Abstract
We used a new computer-assisted method to precisely localize and efficiently quantify increases in NPY immunoreactivity (NPY-ir) along the mediolateral axis of the L4 dorsal horn following transection of either the tibial and common peroneal nerves (thus sparing the sural branch, spared nerve injury, SNI), the tibial nerve, or the common peroneal and sural nerves. Two weeks after SNI, NPY-ir increased within the tibial and peroneal innervation territories; however, NPY-ir in the central-lateral region (innervated by the spared sural nerve) was indistinguishable from that of SHAM. Conversely, transection of the sural and common peroneal nerved induced an increase in NPY-ir in the central-lateral region, while leaving the medial region (innervated by the tibial nerve) unaffected. All nerve injuries increased NPY-ir in dorsal root ganglia (DRG) and nucleus gracilis (NG). By 24 wk, both NPY-ir up-regulation in the dorsal horn and hyper-responsivity to cold and noxious mechanical stimuli had resolved. Conversely, NPY-ir in DRG and NG, and hypersensitivity to non-noxious static mechanical stimuli, did not resolve within 24 wk. Over this time course, the average cross-sectional area of NPY-immunoreactive DRG neurons increased by 150 μm2. We conclude that the up-regulation of NPY after SNI is restricted to medial zones of the dorsal horn, and therefore cannot act directly upon synapses within the more lateral (sural) zones to control sural nerve hypersensitivity. Instead, we suggest that NPY in the medial dorsal horn tonically inhibits hypersensitivity by interrupting mechanisms of central sensitization and integration of sensory signals at the spinal and supraspinal levels.
Keywords: dorsal root ganglia, allodynia, pain, neuropathy, hyperalgesia, rat
INTRODUCTION
Pathological changes in the gene expression of peptidergic neurotransmitters and receptors in primary afferent neurons, spinal cord dorsal horn, and brain are thought to determine whether or not traumatic nerve injury will lead to the development of chronic pain (Hokfelt et al., 1994, Iadarola, 1997). Key candidates of facilitatory and inhibitory modulation include neuropeptide Y (NPY) and its cognate receptors. They are highly expressed at crucial sites of pain transmission, including lamina II of the spinal cord dorsal horn (Gibson et al., 1984, Ji et al., 1994, Brumovsky et al., 2007). Intrathecal administration of NPY receptor agonists reduces behavioral and molecular signs of neuropathic pain (Smith et al., 2007, Taylor et al., 2007, Intondi et al., 2008, Kuphal et al., 2008), implicating spinal NPY as a major player in neuropathic pain control.
Within the cell bodies of large myelinated primary afferent neurons in the dorsal root ganglia (DRG) and their central terminals in the dorsal column nuclei (DCN), the expression of NPY increases after axotomy (Wakisaka et al., 1991), chronic sciatic nerve constriction injury (CCI) (Brumovsky et al., 2004), partial sciatic nerve ligation (pSNL) (Ma and Bisby, 2000), median nerve transection (Tsai et al., 2007) and L5/L6 spinal nerve ligation (SNL) (Ossipov et al., 2002). The dorsal horn, which receives NPY terminals from intrinsic neurons as well as collaterals from large DRG neuron, also exhibits NPY up-regulation after CCI (Brumovsky et al., 2004), pSNL (Shi et al., 1999), and SNL (Ossipov et al., 2002). The primary goal of this study was to determine for the first time whether the spinal up-regulation of NPY is restricted to the central innervation territories of injured nerves, or spreads laterally, to adjacent territories in the dorsal horn which receive input from uninjured nerves. To address this question, we used the spared nerve injury model (SNI, transection of the tibial and common peroneal branches of the sciatic nerves, leaving the sural branch intact (Decosterd and Woolf, 2000, Lee et al., 2000), because it allows for the direct comparison of immunohistochemical staining of injured and uninjured afferent terminals within the same transverse section (Robinson et al., 2003, Beggs and Salter, 2007). Because the central termination sites of each branch of the sciatic nerve can be accurately and reliably mapped within the mediolateral axis of the dorsal horn, we can use the SNI model to understand the spatial distribution of nerve injury-induced changes in spinal cord NPY expression. At the L4 segment, the tibial, peroneal, and sural branches innervate the medial, medial-central and central-lateral aspects of the dorsal horn, respectively (Swett and Woolf, 1985, Shields et al., 2003). We used this anatomical information, together with a new computer-assisted method (see Corder et al, Journal of Pain, in press) to precisely distinguish and quantify immunohistochemical staining patterns across the mediolateral extent of the dorsal horn. This enabled us to evaluate the effect of nerve injury on NPY expression in the central terminal zones of both injured and uninjured primary afferent neurons.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (Charles Rivers Laboratories, Inc) were housed two per cage in a temperature controlled room on a 12-hour light/dark cycle (6am/6pm), and were given food and water ad libitum. All animal use protocols were approved by the Institutional Animal Care and Use Committee of both Tulane University and the University of Kentucky.
Spared Nerve Injury (SNI) surgery
Anesthesia was induced and maintained throughout surgery with 5% and ~2% isoflurane, respectively. The left hind-leg area was shaved and wiped clean with alcohol and betadine. An incision was made in the skin at the level of the trifurcation of the left sciatic nerve. The overlying muscles were retracted, exposing the common peroneal (CP), tibial (T), and sural (S) nerves. CP and T were ligated with 6.0 silk (Ethicon, Somerville, NJ) and then the knot and adjacent nerve (2 mm) were transected (TxCPx) as described previously (Decosterd and Woolf, 2000) . Two additional models were tested in which the tibial nerve alone or the common peroneal and sural nerves were transected (Tx and CPxSx, respectively). Care was always taken to avoid touching the spared branch(es). The muscle was sutured with absorbable 5-0 sutures (Ethicon) and the wound was closed with 9 mm metal clips. Sham surgery was produced by skin incision at the level of the trifurcation, and by exposing but not touching the three sciatic branches.
Behavioral Testing
Tactile threshold
Tactile threshold was assessed with an incremental series of 8 von Frey filaments of logarithmic stiffness (Stoelting, Inc.; approximately 0.7 - 11.8 g). The 50% withdrawal threshold was determined using the up-down method of Dixon, modified by Chaplan et al (Chaplan et al., 1994). First, an intermediate von Frey filament (2.0 g) was applied perpendicular to the hind-paw with sufficient force to cause a slight bending of the filament. The stimulus was applied to the plantar skin of the sural (lateral) and/or tibial (central) innervation territories. In case of a positive response (rapid withdrawal of the paw within 3 sec), the next smaller filament was tested. In case of a negative response, the next larger filament was tested.
Response to cool temperature
Using a syringe connected to PE-90 tubing, flared at the tip to a diameter of 3½ mm, we applied a drop of acetone to the plantar paw. Surface tension maintained the volume of the drop to 10-12 μl. The length of time the animal lifted or shook its paw was recorded. The duration of paw response was recorded for 30 sec. Three observations were averaged for analysis.
Response to noxious mechanical stimulus
We gently applied the tip of a diaper pin to the footpad, avoiding damage to the skin. The stimulus was applied to the plantar skin of the sural (lateral) and/or tibial (central) innervation territories. The duration of paw withdrawal was recorded for 30 sec. Three observations were averaged for analysis.
Perfusion
Animals were deeply anesthetized with an overdose of ketamine/xylazine (Vedco, St. Joseph, MO, Henry Schein, Melville, NY, respectively) by intramuscular injection (1 ml/kg of 88.9 mg/ml ketamine/11.1 mg/ml xylazine). All spinal cord and 50% of the nucleus gracilis samples were harvested following transcardial perfusion with ~200 ml chilled 2% sodium nitrate and then ~400 ml 4% paraformaldehyde with acrolein (2.5 ml/100 ml paraformaldehyde). After post-fixation in 4% paraformaldehyde at 4° for 3 hrs, tissues were transferred to 0.05 M PBS overnight, and then sectioned at 50 μm on a vibratome (Lancer Series 3000, St. Louis, MO). All DRG and the remaining nucleus gracilis samples were harvested following perfusion with ~200 ml PBS and then ~400 ml 10% buffered formalin. After post-fixation for 2 hr and cryoprotection in 30% sucrose overnight, NG sections were post-fixed and sectioned as described above, while DRG were embedded in OCT compound (Sakura Finetek USA, Inc., Torrance, CA), flash-frozen in isopentane (Fisher Scientific, Houston, Tx) and stored at −20° until use (<24 wk). DRG sections (16 μm) were cut on a cryostat (Microm International HM550, Walldorf, Germany).
Immunohistochemistry
Tissues from lesioned and sham rats were processed in parallel to ensure comparable staining across treatment.
Spinal Cord and Nucleus Gracilis
Tissue sections were washed 3 × 5 min in 1% sodium borohydride (1g NaBH4/100 mls PBS) and then 2×10min in PBS. Next they were blocked in PBS with 1% H2O2, 0.02% Triton-X 100, and 1% normal goat serum (NGS) for 10 min, and then again in PBS with 0.4% Triton-X 100 and 2% NGS for 15 min (in PBS). Next, they were incubated in primary antibody solution (NPY 1:6000, anti-rabbit, Peninsula Labs, CA, 2% NGS, 0.4% Triton-X 100, 0.05% NaN3) for 48-72 hr at 4°. After 4 × 15 min washes in PBS, sections were incubated for 2 hrs at RT in secondary antibody (1:200, anti-goat). After further washes in PBS, signal was enhanced with the ABC method and visualized with diaminobenzidine (DAB) according to the manufacturer’s instructions (Vectastain Elite ABC kit, PK-6100, Vector Labs) for 1 h at room temperature. After final washes in phosphate buffer (PB), sections were allowed to dry, dehydrated in an alcohol series, mounted on Superfrost/Plus glass slides (Fisherbrand, Houston, TX) and then cover-slipped using Permount (Fisherbrand, Houston, TX).
DRG
Slides were washed 15 min in 0.1 M PBS containing 1% Normal Donkey Serum and 0.3% Triton-X 100 in (1% NDST), blocked for 60 min with 5% NDST, and then incubated at 4° for 72 hrs in primary NPY antisera (1:10,000 in 1% NDST). Slides were then rinsed 3 × 15 min in 1% NDST, re-blocked for 30 min with 5% NDST, and incubated overnight at 4° with a donkey anti-rabbit secondary antibody conjugated to Alexa 488 (Invitrogen, Eugene, OR). The slides were then rinsed 3 × 10 min in 0.1 PB, and cover-slipped with Prolong Gold Anti-Fade Reagent (Invitrogen).
Image Acquisition and Quantification of NPY-ir in Dorsal Horn
Digitized images of immunostained sections taken from lumbar segment L4 and the rostral part of L5 were captured on a Nikon TE2000-E microscope with a 4X objective on black and white Roper Scientific camera at 12-bit resolution (4096 intensity levels), using Metamorph Imaging software (Version 6.1r4, Universal Imaging Corp.). To standardize image acquisition across staining sessions, we first used the “Shading Correction” tool to normalize light intensity across the field of view. More importantly, we captured images only after adjusting exposure time with the “Image Gamma” tool. This resulted in essentially identical contrast from picture to picture. All procedures were performed blind to treatment.
We faced a unique challenge in the design of an automated, computer-assisted method to quantify staining intensity within the T, CP, and S sub-regions of the dorsal horn: the width of the SG is highly variable between sections. To solve this problem, we used Metamorph imaging software to develop a normalization procedure. First, each image was digitized, and as previously described (Smith et al., 2001), the upper and lower threshold optical densities were adjusted to encompass and match the immunoreactivity, providing an image with immunoreactive material appearing red. The area and density of pixels within the threshold values representing immunoreactivity were calculated and the integrated density was the product of the area and density. Second, we created a 900 μm in width template consisting of 224 narrow adjacent boxes, 260 μm in height and 4.0178 μm in width, and manually placed it over the left or right digitized image of the dorsal horn such that the right-most or left-most box overlaid the most medial edge of contralateral or ipsilateral SG, respectively. Even if nerve injury abolished medial staining (as is often the case following transection of the tibial nerve), the experimenter was easily able to distinguish between the labeled gray matter of the dorsal horn from the white matter of the dorsal columns. Third, all boxes that extended beyond the lateral edge of the gray matter were deleted with a computerized algorithm. The lateral edge of the gray matter was always strongly stained following tibial, common peroneal, and/or sural nerve transection, because these surgeries do not target the posterior cutaneous nerve (which innervates the most lateral aspect of the L4 SG). By contrast, NPY immunohistochemistry never yielded much reaction product within the white matter. Therefore, boxes whose measured intensity value was less than 30% of the overall average intensity were reliably and accurately removed in an automated, computer-assisted fashion. Finally, using boundaries calculated with TMP histochemistry and tibial/common peroneal/sural nerve transection studies [manuscript accepted with minor revision at Journal of Pain], the remaining boxes were divided into tibial (most medial 30.5% of boxes, common peroneal (the next medial 18.8% of boxes) or sural (the next lateral 24.6% of boxes) sectors of the SG. We refer to these sectors as the “medial”, “medial-central”, and “central-lateral” subregions, respectively. Using an automated Metamorph procedure, integrated density was calculated for medial and central-lateral sub-regions, and then imported into a Microsoft Excel spread sheet. We evaluated staining on both sides of the dorsal horn, ipsilateral and contralateral to treatment. We averaged the results of 4-6 sections per rat. Data are presented as the ratio (%) of ipsilateral side / contralateral side.
Image Acquisition and Quantification of NPY-ir in DRG
Using a Nikon TE2000-E microscope with Metamorph software, digital photomicrographs (100×: 10× ocular, 10× objective) of at least 5 randomly selected sections were taken from both L4 and L5 DRGs from a minimum of 3 animals. To standardize exposure time, we used the ‘autoexpose’ function on 3 stained photomicrographs; the average exposure time was applied to all further image captures. Only cells with a visible nucleus were analyzed. Background luminosity was determined by averaging the maximum luminosity of five clearly unlabeled cells. Only cells with luminosity at least twice the background luminosity were counted as labeled cells. To ensure that cells were not counted more than once, we ensured at least 144 μm intervals between analyzed sections. The percentages of labelled profiles were calculated for each DRG and averaged for each group. The percentage of labelled cells was similar in L4 and L5 DRG, so this data was combined for graphical presentation.
To obtain size distribution histograms in randomly-selected DRG sections, Metamorph software was used to calculate the area of each NPY-positive profile. Sections from at least 3 rats provided dozens to hundreds of profiles for analysis within each Surgery group. Size data was binned into 100 um size intervals for graphical presentation.
Image Acquisition and Quantification of NPY staining in the Nucleus Gracilis
Seven sections were randomly selected from each animal for analysis. Using a Nikon T2000 microscope, photomicrographs were captured using Spot Advanced software and analyzed with NIH Image J. A standardized rectangle was placed over the ipsilateral or contralateral NG, and the integrated density (Density × Area) was calculated. Data is presented as a percentage of ipsilateral integrated density compared to contralateral side. Global differences in NPY-ir in the NG due to perfusion method were normalized using a correction factor of 0.4724 (applied to formalin group). This value was calculated by dividing the total means of the groups perfused with acrolein by the total means of the groups perfused with formalin. We then combined the data from formalin-perfused animals with the data from acrolein-perfused animals.
Data Analysis
We conducted two-way ANOVA using Surgery (SHAM, TxCPx, Tx and CPxSx) and Time (2 wk vs 24 wk) as grouping factors. If the overall F-value was significant (p<0.05), then subsequent post hoc Tukey’s or Student’s t-tests were used to determine individual differences between Time for each Surgery group and between Surgery group at each Time. For DRG cell size distribution comparisons, Chi-square tests were conducted to test for a shift in distribution between 2 wk and 24 wk.
RESULTS
Characterization of hypersensitivity in terms of time, modality, and nerve injury variant
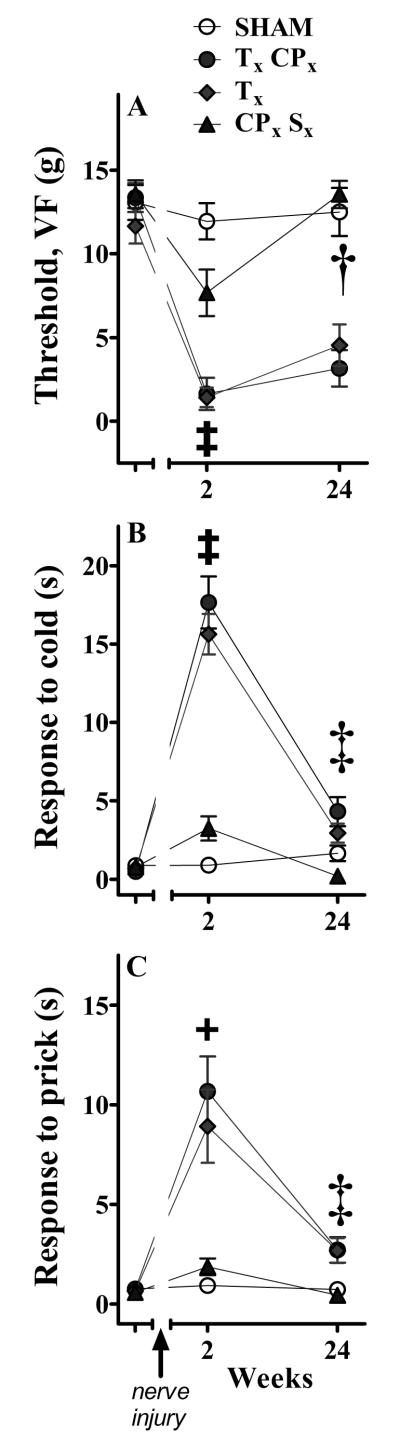
To determine the relationship between transection of specific branches of the sciatic nerve and behavioral signs of neuropathic pain, we evaluated behavioral responses evoked fromthe distal innervation territory of the sural nerve (after TxCPx or Tx) or tibial nerve (after CPxSx)before and at 2 and 24 wk after nerve injury. Two-way ANOVA revealed a main effect of Surgery on von Frey threshold [F(3,116) = 57, p<0.0001], cold response duration [F(3,135) = 60, p<0.0001)], and prick response duration ([F(3,134) = 18, p<0.0001). Subsequent one-way ANOVA revealed a main effect of Surgery (Sham vs TxCPx vs Tx vs CPxSx) on: VF threshold at 2 wk [F(3,57) = 24, p<0.0001)] and 24 wk [F(3,59) = 36, p<0.0001)]; cold response duration at 2 wk [F(3,68) = 55, p<0.0001)] and 24 wk [F(3,67) = 8.5, p<0.0001)]; and prick response duration at 2wk [F(3,67) = 13, p<0.0001)] and 24 wk [F(3,67) = 6.7, p<0.0001)]. Likewise, one-way ANOVA revealed a main effect of Time (2 wk vs 24 wk) on: VF thresholds after CPxSx [F(1,28) = 13, p<0.005)] but neither Sham [F=0.1] nor TxCPx [F=1.1] nor Tx [F=3.2]; cold response duration after TxCPx [F(1,31) = 47, p<0.0001)] and Tx [F(1,38) = 79, p<0.0001)] and CPxSx [F(1,34) = 15, p<0.0001)] but not Sham [F=1.1]; and prick response duration after TxCPx [F(1,32) = 18, p<0.0001)] and Tx [F(1,38) = 10, p<0.005)] and CPxSx [F(1,34) = 11, p<0.005)] but not Sham [F=0.6]. Figure 1 provides the results of post-hoc tests. In summary, transection of the peroneal and/or sural nerve, sparing the tibial nerve, yielded only weak responses to VF, prick, and acetone stimuli. By contrast, transection of the tibial nerve, either alone or in combination with the common peroneal nerve, generated robust hypersensitivity. These results are consistent with and extend previous studies in the rat (Decosterd and Woolf, 2000, Lee et al., 2000), and might reflect the fact that the tibial nerve is by far the largest primary distal branch of the rat sciatic nerve (Schmalbruch, 1986).
Figure 1. Behavioral hypersensitivity after variants of spared nerve injury (SNI).
Von Frey (VF) or prick stimulation at the distal innervation territory of the spared sural nerve, or acetone application at the plantar hindpaw, was delivered at 2 and 24 wk after transection of the tibial (Tx), common peroneal (CPx) and/or sural (Sx) branches of the sciatic nerve. Panel A illustrates that all three lesions decreased VF threshold at 2 wk (‡p<0.05 TxCPx and Tx and CPxSx different from SHAM at 2 wk), but the hypersensitivity produced by CPxSx was relatively small and returned to baseline by 24 wk († p<0.05 2 wk different from 24 wk in CPxSx). By contrast, the dramatic hypersensitivity produced by TxCPx and Tx remained significantly different from that of sham animals at 24 wk, and showed no significant effect of Time. Panels B and C illustrate that CPxSx produced less cold hyper-responsiveness and noxious mechanical hyper-responsiveness than either TxCPx or Tx at 2 wk. Indeed, only TxCPx and Tx produced significant hyper-responsiveness to noxious mechanical prick (+p<0.05 TxCPx and Tx different from SHAM at 2 wk). Cold hyper-responsiveness and noxious mechanical hyper-responsiveness decreased from 2 wk to 24 wk after each lesion, indicating a significant effect of Time (‡ p<0.05, 2 wk different from 24 wk in TxCPx, Tx, and CPxSx). n = 13-20. Values represent mean ± SEM.
We found that VF hypersensitivity after TxCPx and Tx was sustained for 24 wk, consistent with the 31 wk period originally reported by Decosterd and Woolf (Decosterd and Woolf, 2000). By contrast, hypersensitivity to acetone and prick stimuli had largely dissipated at 24 wk. We conclude that the modality of the test stimulus must be taken into account when establishing the temporal resolution of hypersensitivity after peripheral nerve injury.
Short-term up-regulation of NPY in the dorsal horn after SNI after various nerve injuries
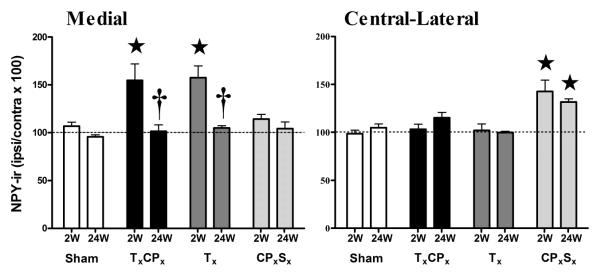
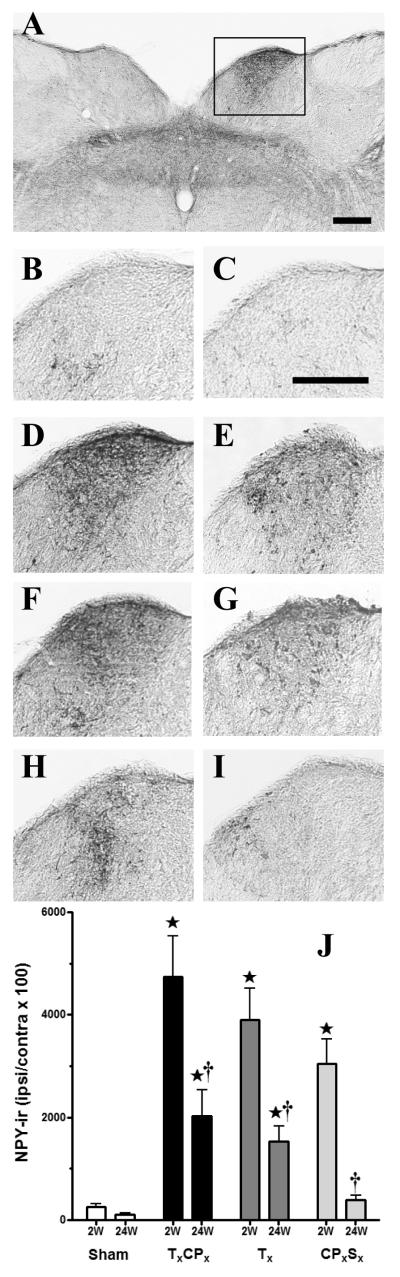
Although previous studies have demonstrated that peripheral nerve injury up-regulates the terminal staining of NPY-ir in dorsal horn (Ma and Bisby, 2000, Brumovsky et al., 2004, Smith et al., 2007), the source of this increase (injured vs uninjured DRG) is unclear. To determine whether transection of selected sciatic nerve branches increases NPY in injured or uninjured nerve innervation territories of the dorsal horn, we used a new method to quantify NPY-ir in the medial and central-lateral subregions along the mediolateral axis (Corder et al.). As illustrated in Figs 2-3, Sham surgery did not change NPY-ir compared to the contralateral side. At 2 wk post-surgery, TxCPx or Tx increased NPY-ir in the medial subregion (which is innervated by the injured tibial nerve, p<0.05 vs Sham) but not the central-lateral subregion (which is innervated by the uninjured sural nerve). Conversely, CPxSx increased NPY-ir in the central-lateral subregion (which is innervated by the injured sural nerve, p<0.05 vs Sham) but not the medial subregion (which is innervated by the uninjured tibial nerve). NPY up-regulation was most apparent in the terminals of lamina III-IV. This was not surprising, because nerve injury predominantly if not exclusively increases NPY expression within the large diameter cell bodies of sensory neurons in DRG that likely give rise to these terminals (Wakisaka et al., 1992, Brumovsky et al., 2004).
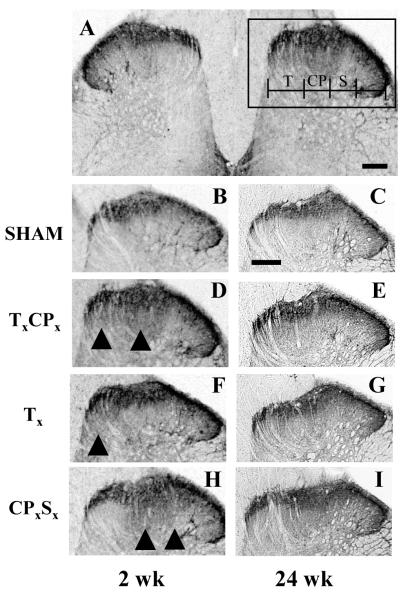
Figure 2. Up-regulation of NPY in medial dorsal horn after SNI.
Panel A illustrates a representative photomicrograph of NPY immunoreactivity (NPY-ir) in ipsilateral dorsal horn of a TxCPx (SNI) rat taken two weeks after nerve injury. The remaining photomicrographs depict higher power images of the ipsilateral lumbar dorsal horn at 2 wk (A,B,D,F,H) or 24 wk (C,E,G, I) after SHAM (B,C), TxCPx (A,D,E), Tx (F,G), or CPxSx (H,I). Panel D represents a higher magnification image of the ipsilateral dorsal horn from an SNI rat, as indicated by the box in Panel A. Scalebar in Panel A = 200 μm, Panel C = 100 μm.
Figure 3. Up-regulation of NPY in medial dorsal horn after SNI.
NPY immunoreactivity in the medial and central-lateral subregions of the dorsal horn at 2 or 24 wk after transection of the tibial (Tx), common peroneal (CPx) and/or sural (Sx) branches of the sciatic nerve, expressed as a percentage of NPY-ir at the ipsilateral side / contralateral side. ⋆p<0.05 compared to respective Sham group. †p<0.05 compared to respective 2 wk group. Values represent mean ± SEM. n = 4-7.
Long-term up-regulation of NPY in DRG and NG but not DH
By 24 wk after Tx or TxCPx, NPY-ir in the medial DH had completely recovered to levels indistinguishable from pre-injury or Sham levels (P<0.05 vs 2 wk). Since NPY-ir terminals in lamina III-IV arise from collaterals of large diameter primary afferent axons as they ascend along the medial leminscus to dorsal column nuclei, we asked whether a similar loss NPY-ir occurred within L4/L5 DRG and their primary termination sites within the nucleus gracilis (NG).
DRG
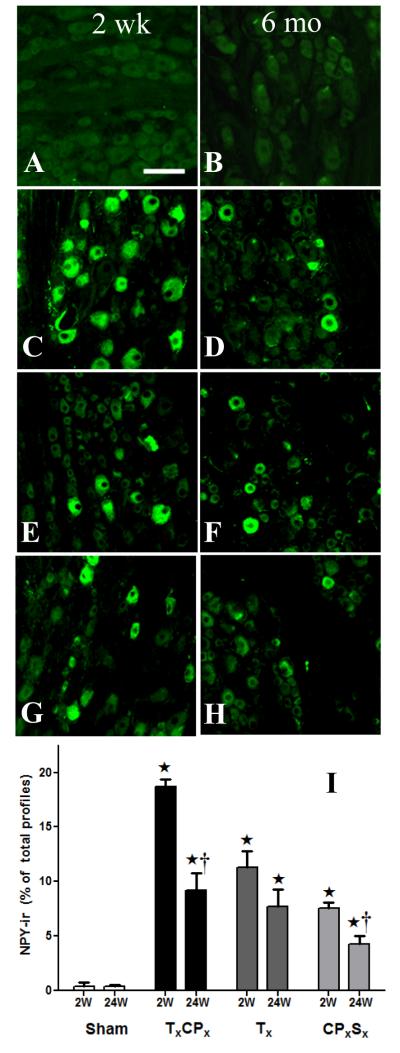
Consistent with previous reports (Wakisaka et al., 1992, Brumovsky et al., 2004) (Tsai et al., 2007), NPY-ir in uninjured Sham (Fig 4A-B) and contralateral DRG (not shown) was extremely low (<1%). As in other models of traumatic nerve damage, Figs 4C-H illustrates that transection of 1-2 branches of the sciatic nerve increased NPY-ir [F(3,18) = 53, P<0.0001]. TxCPx produced a greater increase in NPY-ir as compared to Tx or CPxSx at the 2 wk time point (P<0.001). Two-way ANOVA also revealed that NPY-ir decreased from 2 wk to 24 wk [F(1,18) = 29, P<0.0001]. Unlike the DH, however, NPY-ir did not completely return to control values at 24 wk after Tx or TxCPx.
Figure 4. Up-regulation of NPY in DRG after nerve injury.
Representative photomicrographs of NPY immunoreactivity (NPY-ir) in lumbar DRG at two weeks (A,C,E,G) or 24 weeks (B,D,F,H) after SHAM (A,B), TxCPx (SNI)(C,D), Tx (E,F), or CPxSx (G,H). Panel I illustrates averaged NPY-ir for each condition. ⋆p<0.05 compared to respective Sham group. †p<0.05 compared to respective 2 wk group. Values represent mean ± SEM of 3-4 rats/group. Scalebar = 100 μm.
NG
Consistent with previous reports (Ossipov et al., 2002, and others), NPY-ir was virtually absent on the side contralateral to SNI (Fig 5A) in the Sham groups (Fig 5B-C). As in other models of traumatic nerve damage, Figs 5D-J illustrates that NPY-ir increased 2 wk after TxCPx, Tx and CPxSx [F(3,86) = 19, P<0.0001, main effect of Surgery]. As observed in DH and DRG, two-way ANOVA revealed that NPY-ir decreased from 2wk to 24 wk in all nerve injury groups [F(1,86) = 38, P<0.0001, main effect of Time]. Unlike the DH, however, NPY-ir did not completely return to control values at 24 wk after Tx or TxCPx.
Figure 5. Up-regulation of NPY in nucleus gracilis after nerve injury.
Panel A illustrates a representative photomicrograph of NPY immunoreactivity (NPY-ir) in nucleus gracilis of a TxCPx (SNI) rat taken two weeks after nerve injury. The remaining photomicrographs depict higher power images of the ipsilateral nucleus gracilis at two weeks (A,B,D,F,H) or 24 weeks (C,E,G, I) after SHAM (B,C), TxCPx (A,D,E), Tx (F,G), or CPxSx (H,I). Panel D represents a higher magnification image of the ipsilateral dorsal horn from an SNI rat, as indicated by the box in Panel A. Panel J illustrates averaged NPY-ir for each condition. ⋆p<0.05 compared to respective Sham group. †p<0.05 compared to respective 2 wk group. n = 7. Scalebars = 250 μm.
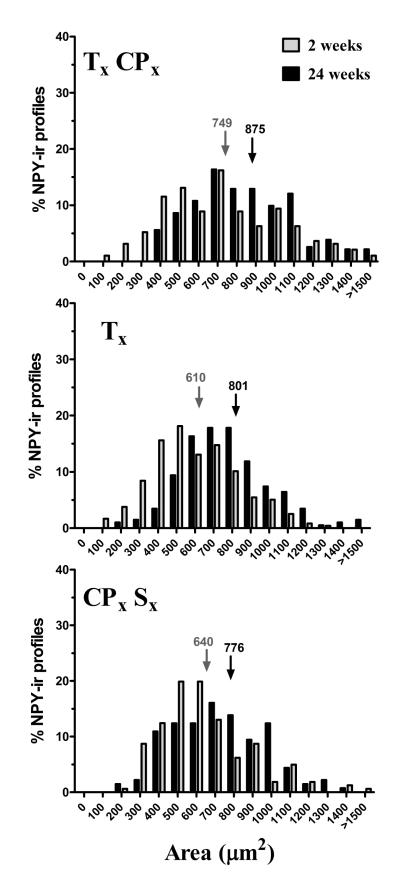
Size of NPY-ir profiles 2 wk or 24 wk after various nerve injuries
Tsai et al reported that median nerve transection increased NPY-ir in large (20-27%) and medium (73-79%) neurons but only rarely in small (0-2%) neurons when measured 1 d to 20 wk after surgery (Tsai et al., 2007). Here we extend this analysis to multiple models of sciatic nerve branch transection, with a more complete analysis of the size distribution of NPY-ir profiles at the 2 and 24 wk time points. As expected, Fig 6 illustrates that NPY-ir was induced in medium to large profiles 2 wk after each of the nerve injuries. NPY SHAM, like naïve rats, did not yield enough NPY-positive profiles to generate a histogram. At 24 wk, the distribution of NPY-positive profiles shifted towards an even larger size in TxCPx (χ2 = 34.46; P<0.01), Tx (χ2 = 55.81; P<0.001) and CPxSx all (χ2 = 25.55; P<0.05). Median profile size increased by 126 μm2 after TxCPx, 191 μm2 after Tx, and 136 μm2 after CPxSx (mean of 3 surgeries = 151 μm2).
Figure 6. Size of NPY-ir profiles 2 wk or 24 wk after nerve injury.
From 2 to 24 wk after transection of tibial, common peroneal, and/or sural branches of the sciatic nerve, the distribution of NPY-ir profiles shifted to larger cells (p>0.05). Arrows indicate the median cell size.
DISCUSSION
Spinal up-regulation of NPY is restricted to the innervation territories of injured nerves
Numerous studies demonstrate that various types of traumatic sciatic nerve injury increase the dorsal horn expression of NPY (Zhang et al., 1994, Shi et al., 1999, Ossipov et al., 2002, Brumovsky et al., 2004). Our aim was to more precisely localize these changes along the mediolateral axis following SNI and two of its variants, so as to more precisely identify the source of injury-induced spinal NPY. We found that Tx and TxCPx increased NPY only in the medial and medial-central regions of the DH; NPY staining in the central-lateral region (innervated by the spared sural nerve) was indistinguishable from that of SHAM. Conversely, CPxSx induced an increase in NPY in the central-lateral region, while leaving the medial region unaffected. Thus, NPY up-regulation is not due to a generalized up-regulation across the entire mediolateral axis of the dorsal horn, but rather is localized to the areas of the horn innervated by the injured nerve branches.
We conclude that sciatic nerve injury-induced increase in spinal NPY does not extend beyond somatotopic boundaries along the mediolateral axis. This may hold true along the other three-dimensional axes. For example, Ossipov et al briefly reported that the spinal up-regulation of NPY did not extend beyond the rostral-caudal borders of segments L5 and L6 following ligation of spinal nerves L5 and L6 (Ossipov et al., 2002). Furthermore, the up-regulation of NPY-ir in lamina III-IV does not appear to spill over ventrally into lamina V (Zhang et al., 1994, Shi et al., 1999, Ossipov et al., 2002, Brumovsky et al., 2004). Whether NPY up-regulation extends dorsally (e.g. from lamina III to lamina II) after nerve injury is a difficult question, because intrinsic substantia gelatinosa neurons are very NPY-immunoreactive in the absence of nerve injury.
Studies involving the application of retrograde fluorescent tracers at the distal stump of severed axons clearly demonstrate that the cell bodies of injured nerves express NPY (Ma and Bisby, 2000) (Tsai et al., 2007). In uninjured neurons, however, the available literature yields mixed results: NPY-ir profiles were reported in a significant proportion of unlabelled DRG after pSNL (~15%) (Ma and Bisby, 2000), but not after medial nerve transection (< 1%) (Tsai et al., 2007). The latter study indicates that peripheral nerve injury-induced up-regulation of NPY is restricted to injured sensory neurons and their terminals, which is consistent with our current findings in the dorsal horn after spared nerve injury.
Long-term up-regulation of NPY in DRG and NG but not DH
Two weeks after spared nerve injury or two of its variants, NPY-ir increased not only in dorsal horn but also in DRG and NG. This adds SNI and two of its variants to the long list of peripheral nerve injuries that induce NPY expression. By 24 wk, NPY-ir returned to normal levels in the dorsal horn. Surprisingly, NPY-ir in neither DRG cell bodies nor NG terminals completely recovered to control values at 24 wk after TxCPx, Tx or CPxSx. Although previous studies have not evaluated NPY-ir for as long as 24 wk after nerve injury, they are consistent with the idea that injury-induced NPY expression in DRG and NG persists for at least several months (Munglani et al., 1996) (Tsai et al., 2007). Our findings suggest that the long-term consequences of nerve injury on NPY expression vary between the termination sites of sensory neurons in the DH (complete recovery) and those in the cell bodies and their terminal fields in the NG (partial recovery). Further studies beyond the scope of this paper will be required to explain this surprising secondary finding. For now, we can only speculate that NPY packaging or transport differs between spinal cord and brainstem circuits, or that intrinsic neurons contribute to injury-induced NPY up-regulation in the DH. This latter idea has not been rigorously tested, despite the widely-held view that NPY up-regulation in the DH is restricted to collaterals arising from dorsal column-medial lemniscus neurons (Brumovsky et al., 2007). Such studies may contribute to a better understanding of the functional significance of NPY to neuropathic pain.
Indeed, as suggested in a review by Taylor and colleagues, neuropathic pain may be increased or decreased by activation of brainstem or spinal NPY receptors, respectively (Smith et al., 2007). On one hand, induction of NPY expression in DRG and NG appears to facilitate neuropathic pain. For example, microinjection of NPY Y1 receptor antagonists into the NG reduces behavioral signs neuropathic pain, suggesting that NPY release from large diameter primary afferents acts in the dorsal column nuclei to drive neuropathic pain (Ossipov et al., 2002). Alternatively, NPY increases neurite growth in cultured DRG neurons, suggesting that NPY induces sprouting, cross-excitation, and ultimately hypersensitivity (White and Mansfield, 1996). On the other hand, a substantial body of work indicates that induction of NPY expression in DRG and DH reduces neuropathic pain (Smith et al., 2007). For example, Ruscheweyh et al found an inverse correlation between NPY expression in the DRG and heat hypersensitivity after CCI (Ruscheweyh et al., 2007). Furthermore, spinally-directed administration of NPY acts at Y1 and or Y2 receptors to inhibit behavioral signs of neuropathic pain after SNI (Intondi et al., 2008).
Shift in Size Distribution of DRG neurons from 2 wk to 24 wk
As previously described in other models of peripheral nerve injury (Wakisaka et al., 1992, Shi et al., 1999, Ruscheweyh et al., 2007), we found that 2 wk after SNI or variants thereof, NPY was preferentially up-regulated in medium to large cell bodies, with an average cross-sectional area of 666 um2, which is comparable to the 714 um2 area reported previously (Ruscheweyh et al., 2007). Here we report for the first time that by 6 mo after nerve injury, the average cross-sectional area increased by 150 μm2, to 817 μm2. Coggeshall and colleagues reported that sciatic nerve transection slightly reduced cell volume of large diameter neurons with myelinated axons in the L5 DRG at 4 wk, but not at 2, 8, or 32 wk (Tandrup et al., 2000); therefore, it is unlikely that a non-specific retrograde reaction to the lesion contributes to an increase in the size of NPY-ir profiles at 24 wk. Ruscheweyh et al recently segregated primary afferent neurons by conduction velocity, and found that NPY-immunoreactive neurons were expressed in both Aδ- and Aβ-fiber neurons after CCI in mouse (Ruscheweyh et al., 2007). It is intriguing to speculate that, over several months after nerve injury, the expression of NPY shifts from Aδ- to Aβ-fiber neurons. Assuming that Aδ-fibers (along with C-nociceptors) mediate hyper-responsiveness to cold and mechanical stimuli, while Aβ-fibers mediate von Frey hypersensitivity (Ossipov et al., 2000), this could explain our observation that the hypersensitivity phenotype shifts from 2 wk to 6 mo after SNI, so as to favour hypersensitivity to von Frey stimulation. Further conduction velocity experiments similar to Ruscheweyh et al could test these hypotheses.
Does endogenous spinal NPY release reduce hyperalgesia in the SNI model?
As alluded to above, an important question is whether extracellular NPY concentrations in the dorsal horn inhibit abnormal sensory function and thus reduce the neural pathophysiology underlying chronic pain (Taylor, 2005, Brumovsky et al., 2007, Smith et al., 2007). Comparison of the time course of hypersensitivity and peptide immunoreactivity in the current study suggests that NPY expression in DH best matches the duration of cold and mechanical hyper-responsiveness, but not hypersensitivity to innocuous von Frey stimulation. Caution must be taken when addressing the physiological significance of this correlation, however, because the intensity of NPY-ir does not necessarily reflect NPY release. With this in mind, our laboratory has recently presented data in abstract form indicating that conditional knockdown of NPY is associated with the facilitation of hypersensitivity (Solway and Taylor, 2007). Furthermore, Ruscheweyh and Sandkuhler recently reported that the extent of up-regulation of NPY in DRG showed a statistically significant inverse correlation with heat hyperalgesia (Ruscheweyh et al., 2007).
We conclude that the up-regulation of NPY after SNI is restricted to medial zones of the dorsal horn, and therefore cannot act directly upon synapses within the more lateral (sural) zones to control sural nerve hypersensitivity. Instead, we suggest that NPY in the medial dorsal horn tonically inhibits hypersensitivity by interrupting mechanisms of central sensitization and the integration of sensory signals at the spinal and supraspinal levels.
Acknowledgements
Supported by grants R01NS45954 and K02DA19656 to BKT and the VA and ONR to JEZ. Contents do not represent the views of the VA or the US Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Beggs S, Salter MW. Stereological and somatotopic analysis of the spinal microglial response to peripheral nerve injury. Brain, behavior, and immunity. 2007;21:624–633. doi: 10.1016/j.bbi.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumovsky P, Shi TS, Landry M, Villar MJ, Hokfelt T. Neuropeptide tyrosine and pain. Trends in pharmacological sciences. 2007;28:93–102. doi: 10.1016/j.tips.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Brumovsky PR, Bergman E, Liu HX, Hokfelt T, Villar MJ. Effect of a graded single constriction of the rat sciatic nerve on pain behavior and expression of immunoreactive NPY and NPY Y1 receptor in DRG neurons and spinal cord. Brain Res. 2004;1006:87–99. doi: 10.1016/j.brainres.2003.09.085. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of neuroscience methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Corder G, Siegel A, Intondi AB, Zhang X, Zadina JE, Taylor BK. A novel method to quantify histochemical changes throughout the mediolateral axis of the substantia gelatinosa after spared nerve injury: characterization with TRPV1 and substance P. Journal of Pain. doi: 10.1016/j.jpain.2009.09.008. (accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Gibson SJ, Polak JM, Allen JM, Adrian TE, Kelly JS, Bloom SR. The distribution and origin of a novel brain peptide, neuropeptide Y, in the spinal cord of several mammals. Journal of Comparative Neurology. 1984;227:78–91. doi: 10.1002/cne.902270109. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Zhang X, Wiesenfeld-Hallin Z. Messenger plasticity in primary sensory neurons following axotomy and its functional implications. Trends in neurosciences. 1994;17:22–30. doi: 10.1016/0166-2236(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Iadarola M. Good Pain, Bad Pain. Science. 1997;278:353. doi: 10.1126/science.278.5336.239. [DOI] [PubMed] [Google Scholar]
- Intondi AB, Dahlgren MN, Eilers MA, Taylor BK. Intrathecal neuropeptide Y reduces behavioral and molecular markers of inflammatory or neuropathic pain. Pain. 2008;137:352–365. doi: 10.1016/j.pain.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Zhang X, Wiesenfeld-Hallin Z, Hokfelt T. Expression of neuropeptide Y and neuropeptide Y (Y1) receptor mRNA in rat spinal cord and dorsal root ganglia following peripheral tissue inflammation. J Neurosci. 1994;14:6423–6434. doi: 10.1523/JNEUROSCI.14-11-06423.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuphal KE, Solway B, Pedrazzini T, Taylor BK. Nutrition. Vol. 24. Burbank; Los Angeles County, Calif: 2008. Y1 receptor knockout increases nociception and prevents the anti-allodynic actions of NPY; pp. 885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Won R, Baik EJ, Lee SH, Moon CH. An animal model of neuropathic pain employing injury to the sciatic nerve branches. Neuroreport. 2000;11:657–661. doi: 10.1097/00001756-200003200-00002. [DOI] [PubMed] [Google Scholar]
- Ma W, Bisby MA. Partial sciatic nerve ligation induced more dramatic increase of neuropeptide Y immunoreactive axonal fibers in the gracile nucleus of middle-aged rats than in young adult rats. Journal of neuroscience research. 2000;60:520–530. doi: 10.1002/(SICI)1097-4547(20000515)60:4<520::AID-JNR11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Munglani R, Harrison SM, Smith GD, Bountra C, Birch PJ, Elliot PJ, Hunt SP. Neuropeptide changes persist in spinal cord despite resolving hyperalgesia in a rat model of mononeuropathy. Brain research. 1996;743:102–108. doi: 10.1016/s0006-8993(96)01026-8. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lai J, Malan TP, Jr., Porreca F. Spinal and supraspinal mechanisms of neuropathic pain. Ann N Y Acad Sci. 2000;909:12–24. doi: 10.1111/j.1749-6632.2000.tb06673.x. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Zhang ET, Carvajal C, Gardell L, Quirion R, Dumont Y, Lai J, Porreca F. Selective mediation of nerve injury-induced tactile hypersensitivity by neuropeptide Y. J Neurosci. 2002;22:9858–9867. doi: 10.1523/JNEUROSCI.22-22-09858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CM, Kriedt CL, Mosley LD, Taylor BK. Comparison Of Hyperalgesia And Allodynia In Spared Sural, Spared Tibial, And Tibial Transection Models Of Neuropathic Pain. Society for Neuroscience Abstracts. 2003:29. [Google Scholar]
- Ruscheweyh R, Forsthuber L, Schoffnegger D, Sandkuhler J. Modification of classical neurochemical markers in identified primary afferent neurons with Abeta-, Adelta-, and C-fibers after chronic constriction injury in mice. The Journal of comparative neurology. 2007;502:325–336. doi: 10.1002/cne.21311. [DOI] [PubMed] [Google Scholar]
- Schmalbruch H. Fiber composition of the rat sciatic nerve. Anat Rec. 1986;215:71–81. doi: 10.1002/ar.1092150111. [DOI] [PubMed] [Google Scholar]
- Shi TJ, Cui JG, Meyerson BA, Linderoth B, Hokfelt T. Regulation of galanin and neuropeptide Y in dorsal root ganglia and dorsal horn in rat mononeuropathic models: possible relation to tactile hypersensitivity. Neuroscience. 1999;93:741–757. doi: 10.1016/s0306-4522(99)00105-0. [DOI] [PubMed] [Google Scholar]
- Shields SD, Eckert WA, 3rd, Basbaum AI. Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. J Pain. 2003;4:465–470. doi: 10.1067/s1526-5900(03)00781-8. [DOI] [PubMed] [Google Scholar]
- Smith PA, Moran TD, Abdulla F, Tumber KK, Taylor BK. Spinal mechanisms of NPY analgesia. Peptides. 2007;28:464–474. doi: 10.1016/j.peptides.2006.09.029. [DOI] [PubMed] [Google Scholar]
- Smith RR, Martin-Schild S, Kastin AJ, Zadina JE. Decreases in endomorphin-2-like immunoreactivity concomitant with chronic pain after nerve injury. Neuroscience. 2001;105:773–778. doi: 10.1016/s0306-4522(01)00228-7. [DOI] [PubMed] [Google Scholar]
- Solway BM, Taylor BK. Contribution of endogenous NPY signaling to chronic pain: a transgenic approach. American Pain Society abstracts. 2007 [Google Scholar]
- Swett JE, Woolf CJ. The somatotopic organization of primary afferent terminals in the superficial laminae of the dorsal horn of the rat spinal cord. The Journal of comparative neurology. 1985;231:66–77. doi: 10.1002/cne.902310106. [DOI] [PubMed] [Google Scholar]
- Tandrup T, Woolf CJ, Coggeshall RE. Delayed loss of small dorsal root ganglion cells after transection of the rat sciatic nerve. The Journal of comparative neurology. 2000;422:172–180. doi: 10.1002/(sici)1096-9861(20000626)422:2<172::aid-cne2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Taylor BK. NPY analgesia: moving from acute to chronic pain. In: Feuerstein GZ, Zukowska Z, editors. NPY family of peptides in inflammation, immune disorders, angiogenesis and cancer: genes, diseases and therapeutics. Birkhaeuser Publishing Ltd.; Basel: 2005. [Google Scholar]
- Taylor BK, Abhyankar SS, Vo NT, Kriedt CL, Churi SB, Urban JH. Neuropeptide Y acts at Y1 receptors in the rostral ventral medulla to inhibit neuropathic pain. Pain. 2007;131:83–95. doi: 10.1016/j.pain.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai YJ, Lin CT, Lue JH. Characterization of the induced neuropeptide Y-like immunoreactivity in primary sensory neurons following complete median nerve transection. J Neurotrauma. 2007;24:1878–1888. doi: 10.1089/neu.2007.3488. [DOI] [PubMed] [Google Scholar]
- Wakisaka S, Kajander KC, Bennett GJ. Increased neuropeptide Y (NPY)-like immunoreactivity in rat sensory neurons following peripheral axotomy. Neurosci Lett. 1991;124:200–203. doi: 10.1016/0304-3940(91)90093-9. [DOI] [PubMed] [Google Scholar]
- Wakisaka S, Kajander KC, Bennett GJ. Effects of peripheral nerve injuries and tissue inflammation on the levels of neuropeptide Y-like immunoreactivity in rat primary afferent neurons. Brain research. 1992;598:349–352. doi: 10.1016/0006-8993(92)90206-o. [DOI] [PubMed] [Google Scholar]
- White DM, Mansfield K. Vasoactive intestinal polypeptide and neuropeptide Y act indirectly to increase neurite outgrowth of dissociated dorsal root ganglion cells. Neuroscience. 1996;73:881–887. doi: 10.1016/0306-4522(96)00055-3. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wiesenfeld-Hallin Z, Hokfelt T. Effect of peripheral axotomy on expression of neuropeptide Y receptor mRNA in rat lumbar dorsal root ganglia. European Journal of Neuroscience. 1994;6:43–57. doi: 10.1111/j.1460-9568.1994.tb00246.x. [DOI] [PubMed] [Google Scholar]