Summary
Prior research has linked heightened cortisol reactivity to stress with increased food consumption. This pilot study tested corollaries of the hypothesis that cortisol stress reactivity promotes obesity. Thirty-four lean and obese women completed an acute stress task and a non-stressful control task in counterbalanced order. Contrary to expectations, higher post-stress cortisol was associated with decreased post-stress snack intake in obese women but was unrelated to snack intake in lean women. Stress also blunted an expected rise in hunger only among obese women. Findings suggest that some obese women may be more sensitive to short-term anorectic effects of HPA axis activation.
Keywords: HPA axis, Cortisol, Stress, Eating behavior
Introduction
Despite long-standing interest in the relations between stress and eating behavior, the true nature of these associations and the underlying biological mechanisms involved remain poorly understood. Stress is associated with both increased and decreased food intake in humans (Greeno & Wing, 1994; Oliver & Wardle, 1999; Torres & Nowson, 2007), and many studies have attempted to identify factors that account for this variability. Stress-induced elevation in cortisol, a glucocorticoid hormone secreted by the hypothalamic-pituitary-adrenocortical (HPA) axis, has been linked to increased energy intake following stress. Surgical removal of the adrenal glands (a procedure which eliminates cortisol secretion) results in decreased food intake in rodents, and administration of glucocorticoids reverses this effect (Freedman, Horwitz, & Stern, 1986). In humans, therapeutic administration of glucocorticoids stimulates food intake (Tataranni et al., 1996) and Cushing's Disease (hypercortisolism induced by a pituitary tumor) is associated with weight gain and central obesity (Orth, 1995). These findings have led some to hypothesize that stress-induced cortisol secretion contributes to obesity through its effects on eating behavior (Dallman et al., 2003; Rosmond, 2005). Partial support for this notion was found in two studies showing that elevated cortisol stress reactivity is associated with increased food intake inside and outside the laboratory among women (Epel, Lapidus, McEwen, & Brownell, 2001; Newman, O'Connor, & Conner, 2007).
If cortisol reactivity promotes obesity by stimulating food intake following stress, several findings would be anticipated. First, it could be expected that obese women would engage in stress eating to a greater extent than lean women. Research on this topic has been quite mixed (reviewed in Torres & Nowson, 2007), with findings of both increased and decreased food intake following stress in obese but not lean individuals, and some studies reporting no effect of stress on food intake in either group. Larger magnitude cortisol stress responses among the obese might also be expected if cortisol reactivity promotes obesity. Though a few studies have linked the magnitude of cortisol stress responses to overall adiposity (Benson et al., 2009), associations have been somewhat more consistent with central obesity (Epel et al., 2001; Mårin et al., 1992). If cortisol reactivity is indeed associated with central obesity, this association might be interpreted in terms of cortisol's role in redistributing fat to central stores (Bjorntorp & Rosmond, 2000) rather than through an effect on food intake since the latter would be equally likely to promote peripherally-distributed obesity. A third possibility is that HPA axis responses to stress, including cortisol elevations, influence food intake differently in obese individuals. There is some evidence that the HPA axis may influence eating through pathways that more prominently control eating among obese individuals. For example, HPA axis activation modifies the incentive salience of food rewards (Adam & Epel, 2007) which is believed to be a stronger influence on energy intake among obese individuals (Mela, 2006). Cortisol also dampens the appetite-suppressing effect of leptin (Zakrzewska, Cusin, Sainsbury, Rohner-Jeanrenaud, & Jeanrenaud, 1997), an adiposity signal that is elevated and functionally resistant in obesity (Morrison, 2008).
This pilot study tested several corollaries of the hypothesis that cortisol stress reactivity promotes obesity. Lean and obese women completed a standardized laboratory stressor and a control task on two consecutive days. Task-related changes in cortisol levels and snack intake were measured. We hypothesized that relative to lean women, obese women would show 1) greater snack intake and hunger following stress, 2) greater cortisol stress reactivity, and 3) a stronger positive association between cortisol and snack intake.
Method
Participants
Healthy lean and obese women between ages 25–45 were recruited through community advertisements for a study on “stress physiology”. Exclusion criteria included lactation, pregnancy, hormonal contraceptive use (Kudielka, Hellhammer, & Wust, 2009), peri- or post-menopausal status, active dieting, food allergies, and the use of medications affecting neuroendocrine function, metabolism, or appetite. Those who met eligibility criteria were screened to verify that their BMI was in the lean (18.5–24.9) or class I obese (30–34.9) range. The final sample consisted of 16 lean and 18 obese women. Participants were compensated $50 (US). The Northwestern University Institutional Review Board approved this study.
Procedure
Two laboratory sessions were completed on consecutive weekdays. Participants were instructed to fast and refrain from alcohol, caffeine, energy drinks, non-prescription medication, and physical activity after midnight on both days. Two participants (one lean, one obese) reported smoking more than five cigarettes per day. Nicotine consumption was not allowed within two hours of participation. A 430-kcal breakfast consisting of a nutrition shake and a granola bar was provided for participants to consume between 08:30 and 09:00. Experimental sessions were scheduled to begin at 11:30 (+/− 30 mins) to minimize the effects of diurnal rhythms on HPA axis reactivity (Kudielka et al., 2009) and to allow control of snack intake prior to the experimental sessions without requiring an extended fast.
Participants completed questionnaires for the first 45 mins of both experimental sessions, followed by either a 30-min stress task or a 30-min control task. Stratified randomization was used to counterbalance task order in the overall sample and within BMI groups. The stress task was a modified version of the Trier Social Stress Task (Kirschbaum, Pirke, & Hellhammer, 1993), which has been shown to elicit robust cortisol reactivity and has been used in previous studies of the role of HPA axis function in eating behavior. Briefly, the task consisted of preparing and delivering a videotaped speech (15 mins), completing a serial subtraction task (5 mins), and solving highly challenging visuospatial puzzles under time pressure (10 mins). Participants were told that their performance on all 3 tasks would be evaluated and compared to that of other women their age. The control task consisted of viewing and evaluating a nature documentary film. Participants had access to four snacks very shortly after completing the tasks: caramel flavored miniature rice cakes (24g), low-fat butter popcorn (20g), miniature chocolate chip cookies (50g), and potato chips (40g). To encourage snack intake, participants were reminded that they had not eaten since morning and told that uneaten food would be discarded. Participants were left alone to eat for 10 mins and were presumably unaware that snack intake was being monitored.
Saliva samples were collected at four time points on each day of the study. Participants chewed wax film to stimulate saliva flow and passively drooled through a straw into a graduated collection cup until at least 1.5 ml of saliva was obtained. Saliva was stored at −80°C within 30 mins of collection. Two pre-task samples were collected during questionnaire completion, approximately 10 mins and 20 mins after participants arrived. Additional saliva samples were obtained 20 mins after the tasks began (after the serial subtraction component of the stress task, or 20 minutes into the documentary film on the control day), and immediately after task completion. Affect and hunger ratings were collected upon arrival, pre-task, and post-task on both days.
Measures
Body mass index (BMI) [weight (kg) / height2 (m)] was derived from height and weight measurements taken in light clothing and stocking feet.
Salivary cortisol was analyzed with a high-sensitivity enzyme immunoassay (Alpco model 11-CORHU-E01-SLV; Salem, NH). Values from the two pre-task and the two post-task samples were averaged. Cortisol secretion follows a pronounced circadian rhythm characterized by a spike in cortisol upon awakening and a subsequent decline that is most pronounced at midday (Stone et al., 2001). This diurnal decrease in cortisol can easily mask stress-related increases in cortisol at midday, and the use of pre-to-post cortisol change scores to quantify HPA axis reactivity was precluded in this study. Instead, cortisol reactivity was operationalized as the magnitude of change in post-task cortisol levels from the control day to the stress day.
Snack intake was quantified as the weight of each food item consumed in grams because food weight has been shown to be a stronger determinant of intake during an episode of eating than energy content (Westerterp-Plantenga, 2004). Plates of food were weighed with a high-sensitivity laboratory balance (model SP402, Ohaus Corporation, Pinebrook, NJ, USA) before and after food consumption.
Participants completed two self-report measures for use as potential covariates in statistical analyses. The Beck Depression Inventory II (Beck, 1996) includes 21 items (α=0.89) assessing the presence and severity of different depressive symptoms. The Dutch Eating Behavior Questionnaire (Van Strien, Frijters, Bergers, & Defares, 1986) is a 33-item measure of influences on typical eating behavior, with scales for restrained (α=.87), emotional (α=.95), and external (α=.82) eating. This scale was administered at the conclusion of the study to keep participants blind to the fact that eating behavior was being evaluated. Sociodemographic and medical data were also collected by self-report.
The Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988) was administered upon arrival, immediately pre-task, and immediately post-task both days. Participants indicated the degree to which they were experiencing 10 positive and 10 negative affect descriptors on a 1 (very slightly or not at all) to 5 (extremely) scale. The descriptor “hungry” was inserted among the PANAS items so that hunger could be evaluated without alerting participants to the true focus of the study.
Analysis
Repeated measures ANCOVAs were used to model change in snack intake, post-task cortisol levels, and hunger from BMI group, condition, and the BMI group × condition interaction while controlling for relevant covariates described below. A univariate general linear model was used to model snack intake following the stress task from BMI group, a cortisol reactivity change score, and the BMI group × cortisol reactivity interaction. Finally, we conducted “focused” analyses comparing BMI groups on the temporal pattern of hunger changes on the stress day modeled through orthogonal a priori polynomial contrasts.
Results
BMI groups were compared on anthropometric and self-report measures through independent samples t-tests. The obese group was slightly older than the lean group (35.6 vs. 31.2 years, t(32)=2.27, p=.03) and scored higher on Emotional (t(32)=2.90, p<.01) and Restrained (t(32)=2.71, p=.01) Eating. Obese women had slightly higher depression scores (t(32)=2.24, p=.03), but both groups' means fell within the “not depressed/minimal” range suggested by the test developers (Beck, 1996). Including these variables as covariates did not alter the statistical significance of results, so we report final models without covariates for simplicity. Total snack intake was similar (control: M=31.2g, SD=29.5g; stress: M=32.1g, SD=32.0g) and highly correlated (r(33)=.75, p<.001) across both days. All cortisol values were within normal physiological limits for healthy individuals and were retained in analyses. Snack intake data were lost for one lean participant who took food from the lab on the stress day. Another lean participant's post-stress snack intake was more than 3 SD above the mean and more than 1 SD above than the next highest value. These participants were excluded from analyses involving snack intake.
Two manipulation checks of the stress task were performed. The stress task elicited significant cortisol reactivity, with post-task cortisol levels significantly higher on the stress day than the control day (4.06 vs. 3.56 ng/ml; F(1,31)=13.33, p<.001), adjusting for pre-task levels. There was also an effect of the stress task on the repeated measures pattern of negative affect (F(2,62)=10.23, p<.001), with negative affect increasing linearly over time on the stress day (F(1,31)=11.04, p<.01), but decreasing linearly on the control day (F(1,33)=8.57, p<.01).
The BMI group × condition interaction was not a significant predictor of the within-subjects change in post-task cortisol from the control day to the stress day adjusting for baseline levels, indicating that the BMI groups did not differ in their HPA axis stress responses. Lean women had slightly higher mean cortisol levels than obese women (F(1,32)=4.90, p=.03). A second model indicated that there was no difference between lean and obese women in the effect of condition on snack intake (Lean, control: M=24.8g, SD=21.7g; Lean, stress: M=24.9, SD=19.4 g; Obese, control: M=32.2g, SD=30.3g; Obese, stress: M=24.1g, SD=22.6g). The lower order terms representing BMI group and condition were also non-significant, indicating that snack intake was unaffected by the stressor and did not differ overall between lean and obese participants.
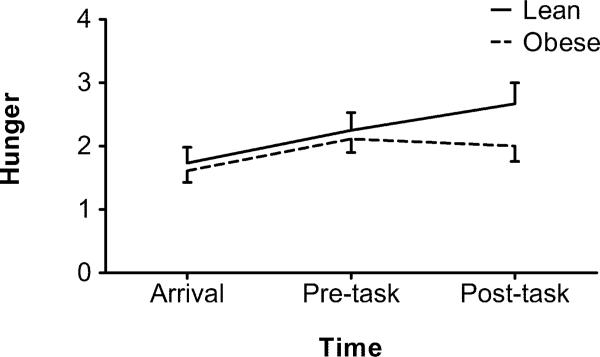
The BMI groups were found to differ on the temporal pattern of hunger ratings on the stress day (F(2,60)=3.73, p=.03). Though both groups showed an overall linear increase in hunger ratings on the stress day (lean: F(1,13)=14.36, p=.01; obese: F(1,17)=7.37, p=.02), the increase in hunger was blunted following the stress task only in the obese group (quadratic trend: F(1,17)=6.25, p=.02; Figure 1). BMI groups did not differ in overall hunger levels on either day. Correlations between snack intake and hunger ratings at all time points were non-significant.
Figure 1.
Change in hunger ratings on the stress day by BMI group. Bars represent SEM.
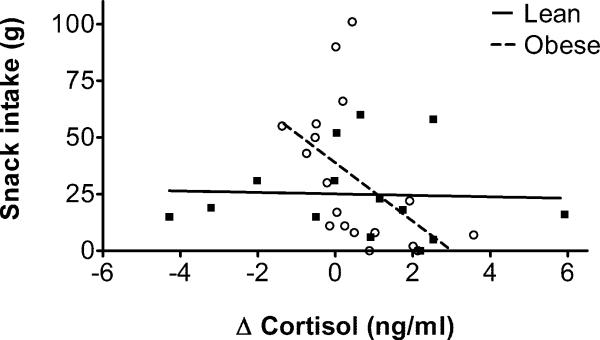
Our final analysis tested whether the association between cortisol reactivity and food intake on the stress day differed between BMI groups. The BMI group × cortisol reactivity interaction significantly predicted snack intake following the stressor (F(1,28)=5.00,p=.03; Figure 2). Analysis of simple effects within each group showed that higher cortisol reactivity was associated with lower snack intake in obese women (F(1,16)=5.39, p=.03), but was unrelated to intake in lean women (p=.88). The interaction term and the simple effect in the obese group remained statistically significant when controlling for pre-task cortisol levels, age, depressive symptoms, and emotional and restrained eating.
Figure 2.
Associations of cortisol reactivity and snack intake on the stress day in lean (■) and obese (○) women. Positive Δ cortisol values indicate an increase in post-task cortisol from the control day to the stress day.
Discussion
Cortisol stress reactivity has been linked to increased food intake in prior studies. This pilot study examined whether associations between stress, HPA axis reactivity, and food intake differ between lean and obese women. Contrary to expectations, lean and obese women did not differ in the effect of a laboratory stressor on snack intake or cortisol reactivity. These null effects cannot be explained by a lack of affective or physiological reactivity to the stress task. A number of studies have failed to demonstrate main effects of stress on food intake, and differences in stress eating between lean and obese individuals have been inconsistent (Torres & Nowson, 2007). It is worth noting that the effects of stress on food intake have been most consistent among women reporting high levels of dietary restraint (Greeno & Wing, 1994), and current dieters were excluded from participation in this study. As mentioned in the introduction, cortisol reactivity has been somewhat more consistently associated with central rather than total adiposity (Bjorntorp & Rosmond, 2000; Epel et al., 2000). Though BMI is strongly correlated with measures of central adiposity, our analysis comparing groups of lean and obese women (overweight women were excluded from participation) would be unlikely to capture the effects of central adiposity on cortisol stress responses. The current findings suggest that non-dieting lean and obese women, on average, do not meaningfully differ in their snack intake immediately following acute stress or the magnitude of HPA axis stress reactivity.
Greater cortisol reactivity was associated with reduced snack intake following stress in obese women but not lean women. One explanation for this unexpected finding is that snack intake among some obese women was influenced by stress-related secretion of corticotropin-releasing hormone (CRH), a hormone released early in the HPA axis cascade that has potent anorectic effects (Mastorakos & Zapanti, 2004). CRH is rapidly released from the paraventricular nucleus of the hypothalamus with the onset of stress. CRH initiates the rest of the HPA axis cascade that ultimately results in the secretion of cortisol from the adrenal cortex approximately 15 minutes later. Circulating cortisol reaches the hypothalamus and pituitary and acts to suppress further HPA axis activation, and it has been suggested that rapid feedback inhibition of CRH by cortisol may be responsible for the orexigenic effects of cortisol following stress (Dallman et al., 2003). The timing and mechanisms by which cortisol might affect hypothalamic CRH activity are still being elucidated, but preliminary evidence suggests that glucocorticoid suppression of CRH begins to occur 5–15 minutes after cortisol levels have risen (Hinz & Hirschelmann, 2000), or about 35–45 minutes after stressor onset (Dallman, 2003). Thus, it is very likely that there was still meaningful stress-induced hypothalamic CRH activity at the time participants were exposed to food in this study. As both CRH and cortisol reflect HPA axis activation, the observed negative association between post-stress cortisol level and snack intake in obese women may actually reflect the anorectic effects of CRH.
Though stress did not affect snack intake, stress appeared to selectively suppress a gradual increase in hunger among obese women. It is interesting that hunger ratings and snack intake were uncorrelated in this study, which highlights the dissociation between subjective hunger and eating behavior. A sizable literature exists supporting the role of non-homeostatic factors in eating behavior, with aspects of the environment and food palatability being capable of overriding or circumventing neural hunger and satiety mechanisms (Berthoud, 2007). Despite the discrepancy between subjective ratings and snack intake, the apparent suppression of hunger following stress in obese women is consistent with the view that a subset of non-dieting, obese women are sensitive to the appetite-suppressing effects of CRH immediately after stress, as discussed above.
This pilot study was conducted in a small and narrowly selected sample of healthy lean and class I obese women, which limits the generalization of findings to other populations (e.g., men, different BMI categories). The association between cortisol stress responses and snack intake was evaluated at midday in order to minimize effects of diurnal cortisol rhythms, and it is possible that different patterns of association between cortisol reactivity and snack intake would be observed in the afternoon when cortisol responses are more pronounced (Kudielka et al., 2009). Additionally, our decision to evaluate eating immediately following the termination of stress rather than after a delay may explain the discrepancy between our findings and those of prior studies (e.g., Epel et al., 2001). The effect of the post-stress interval on food intake has not yet been studied systematically.
Contrary to our initial hypotheses, lean and obese women showed similar cortisol responses to a laboratory stressor and snack intake was unaffected by the stressor in both groups. In obese women, greater cortisol reactivity was associated with decreased snack intake following stress, and stress appeared to suppress a gradual rise in hunger. These findings suggest that variability in the short-term appetite-suppressing effects of CRH may account for individual differences in the eating behavior of non-dieting obese women following stress, and that any orexigenic effect of HPA axis stress responses would occur at longer intervals following stress. The underlying physiological mechanisms of these effects and the influence of the post-stress interval deserve further attention.
Acknowledgements
We thank Dr. Susan Gapstur for methodological guidance, and Sue Giovanazzi, Claudia Chambers, Brigitte Salembier, Cheryl Westbrook, and Dr. Robert Chatterton for their technical assistance. This research was supported by US Public Health Service Grant R25 CA100600 from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam T, Epel E. Stress, eating and the reward system. Physiology & Behavior. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Beck AT. The Beck Depression Inventory II. Harcourt Brace & Co; San Antonio: 1996. [Google Scholar]
- Benson S, Arck PC, Tan S, Mann K, Hahn S, Janssen OE, et al. Effects of obesity on neuroendocrine, cardiovascular, and immune cell responses to acute psychosocial stress in premenopausal women. Psychoneuroendocrinology. 2009;34:181–189. doi: 10.1016/j.psyneuen.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Interactions between the “cognitive” and “metabolic” brain in the control of food intake. Physiology & Behavior. 2007;91:486–498. doi: 10.1016/j.physbeh.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P, Rosmond R. Obesity and cortisol. Nutrition. 2000;16:924–936. doi: 10.1016/s0899-9007(00)00422-6. [DOI] [PubMed] [Google Scholar]
- Dallman MF. Fast glucocorticoid feedback favors `the munchies'. Trends in Endocrinology and Metabolism: TEM. 2003;14:394–396. doi: 10.1016/j.tem.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, et al. Chronic stress and obesity: A new view of “comfort food.”. Proceedings of the National Academy of Sciences. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: A laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- Epel ES, McEwen B, Seeman T, Matthews K, Castellazzo G, Brownell KD, et al. Stress and body shape: Stress-induced cortisol secretion is consistently greater among women with central fat. Psychosomatic Medicine. 2000;62:623–632. doi: 10.1097/00006842-200009000-00005. [DOI] [PubMed] [Google Scholar]
- Freedman MR, Horwitz BA, Stern JS. Effect of adrenalectomy and glucocorticoid replacement on development of obesity. The American Journal of Physiology. 1986;250:R595–607. doi: 10.1152/ajpregu.1986.250.4.R595. [DOI] [PubMed] [Google Scholar]
- Greeno CG, Wing RR. Stress-induced eating. Psychological Bulletin. 1994;115:444–464. doi: 10.1037/0033-2909.115.3.444. [DOI] [PubMed] [Google Scholar]
- Hinz B, Hirschelmann R. Rapid non-genomic feedback effects of glucocorticoids on CRF-induced ACTH secretion in rats. Pharmaceutical Research. 2000;17:1273–1277. doi: 10.1023/a:1026499604848. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The `Trier Social Stress Test' - a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Wust S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34:2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Mårin P, Darin N, Amemiya T, Andersson B, Jern S, Bjorntorp P. Cortisol secretion in relation to body fat distribution in obese premenopausal women. Metabolism: Clinical and Experimental. 1992;41:882–886. doi: 10.1016/0026-0495(92)90171-6. [DOI] [PubMed] [Google Scholar]
- Mastorakos G, Zapanti E. The hypothalamic-pituitary-adrenal axis in the neuroendocrine regulation of food intake and obesity: The role of corticotropin releasing hormone. Nutritional Neuroscience. 2004;7:271–280. doi: 10.1080/10284150400020516. [DOI] [PubMed] [Google Scholar]
- Mela DJ. Eating for pleasure or just wanting to eat? Reconsidering sensory hedonic responses as a driver of obesity. Appetite. 2006;47:10–17. doi: 10.1016/j.appet.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Morrison CD. Leptin resistance and the response to positive energy balance. Physiology & Behavior. 2008;94:660–663. doi: 10.1016/j.physbeh.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E, O'Connor DB, Conner M. Daily hassles and eating behaviour: the role of cortisol reactivity status. Psychoneuroendocrinology. 2007;32:125–132. doi: 10.1016/j.psyneuen.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Oliver G, Wardle J. Perceived effects of stress on food choice. Physiology & Behavior. 1999;66:511–515. doi: 10.1016/s0031-9384(98)00322-9. [DOI] [PubMed] [Google Scholar]
- Orth DN. Cushing's syndrome. The New England Journal of Medicine. 1995;332:791–803. doi: 10.1056/NEJM199503233321207. [DOI] [PubMed] [Google Scholar]
- Rosmond R. Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology. 2005;30:1–10. doi: 10.1016/j.psyneuen.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Stone AA, Schwartz JE, Smyth J, Kirschbaum C, Cohen S, Hellhammer D, et al. Individual differences in the diurnal cycle of salivary free cortisol: A replication of flattened cycles for some individuals. Psychoneuroendocrinology. 2001;26:295–306. doi: 10.1016/s0306-4530(00)00057-3. [DOI] [PubMed] [Google Scholar]
- Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, Ravussin E. Effects of glucocorticoids on energy metabolism and food intake in humans. The American Journal of Physiology. 1996;271:E317–25. doi: 10.1152/ajpendo.1996.271.2.E317. [DOI] [PubMed] [Google Scholar]
- Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23:887–894. doi: 10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Van Strien T, Frijters JE, Bergers GP, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. International Journal of Eating Disorders. 1986;5:295–315. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Westerterp-Plantenga MS. Effects of energy density of daily food intake on long-term energy intake. Physiology & Behavior. 2004;81:765–771. doi: 10.1016/j.physbeh.2004.04.030. [DOI] [PubMed] [Google Scholar]
- Zakrzewska KE, Cusin I, Sainsbury A, Rohner-Jeanrenaud F, Jeanrenaud B. Glucocorticoids as counterregulatory hormones of leptin: Toward an understanding of leptin resistance. Diabetes. 1997;46:717–719. doi: 10.2337/diab.46.4.717. [DOI] [PubMed] [Google Scholar]