Abstract
The most common test to identify latent tuberculosis is the Tuberculin skin test that detects T cell responses of delayed type hypersensitivity type IV. Since it produces false negative reactions in active tuberculosis or in high-risk persons exposed to tuberculosis patients as shown in this report, we studied antibody profiles to explain the anergy of such responses in high-risk individuals without active infection.
Our results showed that humoral immunity against Tuberculin, regardless of the result of the Tuberculin skin test is important for protection from active tuberculosis and that the presence of high antibody titers is a more reliable indicator of infection latency suggesting that latency can be based on the levels of antibodies together with in vitro proliferation of peripheral blood mononuclear cells in the presence of the purified protein derivative. Importantly, anti-Tuberculin IgG antibody levels mediate the anergy described herein, which could also prevent reactivation of disease in high-risk individuals with high antibody titers. Such IgG Tuberculin antibodies were also found associated with blocking and/or stimulation of in vitro cultures of PBMC with Tuberculin. In this regard, future studies need to establish if immune responses to Mycobacterium tuberculosis can generate a broad spectrum of reactions either toward Th1 responses favoring stimulation by cytokines or by antibodies and those toward diminished responses by Th2 cytokines or blocking by antibodies; possibly involving mechanisms of antibody dependent protection from Mtb by different subclasses of IgG.
Introduction
Mycobacterium tuberculosis (Mtb) infection is a major world public health problem; over 2.0 million people die every year from this common infection. One third of the world’s population is thought to have latent tuberculosis (LTBI) [Smith. 2003], a condition where individuals are infected by the intracellular bacteria without exhibiting the active disease but are at risk for reactivation, if their immune system fails. The infection by Mtb is accompanied by non-specific inflammatory responses regulated by cytokines and chemokines produced by macrophages which are activated by toll-like receptors and dendritic cells [Gehring et al, 2003, Lin. 2005]. Also, interferon (IFNγ), an inflammatory cytokine, stimulates the antimicrobial activity of macrophages and regulates their antigen presentation through the MHC class II molecules by up-regulating their mRNA and protein expression [Pier, 2004]. As well, IFNγ can induce autophagy, a mechanism that plays an important role in the innate immunity against intracellular microorganisms [Harris et al, 2007 and Vergne et al, 2006]; MHC type II restricted CD4+T cells, MHC class I CD8+T cells and macrophages are important in the protective immunity against Mtb where a decrease of the number or function of these cells results in the reactivation of the infection [Tully et al, 2005]. And, γ/δ T cells play an important role in the protective immune response to tuberculosis (TB) [Szereday et al, 2003].
The most common screening for Mtb infection in asymptomatic patients (LTBI) are the Tuberculin skin test (TST) and chest × rays to detect the evidence of the Ghon complex (a granuloma that contains an organized collection of immune cells, predominantly macrophages). The TST is performed by intradermal injection in the anterior forearm of 5 units (0.1 ml) of Tuberculin. Reaction in the skin to Mtb, purified protein derivative (PPD) also named Tuberculin begins when T cells, sensitized by vaccination or infection, are recruited to the intradermal site and lymphokines are locally secreted. These lymphokines cause vasodilatation and edema plus recruitment of additional inflammatory cells. A positive reaction usually begins 5–6 hours after injection, reaching a maximum point at 48–72 hours and continues over a few days [Pier, 2004]. The results of the TST are based on the immune status of the individual and three cut off points have been recommended for a positive reaction to Tuberculin based on the size of the indurations seen after injection of the antigen: 1) 5 mm or more: individuals with HIV infection, recent contacts of TB patients, LTBI in patients with organ transplants, and other immuno-suppressed patients receiving corticosteroids (i.e., prednisone) for at least one month, 2) 10 mm or more: recent immigrants (within 5 years) from countries with high TB prevalence, recent infection with Mtb, immuno-compromised individuals other than HIV positive individuals, intravenous drug users, and health care workers with exposure to TB, and 3) 15 mm and greater: people with no risk to TB [American Thoracic Society, 2000]. Unfortunately in the absence of chest X-rays, which unequivocally show the absence of Ghon complexes the TST, is not reliable to detect LTBI, to predict disease progression, nor to determine the risk of disease reactivation [Chee et al, 2007]. Chest X-rays may not unveil the Ghon complexes that help to contain the spread of Mtb [Pier, 2004] and more sensitive radiological techniques are frequently unavailable in areas were Mtb infections are common. In 2001, the IFNγ release assays (IGRAs) [Chee et al, 2007] were developed with the advantage of no false positives secondary to BCG or exposure to non-tuberculosis mycobacterial strains.
The absence of a positive TST has also been described in immunocompetent anergic patients diagnosed with active TB; inability to mount an antigen specific DTH response to PPD was shown to be associated with a defective T cell response including an antigen-specific impaired inability to produce IL-2 and to proliferate in response to the PPD [Delgado et al, 2002 and Sousa et al, 1997] regardless of prior BCG vaccination status [Jasmer, 2002]. These findings could be due to a defective phosphorylation of T cell receptor (TCR) leading to a defective activation of ZAP-70 and MAPK proteins related to IL2 gene transcription and protein production after antigen stimulation of T cells [Boussiotis et al, 2000]. Or, decreases on the γ/δ T cells subset [Szereday, 2003], decreases of IFN-γ production, increase of IL-10 in response to PPD [Boussiotis et al, 2000], and a failure of the autophagic control by macrophages have been associated with anergy in ATBI [Harris et al, 2007]. However, studies of anergy in LTBI have not been performed before. In this regard, we studied individuals without ATBI, with different degrees of exposure to patients with ATBI from a tuberculosis specialized hospital in Bogota, Colombia. In a first cohort we observed a high incidence of negative TST in high-risk contacts. Based on these preliminary results (Cohort I) we investigated a second cohort for TST, in-vitro proliferation of peripheral blood leukocytes (PBMC) with Tuberculin to establish anergy more frequently in high-risk contacts. Also, we studied the role antituberculin and anti-HLA antibodies in anergic and non-anergic contacts or hospital employees since humoral immune responses had been described in anergic individuals diagnosed with ATBI (Sousa et al, 1997).
Methods
Baseline characteristics of subjects studied
The two cohorts of study subjects are shown in Table A. Cohort I was recruited between the years of 1999 and 2001 from a community hospital specialized in the treatment of tuberculosis in Bogotá, Colombia. The majority of these individuals had received BCG vaccination shortly after birth. All subjects were studied by the TST. We did not include immunocompromised individuals such as HIV positive patients, patients using steroids, or patients with neoplasias that could affect the TST [Chee et al, 2007]. The subjects of cohort I included 29 high-risk personal contacts (spouses and significant others) with patients with established diagnosis of active tuberculosis infection (ATBI) and compared them with 71 employees with a range of service of 5 to 23 years (nurses, administrators, general services, and security). Based on the assumption that nurses have higher risk due to high patient contact, we analyzed the 40 nurses as a separate group A, and the 31 other employees as group B. All three groups showed a higher percentage of women and the mean age was 40 years. Cohort 2 was recruited from 2002 to 2006 from the same hospital and consisted of 91 individuals, 12 high-risk, 30 nurses, and 49 hospital employees. The gender distribution and mean age was comparable to Cohort 1. We also tested 6 healthy Colombians and 43 Mexicans as controls. All subjects signed a consent form and the institutional review board at Dana-Farber Cancer Institute approved the protocol for the studies. After consent was obtained, 0.1 ml of Tubersol (5 Tuberculin units (TU) PPD; Aventis-Pasteur, Swiftwater, PA) was injected intradermally in the forearms of the subjects who were then evaluated for induration 48 hours later and scored as positive if the diameter of induration was 5 mm or greater.
Table A. Baseline characteristics of individuals exposed to patients with active tuberculosis (ATBI).
Skin test reactions were measured in all individuals and responsiveness was assessed based on the diameter (mm) of induration after PPD antigen intradermal administration. Cohort 1 shown in the left was used as exploratory to determine the frequency of negativity of TST in the high-risk group. Cohort 2 shown in the right risk confirmed the high frequency of TST negatives in the high-risk group, compared with the group B of the hospital employees.
| Cohort 1 | Cohort 2 | Controls | |||||
|---|---|---|---|---|---|---|---|
| High Risk | Hospital Employees | High Risk | Hospital Employees | Unknown exposure to ATBI |
|||
| Personal Contacts |
Hospital(Nurses) | Hospital employees |
Personal Contacts |
Hospital (Nurses) |
Hospital employees |
||
| Group A | Group B | Group A | Group B | ||||
| n=29 | n=40 | n=31 | n=12 | n=30 | n=49 | n=49 | |
| Age (mean, +/− SD) | 43, +/− 16.4 | 40, +/− 7.9 | 43, +/− 9.9 | 47 +/− 17.6 | 37 +/− 11.8 | 38 +/− 8.1 | 33 +/− 10 |
| Gender (n, %) | |||||||
| Male | (7) 24 % | (4) 7 % | (14) 45% | (7) 58% | (6) 20% | (16) 33% | (22) 45% |
| Female | (22) 76 % | (37) 93% | (17) 55% | (5) 42% | (24) 80% | (33) 67% | (27) 55% |
| BCG Vaccine (n, %) | |||||||
| Yes | (12) 75% | (34) 95% | (29) 94% | (10) 83% | (29) 97% | (43) 82% | (49) 100% |
| No | (4) 25% | (2) 5% | (2) 6% | (2) 17% | (1) 3% | (6) 12% | (0) 0% |
| TST (n, %) | |||||||
| Positive | (8) 28% | (22) 55% | (18) 58% | (2) 17% | (12) 40% | (31) 51% | N.D. |
| 5–9 mm | 3 | 3 | 3 | 0 | 3 | 6 | N.D. |
| 10–14 mm | 4 | 8 | 5 | 0 | 5 | 10 | N.D. |
| 15 or more mm | 1 | 11 | 10 | 2 | 4 | 14 | N.D. |
| Negative | (21) 72% | (18) 45% | (13) 42% | (10) 83% | (18) 60% | (18) 37% | N.D. |
HLA typings
HLA-A, B, and C alleles were typed using polymerase chain reaction and sequence-specific oligonucleotide probes hybridization techniques (PCR-SSOP) as described previously [Cao, 1999]. Briefly extracted DNA was amplified using intronic locus-specific and exonic group-specific primers. The PCR products were immobilized onto nylon membranes that were hybridized with sets of probes matching polymorphic sequences of HLA-A, B, and C alleles. The hybridization signal was detected with a chemiluminescent substrate. Alleles were assigned based on the hybridization patterns with the sets of oligonucleotide probes.
Serology testing
The serum from each subject was screened for anti-HLA antibodies using beads coated with soluble HLA class I and II antigens and was analyzed with luminex (an instrument based on indirect immunofluorescence) using pre-screened positive sera for HLA class I or II antibodies as a positive control and normal human serum as a negative control. We also screened for antibodies anti IgM and IgG against the PPD antigen. Sera from the 4 panels were tested for IgM and IgG antibodies against PPD antigen by enzyme-linked immuno-absorbent assay (ELISA) as follows: 96 wells microtiter plates were coated with 25 ng/well of PPD antigen (Aventis-Pasteur, Swiftwater, PA) overnight at 4°C. Excess protein binding sites were blocked by incubation with 0.5% BSA in PBS-Tween 20 0.5% at 37°C for 1 hour. The plates were washed 4 times with PBS containing 0.5% Tween 20. Sera samples were diluted 1:50 in PBS containing 0.5% BSA and added to the plates for 1 hour of incubation at 37°C. The plates were washed 4 times and then incubated with a second antibody (peroxidase-conjugated monoclonal antibody anti-alpha chain IgM diluted 1:5000 or IgG diluted 1:10000 in blocking solution for 1 hour at 37°C) and washed 4 times (Sigma-Aldrich, US). A substrate solution containing 10 µl of 30% H2O2 and 25 mg o-phenylenediamine (OPD) dihydrochloride (Sigma-Aldrich) in 25 ml citrate buffer at pH 5 was added and incubated for 15 min at room temperature. Fifty microliters of 1N H2SO4 solution were used to stop the reaction. Color development was measured in an ELISA reader at 492 nm [Araujo et al, 2004].
Antigen presentation assays
Subjects from cohort 2 were tested by the TST twice during a period of 3 months and the samples for in vitro proliferation assays from this group were obtained 7–10 days following the last TST. Samples from individuals were processed in Boston, MA 12 hours after collection. (The blood samples were collected in EDTA and an investigator carried them by air from Colombia). Peripheral blood mononuclear cells (PBMC) and plasma were separated from whole blood samples from each subject. In this experiment we measured the ability of leucocytes to proliferate in vitro after stimulation with antigens. Triplicates of individual’s PBMC (1 × 105cells) were incubated with three different antigens: 1) Phytoheamagglutinin (PHA, 2ug/ml, Sigma-Aldrich, St. Louis, MO), a mitogen that stimulates T lymphocytes proliferation used as a control test; 2) Candida albicans (20ug/ml, Green laboratories, Lenoir, NC), used to test each individual’s antigen-specific T cell proliferation and to evaluate the possibility of anergy; and 3) Tuberculin (10ug/ml, Mycos Research LLC, Loveland, CO). The cells were incubated with human group AB or with autologous sera to demonstrate the cellular proliferation in these subjects for 3 days with PHA and 5 days with Candida or PPD at 37°C, 5% CO2. The cells were labeled with [H3] thymidine (1 µCi/well) 6 hours prior to harvesting. Plates were harvested and the radioactivity was monitored by the Wallac liquid scintillation counter (Perkin-Elmer, Boston, MA). PBMC from each subject was isolated and cultured with PHA which served to determine the cell viability, and with Tuberculin (PPD) or Candida albicans to test antigen-specific T cell proliferation measured by the intake of thymidine (H3) as counts per minute (cpm) of the mean of triplicate cultures and expressed by stimulation index (SI) = cpm in cultures with each antigen divided by cpm in culture without antigen.
Statistical methods
Differences between groups were determined using the Mann-Whitney U test and the chi-square or Fischer exact method when needed with STATA version 9.0. The statistical difference was considered significant when P values were less or equal to 0.05.
Results
Frequency distribution of TST of contacts exposed to patients with ATBI
Table A showed the two cohorts of individuals exposed to ATBI. Cohort I was used to determine the frequency of negative results from the TST in the high-risk group and showed an increased proportion of negative TST in personal contacts, compared to those of group B: 72% versus 42 %, p= .01. Cohort I produced the hypothesis that personal contacts to patients with ATBI had a high incidence of false negative TSTs. We confirmed these results in a second cohort, the group of high-risk showed higher frequency of negative TST (83%) compared to Group B (37%), p=. 01. Of interest, the group A composed of nurses showed intermediate frequency of TST negativity between the high risk group and the group B of hospital employees, but not significantly different than that of group B. The number of years employees were exposed to patients were, 12 to 20 for physicians, 15 to 23 for microbiologists, 5 to 22 for nurses, 12 for social workers, 6 to 27 for ambulance drivers, 1 to 10 for security, 3 to 19 for X-ray techs, 1 to 23 for administration and 6 to 20 for general services. Furthermore, the exposure contacts for spouses, significant others and siblings that were included had a minimum of 6 months since diagnosis of ATBI.
Antituberculin (IgG and IgM) studies of contacts to patients with ATBI
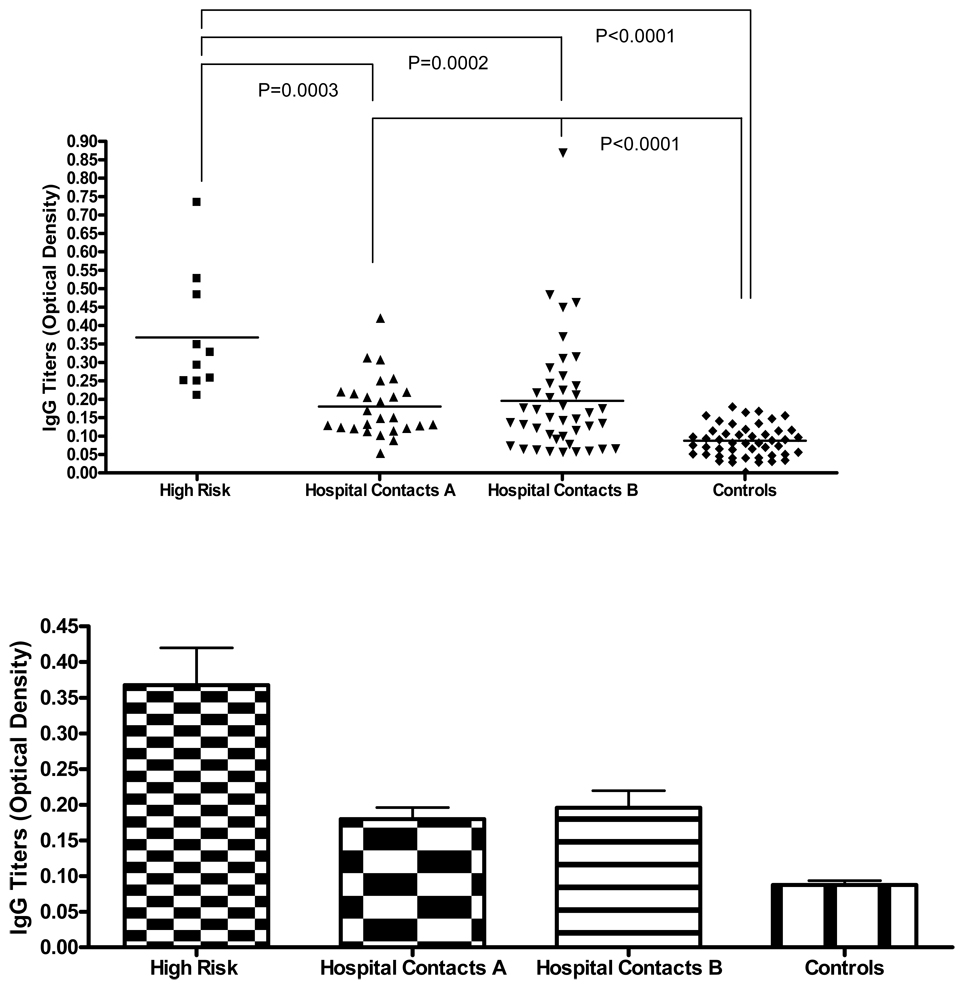
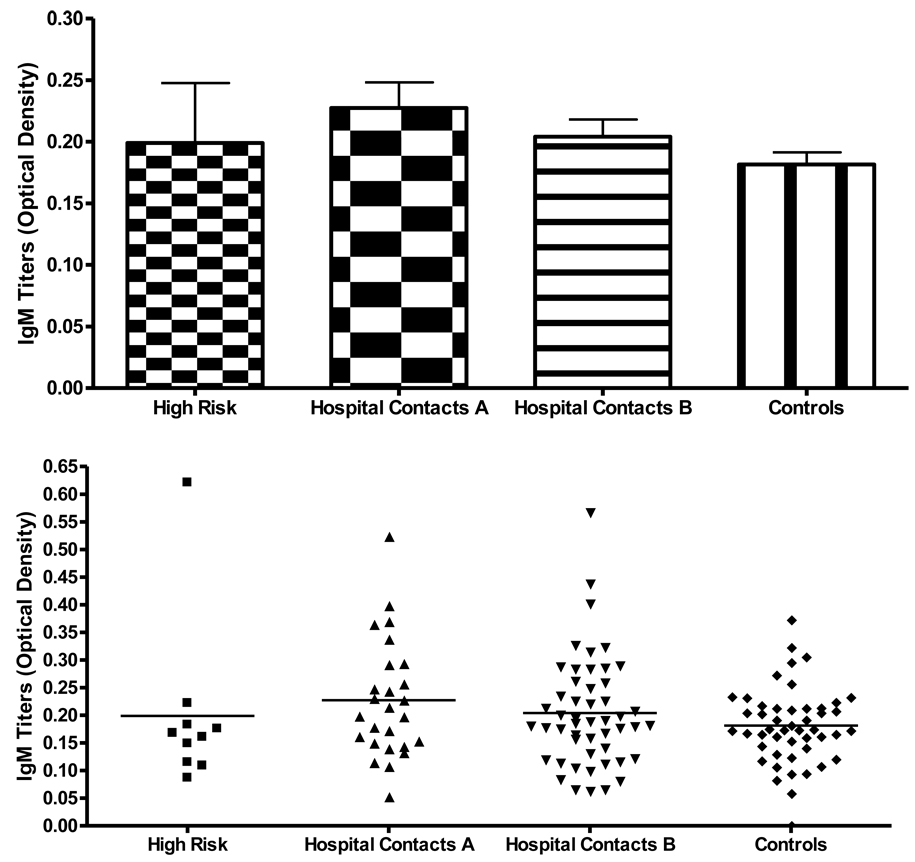
86 out of 104 individuals of the second cohort from Bogotá, Colombia and 49 controls from Mexico City and from Bogotá were tested for IgG and IgM antibodies to Tuberculin antigen that was used for TST. All individuals were tested for TST at least twice before their serum samples were taken. Sera separated 7–10 days after the last phlebotomy were analyzed for specific antibodies reactive to Tuberculin antigen in order to investigate the role of IgG and/or IgM antibodies against Tuberculin. The results were expressed as mean optical density (O.D.). Data from 10 of the high risk group, 26 of group A, 43 of group B, that included 8 friends of patients with ATBI were analyzed for IgG and IgM antibodies against Tuberculin, they are shown in Figure 1 and Figure 2. Fig 1 shows the means and S.D. (standard deviations) of IgG levels as follows: high risk as .39 +/− .42, group A as .18 +/− .03, group B as .18+/− .03. The mean of the controls was .08 +/− .037. The mean values for IgM were comparable in the four groups.
Figure 1. IgG anti-PPD titers in different groups of individuals exposed and non exposed to Mtb.
* IgG titers are significant decreased in controls compared to low and medium risk individuals.
Figure 2. IgM anti-PPD titers in different groups of individuals exposed and non-exposed to Mtb.
The high-risk group (10) had titers of more than .21 O.D. which is more than three standard deviations higher than the mean for IgG compared to the controls. The figure also shows the significant p values when comparing the high-risk group versus Groups A and B and the controls. Also, the combined comparison between Groups A and B had higher titers than the control groups p=.0001.
In vitro proliferation studies from contacts to patients with ATBI; studies of anergy in individuals with a negative TST and positive in vitro proliferation with Tuberculin
Table B showed 9 high-risk, 5 group A and 4 group B of hospital employees with anergy and 3 high risk, 3 group A and 6 group B TST positives without anergy. The PBMC cultures incubated with Tuberculin (PPD) antigen showing significant stimulation indexes (SI) in 18 anergic [negative TST individuals showing significant proliferation (S.I. ranging from 7.9 to 561.1) in the presence of Tuberculin] and 7 TST negative non-anergic, p=.0001.
Table B. PBMC proliferation and tuberculin antibody studies in 37 individuals with different degrees of exposure to ATBI patients.
There were 18 anergic (TST negative, positive proliferation in tuberculin cultures), 12 controls (positive TST, positive proliferation in tuberculin cultures) and 7 non anergic (Negative TST, and negative proliferation).
| Subjects No. |
Risk | TST mm |
Tuberculin Cultures SI |
Candida Cultures SI |
IgG Anti-Tuberculin (O.D.) |
||
|---|---|---|---|---|---|---|---|
| 1* | High | 0 | 144 | 115.8 | 0.25 | ||
| 2* | High | 0 | 301.2 | 89.7 | 0.29 | ||
| (3*) | High | 0 | 31.7 | 42.5 | 0.35 | ||
| 4* | High | 0 | 78.8 | 49.9 | 0.73 | ||
| 5 | High | 0 | 78 | 55 | 0.21 | ||
| 6 | High | 0 | 20.1 | 12.8 | ND | ||
| 7 | High | 0 | 17.1 | 39.8 | 0.25 | ||
| 8 | High | 0 | 7.9 | 1 | 0.33 | ||
| 9* | High | 0 | 561.1 | 37.4 | 0.35 | ||
| 10* | Group A | 0 | 200.4 | 120.1 | 0.22 | ||
| 11 | Group A | 0 | 140.7 | 37.9 | ND | ||
| 12 | Group A | 0 | 19.5 | 41 | 0.15 | ||
| 13 | Group A | 0 | 45.7 | 43.9 | 0.42 | ||
| 14 | Group A | 0 | 15 | 48 | 0.21 | ||
| 15* | Group B | 0 | 309.6 | 30.1 | 0.21 | ||
| 16* | Group B | 0 | 53.9 | 56.2 | 0.37 | ||
| 17* | Group B | 0 | 19.3 | 42.1 | 0.31 | ||
| {18*} | Group B | 0 | 27.7 | 17.9 | 0.89 | ||
| 19* | High | 13 | 42.2 | 20.4 | 0.53 | ||
| 20* | High | 25 | 44.4 | 16.8 | 0.25 | ||
| 21 | High | 25 | 218 | 18.2 | ND | ||
| 22 | Group A | 14 | 80 | 65 | 0.11 | ||
| 23 | Group A | 7 | 3.6 | 1.2 | 0.15 | ||
| 24 | Group A | 23 | 142.2 | 38.1 | ND | ||
| 25* | Group B | 25 | 279.7 | 58.8 | 0.26 | ||
| 26* | Group B | 7 | 43.9 | 19.3 | 0.32 | ||
| [27*] | Group B | 10 | 51.1 | 37.7 | 0.22 | ||
| 28* | Group B | 12 | 18 | 17.3 | 0.45 | ||
| 29 | Group B | 16 | 61.2 | 28.3 | ND | ||
| 30 | Group B | 7 | 0.63 | 2.9 | 0.13 | ||
| 31 | Group A | 0 | 0.5 | 0.1 | ND | ||
| 32 | Group B | 0 | 1.1 | 1.2 | 0.17 | ||
| 33 | Group B | 0 | 0.8 | 0.7 | 0.08 | ||
| 34 | Group B | 0 | 1 | 5.2 | 0.09 | ||
| 35 | Group B | 0 | 0.66 | 0.7 | 0.11 | ||
| 36 | Group B | 0 | 0.97 | 0.8 | 0.10 | ||
| 37 | Group B | 0 | 0.65 | 0.9 | 0.11 | ||
The * were HLA antibody tested. HLA class I antibodies found: (HLA-B7, B81, and B50) identified as [27], (HLA-A80, B50) as (3) and (A80) as {18}.
IgG antituberculin levels in O.D were given.
SI: stimulation index, 3Hthymidine incorporation of PBMC cultures with tuberculin or Candida.
TST: tuberculin skin test. Diameter in mm of induration at 48 hours after intradermal injection
IgG antituberculin antibody studies of anergic individuals
Table B also showed data of hospital employees that included the anergics with comparable levels of IgG to those with positive TST; the control group of TST positive non-anergics showed 6 of 9 with high antituberculin antibodies, which was not different from individuals of the anergic group confirming that antibody titers (IgG against Tuberculin) is a more accurate measurement of immunity against Tuberculin than the TST. Also, 15 of 16 among the 18 anergic individuals tested, had high antibody titers against Tuberculin; O.D 0.350 of anergics versus 0.110, of the TST negative non-anergic group (p= .0001).
HLA antibody studies of anergic individuals
We studied whether anti-HLA antibodies can cross-react with Tuberculin if they share homologous sequences that could possibly lead to molecular mimicry, decreasing the recognition of the processed antigen via MHC class II to CD4+ T cells receptors. A total of 16, identified in table B with *, included 10 anergic individuals that were screened for such antibodies. 2 of the 10 anergic individuals with HLA antibodies against class I antigens were genotyped: the first HLA-A*301, C*0701, B*0801, A*2601, C*1203, B*3801; the second A*0201, C*0102, B*3501, A*2402, C*0501, B*44-2 and a third which was TST positive had the phenotype A*2902, C*1601, B*4403, A*0301, A*0301, C*702, B*0702. These three individuals demonstrated antibodies against class I: HLA (A80, B50), (HLA A80) and (B7, B81 and B50) alternatively. Importantly, these HLA phenotypes were not present in the individuals producing the antibodies suggesting a possible cross-reactivity between HLA antigens and Tuberculin, and also suggested they are not responsible for the production of their anergy, Table B.
BCG vaccination and the levels of IgG and IgM
94% of the individuals tested had a history of BCG vaccination but those with measurements of antibodies against PPD were 92% (79) with history of BCG, mean of O.D. (.214) vaccination, 8% without with BCG vaccination and a mean of O.D. (.148) without significant difference.
Blocking and stimulation of in vitro proliferation studies
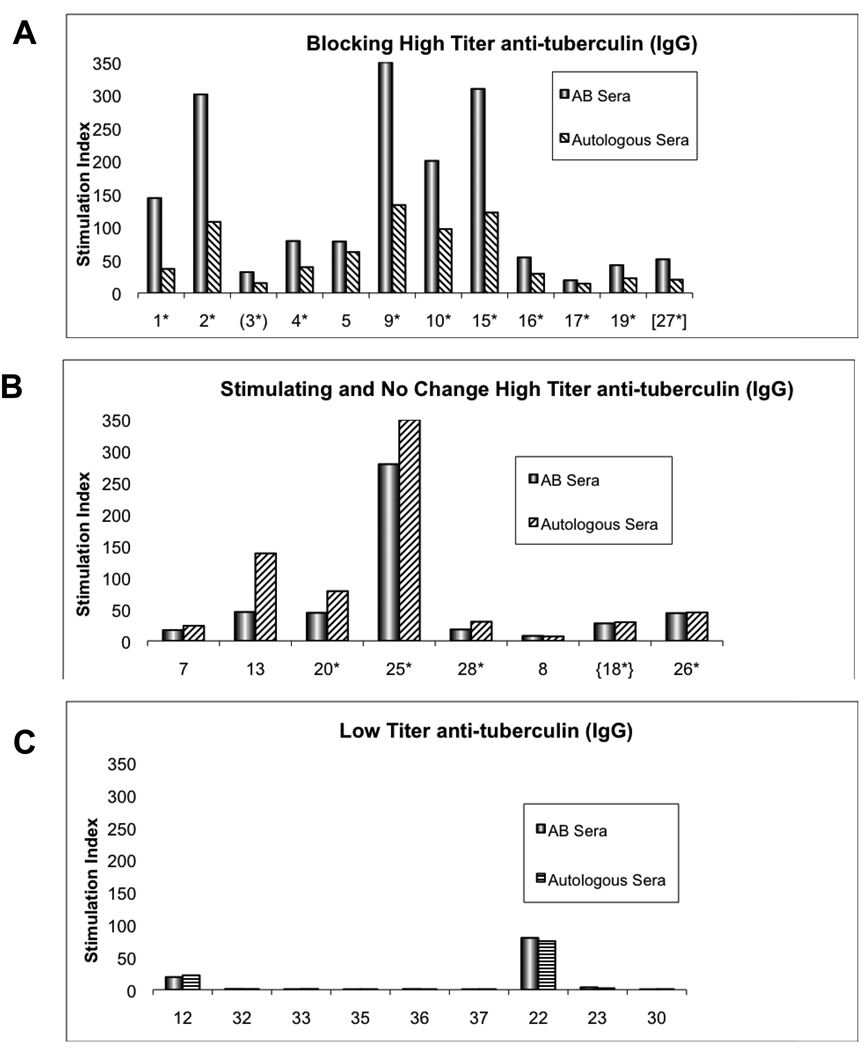
PBMC cultures incubated with Tuberculin (PPD) or Candida antigens were compared using AB versus autologous sera. Figure 3 showed 20 of such cultures in individuals with high IgG titers of Tuberculin antibodies; the proliferation by AB sera showed significant changes by autologous sera, 12 of these showed significant blocking, 5 stimulation and 3 with no changes. 10 cultures of contacts with low antibody titers (IgG) did not show any blocking or stimulation. Not shown were the cultures of 6 of the hospital employees that were not analyzed because they did not have antituberculin antibody measurements. These corresponded to individuals listed in Table B, numbers 11 and 29 with blocking, numbers 6, 24 and 31 with stimulation and number 21 without changes in the cultures with AB and autologous plasma. There was significant correlation between blocking of high titers versus low antibody titers, 12/12 versus 0/10 respectively, p=. 0.0001. Also, the high antibody titers were correlated with stimulation in 5/5 individuals versus the individuals with low antibody titers (0/10,) p=.001. All cultures in table B demonstrated significant proliferation with PHA with S.I. of 8 or higher. Comparable results of blocking and stimulation were obtained by cultures with Candida (data not shown).
Figure 3. Comparison of PBMC cultures with tuberculin; AB versus autologous sera.
The columns are expressed in stimulation indexes (SI) demonstrating that 12 cultures showed blocking (graph A) ranging from .18 to .76 fold decreases and 5 showed stimulation (graph B) from .52 to 7.45 fold increases. There were 10 cultures in low antibody titers against tuberculin (IgG) (graph C) without blocking or stimulation. The levels of IgG antituberculin antibodies are given in O.D. Numbers 1–5,7–13,15–18 and 32–37 were TST (−). Numbers 19, 20, 22, 23, 25, 26, 27, 28 and 30 were TST (+).
These cultures demonstrated a spectrum of immune responses in individuals with high antituberculin IgG antibodies, some producing blocking of proliferation by Tuberculin or Candida while others stimulation.
Discussion
LTBI is a condition wherein individuals are infected by Mycobacterium tuberculosis without having an active disease but are at risk for reactivation of their infection if their immune system fails as in AIDS [Havlir et al, 1999]. LTBI is identified by a positive TST and the presence of the Ghon complex evidenced by chest X rays, however, in many regions of the world access to X rays is somewhat difficult especially in high prevalence areas. The method employed for patient assessment is the TST, which is not accurate enough since it can be altered by the host immune status. Also, it has been demonstrated that even among patients diagnosed with ATBI 10 to 20 percent of these individuals are hypo-responsive to TST [Jasmer, 2002]. But as mentioned before while anergy has been only studied in ATBI, anergy of high-risk contacts with absence of active infection or the mechanism that produces such hypo-responsiveness is unknown. For these reasons we tested a group of individuals (cohort 1) who were exposed to active TB patients. These individuals included personal contacts (spouses and significant others of TB patients) that demonstrated a high incidence of negative TST and employees of a TB hospital from Bogota, Colombia (nurses, bacteriology laboratory technicians, doctors) exposed to patients with active disease for more than 5 years to them. These preliminary studies were corroborated in a second cohort of contacts that were anergic because they demonstrated in vitro responses in cultures of PBMC with Tuberculin.
Importantly, one of the first descriptions of the role of humoral and cell mediated immunity was demonstrated by the normal lymphocyte transfer test (NLT). In this test live lymphocytes were injected intradermally to compare the rejection of allografts with the size of the induration. A positive alloreactive reaction was determined by the skin induration after 24–72 hours of injection [Brent, 1963 and Gray, 1963]. These tests were not reliable since the reactions could not be reproduced during retesting with the same panel of lymphocytes. When their own serum samples were tested, high levels of HLA cytotoxic antibodies were found which correlated with the reduced reactivity to the skin test [Buckley, 1972 and Yunis, 2004] suggesting that antibodies could interfere with the recognition of antigenic determinants by lymphocytes. The antibodies are probably masking the antigen leading to cross reaction as described in association to infectious agents and autoimmune diseases [Oldstone, 1998 and Wucherpfennig, 2001] through the molecular mimicry model. In this model, similar protein structures are shared by molecules or by their protein products from different genes causing a cross reaction between the infectious agent at the T cell level or with antibodies, possibly because of the lack of specificity of the T cell receptor (TCR) recognition [Wucherpfennig, 2001]. This cross reactivity may lead to an immune response directed against autologous molecules involving auto reactive T lymphocytes if the shared epitope belongs to an antigen binding to MHC class II [Aguas et al, 1990]. Therefore, we analyzed the role antibodies have in the TST anergy and found the following: A) anti-HLA antibodies or antituberculin antibodies, which could possibly decrease the recognition of the processed antigen via MHC class II to CD4+ T cell receptors leading to anergy in the high-risk contacts to ATBI. B) IgM and IgG antibodies against to M. tuberculosis antigens in these individuals at risk. Although 2 of the 10 anergic individuals and 1 of the controls tested, demonstrated anti-HLA class 1 antibodies; HLA-A*80 and B*50 when compared to PPD positive subgroup, they could not explain the anergy because their reactivity was not against the phenotypes of the individuals producing the antibodies. However, they possibly resulted from cross-reactivity between class I HLA antigens and Tuberculin [Aguas et al, 1990]. Importantly it is believed that cell mediated immunity is the main mechanism of control in Tuberculosis; however we can infer from our results that immune responses to mycobacterium infection, in high-risk individuals, are controlled by both cell mediated and humoral immunity [Teitelbaum et al, 1998].
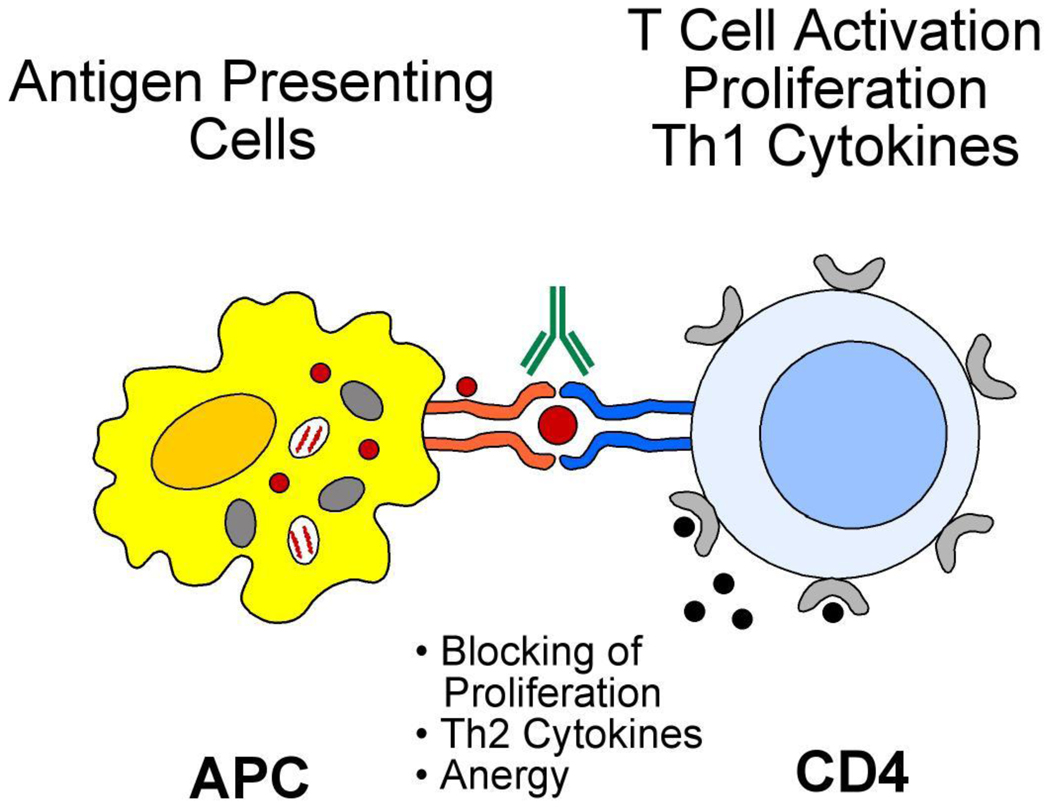
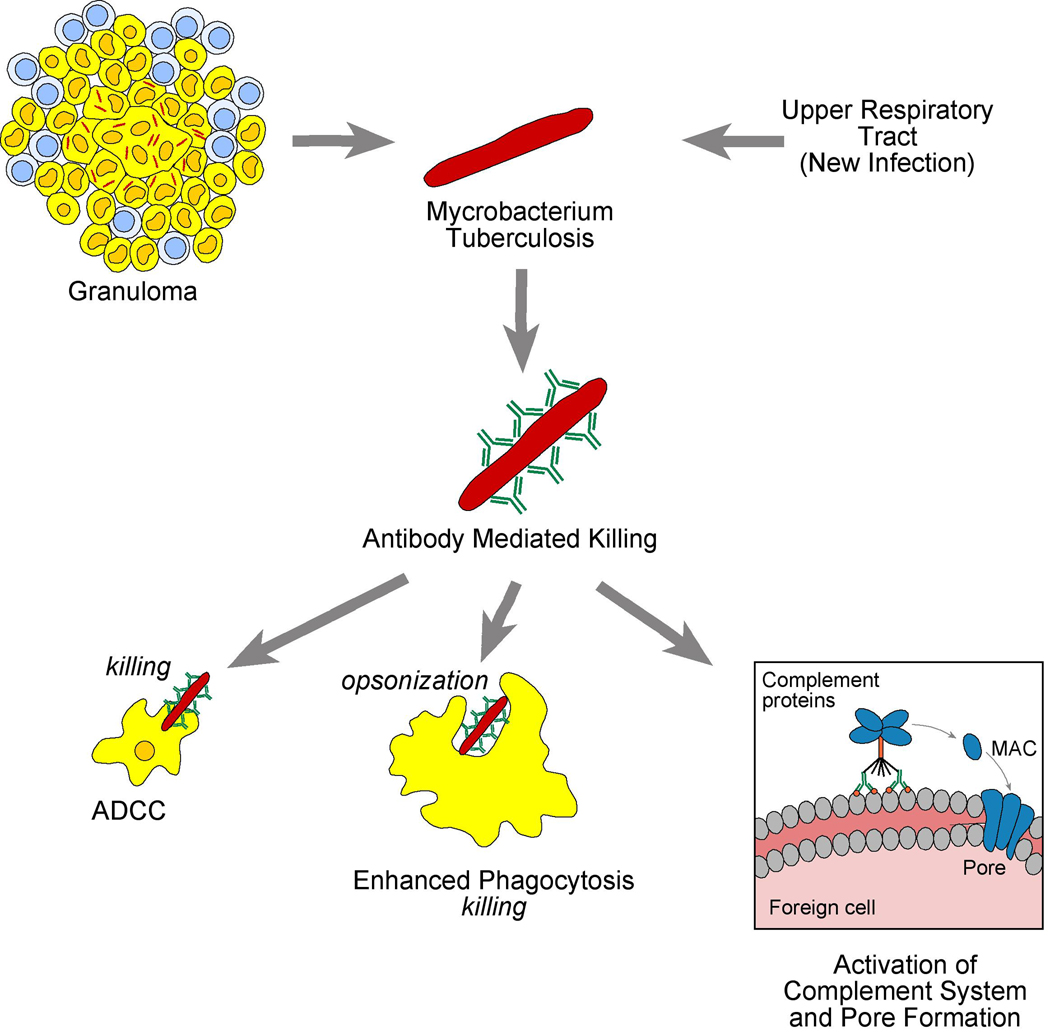
Higher risk contacts to TB with negative or positive TST had significantly higher IgG mean levels as compared to lower-risk contacts indicating that LTBI diagnosis can be based on the levels of antibodies (IgG) against Tuberculin in the absence of X rays regardless of their TST status, together with the in vitro proliferation of PBMC in the presence of PPD. IgG anti Tuberculin levels mediate a new form of anergy in which antibody to Tuberculin impairs the delayed type IV hypersensitivity in skin via macrophages and T cells (Fig 4).
Figure 4. Cellular immunity against Mtb.
Mechanism of antibody mediated anergy.
Our experiments of in-vitro cultures of PBMC with Tuberculin comparing the role of autologous sera with that of AB sera in ATBI contacts, demonstrated that the levels of IgG antituberculin antibodies correlated with blocking of the proliferation responses, although there was a spectrum of responses from blocking to stimulation (Fig 3). Such results suggested the polarity responses that could be produced by either Th1 to Th2 responses. In this regard, two different forms of leprosy correlate with Th1 or Th2 polarity of the immune response [Pier, 2004]. In tuberculoid leprosy, patients mount a strong Th1 type cell-mediated immune response producing interferon γ and interleukin 2 in lesions and localize the infection, restricting the growth of the bacteria. While in lepromatous leprosy, a widely disseminated form of infection is associated with a Th2 cytokine response with diminished cell-mediated immunity [Modlin et al, 1986]. It remains to be determined if the same polarity exists in contacts with ATBI that produce high levels of antituberculin IgG and can generate a broad spectrum of immune reactions with those toward Th1 responses favoring stimulation by autologous sera [Clay, 2008] and those toward decreased responses produced either blocking by antibodies [Aguas et al, 1990] or by Th2 cytokines [Delgado, et a 2,002] (figure 4). In this regard, our blocking or stimulation studies of in vitro proliferation by autologous sera could be due to different subclass of IgG that mediate reactions against Mtb that need to be investigated by: 1) increased killing by phagocytosis by the process of opsonization, 2) Type II hypersensitivity reaction by activation of complement possibly by IgG3, and 3) involvement of NK cells by ADCC, which suggests a possible interaction between antibodies and cell mediated immunity [Glatman-Freedman, 1998].
One interesting aspect of our studies was the possibility that the individuals studied have immunity to both Candida and Mycobacterium and that most individuals studied have a pro-inflammatory state. In the future it will be important to investigate the role of immunity to Candida and other antigens such as bacterial lipopolysaccharide (LPS) in relation to possible cross-reactivity or cross-stimulation by Tuberculin or Candida antigens. Previously, we had shown that up-regulation of the same pro-inflammatory genes in cultures with PPD or Candida in ATBI [Stern et al, 2008], which, is consistent with a report that immunity to Tuberculin is influenced by LPS antigens particularly in relation to protection of latency in HIV infection [Brenchley et al, 2006].
Conclusions
Our results represent an important advance in the detection of previous TB infection in anergic highly exposed individuals. They suggest screening for anti Tuberculin antibodies (IgG) to determine previous TB infection together with TST, chest x-rays and in vitro tests of cellular proliferation against Tuberculin. Furthermore investigations should particularly focus on Th1 and Th2 cytokine responses in high-risk individuals with low IgG titers against PPD, in order to develop novel immunization protocols that will increase humoral immunity against TB in individuals with or without BCG vaccination. This will in turn could produce protection against new infection or from reactivation of the disease [Havlir, 1999], in either immunocompromised patients or patients treated with immunosuppressors in order to avoid reactivation of the disease for example in patients with rheumatoid arthritis treated with anti TNF alpha [Hamdi et al, 2006].
Figure 5.
Acknowledgements
This work was supported by NIH grants AI49213, HL29583 and HL59838
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Thoracic Society. Targeted Tuberculin Testing and Treatment of Latent Tuberculosis Infection. American J Respir Crit Care Med. 2000;161:S221–S247. doi: 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- 2.Aguas A, Esaguy N, Sunkel CE, Silva MT. Cross-Reactivity and sequence homology between the 65-Kilodalton mycobacterial heat shock protein and human lactoferrin, transferrin, and DR beta Subsets of major histocompatibility complex class II molecules. Infection and Immunity. 1990;58:1461–1470. doi: 10.1128/iai.58.5.1461-1470.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araujo Z, Waard JH, Fernández de Larrea C, López D, Fandiño C, Maldonado A, Hernández E, Ocaña Y, Ortega R, Singh M, Ottenhoff TH, Arend SM, Convit J. Study of the antibody response against Mycobacterium tuberculosis antigens in Warao Amerindian children in Venezuela. Mem Inst Oswaldo Cruz. 2004;99:517–524. doi: 10.1590/s0074-02762004000500011. [DOI] [PubMed] [Google Scholar]
- 4.Boussiotis VA, Tsai EY, Yunis EJ, Thim S, Delgado JC, Dascher CC, Berezovskaya A, Rousset D, Reynes JM, Goldfeld AE. IL-10 producing T cells suppress immune responses in anergic tuberculosis patients. Journal of Clinical Investigation. 2000;105:1317–1325. doi: 10.1172/JCI9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek D. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Medicine. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 6.Brent L, Medawar PB. Tissue transplantation: a new approach to the “typing” problem. Br Med J. 1963;2:269–272. doi: 10.1136/bmj.2.5352.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckley RH, Schiff RI, Amos DB. Blocking of autologous and homologous leukocyte responses by human alloimmune plasmas: A possible in vitro correlate of enhancement. J Immunol. 1972;108:34–44. [PubMed] [Google Scholar]
- 8.Cao K, Chopek M, Fernandez-Vina M. High and intermediate resolution DNA typing systems for class I HLA-A, B, C genes by hybridization with sequence-specific oligonucleotide probes (SSOP) Rev Immunogenet. 1999;1:177–208. [PubMed] [Google Scholar]
- 9.Chee CB, KhinMar KW, Gan SH, Barkham TM, Pushparani M, Wang YT. Latent tuberculosis infection treatment and T-cell responses to Mycobacterium tuberculosis-specific antigens. Am J Respir Crit Care Med. 2007;175:282–287. doi: 10.1164/rccm.200608-1109OC. [DOI] [PubMed] [Google Scholar]
- 10.Clay H, Volkman HE, Ramakrishnan L. Tumor necrosis factor signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death. Immunity. 2008;29:283–294. doi: 10.1016/j.immuni.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delgado JC, Tsai EY, Thim S, Baena A, Boussiotis VA, Reynes JM, Sath S, Grosjean P, Yunis EJ, Goldfeld AE. Antigen-specific and persistent tuberculin anergy in a cohort of pulmonary tuberculosis patients from rural Cambodia. Proc Natl Acad Sci. 2002;99:7576–7581. doi: 10.1073/pnas.062056099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gehring AJ, Rojas RE, Canaday DH, Lakey DL, Harding CV, Boom WH. The Mycobacterium tuberculosis 19-kilodalton Lipoprotein Inhibits Gamma Interferon-Regulated HLA-DR and FC gamma R1 on human macrophages through Toll-Like Receptor. Infection and Immunity. 2003;71:4487–4497. doi: 10.1128/IAI.71.8.4487-4497.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glatman-Freedman A, Cassadevall A. Serum therapy for tuberculosis revisited: reappraisal of the role of antibodies-mediated immunity against Mycobacterium tuberculosis. Clinical microbiology reviews. 1998;11:514–532. doi: 10.1128/cmr.11.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray JG, Russell PS. Donor selection in human organ transplantation. A possible screening test. Lancet. 1963;2:863–865. doi: 10.1016/s0140-6736(63)92749-1. [DOI] [PubMed] [Google Scholar]
- 15.Hamdi H, Marriette X, Godot V, Weldingh K, Hamid A, Prejean M-V, Baron G, Emilie Inhibition of anti-tuberculosis T-lymphocyte function with tumour necrosis factor antagonists. Arthritis Research & Therapy. 2006;8:R114. doi: 10.1186/ar1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris J, De Haro SA, Master SS, Keane J, Roberts EA, Delgado M, Deretic V. T helper 2 cytokines inhibit autophagic control of intracellular Mycobacterium tuberculosis. Immunity. 2007;27:505–517. doi: 10.1016/j.immuni.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 17.Havlir DV, Barnes PF. Tuberculosis in patients with human immunodeficiency virus infection. New England J Med. 1999;340:367–373. doi: 10.1056/NEJM199902043400507. [DOI] [PubMed] [Google Scholar]
- 18.Jasmer RM, Nahid P, Hopewell PC. Clinical practice. Latent tuberculosis infection. N Engl J Med. 2002;347:1860–1866. doi: 10.1056/NEJMcp021045. [DOI] [PubMed] [Google Scholar]
- 19.Lin FC, Chen YC, Chen FJ, Chang SC. Cytokines and fibrinolytic enzymes in tuberculous and parapneumonic effusions. Clin Immunol. 2005;116:166–173. doi: 10.1016/j.clim.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 20.Modlin RI, Mehra V, Wong L, Fujimiya Y, Chang WC, Horwitz DA, Bloom BR, Rea TH, Pattengale PK. Suppressor T lymphocytes from lepromatous leprosy skin lesion. J Immunology. 1986;137:2831–2834. [PubMed] [Google Scholar]
- 21.Oldstone MB. Molecular mimicry and immune-mediated diseases. FASEB J. 1998;12:1255–1265. doi: 10.1096/fasebj.12.13.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pier G, Lyczak J, Wetzler L. Cell mediated immunity in Blackwell publishing . Immunology, Infection, and Immunity. Washington, D.C.: ASM Press; 2004. [Google Scholar]
- 23.Smith I. Mycobacterium tuberculosis Pathogenesis and Molecular Determinants of Virulence. Clin Microbiol Rev. 2003;16:463–496. doi: 10.1128/CMR.16.3.463-496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sousa AO, Salem JI, Lee FK, Verçosa MC, Cruaud P, Bloom BR, Lagrange PH, David HL. An epidemic of tuberculosis with a high rate of tuberculin anergy among a population previously unexposed to tuberculosis, the Yanomami Indians of the Brazilian Amazon. Proc Natl Acad Sci. 1997;94:13227–12232. doi: 10.1073/pnas.94.24.13227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stern J, Keskin DB, Romero V, Zuniga J, Encinales L, Li C, Awad C, Yunis EJ. Molecular signatures distinguishing active from latent tuberculosis in peripheral blood mononuclear cells, after in vitro antigenic stimulation with purified protein derivative of tuberculin (PPD) or Candida: a preliminary report. Immunol Res. 2008 doi: 10.1007/s12026-008-8024-2. 0257-277X(print) [DOI] [PubMed] [Google Scholar]
- 26.Szereday L, Baliko Z, Szekeres-Bartho J. Gamma/delta T cell subsets in patients with active Mycobacterium tuberculosis infection and tuberculin anergy. Clin Exp Immunol. 2003;131:287–291. doi: 10.1046/j.1365-2249.2003.02063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teitelbaum R, Glatman-Freedman A, Chen B, Robbins JB, Unanue E, Casadevall A, Bloom BR. A mAB recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Pro. Natl. Acad. Sic U S A. 1998;95:15688–15693. doi: 10.1073/pnas.95.26.15688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tully G, Kortsik C, Höhn H, Zehbe I, Hitzler WE, Neukirch C, Freitag K, Kayser K, Maeurer MJ. Highly focused T cell responses in Latent human pulmonary Mycobacterium tuberculosis infection. Journal of Immunology. 2005;174:2174–2184. doi: 10.4049/jimmunol.174.4.2174. [DOI] [PubMed] [Google Scholar]
- 29.Vergne I, Singh S, Roberts E, Kyei G, Master S, Harris J, de Haro S, Naylor J, Davis A, Delgado M, Deretic V. Autophagy in immune defense against Mycobacterium tuberculosis. Autophagy. 2006;2:175–178. doi: 10.4161/auto.2830. [DOI] [PubMed] [Google Scholar]
- 30.Wucherpfennig KW. Structural basis of molecular mimicry. J Autoimmun. 2001;16:293–302. doi: 10.1006/jaut.2000.0499. [DOI] [PubMed] [Google Scholar]
- 31.Yunis E. Bernard Amos A biographical memoir. Biographical memoirs. National academy of sciences. 2004:287. [Google Scholar]