Abstract
Vaccines represent a new and promising avenue of treatment for drug abuse but also pose new medication adherence challenges due to prolonged and widely-spaced administration schedules. This study examined effects of prize-based incentives on retention and medication adherence among 26 cocaine users involved in a six-month hepatitis B vaccination series. Participants could meet with research staff weekly for 24 weeks and receive seven injections containing either the Hepatitis B vaccine or a placebo. All participants received $10 at each weekly visit (maximum of $240). Those randomly assigned to the incentive program received additional monetary payments on an escalating schedule for attendance at weekly monitoring and vaccination visits with maximum possible earnings of $751. Group attendance diverged after study week 8 with attendance better sustained in the incentive than control group (group by time interaction, p = .035). Overall percent of weekly sessions attended was 82% for incentive versus 64% for control (p = .139). Receiving all scheduled injections were 77% of incentive versus 46% of control participants (p = .107). A significantly larger percentage (74% versus 51%; p = .016) of injections were received by incentive versus control participants on the originally scheduled day. Results suggest that monetary incentives can successfully motivate drug users to attend sessions regularly and to receive injected medications in a more reliable and timely manner than may be seen under usual care procedures. Thus, incentives may be useful for addressing adherence and allowing participants to reap the full benefits of newly developed medications.
Keywords: medication adherence, contingency management, motivational incentives, vaccines, cocaine abuse
1. Introduction
There has been a long and largely unsuccessful history of medication development for the treatment of cocaine dependence (Kampman, 2008; Preti, 2007). However, the recent development of a vaccine that binds to the drug before it can exert its reinforcing effects in the brain represents a new and promising avenue that may aid in treatment by promoting cessation and preventing relapse (Martell et al., 2005; Orson et al., 2009). The advent of vaccine therapies as well as the development of other long-acting formulations for drug abuse pharmacotherapy, including implantable naltrexone (Comer et al., 2007; Johansson et al., 2006) and buprenorphine (Mattick et al., 2008; Raisch et al., 2002; Sigmon et al., 2004), suggests that a paradigm shift in medication administration schedules may be taking place with a concomitant need for methods to ensure adherence to schedules that involve infrequent administrations stretched over lengthy periods of time.
Contingency management, a procedure that reinforces specific desired behavior with delivery of tangible rewards, has previously been shown efficacious for promoting adherence to naltrexone (Carroll et al., 2001; Preston et al. 1999), HIV/AIDS medications (Rigsby et al., 2000; Rosen et al., 2007; Sorenson et al., 2006) and tuberculosis testing (Malotte et al., 2001) in drug users. The present study investigates the impact of contingent monetary incentives on adherence of cocaine abusers to a 6-month regimen of weekly clinical care meetings and once monthly vaccinationa. A Hepatitis B (HBV) vaccine series with additional placebo injections was used to simulate a cocaine vaccination series. Although only 3 of the scheduled inoculations contained active Hepatitis B vaccine (HBV), 100% adherence to the injection schedule was deemed important in order to model an effective cocaine vaccination protocol. We extend the findings of Seal et al. (2003) who also used the HBV injection series as a model for future injection medication regimens requiring widely-spaced medication administration visits.
2. Methods
2.1. Recruitment
Study participants were recruited primarily through referrals from the Michael E. DeBakey Veterans Administration Medical Center (34% of participants) and Ben Taub General Hospital (BTGH; 37% of participants), with the remaining 29% coming from advertisements, websites and word of mouth. Eligibility criteria included age18 – 64 years, meets diagnostic criteria for cocaine abuse or dependence, agrees to a six-month regimen of the HBV vaccine, and reads English. Individuals were excluded if they were opiate-dependent and/or received methadone treatment, had a current need for alcohol detoxification treatment, or were dually diagnosed with schizophrenia, bipolar disorder, or dementia.
A total of 115 calls were received from individuals interested in the study. Of these 115 individuals, 32% (n = 37) were excluded due to co-morbid psychiatric conditions, failure to meet cocaine abuse or dependence criteria, or participation in an alternative HBV study; 35% (n = 40) were not interested in participating; and 10% (n = 12) did not show up for their intake appointments.
Twenty-six participants were included in the study. The majority were African-American (73%), male (81%) and unemployed (77%) with a mean education of 12.5 (SD = 1.8) years. Average age was 45 years with a significant (p = .05) difference in age between incentive (mean = 48 SD = 7.9) and control (mean age = 41 SD = 11.7) participants. All but 2 (1 incentive, 1 control) reported using cocaine in the 30 days prior to study intake with mean days of cocaine use in the past 30 days being 11.0 (SD = 9.3).
2.2. Procedures
Following an initial screening visit, those who met eligibility criteria and signed informed consent were randomly assigned to incentive or usual care conditions. All participants were expected to meet with research staff for one hour each week for 24 weeks. Telephone appointment reminders were routinely made prior to weekly visits so long as the participant was receptive and could be contacted. During weekly visits, research staff questioned participants about their recent (past 7 day) drug and alcohol use and provided support related to substance abuse problems. Specifically, participants could either use a module-based computerized counseling program (Bickel et al., 2008) or meet with the research coordinator for a non-directive, client-centered discussion regarding current problems and goals. The majority of participants opted to talk with the research coordinator.
The HBV series injections were scheduled during the intake visit and study weeks 4 and 20, consistent with CDC recommendations for this series (Centers for Disease Control and Prevention, 2001). Four additional placebo injections were scheduled at weeks 2, 8, 12 and 16 to more closely model a likely cocaine vaccine schedule (Haney & Kosten, 2004; Martell et al., 2005). Participants were informed as to the active versus placebo status of the injections during the consent process and again upon administration. Injections were administered at a nearby addiction medicine clinic by licensed research nurses. Participants were monitored on a weekly basis for adverse reactions, though none were reported. Because of the coordination between clinical and research staff required to administer injections, it was not always possible to administer injections at visits as scheduled per protocol. Every attempt was made to make up any vaccinations missed due either to staff or participant (missed session) factors as long as CDC recommended HBV vaccination intervals were maintained for the active injections.
Participants assigned to both groups received $20 for completing study intake procedures (screening, consent and initial injection) and $10 after each weekly visit to cover transportation costs. For incentive participants, draws for attendance were awarded at each weekly visit with the chance to win prizes (Petry & Martin, 2002). The draw bowl contained 500 tickets, 50% “Good job,” indicating that no prize had been won; 41% indicating a small ($1) prize; 9% a large ($20) prize and 0.2% (single ticket) a jumbo ($80) prize. Small prizes were dollar store items; large and jumbo prizes were awarded in the form of Walmart gift cards. An escalating draw schedule (alternate week increases from 2 to 13 draws over 24 weeks) with reset for missed sessions was employed and three bonus draws were awarded after each 3 consecutive weekly attendances. An average total of $486 in prizes could be earned for perfect attendance over 24 weeks.
Incentive participants also received cash bonuses for attending injection visits. These started at $20 (intake visit) and increased by $5 each month, up to $50 with (total possible = $265). Injection visit bonuses were given if the participant was present for their scheduled injection visit, but unable to receive the injection due to staffing issues and also if they missed the scheduled injection but received their injection at a later date. Overall incentive group earnings averaged $501 out of $751 possible.
2.3. Outcome Measures
Three dichotomous outcome variables were assessed: 1) weekly attendance (Y/N at each of 24 weekly time points), 2) injections received (Y/N for each of 7 scheduled), and 3) injections received on-time (i.e. at the scheduled injection visit; Y/N for each of 7 scheduled). In five ambiguous cases (3% of total injections, 1 from the incentive group, 4 from the comparison group) participants were present for a scheduled injection visit, but never received that scheduled injection either because they subsequently dropped out of the study, refused a placebo injection or did not have another visit prior to the next scheduled injection. These 5 cases were included in analysis as missed injections. Overall, participants received 63% of the 182 total scheduled injections on-time with 19% received late and 19% never received.
2.4. Data Analysis
Demographic variables were first examined for between group differences. Since age was found to differ significantly across groups, subsequent analyses were conducted using age as a covariate. Four summary outcome variables were analyzed by condition while controlling for age (ANCOVA): 1) percent of weekly sessions attended, 2) percent of injections received, 3) percent of injections received on time and 4) longest number of consecutive sessions attended without a miss. The first 3 variables were also subject to condition by time (ANCOVA) to examine for main effects of the intervention and interactions with time over the course of the lengthy 6-month protocol.
3. Results
Table 1 shows outcomes by condition collapsed across time. Several outcomes including percent receiving all injections, percent of weekly visits attended and longest consecutive weeks attended showed trends in favor of the incentive condition. Only group differences in injections received on time (74% vs 51%) reached accepted levels of statistical significance.
Table 1.
Study outcomes by condition
| Age Adjusted Data (mean + sem) | Unadjusted Data | ||||
|---|---|---|---|---|---|
| Outcome measure | Incentive group (N = 13) | Control group (N = 13) | P value | Incentive Group (N = 13) | Control group (N = 13) |
| Injections received (%)a | 91 (.07) | 78 (.07)) | 0.219 | 88 | 80 |
| Received on time (%)b | 74 (.06) | 51 (.06) | .016 | 71 | 54 |
| Received all injections (%) | 77 | 46 | .107 | 77 | 46 |
| Weeks attended (%)c | 82 (.08) | 64 (.08) | .139 | 77 | 69 |
| Consecutive weeks of attendanced | 17 (2.06) | 12 (2.06) | .097 | 16 | 13 |
average percent received out of 7 scheduled injections
average percent of injections received on the day scheduled.
average percent of weeks attended out of 25 scheduled
longest number of consecutive sessions attended without a miss
Figure 1 shows both injection and attendance data as a function of time. Groups started the study with very similar high rates of attendance and receipt of injections. However, group performance diverged after the fourth injection, at study week 8. Divergence between the groups for injections received on-time was apparent throughout the study. The condition by time interaction term was significant for sessions attended (p =.035) but not for total injections received (p = .178) or on-time injections (p = .527).
Fig. 1.
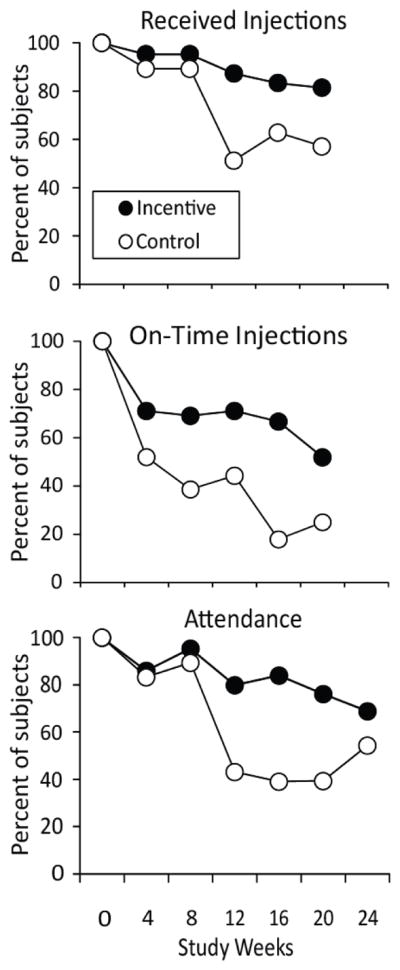
Top panel (received injections) shows percent of participants ultimately receiving six of seven scheduled injections containing either Hepatitis B vaccine (weeks 0, 4, and 20) or placebo (weeks 8, 12, and 16). Middle panel (on-time injections) shows percent receiving the injection during the weekly visit on which it was scheduled (weeks 0, 4, 8, 12, 16, 20). Week 2 placebo injection is omitted from these figures. Bottom panel shows percent of incentive (N = 13) and control (N = 13) participants attending weekly study visits during a 24-week protocol.
4. Discussion
This study demonstrated the ability of an incentive procedure with an escalating schedule of payments, when added to a $10 per visit travel reimbursement, to sustain better weekly attendance as well as better adherence to an injected medication administration schedule compared to the travel reimbursement only in a sample of cocaine abusers studied over a lengthy (6-month) and demanding (once weekly attendance requested) protocol. We extend the findings of Seal et al. (2003) who also used a HBV vaccination series as a model for new medication administration protocols that will require injected administrations spaced over lengthy periods of time. They found significant effects for a contingent payment intervention ($20 per month for monthly visits to the injection site) compared with a street outreach intervention in homeless, injection drug users. The percent of participants receiving their final injections at week 20 in the present study (81%) is similar to the percent (69%) receiving their final injections in the Seal et al. (2003) study. The slightly better results in the present study could be due to the higher incentive values provided. What differs more markedly across studies is the outcome for the comparison groups. The higher percentage receiving their final injection In this (57%) versus the Seal et al. study (23%) could be due to differences in the stability of the samples enrolled as well as differences in the protocols. In particular, control participants in the present study received $10 in transportation money for attending weekly visits and were part of a very small study cohort that may have received more appointment reminders and within-session support than was delivered to outreach participants in the Seal et al. study.
The condition by time interactions displayed in Fig. 1 reveal the specific benefit conferred by the use of an escalating incentive procedure since attendance dropped off over time for the control group, but was well sustained over time in the incentive group. Further, the incentive participants were more likely to receive injections on the day they were scheduled. A lenient philosophy was adopted in the study such that missed injections were provided at the next visit whenever possible; this may have contributed to the number of late injections observed. However, the significant group effect for on-time injections suggests that the incentive may have promoted more timely or responsible attendance behavior in addition to better acceptance of the injection regimen and retentiton in the protocol. The fact that group differences in total injections received did not reach statistical significance (91% vs 78%; p = .219) may be due in part to the study’s very small sample size and to the control group’s excellent adherence to the injection schedule over the first 4 injections in the series (Fig. 1). More resolution in incentive versus control performance might be seen in an even lengthier injection series or under conditions where the medication being delivered is inherently less desirable to participants, where there is less opportunity for flexibility in the injection administration schedule (in which case, on-time adherence to the schedule becomes increasingly important), or where control participants receive no monetary benefits for participation.
The most important limitation of this study was its small sample size, a feature that may have prevented detection of statistically significant between group differences on several outcome variables. Homogeneity of the sample may limit generalizability of results. A third limitation is that the study protocol was very intensive, with once weekly visits requested in addition to the spaced injections. It would have been useful if the study design had separated out the impact of reinforcing vaccine injections per se in order to broaden the generality of findings beyond a clinical trials context. A final limitation is that the study used a fairly rich and complex incentive schedule, thus it cannot speak to whether a less costly or less complicated schedule would suffice to sustain prolonged attendance and injection-schedule adherence.
Despite these limitations, the study adds to a growing body of literature demonstrating the value of contingent incentives for improving medication adherence among disadvantaged populations, including drug users. There is an exciting array of new long-acting medications being developed as well as vaccines designed to aid the prolonged recovery from drug use. Such medications, that typically require widely-spaced administrations over prolonged periods of time, may promote better adherence than do short-acting medications simply because they reduce the need for medication visits and prescription refills. Nevertheless, adherence to these new medication regimens poses new challenges and requires altered protocols for behavioral support. The present study supports the conclusion that adherence to new medications requiring prolonged injection visit schedules can be improved with the use of behaviorally contingent monetary or prize-based incentives especially when an escalating schedule is employed.
Acknowledgments
Role of funding source
Funding was provided by NIDA grants K05DA0454, P50DA18197 and U10 DA013134. The NIDA had no further role in design of the study, collection, analysis and interpretation of the data, writing of the report and the decision to submit the report for publication.
Footnotes
Contributors
Authors Stitzer and Kosten designed the study and the protocol. Author Polk oversaw all study operations, recruited participants, implemented all aspects of the protocol and performed initial data analysis. Author Bowles analyzed data for the publication and wrote a first draft of the paper. All authors have approved the final paper.
Conflict of interest
No authors have a conflict of interest to declare in relation to this particular study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bickel WK, Marsch LA, Buchhalter AR, Badger GJ. Computerized behavior therapy for opioid-dependent outpatients: A randomized controlled trial. Exp Clin Psychopharm. 2008;16:132–143. doi: 10.1037/1064-1297.16.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Nich C, O’Connor PG, Eagan DA, Frankforter TL, Triffleman EG, Shi J, Rounsaville BJ. Targeting behavioral therapies to enhance Naltrexone treatment of opioid dependence. Arch Gen Psychiatry. 2001;58:755–761. doi: 10.1001/archpsyc.58.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Hepatitis B vaccine: What you need to know. (DHHS Publication No. 42 U.S.C. § 300aa-26) Atlanta, GA: U.S. Gov. Printing Office; 2001. [Google Scholar]
- Comer SD, Sullivan MA, Hulse GK. Sustained-release naltrexone: Novel treatment for opioid dependence. Expert Opin Investig Drugs. 2007;16:1285–1294. doi: 10.1517/13543784.16.8.1285. [DOI] [PubMed] [Google Scholar]
- Haney M, Kosten TR. Therapeutic vaccines for substance dependence. Expert Rev Vaccines. 2004;3:11–18. doi: 10.1586/14760584.3.1.11. [DOI] [PubMed] [Google Scholar]
- Johansson BA, Berglund M, Lindgren A. Efficacy of maintenance treatment with naltrexone for opioid dependence: a meta-analytical review. Addiction. 2006;101:491–503. doi: 10.1111/j.1360-0443.2006.01369.x. [DOI] [PubMed] [Google Scholar]
- Kampman KM. The search for medications to treat stimulant dependence. Addict Sci Clin Pract. 2008;4:36–38. doi: 10.1151/ascp084228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malotte CK, Hollingshead JR, Rhodes F. Monetary versus nonmonetary incentives for TB skin test reading among drug users. Am J Prevent Med. 2001;16:182–188. doi: 10.1016/s0749-3797(98)00093-2. [DOI] [PubMed] [Google Scholar]
- Martell BA, Mitchell E, Poling J, Gonsal K, Kosten TR. Vaccine pharmacotherapy for the treatment of cocaine dependence. Biol Psychiatry. 2005;58:158–164. doi: 10.1016/j.biopsych.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2008:CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- Orson FM, Kinsey BM, Singh RA, Wu Y, Kosten TR. Vaccines for cocaine abuse. Hum Vaccin. 2009;5:194–199. doi: 10.4161/hv.5.4.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Martin B. Low-cost contingency management for treating cocaine- and opioid-abusing methadone patients. J Consult Clin Psychol. 2002;70:398–405. doi: 10.1037//0022-006x.70.2.398. [DOI] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Umbricht A, DeJesus A, Montoya ID, Schuster CR. Improvement in naltrexone treatment compliance with contingency management. Drug Alcohol Depend. 1999;54:127–135. doi: 10.1016/s0376-8716(98)00152-5. [DOI] [PubMed] [Google Scholar]
- Preti A. New Developments in the pharmacotherapy of cocaine abuse. Addict Biol. 2007;12:133–151. doi: 10.1111/j.1369-1600.2007.00061.x. [DOI] [PubMed] [Google Scholar]
- Raisch DW, Fye CL, Boardman KD, Sather MR. Opioid dependence treatment, including buprenorphine/naloxone. Ann Pharmacother. 2002;36:312–321. doi: 10.1345/aph.10421. [DOI] [PubMed] [Google Scholar]
- Rigsby MO, Rosen MI, Beauvais JE, Cramer JA, Rainey PM, O’Malley SS, Dieckhaus KD, Rounsaville BJ. Cue dose training with monetary reinforcement: Pilot study of an antiretroviral adherence intervention. J Gen Int Med. 2000;15:841–847. doi: 10.1046/j.1525-1497.2000.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MJ, Dieckhaus K, McMahon TJ, Valdes B, Petry NM, Cramer J, Rounsaville B. Improved adherence with contingency management. AIDS Patient Care STDS. 2007;21:30–40. doi: 10.1089/apc.2006.0028. [DOI] [PubMed] [Google Scholar]
- Seal KH, Kral AH, Lorvick J, McNees A, Gee L, Edlin BR. A randomized controlled trial of monetary incentives vs. outreach to enhance adherence to the hepatitis B vaccine series among injection drug users. Drug Alcohol Depend. 2003;71:127–131. doi: 10.1016/s0376-8716(03)00074-7. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Wong CJ, Chausmer AL, Liebson IA, Bigelow GE. Evaluation of an injection depot formulation of buprenorphine: placebo comparison. Addiction. 2004;99:1429–1449. doi: 10.1111/j.1360-0443.2004.00834.x. [DOI] [PubMed] [Google Scholar]
- Sorensen JL, Haug NA, Delucchi KL, Gruber V, Kletter E, Batki SL, Tulsky JP, Barnett P, Hall S. Voucher reinforcement improves medication adherence in HIV-positive methadone patients: A randomized trial. Drug Alcohol Depend. 2006;88:54 –63. doi: 10.1016/j.drugalcdep.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]


