Abstract
The precise interplay of hormonal influences that governs gonadotropin hormone production by the pituitary includes endocrine, paracrine and autocrine actions of hypothalamic gonadotropin-releasing hormone (GnRH), activin and steroids. However, most studies of hormonal regulation of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in the pituitary gonadotrope have been limited to analyses of the isolated actions of individual hormones. LHβ and FSHβ subunits have distinct patterns of expression during the menstrual/estrous cycle as a result of the integration of activin, GnRH, and steroid hormone action. In this review, we focus on studies that delineate the interplay among these hormones in the regulation of LHβ and FSHβ gene expression in gonadotrope cells and discuss how signaling cross-talk contributes to differential expression. We also discuss how recent technological advances will help identify additional factors involved in the differential hormonal regulation of LH and FSH.
Keywords: Luteinizing Hormone, Follicle-stimulating Hormone, Gonadotropin-releasing Hormone, Pituitary, Reproduction
1. Introduction to the gonadotropins
The hypothalamic-pituitary-gonadal (HPG) axis plays a pivotal role in every phase of mammalian reproduction including fetal development, puberty, the menstrual cycle, pregnancy, postpartum, and menopause. Fertility depends on precise hormonal regulation of this axis. Two of the most critical hormones, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), are produced exclusively in the gonadotrope cells of the anterior pituitary. They are secreted into the blood stream, primarily in response to gonadotropin-releasing hormone (GnRH) from the hypothalamus (Seeburg et al., 1987; Vale et al., 1977). Gonadal steroid hormones, such as estrogen, progesterone and testosterone, as well as peptide hormones, such as activin, inhibin, and follistatin, modulate LH and FSH levels via feedback to the anterior pituitary, as well as to the hypothalamus. LH and FSH regulate critical aspects of reproduction in the gonads, including steroidogenesis, gametogenesis and ovulation (Burns and Matzuk, 2002).
LH and FSH are heterodimeric glycoproteins composed of a common alpha subunit (α-GSU) dimerized with unique beta subunits (LHβ and FSHβ) (Pierce and Parsons, 1981). Since the β subunits confer the biological specificity of LH and FSH and synthesis of these subunits is the rate-limiting step for production of the mature hormones (Kaiser et al., 1997b; Papavasiliou et al., 1986), many studies have focused on the mechanisms of hormonal signaling involved in the regulation of LHβ and FSHβ gene expression, though most have addressed the actions of the individual hormones. Recently, a number of studies have focused on the interactions of hormones within the pituitary to control LH and FSH synthesis. Comprehension of these molecular mechanisms not only provides insight into the physiology and pathology of the mammalian reproductive system, but may lead to the development of novel contraceptive methods or conversely, infertility treatments.
LH is required for reproduction in mice, since absence of the LHβ gene results in hypogonadism and infertility in both sexes (Ma et al., 2004). Humans with inactivating mutations in LHβ have normal intrauterine development due to a compensatory role of placental human chorionic gonadotropin hormone, but lack pubertal development and are infertile (Huhtaniemi, 2006; Huhtaniemi et al., 2006). FSH is essential for the fertility of female mice and plays an important, though not essential, role in male fertility. Female mice deficient in FSHβ have a block in folliculogenesis prior to the antral stage, while males are fertile but have impaired reproductive function (Kumar et al., 1997). The human FSHβ gene is critical for reproductive function in both genders. A variety of non-synonymous mutations result in absent or incomplete pubertal development in women, relatively normal pubertal development but azoospermia in men, and infertility in both women and men (Lamminen et al., 2005).
2. Differential regulation of LH and FSH synthesis
During the rodent estrous cycle, a surge of GnRH during the afternoon of proestrus triggers a surge in both LH and FSH, resulting in ovulation of the mature follicle in response to LH (Jones, 1997). Several hours later, during the morning of estrus, a secondary FSH surge occurs that does not correspond to a surge in LH (Besecke et al., 1997; Woodruff et al., 1996). In humans the FSH bioactivity levels also increase at mid to late luteal phase through the early to mid-follicular phase of the menstrual cycle. This secondary FSH surge in rodents is essential for follicular development for the subsequent cycle (DePaolo et al., 1979; Hoak and Schwartz, 1980; Papavasiliou et al., 1986). In humans, the increase in the FSH level coincides with a critical time during folliculogenesis when the next cohort of follicles is being recruited. Transcription of the gonadotropin β-subunits both precedes and correlates with LH and FSH levels in the blood. LHβ gene expression is upregulated three fold in the afternoon of proestrus prior to the onset of the surge (Butcher et al., 1974; Zmeili et al., 1986). FSHβ mRNA levels increase four to five fold during the afternoon of proestrus and three fold during estrus (Halvorson et al., 1994; Ortolano et al., 1988). Thus, despite receiving identical input from autocrine, paracrine and endocrine hormonal signals, LH and FSH synthesis and secretion are differentially regulated during the estrous cycle and this regulation appears to occur primarily at the level of transcription of the β subunits.
As we discuss below, numerous studies have documented differential hormonal regulation of LHβ and FSHβ gene expression. Several mechanisms have been proposed to explain the means by which variations or alterations in gonadotropin synthesis occur. GnRH is released in a pulsatile fashion from hypothalamic neurons into the hypophyseal portal system and differing GnRH pulse frequencies have been found to participate in differential regulation of LHβ and FSHβ [reviewed recently in (Ferris and Shupnik, 2006)]. Multiple studies have shown that an increase in GnRH pulse frequency favors LHβ (Burger et al., 2008; Haisenleder et al., 2008), while a decrease favors FSHβ gene expression (Dalkin et al., 1989; Haisenleder et al., 1991; Haisenleder et al., 1988; Papavasiliou et al., 1986). LHβ seems to be especially sensitive to GnRH pulse frequency and amplitude, while FSHβ less so (Burger et al., 2008). In addition to GnRH, regulation of the activin/follistatin/inhibin feedback loop is thought to be important for differential LHβ and FSHβ synthesis. Follistatin synthesis in the pituitary is inversely correlated with FSHβ expression during the estrous cycle (Besecke et al., 1997; Halvorson et al., 1994). Follistatin transcription may also be regulated by differential GnRH pulse frequencies, again in the opposite manner to FSHβ (Burger et al., 2002; Kirk et al., 1994). Ovarian-derived inhibin has been shown to suppress FSH production during metestrus and diestrus (Woodruff et al., 1993), but its expression is decreased during estrus (Woodruff et al., 1996). Therefore, higher levels of bio-available activin, due to a drop in follistatin and/or inhibin levels in estrus, may selectively favor FSHβ synthesis (Besecke et al., 1997; Woodruff et al., 1996). Finally, steroid hormones, such as progesterone, testosterone, and glucocorticoids have all been shown to induce FSHβ but inhibit LHβ expression (Burger et al., 2004b; Thackray et al., 2006). In this review, we discuss the evidence that differential LHβ and FSHβ synthesis is modulated by interactions among these hormones, in concert with differential GnRH pulse control. Understanding how these signals are conveyed and integrated may be especially important in pathological conditions associated with a disturbed LH to FSH ratio such as polycystic ovary syndrome (PCOS) or obesity.
PCOS is a clinical disorder affecting ~10% of reproductive-age women. Characteristics of PCOS include increased LH pulsatility and reduced FSH levels, which lead to increased androgen production and anovulation, respectively (Chang, 2007; Hall et al., 1998; Morales et al., 1996). Hyperandrogenemia, in turn, may impair hypothalamic progesterone sensitivity and prevent slowing of GnRH pulses (Blank et al., 2006). Obesity is the most common chronic disease in the United States today, and contributes to menstrual cycle disturbances, anovulation and reduced rates of conception (Zain and Norman, 2008). Similarly to PCOS, obesity correlates with increased LH pulse frequency (Yoo et al., 2006). An increase in the frequency of LH pulses and disruption of circadian fluctuations in LH secretion have been observed in obese girls by late puberty (McCartney et al., 2009). Although it is still not completely understood how signals from GnRH neurons in the hypothalamus and paracrine/endocrine feedback from the anterior pituitary/gonads are interpreted by gonadotrope cells, it is clear that proper functioning of the HPG axis and maintenance of a normal LH/FSH ratio is critical for reproductive health.
3. Approaches for analyses of hormonal regulation in gonadotrope cells
Although there are distinct differences in the hormonal regulation of the HPG axis among mammalian species, there is also a great deal of conservation. For instance, FSHβ null mice, which are infertile, can have their fertility restored by replacing the mouse FSHβ gene with 10 kb of the human one containing 4 kb of the 5′ regulatory region, all exons and introns and 2 kb of the 3′ flanking region (Kumar et al., 1998). Several experimental systems have been used extensively to examine hormonal regulation of the gonadotropin genes in rodents. These include whole animals, primary pituitary cell culture and immortalized cell culture. Conclusions derived from physiological studies in rats and mice in vivo obviously benefit from the fact that the data are obtained within the endogenous hormonal setting. However, it can be cumbersome to differentiate the sites of hormone action because hormonal feedback occurs at the level of both the hypothalamus and the anterior pituitary. In rodents, separation of GnRH input from the pituitary can be accomplished biochemically with continuous GnRH agonist or antagonist administration. However, cross-talk between GnRH and other hormone signaling pathways cannot be studied effectively in this setting.
Primary pituitary cell culture is also frequently employed to study hormonal effects on gonadotropin synthesis. However, there are several caveats to these models that are worth mentioning. First, the endocrine milieu at the time of harvest can impact the results (Fallest and Schwartz, 1991), so caution must be used when interpreting data from animals in different estrous stages. Second, gonadotropes only comprise 5–15% of the cells in the anterior pituitary (Ooi et al., 2004). Four other types of secretory cells including thyrotropes, somatotropes, lactotropes and corticotropes are also present, as well as folliculostellate cells. Many hormones secreted from these cells, such as prolactin and oxytocin from lactotropes, have paracrine effects on gonadotrope cells [recently reviewed in (Denef, 2008)]. Folliculostellate cells have been reported to rapidly overgrow other pituitary cells in culture (Kawakami et al., 2002) and can impact the experimental outcome since they produce paracrine factors such as follistatin and PACAP [recently reviewed in (Winters and Moore, 2007)].
Gonadotrope cell culture is ideal for analyzing the mechanisms of individual and combined hormonal regulation of gonadotropin gene expression. Several reports describe methods to enrich gonadotropes from the mixed cell population of the pituitary. For instance, gonadotropes have been successfully enriched from rat pituitaries, though the procedure is time consuming (Childs and Unabia, 2001). More recently, gonadotropes have been purified from transgenic mice containing 4.7 kb of the ovine FSHβ promoter linked to the cell surface antigen, H-2Kk (Wu et al., 2004). However, these gonadotrope cells are only efficiently purified from the pituitaries of male or ovariectomized female mice and the relatively low yield makes it difficult to perform detailed characterization of the molecular mechanisms involved in hormonal regulation of gonadotropin gene expression.
Two immortalized clonal cell lines were developed in our laboratory using targeted tumorigenesis with SV40 T-antigen linked to the 5′ regulatory regions of α-GSU or LHβ (Alarid et al., 1998; Alarid et al., 1996). The αT3-1 cell line approximates an immature gonadotrope cell since it expresses α-GSU, GnRH receptor and SF-1. On the other hand, the LβT2 cell line displays a more mature phenotype since it expresses LHβ and FSHβ and secretes LH and FSH in response to hormonal cues (Graham et al., 1999; Pernasetti et al., 2001). LβT2 cells also express activin, follistatin, and inhibin, as well as the receptors for activin, inhibin and steroid hormones (Lawson et al., 2001; Lewis et al., 2000; Schreihofer et al., 2000; Thackray et al., 2006; Turgeon et al., 1996). Thus, the development of these immortalized cell lines set the stage for an intensive characterization of the hormonal signaling mechanisms that mediate transcription of the gonadotropin genes. Given the comprehensive experimental tools available for studying gonadotropin biology in rodents, we have focused this review on studies performed in rodents or tissues derived from them with only occasional comparisons to studies in other mammalian species.
4. Hormonal regulation of LHβ and FSHβ gene expression
4.1. GnRH
The GnRH receptor is a member of the rhodopsin family of G protein-coupled, seven-transmembrane receptors and is expressed specifically in pituitary gonadotropes (Kaiser et al., 1997a; Sealfon et al., 1997; Stojilkovic et al., 1994; Tsutsumi et al., 1992). However, the GnRH receptor has a unique structure, because it lacks an intracellular, cytoplasmic tail; a feature that allows for a much slower internalization (Hislop et al., 2001). Slower internalization may play in role in the necessity for pulsatile release of GnRH into the hypophysial-portal system, since long-term tonic exposure adversely affects gonadotrope function (Belchetz et al., 1978; Burrin and Jameson, 1989; Shupnik, 1990). Ligand binding to the GnRH receptor causes activation of G-proteins, primarily Gq and G11 (Stanislaus et al., 1997), with the subsequent activation of phospholipase Cβ, release of calcium from intracellular stores, and increase in the activity of protein kinase C (PKC) and calcium/calmodulin kinase II (Haisenleder et al., 2003; Liu et al., 2002). GnRH receptor activation also leads to activation of three branches of the mitogen-activated protein kinase (MAPK) pathway: ERK1/2, JNK, and p38 (Liu et al., 2002; Roberson et al., 1995; Sundaresan et al., 1996), via PKC (Naor, 2009) or via association of GnRH receptor with Raf and calmodulin in lipid rafts (Roberson et al., 2005).
Induction of LHβ gene expression by GnRH is dependent upon PKC signaling, since the PKC inhibitor Bis-Indolyl Maleimide prevents LHβ induction (Vasilyev et al., 2002a; Harris et al., 2002). A role for calcium has been documented (Weck et al., 1998; Yamada et al., 2004), and may correlate with its role in the activation of conventional PKC isoforms, since calcium increase by itself after ionophore treatment is not sufficient to induce LHβ (Harris et al., 2002). The role of MAPK in LHβ induction was examined in a number of studies, but it is still inconclusive which branches of the MAPK pathway contribute to LHβ induction. The discrepancies in these studies may arise from different experimental paradigms. For instance, the length of GnRH treatment may be important, since ERK1/2 are activated rapidly after 2–5 minutes, while p38 and JNK are maximally activated after 30–60 minutes (Yokoi et al., 2000; Harris et al., 2002). Most studies agree that the ERK1/2 and JNK branches of the MAPK pathway are required for LHβ induction by GnRH, while a role of p38 is controversial (Yokoi et al., 2000; Harris et al., 2002; Yamada et al., 2004). These results correlate with the fact that EKR1/2 and JNK are involved in the growth factor induction of early growth response-1 (Egr-1) which is the only known factor induced by GnRH that regulates transcription of LHβ.
GnRH induces mammalian LHβ gene expression through rapid induction of the immediate-early gene, Egr-1 (Kaiser et al., 2000; Weck et al., 2000). Upon induction by GnRH, Egr-1 interacts with both tissue-specific, and ubiquitous, basal factors to regulate the LHβ promoter [reviewed in (Jorgensen et al., 2004)]. Female Egr-1 null mice are infertile and lack expression of the LHβ gene, indicating that other Egr family members (Egr-2,3,4) are unable to compensate (Lee et al., 1996; Topilko et al., 1998). The proximal promoter region contains two Egr-1 sites in tandem with two sites for steroidogenic factor-1 (SF-1) located on either side of a homeodomain element (HD) (Halvorson et al., 1996; Quirk et al., 2001) (Fig. 1). The pituitary-specific HD protein, Ptx1, has been shown to bind the homeodomain element in heterologous cells (Tremblay and Drouin, 1999), while in LβT2 cells, the protein binding to the site is related to Otx1, rather than Ptx1 (Rosenberg and Mellon, 2002). Synergistic interactions among SF-1, Egr-1 and Ptx1 are essential for GnRH induction of LHβ gene expression (Dorn et al., 1999; Tremblay and Drouin, 1999). The distal region of the rodent promoter contains several Sp1 binding sites (Kaiser et al., 1998) and an overlapping CArG element, important for pulsatile GnRH induction in LβT2 cells (Weck et al., 2000) (Fig. 1). Sp1 interacts with the Egr-1/SF-1/HD complex in a spatially-dependent manner (Kaiser et al., 2000; Weck et al., 2000). The co-activator, SNURF (Curtin et al., 2004) and the scaffold protein, p300 (Mouillet et al., 2004) bridge distal and proximal promoter regions and may potentiate Egr-1 action, and therefore, the GnRH effect. β-catenin has also been reported to play a role in GnRH induction of LHβ, since it accumulates in the nucleus following GnRH treatment and increases SF-1 and Egr-1-mediated LHβ transcription through an interaction with SF-1 (Gardner et al., 2007; Salisbury et al., 2007).
Fig. 1.
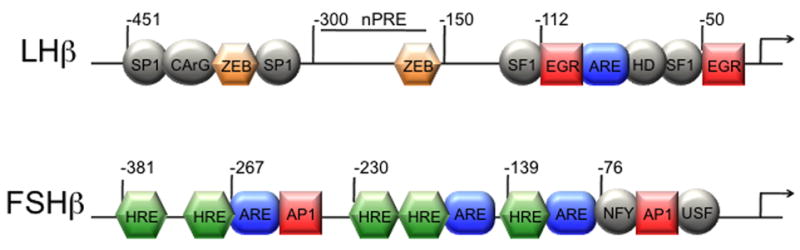
Transcription factors and associated binding sites involved in hormonal induction of the rat LHβ and mouse FSHβ promoters. Binding sites for factors involved in basal expression, which interact with factors or sites involved in hormonal regulation, are illustrated with circles or ovals. Squares indicate sites involved in GnRH induction. Hexagons indicate sites involved in steroid hormone regulation, and are labeled as hormone response elements (HREs) if they play a role in regulation by 3-keto steroid hormones or as a negative progesterone response element (nPRE). Rounded rectangles indicate activin responsive elements (AREs). Smad transcription factors bind some of these elements (−116 in LHβ and −267 in FSHβ), while others bind different factors (i.e. Pbx and Prep at −120 in the FSHβ promoter), which may interact with Smads and help stabilize Smad-DNA interactions.
Recent studies have also analyzed mechanisms involved in the responsiveness to pulsatile GnRH treatment and frequency-dependent expression of the LHβ gene. For instance, proteasome function was reported to be critical for LHβ responsiveness and GnRH treatment was shown to mediate ubiquitination of SF-1 and Egr-1 which results in the constant cycling of these factors on and off of the LHβ promoter (Walsh and Shupnik, 2009). This may allow the LHβ gene to respond to each pulse of GnRH. Sustained induction of Egr repressor proteins from the Nab family at low GnRH frequency may also be critical in turning off the signal and/or adjusting the LHβ response to high pulse frequency of GnRH (Lawson et al., 2007).
GnRH regulates FSH at the transcriptional level, since serum FSH levels are reduced 60–90% in mice lacking GnRH compared to wild-type mice (Mason et al., 1986) and one pulse of GnRH administered to castrated, testosterone-replaced rats increased FSHβ gene expression four fold (Dalkin et al., 2001). GnRH regulation of FSHβ gene expression is mediated by the PKC and MAPK signaling pathways (Coss et al., 2004; Liu et al., 2005; Vasilyev et al., 2002b; Bonfil et al., 2004).
GnRH regulation of the FSHβ gene also occurs through the induction of intermediate, immediate-early genes, which constitute activator protein-1 (AP-1). AP-1 consists of a variety of dimers of Fos isoforms (c-Fos, FosB, Fra-1 and Fra-2) and Jun isoforms (c-Jun, JunB and JunD), most of which are induced by GnRH (Kakar et al., 2003; Wurmbach et al., 2001). While Fos proteins can only heterodimerize with members of the Jun family to form transcriptionally active complexes, Jun proteins can also make homodimers. In the ovine FSHβ promoter, two putative AP-1 binding sites were identified using JAR placental cells (Strahl et al., 1997; Strahl et al., 1998). More recently, we demonstrated that AP-1 plays a role in GnRH induction of the murine FSHβ gene (Fig. 1). Specifically, we showed that the role of MAPK, in the GnRH induction of FSHβ gene, is via the induction of c-Fos and JunB (Coss et al., 2004; Liu et al., 2002), which, with c-Jun and FosB, bind and induce FSHβ promoter. The human FSHβ promoter has also been shown to be regulated by AP-1 proteins through two AP-1 response elements (Wang et al., 2008). AP-1, like Egr-1 on the LHβ gene, interacts with factors involved in basal expression of FSHβ, such as NF-Y, in the mouse and possibly USF1, in the rat promoter (Ciccone et al., 2008; Coss et al., 2004) to integrate GnRH responsiveness. A study by Johnson et al. (1992) indicated that c-Fos may play an important role in reproduction, since c-Fos knockout animals were reported to have small ovaries with atretic follicles (Johnson et al., 1992). Although the levels of gonadotropin hormones were not measured in these animals, the phenotype is reminiscent of FSHβ knock-out mice (Kumar et al., 1997). GnRH regulation of the rat FSHβ gene also involves the CREB transcription factor (Ciccone et al., 2008), which binds the homologous CRE/AP-1 half-site. Although CREB deficient mice do not exhibit changes in FSH levels, other family members, such as CREM or ATF, may compensate for CREB deficiency (Hummler et al., 1994), or, alternatively, CREB may play a role only in rat FSHβ expression in a species-specific manner.
4.2. Activin
Activin was originally identified as a gonadal peptide that stimulates FSH production (Ling et al., 1986). It is a dimer of two β subunits, of which there are two critical isoforms, A and B. Activin binds the type II receptor, which leads to heterodimerization and phosphorylation of the type I receptor (Attisano and Wrana, 2002). Activin has also been shown to be critical for embryonic development. Knock-in of activin B into the activin A locus rescued neonatal lethality in mice, but revealed that activin A is required for ovarian and testicular growth (Chang et al., 2002). Mice deficient in the activin type II receptor survive to adulthood, as opposed to mice lacking the type I receptor, but have lower FSH levels and the females are infertile (Matzuk et al., 1995). Following receptor binding and phosphorylation of type I receptors, the type I receptor then phosphorylates Smad2 and Smad3. The phosphorylated Smads bind to Smad4 and translocate into the nucleus to activate transcription of target genes (Attisano and Wrana, 2002; Massague, 1998). Smad3 and 4 can bind DNA directly at Smad-binding elements (SBE), although their binding affinity is rather weak, while Smad2 does not bind directly to DNA (Massague, 1998; Shi et al., 1998). Inhibin (a heterodimer of one of the activin subunits and a unique α subunit) (Nakamura et al., 1990) and follistatin (a potent activin-binding protein) (Shimasaki et al., 1988) antagonize activin action. Interestingly, activin, follistatin and inhibin are all expressed in the mature pituitary gonadotrope and can function in an autocrine manner (Kaiser et al., 1992; Kogawa et al., 1991; Roberts et al., 1989). Follistatin is also synthesized by folliculostellate cells in the pituitary and can regulate activin availability in a paracrine manner (Gospodarowicz and Lau, 1989; Kawakami et al., 2002).
LHβ gene expression and secretion has been shown to be regulated by activin in rodents (Attardi and Miklos, 1990; Burger et al., 2002), monkeys (McLachlan et al., 1989; Stouffer et al., 1993), human fetal cells (Blumenfeld and Ritter, 2001), and mouse primary pituitary cultures (Coss et al., 2005), although far less potently than FSHβ. Our studies demonstrated that activin induction of LHβ occurs through three adjacent activin-response elements (ARE, Fig 1), which are juxtaposed to the SF-1, Egr-1 and homeodomain elements in the proximal region of the LHβ promoter (Coss et al., 2005). Upon activation, Smad3 and 4 bind the middle ARE element (Fig. 1) to induce the LHβ gene. Similarly to other Smad-regulated genes, the interaction of Smads with the LHβ promoter is probably strengthened by their interaction with a homeodomain protein such as Ptx1 or Otx1 (Datta et al., 2000; Germain et al., 2000). Alternatively, multiple activin-response elements in close proximity, such as the three in the LHβ promoter, permit cooperative binding of Smad proteins and allow them to overcome their low binding affinity (Lopez-Rovira et al., 2002). Smad3-deficient male mice were analyzed for gonadotropin gene expression, since females have an ovarian phenotype that disregulates the feedback (Tomic et al., 2002). We demonstrated that Smad3-null mice express lower levels of both LHβ and FSHβ (Coss et al., 2005), substantiating a role of activin in LHβ expression.
Activin is a potent inducer of the rodent FSHβ gene. Activin increases the release of FSH from the pituitary (Ling et al., 1986) and induces FSHβ expression in gonadotrope cells (Weiss et al., 1995). For activin induction of FSHβ, Smad3 seems to be a limiting factor, since its overexpression can induce a FSHβ luciferase reporter (Gregory et al., 2005; Lamba et al., 2006). The FSHβ promoter contains three activin-response elements, identified to date. One of them, located at −267 in the mouse FSHβ promoter, is a classical consensus Smad binding site, comprised of a palindrome sequence GTCTAGAC (Bernard, 2004; Gregory et al., 2005; Suszko et al., 2005) (Fig. 1), but it does not appear to play a major role in the overall activin induction (Coss et al., 2007; McGillivray et al., 2007) and is not present in the human promoter. Two other AREs, containing a half-site AGAC motif, were identified in the FSHβ proximal promoter and are critical for activin induction in all species examined (Bailey et al., 2004) (Fig. 1). These elements are likely bound by other proteins, such as Pbx and Prep, which can interact with Smads and tether them to the promoter following their activation (Bailey et al., 2004; Suszko et al., 2003). These proteins may not only provide higher affinity binding, but may also afford signal specificity, if they are tissue-restricted binding partners. In addition to the Smad signaling pathway, the TAK1 pathway has been identified as another potential player in activin induction of FSHβ transcription (Safwat et al., 2005). Various BMPs can also stimulate FSHβ expression (although not as potently as activin) or can potentiate activin stimulation of FSHβ expression (Lee et al., 2007; Nicol et al., 2008; Otsuka and Shimasaki, 2002). However, the mechanism of their action is not known, nor is it understood whether Smads1/5/8 when activated by BMP signaling, function through the same sites as Smads2/3 activated by activin.
4.3 Steroids
The ovarian steroid hormones, estrogen and progesterone, regulate gonadotropin gene expression, as well as secretion, by negative and positive feedback to the hypothalamus and the anterior pituitary. Removal of ovarian hormones via ovariectomy in rodents results in increased levels of LH and FSH. For LH, these levels return to baseline after exogenous estrogen treatment (Shupnik et al., 1988). This negative feedback appears to occur via estrogen receptor (ER) α since knockout of the receptor in mice results in increased LH levels (Couse et al., 2003; Glidewell-Kenney et al., 2008). LH suppression by estrogen is also abolished by treatment with a GnRH antagonist, indicating the hypothalamic nature of the estrogen negative feedback (Shupnik and Fallest, 1994). In contrast to its action in the hypothalamus, estrogen has been reported to directly induce LHβ gene expression in gonadotropes (Shupnik et al., 1989a; Shupnik et al., 1989b). The mechanism of estrogen action on the LHβ promoter is still unclear. ER was shown to bind an imperfect estrogen response element at −1189 in the distal rat LHβ promoter (Shupnik and Rosenzweig, 1991; Shupnik et al., 1989b), but it is not conserved in other mammalian species. Alternatively, ER can be recruited to the LHβ promoter through interactions with SF-1 and Ptx1 (Luo et al., 2005). Finally, estrogen has been shown to potentiate GnRH induction of LHβ indirectly by enhancing the expression of Egr-1 and suppressing the expression of ZEB, a transcriptional repressor that binds at −381 and −182 on the rat LHβ promoter (Kowase et al., 2007).
Estrogen appears to play only a partial role in suppressing FSH levels since its addition after ovariectomy does not result in full suppression (Shupnik et al., 1988). Studies provide evidence that another ovarian hormone such as inhibin is important for suppression of FSH levels (Dalkin et al., 1990; Dalkin et al., 1993; Gharib et al., 1987). There is very little evidence that estrogen directly modulates FSHβ gene expression in rodents. Estrogen did not alter the expression of FSHβ mRNA in ovariectomized rats treated with a GnRH antagonist (Dalkin et al., 1993; Shupnik et al., 1989a) or in female rat pituitary fragments (Shupnik and Fallest, 1994). Our experiments with the murine FSHβ promoter in LβT2 cells agree with these studies because estrogen did not stimulate FSHβ gene expression even in the presence of transfected ERα or ERβ (Thackray et al., 2006). Thus, instead of a direct effect, estrogen may regulate FSH indirectly by modulating GnRH or activin action. Interestingly, activin B expression was elevated in pituitary tissues from ERαKO mice (Couse et al., 2003).
Progesterone exerts both inhibitory and facilitating feedback effects on gonadotropin synthesis and secretion, through indirect regulation of GnRH production in the hypothalamus and direct regulation in the anterior pituitary [reviewed in (Levine et al., 2001)]. Studies on the role of progesterone in reproductive tissues, including the gonadotrope cell, are complicated by the fact that one of the functions of estrogen is to stimulate the expression of the progesterone receptor (PR) (Romano et al., 1989). PR is essential for female reproduction. Female mice carrying a null mutation of PR are infertile; they cannot respond to GnRH surge conditions and do not experience an elevation of LH and FSH levels prior to ovulation (Chappell et al., 1997).
Since LHβ mRNA levels decrease during estrus when progesterone levels are at their highest, progesterone is an excellent candidate for countering the stimulatory effects of GnRH and activin on LHβ gene expression. Contrary to studies measuring LHβ mRNA levels in ovariectomized rats or in primary pituitary cells treated with estrogen and progesterone (Kerrigan et al., 1993; Park et al., 1996), we recently demonstrated that progesterone can suppress both basal transcription and GnRH- or activin-stimulated LHβ gene expression in gonadotrope cells (Thackray et al., 2006; Thackray and Mellon, 2008). It is possible that earlier studies did not observe progesterone suppression because of the stimulatory effect of estrogen on LHβ gene expression. In our studies, we enhanced PR levels directly by overexpressing the receptor in LβT2 cells instead of using estrogen to induce PR, thus avoiding the effects of estrogen on LHβ gene expression. The suppressive effects of progesterone mapped to a region between −300 to −150 of the LHβ promoter (Thackray et al., 2009) (Fig. 1). This region was shown to be both necessary and sufficient for the suppression, since deletion of this region abrogated the progesterone suppression and fusing this region to a heterologous promoter recapitulated the suppression. Additionally, chromatin immunoprecipitation (ChIP) analysis revealed that PR was recruited to the LHβ promoter, though gel-shift assays did not detect direct binding of PR to the necessary regions of the promoter. Since the progestin suppression of LHβ depended on the DNA-binding domain of PR, these results suggest that suppression is mediated by indirect binding of PR or “tethering” to the LHβ promoter.
In contrast to the LHβ promoter, many studies support the hypothesis that progestins induce FSHβ gene expression in the anterior pituitary [reviewed in (Burger et al., 2004b)]. Both basal and GnRH-induced FSHβ mRNA levels increased in rats treated with estrogen and progesterone (Attardi and Fitzgerald, 1990; Kerrigan et al., 1993). Progesterone antagonists also blocked FSH secretion and mRNA expression during the pre-ovulatory FSH surge (Ringstrom et al., 1997) and the secondary FSH surge (Knox and Schwartz, 1992; Szabo et al., 1998). Reporter genes containing either the proximal rat or ovine FSHβ promoter responded to progestin treatment in primary rat or ovine pituitary cultures, respectively (O’Conner et al., 1997; Webster et al., 1995). Our studies also demonstrated that progesterone induced the murine FSHβ promoter in LβT2 cells (Thackray et al., 2006). In contrast to LHβ, progesterone induction of the mouse FSHβ promoter required direct binding and transactivation of PR. ChIP and gel-shift analysis demonstrated that PR bound to the proximal mouse FSHβ promoter in vivo and in vitro (Thackray et al., 2006). The progesterone responsiveness on the FSHβ promoter mapped to a region between −500 and −95 of the proximal mouse promoter; this region contains six putative hormone response elements (HREs) previously described in the rat and ovine promoters. Five of these HREs appear to play a functional role, including a consensus direct repeat element at −381 (Fig. 1). Collectively, our studies provide strong evidence that progesterone can directly modulate LHβ and FSHβ synthesis, as opposed to indirect means such as altering the balance of the activin/inhibin/follistatin feedback loop. These results also support the hypothesis that progesterone differentially regulates gonadotropin gene expression by inhibiting LHβ and inducing FSHβ gene expression in the pituitary, and, thus, progesterone may modulate the secondary FSH surge.
In addition to progestins, there is considerable evidence that androgens directly modulate the levels of LHβ and FSHβ mRNA in the anterior pituitary. Androgens have been reported to repress LHβ gene expression in both castrated, GnRH antagonist-treated rats (Burger et al., 2004a; Wierman and Wang, 1990) and primary pituitary cell culture (Winters et al., 1992). Additionally, androgens have been shown to suppress transcription of LHβ in immortalized gonadotrope cells. Androgen treatment suppressed GnRH-induced transcription of the rat LHβ promoter, likely through a direct protein-protein interaction between the androgen receptor (AR) and Sp1 (Curtin et al., 2001), while androgens were reported to suppress the bovine LHβ promoter through a protein-protein interaction between AR and SF-1 (Jorgensen and Nilson, 2001). Like progestins, androgens upregulate FSHβ mRNA levels. For example, castrated GnRH antagonist-treated rats exhibited a selective increase in FSHβ mRNA upon treatment with testosterone (Burger et al., 2004a; Dalkin et al., 1992; Paul et al., 1990; Wierman and Wang, 1990). Studies in primary pituitary cells confirmed that the activation of FSHβ expression by testosterone occurs at the level of the pituitary (Gharib et al., 1990; Leal et al., 2003; Winters et al., 1992). Our studies using LβT2 cells also demonstrated that the gonadotrope cell was sufficient for this response and that activation of both the murine and ovine FSHβ promoter by androgens was due to direct androgen receptor binding to HREs in the proximal promoter (Spady et al., 2004; Thackray et al., 2006) (Fig. 1).
Similarly to progestins and androgens, glucocorticoids can regulate LHβ and FSHβ synthesis in the anterior pituitary. A suppressive effect of glucocorticoids on GnRH-induced LHβ gene expression, as well as LH secretion, was observed in castrated rats (Rosen et al., 1991). Our recent studies showed that LHβ mRNA levels were inhibited by glucocorticoids in LβT2 cells (Thackray et al., 2006). In contrast to LHβ, a selective increase in FSHβ gene expression in response to glucocorticoids has been demonstrated in rats (McAndrews et al., 1994; Ringstrom et al., 1991), as well as in primary pituitary cells (Bohnsack et al., 2000; Kilen et al., 1996; Leal et al., 2003). We also demonstrated glucocorticoid induction of the murine FSHβ promoter in LβT2 cells (Thackray et al., 2006).
5. Synergy between hormonal signaling pathways modulates FSHβ gene expression
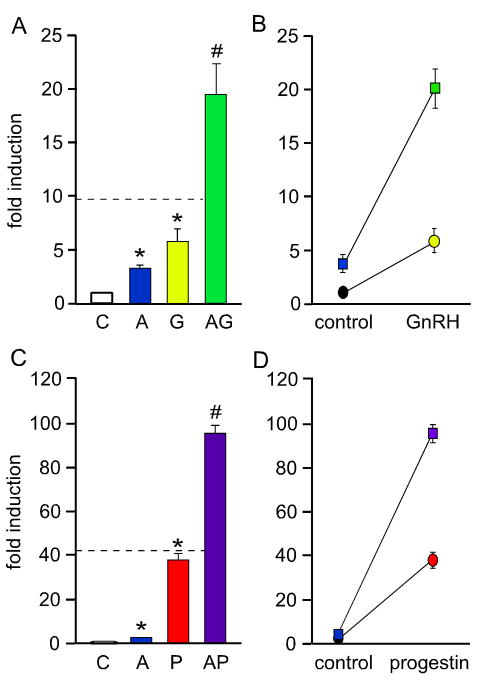
Levels of bio-available activin increase during estrus, presumably due to decreases in follistatin and inhibin levels. GnRH and 3-keto steroid hormones also convey signals to the pituitary gonadotrope during this time. Thus, we propose that interactions between activin and these other hormonal signaling pathways are responsible for differential LHβ and FSHβ synthesis and the secondary FSH surge. In particular, our recent studies have demonstrated that GnRH and steroid hormones, such as progestins, androgens and glucocorticoids, can synergistically cooperate with activin to specifically induce FSHβ mRNA levels (Fig. 2). Synergy is defined as an interaction or cooperation of two hormones resulting in a combined effect greater than the sum of their individual effects. In Figure 2A–B, activin and GnRH synergize while in C–D, activin and progesterone synergize. See Slinker (1998) for a succinct and useful discussion about synergy and the use of 2-way ANOVA to identify an interaction between two treatments (Slinker, 1998). Confusion in the literature about interactions between hormonal signaling pathways mostly results from incorrect statistical analysis of the data. For instance, data is sometimes incompletely analyzed by one-way ANOVA despite the inherent two-way factorial design of the experiment. One-way ANOVA analysis can demonstrate that the combined effect of the two treatments is statistically different from either treatment alone, but not whether the interaction is additive or synergistic. We have used a dashed line in Figure 2A and C to represent the level at which the hormone co-treatment would be additive rather than synergistic, which can be determined using 2-way ANOVA. Figure 2B and D illustrate this synergistic interaction with non-parallel lines.
Fig. 2.
Activin synergizes with GnRH (A–B) or progesterone (C–D) to induce FSHβ gene expression. A luciferase reporter containing 1 kb of the mouse FSHβ promoter was transiently transfected into LβT2 cells. After overnight starvation in serum-free media, the cells were treated with vehicle control (C), 10 ng/μl activin (A), 10 nM GnRH (G), 100 nM R5020 (a synthetic progestin; P), activin and GnRH co-treatment (AG) or activin and progestin (AP), as indicated. The asterisks indicate significant differences from the vehicle-treated control, while pound signs indicate a synergistic interaction as defined by a two-way ANOVA (p<0.05). The dashed line in A and C represents the level at which the hormone co-treatment is additive rather than synergistic. In B and D, synergy is demonstrated graphically as described in Slinker et al. (Slinker, 1998). If the lines diverge, there is synergy, whereas if the lines remain parallel, there is no interaction. The circles represent control and GnRH (B) or progestin (D) treatment alone. The squares represent activin treatment, both alone and with GnRH (B) or progestin (D).
5.1. Synergy between activin and GnRH
Activin and GnRH have been shown to synergistically induce rodent FSHβ gene expression (Coss et al., 2007; Gregory et al., 2005) and this synergism is specific for FSHβ. Follistatin diminishes the level of FSHβ mRNA levels in rat primary pituitary cells (Besecke et al., 1996) and reduces FSHβ promoter activity (Coss et al., 2007; Pernasetti et al., 2001). Follistatin also reduces the GnRH induction of FSHβ. Since gonadotrope cells secrete activin, these results support the concept that autocrine activin can synergize with GnRH and potentiate its effect. As we discussed previously, both GnRH and activin induce LHβ. However, co-treatment of LβT2 cells with activin and GnRH results in an additive LHβ induction (Coss et al., 2007; Yamada et al., 2004), suggesting independent and separate molecular mechanisms for induction, as opposed to their synergistic interaction on FSHβ. Therefore, synergistic induction of the FSHβ gene by activin and GnRH is not replicated on the LHβ gene, indicating that synergy is not due to an induction of the GnRH receptor and is likely an effect of direct action of these two hormones on the FSHβ promoter. The mechanism of the synergy between activin and GnRH on FSHβ entails cross-talk between signaling pathways that impinge on p38 MAPK and result in an increased amount of c-Fos protein and Smad3 phosphorylation (Coss et al., 2007). The human FSHβ gene was also reported to be synergistically induced by activin and GnRH (Wang et al., 2008), although the mechanism of this synergistic induction has yet to be elucidated. In the murine promoter, mutation of the −267 consensus SBE did not affect the level of synergy, while mutation of the AP-1 sites, both in the mouse (Coss et al., 2007) and human promoters (Wang et al., 2008) reduced the synergism. Only when the Smad site was mutated together with the AP-1 sites, in the mouse FSHβ promoter, was the synergy completely abrogated. On multimerized AP-1 elements, which lack activin responsiveness, there was also an increase in expression with activin and GnRH co-treatment, compared to GnRH treatment alone (Coss et al., 2007). The minor contribution of the −267 Smad site is not that surprising, since it has been shown that Smads and AP-1 can interact and induce the AP-1-response element of the human collagenase promoter (Qing et al., 2000). It is likely that Smads can be tethered to the FSHβ promoter through interaction with other transcription factors such as AP-1. On the other hand, Smad DNA binding may still play a role, since there was a significant increase in synergy when both AP-1 and Smad elements were linked together on a heterologous promoter (Coss et al., 2007). Thus, during estrus and the secondary FSHβ surge, which is characterized by a slower GnRH pulse frequency, synergy between GnRH and activin may specifically induce FSHβ gene expression to promote folliculogenesis (Fig. 3).
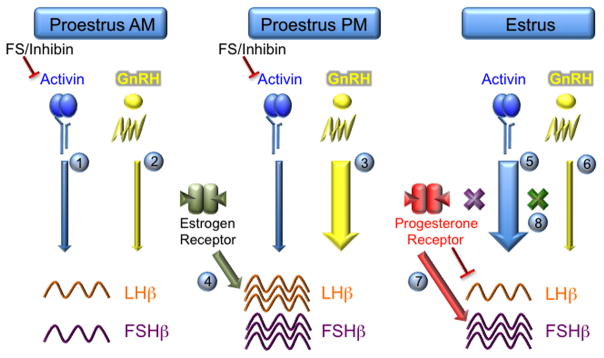
Fig. 3.
A model of hormonal regulation of differential LH and FSH synthesis in pituitary gonadotrope cells. There are multiple ways that peptide and steroid hormones can influence gonadotropin transcription. On the morning of proestrus, activin signaling is minimized due to high levels of the inhibitory molecules, follistatin (FS) and inhibin (1). GnRH signaling is also muted, resulting in low levels of both LHβ and FSHβ mRNA (2). On the afternoon of proestrus, GnRH pulsatility and amplitude increase dramatically prior to ovulation leading to increased transcription of LHβ and FSHβ (3). Estrogen may also play a direct role in inducing LHβ gene expression (4). During estrus, the levels of bio-available activin increase due to a reduction in follistatin and inhibin levels, resulting in increased levels of FSHβ mRNA (5). Concurrently, GnRH pulses become slower, which may favor FSHβ transcription directly and indirectly via reduced induction of follistatin (6). Moreover, progesterone action leads to induction of FSHβ but suppression of LHβ gene expression (7). Finally, synergy between activin and GnRH or progesterone signaling pathways enhances the positive action of these hormones on FSHβ transcription (8) and, thus, may help regulate the secondary FSH surge.
5.2. Synergy between activin and steroid hormones
Gonadal steroids and glucocorticoids differentially modulate LH and FSH synthesis by suppressing LHβ and inducing FSHβ gene expression. It has also been suggested that activin action in the pituitary gonadotrope contributes to steroid induction of the FSHβ promoter. For example, Miyake et al. (1993) observed increased levels of FSH in primary pituitary cells after co-treatment with activin and gonadal steroid hormones (Miyake et al., 1993). Furthermore, steroid enhancement of FSH secretion was blunted by inhibin (Dahl et al., 1992) and follistatin (Bohnsack et al., 2000). Several studies also indicated that increased secretion of FSH resulted from a combinatorial effect of activin and steroids on FSHβ gene expression. The anti-progestin and anti-glucocorticoid, RU-486, suppressed activin induction of FSHβ gene expression in primary pituitary cell culture (Szabo et al., 1998). In addition, co-treatment of primary pituitary cells with activin and testosterone or glucocorticoids enhanced FSHβ mRNA levels above those observed with activin alone (Leal et al., 2003).
Regulation of FSH transcription by interactions between activin and steroid hormones could occur through direct or indirect means. Steroids may modify the activin/follistatin/inhibin feedback loop such that activin action on the FSHβ promoter is enhanced. Alternatively, components of the activin and steroid hormone signaling pathways could directly interact on the FSHβ promoter. In support of an indirect mechanism, testosterone has been shown to suppress follistatin mRNA and primary transcripts in rat primary cell cultures (Bilezikjian et al., 1996; Burger et al., 2004a). Smad mRNA and Smad phosphorylation have also been reported to change after testosterone treatment in primary pituitary cells (Burger et al., 2007). However, if steroid action were simply indirect, we would expect that all regulatory regions responsive to activin would be affected and this is not what is observed. Both the LHβ and FSHβ promoters are activin responsive, yet only FSHβ is induced in LβT2 cells after activin and steroid hormone co-treatment, while LHβ is suppressed (McGillivray et al., 2007; Thackray and Mellon, 2008). These experiments demonstrated that the gonadotrope itself is sufficient for synergy between activin and progestins, androgens or glucocorticoids. Smad transcription factors can interact directly with steroid receptors (Chipuk et al., 2002; Hayes et al., 2001; Kang et al., 2002; Li et al., 2003; Song et al., 1999; Thackray and Mellon, 2008). Moreover, we showed that synergy between activin and 3-keto steroid hormones occurs directly on the FSHβ promoter and requires Smad and steroid receptor signaling, as well as DNA binding to the FSHβ promoter (Thackray and Mellon, 2008). This synergistic interaction between activin and 3-keto steroid hormones may play an important physiological role by participating in the generation of the secondary FSH surge that occurs during the early morning of estrus in rodents (Fig. 3).
6. Conclusions and future directions
In the female, normal reproductive function involves a pattern of regular ovulatory cycles that is achieved through precise integration of stimulatory and inhibitory signals between the hypothalamus, pituitary and ovary. Insight into the molecular mechanisms of hormonal regulation of LH and FSH synthesis may be critical for understanding not only the ovulatory cycle, but also fetal development, puberty, pregnancy, postpartum, and menopause. In this review, we have highlighted recent studies demonstrating that synergy between activin and GnRH, as well as activin and steroid hormones, in concert with variations in GnRH amplitude and frequency, may be responsible for the differential regulation of LH and FSH synthesis, particularly during the secondary FSH surge.
As illustrated in Figure 1, hormonal regulation of the LHβ and FSHβ promoters is extremely complex. Although numerous molecular mechanisms of hormone action on these promoters have been elucidated over the past decade, there are potentially many more factors that may play important roles. For instance, factors important in pituitary gland development such as LIM homeodomain proteins have been shown to regulate FSHβ gene expression (West et al., 2004), but whether they also convey hormone responsiveness or interact with hormone-induced factors has not been examined. It is also true that we are just beginning to understand the interactions between transcription factors involved in hormonal regulation of gonadotropin synthesis and cofactors involved in chromatin remodeling or the activation of basal transcriptional machinery.
The use of more physiologically relevant model systems is also required to confirm results obtained in immortalized gonadotrope cells. The generation of a mouse co-expressing the GnRH receptor and Cre recombinase (GRIC) (Wen et al., 2008) that can be crossed to a ROSA 26-yellow fluorescent protein mouse to generate purified gonadotropes by fluorescence-activated cell sorting may be particularly useful in this regard. Gonadotropes purified from transgenic mice containing 4.7 kb of the ovine FSHβ promoter linked to the cell surface antigen, H-2Kk (Wu et al., 2004) may be an alternative. Moreover, genetically modified mice can be used to test the necessity of specific regulatory elements or transcription factors in hormonal regulation of the LHβ and FSHβ promoters (Ferris et al., 2007; Huang et al., 2001; Su et al., 2007).
Just as the gonadotropin β-subunits are regulated by crosstalk among GnRH, activin and steroid hormone signaling pathways, it is highly likely that other proteins important for gonadotrope function are regulated as well. Several microarray studies have defined transcriptional targets of GnRH in LβT2 cells (Kakar et al., 2003; Lawson et al., 2007; Wurmbach et al., 2001). We and others have also recently demonstrated that activin substantially modulates the transcriptional response to GnRH (Mazhawidza et al., 2006; Zhang et al., 2006). Defining the complete transcriptome for these hormones, both alone and in combination, will not be trivial. However, the application of ChIP-chip (Ren et al., 2000) and ChIP-sequencing techniques [reviewed in (Shendure and Ji, 2008)] will expedite the process. The use of tissue-targeted and conditional knockout mice may also help identify additional hormonally regulated genes. In addition to the GRIC mouse discussed above, several transgenic mice have been developed that express Cre recombinase in a pituitary and gonadotrope-specific manner (Charles et al., 2008; Cushman et al., 2000; Naik et al., 2006), and can be used to analyze the role of specific transcription factors or signaling molecules exclusively in gonadotropes.
Acknowledgments
We thank Scott Kelley, Rachel Larder and Kellie Breen for critical reading of the manuscript and editorial comments. This work was supported by NICHD/NIH through a cooperative agreement (U54 HD012303) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (P.L.M.). This work was also supported by NIH grants R01 HD020377 and R01 DK044838 (to P.L.M.). D.C. was partially supported by NIH grants F32 HD41301, R03 HD054595 and R01 HD057549. V.G.T. was partially supported by NIH grants F32 DK065437 and K01 DK080467.
Abbreviations
- AP-1
activator protein-1
- AR
androgen receptor
- ChIP
chromatin immunoprecipitation
- Egr-1
early growth response-1
- ER
estrogen receptor
- FSH
follicle-stimulating hormone
- GnRH
gonadotropin-releasing hormone
- HD
homeodomain element
- HRE
hormone response element
- LH
luteinizing hormone
- HPG
hypothalamic-pituitary-gonadal
- MAPK
mitogen-activated protein kinase
- PCOS
polycystic ovary syndrome
- PR
progesterone receptor
- PKC
protein kinase C
- SBE
smad-binding element
- SF-1
steroidogenic factor-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alarid ET, Holley S, Hayakawa M, Mellon PL. Discrete stages of anterior pituitary differentiation recapitulated in immortalized cell lines. Mol Cell Endocrinol. 1998;140:25–30. doi: 10.1016/s0303-7207(98)00025-2. [DOI] [PubMed] [Google Scholar]
- Alarid ET, Windle JJ, Whyte DB, Mellon PL. Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development. 1996;122:3319–3329. doi: 10.1242/dev.122.10.3319. [DOI] [PubMed] [Google Scholar]
- Attardi B, Fitzgerald T. Effects of progesterone on the estradiol-induced follicle-stimulating hormone (FSH) surge and FSH beta messenger ribonucleic acid in the rat. Endocrinology. 1990;126:2281–2287. doi: 10.1210/endo-126-5-2281. [DOI] [PubMed] [Google Scholar]
- Attardi B, Miklos J. Rapid stimulatory effect of activin-A on messenger RNA encoding the follicle-stimulating hormone beta-subunit in rat pituitary cell cultures. Mol Endocrinol. 1990;4:721–726. doi: 10.1210/mend-4-5-721. [DOI] [PubMed] [Google Scholar]
- Attisano L, Wrana JL. Signal transduction by the TGF-beta superfamily. Science. 2002;296:1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- Bailey JS, Rave-Harel N, Coss D, McGillivray SM, Mellon PL. Activin regulation of the follicle-stimulating hormone -subunit gene involves Smads and the TALE homeodomain proteins Pbx1 and Prep1. Mol Endocrinol. 2004;18:1158–1170. doi: 10.1210/me.2003-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978;202:631–633. doi: 10.1126/science.100883. [DOI] [PubMed] [Google Scholar]
- Bernard DJ. Both SMAD2 and SMAD3 mediate activin-stimulated expression of the follicle-stimulating hormone beta subunit in mouse gonadotrope cells. Mol Endocrinol. 2004;18:606–623. doi: 10.1210/me.2003-0264. [DOI] [PubMed] [Google Scholar]
- Besecke LM, Guendner MJ, Schneyer AL, Bauer-Dantoin AC, Jameson JL, Weiss J. Gonadotropin-releasing hormone regulates follicle-stimulating hormone-beta gene expression through an activin/follistatin autocrine or paracrine loop. Endocrinology. 1996;137:3667–3673. doi: 10.1210/endo.137.9.8756531. [DOI] [PubMed] [Google Scholar]
- Besecke LM, Guendner MJ, Sluss PA, Polak AG, Woodruff TK, Jameson JL, Bauer-Dantoin AC, Weiss J. Pituitary follistatin regulates activin-mediated production of follicle-stimulating hormone during the rat estrous cycle. Endocrinology. 1997;138:2841–2848. doi: 10.1210/endo.138.7.5279. [DOI] [PubMed] [Google Scholar]
- Bilezikjian LM, Corrigan AZ, Blount AL, Vale WW. Pituitary follistatin and inhibin subunit messenger ribonucleic acid levels are differentially regulated by local and hormonal factors. Endocrinology. 1996;137:4277–4284. doi: 10.1210/endo.137.10.8828487. [DOI] [PubMed] [Google Scholar]
- Blank SK, McCartney CR, Marshall JC. The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome. Hum Reprod Update. 2006;12:351–361. doi: 10.1093/humupd/dml017. [DOI] [PubMed] [Google Scholar]
- Blumenfeld Z, Ritter M. Inhibin, activin, and follistatin in human fetal pituitary and gonadal physiology. Ann NY Acad Sci. 2001;943:34–48. doi: 10.1111/j.1749-6632.2001.tb03788.x. [DOI] [PubMed] [Google Scholar]
- Bohnsack BL, Szabo M, Kilen SM, Tam DH, Schwartz NB. Follistatin suppresses steroid-enhanced follicle-stimulating hormone release in vitro in rats. Biol Reprod. 2000;62:636–641. doi: 10.1095/biolreprod62.3.636. [DOI] [PubMed] [Google Scholar]
- Bonfil D, Chuderland D, Kraus S, Shahbazian D, Friedberg I, Seger R, Naor Z. Extracellular signal-regulated kinase, Jun N-terminal kinase, p38, and c-Src are involved in gonadotropin-releasing hormone-stimulated activity of the glycoprotein hormone follicle-stimulating hormone beta-subunit promoter. Endocrinology. 2004;145:2228–2244. doi: 10.1210/en.2003-1418. [DOI] [PubMed] [Google Scholar]
- Burger LL, Dalkin AC, Aylor KW, Haisenleder DJ, Marshall JC. GnRH pulse frequency modulation of gonadotropin subunit gene transcription in normal gonadotropes-assessment by primary transcript assay provides evidence for roles of GnRH and follistatin. Endocrinology. 2002;143:3243–3249. doi: 10.1210/en.2002-220216. [DOI] [PubMed] [Google Scholar]
- Burger LL, Haisenleder DJ, Aylor KW, Dalkin AC, Prendergast KA, Marshall JC. Regulation of luteinizing hormone-beta and follicle-stimulating hormone (FSH)-beta gene transcription by androgens: testosterone directly stimulates FSH-beta transcription independent from its role on follistatin gene expression. Endocrinology. 2004a;145:71–78. doi: 10.1210/en.2003-1047. [DOI] [PubMed] [Google Scholar]
- Burger LL, Haisenleder DJ, Aylor KW, Marshall JC. Regulation of intracellular signaling cascades by GNRH pulse frequency in the rat pituitary: roles for CaMK II, ERK, and JNK activation. Biol Reprod. 2008;79:947–953. doi: 10.1095/biolreprod.108.070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger LL, Haisenleder DJ, Dalkin AC, Marshall JC. Regulation of gonadotropin subunit gene transcription. J Mol Endocrinol. 2004b;33:559–584. doi: 10.1677/jme.1.01600. [DOI] [PubMed] [Google Scholar]
- Burger LL, Haisenleder DJ, Wotton GM, Aylor KW, Dalkin AC, Marshall JC. The regulation of FSHbeta transcription by gonadal steroids: testosterone and estradiol modulation of the activin intracellular signaling pathway. Am J Physiol Endocrinol Metab. 2007;293:E277–285. doi: 10.1152/ajpendo.00447.2006. [DOI] [PubMed] [Google Scholar]
- Burns KH, Matzuk MM. Minireview: genetic models for the study of gonadotropin actions. Endocrinology. 2002;143:2823–2835. doi: 10.1210/endo.143.8.8928. [DOI] [PubMed] [Google Scholar]
- Burrin JM, Jameson JL. Regulation of transfected glycoprotein hormone -gene expression in primary pituitary cell cultures. Mol Endocrinol. 1989;3:1643–1651. doi: 10.1210/mend-3-10-1643. [DOI] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- Chang H, Brown CW, Matzuk MM. Genetic analysis of the mammalian transforming growth factor-beta superfamily. Endocr Rev. 2002;23:787–823. doi: 10.1210/er.2002-0003. [DOI] [PubMed] [Google Scholar]
- Chang RJ. The reproductive phenotype in polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab. 2007;3:688–695. doi: 10.1038/ncpendmet0637. [DOI] [PubMed] [Google Scholar]
- Chappell PE, Lydon JP, Conneely OM, O’Malley BW, Levine JE. Endocrine defects in mice carrying a null mutation for the progesterone receptor gene. Endocrinology. 1997;138:4147–4152. doi: 10.1210/endo.138.10.5456. [DOI] [PubMed] [Google Scholar]
- Charles MA, Mortensen AH, Potok MA, Camper SA. Pitx2 deletion in pituitary gonadotropes is compatible with gonadal development, puberty, and fertility. Genesis. 2008;46:507–514. doi: 10.1002/dvg.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs GV, Unabia G. The use of counterflow centrifugation to enrich gonadotropes and somatotropes. J Histochem Cytochem. 2001;49:663–664. doi: 10.1177/002215540104900514. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Cornelius SC, Pultz NJ, Jorgensen JS, Bonham MJ, Kim SJ, Danielpour D. The androgen receptor represses transforming growth factor-beta signaling through interaction with Smad3. J Biol Chem. 2002;277:1240–1248. doi: 10.1074/jbc.M108855200. [DOI] [PubMed] [Google Scholar]
- Ciccone NA, Lacza CT, Hou MY, Gregory SJ, Kam KY, Xu S, Kaiser UB. A composite element that binds basic helix loop helix and basic leucine zipper transcription factors is important for gonadotropin-releasing hormone regulation of the follicle-stimulating hormone beta gene. Mol Endocrinol. 2008;22:1908–1923. doi: 10.1210/me.2007-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coss D, Hand CM, Yaphockun KK, Ely HA, Mellon PL. p38 mitogen-activated kinase is critical for synergistic induction of the FSH beta gene by gonadotropin-releasing hormone and activin through augmentation of c-Fos induction and Smad phosphorylation. Mol Endocrinol. 2007;21:3071–3086. doi: 10.1210/me.2007-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coss D, Jacobs SB, Bender CE, Mellon PL. A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone beta gene by gonadotropin-releasing hormone. J Biol Chem. 2004;279:152–162. doi: 10.1074/jbc.M304697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coss D, Thackray VG, Deng CX, Mellon PL. Activin regulates luteinizing hormone beta-subunit gene expression through smad-binding and homeobox elements. Mol Endocrinol. 2005;19:2610–2623. doi: 10.1210/me.2005-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta. Mol Endocrinol. 2003;17:1039–1053. doi: 10.1210/me.2002-0398. [DOI] [PubMed] [Google Scholar]
- Curtin D, Ferris HA, Hakli M, Gibson M, Janne OA, Palvimo JJ, Shupnik MA. Small nuclear RING finger protein stimulates the rat luteinizing hormone-beta promoter by interacting with Sp1 and steroidogenic factor-1 and protects from androgen suppression. Mol Endocrinol. 2004;18:1263–1276. doi: 10.1210/me.2003-0221. [DOI] [PubMed] [Google Scholar]
- Curtin D, Jenkins S, Farmer N, Anderson AC, Haisenleder DJ, Rissman E, Wilson EM, Shupnik MA. Androgen suppression of GnRH-stimulated rat LHbeta gene transcription occurs through Sp1 sites in the distal GnRH-responsive promoter region. Mol Endocrinol. 2001;15:1906–1917. doi: 10.1210/mend.15.11.0723. [DOI] [PubMed] [Google Scholar]
- Cushman LJ, Burrows HL, Seasholtz AF, Lewandoski M, Muzyczka N, Camper SA. Cre-mediated recombination in the pituitary gland. Genesis. 2000;28:167–174. doi: 10.1002/1526-968x(200011/12)28:3/4<167::aid-gene120>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Dahl KD, Campen CA, McGuinness DM, Vale W. Differential regulation in the release of bioactive versus immunoactive gonadotropins from cultured rat pituitary cells by inhibin and androgens. J Androl. 1992;13:526–533. [PubMed] [Google Scholar]
- Dalkin AC, Burger LL, Aylor KW, Haisenleder DJ, Workman LJ, Cho S, Marshall JC. Regulation of gonadotropin subunit gene transcription by gonadotropin- releasing hormone: measurement of primary transcript ribonucleic acids by quantitative reverse transcription-polymerase chain reaction assays. Endocrinology. 2001;142:139–146. doi: 10.1210/endo.142.1.7881. [DOI] [PubMed] [Google Scholar]
- Dalkin AC, Haisenleder DJ, Ortolano GA, Ellis TR, Marshall JC. The frequency of gonadotropin-releasing-hormone stimulation differentially regulates gonadotropin subunit messenger ribonucleic acid expression. Endocrinology. 1989;125:917–924. doi: 10.1210/endo-125-2-917. [DOI] [PubMed] [Google Scholar]
- Dalkin AC, Haisenleder DJ, Ortolano GA, Suhr A, Marshall JC. Gonadal regulation of gonadotropin subunit gene expression: evidence for regulation of follicle-stimulating hormone-beta messenger ribonucleic acid by nonsteroidal hormones in female rats. Endocrinology. 1990;127:798–806. doi: 10.1210/endo-127-2-798. [DOI] [PubMed] [Google Scholar]
- Dalkin AC, Knight CD, Shupnik MA, Haisenleder DJ, Aloi J, Kirk SE, Yasin M, Marshall JC. Ovariectomy and inhibin immunoneutralization acutely increase follicle-stimulating hormone-beta messenger ribonucleic acid concentrations: evidence for a nontranscriptional mechanism. Endocrinology. 1993;132:1297–1304. doi: 10.1210/endo.132.3.7679976. [DOI] [PubMed] [Google Scholar]
- Dalkin AC, Paul SJ, Haisenleder DJ, Ortolano GA, Yasin M, Marshall JC. Gonadal steroids effect similar regulation of gonadotrophin subunit mRNA expression in both male and female rats. J Endocrinol. 1992;132:39–45. doi: 10.1677/joe.0.1320039. [DOI] [PubMed] [Google Scholar]
- Datta PK, Blake MC, Moses HL. Regulation of plasminogen activator inhibitor-1 expression by transforming growth factor-beta -induced physical and functional interactions between smads and Sp1. J Biol Chem. 2000;275:40014–40019. doi: 10.1074/jbc.C000508200. [DOI] [PubMed] [Google Scholar]
- Denef C. Paracrinicity: the story of 30 years of cellular pituitary crosstalk. J Neuroendocrinol. 2008;20:1–70. doi: 10.1111/j.1365-2826.2007.01616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePaolo LV, Hirshfield AN, Anderson LD, Barraclough CA, Channing CP. Suppression of pituitary secretion of follicle-stimulating hormone by porcine follicular fluid during pro-oestrus and oestrus in the rat: effects on gonadotrophin and steroid secretion, follicular development and ovulation during the following cycle. J Endocrinol. 1979;83:355–368. doi: 10.1677/joe.0.0830355. [DOI] [PubMed] [Google Scholar]
- Dorn C, Ou Q, Svaren J, Crawford PA, Sadovsky Y. Activation of luteinizing hormone beta gene by gonadotropin-releasing hormone requires the synergy of early growth response-1 and steroidogenic factor-1. J Biol Chem. 1999;274:13870–13876. doi: 10.1074/jbc.274.20.13870. [DOI] [PubMed] [Google Scholar]
- Fallest PC, Schwartz NB. Acute inhibitory effects of 17 beta-estradiol are observed on gonadotropin secretion from perifused pituitary fragments of metestrous, but not proestrous, rats. Endocrinology. 1991;128:273–279. doi: 10.1210/endo-128-1-273. [DOI] [PubMed] [Google Scholar]
- Ferris HA, Shupnik MA. Mechanisms for pulsatile regulation of the gonadotropin subunit genes by GnRH1. Biol Reprod. 2006;74:993–998. doi: 10.1095/biolreprod.105.049049. [DOI] [PubMed] [Google Scholar]
- Ferris HA, Walsh HE, Stevens J, Fallest PC, Shupnik MA. Luteinizing hormone beta promoter stimulation by adenylyl cyclase and cooperation with gonadotropin-releasing hormone 1 in transgenic mice and LBetaT2 Cells. Biol Reprod. 2007;77:1073–1080. doi: 10.1095/biolreprod.107.064139. [DOI] [PubMed] [Google Scholar]
- Gardner S, Maudsley S, Millar RP, Pawson AJ. Nuclear stabilization of beta-catenin and inactivation of glycogen synthase kinase-3beta by gonadotropin-releasing hormone: targeting Wnt signaling in the pituitary gonadotrope. Mol Endocrinol. 2007;21:3028–3038. doi: 10.1210/me.2007-0268. [DOI] [PubMed] [Google Scholar]
- Germain S, Howell M, Esslemont GM, Hill CS. Homeodomain and winged-helix transcription factors recruit activated Smads to distinct promoter elements via a common Smad interaction motif. Genes Dev. 2000;14:435–451. [PMC free article] [PubMed] [Google Scholar]
- Gharib SD, Leung PC, Carroll RS, Chin WW. Androgens positively regulate follicle-stimulating hormone beta-subunit mRNA levels in rat pituitary cells. Mol Endocrinol. 1990;4:1620–1626. doi: 10.1210/mend-4-11-1620. [DOI] [PubMed] [Google Scholar]
- Gharib SD, Wierman ME, Badger TM, Chin WW. Sex steroid hormone regulation of follicle-stimulating hormone subunit messenger ribonucleic acid (mRNA) levels in the rat. J Clin Invest. 1987;80:294–299. doi: 10.1172/JCI113072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glidewell-Kenney C, Weiss J, Hurley LA, Levine JE, Jameson JL. Estrogen receptor alpha signaling pathways differentially regulate gonadotropin subunit gene expression and serum follicle-stimulating hormone in the female mouse. Endocrinology. 2008;149:4168–4176. doi: 10.1210/en.2007-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D, Lau K. Pituitary follicular cells secrete both vascular endothelial growth factor and follistatin. Biochem Biophys Res Commun. 1989;165:292–298. doi: 10.1016/0006-291x(89)91068-1. [DOI] [PubMed] [Google Scholar]
- Graham KE, Nusser KD, Low MJ. L T2 gonadotroph cells secrete follicle stimulating hormone (FSH) in response to activin A. J Endocrinol. 1999;162:R1–R5. doi: 10.1677/joe.0.162r001. [DOI] [PubMed] [Google Scholar]
- Gregory SJ, Lacza CT, Detz AA, Xu S, Petrillo LA, Kaiser UB. Synergy between activin A and gonadotropin-releasing hormone in transcriptional activation of the rat follicle-stimulating hormone-beta gene. Mol Endocrinol. 2005;19:237–254. doi: 10.1210/me.2003-0473. [DOI] [PubMed] [Google Scholar]
- Haisenleder D, Dalkin A, Ortolano G, Marshall J, Shupnik M. A pulsatile gonadotropin-releasing hormone stimulus is required to increase transcription of the gonadotropin subunit genes: Evidence for differential regulation of transcription by pulse frequency in vivo. Endocrinology. 1991;128:509–517. doi: 10.1210/endo-128-1-509. [DOI] [PubMed] [Google Scholar]
- Haisenleder DJ, Burger LL, Walsh HE, Stevens J, Aylor KW, Shupnik MA, Marshall JC. Pulsatile gonadotropin-releasing hormone stimulation of gonadotropin subunit transcription in rat pituitaries: evidence for the involvement of Jun N-terminal kinase but not p38. Endocrinology. 2008;149:139–145. doi: 10.1210/en.2007-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haisenleder DJ, Ferris HA, Shupnik MA. The calcium component of gonadotropin-releasing hormone-stimulated luteinizing hormone subunit gene transcription is mediated by calcium/calmodulin-dependent protein kinase type II. Endocrinology. 2003;144:2409–2416. doi: 10.1210/en.2002-0013. [DOI] [PubMed] [Google Scholar]
- Haisenleder DJ, Katt JA, Ortolano GA, el-Gewely MR, Duncan JA, Dee C, Marshall JC. Influence of gonadotropin-releasing hormone pulse amplitude, frequency, and treatment duration on the regulation of luteinizing hormone (LH) subunit messenger ribonucleic acids and LH secretion. Mol Endocrinol. 1988;2:338–343. doi: 10.1210/mend-2-4-338. [DOI] [PubMed] [Google Scholar]
- Hall JE, Taylor AE, Hayes FJ, Crowley WF., Jr Insights into hypothalamic-pituitary dysfunction in polycystic ovary syndrome. J Endocrinol Invest. 1998;21:602–6011. doi: 10.1007/BF03350785. [DOI] [PubMed] [Google Scholar]
- Halvorson LM, Kaiser UB, Chin WW. Stimulation of luteinizing hormone beta gene promoter activity by the orphan nuclear receptor, steroidogenic factor-1. J Biol Chem. 1996;271:6645–6650. doi: 10.1074/jbc.271.12.6645. [DOI] [PubMed] [Google Scholar]
- Halvorson LM, Weiss J, Bauer-Dantoin AC, Jameson JL. Dynamic regulation of pituitary follistatin messenger ribonucleic acids during the rat estrous cycle. Endocrinology. 1994;134:1247–1253. doi: 10.1210/endo.134.3.8119165. [DOI] [PubMed] [Google Scholar]
- Harris D, Bonfil D, Chuderland D, Kraus S, Seger R, Naor Z. Activation of MAPK cascades by GnRH: ERK and Jun N-terminal kinase are involved in basal and GnRH-stimulated activity of the glycoprotein hormone LHbeta-subunit promoter. Endocrinology. 2002;143:1018–1025. doi: 10.1210/endo.143.3.8675. [DOI] [PubMed] [Google Scholar]
- Hayes SA, Zarnegar M, Sharma M, Yang F, Peehl DM, ten Dijke P, Sun Z. SMAD3 represses androgen receptor-mediated transcription. Cancer Res. 2001;61:2112–2118. [PubMed] [Google Scholar]
- Hislop JN, Everest HM, Flynn A, Harding T, Uney JB, Troskie BE, Millar RP, McArdle CA. Differential internalization of mammalian and non-mammalian gonadotropin-releasing hormone receptors. Uncoupling of dynamin-dependent internalization from mitogen-activated protein kinase signaling. J Biol Chem. 2001;276:39685–39694. doi: 10.1074/jbc.M104542200. [DOI] [PubMed] [Google Scholar]
- Hoak DC, Schwartz NB. Blockade of recruitment of ovarian follicles by suppression of the secondary surge of follicle-stimulating hormone with porcine follicular field. Proc Natl Acad Sci USA. 1980;77:4953–4956. doi: 10.1073/pnas.77.8.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HJ, Sebastian J, Strahl BD, Wu JC, Miller WL. Transcriptional regulation of the ovine follicle-stimulating hormone-beta gene by activin and gonadotropin-releasing hormone (GnRH): involvement of two proximal activator protein-1 sites for GnRH stimulation. Endocrinology. 2001;142:2267–2274. doi: 10.1210/endo.142.6.8203. [DOI] [PubMed] [Google Scholar]
- Huhtaniemi I. Mutations along the pituitary-gonadal axis affecting sexual maturation: novel information from transgenic and knockout mice. Mol Cell Endocrinol. 2006:254–255. 84–90. doi: 10.1016/j.mce.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Huhtaniemi I, Ahtiainen P, Pakarainen T, Rulli SB, Zhang FP, Poutanen M. Genetically modified mouse models in studies of luteinising hormone action. Mol Cell Endocrinol. 2006;252:126–135. doi: 10.1016/j.mce.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Hummler E, Cole TJ, Blendy JA, Ganss R, Aguzzi A, Schmid W, Beermann F, Schutz G. Targeted mutation of the CREB gene: compensation within the CREB/ATF family of transcription factors. Proc Natl Acad Sci USA. 1994;91:5647–5651. doi: 10.1073/pnas.91.12.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RS, Spiegelman BM, Papaioannou V. Pleiotropic effects of a null mutation in the c-fos proto-oncogene. Cell. 1992;71:577–586. doi: 10.1016/0092-8674(92)90592-z. [DOI] [PubMed] [Google Scholar]
- Jones RE. Human Reproductive Biology. Academic Press; San Diego, CA: 1997. p. 581. [Google Scholar]
- Jorgensen JS, Nilson JH. AR suppresses transcription of the LHbeta subunit by interacting with steroidogenic factor-1. Mol Endocrinol. 2001;15:1505–1516. doi: 10.1210/mend.15.9.0691. [DOI] [PubMed] [Google Scholar]
- Jorgensen JS, Quirk CC, Nilson JH. Multiple and overlapping combinatorial codes orchestrate hormonal responsiveness and dictate cell-specific expression of the genes encoding luteinizing hormone. Endocr Rev. 2004;25:521–542. doi: 10.1210/er.2003-0029. [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Conn PM, Chin WW. Studies of gonadotropin-releasing hormone (GnRH) action using GnRH receptor-expressing pituitary cell lines. Endocr Rev. 1997a;18:46–70. doi: 10.1210/edrv.18.1.0289. [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Halvorson LM, Chen MT. Sp1, steroidogenic factor 1 (SF-1), and early growth response protein 1 (egr-1) binding sites form a tripartite gonadotropin-releasing hormone response element in the rat luteinizing hormone-beta gene promoter: an integral role for SF-1. Mol Endocrinol. 2000;14:1235–1245. doi: 10.1210/mend.14.8.0507. [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Jakubowiak A, Steinberger A, Chin WW. Differential effects of gonadotropin-releasing hormone (GnRH) pulse frequency on gonadotropin subunit and GnRH receptor messenger ribonucleic acid levels in vitro. Endocrinology. 1997b;138:1224–1231. doi: 10.1210/endo.138.3.4968. [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Lee BL, Carroll RS, Unabia G, Chin WW, Childs GV. Follistatin gene expression in the pituitary: Localization in the gonadotropes and folliculostellate cells in diestrous rats. Endocrinology. 1992;130:3048–3056. doi: 10.1210/endo.130.5.1572312. [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Sabbagh E, Chen MT, Chin WW, Saunders BD. Sp1 binds to the rat luteinizing hormone beta (LHbeta) gene promoter and mediates gonadotropin-releasing hormone-stimulated expression of the LHbeta subunit gene. J Biol Chem. 1998;273:12943–12951. doi: 10.1074/jbc.273.21.12943. [DOI] [PubMed] [Google Scholar]
- Kakar SS, Winters SJ, Zacharias W, Miller DM, Flynn S. Identification of distinct gene expression profiles associated with treatment of LbetaT2 cells with gonadotropin-releasing hormone agonist using microarray analysis. Gene. 2003;308:67–77. doi: 10.1016/s0378-1119(03)00446-3. [DOI] [PubMed] [Google Scholar]
- Kang HY, Huang KE, Chang SY, Ma WL, Lin WJ, Chang C. Differential modulation of androgen receptor-mediated transactivation by Smad3 and tumor suppressor Smad4. J Biol Chem. 2002;277:43749–43756. doi: 10.1074/jbc.M205603200. [DOI] [PubMed] [Google Scholar]
- Kawakami S, Fujii Y, Okada Y, Winters SJ. Paracrine regulation of FSH by follistatin in folliculostellate cell- enriched primate pituitary cell cultures. Endocrinology. 2002;143:2250–2258. doi: 10.1210/endo.143.6.8857. [DOI] [PubMed] [Google Scholar]
- Kerrigan JR, Dalkin AC, Haisenleder DJ, Yasin M, Marshall JC. Failure of gonadotropin-releasing hormone (GnRH) pulses to increase luteinizing hormone beta messenger ribonucleic acid in GnRH-deficient female rats. Endocrinology. 1993;133:2071–2079. doi: 10.1210/endo.133.5.8404655. [DOI] [PubMed] [Google Scholar]
- Kilen SM, Szabo M, Strasser GA, McAndrews JM, Ringstrom SJ, Schwartz NB. Corticosterone selectively increases follicle-stimulating hormone beta-subunit messenger ribonucleic acid in primary anterior pituitary cell culture without affecting its half-life. Endocrinology. 1996;137:3802–3807. doi: 10.1210/endo.137.9.8756550. [DOI] [PubMed] [Google Scholar]
- Kirk SE, Dalkin AC, Yasin M, Haisenleder DJ, Marshall JC. Gonadotropin-releasing hormone pulse frequency regulates expression of pituitary follistatin messenger ribonucleic acid: a mechanism for differential gonadotrope function. Endocrinology. 1994;135:876–880. doi: 10.1210/endo.135.3.8070381. [DOI] [PubMed] [Google Scholar]
- Knox KL, Schwartz NB. RU486 blocks the secondary surge of follicle-stimulating hormone in the rat without blocking the drop in serum inhibin. Biol Reprod. 1992;46:220–225. doi: 10.1095/biolreprod46.2.220. [DOI] [PubMed] [Google Scholar]
- Kogawa K, Nakamura T, Sugino K, Takio K, Titani K, Sugino H. Activin-binding protein is present in pituitary. Endocrinology. 1991;128:1434–1440. doi: 10.1210/endo-128-3-1434. [DOI] [PubMed] [Google Scholar]
- Kowase T, Walsh HE, Darling DS, Shupnik MA. Estrogen enhances gonadotropin-releasing hormone-stimulated transcription of the luteinizing hormone subunit promoters via altered expression of stimulatory and suppressive transcription factors. Endocrinology. 2007;148:6083–6091. doi: 10.1210/en.2007-0407. [DOI] [PubMed] [Google Scholar]
- Kumar TR, Low MJ, Matzuk MM. Genetic rescue of follicle-stimulating hormone beta-deficient mice. Endocrinology. 1998;139:3289–3295. doi: 10.1210/endo.139.7.6111. [DOI] [PubMed] [Google Scholar]
- Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- Lamba P, Santos MM, Philips DP, Bernard DJ. Acute regulation of murine follicle-stimulating hormone beta subunit transcription by activin A. J Mol Endocrinol. 2006;36:201–220. doi: 10.1677/jme.1.01961. [DOI] [PubMed] [Google Scholar]
- Lamminen T, Jokinen P, Jiang M, Pakarinen P, Simonsen H, Huhtaniemi I. Human FSH beta subunit gene is highly conserved. Mol Hum Reprod. 2005;11:601–605. doi: 10.1093/molehr/gah198. [DOI] [PubMed] [Google Scholar]
- Lawson MA, Li D, Glidewell-Kenney CA, Lopez FJ. Androgen responsiveness of the pituitary gonadotrope cell line LbetaT2. J Endocrinol. 2001;170:601–607. doi: 10.1677/joe.0.1700601. [DOI] [PubMed] [Google Scholar]
- Lawson MA, Tsutsumi R, Zhang H, Talukdar I, Butler BK, Santos SJ, Mellon PL, Webster NJ. Pulse sensitivity of the luteinizing hormone beta promoter is determined by a negative feedback loop Involving early growth response-1 and Ngfi-A binding protein 1 and 2. Mol Endocrinol. 2007;21:1175–1191. doi: 10.1210/me.2006-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal AM, Blount AL, Donaldson CJ, Bilezikjian LM, Vale WW. Regulation of follicle-stimulating hormone secretion by the interactions of activin-A, dexamethasone and testosterone in anterior pituitary cell cultures of male rats. Neuroendocrinology. 2003;77:298–304. doi: 10.1159/000070896. [DOI] [PubMed] [Google Scholar]
- Lee KB, Khivansara V, Santos MM, Lamba P, Yuen T, Sealfon SC, Bernard DJ. Bone morphogenetic protein 2 and activin A synergistically stimulate follicle-stimulating hormone beta subunit transcription. J Mol Endocrinol. 2007;38:315–330. doi: 10.1677/jme.1.02196. [DOI] [PubMed] [Google Scholar]
- Lee SL, Sadovsky Y, Swirnoff AH, Polish JA, Goda P, Gavrilina G, Milbrandt J. Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1) Science. 1996;273:1219–1221. doi: 10.1126/science.273.5279.1219. [DOI] [PubMed] [Google Scholar]
- Levine JE, Chappell PE, Schneider JS, Sleiter NC, Szabo M. Progesterone receptors as neuroendocrine integrators. Front Neuroendocrinol. 2001;22:69–106. doi: 10.1006/frne.2001.0210. [DOI] [PubMed] [Google Scholar]
- Lewis KA, Gray PC, Blount AL, MacConell LA, Wiater E, Bilezikjian LM, Vale W. Betaglycan binds inhibin and can mediate functional antagonism of activin signalling. Nature. 2000;404:411–414. doi: 10.1038/35006129. [DOI] [PubMed] [Google Scholar]
- Li G, Wang S, Gelehrter TD. Identification of glucocorticoid receptor domains involved in transrepression of TGFbeta action. J Biol Chem. 2003;278:41779–41788. doi: 10.1074/jbc.M305350200. [DOI] [PubMed] [Google Scholar]
- Ling N, Ying SY, Ueno N, Shimasaki S, Esch F, Hotta O, Guillemin R. Pituitary FSH is released by a heterodimer of the -subunits from the two forms of inhibin. Nature. 1986;321:779–782. doi: 10.1038/321779a0. [DOI] [PubMed] [Google Scholar]
- Liu F, Austin DA, DiPaolo D, Mellon PL, Olefsky JM, Webster NJG. GnRH activates ERK1/2 leading to the induction of c-fos and LH protein expression in L T2 cells. Mol Endocrinol. 2002;16:419–434. doi: 10.1210/mend.16.3.0791. [DOI] [PubMed] [Google Scholar]
- Liu F, Ruiz MS, Austin DA, Webster NJ. Constitutively active Gq impairs gonadotropin-releasing hormone-induced intracellular signaling and luteinizing hormone secretion in LbetaT2 cells. Mol Endocrinol. 2005;19:2074–2085. doi: 10.1210/me.2004-0145. [DOI] [PubMed] [Google Scholar]
- Lopez-Rovira T, Chalaux E, Massague J, Rosa JL, Ventura F. Direct binding of Smad1 and Smad4 to two distinct motifs mediates bone morphogenetic protein-specific transcriptional activation of Id1 gene. J Biol Chem. 2002;277:3176–3185. doi: 10.1074/jbc.M106826200. [DOI] [PubMed] [Google Scholar]
- Luo M, Koh M, Feng J, Wu Q, Melamed P. Cross talk in hormonally regulated gene transcription through induction of estrogen receptor ubiquitylation. Mol Cell Biol. 2005;25:7386–7398. doi: 10.1128/MCB.25.16.7386-7398.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Dong Y, Matzuk MM, Kumar TR. Targeted disruption of luteinizing hormone beta-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc Natl Acad Sci USA. 2004;101:17294–17299. doi: 10.1073/pnas.0404743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason AJ, Hayflick JS, Zoeller RT, Young WS, Phillips HS, Nikolics K, Seeburg PH. A deletion truncating the gonadotropin-releasing hormone gene is responsible for hypogonadism in the hpg mouse. Science. 1986;234:1366–1371. doi: 10.1126/science.3024317. [DOI] [PubMed] [Google Scholar]
- Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Kumar TR, Bradley A. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature. 1995;374:356–560. doi: 10.1038/374356a0. [DOI] [PubMed] [Google Scholar]
- Mazhawidza W, Winters SJ, Kaiser UB, Kakar SS. Identification of gene networks modulated by activin in LbetaT2 cells using DNA microarray analysis. Histol Histopathol. 2006;21:167–178. doi: 10.14670/HH-21.167. [DOI] [PubMed] [Google Scholar]
- McAndrews JM, Ringstrom SJ, Dahl KD, Schwartz NB. Corticosterone in vivo increases pituitary follicle-stimulating hormone (FSH)-beta messenger ribonucleic acid content and serum FSH bioactivity selectively in female rats. Endocrinology. 1994;134:158–163. doi: 10.1210/endo.134.1.8275929. [DOI] [PubMed] [Google Scholar]
- McCartney CR, Prendergast KA, Blank SK, Helm KD, Chhabra S, Marshall JC. Maturation of luteinizing hormone (gonadotropin-releasing hormone) secretion across puberty: Evidence for altered regulation in obese peripubertal girls. J Clin Endocrinol Metab. 2009;94:56–66. doi: 10.1210/jc.2008-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGillivray SM, Thackray VG, Coss D, Mellon PL. Activin and glucocorticoids synergistically activate follicle-stimulating hormone β-subunit gene expression in the immortalized LβT2 gonadotrope cell line. Endocrinology. 2007;148:762–773. doi: 10.1210/en.2006-0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan RI, Dahl KD, Bremner WJ, Schwall R, Schmelzer CH, Mason AJ, Steiner RA. Recombinant human activin-A stimulates basal FSH and GnRH-stimulated FSH and LH release in the adult male macaque, Macaca fascicularis. Endocrinology. 1989;125:2787–2789. doi: 10.1210/endo-125-5-2787. [DOI] [PubMed] [Google Scholar]
- Miyake T, Irahara M, Shitukawa K, Yasui T, Aono T. Interaction of activin A and gonadal steroids on FSH secretion from primary cultured rat anterior pituitary cells. Biochem Biophys Res Commun. 1993;194:413–419. doi: 10.1006/bbrc.1993.1835. [DOI] [PubMed] [Google Scholar]
- Morales AJ, Laughlin GA, Butzow T, Maheshwari H, Baumann G, Yen SS. Insulin, somatotropic, and luteinizing hormone axes in lean and obese women with polycystic ovary syndrome: common and distinct features. J Clin Endocrinol Metab. 1996;81:2854–2864. doi: 10.1210/jcem.81.8.8768842. [DOI] [PubMed] [Google Scholar]
- Mouillet JF, Sonnenberg-Hirche C, Yan X, Sadovsky Y. p300 regulates the synergy of steroidogenic factor-1 and early growth response-1 in activating luteinizing hormone-beta subunit gene. J Biol Chem. 2004;279:7832–7839. doi: 10.1074/jbc.M312574200. [DOI] [PubMed] [Google Scholar]
- Naik K, Pittman It, Wolfe A, Miller RS, Radovick S, Wondisford FE. A novel technique for temporally regulated cell type-specific Cre expression and recombination in the pituitary gonadotroph. J Mol Endocrinol. 2006;37:63–69. doi: 10.1677/jme.1.02053. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Takio K, Eto Y, Shibai H, Titani K, Sugino H. Activin-binding protein from rat ovary is follistatin. Science. 1990;247:836–838. doi: 10.1126/science.2106159. [DOI] [PubMed] [Google Scholar]
- Naor Z. Signaling by G-protein-coupled receptor (GPCR): studies on the GnRH receptor. Front Neuroendocrinol. 2009;30:10–29. doi: 10.1016/j.yfrne.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Nicol L, Faure MO, McNeilly JR, Fontaine J, Taragnat C, McNeilly AS. Bone morphogenetic protein-4 interacts with activin and GnRH to modulate gonadotrophin secretion in LbetaT2 gonadotrophs. J Endocrinol. 2008;196:497–507. doi: 10.1677/JOE-07-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Conner JL, Wade MF, Prendergast P, Edwards DP, Boonyaratanakornkit V, Mahesh VB. A 361 base pair region of the rat FSH-beta promoter contains multiple progesterone receptor-binding sequences and confers progesterone responsiveness. Mol Cell Endocrinol. 1997;136:67–78. doi: 10.1016/s0303-7207(97)00216-5. [DOI] [PubMed] [Google Scholar]
- Ooi GT, Tawadros N, Escalona RM. Pituitary cell lines and their endocrine applications. Mol Cell Endocrinol. 2004;228:1–21. doi: 10.1016/j.mce.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Ortolano GA, Haisenleder DJ, Dalkin AC, Iliff-Sizemore SA, Landefeld TD, Maurer RA, Marshall JC. Follicle-stimulating hormone beta subunit messenger ribonucleic acid concentrations during the rat estrous cycle. Endocrinology. 1988;123:2946–2948. doi: 10.1210/endo-123-6-2946. [DOI] [PubMed] [Google Scholar]
- Otsuka F, Shimasaki S. A novel function of bone morphogenetic protein-15 in the pituitary: selective synthesis and secretion of FSH by gonadotropes. Endocrinology. 2002;143:4938–4341. doi: 10.1210/en.2002-220929. [DOI] [PubMed] [Google Scholar]
- Papavasiliou SS, Zmeili S, Khoury S, Landefeld TD, Chin WW, Marshall JC. Gonadotropin-releasing hormone differentially regulates expression of the genes for luteinizing hormone alpha and beta subunits in male rats. Proc Natl Acad Sci USA. 1986;83:4026–4029. doi: 10.1073/pnas.83.11.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Cheon M, Kim C, Kim K, Ryu K. Progesterone together with estradiol promotes luteinizing hormone beta-subunit mRNA stability in rat pituitary cells cultured in vitro. Eur J Endocrinol. 1996;134:236–242. doi: 10.1530/eje.0.1340236. [DOI] [PubMed] [Google Scholar]
- Paul SJ, Ortolano GA, Haisenleder DJ, Stewart JM, Shupnik MA, Marshall JC. Gonadotropin subunit messenger RNA concentrations after blockade of gonadotropin-releasing hormone action: testosterone selectively increases follicle-stimulating hormone beta-subunit messenger RNA by posttranscriptional mechanisms. Mol Endocrinol. 1990;4:1943–1955. doi: 10.1210/mend-4-12-1943. [DOI] [PubMed] [Google Scholar]
- Pernasetti F, Vasilyev VV, Rosenberg SB, Bailey JS, Huang HJ, Miller WL, Mellon PL. Cell-specific transcriptional regulation of FSH by activin and GnRH in the L T2 pituitary gonadotrope cell model. Endocrinology. 2001;142:2284–2295. doi: 10.1210/endo.142.6.8185. [DOI] [PubMed] [Google Scholar]
- Pierce JG, Parsons TF. Glycoprotein hormones: structure and function. Ann Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- Qing J, Zhang Y, Derynck R. Structural and functional characterization of the transforming growth factor-beta -induced Smad3/c-Jun transcriptional cooperativity. J Biol Chem. 2000;275:38802–38812. doi: 10.1074/jbc.M004731200. [DOI] [PubMed] [Google Scholar]
- Quirk CC, Lozada KL, Keri RA, Nilson JH. A single Pitx1 binding site is essential for activity of the LHbeta promoter in transgenic mice. Mol Endocrinol. 2001;15:734–746. doi: 10.1210/mend.15.5.0628. [DOI] [PubMed] [Google Scholar]
- Ren B, Robert F, Wyrick JJ, Aparicio O, Jennings EG, Simon I, Zeitlinger J, Schreiber J, Hannett N, Kanin E, Volkert TL, Wilson CJ, Bell SP, Young RA. Genome-wide location and function of DNA binding proteins. Science. 2000;290:2306–2309. doi: 10.1126/science.290.5500.2306. [DOI] [PubMed] [Google Scholar]
- Ringstrom SJ, McAndrews JM, Rahal JO, Schwartz NB. Cortisol in vivo increases FSH beta mRNA selectively in pituitaries of male rats. Endocrinology. 1991;129:2793–2795. doi: 10.1210/endo-129-5-2793. [DOI] [PubMed] [Google Scholar]
- Ringstrom SJ, Szabo M, Kilen SM, Saberi S, Knox KL, Schwartz NB. The antiprogestins RU486 and ZK98299 affect follicle-stimulating hormone secretion differentially on estrus, but not on proestrus. Endocrinology. 1997;138:2286–2290. doi: 10.1210/endo.138.6.5161. [DOI] [PubMed] [Google Scholar]
- Roberson MS, Bliss SP, Xie J, Navratil AM, Farmerie TA, Wolfe MW, Clay CM. Gonadotropin-releasing hormone induction of extracellular-signal regulated kinase is blocked by inhibition of calmodulin. Mol Endocrinol. 2005 doi: 10.1210/me.2005-0094. [DOI] [PubMed] [Google Scholar]
- Roberson MS, Misra-Press A, Laurance ME, Stork PJ, Maurer RA. A role for mitogen-activated protein kinase in mediating activation of the glycoprotein hormone alpha-subunit promoter by gonadotropin-releasing hormone. Mol Cell Biol. 1995;15:3531–3519. doi: 10.1128/mcb.15.7.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts V, Meunier H, Vaughan J, Rivier J, Rivier C, Vale W, Sawchenko P. Production and regulation of inhibin subunits in pituitary gonadotropes. Endocrinology. 1989;124:552–554. doi: 10.1210/endo-124-1-552. [DOI] [PubMed] [Google Scholar]
- Romano GJ, Krust A, Pfaff DW. Expression and estrogen regulation of progesterone receptor mRNA in neurons in the mediobasal hypothalamus: An in situ hybridization study. Mol Endocrinol. 1989;3:1295–1300. doi: 10.1210/mend-3-8-1295. [DOI] [PubMed] [Google Scholar]
- Rosen H, Dalkin A, Haisenleder D, Friberg RD, Ortolano G, Barkan A. Dexamethasone alters responses of pituitary gonadotropin-releasing hormone (GnRH) receptors, gonadotropin subunit messenger ribonucleic acids, and gonadotropins to pulsatile GnRH in male rats. Endocrinology. 1991;128:654–660. doi: 10.1210/endo-128-2-654. [DOI] [PubMed] [Google Scholar]
- Rosenberg SB, Mellon PL. An Otx-related homeodomain protein binds an LH promoter element important for activation during gonadotrope maturation. Mol Endocrinol. 2002;16:1280–1298. doi: 10.1210/mend.16.6.0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safwat N, Ninomiya-Tsuji J, Gore AJ, Miller WL. Transforming growth factor beta activated kinase1 (TAK1) is a key mediator of ovine follicle stimulating hormone beta subunit expression. Endocrinology. 2005;146:4814–4824. doi: 10.1210/en.2005-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury TB, Binder AK, Grammer JC, Nilson JH. Maximal activity of the luteinizing hormone beta-subunit gene requires beta-catenin. Mol Endocrinol. 2007;21:963–971. doi: 10.1210/me.2006-0383. [DOI] [PubMed] [Google Scholar]
- Schreihofer DA, Stoler MH, Shupnik MA. Differential expression and regulation of estrogen receptors (ERs) in rat pituitary and cell lines: estrogen decreases ERalpha protein and estrogen responsiveness. Endocrinology. 2000;141:2174–2184. doi: 10.1210/endo.141.6.7505. [DOI] [PubMed] [Google Scholar]
- Sealfon SC, Weinstein H, Millar RP. Molecular mechanisms of ligand interaction with the gonadotropin-releasing hormone receptor. Endocrine Rev. 1997;18:180–205. doi: 10.1210/edrv.18.2.0295. [DOI] [PubMed] [Google Scholar]
- Seeburg PH, Mason AJ, Stewart TA, Nikolics K. The mammalian GnRH gene and its pivotal role in reproduction. Recent Prog Horm Res. 1987;43:69–98. doi: 10.1016/b978-0-12-571143-2.50008-3. [DOI] [PubMed] [Google Scholar]
- Shendure J, Ji H. Next-generation DNA sequencing. Nat Biotechnol. 2008;26:1135–1145. doi: 10.1038/nbt1486. [DOI] [PubMed] [Google Scholar]
- Shi Y, Wang YF, Jayaraman L, Yang H, Massague J, Pavletich NP. Crystal structure of a Smad MH1 domain bound to DNA: insights on DNA binding in TGF-beta signaling. Cell. 1998;94:585–594. doi: 10.1016/s0092-8674(00)81600-1. [DOI] [PubMed] [Google Scholar]
- Shimasaki S, Koga M, Esch F, Cooksey K, Mercado M, Koba A, Ueno N, Ying SY, Ling N, Guillemin R. Primary structure of the human follistatin precursor and its genomic organization. Proc Natl Acad Sci USA. 1988;85:4218–4222. doi: 10.1073/pnas.85.12.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shupnik MA. Effects of gonadotropin-releasing hormone on rat gonadotropin gene transcription in vitro: Requirement for pulsatile administration for luteInizing hormone-beta gene stimulation. Mol Endocrinol. 1990;4:1444–1450. doi: 10.1210/mend-4-10-1444. [DOI] [PubMed] [Google Scholar]
- Shupnik MA, Fallest PC. Pulsatile GnRH regulation of gonadotropin subunit gene transcription. Neurosci Biobehav Rev. 1994;18:597–599. doi: 10.1016/0149-7634(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Shupnik MA, Gharib SD, Chin WW. Estrogen suppresses rat gonadotropin gene transcription in vivo. Endocrinology. 1988;122:1842–1846. doi: 10.1210/endo-122-5-1842. [DOI] [PubMed] [Google Scholar]
- Shupnik MA, Gharib SD, Chin WW. Divergent effects of estradiol on gonadotropin gene transcription in pituitary fragments. Mol Endocrinol. 1989a;3:474–480. doi: 10.1210/mend-3-3-474. [DOI] [PubMed] [Google Scholar]
- Shupnik MA, Rosenzweig BA. Identification of an estrogen-responsive element in the rat LH beta gene. DNA-estrogen receptor interactions and functional analysis. J Biol Chem. 1991;266:17084–17091. [PubMed] [Google Scholar]
- Shupnik MA, Weinmann CM, Notides AC, Chin WW. An upstream region of the rat luteinizing hormone beta gene binds estrogen receptor and confers estrogen responsiveness. J Biol Chem. 1989b;264:80–86. [PubMed] [Google Scholar]
- Slinker BK. The statistics of synergism. J Mol Cell Cardiol. 1998;30:723–731. doi: 10.1006/jmcc.1998.0655. [DOI] [PubMed] [Google Scholar]
- Song CZ, Tian X, Gelehrter TD. Glucocorticoid receptor inhibits transforming growth factor-beta signaling by directly targeting the transcriptional activation function of Smad3. Proc Natl Acad Sci USA. 1999;96:11776–11781. doi: 10.1073/pnas.96.21.11776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady TJ, Shayya R, Thackray VG, Ehrensberger L, Bailey JS, Mellon PL. Androgen regulates FSH gene expression in an activin-dependent manner in immortalized gonadotropes. Mol Endocrinol. 2004;18:925–940. doi: 10.1210/me.2003-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislaus D, Janovick JA, Brothers S, Conn PM. Regulation of G(q/11)alpha by the gonadotropin-releasing hormone receptor. Mol Endocrinol. 1997;11:738–746. doi: 10.1210/mend.11.6.0005. [DOI] [PubMed] [Google Scholar]
- Stojilkovic SS, Reinhart J, Catt KJ. Gonadotropin-releasing hormone receptors: Structure and signal transduction pathways. Endocrine Rev. 1994;15:462–499. doi: 10.1210/edrv-15-4-462. [DOI] [PubMed] [Google Scholar]
- Stouffer RL, Woodruff TK, Dahl KD, Hess DL, Mather JP, Molskness TA. Human recombinant activin-A alters pituitary luteinizing hormone and follicle-stimulating hormone secretion, follicular development, and steroidogenesis, during the menstrual cycle in rhesus monkeys. J Clin Endocrinol Metab. 1993;77:241–248. doi: 10.1210/jcem.77.1.8325947. [DOI] [PubMed] [Google Scholar]
- Strahl BD, Huang HJ, Pedersen NR, Wu JC, Ghosh BR, Miller WL. Two proximal activating protein-1-binding sites are sufficient to stimulate transcription of the ovine follicle-stimulating hormone-beta gene. Endocrinology. 1997;138:2621–2631. doi: 10.1210/endo.138.6.5205. [DOI] [PubMed] [Google Scholar]
- Strahl BD, Huang HJ, Sebastian J, Ghosh BR, Miller WL. Transcriptional activation of the ovine follicle-stimulating hormone beta-subunit gene by gonadotropin-releasing hormone: involvement of two activating protein-1-binding sites and protein kinase C. Endocrinology. 1998;139:4455–4465. doi: 10.1210/endo.139.11.6281. [DOI] [PubMed] [Google Scholar]
- Su P, Shafiee-Kermani F, Gore AJ, Jia J, Wu JC, Miller WL. Expression and regulation of the beta-subunit of ovine follicle-stimulating hormone relies heavily on a promoter sequence likely to bind Smad-associated proteins. Endocrinology. 2007;148:4500–4508. doi: 10.1210/en.2006-1635. [DOI] [PubMed] [Google Scholar]
- Sundaresan S, Colin IM, Pestell RG, Jameson JL. Stimulation of mitogen-activated protein kinase by gonadotropin-releasing hormone: evidence for the involvement of protein kinase C. Endocrinology. 1996;137:304–311. doi: 10.1210/endo.137.1.8536629. [DOI] [PubMed] [Google Scholar]
- Suszko MI, Balkin DM, Chen Y, Woodruff TK. Smad3 mediates activin-induced transcription of follicle-stimulating hormone beta-subunit gene. Mol Endocrinol. 2005;19:1849–1858. doi: 10.1210/me.2004-0475. [DOI] [PubMed] [Google Scholar]
- Suszko MI, Lo DJ, Suh H, Camper SA, Woodruff TK. Regulation of the rat follicle-stimulating hormone beta-subunit promoter by activin. Mol Endocrinol. 2003;17:318–332. doi: 10.1210/me.2002-0081. [DOI] [PubMed] [Google Scholar]
- Szabo M, Kilen SM, Saberi S, Ringstrom SJ, Schwartz NB. Antiprogestins suppress basal and activin-stimulated follicle-stimulating hormone secretion in an estrogen-dependent manner. Endocrinology. 1998;139:2223–2228. doi: 10.1210/endo.139.5.6015. [DOI] [PubMed] [Google Scholar]
- Thackray VG, Hunnicutt JL, Memon AK, Ghochani Y, Mellon PL. Progesterone inhibits basal and GnRH induction of luteinizing hormone β-subunit gene expression. Endocrinology. 2009;150:2395–2403. doi: 10.1210/en.2008-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackray VG, McGillivray SM, Mellon PL. Androgens, progestins and glucocorticoids induce follicle-stimulating hormone β-subunit gene expression at the level of the gonadotrope. Mol Endocrinol. 2006;20:2062–2079. doi: 10.1210/me.2005-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackray VG, Mellon PL. Synergistic induction of follicle-stimulating hormone beta-subunit gene expression by gonadal steroid hormone receptors and smad proteins. Endocrinology. 2008;149:1091–1102. doi: 10.1210/en.2007-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomic D, Brodie SG, Deng C, Hickey RJ, Babus JK, Malkas LH, Flaws JA. Smad 3 may regulate follicular growth in the mouse ovary. Biol Reprod. 2002;66:917–923. doi: 10.1095/biolreprod66.4.917. [DOI] [PubMed] [Google Scholar]
- Topilko P, Schneider-Maunoury S, Levi G, Trembleau A, Gourdji D, Driancourt MA, Rao CV, Charnay P. Multiple pituitary and ovarian defects in Krox-24 (NGFI-A, Egr-1)-targeted mice. Mol Endocrinol. 1998;12:107–122. doi: 10.1210/mend.12.1.0049. [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Drouin J. Egr-1 is a downstream effector of GnRH and synergizes by direct interaction with Ptx1 and SF-1 to enhance luteinizing hormone gene transcription. Mol Cell Biol. 1999;19:2567–2576. doi: 10.1128/mcb.19.4.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi M, Zhou W, Millar RP, Mellon PL, Roberts JL, Flanagan CA, Dong K, Gillo B, Sealfon SC. Cloning and functional expression of a mouse gonadotropin-releasing hormone receptor. Mol Endocrinol. 1992;6:1163–1169. doi: 10.1210/mend.6.7.1324422. [DOI] [PubMed] [Google Scholar]
- Turgeon JL, Kimura Y, Waring DW, Mellon PL. Steroid and pulsatile gonadotropin-releasing hormone (GnRH) regulation of luteinizing hormone and GnRH receptor in a novel gonadotrope cell line. Mol Endocrinol. 1996;10:439–450. doi: 10.1210/mend.10.4.8721988. [DOI] [PubMed] [Google Scholar]
- Vale W, Rivier C, Brown M. Regulatory peptides of the hypothalamus. Ann Rev Physiol. 1977;39:473–527. doi: 10.1146/annurev.ph.39.030177.002353. [DOI] [PubMed] [Google Scholar]
- Vasilyev VV, Lawson MA, DiPaolo D, Webster NJG, Mellon PL. Different signaling pathways control acute induction versus long-term repression of LH transcription by GnRH. Endocrinology. 2002a;143:3414–1426. doi: 10.1210/en.2001-211215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilyev VV, Pernasetti F, Rosenberg SB, Barsoum MJ, Austin DA, Webster NJG, Mellon PL. Transcriptional activation of the ovine follicle-stimulating hormone- gene by gonadotropin-releasing hormone involves multiple signal transduction pathways. Endocrinology. 2002b;143:1651–1659. doi: 10.1210/endo.143.5.8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh HE, Shupnik MA. Proteasome regulation of dynamic transcription factor occupancy on the GnRH-stimulated luteinizing hormone beta-subunit promoter. Mol Endocrinol. 2009;23:237–250. doi: 10.1210/me.2008-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Fortin J, Lamba P, Bonomi M, Persani L, Roberson MS, Bernard DJ. Activator protein-1 and smad proteins synergistically regulate human follicle-stimulating hormone beta-promoter activity. Endocrinology. 2008;149:5577–5591. doi: 10.1210/en.2008-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster JC, Pedersen NR, Edwards DP, Beck CA, Miller WL. The 5′-flanking region of the ovine follicle-stimulating hormone-beta gene contains six progesterone response elements: three proximal elements are sufficient to increase transcription in the presence of progesterone. Endocrinology. 1995;136:1049–1058. doi: 10.1210/endo.136.3.7867558. [DOI] [PubMed] [Google Scholar]
- Weck J, Anderson AC, Jenkins S, Fallest PC, Shupnik MA. Divergent and composite gonadotropin-releasing hormone-responsive elements in the rat luteinizing hormone subunit genes. Mol Endocrinol. 2000;14:472–485. doi: 10.1210/mend.14.4.0453. [DOI] [PubMed] [Google Scholar]
- Weck J, Fallest PC, Pitt LK, Shupnik MA. Differential gonadotropin-releasing hormone stimulation of rat luteinizing hormone subunit gene transcription by calcium influx and mitogen-activated protein kinase-signaling pathways. Mol Endocrinol. 1998;12:451–457. doi: 10.1210/mend.12.3.0070. [DOI] [PubMed] [Google Scholar]
- Weiss J, Guendner MJ, Halvorson LM, Jameson JL. Transcriptional activation of the follicle-stimulating hormone beta-subunit gene by activin. Endocrinology. 1995;136:1885–1891. doi: 10.1210/endo.136.5.7720634. [DOI] [PubMed] [Google Scholar]
- Wen S, Schwarz JR, Niculescu D, Dinu C, Bauer CK, Hirdes W, Boehm U. Functional characterization of genetically labeled gonadotropes. Endocrinology. 2008;149:2701–2711. doi: 10.1210/en.2007-1502. [DOI] [PubMed] [Google Scholar]
- West BE, Parker GE, Savage JJ, Kiratipranon P, Toomey KS, Beach LR, Colvin SC, Sloop KW, Rhodes SJ. Regulation of the follicle-stimulating hormone beta gene by the LHX3 LIM-homeodomain transcription factor. Endocrinology. 2004;145:4866–4879. doi: 10.1210/en.2004-0598. [DOI] [PubMed] [Google Scholar]
- Wierman ME, Wang C. Androgen selectively stimulates follicle-stimulating hormone-beta mRNA levels after gonadotropin-releasing hormone antagonist administration. Biol Reprod. 1990;42:563–571. doi: 10.1095/biolreprod42.3.563. [DOI] [PubMed] [Google Scholar]
- Winters SJ, Ishizaka K, Kitahara S, Troen P, Attardi B. Effects of testosterone on gonadotropin subunit messenger ribonucleic acids in the presence or absence of gonadotropin-releasing hormone. Endocrinology. 1992;130:726–734. doi: 10.1210/endo.130.2.1370794. [DOI] [PubMed] [Google Scholar]
- Winters SJ, Moore JP. Paracrine control of gonadotrophs. Semin Reprod Med. 2007;25:379–387. doi: 10.1055/s-2007-984744. [DOI] [PubMed] [Google Scholar]
- Woodruff TK, Besecke LM, Groome N, Draper LB, Schwartz NB, Weiss J. Inhibin A and inhibin B are inversely correlated to follicle-stimulating hormone, yet are discordant during the follicular phase of the rat estrous cycle, and inhibin A is expressed in a sexually dimorphic manner. Endocrinology. 1996;137:5463–5467. doi: 10.1210/endo.137.12.8940372. [DOI] [PubMed] [Google Scholar]
- Woodruff TK, Krummen LA, Lyon RJ, Stocks DL, Mather JP. Recombinant human inhibin A and recombinant human activin A regulate pituitary and ovarian function in the adult female rat. Endocrinology. 1993;132:2332–2341. doi: 10.1210/endo.132.6.8504739. [DOI] [PubMed] [Google Scholar]
- Wu JC, Su P, Safwat NW, Sebastian J, Miller WL. Rapid, efficient isolation of murine gonadotropes and their use in revealing control of follicle-stimulating hormone by paracrine pituitary factors. Endocrinology. 2004;145:5832–5839. doi: 10.1210/en.2004-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurmbach E, Yuen T, Ebersole BJ, Sealfon SC. Gonadotropin-releasing hormone receptor-coupled gene network organization. J Biol Chem. 2001;276:47195–47201. doi: 10.1074/jbc.M108716200. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Yamamoto H, Yonehara T, Kanasaki H, Nakanishi H, Miyamoto E, Miyazaki K. Differential activation of the luteinizing hormone beta-subunit promoter by activin and gonadotropin-releasing hormone: a role for the mitogen-activated protein kinase signaling pathway in LbetaT2 gonadotrophs. Biol Reprod. 2004;70:236–243. doi: 10.1095/biolreprod.103.019588. [DOI] [PubMed] [Google Scholar]
- Yokoi T, Ohmichi M, Tasaka K, Kimura A, Kanda Y, Hayakawa J, Tahara M, Hisamoto K, Kurachi H, Murata Y. Activation of the luteinizing hormone beta promoter by gonadotropin- releasing hormone requires c-Jun NH2-terminal protein kinase. J Biol Chem. 2000;275:21639–21647. doi: 10.1074/jbc.M910252199. [DOI] [PubMed] [Google Scholar]
- Yoo RY, Dewan A, Basu R, Newfield R, Gottschalk M, Chang RJ. Increased luteinizing hormone pulse frequency in obese oligomenorrheic girls with no evidence of hyperandrogenism. Fertil Steril. 2006;85:1049–1056. doi: 10.1016/j.fertnstert.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Zain MM, Norman RJ. Impact of obesity on female fertility and fertility treatment. Womens Health (Lond Engl) 2008;4:183–194. doi: 10.2217/17455057.4.2.183. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bailey JS, Coss D, Lin B, Tsutsumi R, Lawson MA, Mellon PL, Webster NJ. Activin modulates the transcriptional response of LβT2 cells to GnRH and alters cellular proliferation. Mol Endocrinol. 2006;20:2909–2930. doi: 10.1210/me.2006-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmeili SM, Papavasiliou SS, Thorner MO, Evans WS, Marshall JC, Landefeld TD. Alpha and luteinizing hormone beta subunit messenger ribonucleic acids during the rat estrous cycle. Endocrinology. 1986;119:1867–1869. doi: 10.1210/endo-119-4-1867. [DOI] [PubMed] [Google Scholar]