialis‡, heart normal | 34 wks, neonatal | 46,XY | Ozden et al., 2002 |
| 26 | M | NS | Agnathia | Cyclopia, proboscis, fused zygomatic bones, fused ears | Trigonocephalic microcephaly, “protruding red-brown brain substance,” pes equino-varus | “Term,” neonatal | NS | Schiffer et al., 2002, #3 |
| 27 | F | Alobar | Agnathia | Cyclopia, proboscis, astomia | Isolated TEF, +consanguinity | 37 wks, NS | 46,XX | Sezgin et al., 2002 |
| 28 | F | Alobar | Agnathia | Hypotelorism, melotia, cleft lip and palate, single nare | “Nearly complete” situs inversus‡ | 31 wks, neonatal | 46,XX | Faye-Petersen et al., 2006, #2 |
| 29 | F | Lobar | Agnathia | Hypotelorism, microstomia, hypoglossia, asymmetric microtia with synotia, right anophthalmia | Hypoplastic hypopharynx, isolated atrial septal defect, small adrenals | 29 wks, neonatal | 46,XX | Faye-Petersen et al., 2006, #5 |
| 30 | NS | NS | Agnathia | NS | NS | NS | NS | Orioli and Castilla, 2007, #52406 |
| 31 | F | Semilobar | “Extreme micrognathia” | Down-slanting palpebral fissures, microstomia, normal position of ears | Camptodactyly, probable choanal atresia | 24 wks, NS | 46,XX; normal microarray | This report |
| 32 | F | XK aprosencephaly | Agnathia | Proptotic eyes, single nare, no mouth, ears fused in midline, absent zygomatic arches and maxilla | Situs inversus‡, absent right radius and thumb, maternal eclampsia, +consanguinity | ~35 wks, neonatal | Normal FISH of amniocentesis | This report |
Fetal demise includes stillbirths, spontaneous abortions, and terminations of pregnancy; all neonatal deaths occurred by 24 hours.
Paper reports 18 total patients with cyclopia and agnathia, however clinical illustration and description were only available for this one.
Situs inversus terminology is used as reported by original references; situs findings and cardiac variants further described in Table II.
GA = gestational age; GI = gastrointestinal system; GU = genitourinary system; NS = Not Specified; TEF = tracheoesophageal fistula.
Table II.
Patients with Situs Abnormalities and Cardiac Variants
| Pt | Sex | Cardiac Findings‡ | Other Findings‡ | Reference, Pt # | |
|---|---|---|---|---|---|
| SITUS * | |||||
|
Situs inversus - Thorax and abdomen reversed |
13 | M | -Dextrocardia -Heart otherwise normal with pattern of coronary arteries reversed and aortic arch descending to right side |
-Bilobed right lung, trilobed left lung; diaphragm intact -GI system normal except for full rotation in reverse with stomach and spleen on right; cecum, duodenum, appendix, liver and gallbladder on left; head of pancreas in right retroperitoneum -GU system, bladder, thymus, and bone marrow unremarkable |
Leech et al., 1988 |
|
Situs ambiguus - Bilateral left-sidedness in thorax and abdomen |
14 | F | -Persistent left-sided superior vena cava entering coronary sinus -Chambers not specified |
-Pulmonary levoisomerism (bilobed lungs bilaterally) -Heterotaxia of stomach and cecum; midline gallbladder, duodenal atresia, polysplenia -No internal carotid arteries; vertebral arteries as entire blood supply to brain; middle and anterior cerebral arteries “thread-like” |
Robinson and Lenke, 1989; Persutte et al., 1990 |
|
Situs inversus - Thorax and abdomen reversed |
15 | M | -Dextrocardia -Chambers not specified |
-Bilobed right lung, trilobed left lung -Stomach, pancreas, and spleen on right; anal atresia -Horseshoe-kidneys with normal ureters, bladder, and urethra |
Meinecke et al., 1990 |
|
Situs inversus - Thorax and abdomen reversed |
21 | M | -Dextrocardia -Four chambers normal |
-Bilobed right lung, trilobed left lung; diaphragm normal -Spleen and pancreas on right; liver and cecum on left |
Ozden et al., 2000 |
|
Situs ambiguus - Thorax normal, abdomen reversed |
25 | M | -Heart localization normal -Four chambers normal, no ASD or VSD -Normal aortic arch, descending aorta, superior and inferior vena cavae, and brachio-cephalic vessels |
-Trilobed right lung, bilobed left lung -Stomach and multilobulated spleen on right; liver, cecum, and appendix on left; sigmoid colon in midline of pelvis |
Ozden et al., 2002 |
|
Situs inversus - Thorax and abdomen reversed |
28 | F | -Dextrocardia -Chambers not specified |
-“Inverted lungs” (presumably bilobed right lung, trilobed left lung) -Stomach and spleen on right; “hepatic inversion” (presumably liver on left); intestinal nonrotation with midline appendix; gastric serosal foci of pancreatic heterotopia |
Faye-Petersen et al., 2006, #2 |
|
Situs inversus - Thorax and abdomen reversed |
32 | F | -Dextrocardia -Four chambers normal, ductus arteriosus and foramen ovale patent |
-Bilobed right lung, trilobed left lung; trachea normal -Spleen and pancreas on right; liver on left; intestinal nonrotation with appendix on left and sigmoid colon on right; anus patent -Single low abdominal midline kidney with left ureter, no right ureter; bladder and urethra unremarkable; adrenal glands normal -Normal female external genitalia; single left cornus uterus, no right cornus uterus; bilateral tubes and ovaries present; cervix and vagina normal -Right radial absence with camptodactyly of remaining right four fingers |
This report |
| CONGENITAL HEART DEFECTS | |||||
| Consistent with situs ambiguus | 8 | F | -Double outlet right ventricle with subaortic VSD and complete pulmonary stenosis -RA dilated, RV hypertrophied -Foramen ovale and ductus arteriosus patent -Only left coronary artery present |
-Right lung half the size of left lung; right pulmonary artery and vein atretic -Tracheoesophageal fistula, proximal end of esophagus terminates as a fibrotic cord and distal segment is in continuity with trachea, entering just above the carina -Hypoplastic adrenal glands; pituitary gland not identified |
Gaba et al., 1982 |
| Consistent with situs ambiguus | 9 | F | -“Tetralogy defect” with common atrium, overriding aorta, small pulmonary trunk, and a membranous interventricular septal defect -Hypoplastic cusps of aortic and pulmonary valves, one well-formed cusp for each valve; both auricles enlarged -Common AV canal predominately open into RV; thick aortic walls; LV small but “unusually thick” muscular wall |
-Stage 2 gut malrotation with common mesentery; cecum and appendix in lower left quadrant medial to sigmoid colon; small intestine entirely on right side -Hypoplastic right kidney; retroaortic left renal vein; right renal artery located cranial to left renal artery -Remaining abdominopelvic cavity normal |
Pauli et al., 1983, #1 |
| 17 | F | - Hypoplastic left atrium | -Bilateral lung hypoplasia -Imperforate anus; short aganglionic segment of rectum -Bilateral forearm hypoplasia; single rudimentary digits on each hand -Lower portion of Mullerian ducts absent; left kidney with cystic-dysplastic changes |
Ades and Sillence, 1992 | |
| Consistent with situs ambiguus | 18 | F | -Truncus arteriosus -Absent pulmonary arteries and veins -VSD |
-Bronchi and lungs absent -Intestinal malrotation; cecum and appendix in left upper quadrant -Single irregular nodule of hypoplastic renal tissue on right, drained by two ureters passing to right and left sides of bladder, respectively; no renal tissue on left -Both adrenal glands normally situated |
Porteous et al., 1993, #1 |
| 29 | F | - Isolated ASD in normally sized heart | - Atresia of upper pharynx; larynx and trachea normally formed; normal lung:body weight ratio | Faye-Petersen et al., 2006, #5 | |
Situs classifications according to Ware and Belmont, 2004, using descriptions by referenced authors.
Findings as described by referenced authors.
ASD = atrial septal defect; AV = atrioventricular; GI = gastrointestinal system; GU = genitourinary system; LV = left ventricle; RV = right ventricle; VSD = ventricular septal defect.
Fig. 2.
Photographs of two previously unreported patients with holoprosencephaly (HPE) and agnathia spectrum, Patient 31 (A-B) and Patient 32 (C-E). A: Patient 31 with microcephaly, extreme micrognathia, microstomia, and normal position of the ears. B: X-ray showing extreme micrognathia. C: Patient 32 with microcephaly, hypoplastic supraorbital ridges, absent zygomatic arches and maxilla, agnathia, a single nostril with a dimple indicating the site of the mouth, and otocephaly with ears fused in the midline. D: Autopsy photograph showing situs inversus with a left-sided liver. E: Right hand showing an absent thumb and camptodactyly of the remaining four fingers.
Aggregate Results
Here we review clinical findings of HPE and agnathia in two previously unreported patients and 30 patients described in the literature. (See Table I for clinical information on all reviewed patients and Table II for detailed cardiac and situs abnormality findings.) For patients in whom sufficient information was available, the following is an analysis of pertinent findings. For calculations, denominators were determined by the total number of patients for whom that finding was adequately described. There was a statistically significant overrepresentation of females, with 64% of patients female (16/25) and 36% male (9/25) (by proportion test, χ2(1) = 3.92, p-value 0.048). Alobar HPE was found in 59% of patients (13/22), semilobar in 9% (2/22), lobar in 4.5% (1/22), hydranencephaly in 9% (2/22), anencephaly in 4.5% (1/22), XK aprosencephaly in 4.5% (1/22), and agenesis of the olfactory bulbs in 9% (2/22). If the type of HPE was not specified, we did not presume the type based on facial findings in order to avoid oversimplifying the relationship between brain and face. However, the presence of cyclopia in many of these cases suggests a relatively severe form of HPE, even if we cannot fully characterize the specific type. Cyclopia or synophthalmia was found in 53% of patients (16/30), two distinct eyes in 43% (13/30), some of which showed hypotelorism, and no identifiable eyes in 3% (1/30). Interestingly, the overall prevalence of cyclopia in cohorts of patients with HPE ranges from 7.4% (9/121) [Croen et al., 1996] to 27% (8/30) [Blaas et al., 2002].
Of the patients for whom visceral findings were reported, situs inversus was reported in 41% of patients (7/17), with four classified as totalis or nearly complete (Patients 13, 21, 28, 32), one as partialis (Patient 25), one as showing “visceral heterotaxia” (Patient 14), and one with unspecified situs inversus (Patient 15). Further classification of these patients according to the situs terminology described above [Ware and Belmont, 2004] revealed five patients with situs inversus (Patients 13, 15, 21, 28, 32) and two patients with situs ambiguus based on orientation of viscera (Patients 14, 25). Analysis was then performed on five additional patients with congenital heart defects (CHD), given that the heart is the first organ to demonstrate clear differences along the left-right axis in the developing embryo [Ware and Belmont, 2004]. Of the five patients with CHD, three had heart defects consistent with the spectrum of CHD found in patients with heterotaxy or situs ambiguus. These include Patient 8 with a double outlet right ventricle, Patient 9 with a common atrium and AV canal, and Patient 18 with truncus arteriosus and a ventricular septal defect. When combined, this analysis reveals a total of 59% of patients (10/17) having situs abnormalities: five with situs inversus and five with situs ambiguus. Although the numbers in this cohort are small, it is interesting to note that 3/5 of the patients with situs inversus and only 1/5 of the patients with situs ambiguus were male. While there are no known X-linked causative genes for HPE, there is a well-recognized form of X-linked situs abnormalities in humans that involves ZIC3 [Gebbia et al., 1997].
Pregnancy resulted in live births in 41% of patients (9/22), ranging from 26 – 41 weeks gestational age. All died soon after birth, with 24 hours as the longest reported survival period. Pregnancy resulted in fetal demise in 59% (13/22), including stillbirths, terminations of pregnancy, and spontaneous abortions. For three patients, details about the timing of demise were not available, however the gestational ages ranged from 24-37 weeks. Two patients were each a member of a set of twins discordant for findings of HPE and agnathia. Patient 23 was a conjoined twin, dicephalus dipus tribrachius, with the other twin's craniofacial structure described as “normal” [Blaas et al., 2002]. Patient 11 was a monochorionic, diamniotic (monozygotic) twin, with her twin reported as anatomically normal on external examination with no evidence of pharyngeal arch malformation but with subtle histologic features of HPE, specifically ectopic adenohypophyseal tissue in the pharyngeal roof [Machin et al., 1985]. Two sibling pairs (Patients 9, 10 and Patients 18, 19) had recurrence of HPE and agnathia.
Of 18 patients in whom genetic testing was performed, 17 had normal karyotypes and one (Patient 10) had an unbalanced translocation, 46,XX,der(18)t(6;18)(p24.1;p11.2)pat, resulting in a partial deletion of 18p and a partial duplication of 6p. This is presumed to be the same in the sibling (Patient 9) given that the father had a balanced translocation [Pauli et al., 1983; Krassikoff and Sekhon, 1989; Overhauser et al., 1995]. Variable expressivity is particularly notable in this set of siblings. Patient 10 had facial findings consistent with agnathia and a “facial appearance [that] did not imply the presence of forebrain abnormalities.” However, a mild form of HPE was identified, with agenesis of both olfactory bulbs. Patient 9 had hypotelorism and alobar HPE with cebocephaly, and the “extremely small mandible” was “not recognized until anatomic dissection was completed” [Pauli et al., 1983]. Genetic testing was unable to be performed on the second set of siblings though their parents had normal karyotypes. To our knowledge, no specific genetic test results are currently available for any of the patients for the four main HPE genes, SHH, ZIC2, SIX3, and TGIF [Dubourg et al., 2007]. Other than the likely cytogenetic etiology of Patients 9 and 10, there are no consistent cytogenetic findings amongst the cohort of patients reported here, and the majority of cases appear to be sporadic. One patient (Patient 31) had a normal microarray. Consanguinity was reported for two patients; the parents of Patient 27 were first cousins, and the details regarding the parents of Patient 32 are not known.
DISCUSSION
Nearly 100 patients are reported as having HPE and features consistent with the spectrum of agnathia. Thirty-two patients, who have adequate information for analysis of findings, are reported here. Although we do not have sufficient details on the remaining patients, it is reasonable to believe that our review is a representative sample. Moreover, while the overall prevalence of HPE and agnathia is relatively infrequent, this association may offer insight into development of forebrain and craniofacial structures, and how the two are likely related. While the mechanism(s) by which HPE and agnathia occur in humans are still not understood, both with and without laterality defects, careful review of the clinical characteristics of patients with HPE and agnathia spectrum, in conjunction with a review of animal studies, indicates various clues that may relate to underlying etiologies for humans.
Variable expressivity
Variable expressivity and pleiotropic expression are typically seen in both animal and human studies. In guinea pigs, variable expressivity is demonstrated by inbred guinea pigs, which are all of a particular strain, and yet display varying grades of otocephaly, both with and without HPE [Wright, 1923; Wright and Wagner, 1934]. In humans, this is demonstrated by two sets of discordant twins, each set of which is presumed to be derived from one zygote [Kaufman, 2004]: a monozygotic twin with HPE and agnathia (Patient 11), whose twin only showed subtle findings of HPE as discussed in results, and a conjoined twin with HPE and agnathia (Patient 23), whose twin had a normal appearing craniofacial structure. This is further illustrated by Patients 9 and 10, siblings with recurrence of HPE and agnathia, who presumably had the same unbalanced translocation but with different phenotypes, as described earlier. Patients 18 and 19, also siblings, had similar phenotypes to one another but no common etiology has yet been identified.
Variation according to genetic background
The manifestations of agnathia and HPE appear to vary according to genetic background. This observation was first described in guinea pigs. Of 14 inbred mating pairs, referred to as “families” by the authors, one pair (family 13) produced 50 otocephalic guinea pigs, and the remaining 13 families produced 28 combined cases [Wright, 1923]. Furthermore, twice as many female guinea pigs as males were affected [Wright, 1933], which is consistent with the gender difference seen in the patients included here. More recently, mouse studies have demonstrated a role for Twsg1 in forebrain and craniofacial development that is more prominent against a specific genetic background, C57BL/6. Mice with a C57BL/6 background and a loss of Twsg1 function (Twsg1−/−) show a continuum of craniofacial phenotypes, ranging from a normal jaw to deficient development of the lower jaw, frequently also with a single nostril and defects in eye development. Furthermore, on histological examination, Twsg1−/− mice demonstrate alobar HPE. Against a 129/SvEv background after three additional generations, the prevalence of craniofacial abnormalities decreases, with far fewer mice showing a reduced jaw phenotype [Petryk et al., 2004].
As discussed earlier, Twsg1 is reported to be involved in BMP signaling. Of note, Patient 10 had an unbalanced translocation (6;18), with deletion of an HPE minimal critical region reported to include TGIF [Gripp et al., 2000; Overhauser et al., 1995] and a breakpoint that is also near TWSG1, the locus of which is 18p11.3-p11.2 [OMIM 605049]. These findings are consistent with the notion that TWSG1 is a potential candidate gene. Twsg1 has also been shown to participate in mouse embryonic salivary gland development [Melnick et al., 2006], and either all or a portion of the salivary glands in Patients 9 and 10 were absent. Shh and Fibroblast growth factor 8 (Fgf8), downstream targets of Hedgehog (Hh) signaling, are also both involved in salivary gland development [Jaskoll et al., 2004a; Jaskoll et al., 2004b], and they are discussed below.
Interrelated signaling pathways
There appears to be a delicate balance between complex interrelated signaling pathways required for development of essential structures such as the forebrain and the surrounding craniofacial form. Context-dependent consequences are observed due to disruption of key elements in pathways that can initially be traced to the establishment of midline, as well as to later downstream functional components. In mice, Shh contributes to establishment of the ventral midline of the brain and to subdivision of the eye field. Mouse embryos deficient in Shh have cyclopia and nearly complete absence of craniofacial bones, though it is thought that the latter may be secondary to the midbrain and forebrain defects. These embryos are reported to have branchial arches with “nearly normal appearance” at embryonic day 9.5 (E9.5), however by E15.5, they display a long proboscis-like extension with no normally identifiable eyes, nose, and oral structures [Figure 2e in Chiang et al., 1996]. These photographs appear to be consistent with mice at E18.5 with HPE and mandibular hypoplasia [Figure 2a in Melnick et al., 2005].
Furthermore, key to the development of the mandible is Meckel's cartilage. In the absence of Shh in mice, a small mesenchymal condensation forms in the region of the presumptive Meckel's cartilage; however the result is a remnant mandibular arch with no differentiated structures. In Shh null mouse, the mandibular bone, Meckel's cartilage, tongue primordium, and tooth buds are absent. These in vivo results are supported by in vitro studies which demonstrate that cyclopamine, an inhibitor of Hh signaling, produces stage-dependent inhibition of Meckel's cartilage chondroblast differentiation into mature chondrocytes. Exogenous Fgf8 is able to rescue the phenotype [Melnick et al., 2005]. In turn, Shh and Fgf8 are inhibited by BMPs, which are modulated by other signals such as Twsg1, described by Petryk et al. [2004], further supporting a complex interplay of signaling mechanisms. Avian embryos have shown that the lower jaw appears to be less sensitive than the premaxilla to the effects of cyclopamine [Cordero et al., 2004], which supports the finding that agnathia is not more commonly associated with HPE.
In addition, potential molecular mechanisms are suggested by animal models to help explain the constellation of laterality and situs abnormalities in a fairly significant number of patients with HPE and agnathia. Nodal signaling, which is involved in positioning of the anterior-posterior axis and left-right patterning [Lowe et al., 2001], also produces abnormalities in gastrulation, left-right axis patterning, and craniofacial defects in mice with trans-heterozygosity of Smad2 and Nodal mutations [Nomura and Li, 1998]. Smad2 is a member of the SMAD family, which is a group of signal transducer proteins involved in TGF-β signaling [Heldin et al., 1997; Massagué et al., 2005]. A synergistic genetic interaction between Gdf1, involved in left-right patterning [Rankin et al., 2000], and Nodal has been illustrated in anterior axis development in mice. Mice show a spectrum of phenotypes that includes HPE, first branchial arch abnormalities, and left-right patterning abnormalities. Interestingly, however, the first branchial arch malformations do not always coincide with HPE [Andersson et al., 2006].
In humans, variants in the NODAL signaling pathway have been identified in patients with CHD, most commonly Tetralogy of Fallot and related conotruncal malformations, and less commonly in patients with laterality defects or HPE [Roessler et al., 2008; Roessler et al., 2009]. Taken together, these animal models and human examples implicate disturbances in the establishment of the vertebrate axial midline, a conserved developmental process in vertebrates, which in turn can link laterality disturbances, including cardiac defects, with abnormal development of the face and brain [Roessler et al., 2008].
While this review highlights identifiable trends in a narrowly-defined cohort of patients, it is limited by the criterion set for inclusion, which required the presence of agnathia spectrum and HPE before further analyzing clinical details. Thus, we did not review patients with isolated agnathia, isolated situs inversus, agnathia alone with situs inversus, or HPE alone with situs inversus.
HPE and agnathia present a challenging area of study given the likely complex etiology, and we propose that a deeper understanding of the association will enable us to offer more informed genetic counseling for families of such patients.
ACKNOWLEDGMENTS
We appreciate the many families who have enabled physicians and researchers to advance our understanding of holoprosencephaly and agnathia. Thank you to all of those who have contributed to the literature on this topic. Thank you to the staff of the National Institutes of Health Library for their assistance in obtaining the included articles.
This research was supported by the Division of Intramural Research, National Human Genome Research Institute, National Institutes of Health, Department of Health and Human Services, United States of America.
Biographies
Emily Kauvar is a medical student, currently participating in the Howard Hughes Medical Institute – National Institutes of Health Research Scholars Program, in the Medical Genetics Branch of the National Human Genome Research Institute, National Institutes of Health, Bethesda, MD, USA. Her interests include pediatrics and development.
Benjamin Solomon is a Clinical Genetics Fellow in the Medical Genetics Branch of the National Human Genome Research Institute, National Institutes of Health, Bethesda, MD, USA. His research interests include holoprosencephaly and VACTERL association.
Cynthia Curry is a Pediatrician and Clinical Geneticist at Genetic Medicine of Central California, University of California, San Francisco, Fresno, CA, USA. Her research interests include the causes of mental retardation and birth defects.
Anthonie van Essen is a Clinical Geneticist at the University Medical Center Groningen, the Netherlands.
Nicole Janssen is a resident in Clinical Genetics at the University Medical Center Groningen, the Netherlands.
Amalia Dutra is the Director of the Cytogenetics and Microscopy Core in the Genetic Disease Research Branch of the National Human Genome Research Institute, National Institutes of Health, Bethesda, MD, USA.
Erich Roessler is a faculty member of the Medical Genetics Branch of the National Human Genome Research Institute, National Institutes of Health, Bethesda, MD, USA. His research interests include holoprosencephaly and disturbances of organ sidedness, or laterality.
Max Muenke is the Branch Chief of the Medical Genetics Branch of the National Human Genome Research Institute, National Institutes of Health, Bethesda, MD, USA. His research interests include holoprosencephaly, craniofacial malformation syndromes, and Attention Deficit Hyperactivity Disorder.
REFERENCES
- Ades LC, Sillence DO. Case Report. Agnathia-holoprosencephaly with tetramelia. Clin Dysmorphol. 1992;1:182–184. [PubMed] [Google Scholar]
- Anderson RM, Lawrence AR, Stottmann RW, Bachiller D, Klingensmith J. Chordin and noggin promote organizing centers of forebrain development in the mouse. Development. 2002;129:4975–4987. doi: 10.1242/dev.129.21.4975. [DOI] [PubMed] [Google Scholar]
- Andersson O, Reissmann E, Jörnvall H, Ibáñez CF. Synergistic interaction between Gdf1 and Nodal during anterior axis development. Dev Biol. 2006;293:370–381. doi: 10.1016/j.ydbio.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Baker PA, Aftimos S, Anderson BJ. Case report. Airway management during an EXIT procedure for a fetus with dysgnathia complex. Pediatric Anesthesia. 2004;14:781–786. doi: 10.1111/j.1460-9592.2004.01284.x. [DOI] [PubMed] [Google Scholar]
- Ballantyne JM. Manual of Antenatal Pathology and Hygiene. Edinburgh: Green. 1902:423–433. [Google Scholar]
- Blaas HGK, Eriksson AG, Salvesen KA, Isaksen CV, Christensen B, Møllerløkken G, Eik-Nes SH. Brains and faces in holoprosencephaly: pre- and postnatal description of 30 cases. Ultrasound Obstet Gynecol. 2002;19:24–38. doi: 10.1046/j.0960-7692.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- Carles D, Serville F, Mainguené M, Dubecq JP. Cyclopia-otocephaly association: a new case of the most severe variant of agnathia-holoprosencephaly complex. J Craniofac Genet Dev Biol. 1987;7:107–113. [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Cohen MM, Jr, Sulik KK. Perspectives on holoprosencephaly: Part II. Central nervous system, craniofacial anatomy, syndrome commentary, diagnostic approach, and experimental studies. J Craniofac Genet Dev Biol. 1992;12:196–244. [PubMed] [Google Scholar]
- Cordero D, Marcucio R, Hu D, Gaffield W, Tapadia M, Helms JA. Temporal perturbations in sonic hedgehog signaling elicit the spectrum of holoprosencephaly phenotypes. J Clin Invest. 2004;114:485–494. doi: 10.1172/JCI19596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen LA, Shaw GM, Lammer EJ. Holoprosencephaly: epidemiologic and clinical characteristics of a California population. Am J Med Genet. 1996;64:465–472. doi: 10.1002/(SICI)1096-8628(19960823)64:3<465::AID-AJMG4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Dubourg C, Bendavid C, Pasquier L, Henry C, Odent S, David V. Holoprosencephaly. Orphanet J Rare Dis. 2007;2:8. doi: 10.1186/1750-1172-2-8. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faye-Petersen O, David E, Rangwala N, Seaman JP, Hua Z, Heller DS. Otocephaly: report of five new cases and a literature review. Fetal Pediatr Pathol. 2006;25:277–296. doi: 10.1080/15513810601123417. [DOI] [PubMed] [Google Scholar]
- Fenton TR. A new growth chart for preterm babies: Babson and Benda's chart updated with recent data and a new format. BMC Pediatr. 2003;3:13. doi: 10.1186/1471-2431-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaba AR, Anderson GJ, VanDyke DL, Chason JL. Alobar holoprosencephaly and otocephaly in a female infant with a normal karyotype and placental villitis. J Med Genet. 1982;19:78. doi: 10.1136/jmg.19.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner S. Cyclopia. Arch Ophthalmol. 1947;37:220–231. doi: 10.1001/archopht.1947.00890220229014. [DOI] [PubMed] [Google Scholar]
- Gebbia M, Ferrero GB, Pilia G, Bassi MT, Aylsworth A, Penman-Splitt M, Bird LM, Bamforth JS, Burn J, Schlessinger D, Nelson DL, Casey B. X-linked situs abnormalities from mutations in ZIC3. Nat Genet. 1997;17:305–308. doi: 10.1038/ng1197-305. [DOI] [PubMed] [Google Scholar]
- Gripp KW, Wotton D, Edwards MC, Roessler E, Ades L, Meinecke P, Richieri-Costa A, Zackai EH, Massagué J, Muenke M, Elledge SJ. Mutations in TGIF cause holoprosencephaly and link NODAL signaling to human neural axis determination. Nat Genet. 2000;25:205–208. doi: 10.1038/76074. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Miyazono K, ten Dijke P. TGF-beta signaling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- Hersh JH, McChane RH, Rosenberg EM, Powers WH, Jr, Corrigan C, Pancratz L. Otocephaly-midline malformation association. Am J Med Genet. 1989;34:246–249. doi: 10.1002/ajmg.1320340223. [DOI] [PubMed] [Google Scholar]
- Jaskoll T, Leo T, Witcher D, Ormestad M, Astorga J, Bringas P, Jr, Carlsson P, Melnick M. Sonic hedgehog signaling plays an essential role during embryonic salivary gland epithelial branching morphogenesis. Dev Dyn. 2004a;229:722–732. doi: 10.1002/dvdy.10472. [DOI] [PubMed] [Google Scholar]
- Jaskoll T, Witcher D, Toreno L, Bringas P, Moon AM, Melnick M. FGF8 dose-dependent regulation of embryonic submandibular salivary gland morphogenesis. Dev Biol. 2004b;268:457–469. doi: 10.1016/j.ydbio.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Jones KL, Higginbottom MC, Smith DW. Determining role of the optic vesicle in orbital and periocular development and placement. Pediatr Res. 1980;14:703–708. doi: 10.1203/00006450-198005000-00001. [DOI] [PubMed] [Google Scholar]
- Kaufman MH. The embryology of conjoined twins. Childs Nerv Syst. 2004;20:508–525. doi: 10.1007/s00381-004-0985-4. [DOI] [PubMed] [Google Scholar]
- Keith A. Three demonstrations on congenital malformations of palate, face, and neck. Br Med J. 1909;2:363–367. doi: 10.1136/bmj.2.2537.363-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krassikoff N, Sekhon GS. Familial agnathia-holoprosencephaly caused by an inherited unbalanced translocation and not autosomal recessive inheritance. Am J Med Genet. 1989;34:255–257. doi: 10.1002/ajmg.1320340227. [DOI] [PubMed] [Google Scholar]
- Leech RW, Bowlby LS, Brumback RA, Schaefer GB., Jr Agnathia, holoprosencephaly, and situs inversus: report of a case. Am J Med Genet. 1988;29:483–490. doi: 10.1002/ajmg.1320290303. [DOI] [PubMed] [Google Scholar]
- Lowe LA, Yamada S, Kuehn MR. Genetic dissection of nodal function in patterning the mouse embryo. Development. 2001;128:1831–1843. doi: 10.1242/dev.128.10.1831. [DOI] [PubMed] [Google Scholar]
- Machin GA, Sperber GH, Wootliffe J. Monozygotic twin aborted fetuses discordant for holoprosencephaly/synotia. Teratology. 1985;31:203–215. doi: 10.1002/tera.1420310205. [DOI] [PubMed] [Google Scholar]
- Massagué J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- Matsunaga E, Shiota K. Holoprosencephaly in human embryos: epidemiologic studies of 150 cases. Teratology. 1977;16:261–272. doi: 10.1002/tera.1420160304. [DOI] [PubMed] [Google Scholar]
- Meinecke P, Padberg B, Laas R. Agnathia, holoprosencephaly, and situs inversus: a third report. Am J Med Genet. 1990;37:286–287. doi: 10.1002/ajmg.1320370226. [DOI] [PubMed] [Google Scholar]
- Melnick M, Witcher D, Bringas P, Jr, Carlsson P, Jaskoll T. Meckel's cartilage differentiation is dependent on Hedgehog signaling. Cells Tissues Organs. 2005;179:146–157. doi: 10.1159/000085950. [DOI] [PubMed] [Google Scholar]
- Melnick M, Petryk A, Abichaker G, Witcher D, Person AD, Jaskoll T. Embryonic salivary gland dysmorphogenesis in Twisted gastrulation deficient mice. Arch Oral Biol. 2006;51:433–438. doi: 10.1016/j.archoralbio.2005.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollica F, Pavone L, Nuciforo G, Sorge G. A case of cyclopia. Role of environmental factors. Clin Genet. 1979;16:69–71. doi: 10.1111/j.1399-0004.1979.tb00854.x. [DOI] [PubMed] [Google Scholar]
- Nomura M, Li E. Smad2 role in mesoderm formation, left-right patterning and craniofacial development. Nature. 1998;393:786–790. doi: 10.1038/31693. [DOI] [PubMed] [Google Scholar]
- Okuno S, Hamada H, Fujiki Y, Yamada N, Sohda S, Kubo T. Two cases of agnathia-holoprosencephpaly complex. Nippon Sanka Fujinka Gakkai Zasshi. 1996;48:237–239. [PubMed] [Google Scholar]
- Orioli IM, Castilla EE. Clinical epidemiologic study of holoprosencephaly in South America. Am J Med Genet Part A. 2007;143A:3088–2099. doi: 10.1002/ajmg.a.32104. [DOI] [PubMed] [Google Scholar]
- Overhauser J, Mitchell HF, Zackai EH, Tick DB, Rojas K, Muenke M. Physical mapping of the holoprosencephaly critical region in 18p11.3. Am J Hum Genet. 1995;57:1080–1085. [PMC free article] [PubMed] [Google Scholar]
- Özden S, Fiçicioğlu C, Kara M, Oral Ö , Bigliç R. Agnathia-holoprosencephaly-situs inversus. Am J Med Genet. 2000;91:235–236. doi: 10.1002/(sici)1096-8628(20000320)91:3<235::aid-ajmg16>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Özden S, Bilgiç R, Delikara N, Başaran T. The sixth clinical report of a rare association: agnathia-holoprosencephaly-situs inversus. Prenat Diagn. 2002;22:831–842. doi: 10.1002/pd.401. [DOI] [PubMed] [Google Scholar]
- Pauli RM, Graham JM, Jr, Barr M., Jr Agnathia, situs inversus, and associated malformations. Teratology. 1981;23:85–93. doi: 10.1002/tera.1420230111. [DOI] [PubMed] [Google Scholar]
- Pauli RM, Pettersen JC, Arya S, Gilbert EF. Familial agnathia-holoprosencephaly. Am J Med Genet. 1983;14:677–698. doi: 10.1002/ajmg.1320140411. [DOI] [PubMed] [Google Scholar]
- Persutte WH, Yeasting RA, Kurczynski TW, Lenke RR, Robinson H. Agnathia malformation complex associated with a cystic distention of the oral cavity and hydranencephaly. J of Craniofac Genet Dev Biol. 1990;10:391–397. [PubMed] [Google Scholar]
- Petrikovsky BM. Diagnosis and Management. Wiley-Liss; New York: 1999. Fetal Disorders. p. 43. [Google Scholar]
- Petryk A, Anderson RM, Jarcho MP, Leaf I, Carlson CS, Klingensmith J, Shawlot W, O'Conner MB. The mammalian twisted gastrulation gene functions in foregut and craniofacial development. Dev Biol. 2004;267:374–386. doi: 10.1016/j.ydbio.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Porteous MEM, Wright C, Smith D, Burn J. Agnathia-holoprosencephaly: a new recessive syndrome? Clin Dysmorphol. 1993;2:161–164. [PubMed] [Google Scholar]
- Potter EL, Craig JM. Pathology of the Fetus and the Infant. Second Edition Year Book; Chicago: 1961. p. 532. [Google Scholar]
- Rankin CT, Bunton T, Lawler AM, Lee SJ. Regulation of left-right patterning in mice by growth/differentiation factor-1. Nat Genet. 2000;24:262–265. doi: 10.1038/73472. [DOI] [PubMed] [Google Scholar]
- Robinson HB, Jr, Lenke R. Agnathia, holoprosencephaly, and situs inversus. Am J Med Genet. 1989;34:266–267. doi: 10.1002/ajmg.1320340230. [DOI] [PubMed] [Google Scholar]
- Roessler E, Ouspenskaia MV, Karkera JD, Vélez JI, Kantipong A, Lacbawan F, Bowers P, Belmont JW, Towbin JA, Goldmuntz E, Feldman B, Muenke M. Reduced NODAL signaling strength via mutation of several pathway members including FOXH1 is linked to human heart defects and holoprosencephaly. Am J Hum Genet. 2008;83:18–29. doi: 10.1016/j.ajhg.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler E, Pei W, Ouspenskaia MV, Karkera JD, Vélez JI, Banerjee-Basu S, Gibney G, Lupo PJ, Mitchell LE, Towbin JA, Bowers P, Belmont JW, Goldmuntz E, Baxevanis AD, Feldman B, Muenke M. Cumulative ligand activity of NODAL mutations and modifiers are linked to human heart defects and holoprosencephaly. Mol Genet Metab. 2009;98:225–234. doi: 10.1016/j.ymgme.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers KT. Presence of the pituitary in perfect cyclopia. Anat Rec. 1957;128:213–218. doi: 10.1002/ar.1091280206. [DOI] [PubMed] [Google Scholar]
- Rolland M, Sarramon MF, Bloom MC. Astomia-agnathia-holoprosencephaly association. Prenatal diagnosis of a new case. Prenat Diagn. 1991;11:199–203. doi: 10.1002/pd.1970110310. [DOI] [PubMed] [Google Scholar]
- Sadler TW. Langman's Medical Embryology. Eighth Edition Lippincott Williams and Wilkins; Philadelphia: 2000. pp. 345–347. [Google Scholar]
- Sarma V. Ocular abnormalities of the foetus with special reference to cyclopia. Br J Ophthalmol. 1963;47:193–202. doi: 10.1136/bjo.47.4.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer C, Tariverdian G, Schiesser M, Thomas MC, Sergi C. Agnathia-otocephaly complex: report of three cases with involvement of two different Carnegie stages. Am J Med Genet. 2002;112:203–208. doi: 10.1002/ajmg.10672. [DOI] [PubMed] [Google Scholar]
- Sedano HO, Gorlin RJ. The oral manifestations of cyclopia. Review of the literature and report of two cases. Oral Surg Oral Med Oral Pathol. 1963;16:823–838. doi: 10.1016/0030-4220(63)90321-9. [DOI] [PubMed] [Google Scholar]
- Sezgin I, Süngü S, Bekar E, Çetin M, Ceran H. Cyclopia-astomia-agnathia-holoprosencephaly association: a case report. Clin Dysmorphol. 2002;11:225–226. doi: 10.1097/00019605-200207000-00018. [DOI] [PubMed] [Google Scholar]
- Siebert JR, Kokich VG, Beckwith JB, Cohen MM, Lemire RJ. The facial features of holoprosencephaly in anencephalic human specimens. II. Craniofacial anatomy. Teratology. 1981;23:305–315. doi: 10.1002/tera.1420230305. [DOI] [PubMed] [Google Scholar]
- Ware SM, Belmont JW. ZIC3, CFC1, ACVR2B, and EBAF and the visceral heterotaxies. In: Epstein CJ, Erickson RP, Wynshaw-Boris A, editors. Inborn Errors of Development: The Molecular Basis of Clinical Disorders of Morphogenesis. Oxford University Press; New York: 2004. pp. 300–313. [Google Scholar]
- Wright S. Factors which determine otocephaly in guinea pigs. J Agric Res. 1923;26:161–181. [Google Scholar]
- Wright S. On the genetics of subnormal development of the head (otocephaly) in the guinea pig. Genetics. 1933;19:471–503. doi: 10.1093/genetics/19.6.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S, Wagner K. Types of subnormal development of the head from inbred strains of guinea pigs and their bearing on the classification and interpretation of vertebrate monsters. Am J Anat. 1934;54:383–447. [Google Scholar]