Abstract
Extracellular single unit recording experiments were performed to examine response characteristics of wide dynamic range neurons in the Vc that receive masseter afferent input in Sprague Dawley rats. Capsaicin, or its vehicle, was directly administered into the masseter muscle and changes in resting discharge, responses to mechanical stimulation on the cutaneous receptive field and the electrical threshold for masseter nerve stimulation were assessed. Intramuscular capsaicin induced significant increase in the background discharge and mechanical hypersensitivity to the cutaneous stimulation and lowered the threshold masseter nerve stimulation evoked responses in the majority of neurons. The capsaicin-induced increase in evoked responses, but not the resting discharge, was partially attenuated when the muscle was pretreated with a mGluR antagonist. The present study suggests that injury or inflammation in the masseter muscle induce generalized hyperexcitability of central trigeminal neurons and that the blockade of peripherally localized mGluR5 can effectively attenuate muscular hypersensitivity.
Keywords: Muscle pain, peripheral glutamate receptors, brainstem neurons
INTRODUCTION
Acute injection of various algogenic substances into deep structures such as skeletal muscle tissue has been widely used and contributed significantly to our understanding of deep tissue pain and sensorimotor mechanisms [3]. Injections with capsaicin, a potent agonist for transient receptor potential cation channel 1 (TRPV1), into the human masseter muscle produce intense local pain, referred pain, and mechanical hypersensitivity [1,2,21]. Capsaicin injection in the rat masseter also produces vigorous nociceptive responses followed by prolonged mechanical hyperalgesia [15,18,19]. These behavioral responses likely reflect the activities of masseter afferents as well as activity-dependent changes in functional properties of central trigeminal neurons that process noxious input from the masseter afferents. The responses of nociceptive neurons in the trigeminal subnucleus caudalis (Vc) following capsaicin stimulation of various orofacial tissues have been documented [4,6, 11]. However, the responses of a specific subset of Vc neurons that receive muscle afferent input upon capsaicin stimulation have not been described.
The involvement of peripherally localized glutamate receptors in craniofacial muscle pain and hyperalgesia has been amply demonstrated [17]. Along with NMDA receptors, activation of metabotropic glutamate receptor 5 (mGluR5) in masseter afferents contributes to the development of mechanical hypersensitivity in rats [13,14]. A possible mechanism underlying the behavioral responses is that the acute injury produced by capsaicin can cause the local release of glutamate in the masseter, which then engages in mGluR5-mediated sensitization of Vc neurons. Thus, the objectives of this single unit recording study were: (1) to describe physiological properties of Vc neurons that receive high threshold masseter input with and without the intramuscular capsaicin, (2) to determine whether intramuscular capsaicin induces hyperexcitability of the Vc neurons, and (3) to examine whether the pretreatment of the masseter with an mGluR5 antagonist prevents the capsaicin-induced activation and sensitization of the Vc neurons.
METHODS
All procedures were conducted in accordance with the NIH guide for care and use of Laboratory Animals (NIH Publications No. 80-23) and under a University of Maryland approved Institutional Animal Care and Use Committee protocol. Extracellular single unit recording experiments were carried out on male Sprague-Dawley rats (300–400 g; Harlan; Indianapolis, IN, USA). Rats were initially anesthetized with sodium pentobarbital (40–50 mg/kg, i.p.). One femoral vein was cannulated and connected to an infusion pump to provide additional anesthesia as needed. Rectal temperature was monitored for the duration of the experiment and maintained within normal physiological limits.
Glass microelectrodes pulled to a tip diameter of 1–3µm and filled with 2M NaCl (2–5 MOhms) were used for extracellular recording of Vc neurons. The recording electrodes were targeted for the Vc region 3–5 mm caudal to the obex. Recordings were amplified, band-pass filtered (Grass P511, Grass Instruments; 10 Hz–35 KHz), monitored via oscilloscopes and an audio amplifier, digitized with CED 1401 micro interfaced with a Pentium PC.
The Vc neurons that receive muscle primary afferents were identified by orthodromic responses to electrical stimulation of the masseter nerve. For nerve stimulation, animals were paralyzed with Pancuronium Bromide (2 mg/kg i.v. as needed) and artificially ventilated. Graded electrical stimuli were applied to the masseter nerve with bipolar cuff electrode on the side of the brainstem recording. Single 0.1 msec square wave pulses were delivered at a rate of 0.3–1 Hz. The lowest current necessary to elicit five consecutive responses without a failure was considered as the nerve threshold. Neurons lacking the evidence of muscle afferent input were not studied further. Once a unit responsive to electrical stimulation of the muscle nerve was isolated, its receptive field (RF) was identified by natural stimulation (i.e., palpation over the masseter muscle). If a unit had the cutaneous RF on the area of skin overlying the masseter muscle, additional tests such as stretching the jaw and/or grabbing the buccal mucosa with forceps, were applied in an effort to differentiate the response originating from the skin from that of the muscle.
Additional RFs were tested by mechanical stimulation of the hair, skin oral mucosa, teeth, and palpation of other jaw muscles. A majority of Vc neurons that were activated by the masseter nerve stimulation were wide dynamic range (WDR) neurons as they showed a graded response to innocuous and noxious mechanical stimuli applied to their cutaneous RF. WDR neurons were selectively used in this study. For each neuron, the mechanical threshold for the cutaneous RF was determined with calibrated von Frey (VF) filaments (Stoelting, Chicago, IL). Neuronal responses to the threshold and suprathreshold forces (2 VF filaments with consecutively higher forces) were documented. Each filament was applied for 1–2 seconds three times separated by 5-second intervals. The mean firing rate (MFR) evoked for each filament application was then calculated.
The Vc neurons were identified and studied under three separate conditions. In one condition (n=10), an acute inflammatory agent, capsaicin (0.1%, 100µl dissolved in 7% Tween 80, 20% ethanol, and 73% isotonic saline), was directly injected into the mid-region of the masseter with a 30-gauge needle. The same dose and volume of capsaicin have been shown to reliably induce mechanical hyperalgesia when injected in the rat masseter [18,19]. In the second condition (n=12), the vehicle control was administered into the masseter muscle in the same manner. In the third condition (n=11), the masseter muscle was pre-treated with MPEP (2-methyl-6(phenylethynyl) pyridine hydrochloride; 30 nmol/20 µl), an mGluR5 selective receptor antagonist, 10 minutes prior to the capsaicin injection in the same muscle. The same dose of MPEP significantly attenuated mechanical hyperalgesia in the rat masseter [13]. Only one neuron per animal was documented.
Initial evaluation of neuronal responses to the injection were based on peri-stimulus time histograms (bin width 2 s) compiled for one-minute blocks for 5–10 minutes post-injection to study the temporal pattern of injection-related changes on the resting discharge. The number of spikes in each bin were converted to MFRs and plotted against time. If a neuron did not show any spontaneous activity prior to the injection procedure induction of neuronal activities following the injection procedure were considered evoked and the neuron’s resting discharge considered modulated. For spontaneously active neurons the resting discharge was considered modulated only if the firing rate changed 2 standard deviations and more following the injection procedure.
For each unit, responses to VF stimulation of the cutaneous RFs were assessed 15 minutes prior to and 15, 30, 45 and 60 min post injection. MFRs for threshold and suprathreshold VF stimuli taken at each time point were compared with two-way repeated measures of analysis of variance (ANOVA) or the Friedman repeated measures of ANOVA on ranks depending on the outcome of a normality test. Cells were considered modulated if the MFRs following the injection were significantly different from those of the pre-injection period. The comparison of thresholds for orthodromic electrical nerve stimulation before and after the injection in the three groups of neurons was performed with two-way repeated measures of ANOVA. The significance level of all analyses was set at p=<0.05.
RESULTS
Data were compiled from 33 cells recorded at the level of Vc between 3–5 mm caudal to the obex, 1.5–2.5 mm lateral from the midline, and 1–2 mm ventral from the surface of the brainstem. This region corresponds to the caudal Vc [10, 11]. All units were identified as masseter muscle units on the basis of their response to the electrical nerve stimulation. Muscle RFs were verified by manual palpation of the masseter muscle. The mean response latencies to electrical stimulation of the masseter nerve were 11.8±1.8, 10±2.3, and 11.9±1.04 msec for capsaicin treated group, vehicle treated group, and MPEP pretreated capsaicin group, respectively. There was no significant difference in the mean latency between the groups (F=0.39, p=0.68). All units displayed sufficient variability in the latency to the first spike to suggest that recordings were made from central neurons and not primary afferent fibers in the spinal tract of the trigeminal nerve.
All units were also excited by one or more types of mechanical stimuli applied to orofacial skin. These units showed moderate responses to light touch or brush on their cutaneous RFs and reached maximal discharge rates upon noxious stimulation. The RFs included a zone that responded to tactile and noxious stimuli and areas that were activated only by noxious stimuli. The most common location of the RFs of these units involved the skin extending from the ipsilateral vibrissae pad, to periorbital area, and occasionally up to the skin covering the pinna.
Table 1 summarizes the responses of the three groups of Vc neurons. Eighty percent of the cells showed a significant modulation of the resting discharge upon capsaicin injection. These cells typically responded with rapid increase in their resting discharge in the first minute which lasted between 1–10 minutes. Eight of the ten cells also showed significant modulation of MFRs to mechanical stimulation of the cutaneous RF at one or more time points, of which 7 showed significant facilitation and 1 reduction of MFRs. The two neurons whose resting discharge was unchanged showed a significant increase in MFRs to the mechanical stimulation, whereas the two neurons that did not show significant modulation of their MFRs to the mechanical stimulation were effectively activated by the capsaicin injection. An example of the Vc neurons responsive to intramuscular capsaicin is illustrated in Fig 1. This unit which was reliably activated by electrical stimulation of the masseter nerve and mechanical pressure on the masseter muscle had cutaneous receptive that spanned from the upper lip to the back of the ear on the ipsilateral side. The neuron was unresponsive to mechanical stimulation of the skin overlying the masseter muscle. The VF stimulation with increasing force on the skin superior to the vibrissae pad produced graded neuronal responses characteristic of WDR neurons (Fig 1C). Masseteric injection of the capsaicin intensely activated the neuron in the initial minute that gradually returned to the pre-injection level in approximately 3 minutes. (Fig 1D). The statistical analysis on the MFRs to VF stimulations showed significant main effect (Force: F=86.03, p<0.001) as well as temporal effect at 30 and 45 minutes following the injection (F=4.5, p<0.05). Therefore, this unit was qualified as a WDR neuron and categorized as a capsaicin-sensitive neuron.
Table 1.
Summary of responses in the resting discharge and responses to VF stimulation on the cutaneous receptive fields in Vc neurons following masseter injections.
| Resting Discharge | Responses to VF Stimulation | ||||||
|---|---|---|---|---|---|---|---|
| F | R | N | F | R | N | Total | |
| Capsaicin | 8 | - | 2 | 8 | - | 2 | 10 |
| Vehicle | 3 | 1 | 8 | 3 | 2 | 7 | 12 |
| MPEP+Capsaicin | 7 | 2 | 2 | 4 | 6 | 1 | 11 |
F-facilitated, R-reduced, N-no change
Figure 1.
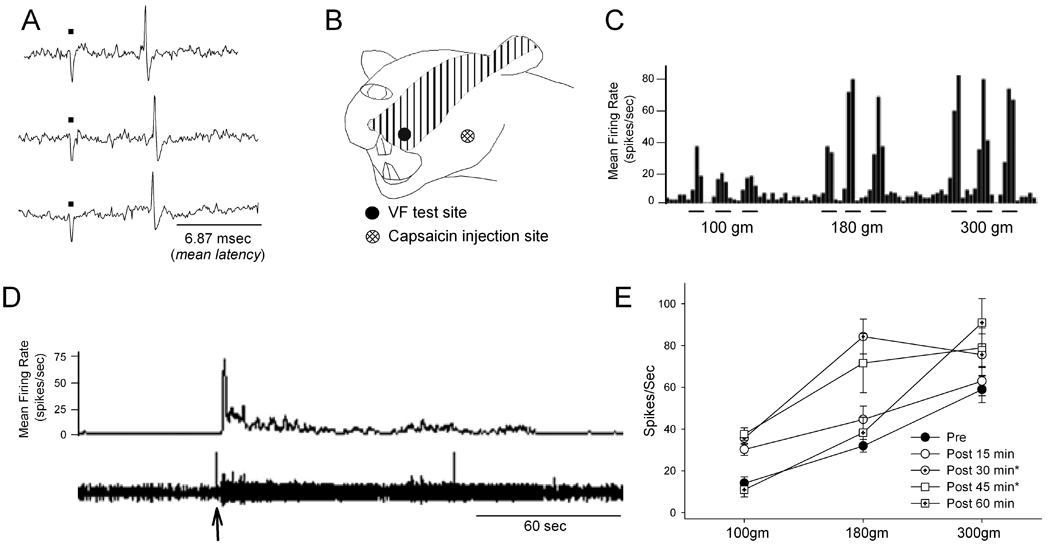
Responses of a Vc unit to three consecutive electrical stimulation of the masseter nerve is shown in A. Electrical traces below black dots show stimulus artifacts. The unit’s cutaneous receptive field was assessed (B) and classified as WDR based on its responses to graded VF stimulation (C). Intramuscular capsaicin injection intensely facilitated the resting discharge of this Vc neuron (D). The arrow indicates when the capsaicin was administered. Threshold and suprathreshold VF stimulations on cutaneous RF evoked increased responses following the capsaicin injection (E). The elevated resting discharge persisted for over 45 min. * p<0.05
The vehicle administration in the masseter muscle did not produce any significant changes in 8 of 12 neurons (67%). Three neurons showed an immediate increase in resting discharge which returned to the baseline level within 1 minute and one neuron responded with the reduction of resting discharge in the first minute (Table 1). Seven of the twelve neurons (58%) did not show any modulation of responses to the VF stimulation following the vehicle injection. Only one neuron showed a significant increase in MFRs at multiple time points. Three neurons (25%) responded with a significant reduction of MFRs at one or more time points.
In the MPEP pretreated group, capsaicin significantly increased the resting discharge of 7 out of the 11 neurons (63.6%). Two neurons (18.2%) showed a slight but significant reduction of resting discharge that returned to the pre-injection level in the first two minutes; and two neurons did not show any changes. The majority of the neurons (54.5%) showed no significant modulation of MFRs to VF stimulation when MPEP was administered prior to capsaicin. Four of the eleven neurons (36%), however, showed a clear facilitation. The rest showed a significant reduction of the MFRs at one or more time points (Table 1).
Thresholds for electrical stimulation of the masseter nerve were assessed before and after the capsaicin injection from Vc neurons in all three groups as another measure of the changes in excitability. The mean threshold changes were significantly different in the three groups of neurons (F=5.67, p<0.05). There was a significant and time-dependent reduction of the mean threshold (F=17.3, p<0.01). The post hoc analysis revealed that the capsaicin, but not the vehicle, treatment significantly reduced the electrical nerve threshold and that the MPEP pretreatment prevented the capsaicin effect (Fig 2).
Figure 2.
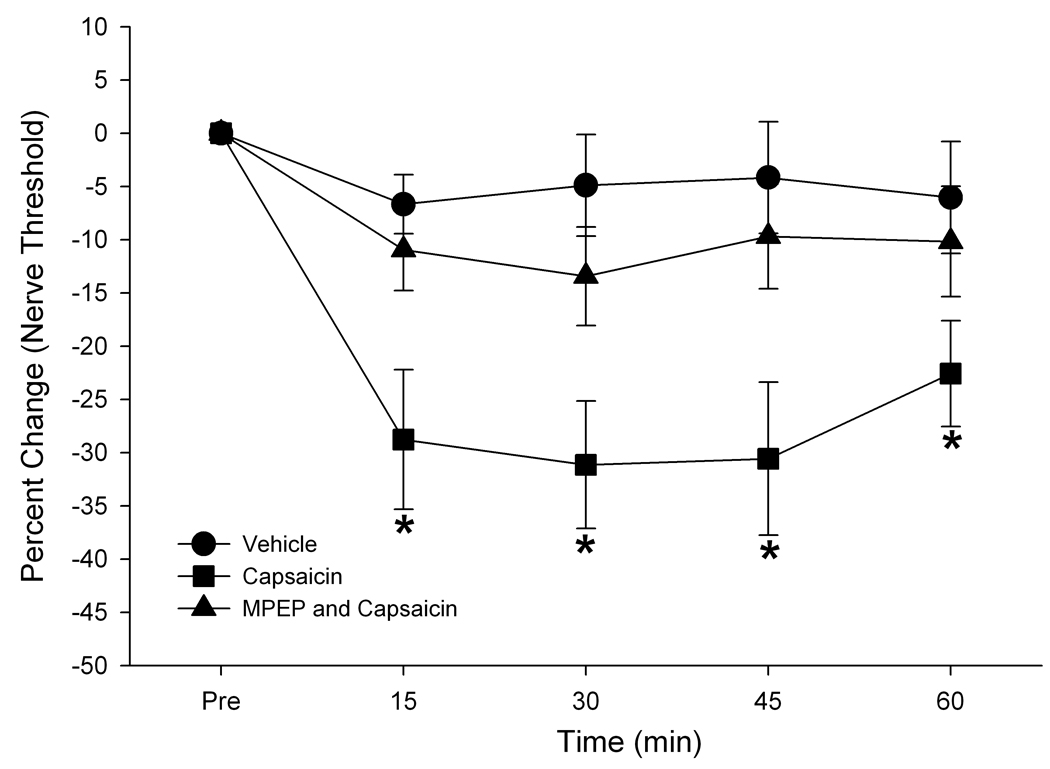
Percent changes in thresholds to masseter nerve stimulation before and after capsaicin injection. The capsaicin-induced reduction in the nerve threshold was not observed when the muscle is pretreated with MPEP. * P<0.05
DISCUSSION
Tissue injury and inflammation can produce neuroplastic changes in functional properties of peripheral nociceptors and spinal dorsal horn neurons [9]. In the trigeminal system, Hu et al [10] presented the first evidence of sensitization of Vc neurons following the masseteric injection of mustard oil. The effect is reflected in the expansion of the cutaneous RF, an increase in spontaneous activity and hypersensitivity to cutaneous stimulation. While the findings are important in that they demonstrated changes in cutaneous sensibilities that often accompany muscle pain, the changes in functional properties of Vc neurons in response to muscle tissue stimulation have not been examined. Therefore, it is unknown whether the masseter inflammation would also produce changes in synaptic efficacy specifically from muscle afferent input. Recently, Lam et al [11] demonstrated that injection of capsaicin into the temporomandibular joint (TMJ) significantly reduces mechanical activation thresholds for both TMJ and cutaneous RF stimulations. The incidence and extent of changes in mechanical threshold between the two sites are comparable suggesting that the acute injury in the peripheral tissue could alter the general excitability of Vc neurons.
The present study provided additional information on the response characteristics of Vc neurons to stimulation of the masseter muscle with capsaicin. Similar to TMJ injection, intramuscular capsaicin activated the majority of the Vc neurons tested (80%). The temporal profiles of capsaicin-evoked neuronal responses were comparable to those described following TMJ and oral mucosa [4,11]. The duration of neuronal responses correlated with the duration of capsaicin-induced nocifensive behavioral responses in the rat under a similar condition [19]. Our data also showed that the peripheral application of capsaicin alters the excitability of Vc neurons to both mechanical stimulation of the cutaneous RF and electrical stimulation of the masseter nerve. The reduction of nerve threshold and facilitation of neuronal responses could be detected as early as 15 minutes following the capsaicin application and lasted as long as 60 minutes. Although we could not document the exact time when the responses return to the pre-injection level due to technical difficulties in holding cells in a prolonged period of time most neurons showed a tendency of recovery between 1–2 hrs. This time course is also similar to that of capsaicin-induced mechanical hyperalgesia in the rat under a similar condition [19]. We did not systematically examine the changes in receptive field size changes in this study, which has been frequently used as an index of central sensitization. In many cases these neurons exhibited the RF spanning a large area of the face along with other orofacial regions which we could not clearly delineate. A documentation of only those Vc neurons with delimited RF would have significantly limited the sampling of Vc neurons. Our study, however, provided a new method of assessing sensitivity changes in central trigeminal neurons following injury or inflammation. The results collectively showed that that intramuscular capsaicin, but not the vehicle, activated a majority of neurons that process masseter afferent input in the Vc, and produced excitability changes in these neurons upon stimulation of the affected nerve as well as the tissue remote from the injured area.
While several studies demonstrated that application of exogenous glutamate in peripheral tissue activates primary afferent nociceptors and produce peripheral sensitization [7,8], little information is available on the source of endogenously released glutamate in the muscle tissue, and how the peripherally released glutamate contributes to muscle pain and hyperalgesia. Since noxious stimulation of nociceptors triggers the endogenous release of glutamate from nerve endings [12], it is possible that activation of muscle nociceptors following capsaicin stimulation promotes the glutamate release, which then produces autogenic activation of glutamate receptors that are located on the peripheral terminals of muscle nociceptors. The prolonged activation of nociceptors by endogenously released glutamate can contribute to the excitability changes of central trigeminal neurons. There is evidence that blockade of peripheral NMDA receptors reduces responses of WDR cells in the spinal cord dorsal horn to both noxious and innocuous stimuli [20]. However, whether the activation of glutamate receptors at the peripheral terminal in muscle nociceptors provides sufficient input to induce hyerpsensitivity of central trigeminal neurons was yet to be determined. Here we provided the first evidence the blockade of peripherally localized mGluR5 significantly reduce the capsaicin-mediated hypersensitivity of Vc neurons to cutaneous RF and masseter nerve stimulations implicating the role of peripheral mGluR5 in the development of hyperalgesia associated with masseter injury. Direct activation of mGluR5 in the masseter produces masseter hypersensitivity with a time course comparable to that shown in these Vc neurons [13]. It was interesting to note that MPEP had little effect on the capsaicin-induced resting discharge, data suggesting that acute nociception following capsaicin stimulation may not involve mGluR5. The blockade of NMDA and AMPA receptors has been shown to effectively attenuate acute nocifensive behaviors [5,16].
In summary, the results from the present study show that masseter injection of capsaicin produces intense activation of Vc neurons that receive muscle afferent input and pronged excitability changes, and suggest that peripheral mGluR5 partially mediates capsaicin-induced masseter hypersensitivity.
ACKNOWLEDGEMENTS
We would like to acknowledge Ms. Youping Zhang for her valuable technical assistance and Ms Jami Saloman for editorial comments on the manuscript. Support for this work was provided by NIH Grant RO1 DE16062 (JYR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest.
REFERENCES
- 1.Arima T, Svensson P, Arendt-Nielsen L. Capsaicin-induced muscle hyperalgesia in the exercised and non-exercised human masseter muscle. J Orofac Pain. 2000;14:213–223. [PubMed] [Google Scholar]
- 2.Arima T, Arendt-Nielsen L, Minagi S, Svensson P. Effect of capsaicin-evoked jaw-muscle pain on intramuscular blood-flow. Arch Oral Biol. 2009;54:241–249. doi: 10.1016/j.archoralbio.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Capra NF, Ro JY. Human and animal experimental models of acute and chronic muscle pain: intramuscular algesic injection. Pain. 2004;110:3–7. doi: 10.1016/j.pain.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 4.Carstens E, Kuenzler N, Handwerker HO. Activation of neurons in rat trigeminal subnucleus caudalis by different irritant chemicals applied to oral or ocular mucosa. J Neurophysiol. 1998;80:465–492. doi: 10.1152/jn.1998.80.2.465. [DOI] [PubMed] [Google Scholar]
- 5.Chun YH, Frank D, Lee JS, Zhang Y, Auh QS, Ro JY. Peripheral AMPA receptors contribute to muscle nociception and c-fos activation. Neurosci Res. 2008;62:97–104. doi: 10.1016/j.neures.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dessirier JM, Simons CT, Sudo M, Sudo S, Carstens E. Sensitization, desensitization and stimulus-induced recovery of trigeminal neuronal responses to oral capsaicin and nicotine. J Neurophysiol. 2000;84:1851–1862. doi: 10.1152/jn.2000.84.4.1851. [DOI] [PubMed] [Google Scholar]
- 7.Du J, Koltzenburg M, Carlton SM. Glutamate-induced excitation and sensitization of nociceptors in rat glabrous skin. Pain. 2001;89:187–198. doi: 10.1016/s0304-3959(00)00362-6. [DOI] [PubMed] [Google Scholar]
- 8.Du J, Zhou S, Coggeshall RE, Carlton SM. N-methyl-D-aspartate-induced excitation and sensitization of normal and inflamed nociceptors. Neuroscience. 2003;118:547–562. doi: 10.1016/s0306-4522(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 9.Dubner R, Ruda MA. Activity-dependent neuronal plasticity following tissue injury and inflammation. Trends Neurosci. 1992;15:96–103. doi: 10.1016/0166-2236(92)90019-5. [DOI] [PubMed] [Google Scholar]
- 10.Hu JW, Sessle BJ, Raboisson P, Dallel R, Woda A. Stimulation of craniofacial muscle afferents induces prolonged facilitatory effects in trigeminal nociceptive brain-stem neurones. Pain. 1992;48:53–60. doi: 10.1016/0304-3959(92)90131-T. [DOI] [PubMed] [Google Scholar]
- 11.Lam DK, Sessle BJ, Hu JW. Glutamate and capsaicin effects on trigeminal nociception II: activation and central sensitization in brainstem neurons with deep craniofacial afferent input. Brain Res. 2009;1253:48–59. doi: 10.1016/j.brainres.2008.11.056. [DOI] [PubMed] [Google Scholar]
- 12.Lawand NB, McNearney T, Westlund KN. Amino acid release into the knee joint: key role in nociception and inflammation. Pain. 2000;86:69–74. doi: 10.1016/s0304-3959(99)00311-5. [DOI] [PubMed] [Google Scholar]
- 13.Lee JS, Ro JY. Peripheral metabotropic glutamate receptor 5 mediates mechanical hypersensitity in craniofacial muscle via protein kinase C dependent mechanisms. Neuroscience. 2007;146:375–383. doi: 10.1016/j.neuroscience.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Lee HJ, Choi HS, Ju JS, Bae YC, Kim SK, Yoon YW, Ahn DK. Peripheral mGluR5 antagonist attenuated craniofacial muscle pain and inflammation but not mGluR1 antagonist in lightly anesthetized rats. Brain Res Bull. 2006;70:78–385. doi: 10.1016/j.brainresbull.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 15.Lee JS, Zhang Y, Ro JY. Involvement of neuronal, inducible, and endothelia nitric oxide synthases in capsaicin-induced muscle hypersensitivity. European Journal of Pain. 2008 doi: 10.1016/j.ejpain.2008.11.009. doi:10.1016/j.ejpain.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ro JY. Contribution of peripheral NMDA receptors in craniofacial muscle nociception and edema formation. Brain Res. 2003;979:78–84. doi: 10.1016/s0006-8993(03)02873-7. [DOI] [PubMed] [Google Scholar]
- 17.Ro JY. Functional role of peripheral glutamate receptors in craniofacial muscle pain and hyperalgesia. In: Graven-Nielsen T, Arendt-Nielsen L, Mense S, editors. Fundamentals of Musculoskeletal Pain. Seattle: IASP Press; 2008. pp. 33–45. [Google Scholar]
- 18.Ro JY, Lee J, Capra NF, Zhang Y. Role of soluble guanylate cyclase in the trigeminal subnucleus caudalis in capsaicin-induced muscle hypersensitivity. Brain Res. 2007;1184:141–148. doi: 10.1016/j.brainres.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 19.Ro JY, Lee JS, Zhang Y. Activation of TRPV1 and TRPA1 leads to muscle nociception and mechanical hyperalgesia. Pain. 2009;144:270–277. doi: 10.1016/j.pain.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ushida T, Tani T, Kawasaki M, Iwatsu O, Yamamoto H. Peripheral administration of an N-methyl-D-aspartate receptor antagonist (MK-801) changes dorsal horn neuronal responses in rats. Neurosci Lett. 1999;260:89–92. doi: 10.1016/s0304-3940(98)00968-9. [DOI] [PubMed] [Google Scholar]
- 21.Wang K, Arendt-Nielsen L, Svensson P. Capsaicin-induced muscle pain alters the excitability of the human jaw-stretch reflex. J Dent Res. 2002;81:650–654. doi: 10.1177/154405910208100915. [DOI] [PubMed] [Google Scholar]


