Abstract
Objective
To determine the concentration of amino acids in women receiving the first course of antenatal betamethasone and to evaluate the umbilical venous and arterial amino acid concentrations at the time of elective caesarean section following betamethasone administration.
Study Design
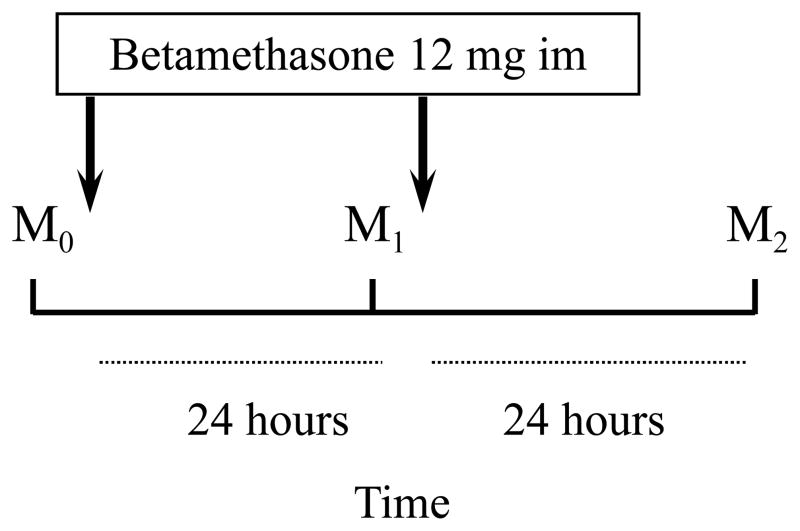
Blood samples were collected from 34 pregnant women at risk of premature delivery before and 24 and 48 hours after the first course of betamethasone. In addition, maternal and cord blood samples were collected in 13 women undergoing an elective cesarean section between 24 and 192 hours after betamethasone.
Results
Maternal amino acid concentrations were significantly increased after the first dose of betamethasone. Overall total amino nitrogen increased 17.5% 24 hours after betamethasone administration and 20.5% after 48 hours.
The concentration of most amino acids was increased both in the umbilical vein and artery after maternal betamethasone administration.
Conclusion
The concentration of maternal and fetal amino acids increases significantly after betamethasone administration.
Keywords: antenatal betamethasone, amino acids, human pregnancy
Introduction
There is no doubt that the administration of antenatal corticosteroids has radically changed the outcome of preterm babies 1 since it was first introduced in 1972.2 Since then, researchers have studied the effects of this treatment for the reduction of the incidence and severity of respiratory distress syndrome as well as for other derivative effects: i.e., beneficial short term effects have been reported associated with a significant reduction of the risk of cerebroventricular haemorrhage, necrotizing enterocolitis and intensive care admission. 3 In addition, an association with less developmental delay and probably less cerebral palsy in childhood have been reported as beneficial long term effects. 3 In the late nineties, however, animal studies began to raise some concerns regarding fetal growth: one to three doses administered to the mother, but not to the fetus, in early gestation have been reported to induce growth restriction in premature lambs 4 although this finding has not been confirmed in mice. 5 In human pregnancies, an independent association between repeated antenatal corticosteroids and impairment of fetal growth, as measured by birth weight, head circumference, and length has been reported. 6 One possible mechanism may be through an influence on the maternal and fetal insulin-IGF-GH axis 7: a single course of antenatal betamethasone has been found to increase maternal glucose, insulin and IGF-I but not IGF binding protein levels, whereas, in the fetal compartment, this treatment was associated with a decrease for GH and IGF-II levels. Recently it has been shown that fetuses exposed ≤ 48 hours to corticosteroids exhibit a significant increase of glucose, insulin, amino acids (AA) and free fatty acid concentrations accompanied by a significant decrease in IGF-I levels. 8 However, in that study the maternal concentration of amino acids was not measured. Hence any possible influence upon umbilical venous concentrations could not be determined.
The aim of the present study was twofold: first, to determine the plasma concentration of amino acids in women receiving the first course of antenatal betamethasone to enhance fetal lung maturation. Second, to evaluate the umbilical venous and arterial AA concentrations with respect to maternal concentrations in a group of women undergoing an elective caesarean section following betamethasone administration.
Materials and methods
The protocol for the study was approved by the Review Board of the Department of Medicine, Surgery, Dentistry of the San Paolo Hospital. Written informed consent was given by all study participants.
Patients
Studies of maternal concentrations in at-risk pregnancies
Thirty four pregnant women at risk of premature delivery who received their first course of antenatal betamethasone at 30.2 ± 3.3 (range 24.6 – 35.5) weeks of gestation (Celestone cronodose, Schering-Plough, 12 mg + 12 mg, 24 hours apart) were recruited for the study. 33/34 women were Caucasian, age was 33 ± 4.5 years, pre pregnancy body mass index was 22.3 ± 3.3 kg/m2. 8 pregnancies were multiple (6 twins and 2 triplets), 14 were complicated by intrauterine growth restriction, 2 by preeclampsia and 1 by preterm premature rupture of membranes: in the remaining 9 pregnancies no other complication but threatened preterm delivery was present.
Three maternal “arterialized” venous samples were collected from an antecubital vein after at least 4 hours of fasting: M0 before the first dose, M1 24 hours after the first dose and M2 48 hours after the first dose. The “arterialization” of the brachial venous samples was obtained by placing the arm between two heated thermophores whose temperature was adjusted until the maternal peripheral blood oxygen saturation was >90%: the sampling site of the brachial vein was proximal to the wrist.
Maternal and fetal studies at cesarean section
Thirteen pregnant women underwent an elective cesarean section between 24 and 192 hours (mean 92 ± 65 hours) after betamethasone administered according to the same protocol (M0 before the first dose, M1 24 hours after the first dose and M2 48 hours after the first dose). Indication for cesarean section was intrauterine growth restriction (7 cases), multiple pregnancy (4 cases), preeclampsia (1 case) and fetal Rh immunization (1 case).
Four patients (two multiple, one IUGR and the one with preeclampsia) are also included in the analysis of maternal concentrations. In all cases, cesarean section was performed under general anesthesia: none of the mothers had entered labor. At the time of fetal extraction, umbilical arterial and venous blood samples were obtained from a doubly clamped segment of the cord. A maternal arterial sample was collected simultaneously.
The data obtained in these patients were compared to historical data collected in two groups of pregnant patients undergoing elective cesarean section under general anesthesia: a first group of 16 pregnant patients at term who did not receive antenatal betamethasone and a second group of 10 pregnant patients receiving an amino acid formulation (Freamine 8.5% III, Baxter) before cesarean section. The latter represent a group with high maternal AA concentrations without exposure to betamethasone. Complete data on these patients have been previously published. 9
Methods
All blood samples were collected into heparinized syringes that were immediately sealed and stored in ice. Plasma for amino acid analyses was separated by centrifugation at −4°C and frozen at −70°C until the time of analysis.
Maternal glucose concentration and umbilical arterial pH at delivery were measured in duplicate on a Radiometer ABL 330 analyzer (Copenhagen, Dk).
Plasma was quickly thawed and deproteinized with a solution of 10% sulfosalycilic acid with nor-leucine added as an internal standard and buffered with LiOH to pH 2.2. After centrifugation at 14,000 rpm for 10 minutes, the supernatant fraction was filtered through a millipore filter and loaded into a Dionex HPLC with refrigerated autosampler.
The samples were analyzed by cation exchange column with three buffers changed by gradient isothermally. Ninhydrin was used as color reagent and a dual wavelength spectrophotometer with 440 nm and 570 nm wavelengths were used for concentration determinations. Column, buffers and ninhydrin reagent were purchased from Pickering laboratory. All the instrument operation and data processing were controlled by DIONIX’S AI-450 software. Samples from each study were analyzed on a single column in the same run, with a variance of ± 2%.
Statistical analysis
Data are presented as mean ± sem. Amino acid concentration differences between the three maternal samples in the study during pregnancy were tested with ANOVA for repeated measures and with the Scheffè multiple comparison procedure.. The Student t test for paired samples was used to test the difference between the umbilical venous and arterial fetal samples in the group receiving antenatal betamethasone and the historical control group. The relationships between fetal and maternal amino acid concentrations were analyzed by linear regression analysis determined by the least-squared method using Statistica for Windows (StatSoft, Tulsa, Okla) software. Differences between slopes were tested with the t-test. P<0.05 were considered significant.
Results
Studies of maternal concentrations in at-risk pregnancies
Table 1 presents the mean concentration of individual amino acids in M0, M1, M2. The concentration of all amino acids except arginine, lysine, histidine, glutamic and aspartic acid, asparagine and taurine was significantly increased after betamethasone administration. Alanine was the non essential AA whose concentration increased the most throughout the study (36.6% from M0 to M1; 44.3% from M0 to M2).
Table 1.
Maternal arterialized amino acid concentrations (μM). Data are mean ± sem.
| Amino acid | M0 | M1 | M2 | p |
|---|---|---|---|---|
| Arginine | 65.5 ± 5.3 | 77.1 ± 5.2 | 75.9 ± 4.1 | |
| Phenylalanine | 52.2 ± 2.1 | 59.3 ± 2.2 | 58.5 ± 2.1 | 0.04 |
| Lysine | 150.6 ± 9.5 | 163.9 ± 10 | 178.9 ± 9.3 | |
| Histidine | 88.6 ± 3.1 | 93.4 ± 4.4 | 90.8 ± 3.1 | |
| Methionine | 21.3 ± 1 | 29.3 ± 1.3 *** | 30.3 ± 1.8 *** | 0.001 |
| Threonine | 187.2 ± 9.2 | 234.4 ± 14.3 ‡ | 266.6 ± 15.9 *** | 0.001 |
| Valine | 154.5 ± 4.9 | 179.7 ± 6.6 ** | 175 ± 5.5 † | 0.01 |
| Leucine | 77.2 ± 2.8 | 89.9 ± 3.7 § | 88.5 ± 3.6 | 0.02 |
| Isoleucine | 46.5 ± 1.7 | 59 ± 2.1 *** | 60 ± 1.9 *** | 0.001 |
| Serine | 95.3 ± 3.5 | 115.6 ± 4.3 ** | 111.3 ± 4.3 * | 0.002 |
| Glycine | 152.7 ± 9 | 190.9 ± 9.6 * | 186.8 ± 9.7 † | 0.01 |
| Alanine | 312.7 ± 15.7 | 493.4 ± 27.2 *** | 561.2 ± 19.3 *** | 0.001 |
| Glutamine | 429 ± 18.6 | 504.6 ± 23.9 | 517.1 ± 25.7 § | 0.02 |
| Glutamate | 59.1 ± 5 | 61.3 ± 7 | 58.5 ± 6 | |
| Asparagine | 49.8 ± 3.6 | 63.8 ± 6.2 | 63.6 ± 5 | |
| Aspartate | 8.2 ± 1 | 9.1 ± 1.2 | 8.5 ± 1 | |
| Proline | 157.8 ± 7.6 | 194.2 ± 10.4 * | 216.1 ± 9 *** | 0.001 |
| Taurine | 26.1 ± 1 | 32.3 ± 2.5 ** | 27.7 ± 2.1 | |
| Tyrosine | 39.3 ± 1.9 | 49.3 ± 3.2 * | 47.4 ± 2.1 | 0.01 |
| N Essential mg/L | 19.1 ± 0.7 | 21.8 ± 0.9 ‡ | 22.5 ± 0.8 ** | 0.01 |
| N non Ess mg/L | 25.2 ± 0.9 | 31.8 ± 1.3 *** | 33.2 ± 1.1 *** | 0.001 |
| Total N mg/L | 44.3 ± 1.3 | 53.7 ± 1.9 *** | 55.7 ± 1.7 *** | 0.001 |
p<0.001;
p<0.01;
p<0.02;
p<0.03;
p<0.04;
p<0.05 for M1 and M2 compared to M0
Overall total amino nitrogen increased 17.5% at 24+ hours after betamethasone administration and 20.5% after 48 hour.
In the same period the mean maternal glucose concentration significantly increased after the first dose (from 4.8 ± 0.9 mM to 5.6 ± 1 mM; p<0.001) and remained higher thereafter (5.2 ± 0.7 mM after 48 hours).
Maternal and fetal studies at cesarean section
Since the study of maternal concentrations had shown that the concentration of most amino acids remained higher than the basal value at 48 hours, to evaluate the fetal concentrations and the fetal-maternal relationships, the patients were separated into two groups: patients undergoing caesarean section within 48 hours (B <48: mean time 40.8 ± 11.6 hours) and after 48 hours (B ≥48; mean time 156 ± 40.6 hours) of betamethasone administration. Table 2 presents the clinical information on the study patients. No significant differences were found for any of the parameters included in the table with the exception of gestational age and fetal weight that were significantly higher in the Control and Freamine groups when compared to the betamethasone groups (p<0.001).
Table 2.
Maternal age, pre pregnancy weight, height, BMI, gestational age at delivery, fetal and placental weight and umbilical arterial pH at delivery. Data are mean ± sem.
| Clinical data | B <48 | B ≥48 | Control | Freamine |
|---|---|---|---|---|
| N= 8 | N = 5 | N= 16 | N= 10 | |
| Maternal age (years) | 34 ± 1.7 | 35.4 ± 2 | 34 ± 0.2 | 32 ± 08 |
| Maternal pre pregnancy weight (kg) | 64.2 ± 4 | 63.1 ± 4.7 | 69.6 ± 1.5 | 69.3 ± 2 |
| Maternal height (cm) | 163.6 ± 2 | 169.4 ± 1.6 | na | na |
| Maternal BMI (kg/m2) | 24 ± 1.5 | 22.1 ± 2 | na | 22.9 ± 1.5 |
| Gestational age at delivery (weeks) | 33 ± 0.8 | 35.2 ± 0.4 | 38.5 ± 0.2 | 38.5 ± 0.2 |
| Fetal weight (grams) | 1763 ± 298 | 2119 ± 125 | 3248 ± 72 | 3173 ± 112 |
| Placental weight (grams) | 391 ± 101 | 621 ± 298 | 432 ± 22 | 440 ± 38 |
| Umbilical artery pH | 7.30 ± 0.01 | 7.31 ± 0.02 | 7.28 ± 0.01 | 7.29 ± 0.01 |
B <48: cases delivered within 48 hours from maternal betamethasone administration; B ≥48: cases delivered after at least 48 hours from maternal betamethasone administration. na= not available
Table 3 presents the concentrations of AA in the umbilical vein and in the umbilical artery, respectively, in the B <48 and B ≥48 and the control groups. The concentrations of phenylalanine, methionine, threonine, valine, leucine, serine, glycine, alanine, glutamine and proline were significantly increased both in the umbilical vein and artery of betamethasone treated pregnancies when compared to controls. In the umbilical vein the concentration of isoleucine and tyrosine was also increased. The umbilical venoarterial total nitrogen concentration difference was not significantly different (B <48: 2.6 ± 2 g/L; B ≥48: 2.4 ± 1.9 g/L) from controls (3 ± 0.7 g/L). In the betamethasone groups however, the umbilical venoarterial concentration differences were not significantly different from 0.
Table 3.
Umbilical venous and arterial amino acid concentrations (μM). Data are mean ± sem
| Amino acid | Cases delivered <48 hours form B | Cases delivered ≥ 48 hours form B | Controls | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Umb vein | Umb Art | p | Umb vein | Umb Art | p | Umb vein | Umb Art | p | |
| Arginine | 129.5 ± 5 | 110.6 ± 8.4 | 0.01 | 112.8 ± | 116.9 ± 8.6 | 0.7 | 110 ± 7.5 | 102.2 ± 4.7 | 0.01 |
| Phenylalanine | 77.3 ± 3.8 | 74.6 ± 2.4 | 0.3 | 64.6 ± 4.6 | 66.8 ± 3.4 | 0.3 | 65.5 ± 1.6 | 63.6 ± 2.1 | 0.05 |
| Lysine | 375.7 ±26.4 | 353.7 ± 22.9 | 0.06 | 378.9 ± | 396.5 ± 26.5 | 0.2 | 345.1 ± 10.9 | 319.1 ± 10.2 | 0.001 |
| Histidine | 104.9 ± 5.4 | 97.8 ± 4.4 | 0.05 | 123.1 ± | 128.9 ± 20.8 | 0.2 | 119.6 ± 3.4 | 108.4 ± 3.1 | 0.001 |
| Methionine | 40.8 ± 2.3 | 37.5 ± 2.4 | 0.1 | 30.9 ± 4.7 | 35.8 ± 2.1 | 0.2 | 25.4 ± 0.9 | 24.6 ± 1.1 | 0.1 |
| Threonine | 362.6 ± 27 | 366.5 ± 30 | 0.7 | 365.3 ± | 373.1 ± 26.5 | 0.5 | 276.1 ± 16.4 | 265.3 ± 16.5 | 0.01 |
| Valine | 257.5 ± 9.1 | 242.8 ± 8.7 | 0.04 | 203.4 ± | 212.1 ± 10.3 | 0.1 | 218.3 ± 8.1 | 202.3 ± 7.9 | 0.001 |
| Leucine | 141.8 ± 6.7 | 129.1 ± 5.6 | 0.01 | 103.5 ± | 113.3 ± 4.7 | 0.02 | 120.3 ± 4.6 | 105.8 ± 3.9 | 0.001 |
| Isoleucine | 88.8 ± 4.8 | 70.8 ± 8.7 | 0.1 | 61.9 ± 5.2 | 69.4 ± 5.4 | 0.05 | 64.1 ± 3.2 | 56.6 ± 2.9 | 0.001 |
| Serine | 169.7 ± 7.3 | 180.3 ± 10.1 | 0.02 | 162.5 ± | 157.9 ± 7.8 | 0.1 | 142.8 ± 4.2 | 145.3 ± 4.2 | 0.1 |
| Glycine | 314.7 ± 28.2 | 326.3 ± 28.5 | 0.3 | 292.8 ± | 289.9 ± 17 | 0.6 | 219.8 ± 8 | 220.1 ± 9.1 | 0.9 |
| Alanine | 510.1 ± 33.6 | 475.9 ± 31.7 | 0.03 | 400.8 ± | 442.9 ± 50.7 | 0.02 | 286.3 ± 12.3 | 241.8 ± 10.2 | 0.001 |
| Glutamine | 768.7 ± 53.7 | 763 ± 50.9 | 0.8 | 555 ± | 546.7 ± 36.6 | 0.7 | 495.7 ± 10.9 | 485.3 ± 13.5 | 0.2 |
| Glutamate | 50.5 ± 11.4 | 78.4 ± 18.7 | 0.05 | 73 ± 15 | 54.7 ± 9.8 | 0.2 | 27.5 ± 7.7 | 43 ± 6.5 | 0.01 |
| Proline | 234.4 ± 19.8 | 220.3 ± 29 | 0.2 | 219.3 ± | 230 ± 24 | 0.3 | 157.1 ± 5.2 | 143.9 ± 6.4 | 0.01 |
| Taurine | 89.6 ± 6.9 | 103.9 ± 7.6 | 0.2 | 84.5 ± | 80.3 ± 7.4 | 0.7 | 96.1 ± 5.6 | 107.3 ± 11.7 | 0.3 |
| Tyrosine | 69.8 ± 3.4 | 70.5 ± 3.4 | 0.7 | 66.2 ± 5.6 | 67.4 ± 4.4 | 0.6 | 59.9 ± 2 | 60 ± 2.3 | 0.9 |
B: betamethasone
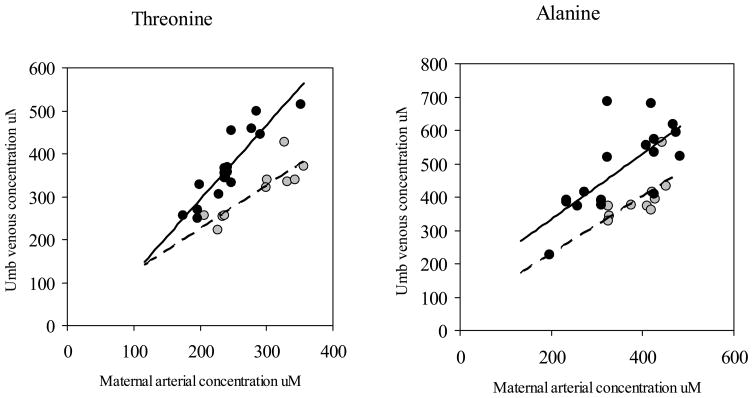
Figure 1 presents the relationship between the umbilical venous and the maternal “arterial” concentration of threonine, an essential amino acid, and alanine, a glucogenic amino acid in patients who received betamethasone and in the historical group of patients in which maternal concentrations were increased by an infusion of an amino acid infusate (Freamine 8.5% III, Baxter). 9 In both group of patients there is a significant relationship between fetal and maternal concentrations (THR betamethasone: umbilical venous concentration (μM)= −53.6 + 1.7 maternal concentration (μM); R2=0.82; p<0.001 - THR Freamine: umbilical venous concentration (μM)= 24.4 + 1 maternal concentration (μM); R2=0.76; p<0.001; ALA betamethasone: umbilical venous concentration (μM)= 137.9 + 0.98 maternal concentration (μM); R2=0.52; p<0.001 - ALA Freamine: umbilical venous concentration (μM)= 57.5 + 0.9 maternal concentration (μM); R2=0.43; p<0.04). However, the regression lines are significantly different in intercept (THR p<0.02; ALA p<0.001) and slope (THR p<0.02) for both amino acids: for the same maternal concentration, the increase in fetal concentrations is significantly greater in women receiving betamethasone compared to those receiving Freamine.
Figure 1. Relationship between fetal and maternal threonine and alanine concentrations.
Relationship between umbilical venous and maternal arterial threonine (left panel) and alanine concentration (right panel) in pregnancies after betamethasone (black circles) and Freamine (grey circles) administration.
THR betamethasone: umbilical venous concentration (μM)= −53.6 + 1.7 maternal concentration (μM); R2=0.82; p<0.001
THR Freamine: umbilical venous concentration (μM)= 24.4 + 1 maternal concentration (μM); R2=0.76; p<0.001
ALA betamethasone: umbilical venous concentration (μM)= 137.9 + 0.98 maternal concentration (μM); R2=0.52; p<0.001
ALA Freamine: umbilical venous concentration (μM)= 57.5 + 0.9 maternal concentration (μM); R2=0.43; p<0.04
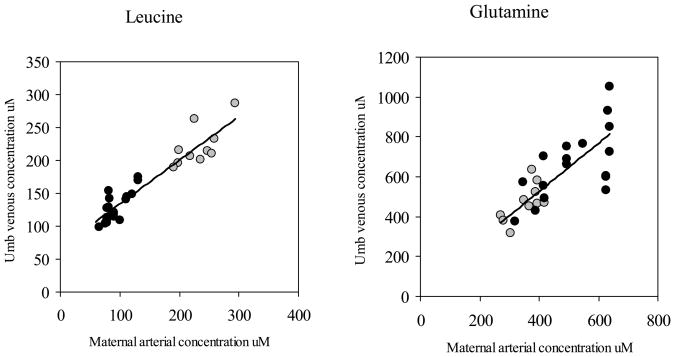
Figure 2 presents the same comparison of the present study and the Freamine study for leucine, an essential amino acid, and glutamine, a gluconeogenic amino acid. In these cases regression lines were not significantly different. Hence, data were pooled together: LEU: umbilical venous concentration (μM)= 65.4 + 0.7 maternal concentration (μM); R2=0.88; p<0.001; GLN: umbilical venous concentration (μM)= 96.5 + 1.1 maternal concentration (μM); R2=0.58; p<0.001.
Figure 2. Relationship between fetal and maternal leucine and glutamine concentrations.
Relationship between umbilical venous and maternal arterial leucine concentration (left panel) and glutamine concentration (right panel) in pregnancies after betamethasone (black circles) and Freamine (grey circles) administration.
LEU: umbilical venous concentration (μM)= 65.4 + 0.7 maternal concentration (μM); R2=0.88; p<0.001
GLN: umbilical venous concentration (μM)= 96.5 + 1.1 maternal concentration (μM); R2=0.58; p<0.001
Discussion
The results of the present study show that the maternal concentration of most amino acids is significantly increased even 48 hours after the first antenatal course of betamethasone which results in a significant increase in total alpha-amino nitrogen. To our knowledge this is the first report on maternal amino acid concentrations following antenatal betamethasone. The catabolic effect of glucocorticoids is well known: they reduce protein synthesis and stimulate protein catabolism in skeletal muscle 10 through a number of mechanisms recently reviewed. 11 The administration of 30 mg twice daily in healthy volunteers determines an increase in protein breakdown which leads to a significant increase in the arterial concentrations of most amino acids with the exception of histidine and glutamate 12: a pattern which is strikingly similar to the present study in pregnant women.
Recently, Verhaeghe et al 8 have reported an increase in fetal amino acid concentrations ≤48 hours from corticosteroid administration. In their study, no maternal AA levels were measured. However, there are many similarities between the two studies: first, the concentration of all AA we measured, with the exception of aspartate, were significantly increased within 48 hours from betamethasone in both studies; second, alanine was the AA whose concentration was increased the most in both studies; third, the increase was more pronounced for the non essential AA than for the essentials.
The significant findings of this study are presented in figures 1 and 2 where the data obtained in the present study are compared with data obtained in an earlier study by Ronzoni et al 9 in which maternal concentrations were increased in pregnant women by an infusion of an amino acid infusate (Freamine 8.5% III, Baxter). These pregnant women had not received betamethasone. The fetal concentration of threonine and alanine (Figure 1) is much greater in women receiving betamethasone, thus, it is clear that the increase in fetal concentrations with betamethasone is not simply due to the increase in maternal concentrations but involves fundamentally different mechanisms. Given the many studies in adults of different species which have shown that corticoids increase protein catabolism, it is likely that the increase in fetal concentrations in the present study reflects an increase in fetal protein catabolism. The increase in fetal alanine concentration is particularly striking. Since animal studies have shown that there is a large fetal hepatic alanine uptake in normal pregnancies, one might speculate that the betamethasone has altered the hepatic uptake of alanine. There are no studies that bear directly on this question, however so this remains speculative at this stage. 13 It is also worth noting that as serine and glutamate concentrations increased there was a significant delivery of these two amino acids from the fetal circulation into the placenta confirming that there is a net delivery of these amino acids into the placenta 14. For serine the umbilical venoarterial difference was −7.9 ± 2.5 μM and for glutamate −23.6 ± 8.8 μM which are significantly different from zero (p<0.01).
Figure 2 also illustrates another finding of the present study. It presents the same comparison of the present study and the Freamine study for leucine, an essential amino acid, and glutamine, a gluconeogenic amino acid. In this case, these amino acids do not demonstrate higher fetal concentrations at the same maternal concentrations, after betamethasone. Similar findings were true for the other branched chain amino acids, isoleucine (R2=0.48; p<0.001) and valine (R2=0.52; p<0.001) and also true for methionine at ≥48 hours (R2=0.72; p<0.01) and phenylalanine (R2=0.62; p<0.001). The significance of this finding is that five of these are amino acids whose placental transport has been studied utilizing stable isotopes, have been shown to cross the placenta in vivo rapidly. 15 Hence, a plausible hypothesis is that these amino acids have relatively large bidirectional fluxes across the human placenta which blocks any large increase in fetal concentrations despite an increase in protein catabolism. By contrast, the amino acids presented in figure 1 may have very little bidirectional flux and hence are trapped within the fetal circulation leading to the large increase in their concentrations.
It is important to note that, associated with the increase in fetal concentrations of the gluconeogenic amino acids, there was a large increase in glutamate concentration and in placental glutamate uptake. Studies in pregnant sheep have clearly demonstrated that in fetal life, net glutamate efflux from the fetal liver, substitutes for the net hepatic output of glucose in postnatal life. 16 The present study provides additional support for this hypothesis in human fetal development.
There are a few additional issues to address. First of all, in the maternal concentration study, eight out of 34 women had multiple gestation, however the statistical analysis did not show any difference between singleton and multiple pregnancies. Secondly, the control group is represented by an historical group of uncomplicated term pregnancies (gestational age 38.5 ± 0.2 weeks) 9 however, given the study design (women at risk of preterm labor) a more appropriate control group would have been impossible to collect since, at our Institution, betamethasone is administered routinely in women with threatened preterm labor. However, we have previously shown that, in human fetuses sampled in utero at the time of cordocentesis 17 there are only minor changes in amino acid concentrations between midgestation and late gestation. Thus it is unlikely that the difference in gestational age might have affected significantly the results. Lastly, we used for comparison of the fetal-maternal amino acid concentration relationships historical data from a group of women where the amino acid concentration had been steadily increased by means of a continuous infusion of an amino acid solution 9 to test possible differences with a situation where the increase in maternal amino acids concentration had been pharmacologically induced. The comparison has shown profound differences for some amino acids most likely due to the fact that betamethasone exerts its catabolic effect not only into the maternal but also in the fetal compartment. In vitro studies in the mouse placenta suggest that these differences might be mediated by a direct effect of betamethasone on the placental amino acid transport systems. 17 These observations need to be confirmed in the human placenta.
In conclusion, this study shows the profound effect on maternal and fetal amino acid concentrations when betamethasone is given prenatally to enhance fetal lung maturation. These changes may be responsible for the impairment in intrauterine growth which has been shown in experimental animals 4 17 through a direct catabolic effect on the growing fetus.
It was not a goal of the study to evaluate how persistent these changes were but in the three studies where the time elapsed was >160 hours, they were still present in the fetal compartment.
Figure 3.
Relationship between fetal and maternal leucine and glutamine concentrations
Acknowledgments
This work was supported by National Institutes of Health Grant 5 RO1 HD34837-06, Fetal Velocimetry & Amino Acid Transport in Pregnancy, General Clinical Research Centers Program, National Centers for Research Resources, NIH, Grant Number MO1 RR00069 and a grant from the Association for Study of Malformations (ASM) (Italy). A preliminary account of this work was presented at the 2002 Society for Gynecologic Investigation Annual Meeting, Los Angeles, CA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.ACOG Committee Opinion. Antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2008;111:805–807. doi: 10.1097/AOG.0b013e318169f722. [DOI] [PubMed] [Google Scholar]
- 2.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972 Oct;50(4):515–25. [PubMed] [Google Scholar]
- 3.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews. 2006;(3) doi: 10.1002/14651858.CD004454.pub2. Art. No.: CD004454. [DOI] [PubMed] [Google Scholar]
- 4.Jobe AH, Newnham J, Willet K, Sly P, Ikegami M. Fetal versus maternal and gestational age effects of repetitive antenatal glucocorticoids. Pediatrics. 1998;102:1116–1125. doi: 10.1542/peds.102.5.1116. [DOI] [PubMed] [Google Scholar]
- 5.Stewart JD, Gonzalez CL, Christensen HD, Rayburn WF. Impact of multiple antenatal doses of betamethasone on growth and development of mice offspring. Am J Obstet Gynecol. 1997;177:1138–44. doi: 10.1016/s0002-9378(97)70030-9. [DOI] [PubMed] [Google Scholar]
- 6.French NP, Hagan R, Evans SF, Godfrey M, Newnham JP. Repeated antenatal corticosteroids: Size at birth and subsequent development. Am J Obstet Gynecol. 1999;180:114–21. doi: 10.1016/s0002-9378(99)70160-2. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad I, Beharry KDA, Valencia AM, et al. Influence of a single course of antenatal betamethasone on the maternal–fetal insulin-IGF-GH axis in singleton pregnancies. Growth Horm IGF Res. 2006;16:267–275. doi: 10.1016/j.ghir.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Verhaeghe J, Vanstapel F, van Bree R, Van Herck E, Coopmans W. Transient Catabolic State with Reduced IGF-I after Antenatal Glucocorticoids. Pediatr Res. 2007;62:295–300. doi: 10.1203/PDR.0b013e318123f72f. [DOI] [PubMed] [Google Scholar]
- 9.Ronzoni S, Marconi AM, Cetin I, et al. Umbilical amino acid uptake at increasing maternal amino acid concentrations: effect of a maternal amino acid infusate. Am J Obstet Gynecol. 1999;181:477–483. doi: 10.1016/s0002-9378(99)70581-8. [DOI] [PubMed] [Google Scholar]
- 10.Hasselgren PO. Glucocorticoids and muscle catabolism. Curr Opin Clin Nutr Metab Care. 1999;2:201–205. doi: 10.1097/00075197-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Menconi M, Fareed M, O’Neal P, Poylin V, Wei W, Hasselgren PO. Role of glucocorticoids in the molecular regulation of muscle wasting. Crit Care Med. 2007;35(Suppl):S602–S608. doi: 10.1097/01.CCM.0000279194.11328.77. [DOI] [PubMed] [Google Scholar]
- 12.Lofberg E, Gutierrez A, Wernerman J, et al. Effects of high doses of glucocorticoids on free amino acids, ribosomes and protein turnover in human muscle. Eur J Clin Invest. 2002;32:345–353. doi: 10.1046/j.1365-2362.2002.00993.x. [DOI] [PubMed] [Google Scholar]
- 13.Teng C, Battaglia FC, Meschia G, Narkewicz MR, Wilkening RB. Fetal hepatic and umbilical uptakes of glucogenic substrates during a glucagon-somatostatin infusion. Am J Physiol. 2002;282:E542–50. doi: 10.1152/ajpendo.00248.2001. [Endocrinol Metab] [DOI] [PubMed] [Google Scholar]
- 14.Marconi AM, Battaglia FC, Meschia G, Sparks JW. A comparison of amino acid arteriovenous differences across the liver and placenta of the fetal lamb. Am J Physiol. 1989;257:E909–E915. doi: 10.1152/ajpendo.1989.257.6.E909. [Endocrinol.Metab.20] [DOI] [PubMed] [Google Scholar]
- 15.Galan HR, Marconi AM, Paolini CL, Cheung A, Battaglia FC. The transplacental transport of essential amino acids in uncomplicated human pregnancies. Am J Obstet Gynecol. 2009;200:91.e1–7. doi: 10.1016/j.ajog.2008.06.054. [DOI] [PubMed] [Google Scholar]
- 16.Vaughn PR, Lobo C, Battaglia FC, Fennessey PV, Wilkening RB, Meschia G. Glutamine-glutamate exchange between placenta and fetal liver. Am J Physiol. 1995 Apr;268(4 Pt 1):E705–11. doi: 10.1152/ajpendo.1995.268.4.E705. [DOI] [PubMed] [Google Scholar]
- 17.Cetin I, Corbetta C, Piceni Sereni L, et al. Umbilical amino acid concentrations in normal and growth retarded fetuses sampled in utero by cordocentesis. Am J Obstet Gynecol. 1990;162:253–261. doi: 10.1016/0002-9378(90)90860-a. [DOI] [PubMed] [Google Scholar]
- 18.Vaughan OR, Coan PM, Fowden AL. Maternal dexamethasone treatment retards growth but increases transport capacity of the mouse placenta at day 16 of pregnancy. Reprod Sci. 2009;16:347A. [Google Scholar]