1.0 Introduction
Venezuelan equine encephalitis virus (VEEV) is a highly infectious agent that can cause severe and frequently fatal encephalitis in equines and is naturally transmitted to humans by mosquitoes [1]. VEEV is considered a potential biological weapon amenable to use in warfare and terrorism due to its high infectivity via aerosol exposure [2, 3]. To address the aerosol threat of VEEV on public health, two vaccines were developed by the U.S. government during the 1960s and 1970s: TC-83, a cell-culture attenuated vaccine developed from the Trinidad donkey (VEEV TrD) strain of subtype IA/B VEEV [4] and a formalin-inactivated vaccine derived from TC-83, designated C-84 [5]. For several decades the TC-83 and C-84 vaccines have been administered by the U.S. Army Special Immunizations Program to laboratory workers and animal health field workers at risk for exposure to VEEV. While TC-83 induces long-lasting immunity against closely related VEEV subtypes [6], major limitations of the vaccine exist including: only an approximately 80% response rate as assessed by plaque reduction neutralization test (PRNT) [7]; a 25% incidence of adverse reactions [8]; and reversion to virulence after mouse brain passages [4]. In addition, as a live virus vaccine, TC-83 cannot be used as a booster for subjects with waning antibody titers [9]. C-84 is currently used to boost antibody titers following vaccination with TC-83 and to immunize TC-83 non-responders. C-84 also has limitations in that protection is of short duration thus requires multiple boosters.
As a result of the limitations of TC-83 and C-84, a new live-attenuated VEEV vaccine candidate was developed, V3526, that contains a deletion of the PE2 cleavage signal (furin cleavage site) combined with a second-site suppressor mutation in the E1 glycoprotein [10]. These attenuating mutations were shown to eliminate the characteristic VEEV disease in animal models inoculated with the parent clone, V3000 [11–14]. In spite of the attenuation, V3526 maintains the ability to elicit a protective immune responses in animals [11, 12, 14]. Due to the success of V3526 in animal models, a Phase 1 clinical trial of V3526 was conducted to evaluate the safety and immunogenicity of this new vaccine candidate. V3526 induced a robust immune response in all vaccines but high frequency of fever and a flu-like syndrome were reported [15] which led to the cessation of further development of V3526 as a live-attenuated vaccine. Next generation vaccine development efforts for VEEV were then redirected toward the development of an inactivated V3526 vaccine. There are several features of V3526 that make it a good candidate for inactivation. First, the molecular basis of attenuation of V3526 is known. Second, extensive nonclinical studies combined with the recent Phase 1 clinical trial demonstrate a higher responder rate and more robust immune response following vaccination with V3526 than observed with TC-83. The superior immunogenicity of V3526 compared to TC-83 suggests a higher rate of success may be achieved using inactivated V3526 compared to C-84, the inactivated version of TC-83. In fact, C-84 has undergone extensive testing in animal models and fails to protect hamsters against aerosol exposure to virulent VEEV suggesting C-84 does not induce strong mucosal immunity [16]. Although studies could have been conducted to optimize C-84 as a protective immunogen by modifying dosage, schedule, route and use of adjuvant, this was not pursued as C-84 is no longer being manufactured and optimization of C-84 as a vaccine would not further our development of a next generation VEE vaccine.
In addition to the well-documented safety and immunological profile of live V3526 in animal and humans, V3526 has advantages over TC-83, C-84 and other attenuated VEE viruses with respect to the manufacturing process. The manufacturing process for V3526 has been developed to meet Good Manufacturing Practice standards and meets the requirements established by the US Food and Drug Administration for biologicals seeking Investigational New Drug status. In contrast, the production of TC-83 and C-84 are based on older technology not compatible with current FDA standards and would require re-derivation of the TC-83 stock, followed by development of a GMP production process for TC-83 in a certifiable cell line and further development of the entire TC-83/C-84 manufacturing process.
Several methods have been used to inactivate infectious agents, including gamma irradiation [17]. In the early 1970s, gamma irradiation was used to inactivate wild-type VEEV [18, 19] with the intent of developing an inactivated vaccine. In these studies, gamma-irradiated VEEV preparations were highly immunogenic and afforded protection against lethal challenge; however, further evaluation was not pursued. To further evaluate gamma-irradiation as an inactivation method for a VEEV vaccine, we optimized the gamma-irradiation process for V3526 with the intent of completely inactivating the virus while preserving immunologically important epitopes [20].
The objective of this study was to evaluate gamma-irradiated V3526 for immunogenicity and efficacy when administered alone and when formulated with adjuvants in BALB/c mice following SC or IM administration. The protective efficacy of the immunological responses was evaluated by challenge with VEEV TrD via the SC and aerosol routes.
2.0 Materials and Methods
2.1 Test Material Information
V3526 bulk drug substances (BDS Lot 220 and 225) were produced by Sigma Aldrich Fine Chemicals (SAFC Pharma), Carlsbad, CA. V3526 BDS was produced by infecting multiple semi-confluent monolayers of human MRC-5 cells grown in 10-layer Nunc Cell Factories (NCF) (Nunc, Inc.) with aliquots of a V3526 working virus bank (multiplicity of infection = 0.005/cell). Prior to infection, V3526 was diluted in phenol-red free Dulbecco’s Minimal Essential Medium (DMEM) containing 0.5% human serum albumin (HSA) (Grifols), 4 mM L-glutamate and 1 × non-essential amino acids (NEAA). The diluted virus inoculum was then added to each NCF and the cultures were incubated for approximately 60 hours. At harvest, supernatant fluids were clarified over a 0.8 micron filter, followed by concentration through a hollow fiber tangential flow filter (TFF) (GE Healthcare) with a 500 kDa molecular weight cutoff membrane. The concentrated bulk was diafiltered with 16 volumes of diafiltration buffer, consisting of phenol-red free DMEM supplemented with 0.5% v/v HSA. The diafiltered material was then filtered over a 0.2 µm membrane and stored at −80°C. Infectivity titers of the BDS Lot 220 and Lot 225 were determined to be 2.9 × 107 pfu/mL and 3.0 × 108 pfu/mL, respectively, by plaque assay on Vero cell monolayers [11]. Process control material (PCM) was produced by essentially the same procedure described above with the exception that MRC-5 cells were mock-infected with phosphate buffered saline (PBS). Throughout the following studies, PCM was used as negative control. The challenge virus, VEEV TrD, was produced by Commonwealth Biotechnologies Incorporated, Richmond, VA. VEEV TrD virus titer was determined by plaque assay on Vero cell monolayers [11]. C-84 (Lot 7 Run 1) was produced by the Salk Institute, Swiftwater, PA.
2.1.1 Gamma Irradiation
V3526 BDS prepared at SAFC Pharma was shipped frozen to Sterigenics, Corona, CA following standard procedures for shipping of infectious materials. The virus remained on dry ice throughout the gamma-irradiation process. Process development studies demonstrated complete inactivation of V3526 BDS by exposure of aliquots (15 mL in 50 mL conical tubes) to 50 kGy gamma irradiation [20]. For all runs, dosimeters were in place to ensure that the calculated irradiation dosage was achieved.
2.1.2 Testing for Residual Infectivity
Inactivated virus preparations were tested for residual infectivity using a standard plaque assay previously described [11] and serial passage on baby hamster kidney (BHK)-21 cells [21]. The presence of residual infectivity was also assessed by intracranial inoculation of suckling mice with 10 µL of test virus. As controls for the assay, additional suckling mice were intracranially inoculated with live V3526 or PCM. The brains from mice surviving 14 days post-inoculation were removed upon euthanasia, homogenized and frozen. A second set of suckling mice were inoculated intracranially with the brain homogenate from the corresponding group and observed for an additional 14 days.
2.1.3 Analysis of Epitope Integrity Following Gamma-Irradiation
A sandwich ELISA was developed utilizing monoclonal antibodies (Mabs) designated 1A4A-1 and 13D4 for the capture of antigen and horse anti-V3526 polyclonal serum for the detection of bound antigen. Mab 1A4A-1 recognizes the E2c epitope on the VEEV IA/B E2 glycoprotein [22]. Mab 1A4A-1 binds to VEEV IA/B viruses including V3526, VEEV TrD and C84 as well as VEEV subtypes IC and ID. Mab 13D4 recognizes an E3 epitope of the PE2 protein of V3526 [23]. Individual Mabs were coated on a 96-well plate overnight at 4°C at 0.5 µg/well. All subsequent incubations were performed at 37°C. Plates were then blocked with PBS containing 0.5% Tween-20 and 5% skim milk (PBSTM) for 2 hours. Samples were diluted in PBSTM containing 1% inactivated fetal bovine serum (FBS), serially diluted 1:2 and incubated for 2 hours. Plates were washed six times with PBS plus Tween-20 (PBST). Bound virus was detected using horse anti-V3526 serum (1:1000) for 2 hours [11]. Following incubation, plates were washed six times with PBST. Bound equine antibody was quantitated by addition of peroxidase-labeled goat anti-horse antibody (KPL, Inc.) incubated for 1hour, followed by six washes with PBST and the addition of ABTS substrate (KPL, Inc). After 30 minutes at room temperature, the optical density (OD) was determined at 410 nm using the SpectraMax 340PC (Molecular Devices). The per well background values were determined at 490 nm and subtracted from the 410 nm value to normalize differences in the non-optical quality of plastic of the round-bottom plates. All data were collected using SoftMaxPro 3.1 (Molecular Devices).
2.1.4 Determination of Neutralizing Antibody Titers
Virus-neutralizing antibody titers in serum obtained from immunized and control mice were determined using the PRNT as previously described [24] using VEEV TrD virus as the target antigen. Sera were serially diluted two-fold and incubated overnight at 4°C with virus. The serum-virus mixtures were further incubated on Vero cell monolayers for one hour at 37°C. The cells were overlaid with 0.6% agarose in Eagle’s basal medium with Earle’s salts supplemented with 10% fetal bovine serum, 200 IU/mL penicillin, 200 µg/mL streptomycin, 2 mM L-glutamine, and 100 µM non-essential amino acids. Cells were stained with 5% Neutral Red one day later and plaques counted the following day. The endpoint titer was determined to be the highest dilution with an 80% or greater reduction of the number of plaques observed compared to control wells. The limit of quantitation for the PRNT was at the initial 1:10 serum dilution (the most concentrated dilution tested) which was 1:20 following dilution of the serum with the virus. The endpoint titer was determined to be the reciprocal of the highest final dilution. Non-responders were assigned a value of one and geometric mean endpoint titers were calculated.
2.1.5 Determination of Serum Binding Antibody Titers
Antibody responses to VEEV TrD were evaluated by ELISA. Plates were coated with 0.5 µg purified VEEV TrD per well and incubated overnight at 4°C. All subsequent incubations were performed at 37°C. The following day, plates were blocked with PBS containing 0.05% Tween-20, 5% nonfat dry milk and 3% normal goat serum (Sigma) (PBSTMG) for 2 hours. The plates were washed three times with PBST. Mouse sera were serially diluted 1:3 in PBSTMG, and incubated for 2 hours. Plates were washed three times with PBST followed by addition of peroxidase-labeled goat anti-mouse IgG (KPL, Inc.). The plates were incubated with secondary antibody for 1 hour and subsequently washed three times with PBST. The ABTS Peroxidase substrate (KLP, Inc.) was applied to each well and color developed for approximately 20 minutes at which time the OD was determined at 410 nm using the SpectraMax 340PC. The per well background value was determined at 490 nm and subtracted from the 410 nm value to normalize differences in the non-optical quality of plastic of the round-bottom plates. All data were collected using SoftMaxPro 3.1. Endpoint titers were determined as the highest serum dilution that produced an optical density greater than the negative control OD (normal mouse serum, KPL, Inc.) plus 3 standard deviations of background values. The endpoint titer was determined to be the reciprocal of the highest final dilution. Non-responders were assigned a value of one and geometric mean endpoint titers were calculated.
2.2 Animal Information and Environmental Conditions
Female BALB/c mice were purchased from the National Cancer Institute, Fort Detrick, MD. The mice were 6 to 8 weeks old upon arrival and weighed between 18 and 20 grams. The room temperature was 23°C ± 1°C and periods of light and dark were maintained on a 12 hour cycle. Animals were provided rodent diet and tap water ad libitum throughout the study. Research was conducted at the United States Army Medical Research Institute of Infectious Diseases (USAMRIID) and was in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals. USAMRIID is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
2.3 Adjuvants
Alhydrogel™ (AlOH) was purchased from Accurate Chemical and Scientific Corporation (Westbury, NY) and diluted with sterile PBS on the day of use to achieve a final concentration of 0.2%/ dose. The CpG oligodeoxynucleotides (ODN) 2395 was purchased from InvivoGen, San Diego, CA, reconstituted on the day of use and diluted in sterile, endotoxin-free water to achieve a final concentration of 20 µg/dose. The concentration of CpG and AlOH when used in combination to formulate test materials was the same as when the adjuvants were used to prepare test materials formulated with a single adjuvant. gV3526 was formulated with adjuvant(s) on the day of inoculation.
2.4 Vaccinations and Challenges
Eight to ten week old mice were vaccinated by either the subcutaneous (SC) or intramuscular (IM) route with gV3526 alone or formulated with adjuvant. For SC vaccination, 0.5 mL of inoculum was administered to the interscapular area whereas for IM vaccination, 0.05 mL of inoculum was administered into the hind limb muscle. Mice receiving gV3526 formulations were vaccinated on a 0 and 28 day schedule or a 0, 7 and 28 day schedule. Mice receiving C-84 were vaccinated on a 0, 7 and 28 day schedule. Historically, C-84 has not been administered with adjuvant therefore was not evaluated with adjuvant in this study. Specific details on dosages and schedules are located in Table 2 and Table 3. On Day 21 and 49 post-primary vaccination, blood was collected from all mice for immunological analysis. Mice were challenged on Day 56 with 1×104 plaque forming units (pfu) VEEV TrD by either aerosol or SC route. Aerosol exposures were conducted as previously described [25]. Mice were placed in wire cages and transferred to a chamber where they were exposed to aerosolized virus for 10 minutes. Virus collected in an all-glass impinger (AGI) was titrated to determine the concentration of virus (pfu/L) in air using a previously described plaque assay method [11] and the volume inhaled was estimated using Guyton’s formula [26]. Mice were monitored daily for signs of illness for 28 days post-challenge at which time surviving mice were euthanized. Each group contained 10 mice. One iteration of each vaccination-challenge study was conducted.
Table 2.
Induction of Immune Responses and Protection Against VEEV TrD Challenges in Mice Immunized with gV3526 Formulations
| Vaccine Formulation |
Route of Vaccination |
Dosaged (µg/dosage) |
Percent Seroconversiona PRN |
|
|---|---|---|---|---|
| Day 21 Post- vaccinationb |
Day 49 Post- vaccinationb |
|||
| gV3526 | SC | 0.2 | 35 | 85 |
| gV3526 | IM IM |
0.02 0.04e |
0 30 |
40 80 |
| gV3526/ CpG | SC | 0.2 | 35 | 75 |
| gV3526/ CpG | IM IM |
0.02 0.04e |
10 30 |
55 78c |
| gV3526/ AlOH | SC | 0.2 | 55 | 90 |
| gV3526/ AlOH | IM IM |
0.02 0.04e |
20 50 |
60 90 |
| gV3526 / CpG + AlOH |
IM | 0.04e | 60 | 100 |
| C-84 (3 dosage) | SC | 4 | 60 | 100 |
| CpG alone AlOH alone CpG + AlOH alone |
SC and IM SC and IM SC and IM |
0 0 0 |
0 0 0 |
0 0 0 |
| Saline | SC IM |
0 0 |
0 0 |
0 0 |
Percent seroconversion is defined as follows: PRNT, the percentage of mice with PRN titers ≥20; ELISA, the percentage of mice with ELISA titers ≥100.
Percent seroconversion was calculated by combining groups of similarly vaccinated mice making a total of 20 mice per group. Groups were later split into 10 mice challenged by the aerosol route and 10 challenged by the SC route.
The total number of mice in this group was 19 at the time of the 49 day blood draw. One mouse succumbed to a non-study related reason between study day 21 and 49.
All dosages were administered on a 2 dose schedule (day 0 and 28) except for C-84 which was administered on a 3 dose schedule (day 0, 7 and 28).
To double the IM dosage, both hind limb muscles were injected with 0.05 mL of inoculum (0.02 µg gV3526).
Table 3.
Dosing and Schedule Study Matrix
| Study # | Group | Treatment | Dosage 1 (µg) |
Dosage 2 (µg) |
Dosage 3 (µg) |
Total Dosage (µg) |
|---|---|---|---|---|---|---|
| 1a | 1 | gV3526/CpG | 0.02 | 0.02 | ND | 0.04 |
| 2 | gV3526/CpG | 0.04 | 0.04 | ND | 0.08 | |
| 3 | gV3526/ CpG + AlOH | 0.04 | 0.04 | ND | 0.08 | |
| 4 | gV3526/CpG | 0.4 | 0.4 | ND | 0.8 | |
| 5 | gV3526/ CpG + AlOH | 0.4 | 0.4 | ND | 0.8 | |
| 6 | gV3526/ CpG | 0.2 | 0.2 | ND | 0.4 | |
| 7 | gV3526/ CpG + AlOH | 0.2 | 0.2 | ND | 0.4 | |
| 8 | gV3526/ CpG | 0.1 | 0.1 | ND | 0.2 | |
| 9 | gV3526/CpG + AlOH | 0.1 | 0.1 | ND | 0.2 | |
| 2b | 10 | gV3526/ CpG | 0.27 | 0.27 | 0.27 | 0.81 |
| 11 | gV3526/ CpG + AlOH | 0.27 | 0.27 | 0.27 | 0.81 | |
| 12 | gV3526/ CpG | 0.15 | 0.25 | 0.4 | 0.8 | |
| 13 | gV3526/ CpG + AlOH | 0.15 | 0.25 | 0.4 | 0.8 | |
| 14 | C-84 | 4 | 4 | 4 | 12 | |
| 15 | PCMc | 0 | 0 | 0 | 0 |
All vaccinations were performed via the IM route.
Study #1 dosages were administered on days 0 and 28.
Study #2 dosages were administered on days 0, 7 and 28.
PCM was administered on days 0 and 28.
To achieve IM dosages of 0.1, 0.2, and 0.4 µg gV3526, both hind limb muscles were injected with 0.05 mL of inoculum (gV3526, BDS Lot 225). IM vaccination with three escalating dosages (0.15, 0.25, 0.4 µg) and three fixed dosages (0.27 µg) were also administered by injecting both hind limb muscles with 0.05 mL of inoculum.
2.5 Statistical Analysis
All ELISA and PRNT determined titers were log10-transformed for analysis. After transformation, the data met assumptions of normality and homogeneity of variance. ELISA and PRNT values were compared between groups using ANOVA with post-hoc Tukey’s tests for pair-wise comparisons. Fisher’s Exact Test was employed to determine statistical significance of difference in survival rates between groups. Correlations between antibody titers and survival were evaluated using logistic regression analysis. All data were analyzed using SAS Version 9.2.
3.0 Results
3.1 Testing of gV3526 for Infectivity and Epitope Integrity
Two lots of V3526 BDS (Lot 220 and 225) were irradiated at 50 kGy. V3526 BDS Lot 225 was concentrated 10-fold following production to allow for administration of higher dosages. We previously demonstrated exposure to 50kGy of gamma radiation was the lowest dosage that effectively destroyed V3526 infectivity [20]. The loss of infectivity following gamma irradiation of V3526 BDS Lots 220 and 225 was confirmed by assaying these materials for infectivity on Vero cells, serial passage of infected cell supernatants on BHK-21 cell monolayers, and intracranial inoculation of suckling mouse brains. By all three methods, the gV3526 preparations were deemed completely inactivated (Table 1). A critical contributor to the efficacy of inactivated vaccines is the retention of immunologically relevant epitopes. Excessive modification by over-inactivation, regardless of inactivation method, may destroy important epitopes thereby reducing vaccine immunogenicity. Using an ELISA to evaluate epitope preservation, the gV3526 preparation showed decreased binding to the E2c and E3 epitopes (Table 1).
Table 1.
Gamma Irradiation of V3526 – Loss of Infectivity and Retention of Epitope Integrity
| V3526 BDSE | Infectivityb of Starting Concentration (pfu/mL) |
Infectivityb Post Irradiation (pfu/mL) |
IC Inoculation (Survived/ Inoculated) |
Epitope Integrity (% of Controld) |
|---|---|---|---|---|
| Untreated | 3 × 107 | 3 × 107 | 0/13 | 100 |
| Irradiated DEVa Lot 220 |
3 × 107 | BLDc | 13/13 | 32 |
| Irradiated DEV Lot 225 |
3 × 108 | BLDc | 13/13 | 50 |
| PCM | 0 | ND e | 12/12 | NDe |
Development (DEV) lots 225 and 220 were each irradiated at 50 kGy gamma irradiation
Plaque assay on Vero cell monolayers
BLD = below limit of detection (< 3 pfu/mL) in Vero cell assay
Untreated V3526 BDS served as control for the ELISA
Not done
3.2 Induction of Immune Response and Protective Efficacy Following Low Dosage Administration of gV3526
The objective of the study was to compare the immunological responses in mice vaccinated either IM or SC with gV3526 formulations and survival following VEEV TrD challenge by either the SC or aerosol route. The dosages of gV3526 in this study were limited by the concentration of V3526 in BDS Lot 220 and Lot 225 and the total volume of inoculum that can be administered to a mouse SC and IM. The initial study evaluated a single IM injection of gV3526 and was followed with a study evaluating administration of 2 times more gV3526 by injecting mice with inoculum into both hind limb muscles on each day of dosing thereby increasing the dosage of gV3526 administered from 0.02 µg to 0.04 µg. The C-84 dosage of 4 µg (0.5 mL) administered SC on Day 0, 7 and 28 is the dosage administered to humans [7], nonhuman primates (NHP) [27] and rodents [13]and was not modified in this study to allow bridging of the data from this study to studies reported in the literature.
Sera were collected three weeks following each vaccination and were tested for the presence of neutralizing antibodies by PRNT. By Day 21, neutralizing antibody was detectable in 35–55% of mice in groups immunized SC with gV3526 formulated with or without adjuvant (Table 2). A trend toward increased seroconversion rates between Day 21 and 49, as measured by PRNT, was observed in each SC vaccinated group. In comparison, neutralizing antibodies were not detected on Day 21 in mice administered 0.02 µg gV3526 by the IM route and only in 40% of the same mice by Day 49. Corresponding groups of mice receiving 0.02 µg gV3526 formulated with adjuvant demonstrated modest (10% for gV3526/CpG and 20% for gV3526/ AlOH) seroconversion by Day 21. By Day 49 additional mice in each of these groups had seroconverted (Table 2). Doubling the dosage administered IM resulted in seroconversion rates after one dose of the vaccine that were similar to seroconversion rates following 2 doses of the 0.2 µg dosage. Seroconversion rates on Day 21 and 49, as measured by ELISA, were equal to or showed a trend toward higher seroconversion rates than those observed for neutralizing antibodies (data not shown).
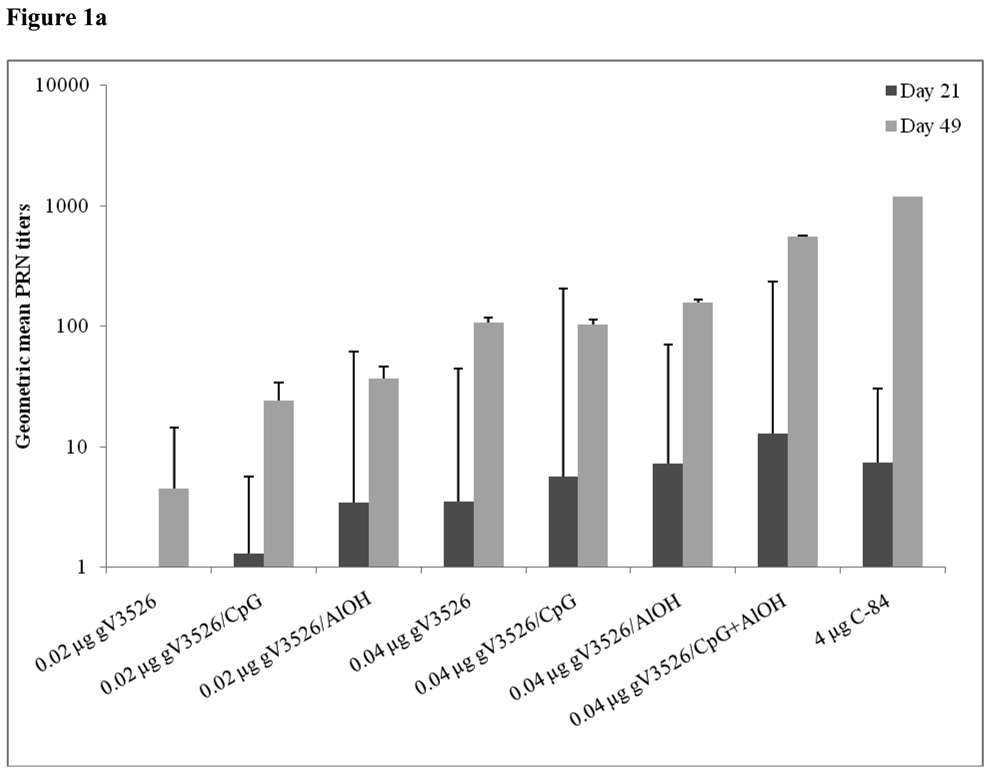
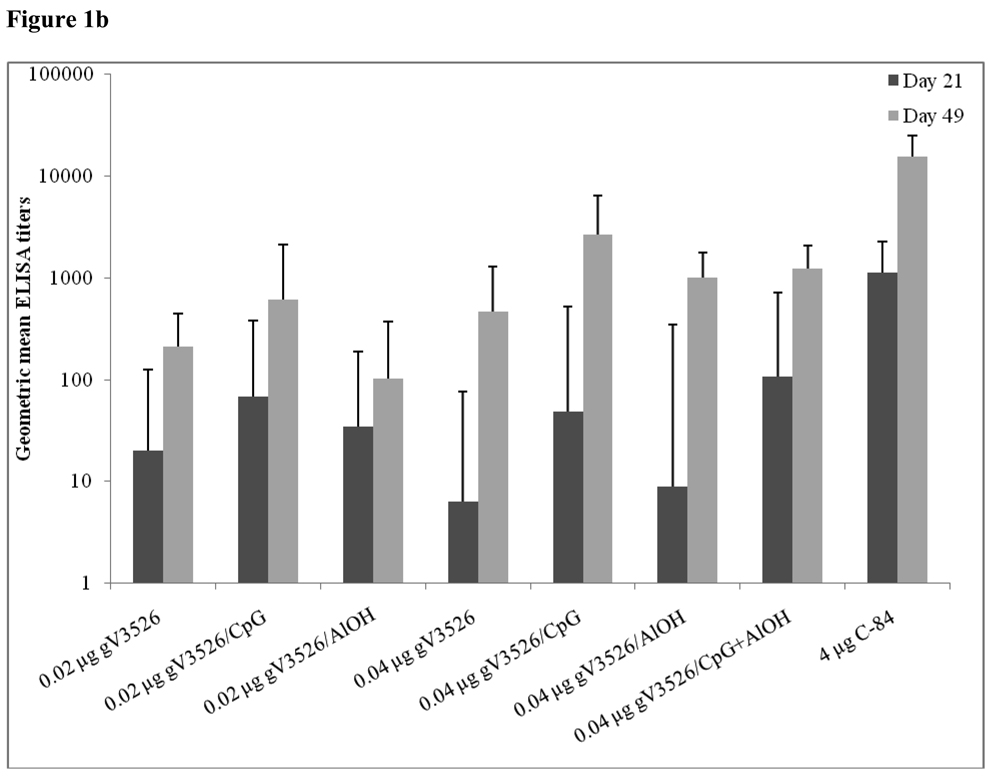
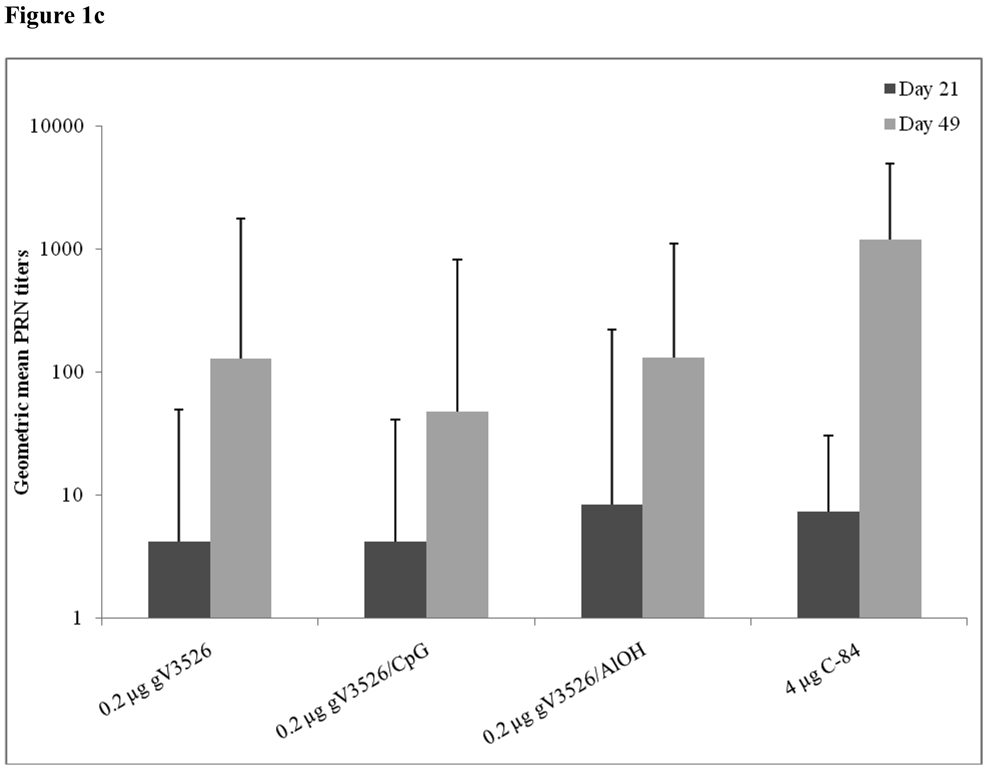
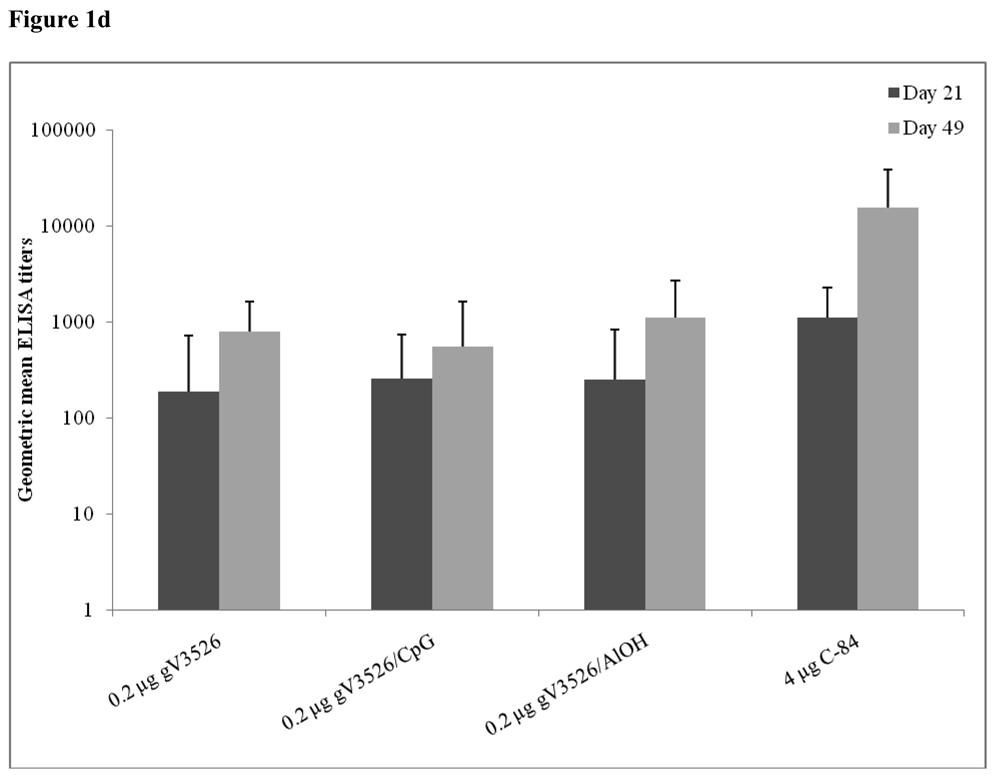
Figure 1a–d summarizes Day 21 and 49 GMT determined by PRNT and ELISA for gV3526 and gV3526 plus adjuvant groups administered IM or SC, demonstrating the enhancement of the antibody responses following a booster vaccination. These data also revealed a trend toward increased antibody titers as the dosage of gV3526 increased from 0.02 µg to 0.04 µg on Day 49 (Figure 1a and 1b). Additionally, 0.04 µg gV3526 formulated with adjuvant when administered IM induced immune responses similar to or greater than those detected in mice receiving adjuvanted gV3526 SC (Figure 1a vs. 1c, 1b vs. 1d). This is an important observation given the amount of viral protein delivered IM was five-fold less than delivered SC.
Figure 1.
Geometric mean PRN and ELISA titers at Day 21 and 49 post-vaccination with gV3526 with and without adjuvant. All mice were vaccinated on a 2 dose schedule except mice vaccinated with C-84 which were vaccinated with 3 doses. The route of administration in 1a and 1b was IM except for C-84 which was administered SC. The route of vaccination in 1c and 1d was SC. The data in 1a and 1c are reported as the geometric mean endpoint titers that inhibit virus plaque formation by 80% and the data reported in 1b and 1d are the geometric mean endpoint titers of immunoglobulin. Error bars represent the 95% confidence level.
3.3 Subcutaneous Administration of gV3526 Formulations Protects Against SC Challenge But Not Aerosol Challenge
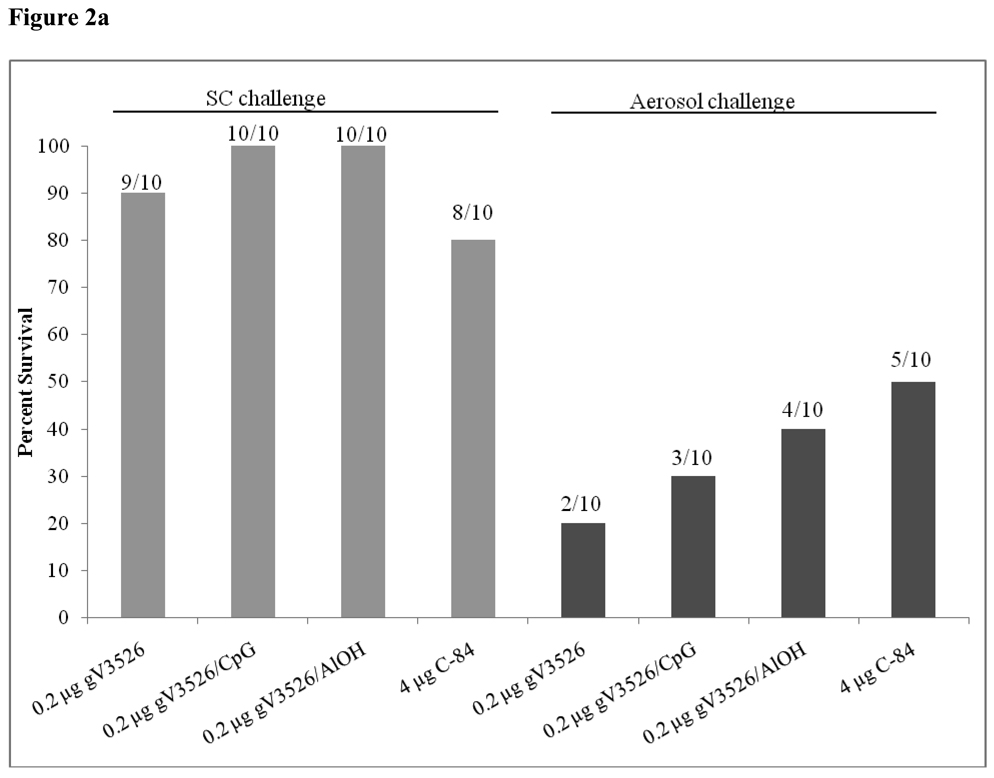
Mice vaccinated with 0.2 µg gV3526 SC either alone or in combination with adjuvant were protected against SC challenge with VEEV TrD (Figure 2a). In contrast, mice vaccinated SC with 0.2 µg gV3526 without adjuvant were not protected against aerosol challenge and gV3526 formulated with adjuvant (CpG or AlOH) given SC only marginally enhanced protection against aerosol challenge. By comparison, C-84 administered SC at 4 µg/dose provided protection against aerosol challenge in 5 of 10 mice.
Figure 2.
Survival rates following SC and aerosol challenge with VEEV TrD in mice vaccinated (a) SC or (b) IM with gV3526 with and without adjuvant. (a) Mice were SC vaccinated on a two dose schedule with 0.2 µg gV3526 or (b) IM vaccinated on a two dose schedule with 0.02 or 0.04 µg gV3526. Four micrograms of C-84 was administered on a three dosage schedule. The number of mice surviving challenge over the total number of mice challenged for each group is indicated above each bar. All sham-vaccinated mice succumbed to infection by Day 7 post-challenge. Gray bars represent survival rates following SC challenge. Black bars represent survival rates following aerosol challenge. A total of 9 mice vaccinated with 0.04 µg gV3526/CpG were challenged as one mouse succumbed to a non-study related illness prior to challenge.
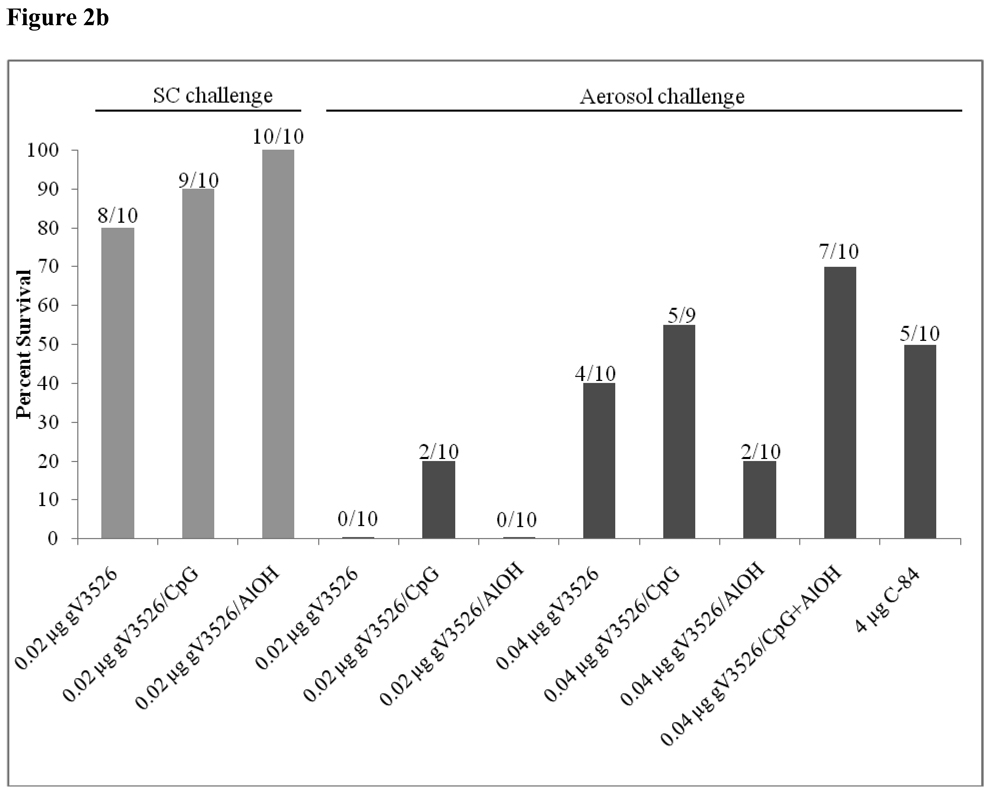
3.4 Intramuscular Administration of Low Dosages of gV3526 Formulations Protect Against SC Challenge But Not Aerosol Challenge
Intramuscular administration of unadjuvanted gV3526 at 0.02 µg/dose induced protective immunity in 8 of 10 mice when challenged SC (Figure 2b). gV3526 formulated with CpG or AlOH administered at the same dosage level also induced a high rate of survival where 9 of 10 and 10 of 10 mice, respectively survived SC challenge. Nevertheless, this dosage level of gV3526 did not protect mice from aerosol challenge and provided only marginal protection (2 of 10) against aerosol challenge when combined with CpG. Mice that received 0.04 µg/dose of gV3526 showed 40% protection against aerosol challenge whereas gV3526/ CpG protected 55% of vaccinated mice following aerosol challenge and gV3526/ CpG + AlOH induced protective immunity in 70% of immunized mice. Logistic regression analysis of antibodies titers from individual mice and their survival outcome post-challenge indicated neither titers of neutralizing antibody nor the serum responses as determined in this study, predicted the survival of vaccinated mice after aerosol challenge.
3.5 Increasing Dosage Enhances Immune Responses and Protection Against Aerosol Challenge
The results summarized in Figure 1 and Figure 2 suggested that increasing the total dosage of gV3526 may enhance antibody responses and/or protection against VEEV TrD aerosol challenge. The 10-fold concentrated gV3526 (BDS Lot 225) was used to evaluate immunogenicity and efficacy of a range of gV3526 dosages on a two or three dose schedule. In that the earlier study demonstrated lower dosages administered IM are equivalent to immune responses induced by higher dosages administered SC, subsequent studies only evaluated the IM route of inoculation. An overview of the respective dosing studies is presented in Table 3.
In dosing study #1, three additional concentrations of gV3526 (0.4 µg, 0.2 µg or 0.1µg) were tested using a two dose vaccination schedule. A significant difference was seen in ELISA and neutralizing antibody GMT in mice vaccinated with gV3526/CpG between the lowest dosage group (0.02 µg/dose) and the higher dosage groups (≥0.1 µg/dose) (p<0.001, ANOVA) (Figure 3 and 4). In contrast, mice vaccinated with gV3526/CpG + AlOH showed a dose-dependent increase in ELISA GMT across all dosages evaluated (p<0.001, ANOVA), except the 0.1 µg dose (Figure 4) but differences in neutralizing antibody GMT were only observed between the lowest dosage group (0.04 µg/dose) and higher dosage groups (≥0.1 µg/dose) (p<0.001, ANOVA). In both formulation groups, a high rate of survival was observed following aerosol challenge in mice vaccinated with 0.04 µg gV3526 or greater (Figure 5). Similar to the low dose study, logistic regression analysis comparing individual mouse PRN and ELISA antibody titers, as determined in this study, prior to challenge and to their survival outcome post-challenge indicated antibody titers were not predictive of survival.
Figure 3.
Neutralizing antibody responses induced to VEEV TrD virus by increased dosages of gV3526 vaccination. Sera were obtained from mice on Day 49 post-primary vaccination, one week prior to challenge. These data are reported as the geometric mean endpoint titers that inhibited plaque formation by 80%. Escalating dosages consisted of 0.15, 0.25 and 0.4 µg gV3526. All neutralizing antibody titers were below detectable levels in sham-vaccinated mice. The 0.02 µg dosage of gV3526 was not tested with CpG + AlOH. Error bars represent the 95% confidence level.
Figure 4.
Virus-binding antibody responses induced to VEEV TrD virus by increased dosages of gV3526 vaccination. Sera were obtained from mice on Day 49 post-primary vaccination, one week prior to challenge. These data are reported as the geometric mean endpoint titers as determined by ELISA. Escalating dosages consisted of 0.15, 0.25 and 0.4 µg gV3526. All antibody titers were below detectable levels in sham-vaccinated mice. The 0.02 µg dosage of gV3526 was not tested with CpG + AlOH. Error bars represent the 95% confidence level.
Figure 5.
Survival rates in mice challenged by the aerosol route with 1×104 pfu VEEV TrD virus four weeks following the final vaccination. All sham-vaccinated mice succumbed to infection by Day 7 post-challenge. Escalating dosages consisted of 0.15, 0.25 and 0.4 µg gV3526. The 0.02 µg dosage of gV3526 was not tested with CpG + AlOH.
It has recently been reported that delivering escalating dosages of antigen stimulates stronger immune responses than multiple administrations of the same dosage [28]. In study #2, the effect of escalating dosages was evaluated by delivering a total dosage of gV3526 equivalent to that of Group 1 (0.8 µg), over a three dose schedule. Administration of three escalating dosages of gV3526 regardless of formulation did not induce higher antibody GMT compared to administration of a fixed dosage on the same schedule. In fact, ELISA and neutralizing antibody GMT were either equivalent to or significantly lower in mice vaccinated with escalating dosages compared to the fixed dosage (Figure 3, p<0.05, ANOVA). Survival rates following aerosol challenge in mice receiving three escalating dosages was also similar to the group of mice receiving three fixed dosages (Figure 5).
3.6 Intramuscular vaccination induces a better protective response against aerosol challenge than SC vaccination
A comparison of survival rates against aerosol challenge in IM and SC vaccinated mice with similar dosages of gV3526 formulated with adjuvants provides additional support to our suggestion that IM vaccination induces a more protective immune response than SC vaccination. In these studies, 10 of 10 mice vaccinated IM with 0.2 µg gV3526 with CpG or CpG + AlOH survived aerosol challenge (Figure 5) whereas 3 of 10 mice vaccinated SC with 0.2 µg gV3526/CpG and 4 of 10 mice vaccinated SC with 0.2 µg gV3526/CpG + AlOH survived aerosol challenge (Figure 2a). The differences in survival rates following aerosol challenge between mice vaccinated with the same dosages by either the IM or SC routes are statistically significant (p<0.01, Fisher’s Exact Test).
3.7 Clinical Observations Post-Challenge
Clinical signs of disease (ruffed fur) were observed 6 to 8 days following aerosol challenge in <20% of mice vaccinated with gV3526 formulated with adjuvant. In all instances except one, mice with signs of illness ultimately succumbed to challenge. In general, observed illnesses were followed by death within 24 to 48 hours. Neither severity nor duration of clinical illness differed depending on the concentration of gV3526 administered. One of 9 C-84 immunized mice displayed similar mild clinical signs of disease. In contrast, 10 of 10 PCM inoculated mice demonstrated more advanced clinical signs as early as Day 4 post-challenge and all succumbed to infection by Day 7 post-challenge.
4.0 Discussion
Previous nonclinical studies with live-attenuated V3526 showed promise [11, 12, 14] for this technology as a replacement vaccine for TC-83 for prevention of VEEV disease. Of particular note was the ability of V3526 to protect against aerosol exposures to closely related VEEV subtypes [13, 29]. However, further development of V3526 was stopped during Phase 1 clinical testing when a significant number of volunteers demonstrated adverse responses to the vaccine. With the intent to reduce the adverse event profile of V3526, while retaining its potential as a protective immunogen against VEEV subtype IA/B and closely related VEEV subtypes, we proceeded to develop V3526 as an inactivated vaccine using gamma radiation.
The use of ionizing radiation has been explored in the development of vaccines for prevention of a broad spectrum of infectious diseases in cattle [30] and humans [31–35]. Several advantages are associated with using gamma radiation to inactivate viruses. The production methodology for gamma irradiation for inactivation of viruses is relatively straightforward in that bulk drug substance (live virus concentrate) does not have to be thawed prior to inactivation. Further, the methodology does not require additional purification steps that are performed when virus or microbe is inactivated via chemical agents such as formalin.
Gamma irradiation abolishes the replication capacity of the agent without affecting its ability to induce antigen-specific T-cell responses and protective immunity [30]. Although T-cell responses were not evaluated in this study, we did observe decreased epitope integrity following gamma irradiation which may decrease antigenicity of the gV3526. A decrease in antigenicity was also observed following gamma irradiation of VEEV performed in the 1970s [19]. Reitman et al. suggested the reduced antigenicity may be related to the virus preparation, specifically the quantity of radioprotective substances in the propagation medium [19]. This observation may provide an explanation for the reduced epitope binding observed in the current study as the V3526 was purified prior to inactivation.
Our initial studies showed SC administration of low dosages of gV3526 were highly effective in protecting mice against SC challenge with 1 × 104 pfu of VEEV TrD but was not as successful in protecting against an aerosol challenge. Similar results were observed in mice vaccinated by the IM route with low dosages of gV3526. These data demonstrate the ease of inducing immune responses protective against a SC challenge and the difficulties in protecting against an aerosol challenge. However, when gV3526 was administered IM at higher dosages and formulated with adjuvants, a trend toward increased protection was observed. These data suggested that both antigen concentration and formulation with an adjuvant are critical factors in inducing sufficient levels of immunity to protect against VEEV aerosol challenge. Although the increase in survival between the 0.04 µg/dose group and the higher dose groups was not significant in this study, the need for higher dosages should continue to be evaluated as higher dosages may be necessary to achieve longer durations of protection.
In these studies, the level of neutralizing antibody titers induced by vaccination did not correlate with survival following challenge with VEEV TrD. This may, in part, be due to the loss of epitope binding observed following inactivation. The E2c epitope is located in the neutralization domain of the E2 glycoprotein and antibodies directed at epitopes within this domain are reported to have virus neutralizing activity [36]. Similarly, antibodies directed at the E3 epitope are also reported as having virus neutralizing activity [23]. Destruction of the E3, E2c and potentially other proximal epitopes may render the inactivated virus ineffective in stimulating the production of high neutralizing antibody titers. The dosing study described in this report supports this idea in that an increase in dosage of gV3526, thus an increase in preserved epitopes, results higher neutralizing antibody titers. However, even at these higher neutralizing antibody titers, a correlation between titer and survival were not found. These findings suggest that while neutralizing antibody is induced via immunization with gV3526, other facets of the immune response are triggered by administration of inactivated virus vaccines [37], particularly those formulated with adjuvant.
Administration of CpG in combination with vaccine antigens is known to activate additional pathways of the innate and adaptive immune systems including, but not limited to, stimulation of antigen presenting cells, cytokine production, and stimulation of humoral as well as cell-mediated responses [38, 39]. The trend of increased immunogenicity and efficacy in mice vaccinated with gV3526 formulated with adjuvants, particularly those containing CpG, further indicates the importance of formulating inactivated whole virus vaccines with adjuvants to engage other immune mechanisms that are necessary in the prevention of VEEV clinical disease.
The use of CpG adjuvants [40], particularly CpG+Alhydrogel™ [41, 42] has been evaluated for immune enhancing activity with vaccines directed against other pathogens including, Plasmodium falciparum, influenza and hepatitis B and has also been shown to induce more robust immune responses compared to other adjuvants evaluated in those studies. Safety studies conducted in animals with CpG ODNs found them to be less reactogenic than other adjuvants [42–44] and has led to the use and testing of CpG ODN 7909 in human clinical trials [45, 46]. Of particular relevance to our study, a mouse study assessing safety of CpG ODNs administered IM reported minimal to no tissue damage in the injected tissue [42]. Similar results were reported in clinical studies assessing CpG safety following IM administration [40, 46]. Collectively, these CpG safety and efficacy studies support the further evaluation of CpG as an adjuvant for gV3526 either alone or in combination with Alhydrogel™.
The current study was designed to compare the induction of a protective immune response following vaccination of mice with multiple dosages of gV3526 when formulated with adjuvants. In this study, we demonstrated a high level of protection against SC challenge using low dosages of gV3526 and that the use of adjuvants was not necessary. On the other hand, protection against aerosol challenge was more difficult to achieve. By increasing the dosage and formulating gV3526 with adjuvants, a high level of protection was observed that was not predicted by neutralizing antibody titers. In summary, the high level of protection afforded by the gV3526 formulations evaluated in this study provide the foundation and rationale for further investigation of gV3526 as a candidate vaccine in the prevention of percutaneous infection to VEEV such as natural infection via mosquitoes or accidental needle stick as well as protection against either accidental or deliberate exposure to VEEV aerosols.
Acknowledgements
The research described herein was sponsored by the National Institute of Allergy and Infectious Diseases Grant Number 1UC1AI062538-01 and the Joint Science and Technology Office-Chemical, Biological Defense Plan1.1C0041_09_RD_B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Weaver SC, Ferro C, Barrera R, Boshell J, Navarro JC. Venezuelan equine encephalitis. Annu.Rev.Entomol. 2004;49:141–174. doi: 10.1146/annurev.ento.49.061802.123422. [DOI] [PubMed] [Google Scholar]
- 2.Dietz WH, Jr., Peralta PH, Johnson KM. Ten clinical cases of human infection with venezuelan equine encephalomyelitis virus, subtype I-D. Am J Trop Med Hyg. 1979;28(2):329–334. doi: 10.4269/ajtmh.1979.28.329. [DOI] [PubMed] [Google Scholar]
- 3.de Mucha-Macias J, Sanchez-Spindola I. Two human cases of laboratory infections with Mucambo virus. American Journal of Hygeine. 1965;14:475–478. [PubMed] [Google Scholar]
- 4.McKinney RW, Berge TO, Sawyer WD, Tigertt WD, Crozier D. Use of an attenuated strain of venezuelan equine encephalomyelitis virus for immunization in man. Am J Trop Med Hyg. 1963;12(4):597–603. doi: 10.4269/ajtmh.1963.12.597. [DOI] [PubMed] [Google Scholar]
- 5.Cole FE, Jr., May SW, Eddy GA. Inactivated Venezuelan equine encephalomyelitis vaccine prepared from attenuated (TC-83 strain) virus. Appl.Microbiol. 1974;27(1):150–153. doi: 10.1128/am.27.1.150-153.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burke DS, Ramsburg HH, Edelman R. Persistence in humans of antibody to subtypes of Venezuelan equine encephalomyelitis (VEE) virus after immunization with attenuated (TC- 83) VEE virus vaccine. J.Infect.Dis. 1977;136(3):354–359. doi: 10.1093/infdis/136.3.354. [DOI] [PubMed] [Google Scholar]
- 7.Pittman PR, Makuch RS, Mangiafico JA, Cannon TL, Gibbs PH, Peters CJ. Long-term duration of detectable neutralizing antibodies after administration of live-attenuated VEE vaccine and following booster vaccination with inactivated VEE vaccine. Vaccine. 1996;14(4):337–343. doi: 10.1016/0264-410x(95)00168-z. [DOI] [PubMed] [Google Scholar]
- 8.McKinney RW. Inactivated and live VEE vaccines - A review; PAHO Scientific Publication: Proc. of the workshop symposium on Venezuelan Encephalitis; 1972. pp. 369–389. [Google Scholar]
- 9.Edelman R, Ascher MS, Oster CN, Ramsburg HH, Cole FE, Eddy GA. Evaluation in humans of a new, inactivated vaccine for Venezuelan equine encephalitis virus (C-84) J.Infect.Dis. 1979;140(5):708–715. doi: 10.1093/infdis/140.5.708. [DOI] [PubMed] [Google Scholar]
- 10.Davis NL, Brown KW, Greenwald GF, et al. Attenuated mutants of Venezuelan equine encephalitis virus containing lethal mutations in the PE2 cleavage signal combined with a second-site suppressor mutation in E1. Virology. 1995;212(1):102–110. doi: 10.1006/viro.1995.1458. [DOI] [PubMed] [Google Scholar]
- 11.Fine DL, Roberts BA, Teehee ML, et al. Venezuelan equine encephalitis virus vaccine candidate (V3526) safety, immunogenicity and efficacy in horses. Vaccine. 2007;25(10):1868–1876. doi: 10.1016/j.vaccine.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 12.Hart MK, Caswell-Stephan K, Bakken R, et al. Improved mucosal protection against Venezuelan equine encephalitis virus is induced by the molecularly defined, live-attenuated V3526 vaccine candidate. Vaccine. 2000;18(26):3067–3075. doi: 10.1016/s0264-410x(00)00042-6. [DOI] [PubMed] [Google Scholar]
- 13.Hart MK, Lind C, Bakken R, Robertson M, Tammariello R, Ludwig GV. Onset and duration of protective immunity to IA/IB and IE strains of Venezuelan equine encephalitis virus in vaccinated mice. Vaccine. 2002;20(3–4):616–622. doi: 10.1016/s0264-410x(01)00337-1. [DOI] [PubMed] [Google Scholar]
- 14.Pratt WD, Davis NL, Johnston RE, Smith JF. Genetically engineered, live attenuated vaccines for Venezuelan equine encephalitis: testing in animal models. Vaccine. 2003;21(25–26):3854–3862. doi: 10.1016/s0264-410x(03)00328-1. [DOI] [PubMed] [Google Scholar]
- 15.Holley P, Fine DL, Terpening SJ, et al. Safety of an Attenuated Venezuelan Equine Encephalititis Virus (VEEV) Vaccine in Humans. 48th ICAAC/IDSA Meeting.2008. [Google Scholar]
- 16.Jahrling PB, Stephenson EH. Protective efficacies of live attenuated and formaldehyde-inactivated Venezuelan equine encephalitis virus vaccines against aerosol challenge in hamsters. J.Clin.Microbiol. 1984;19(3):429–431. doi: 10.1128/jcm.19.3.429-431.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordan RT, Kempe LL. Inactivation of some animal viruses with gamma radiation from cobalt-60. Proc.Soc.Exp Biol Med. 1956;91(2):212–215. doi: 10.3181/00379727-91-22215. [DOI] [PubMed] [Google Scholar]
- 18.Gruber J. Immunogenicity of Purified Venezuelan Equine Encephalitis Virus Inactivated by Ionizing Radiation. Infect Immun. 1971;3(4):574–579. doi: 10.1128/iai.3.4.574-579.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reitman M, Tribble HR, Jr., Green L. Gamma-irradiated Venezuelan equine encephalitis vaccines. Appl.Microbiol. 1970;19(5):763–767. doi: 10.1128/am.19.5.763-767.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenkins E, Parker MD, Bakken R, et al. A Multisystem Approach for the Evaluation of Inactivation Efficiency for Venezuelan Equine Encephalitis Virus (VEEV) Vaccine Candidates; 12th Annual Conference on Vaccine Research; Baltimore, MD. 2009. [Google Scholar]
- 21.Beaty BJ, Calisher CH, Shope RE, Schmidt NJ, Emmons RW, editors. American Public Health Association. 6th ed. Washington, DC: 1989. Diagnostic procedures for viral, rickettsial and chlamydial infections; pp. 797–855. Arboviruses. [Google Scholar]
- 22.Roehrig JT, Mathews JH. The neutralization site on the E2 glycoprotein of Venezuelan equine encephalomyelitis (TC-83) virus is composed of multiple conformationally stable epitopes. Virology. 1985;142(2):347–356. doi: 10.1016/0042-6822(85)90343-5. [DOI] [PubMed] [Google Scholar]
- 23.Buckley M, Hart MK. Characterization of VEE Virus E2-Specific Monoclonal Antibodies [dissertation] Frederick (MD): Hood College; 1997. [Google Scholar]
- 24.Earley E, Peralta PH, Johnson KM. A plaque neutralization method for arboviruses. Proc.Soc.Exp Biol Med. 1967;125(3):741–747. doi: 10.3181/00379727-125-32194. [DOI] [PubMed] [Google Scholar]
- 25.Hart MK, Pratt W, Panelo F, Tammariello R, Dertzbaugh M. Venezuelan equine encephalitis virus vaccines induce mucosal IgA responses and protection from airborne infection in BALB/c, but not C3H/HeN mice. Vaccine. 1997;15(4):363–369. doi: 10.1016/s0264-410x(96)00204-6. [DOI] [PubMed] [Google Scholar]
- 26.Guyton AC. Measurement of the respiratory volumes of laboratory animals. American Journal of Physiology. 1947;150:70–77. doi: 10.1152/ajplegacy.1947.150.1.70. [DOI] [PubMed] [Google Scholar]
- 27.Pratt WD, Gibbs P, Pitt ML, Schmaljohn AL. Use of telemetry to assess vaccine-induced protection against parenteral and aerosol infections of Venezuelan equine encephalitis virus in non-human primates. Vaccine. 1998;16(9–10):1056–1064. doi: 10.1016/s0264-410x(97)00192-8. [DOI] [PubMed] [Google Scholar]
- 28.Johansen P, Storni T, Rettig L, et al. Antigen kinetics determines immune reactivity. Proc.Natl.Acad Sci U.S.A. 2008;105(13):5189–5194. doi: 10.1073/pnas.0706296105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reed DS, Larsen T, Sullivan LJ, et al. Aerosol exposure to western equine encephalitis virus causes fever and encephalitis in cynomolgus macaques. J Infect Dis. 2005;192(7):1173–1182. doi: 10.1086/444397. [DOI] [PubMed] [Google Scholar]
- 30.Sanakkayala N, Sokolovska A, Gulani J, et al. Induction of antigen-specific Th1-type immune responses by gamma-irradiated recombinant Brucella abortus RB51. Clin Diagn.Lab Immunol. 2005;12(12):1429–1436. doi: 10.1128/CDLI.12.12.1429-1436.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alsharifi M, Furuya Y, Bowden TR, et al. Intranasal flu vaccine protective against seasonal and H5N1 avian influenza infections. PLoS.One. 2009;4(4):e5336. doi: 10.1371/journal.pone.0005336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Datta SK, Okamoto S, Hayashi T, et al. Vaccination with irradiated Listeria induces protective T cell immunity. Immunity. 2006;25(1):143–152. doi: 10.1016/j.immuni.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Eisenberg GH, Jr., Osterman JV. Gamma-irradiated scrub typhus immunogens: broad-spectrum immunity with combinations of rickettsial strains. Infect Immun. 1979;26(1):131–136. doi: 10.1128/iai.26.1.131-136.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quan FS, Lee JB, Bae JS, Ohwatari N, Min YK, Yang HM. Resistance to reinfection in rats induced by irradiated metacercariae of Clonorchis sinensis. Mem.Inst.Oswaldo Cruz. 2005;100(5):549–554. doi: 10.1590/s0074-02762005000500016. [DOI] [PubMed] [Google Scholar]
- 35.Chattopadhyay R, Conteh S, Li M, James ER, Epstein JE, Hoffman SL. The Effects of radiation on the safety and protective efficacy of an attenuated Plasmodium yoelii sporozoite malaria vaccine. Vaccine. 2009;27(27):3675–3680. doi: 10.1016/j.vaccine.2008.11.073. [DOI] [PubMed] [Google Scholar]
- 36.Mathews JH, Roehrig JT. Determination of the protective epitopes on the glycoproteins of Venezuelan equine encephalomyelitis virus by passive transfer of monoclonal antibodies. J Immunol. 1982;129(6):2763–2767. [PubMed] [Google Scholar]
- 37.Storni T, Kundig TM, Senti G, Johansen P. Immunity in response to particulate antigen-delivery systems. Adv.Drug Deliv.Rev. 2005;57(3):333–355. doi: 10.1016/j.addr.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Davis HL. Use of CpG DNA for enhancing specific immune responses. Curr.Top Microbiol Immunol. 2000;247:171–183. doi: 10.1007/978-3-642-59672-8_12. [DOI] [PubMed] [Google Scholar]
- 39.Klinman DM, Currie D, Gursel I, Verthelyi D. Use of CpG oligodeoxynucleotides as immune adjuvants. Immunol Rev. 2004;199:201–216. doi: 10.1111/j.0105-2896.2004.00148.x. [DOI] [PubMed] [Google Scholar]
- 40.Cooper CL, Davis HL, Morris ML, et al. Safety and immunogenicity of CPG 7909 injection as an adjuvant to Fluarix influenza vaccine. Vaccine. 2004;22(23–24):3136–3143. doi: 10.1016/j.vaccine.2004.01.058. [DOI] [PubMed] [Google Scholar]
- 41.Mullen GE, Giersing BK, Ajose-Popoola O, et al. Enhancement of functional antibody responses to AMA1-C1/Alhydrogel, a Plasmodium falciparum malaria vaccine, with CpG oligodeoxynucleotide. Vaccine. 2006;24(14):2497–2505. doi: 10.1016/j.vaccine.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 42.Weeratna RD, McCluskie MJ, Xu Y, Davis HL. CpG DNA induces stronger immune responses with less toxicity than other adjuvants. Vaccine. 2000;18(17):1755–1762. doi: 10.1016/s0264-410x(99)00526-5. [DOI] [PubMed] [Google Scholar]
- 43.Ioannou XP, Griebel P, Mena A, et al. Safety of CpG oligodeoxynucleotides in veterinary species. Antisense Nucleic Acid Drug Dev. 2003;13(3):157–167. doi: 10.1089/108729003768247628. [DOI] [PubMed] [Google Scholar]
- 44.Stewart VA, McGrath S, Krieg AM, et al. Activation of innate immunity in healthy Macaca mulatta macaques by a single subcutaneous dose of GMP CpG 7909: safety data and interferon-inducible protein-10 kinetics for humans and macaques. Clin Vaccine Immunol. 2008;15(2):221–226. doi: 10.1128/CVI.00420-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cooper CL, Davis HL, Angel JB, et al. CPG 7909 adjuvant improves hepatitis B virus vaccine seroprotection in antiretroviral-treated HIV-infected adults. AIDS. 2005;19(14):1473–1479. doi: 10.1097/01.aids.0000183514.37513.d2. [DOI] [PubMed] [Google Scholar]
- 46.Ellis RD, Mullen GE, Pierce M, et al. A Phase 1 study of the blood-stage malaria vaccine candidate AMA1-C1/Alhydrogel((R)) with CPG 7909, using two different formulations and dosing intervals. Vaccine. 2009;27(31):4104–4109. doi: 10.1016/j.vaccine.2009.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]