Abstract
As many as one-half of smokers relapse in the first week following a quit attempt, and subjective reports of cognitive deficits in early abstinence are associated with increased relapse risk. This study examined whether objective cognitive performance after three days of abstinence predicts smoking resumption in a 7-day simulated quit attempt. Sixty-seven treatment-seeking smokers received either varenicline or placebo (randomized double-blind) for 21 days. Following medication run-up (days 1-10), there was a 3-day mandatory (biochemically confirmed) abstinence period (days 11-13) during which working memory (Letter-N-Back Task) and sustained attention (Continuous Performance Task) were assessed (day 13). Participants were then exposed to a scheduled smoking lapse and instructed to try to remain abstinent for the next 7 days (days 15-21). Poorer cognitive performance (slower correct reaction time on Letter-N-Back task) during abstinence predicted more rapid smoking resumption among those receiving placebo (p=.038) but not among those receiving varenicline. These data lend further support for the growing recognition that cognitive deficits involving working memory are a core symptom of nicotine withdrawal and a potential target for the development of pharmacological and behavioral treatments.
Keywords: nicotine, addiction, withdrawal, smoking relapse, cognition
1. Introduction
Following a quit attempt, smokers commonly report withdrawal-related cognitive symptoms (Hughes, 2007; Ward et al., 2001), and objective deficits in attention and working memory have been documented in human laboratory studies (Mendrek et al., 2006; Myers et al., 2008). Nicotine re-exposure following deprivation reverses these cognitive deficits in animals (Davis et al., 2005) and humans (Myers et al., 2008), supporting the hypothesis that relapse to smoking may occur as an attempt to ameliorate these deficits. Efficacious medications, such as varenicline, also reverse abstinence-induced cognitive deficits, suggesting that cognition may be a valuable target for the development of pharmacological and behavioral therapies (Lerman et al., 2007; Patterson et al., 2009; Raybuck et al., 2008).
Few studies have examined the relationship between withdrawal-related cognitive deficits and smoking relapse. In smokers with schizophrenia, poor performance on sustained attention (Culhane et al., 2008) and working memory tasks (Dolan et al., 2004) predicts relapse, with similar findings among depressed smokers (Kassel et al., 2007). Only one study has examined the role of cognitive deficits in relapse among healthy smokers (Rukstalis et al., 2005), showing that self-reported inattention predicts relapse.
This human laboratory study examined whether objective cognitive performance after 3-days of abstinence predicts resumption to smoking in a 7-day simulated quit attempt. We hypothesized that slower performance on working memory and sustained attention tasks during abstinence would predict faster smoking resumption among smokers treated with placebo. No such relationship was expected among smokers treated with varenicline, because varenicline attenuates abstinence-induced cognitive deficits (Patterson et al., 2009).
2. Methods
2.1. Study Participants
Treatment-seeking smokers were recruited from September 2006 to August 2007. Eligible participants were ≥ 18 years of age and reported smoking ≥ 10 cigarettes per day for the previous 12 months. Standard exclusion criteria for varenicline were used (Patterson et al., 2009).
2.2. Procedures and Treatment
Study procedures were approved by the University of Pennsylvania Institutional Review Board. Data were collected within a prior human laboratory study of varenicline versus placebo effects on cognitive symptoms (Patterson et al., 2009). This prior study used a within-subject cross-over design to assess medication effects; however, due to the presence of carryover effects of varenicline on quitting in the prior study, the present analysis of the relationship of cognitive deficits to resumption of smoking was conducted using data from the first medication period only; 35 participants received placebo and 32 participants received varenicline in the first period.
Following a baseline assessment of demographics, smoking history and cognitive performance, participants completed a 21-day medication period. Varenicline (or matching placebo) was administered according to standard treatment guidelines (Pfizer, 2007). The 21-day paradigm included a 10 day medication run-up (days 1-10), a 3-day biochemically confirmed (CO ≤ 10ppm) mandatory abstinence period (days 11-13), a programmed lapse during which participants smoked their own brand cigarettes (day 14), and a 7-day observation period during which participants were instructed to try to remain abstinent after receiving a brief (20-minute) counseling session (days 15-21).
Cognitive performance tasks (see below) were administered at baseline (smoking as usual) and on the third day of the mandatory abstinence period. Abstinence during the 7-day observation period was assessed by self-reports of daily smoking, and verified by breath CO on Days 15, 17, 19, and 21 (CO ≤ 10 ppm required). Participants received a small ($15 daily) incentive for meeting abstinence criteria (days 11-13 and 15-21) (Juliano et al., 2006).
2.3. Measures
2.3.1. Demographics and smoking history
Sex, age, race and number of cigarettes smoked per day were assessed at baseline.
2.3.2 Neurocognitive task performance
Sustained attention and working memory were evaluated using the Penn Continuous Performance Task (P-CPT) (Kurtz et al., 2001) and the Letter-N-Back task (Ragland et al., 2002), respectively. Due to limited variability in the number of correct responses, we utilized median reaction time for correct responses as the primary predictor of smoking resumption. This measure is sensitive to abstinence effects and varenicline effects (Patterson et al., 2009). [Please see supplemental material available with the online version of this paper for task descriptions].
2.3.3. Outcome Variable
The outcome variable was days to smoking resumption, defined as the number of consecutive days of abstinence during the 7-day observation period. Abstinence for each day was assessed by self-report and biochemically confirmed as described above.
2.4. Statistical Analysis
Pearson correlations were used to test for associations between cognitive performance on day 13 and days of abstinence within each treatment group; for purposes of illustration, t-tests were used to compare mean cognitive performance scores of participants who were able to remain abstinent for the full seven days to those who were not. Cognitive variables (day 13) with significant univariate associations with subsequent days of abstinence were tested in Cox regression models, separately within the placebo and varenicline groups. Models controlled for sex, baseline smoking rate and baseline task performance. Additional models in the full sample tested for a possible interaction effect of treatment and cognitive performance on days of abstinence.
3. Results
3.1 Study Participants
Of the 67 participants included in the analysis (two were excluded for not achieving abstinence on study days 11-13), 57% percent were female and the mean age was 43.6 years (S.E.=1.42). The mean number of cigarettes smoked per day at baseline was 21.6 (S.E.=1.22). Sixty-one percent reported European ancestry, 37% were African American and 2% reported other ethnicities. The varenicline and placebo groups did not differ significantly with regard to any baseline variables.
3.2. Days to Smoking Resumption following the Programmed Lapse
The mean days to smoking resumption in the varenicline group was 4.19 (S.E.=0.54) and 2.57 days (S.E.=0.50) for those in the placebo group (t=2.21, p=.03).
3.3 Associations of Cognitive Performance with Days to Smoking Resumption
In the placebo group, overall correct reaction time on the Letter-N-back working memory task was significantly associated with days to smoking resumption during the observation phase (r=−0.38, p=.025). Thus, faster reaction time predicted more days to smoking resumption. Post-hoc analyses of memory load blocks indicated that correct reaction time on the 3-back trials (highest working memory load) was most strongly related to days to resumption to smoking (r=−.45, p=.006). Performance on 1-back and 2-back trials exhibited nonsignificant trends for association (r=−.32, p=.058, and r=−.29, p=.10, respectively), as did performance on the Penn Continuous Performance Test (r=−.27, p=.12). The results of the Cox regression are shown in Table 1. Among participants in the placebo group, performance (correct reaction time) on 3-back trials was a significant independent predictor of days to smoking resumption, controlling for baseline performance and covariates (p=.038).
Table 1.
Cox Regression Analysis of Days to Smoking Resumption in the Placebo Group
| Variable | Hazard Ratio | 95% Confidence Interval | p |
|---|---|---|---|
| Sex | 1.256 | (0.551, 2.861) | .59 |
| Baseline cigarettes/day | 0.765 | (0.548, 1.067) | .12 |
| Baseline 3-back performance | 0.986 | (0.661, 1.469) | .94 |
| Abstinent 3-back performance | 1.618 | (1.027, 2.550) | .038 |
As predicted, there were no significant relationships between cognitive performance and days to smoking resumption in the varenicline-treated group. Correlations between Letter-N-Back correct reaction time and days to smoking resumption in this group ranged from r=−0.14 (p=.45) for overall correct reaction time to r=−0.05 (p=.78) for correct reaction time on 3-back trials. The correlation between Continuous Performance Test correct reaction time and days to smoking resumption was r=−0.16 (p=.40). Among participants in the varenicline group, Letter-N-Back performance was not a significant predictor of days to smoking resumption in the Cox regression model (p=.81).
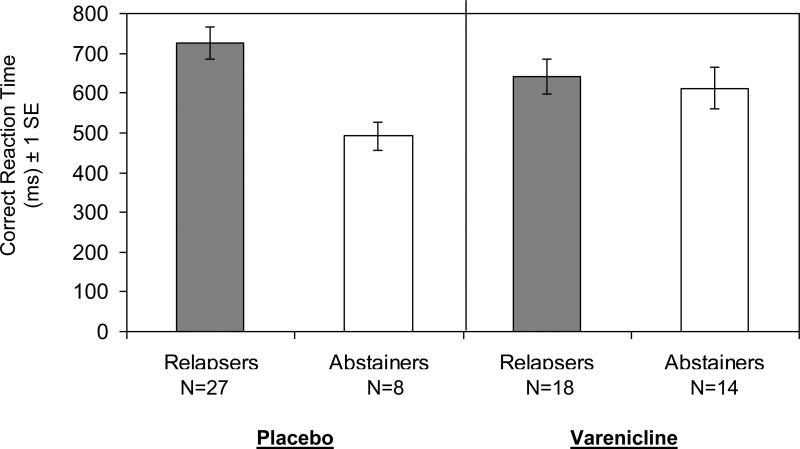
Figure 1 illustrates correct reaction time on 3-back trials among 7-day abstainers versus relapsers on varenicline or placebo. In the placebo group, abstainers had significantly faster correct reaction times on 3-back trials (t (33 df)=4.32, p < .001), consistent with the analysis of days to smoking resumption reported above. In the varenicline group, correct reaction times did not differ between abstainers and relapsers (t (30 df)=0.44, p=.66).
Figure 1.
3-Back Correct Reaction Time for Relapsers and Abstainers in the 7-day Observation Period by Treatment Assignment. The group difference is significant in the placebo condition (p<.001), but not in the varenicline condition (p=.66).
In the Cox regression model including all participants, the interaction of group by Letter-N-back (3-back trials) performance was not significant (p=37).
4. Discussion
There is abundant evidence that smokers experience abstinence-induced deficits in cognitive function (Jacobsen et al., 2005; Mendrek et al., 2006; Myers et al., 2008). Data from the current study extend this work by demonstrating, for the first time in healthy treatment-seeking smokers, that these abstinence-induced cognitive deficits predict short-term smoking resumption. Specifically, among participants receiving placebo (i.e., abstinent, no medication), slower reaction time on the most difficult trials of a working memory task (i.e., the 3-back task) predicted faster smoking resumption during a 7-day simulated quit attempt. Importantly, our model controlled for baseline, “smoking as usual” reaction time, which bolsters our interpretation that the observed deficits are likely to be abstinence-induced.
These data are consistent with prior studies of smokers with comorbid psychiatric illness. For example, among smokers with schizophrenia, slower reaction time at baseline reduced the odds of continuous abstinence 4 weeks after quitting (Culhane et al., 2008). In formerly depressed smokers, slower reaction time predicted increased risk of relapse at 12-month follow-up (Kassel et al., 2007). Our data extend these findings by showing that subtle differences in reaction time after 3 days of abstinence predict fewer days to smoking resumption in a non-psychiatric population of smokers.
While further research is needed, emerging support for the role of cognitive function in smoking relapse has implications for treatment development. Consistent with prior evidence for reversal of withdrawal-related cognitive deficits in animals and human smokers treated with varenicline (Patterson et al., 2009; Raybuck et al., 2008), abstainers and relapsers receiving varenicline in the current trial had comparable reaction times. Thus, part of varenicline's efficacy for smoking cessation may be attributable to its effects on cognitive performance during abstinence (Patterson et al., 2009). Further evidence suggests that some smokers may be more susceptible to cognitive deficits and altered brain function in abstinence, such as those carrying the val allele of the catechol-0-methyltransferase (COMT Val158Met) gene (Loughead et al., 2008). Interestingly, deficits in this prior study were most pronounced during the 3-back task, consistent with the current evidence that 3-back performance is the best predictor of smoking relapse. Taken together, these data support the premise that the development of treatments, both behavioral and pharmacologic, to enhance post-abstinence cognitive performance could be a viable strategy to improve cessation outcomes. Results from this line of research could potentially support the use of cognitive performance tasks as an early screening tool for treatment efficacy and to characterize individual differences in relapse risk.
While this is the first study of cognitive deficits and relapse in healthy treatment-seeking smokers, there are some limitations. The sample size of each group, while large for a human laboratory study, is relatively small for assessing predictors of smoking resumption. Second, this study assessed days to smoking resumption over a brief 7-day period in a simulated quit attempt, and the relationship between cognitive performance and days to relapse during a clinical trial may be different. Third, the definition of abstinence used in this study (CO ≤10ppm), although appropriate for a smoking cessation trial, may also be considered too liberal for a human laboratory of smoking lapses. Finally, only two cognitive tasks were included in this study. Larger studies that include a broader range of cognitive tasks and assess abstinence in a clinical trial would be an important next step to elucidate the role of cognitive deficits in smoking relapse and treatment response.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary information about the methodology for the study is available with the online version of this article at doi:xxx/j.drugalcdep.xxx . . .
References
- Culhane MA, Schoenfeld DA, Barr RS, Cather C, Deckersbach T, Freudenreich O, Goff DC, Rigotti NA, Evins AE. Predictors of early abstinence in smokers with schizophrenia. J. Clin Psychiatry. 2008;69:1743–1750. doi: 10.4088/jcp.v69n1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci. 2005;25:8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan SL, Sacco KA, Termine A, Seyal AA, Dudas MM, Vessicchio JC, Wexler BE, George TP. Neuropsychological deficits are associated with smoking cessation treatment failure in patients with schizophrenia. Schizophrenia Res. 2004;70:263–275. doi: 10.1016/j.schres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9:315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Juliano LM, Donny EC, Houtsmuller EJ, Stitzer ML. Experimental evidence for a causal relationship between smoking lapse and relapse. J Abnorm Psychology. 2006;115:166–173. doi: 10.1037/0021-843X.115.1.166. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Yates M, Brown RA. Baseline reaction time predicts 12-month smoking cessation outcome in formerly depressed smokers. Psychol Addict Behav. 2007;21:415–419. doi: 10.1037/0893-164X.21.3.415. [DOI] [PubMed] [Google Scholar]
- Kurtz MM, Ragland JD, Bilker W, Gur RC, Gur RE. Comparison of the continuous performance test with and without working memory demands in healthy controls and patients with schizophrenia. Schizophrenia Res. 2001;48:307–316. doi: 10.1016/s0920-9964(00)00060-8. [DOI] [PubMed] [Google Scholar]
- Lerman C, LeSage MG, Perkins KA, O'Malley SS, Siegel SJ, Benowitz NL, Corrigall WA. Translational research in medication development for nicotine dependence. Nat Rev Drug Discov. 2007;6:746–762. doi: 10.1038/nrd2361. [DOI] [PubMed] [Google Scholar]
- Loughead J, Wileyto EP, Valdez JN, Sanborn P, Tang K, Strasser AA, Ruparel K, Ray R, Gur RC, Lerman C. Effect of abstinence challenge on brain function and cognition in smokers differs by COMT genotype. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.132. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrek A, Monterosso J, Simon SL, Jarvik M, Brody A, Olmstead R, Domier CP, Cohen MS, Ernst M, London ED. Working memory in cigarette smokers: comparison to non-smokers and effects of abstinence. Addict Behav. 2006;31:833–844. doi: 10.1016/j.addbeh.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CS, Taylor RC, Moolchan ET, Heishman SJ. Dose-related enhancement of mood and cognition in smokers administered nicotine nasal spray. Neuropsychopharmacology. 2008;33:588–598. doi: 10.1038/sj.npp.1301425. [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, Frey JM, Siegel S, Lerman C. Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry. 2009;65:144–149. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfizer [November 16, 2007];2007 http://www.pfizer.com/files/products/uspi_chantix.pdf.
- Ragland JD, Turetsky BI, Gur RC, Gunning-Dixon F, Turner T, Schroeder L, Chan R, Gur RE. Working memory for complex figures: an fMRI comparison of letter and fractal n-back tasks. Neuropsychology. 2002;16:370–379. [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Portugal GS, Lerman C, Gould TJ. Varenicline ameliorates nicotine withdrawal-induced learning deficits in C57BL/6 mice. Behav Neurosci. 2008;122:1166–1171. doi: 10.1037/a0012601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukstalis M, Jepson C, Patterson F, Lerman C. Increases in hyperactive-impulsive symptoms predict relapse among smokers in nicotine replacement therapy. J Sub Abuse Treatment. 2005;28:297–304. doi: 10.1016/j.jsat.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Ward MM, Swan GE, Jack LM. Self-reported abstinence effects in the first month after smoking cessation. Addict Behav. 2001;26:311–327. doi: 10.1016/s0306-4603(00)00107-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.