Abstract
In this study, we demonstrate that protein kinase C (PKC) activators, including phorbol-12-myristate-13-acetate (PMA), 1,2-dioctanoyl-sn-glycerol (DOG), and platelet-derived growth factor α are potent inducers of angiopoietin-like protein 4 (ANGPTL4) expression in several normal lung cell types and carcinoma cell lines. In human airway smooth muscle (HASM) cells induction of ANGPTL4 expression is observed as early as 2 h after the addition of PMA. PMA also increases the level of ANGPTL4 protein released in the medium. PKC inhibitors Ro31-8820 and Gö6983 greatly inhibit the induction of ANGPTL4 mRNA by PMA suggesting that this up-regulation involves activation of PKC. Knockdown of several PKCs by corresponding siRNAs suggest a role for PKCα. PMA does not activate MAPK p38 and p38 inhibitors have little effect on the induction of ANGPTL4 indicating that p38 is not involved in the regulation of ANGPTL4 by PMA. In contrast, treatment of HASM by PMA induces phosphorylation and activation of Ra, MEK1/2, ERK1/2, JNK, Elk-1, and c-Jun. The Ras inhibitor manumycin A, the MEK1/2 inhibitor U0126, and the JNK inhibitor SP600125, greatly reduce the increase in ANGPTL4 expression by PMA. Knock-down of MEK1/2 and JNK1/2 expression by corresponding siRNAs inhibit the induction of ANGPTL4. Our observations suggest that the induction of ANGPTL4 by PMA in HASM involves the activation of PKC, ERK, and JNK pathways. This induction may play a role in tissue remodeling during lung injury and be implicated in several lung pathologies.
Keywords: ANGPTL4, PKC, smooth muscle cells, tissue remodeling, lung, PMA, MAPK
Introduction
Protein kinase C (PKC) comprises a family of serine/threonine kinases that are involved in the regulation of many cellular responses, including proliferation, apoptosis, differentiation, angiogenesis, stress responses, and lipid metabolism [1–4]. The second messenger diacylglycerol (DAG), which is most commonly generated by cellular phosphatidylinositol 4,5-biphosphate-specific phospholipases, is the endogenous activator of PKC enzymes [5–7]. Phorbol esters mimic the action of DAG. Activation of PKC signaling pathways has been implicated in several lung functions and pathologies, including cancer, asthma, fibrosis, chronic obstructive pulmonary disease (COPD), and interstitial lung diseases [2, 8]. Various growth factors and cyto/chemokines, including platelet-derived growth factor α (PDFGα), with established roles in tissue remodeling and inflammation in the airways, mediate their action at least in part through activation of PKC signaling pathways [9, 10]. In the lung, PKCs can also be activated by broncho- and vasoconstriction, hypoxia, and a variety of environmental exposures, such as cigarette smoke and asbestos [2, 11–13].
Angiopoietin-like protein 4 (ANGPTL4), a member of the angiopoietin-like protein subfamily, is a 50-kD secretory protein that functions as an important modulator of glucose and lipid metabolism [14–19]. ANGPTL4 inhibits lipoprotein lipase (LPL)-dependent lipolysis thereby limiting the uptake of free fatty acids. Overexpression of ANGPTL4 results in hypertriglyceridemia, while ANGPTL4 deficiency suppresses foam formation in macrophages and protects against atherosclerosis [18–20]. In addition, ANGPTL4 has been reported to decrease blood glucose and to improve glucose tolerance [17]. ANGPTL4 expression is up-regulated under a variety of conditions, including caloric restriction, and treatment with glucocorticoids, peroxisome proliferator-activated receptor (PPAR) agonists, and transforming growth factor β (TGFβ) [14, 21–23]. Moreover, expression of ANGPTL4 is dramatically induced under ischemic and hypoxic conditions [24, 25]. ANGPTL4 has been reported to positively as well as negatively modulate cellular migration, invasion, and angiogenesis suggesting that it may have a regulatory function in metastasis and tissue remodeling during injury [23, 26–31].
In this study, we demonstrate that several PKC activators, including PMA and PDGFα, are potent inducers of ANGPTL4 mRNA expression in several cell types of the lung and increase the secretion of ANGPTL4 protein. We provide evidence indicating that this induction is mediated through activation of PKC and the extracellular signal-related kinase (ERK) and Jun N-terminal kinase (JNK) pathways. We propose that induction of ANGPTL4 expression through activation of PKC by endogenous factors and exogenous signals play an important role in the regulation of airway remodeling and lipid homeostasis in several lung cell types, and may be implicated in different pathological processes in the lung, including cancer, asthma, and COPD.
Materials and methods
Cell culture
Normal human primary airway smooth muscle (HASM) cells were isolated and cultured as described previously [32]. Cells were grown in Ham’s F12 containing 10% FBS and 25 mM Hepes (pH 7.4). At confluence, 24 h prior to treatment, cells were switched to medium without serum supplemented with 5.7 μg/ml insulin and 5 μg/ml transferrin. Normal primary human bronchial epithelial (HBE) cells were cultured in BEGM medium (Clonetics, Walkersville, MD). Normal primary human lung fibroblasts (HLF) and murine hepatocellular carcinoma Hepa 1-6 were grown in DMEM plus 10% FBS medium. Normal lung microvascular endothelial cells (MVEC) and human umbilical vein endothelial cells (HUVEC) were purchased from Clonetics and cultured in EGM-2-MV and EGM medium, respectively. Human lung carcinoma A549, Calu6, H441, H460, and H82 cells, and mammary carcinoma MCF7 and T47D, ovary carcinoma OVCAR, and squamous cell carcinoma SCC13 cells were grown in RPMI 1640 plus 10% FBS medium.
Materials
1,2-Dioctanoyl-sn-glycerol (DOG), 4α-phorbol-12,13-didecanoate (PDD), Gö6983, bisindolylmaleimide I (BIM I or GF109203X), Gö6976, bryostatin 1 and 2, PD169316, SB203580, and manumycin A were purchased from Calbiochem (La Jolla, CA). Phorbol-12-myristate-13-acetate (PMA) was purchased from Alexis Biochemicals (San Diego, CA). Ro31-8220, U0126 and actinomycin D were purchased from Sigma (St. Louis, MO), and SP600125 from Biosource (Camarillo, CA). PKC activators and inhibitors were dissolved in dimethylsulfoxide (DMSO). DMSO was used in control experiments. PDGFα and interleukin 1β (IL-1β) were obtained from R&D (Minneapolis, MN).
Northern blot analysis
Total RNA was isolated using TriReagent (Sigma, St. Louis, MO) and examined by Northern blot analysis using 32P-labeled probes for mouse or human ANGPTL4. Total RNA (20 μg) or poly(A)+ RNA (2 μg) was separated by electrophoresis on a formaldehyde 1.2% agarose gel, blotted to a Nytran Plus membrane (Schleicher & Schuell, Keene, NH), and UV-crosslinked. Hybridizations were carried out for 1–2 h at 68 °C using QuikHyb™ reagent (Stratagene, La Jolla, CA), blots were washed twice with 2X SSC, 0.05% SDS for 15 min at room temperature and subsequently with 0.5X SSC, 0.1% SDS at 65 °C for 30 min. Autoradiography was carried out with Hyperfilm-MP (Amersham) at −70 °C using double intensifying screens. For human ANGPTL4, the 600 bp NotI/EcoRI insert excised from the IMAGE clone 490413 was used as probe. For mouse Angptl4, the 255 bp KpnI-SacI fragment of the IMAGE clone 329741 was used as probe. Plasmid inserts were verified by sequence analysis.
Western blot analysis
To determine the level of secreted ANGPTL4, conditioned media collected from cells treated with and without PMA were concentrated 100-fold. Whole cell lysates were prepared in sample buffer (60 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 10 mM DTT, 1 mM phenylmethylsulfonyl fluoride, aprotinin, and leupeptin) and phosphatase inhibitor mixture I and II (Sigma). Proteins were examined by Western blot analysis and visualized by the ECL Western blot analysis system (Amersham) using HRP-conjugated anti-rabbit or anti-mouse IgG. Rabbit anti-ANGPTL4 antibody (1:500) was generously provided by Sander Kersten (Nutrition, Metabolism and Genomics Group, Wageningen University, Wageningen, The Netherlands). Other antibodies were purchased from Cell Signaling Technology (Beverly, MA) and used according to the manufacturer’s instructions: p-Raf-1 (# 9424), p-MEK1/2 and total MEK1/2 (#9121 and 9122), p-ERK1/2 and total ERK (#9101 and 9102), p-SEK/MEK4 (#9151), p-JNK and total JNK (#9251 and 9252), p-c-Jun, (#9162), p-p38 and total p38 (#9211 and 9212). Total Raf-1 (sc-133) and c-Jun (sc-1694) was obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA).
Small interfering RNAs (siRNA) knockdown
Knockdown of PKCα, βI, and δ, MEK1/2 and JNK1/2 expression in HASM cells was achieved by transfection of siRNAs for human PKCα, βI, and δ (sc-36243, sc-39168, sc-36253, respectively), MEK-1 (cat# sc-29396), MEK-2 (sc-35905), JNK-1 (sc-29380), and JNK-2 (sc-39101) (Santa Cruz Biotechnology, Santa Cruz, CA). The silencer-negative control siRNA (cat# 4611) was purchased from Ambion (Austin, TX). Transfection of siRNAs was performed using DharmaFECT 1 transfection reagent (Dharmacon, Chicago, IL). HASM cells were plated in 6-well dishes at a density of 3.3 × 105 cells per well. The next day, cells were treated with the siRNA transfection mixtures following the DharmaFECT General Transfection Protocol. After 48 h incubation, cells were treated with or without PMA. Fifteen min or 6 h later cells were harvested for Western and PCR analysis, respectively.
Quantitative Real-Time PCR (QRT-PCR)
Total RNA was reverse transcribed using an Oligo(dT) primer and MuLV reverse transcriptase according to manufacturer’s instructions (Invitrogen). QRT-PCR analysis of ANGPL4 expression was carried out using a POWER SYBER® Green PCR master mix (Applied Biosystems, Foster City, CA) and the following primers: 5′-GCGAATTCAGCATCTGCAAA (forward) and 5′-CTTGGCCACCTCATGGTCTAG (reverse). QRT-PCR reactions were carried out in triplicate in a 7300 Real Time PCR system (Applied Biosystems). Gene expression level was normalized to 18S RNA. Data were analyzed using Sequence Detection Software (version 1.2.2, Applied Biosystems) and are presented as mean ± SD of three independent experiments.
Results
PMA induces ANGPTL4 mRNA expression in several human lung cell types
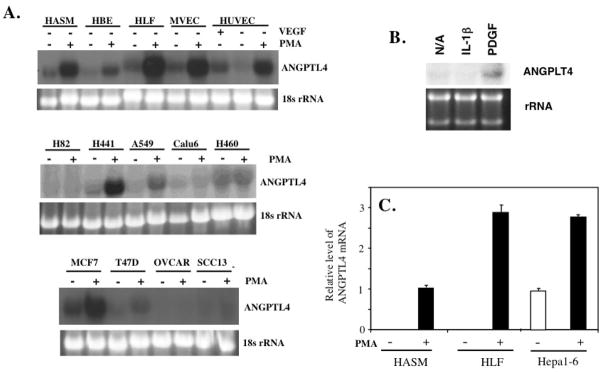
PKC signaling pathways play an important role in the regulation of a number of different functions in the lung and has been implicated in several lung pathologies [2, 6, 8, 33, 34]. In this study, we examined the effect of PMA on the expression of ANGPTL4 in a variety of normal and carcinoma cells. As shown in Fig. 1, ANGPTL4 mRNA expression was dramatically induced in normal HASM, HBE, HLF, MVEC, and HUVEC. Treatment of HASM cells with PDGFα, an activator of PKC, also induced ANGPTL4 mRNA expression whereas addition of IL-1β had no effect (Fig. 1B). MVEC were also treated with VEGF (25 ng/ml for 6 hrs); however, this did not increase ANGPTL4 expression. Quantitation of ANGPTL4 mRNA expression in HASM and HLF cells by QRT-PCR showed that PMA induced ANGPTL4 expression 100-fold or more (Fig. 1C). While several carcinoma cell lines were non-responsive, lung adenocarcinoma cell lines H441 and A549, and mammary carcinoma MCF7 showed a significant induction of ANGPTL4 expression after PMA treatment while a small induction was observed in mammary carcinoma T47D cells (Fig. 1A). PMA also induced Angptl4 expression in murine hepatocellulat carcinoma Hepa 1-6 cells (Fig. 1C). These results demonstrate that PMA is a very effective inducer of ANGPTL4 expression in various normal lung cell types and in a several carcinoma cells.
Fig. 1.
PMA induces ANGPTL4 mRNA expression in a variety of normal and carcinoma cells. (A) Normal human airway smooth muscle (HASM) cells, bronchial epithelial cells (HBE), lung fibroblasts (HLF), microvascular endothelial cells (MVEC), umbilical vein endothelial cells (HUVEC), human lung carcinoma cell lines H82, H441, A549, Calu6, and H460, mammary carcinoma MCF7 and T47D, ovary carcinoma OVCAR, and squamous cell carcinoma SCC13 cells were treated with 30 nM PMA for 6 h. Total RNA was then isolated and examined by Northern blot analysis to determine the level of ANGPTL4 expression. MVEC were also treated with vascular endothelial growth factor (25 ng/ml VEGF for 6 h). (B) ANGPTL4 expression is induced by PDGFα, not by IL-1β. HASM cells were treated with PDGFα or IL-1β (10 ng/ml) for 20 h before cells were collected for Northern blot analysis. (C) QRT-PCR analysis of the induction of ANGPTL4 mRNA by PMA in HASM, HLF, and Hepa 1-6 cells. Cells were treated with 30 nM PMA or vehicle; 6 h later RNA was isolated and ANGPTL4 mRNA expression examined by QRT-PCR.
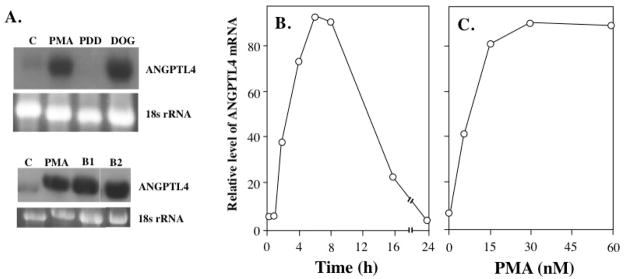
As shown in Fig. 2A, in contrast to PMA, the inactive phorbol ester 4α-phorbol-12,13-didecanoate (PDD) did not enhance ANGPTL4 mRNA expression in HASM cells. Next, we examined the effect of several other agents that bind to and activate members of the PKC and RasGRP families [35, 36] on the induction of ANGPTL4. Similar to PMA, treatment of HASM cells with the PKC activators DOG, bryostatin 1 or 2 also resulted in an up-regulation of ANGPTL4 expression (Fig. 2A).
Fig. 2.
Induction of ANGPTL4 mRNA expression by PMA and other PKC activators. (A) HASM cells were treated with vehicle (DMSO; C), 30 nM PMA, 200 μM 1,2-dioctanoyl-sn-glycerol (DOG), or 30 nM of 4α-phorbol-12,13-didecanoate (PDD), 100 nM bryostatin 1 (B1), or 100 nM bryostatin 2 (B2). After 6 h treatment, RNA was isolated and examined by Northern blot analysis using a radiolabeled probe for ANGPTL4. (B) Time-dependent induction of ANGPTL4 mRNA expression by PMA. HASM cells were treated with 30 nM PMA for the times indicated. (B) Dose-dependent induction of ANGPTL4 mRNA expression by PMA. HASM cells were treated for a 6 h period with PMA at the concentrations indicated. ANGPTL4 mRNA expression was examined by Northern blot analysis. The level of ANGPTL4 mRNA relative to the level of 18S rRNA was calculated and plotted.
Time- and dose-dependent induction of ANGPTL4
As shown in Fig. 2, PMA induced ANGPTL4 mRNA expression in HASM cells in a time- and dose-dependent manner. An increase in ANGPTL4 mRNA expression could be observed as early as 2 h after the addition of PMA and levels peaked after about 6 h (Fig. 2B). Thereafter, expression of ANGPTL4 mRNA gradually diminished and by 24 h returned to basal levels. Induction of ANGPTL4 mRNA expression by PMA was concentration dependent; the calculated EC50 was 8 nM PMA (Fig. 2C).
Induction of ANGPTL4 protein by PMA
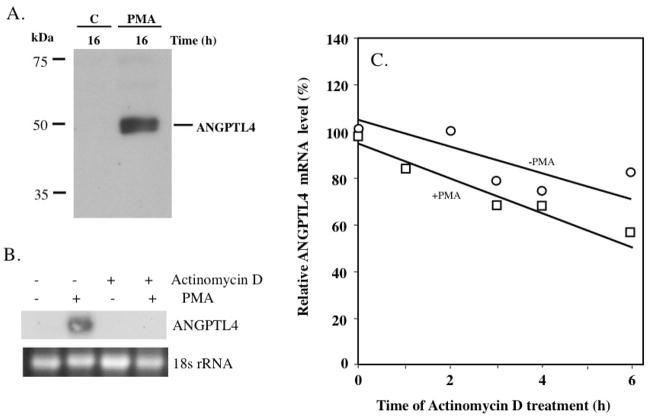
Next, we examined the induction of Angptl4 at the protein level. Western blot analysis with an ANGPTL4-specific antibody recognized a 50 kDa protein in the conditioned medium from HASM cells treated for 16 h with PMA (Fig. 3A). ANGPTL4 was not detectable in conditioned medium from cells treated for 16 h with vehicle. These observations show that the induction of ANGPTL4 mRNA expression by PMA is accompanied by increased synthesis and release of ANGPTL4 protein in the medium. No significant levels of ANGPTL4 were detectable in whole cell protein lysates from either PMA-treated or untreated HASM cells (not shown) suggesting that most of the ANPTL4 is rapidly secreted after being synthesized.
Fig. 3.
(A) PMA causes an increase in secreted ANGPTL4 protein. HASM cells were grown for 24 h in serum-free medium and then treated for 16 h with 30 nM PMA or vehicle (C). Conditioned medium was collected, concentrated, and examined by Western blot analysis using an anti-ANGPTL4 antibody. (B) HASM cells were treated with actinomycin D (2.5 μg/ml) for 30 min prior to the addition of PMA (30 nM) or DMSO and 6 h later, total RNA was isolated and analyzed by Northern Blot analysis for ANGPTL4 expression. (C) Effect of PMA on the stability of ANGPTL4 mRNA. HASM cells were treated with 30 nM PMA or DMSO. After 4 h treatment, actinomycin D (2.5 μg/ml) was added and at the times indicated cells were collected for RNA isolation. Total RNA was isolated from PMA-treated cells and because DMSO-treated cells express very low levels of ANGPTL4, poly(A)+ RNA was isolated from vehicle-treated cells. ANGPTL4 and α-tubulin mRNA expression were examined by Northern Blot analysis. The level of ANGPTL4 mRNA (relative to the level of α-tubulin mRNA) was calculated and the results plotted as the percentage of the RNA level present at time 0 of actinomycin D addition. Data shown is representative of two independent experiments.
Induction of ANGPTL4 mRNA by PMA occurs at the transcriptional level
Phorbol esters can regulate gene expression by transcriptional and post-transcriptional mechanisms. Fig. 3B shows that pretreatment of HASM cells with 2.5 μg/ml actinomycin D blocks the induction of ANGPTL4 mRNA by PMA. To determine whether the induction of ANGPTL4 mRNA was regulated by PMA at the level of ANGPTL4 mRNA stability, HASM cells were treated with PMA or vehicle for 4 h, transcription was then inhibited by the addition of 2.5 μg/ml actinomycin D and at different time intervals the level of ANGPTL4 mRNA was determined by Northern blot analysis. Quantitative analysis of ANGPTL4 mRNA expression, normalized for α-tubulin expression, showed that ANGPTL4 mRNA is quite stable in vehicle-treated HASM cells (Fig. 3C). The calculated half-life of ANGPTL4 mRNA in vehicle- and PMA-treated HASM cells was 9.1 and 7.0 h, respectively. These results indicate that treatment of HASM cells with PMA decreased, rather than increased, the stability of ANGPTL4 mRNA. These data are in agreement with the conclusion that the induction of ANGPTL4 expression by PMA is regulated at the level of transcription.
Induction of ANGPTL4 by PMA by PKC
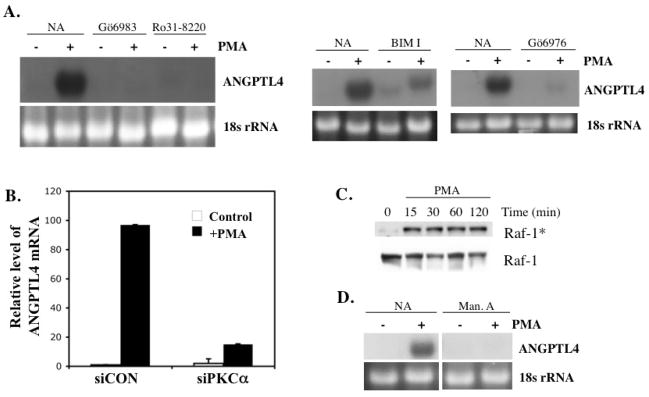
Many of the effects of PMA and DAGs are mediated through the activation of members of the PKC and RasGRP family. We therefore examined the effect of several PKC inhibitors on the induction of ANGPTL4 by PMA. Fig. 4A demonstrates that the PKC inhibitors Ro31-8820 and Gö6983 (selective for PKCα, β, γ, δ, ζ) blocked the induction of ANGPTL4 expression by PMA in HASM cells while Gö6976 (selective inhibitor of PKCα and βI) greatly inhibited its induction. Recent studies have identified Ras guanine nucleotide releasing proteins (RasGRPs), proteins that enhance the dissociation of GDP and favor the association with GTP to Ras, as receptors of phorbol esters and DAGs [35, 37, 38]. Several PKC inhibitors, including Gö6976, also inhibit the activation of RasGRPs. However, Ro31-8820 and BIM I have been reported to inhibit PKC but not RasGRP [37, 39]. Fig. 4A shows that BIM I (selective for PKCα, βI, βII, γ, δ) and Ro31-8820 greatly reduced the PMA-induced increase in ANGPTL4 mRNA. These data suggest that the PMA-induced expression of ANGPTL4 mRNA requires activation of PKC rather than RasGRP.
Fig. 4.
(A) PKC inhibitors block PMA-induced ANGPTL4 expression. HASM cells were pretreated with the PKC inhibitors Gö6983 (10 μM), Ro31-8220 (1 μM), bisindolylmaleimide I (BIM I; 10 μM), and Gö6976 (1 μM) for 30 min prior to the addition of PMA (30 nM). After 6 h of treatment, cells were collected for RNA isolation. RNA was examined by Northern blot analysis using a radiolabeled ANGPTL4 probe. (B) Down regulation of PKCα by corresponding siRNAs inhibit the induction of ANGPTL by PMA. HASM cells were transfected with siPKCα or control (scrambled; siCON) siRNAs for 48 h before they were treated with or without 30 nM PMA. Levels of ANGPTL4 mRNA were determined after 6 h treatment. (C) Role of Ras/Raf activation in the induction of ANGPTL4 mRNA by PMA. HASM cells were treated with 30 nM PMA. At the times indicated cells were collected and protein lysates examined by Western blot analysis using antibodies against total Raf-1 and phosphorylated Raf-1 (Raf*). (D) Effect of manumycin A on ANGPTL4 induction. HASM cells were pretreated for 3 h with or without 3 μM manumycin A (Man. A) prior to the addition of PMA (30 nM). After 6 h of treatment, cells were collected and RNA examined by Northern blot analysis with a radiolabeled probe for ANGPTL4.
Human airway smooth muscle cells have been reported to express several PKC isoforms, PKCα, β, δ, ε, ζ, and ι [40]. To obtain further support for the role of PKC in the induction of ANGPTL4 by PMA, the effect of down-regulation of several specific PKC isoforms by siRNAs on ANGPTL4 induction was examined. Knockdown of PKCα expression (Fig. 4B) significantly reduced ANGPTL4 induction by PMA, while knockdown of PKCβI or PKCδ had no significant effect (not shown). These data support the conclusion that PKCα is at least one of the PKCs involved in the induction of ANGPTL4 by PMA.
Involvement of Ras/Raf/MEK/ERK signaling
Activation of members of the protein kinase C (PKC) family can subsequently lead to activation of a number of other signaling pathways, including Ras, and several mitogen-activated protein kinases (MAPKs) [5, 7, 41–43]. We first investigated the involvement of the Ras/Raf/MEK/ERK cascade in ANGPTL4 induced expression. Fig. 4C shows that Raf was phosphorylated in HASM cells as early as 15 min after the addition of PMA indicating that treatment of HASM with PMA activates Raf. We subsequently examined the role of Ras activation by determining the effect of the Ras inhibitor manumycin A [44] on the induction of ANGPTL4 by PMA. Northern blot analysis shows that manumycin A inhibited the induction of ANGPTL4 expression by PMA (Fig. 4D). These results are in agreement with the hypothesis that PMA activates the Ras/Raf signaling pathway in HASM cells and that its activation is involved in the induction of ANGPTL4 by PMA.
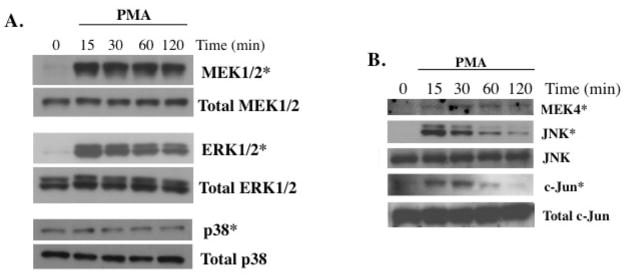
To determine whether activation of MEK and ERK or p38 plays a role in the induction of ANGPTL4 expression by PMA, the phosphorylation of MEK1/2, ERK1/2 and p38 was analyzed at different times after the addition of PMA. As shown in Fig. 5A, PMA induced phosphorylation of both MEK1/2 and ERK1/2 proteins as early as 15 min after the addition, while no changes were observed in total MEK1/2 and ERK1/2 protein levels. In contrast, PMA did not have any effect on the phosphorylation of p38.
Fig. 5.
Activation of the ERK1/2 and JNK pathway in PMA-treated HASM cells. (A) HASM cells were treated with 30 nM PMA and at the indicated times cells were collected for Western blot analysis with antibodies specific for phosphorylated (*) or total MEK1/2, ERK1/2, and p38. (B) HASM cells were treated with 30 nM PMA and at the times indicated cells were collected for Western blot analysis with antibodies specific for phosphorylated (*) MEK4, JNK, and c-Jun and total JNK and c-Jun.
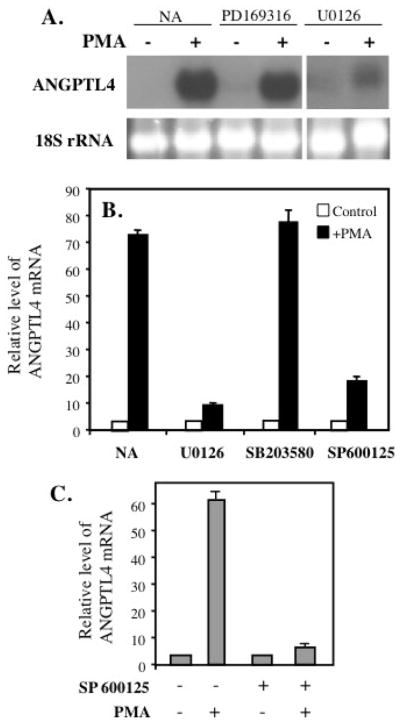
To obtain further insight into the role of these proteins in the induction of ANGPTL4, HASM cells were pre-treated with the MAPK p38 inhibitor PD169316 and the MEK1/2 inhibitor U0126 (Fig. 6A). Treatment with PD169316 had little effect on the induction of ANGPTL4 by PMA. This, in combination with the observation that PMA did not induce activation of p38 in HASM cells, suggested that p38 is not involved in the regulation of ANGPTL4 by PMA. The MEK1/2 inhibitor U0126 almost completely blocked the induction of ANGPTL4 mRNA by PMA. Similar results were obtained by QRT-PCR analysis with samples from HLF cells (Fig. 6B). U0126 significantly reduced the induction of ANGPTL4 mRNA expression in HLF cells while the p38 inhibitor SB203580 had little effect. These data support a role for MEK/ERK1/2 signaling in the induction of ANGPTL4 by PMA.
Fig. 6.
Role of the ERK1/2 and JNK1/2 pathway in PMA-induced expression of ANGPTL4. (A) HASM cells were treated with the p38 inhibitors PD169316 (5 μM) or with the MEK/ERK inhibitor U0126 (10 μM) 30 min before the addition of 30 nM PMA. After 6 h, cells were collected for RNA isolation and examined by Northern blot analysis for ANGPTL4 expression. The results shown are representative of two independent experiments. (B) MEK and JNK inhibitors block PMA-induced ANGPTL4 mRNA expression in HLF cells. Cells were pretreated with MEK inhibitor U0126 (10 μM), p38 inhibitor SB203580 (10 μM), and JNK inhibitor SP600125 (10 μM) for 30 min prior to the addition of PMA (30 nM). After 6 h of treatment, cells were collected for RNA isolation. Expression of ANGPTL4 mRNA was examined by QRT-PCR. (C) HASM cells were pretreated for 30 min with 10 μM of the JNK inhibitor SP600125 prior to the addition of 30 nM PMA. After 6 h incubation, total RNA was isolated and ANGPTL4 expression examined by QRT-PCR analysis.
PMA activates the JNK pathway in HASM
We next examined the role of the Jun-N-terminal kinase (JNK). As shown in Fig. 5B, treatment of HASM cells with PMA caused a rapid and transient phosphorylation of MEK4 and JNK1/2 indicating that PMA activates the JNK signaling pathway in HASM cells. Activation of JNK can subsequently lead to the phosphorylation of c-Jun and other transcription factors [5, 45]. Fig. 5A shows that treatment of HASM cells with PMA transiently induced phosphorylation of c-Jun. Pretreatment of HASM cells with the JNK inhibitor SP600125 blocked the induction of ANGPTL4 mRNA expression by PMA (Fig. 6C). A similar result was obtained with SP600125-treated HLF cells (Fig. 6B). These results are consistent with a role for JNK activation in the induction of ANGPTL4.
Inhibition of PMA-induced ANGPTL4 expression by MEK1/2 and JNK1/2 siRNAs
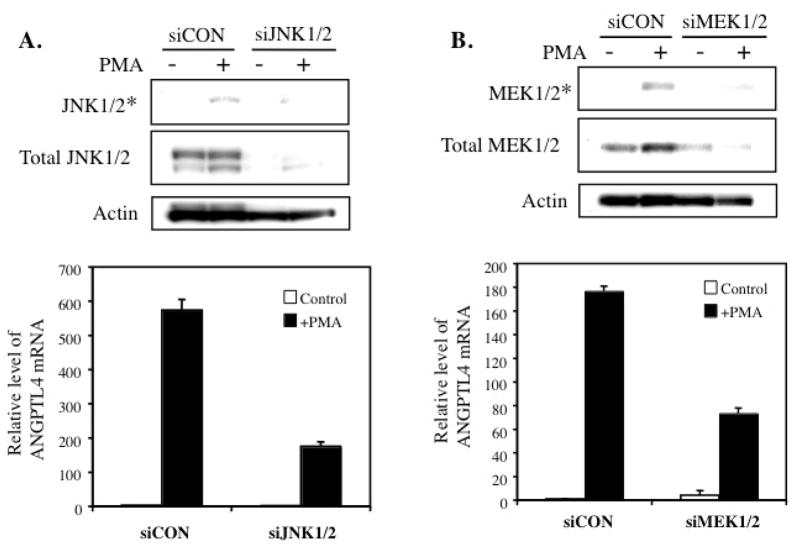
Because SP600125 has been reported to inhibit other kinases, in addition to JNK [46], we investigated the effect of down-regulation of JNK1/2 by corresponding siRNAs on the induction of ANGPTL4 by PMA. As shown in Fig. 7A, transfection with JNK1/2 siRNAs significantly reduced the level of total and phosphorylated JNK1/2 and significantly diminished the induction of ANGPTL4 mRNA expression by PMA. Similarly, transfection with MEK1/2 siRNAs also inhibited the induction of ANGPTL4 mRNA by PMA (Fig. 7B). These data are consistent with a role for the JNK1/2 and MEK1/2/ERK1/2 signaling pathways in PMA-induced ANGPTL4 expression.
Fig. 7.
Knockdown of JNK1/2 or MEK1/2 by corresponding siRNAs inhibits PMA-induced ANGPTL4 mRNA expression. HASM cells were transfected with siJNK1/2 (A) or siMEK1/2 (B), or control (scrambled; siCON) siRNAs for 48 h before they were treated with or without 30 nM PMA. After 15 min of treatment, cells were harvested, protein isolated and examined by Western blot analysis with antibodies specific for phosphorylated (*) or total MEK1/2 and JNK1/2. Actin is shown as a control. RNA was isolated after 6 h of treatment and expression of ANGPTL4 mRNA examined by QRT-PCR analysis (lower panels). Relative expression of ANGPTL4 mRNA was plotted.
Discussion
In this study, we demonstrate that PMA, 1,2-dioctanoyl-sn-glycerol, and bryostatin 1 and 2 are potent inducers of ANGPTL4 expression in human airway smooth muscle cells, in several other normal lung cell types as well as in a number of lung carcinoma cell lines. The induction of ANGPTL4 mRNA is associated with an increase in the level of ANGPTL4 protein in the medium and mediated through activation of PKC, JNK1/2, and MEK1/2/ERK1/2 signaling pathways.
The majority of the actions of phorbol esters and diacylglycerol have been attributed to their interaction with, and activation of, PKC and the subsequent activation of several MAPK pathways [5, 41–43]. However, several studies have demonstrated that PMA, DAG, and bryostatins can also bind RasGRP proteins [35, 37, 38]. Interaction of PMA with RasGRP promotes the binding of GTP to Ras, resulting in its activation and subsequently that of MAPKs. Therefore, the induction of ANGPTL4 by PMA could be mediated through the activation of PKC and/or RasGRP. Our results show that several PKC inhibitors were very effective in inhibiting the induction of ANGPTL4 expression by PMA. The PKC inhibitor Gö6976 has been reported to also inhibit the activation of RasGRPs whereas Ro31-8820 and BIM I selectively inhibit PKC but do not inhibit RasGRP-mediated signaling [37, 39]. Because both Ro31-8820 and BIM I inhibit the induction of ANGPTL4 by PMA, the increased expression of ANGPTL4 appears to be mediated through the activation of PKC rather than RasGRP. This is supported by data showing that down-regulation of of the expression of PKCα, but not of PKCβ or PKCδ, by corresponding siRNAs significantly inhibits the induction of ANGPTL4 by PMA. PDGFα, which plays an important role in several lung diseases, including fibrosis, was also found to induce ANPTL4 expression. Because PDGF has been reported to activate PKC [9, 47], this induction might also be mediated through PKC activation.
Activation of PKC by PMA, DAGs, and bryostatins can subsequently lead to activation of the Ras/Raf, p38, ERK, and JNK pathways [5, 41–43, 48]. Our results indicate that treatment of HASM with PMA induces activation of the Ras/Raf pathway while the inhibition of Ras by manumycin A [44] suggests that activation of Ras is essential for the induction of ANGPTL4 by PMA. This activation might be responsible for the observed activation of MEK1/2 and subsequently the activation of ERK1/2. The MEK1/2 inhibitor U0126 greatly inhibits the increase in ANGPTL4 expression by PMA indicating that activation of ERK1/2 is essential for this induction. This was supported by the decrease in the induction of ANGPTL4 observed after down-regulation of MEK1/2 expression by corresponding siRNAs.
We further demonstrated in HASM cells that PMA treatment induces phosphorylation of MEK4 and JNK1/2. The activation of JNK1 is likely responsible for the observed phosphorylation of c-Jun, a target for JNKs. The JNK inhibitor SP600125 was able to inhibit PMA-induced expression of ANGPTL4. These results suggest that activation of the JNK pathway is required for the induction of ANGPTL4 expression by PMA. This was supported by the decrease in the induction of ANGPTL4 observed after knockdown of JNK1/2 expression by siRNAs. Since both inhibitors of the JNK and ERK signaling pathway inhibit the induction of ANGPTL4 expression, both pathways appear be required for the transcriptional activation of the ANGPTL4 gene by PMA. A role for p38 in PMA-induced ANGPTL4 expression was excluded because PMA did not induce phosphorylation of p38 in HASM cells nor did pretreatment with p38-specific inhibitors block the increase in ANGPTL4 expression.
An increase in ANGPTL4 expression was observed as early as 2 h after the addition of PMA. PMA has been reported to regulate gene expression by transcriptional as well as post-transcriptional mechanisms. For example, the induction of VEGF by PMA in human keratinocytes depends on an AP-1 binding site in its promoter flanking region of the VEGF gene [49] while it regulates the expression of mitochondrial elongation factor Tu in HL60 cells at the level of RNA stability [50]. Our results show that PMA does not induce ANGPTL4 expression by increasing the stability of its respective mRNA. These observations suggest that the induction of ANGPTL4 expression occurs at the level of transcription. Because both the ERK1/2 and JNK signaling pathways appear to be required for the induction of ANGPTL4 by PMA, we examined the 3 kb ANGPTL4 promoter region for the presence of potential binding sites for several transcription factors, such as AP-1, Elk-1, and ets, that act downstream of these MAPKs. Although the 3 kb ANGPTL4 promoter flanking region contained several potential AP-1 and ets binding sites, PMA did not cause transcriptional activation of the Luc reporter under the control of different fragments of this 3 kb region of the ANGPTL4 gene (Stapleton, C., unpublished observations). These results suggest that either the response element does not reside in this region or requires an additional element not included in this promoter region.
In addition to its function in the regulation of glucose homeostasis and lipid metabolism [14–19], studies have demonstrated a role for ANGPTL4 in migration, metastasis, vascular permeability, and angiogenesis [23, 26–31, 51]. Migration, vascular permeability, and angiogenesis are an integral part of lung injury and airway remodeling associated with many lung pathologies [52–54]. This involves cross-talk between a variety of cell types, including (myo)fibroblasts, epithelial, smooth muscle, inflammatory, and endothelial cells. In lung, PKCs are activated by PDGFα, hypoxia, broncho- and vasoconstriction, and a variety of environmental stimuli, including cigarette smoke, and have been implicated in cancer, asthma, fibrosis, COPD, pulmonary hypertension, and interstitial lung diseases [2, 3, 8, 11–13, 55, 56]. The PKC-dependent induction of ANGPTL4 in the several lung cell types described in this study may be part of the response to hypoxia, various environmental exposures, and induction of endogenous factors, such as PDGFα, that activate PKCs. Therefore, ANGPTL4 might have important functions in airway remodeling and lung disease.
In summary, our results demonstrate that activation of PKC by PMA and several other PKC activators leads to increased expression of ANGPTL4. Although we focused in this study largely on airway smooth muscle cells, we show that PMA induces ANGPTL4 in a number of normal and tumor lung cell types. The induction of ANGPTL4 in HASM cells involves activation of the Ras, JNK, and MEK1/2/ERK1/2 pathways. In the lung, ANGPTL4 may play a role in tissue remodeling and lipid metabolism, and be implicated in several lung diseases, including asthma, cancer, and COPD, as well as in hypoxia-induced responses in the lung.
Acknowledgments
This research was supported by the Intramural Research Program of the NIEHS, NIH (Z01-ES-101586). Would like to thank Dr. Xiao-Ping Yang for technical assistance.
Abbreviations
- ANGPTL4
angiopoietin-like 4
- HASM
human airway smooth muscle
- PKC
protein kinase C
- PMA
phorbol-12-myristate-13-acetate
- DAG
diacylglycerol
- PDGFα
platelet-derived growth factor α
- ERK
extracellular signal-related kinase
- JNK
Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- siRNA
small interfering RNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barnett ME, Madgwick DK, Takemoto DJ. Protein kinase C as a stress sensor. Cell Sign. 2007;19:1820–1829. doi: 10.1016/j.cellsig.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dempsey EC, Cool CD, Littler CM. Lung disease and PKCs. Pharmacol Res. 2007;55:545–559. doi: 10.1016/j.phrs.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Mackay HJ, Twelves CJ. Targeting the protein kinase C family: are we there yet? Nat Rev Cancer. 2007;7:554–562. doi: 10.1038/nrc2168. [DOI] [PubMed] [Google Scholar]
- 4.Carmen GY, Victor SM. Signalling mechanisms regulating lipolysis. Cell Signal. 2006;18:401–408. doi: 10.1016/j.cellsig.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Brändlin I, Hübner S, Eiseler T, Martinez-Moya M, Horschinek A, Hausser A, Link G, Rupp S, Storz P, Pfizenmaier K, Johannes FJ. Protein Kinase C (PKC)η-mediated PKCμ activation modulates ERK and JNK signal pathways. J Biol Chem. 2002;277:6490–6496. doi: 10.1074/jbc.M106083200. [DOI] [PubMed] [Google Scholar]
- 6.Ventura C, Maioli M. Protein kinase C control of gene expression. Crit Rev Eukar Gene Expr. 2001;11:243–267. [PubMed] [Google Scholar]
- 7.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward JP, Knock GA, Snetkov VA, Aaronson PI. Protein kinases in vascular smooth muscle tone--role in the pulmonary vasculature and hypoxic pulmonary vasoconstriction. Pharmacol Ther. 2004;104:207–231. doi: 10.1016/j.pharmthera.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Ginnan R, Singer HA. PKC-delta-dependent pathways contribute to PDGF-stimulated ERK1/2 activation in vascular smooth muscle. Am J Physiol Cell Physiol. 2005;288:C1193–1201. doi: 10.1152/ajpcell.00499.2004. [DOI] [PubMed] [Google Scholar]
- 10.Gopalakrishna R, Chen ZH, Gundimeda U. Tobacco smoke tumor promoters, catechol and hydroquinone, induce oxidative regulation of protein kinase C and influence invasion and metastasis of lung carcinoma cells. Proc Natl Acad Sci U S A. 1994;91:12233–12237. doi: 10.1073/pnas.91.25.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shukla A, Stern M, Lounsbury KM, Flanders T, Mossman BT. Asbestos-induced apoptosis is protein kinase C delta-dependent. Am J Respir Cell Mol Biol. 2003;29:198–205. doi: 10.1165/rcmb.2002-0248OC. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Liu HW, McNeill KD, Stelmack G, Scott JE, Halayko AJ. Mechanical strain inhibits airway smooth muscle gene transcription via protein kinase C signaling. Am J Respir Cell Mol Biol. 2004;31:54–61. doi: 10.1165/rcmb.2003-0240OC. [DOI] [PubMed] [Google Scholar]
- 13.Elliott MK, Sisson JH, Wyatt TA. Effects of cigarette smoke and alcohol on ciliated tracheal epithelium and inflammatory cell recruitment. Am J Respir Cell Mol Biol. 2007;36:452–459. doi: 10.1165/rcmb.2005-0440OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kersten S. Regulation of lipid metabolism via angiopoietin-like proteins. Biochem Soc Trans. 2005;33:1059–1062. doi: 10.1042/BST20051059. [DOI] [PubMed] [Google Scholar]
- 15.Oike Y, Akao M, Kubota Y, Suda T. Angiopoietin-like proteins: potential new targets for metabolic syndrome therapy. Trends Mol Med. 2005;11:473–479. doi: 10.1016/j.molmed.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Yin W, Romeo S, Chang S, Grishin NV, Hobbs HH, Cohen JC. Genetic variation in ANGPTL4 provides insights into protein processing and function. J Biol Chem. 2009;284:13213–13222. doi: 10.1074/jbc.M900553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu A, Lam MC, Chan KW, Wang Y, Zhang J, Hoo RL, Xu JY, Chen B, Chow WS, Tso AW, Lam KS. Angiopoietin-like protein 4 decreases blood glucose and improves glucose tolerance but induces hyperlipidemia and hepatic steatosis in mice. Proc Natl Acad Sci U S A. 2005;102:6086–6091. doi: 10.1073/pnas.0408452102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desai U, Lee EC, Chung K, Gao C, Gay J, Key B, Hansen G, Machajewski D, Platt KA, Sands AT, Schneider M, Van Sligtenhorst I, Suwanichkul A, Vogel P, Wilganowski N, Wingert J, Zambrowicz BP, Landes G, Powell DR. Lipid-lowering effects of anti-angiopoietin-like 4 antibody recapitulate the lipid phenotype found in angiopoietin-like 4 knockout mice. Proc Natl Acad Sci U S A. 2007;104:11766–11771. doi: 10.1073/pnas.0705041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koster A, Chao YB, Mosior M, Ford A, Gonzalez-DeWhitt PA, Hale JE, Li D, Qiu Y, Fraser CC, Yang DD, Heuer JG, Jaskunas SR, Eacho P. Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. Endocrinology. 2005;146:4943–4950. doi: 10.1210/en.2005-0476. [DOI] [PubMed] [Google Scholar]
- 20.Adachi H, Fujiwara Y, Kondo T, Nishikawa T, Ogawa R, Matsumura T, Ishii N, Nagai R, Miyata K, Tabata M, Motoshima H, Furukawa N, Tsuruzoe K, Kawashima J, Takeya M, Yamashita S, Koh GY, Nagy A, Suda T, Oike Y, Araki E. Angptl 4 deficiency improves lipid metabolism, suppresses foam cell formation and protects against atherosclerosis. Biochem Biophys Res Commun. 2009;379:806–811. doi: 10.1016/j.bbrc.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 21.Mandard S, Zandenbergen F, Tan NS, Escher P, Patsouris D, Koenig W, Kleeman R, Bakker A, Veenman F, Wahli W, Müller M, Kersten S. The direct PPAR target FIAF/PGAR/ANGPTL4 is present in blood plasma as a truncated protein that is increased by fenofibrate treatment. J Biol Chem. 2004;279:34411–34420. doi: 10.1074/jbc.M403058200. [DOI] [PubMed] [Google Scholar]
- 22.Koliwad SK, Kuo T, Shipp LE, Gray NE, Backhed F, So AY, Farese RV, Jr, Wang JC. Angiopoietin-like 4 (ANGPTL4, fasting-induced adipose factor) is a direct glucocorticoid receptor target and participates in glucocorticoid-regulated triglyceride metabolism. J Biol Chem. 2009;284:25593–25601. doi: 10.1074/jbc.M109.025452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, Massague J. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belanger AJ, Lu H, Date T, Liu LX, Vincent KA, Akita G, Cheng SH, Gregory RJ, Jiang C. Hypoxia up-regulates expression of peroxisome proliferator-activated receptor γ angiopoietin-related gene (PGAR) in cardiomyocytes: role of hypoxia inducible factor 1α. J Mol Cell Cardiol. 2002;34:765–774. doi: 10.1006/jmcc.2002.2021. [DOI] [PubMed] [Google Scholar]
- 25.Le Jan S, Amy C, Cazes A, Monnot C, Lamandé N, Favier J, Philippe J, Sibony M, Gasc JM, Corvol P, Germain S. Angiopoietin-like 4 is a proangiogenic factor produced during ischemia and in conventional renal cell carcinoma. Am J Pathol. 2003;162:1521–1528. doi: 10.1016/S0002-9440(10)64285-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galaup A, Cazes A, Le Jan S, Philippe J, Connault E, Le Coz E, Mekid H, Mir LM, Opolon P, Corvol P, Monnot C, Germain S. Angiopoietin-like 4 prevents metastasis through inhibition of vascular permeability and tumor cell motility and invasiveness. Proc Natl Acad Sci U S A. 2006;103:18721–18726. doi: 10.1073/pnas.0609025103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cazes A, Galaup A, Chomel C, Bignon M, Brechot N, Le Jan S, Weber H, Corvol P, Muller L, Germain S, Monnot C. Extracellular matrix-bound angiopoietin-like 4 inhibits endothelial cell adhesion, migration, and sprouting and alters actin cytoskeleton. Circ Res. 2006;99:1207–1215. doi: 10.1161/01.RES.0000250758.63358.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang YH, Wang Y, Lam KS, Yau MH, Cheng KK, Zhang J, Zhu W, Wu D, Xu A. Suppression of the Raf/MEK/ERK signaling cascade and inhibition of angiogenesis by the carboxyl terminus of angiopoietin-like protein 4. Arterioscler Thromb Vasc Biol. 2008;28:835–840. doi: 10.1161/ATVBAHA.107.157776. [DOI] [PubMed] [Google Scholar]
- 29.Stull RA, Tavassoli R, Kennedy S, Osborn S, Harte R, Lu Y, Napier C, Abo A, Chin DJ. Expression analysis of secreted and cell surface genes of five transformed human cell lines and derivative xenograft tumors. BMC Genomics. 2005;6:55. doi: 10.1186/1471-2164-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ifon ET, Pang AL, Johnson W, Cashman K, Zimmerman S, Muralidhar S, Chan WY, Casey J, Rosenthal LJ. U94 alters FN1 and ANGPTL4 gene expression and inhibits tumorigenesis of prostate cancer cell line PC3. Cancer Cell Int. 2005;5:19. doi: 10.1186/1475-2867-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gealekman O, Burkart A, Chouinard M, Nicoloro SM, Straubhaar J, Corvera S. Enhanced angiogenesis in obesity and in response to PPARgamma activators through adipocyte VEGF and ANGPTL4 production. Am J Physiol Endocrinol Metab. 2008;295:E1056–1064. doi: 10.1152/ajpendo.90345.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shore SA, Laporte J, Hall IP, Hardy E, PRA Effect of IL-1 beta on responses of cultured human airway smooth muscle cells to bronchodilator agonists. Am J Respir Cell Mol Biol. 1997;16:702–712. doi: 10.1165/ajrcmb.16.6.9191472. [DOI] [PubMed] [Google Scholar]
- 33.Kazanietz MG, Caloca MJ, Eroles P, Fujii T, Garcia-Bermejo ML, Reilly M, Wang H. Pharmacology of the receptors for the phorbol ester tumor promoters: multiple receptors with different biochemical properties. Biochem Pharmacol. 2000;60:1417–1424. doi: 10.1016/s0006-2952(00)00470-6. [DOI] [PubMed] [Google Scholar]
- 34.Chaudhary D, Kasaian M. PKCtheta: A potential therapeutic target for T-cell-mediated diseases. Curr Opin Investig Drugs. 2006;7:432–437. [PubMed] [Google Scholar]
- 35.Kazanietz MG. Eyes wide shut: protein kinase C isozymes are not the only receptors for the phorbol ester tumor promoters. Mol Carcinog. 2000;28:5–11. doi: 10.1002/(sici)1098-2744(200005)28:1<5::aid-mc2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 36.Lorenzo PS, Beheshti M, Pettit GR, Stone JC, Blumberg PM. The guanine nucleotide exchange factor RasGRP is a high -affinity target for diacylglycerol and phorbol esters. Mol Pharmacol. 2000;57:840–846. [PubMed] [Google Scholar]
- 37.Lorenzo PS, Kung JW, Bottorff DA, Garfield SH, Stone JC, Blumberg PM. Phorbol esters modulate the Ras exchange factor RasGRP3. Cancer Res. 2001;61:943–949. [PubMed] [Google Scholar]
- 38.Brose N, Rosenmund C. Move over protein kinase C, you’ve got company: alternative cellular effectors of diacylglycerol and phorbol esters. J Cell Sci. 2002;115:4399–4411. doi: 10.1242/jcs.00122. [DOI] [PubMed] [Google Scholar]
- 39.Teixeira C, Stang SL, Zheng Y, Beswick NS, Stone JC. Integration of DAG signaling systems mediated by PKC-dependent phosphorylation of RasGRP3. Blood. 2003;102:1414–1420. doi: 10.1182/blood-2002-11-3621. [DOI] [PubMed] [Google Scholar]
- 40.Sakai H, Yamamoto M, Kozutsumi Y, Chiba Y, Misawa M. Identification of PKC isoforms expressed in human bronchial smooth muscle cell. J Smooth Muscle Res. 2009;45:55–62. doi: 10.1540/jsmr.45.55. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Wang Q, Hu W, Evers BM. Regulation of phorbol ester-mediated TRAF-1 induction in human colon cancer cells through PKC/RAF/ERK/NF-κB -dependent pathways. Oncogene. 2004;23:1885–1895. doi: 10.1038/sj.onc.1207312. [DOI] [PubMed] [Google Scholar]
- 42.Kast C, Wang M, Whiteway M. The ERK/MAPK pathway regulates the activity of the human tissue factor pathway inhibitor-2 promoter. J Biol Chem. 2003;278:6787–6794. doi: 10.1074/jbc.M210935200. [DOI] [PubMed] [Google Scholar]
- 43.Kolch W, Heidecker G, Kochs G, Hummel R, Vahidi H, Mischak H, Finkenzeller G, Marme D, Rapp UR. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993;364:249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- 44.Zhou JM, Zhu XF, Pan QC, Liao DF, Li ZM, Liu ZC. Manumycin inhibits cell proliferation and the Ras signal transduction pathway in human hepatocellular carcinoma cells. Int J Mol Med. 2003;11:767–771. [PubMed] [Google Scholar]
- 45.Schaeffer HJ, Catling AD, Eblen ST, Collier LS, Krauss A, Weber MJ. MP1: A MEK binding partner that enhances enzymatic activation of the MAP kinase cascade. Science. 1998;281:1668–1671. doi: 10.1126/science.281.5383.1668. [DOI] [PubMed] [Google Scholar]
- 46.Bain J, McLauchlan H, Elliot M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J. 2003;371:199–201. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robin P, Boulven I, Bole-Feysot C, Tanfin Z, Leiber D. Contribution of PKC-dependent and -independent processes in temporal ERK regulation by ET-1, PDGF, and EGF in rat myometrial cells. Am J Physiol Cell Physiol. 2004;286:C798–806. doi: 10.1152/ajpcell.00465.2003. [DOI] [PubMed] [Google Scholar]
- 48.Kribben A, Wieder ED, Li X, Van Putten V, Granot Y, Schrider RW, Nemenoff RA. AVP-induced activation of MAP kinase in vascular smooth muscle cells is mediated through protein kinase C. Am J Physiol. 1993;265:C939–345. doi: 10.1152/ajpcell.1993.265.4.C939. [DOI] [PubMed] [Google Scholar]
- 49.Diaz BV, Lenoir MC, Ladoux A, Frelin C, Démarchez M, Michel S. Regulation of vascular endothelial growth factor expression in human keratinocytes by retinoids. J Biol Chem. 2000;275:642–650. doi: 10.1074/jbc.275.1.642. [DOI] [PubMed] [Google Scholar]
- 50.Takeuchi N, Ueda T. Down-regulation of the mitochondrial translation system during terminal differentiation of HL-60 cells by 12-O-tetradecanoyl-1-phorbol-13-acetate: comparison with the cytoplasmic translation system. J Biol Chem. 2003;278:45318–45324. doi: 10.1074/jbc.M307620200. [DOI] [PubMed] [Google Scholar]
- 51.Hermann LM, Pinkerton M, Jennings K, Yang L, Grom A, Sowders D, Kersten S, Witte DP, Hirsch R, Thornton S. Angiopoietin-like-4 is a potential angiogenic mediator in arthritis. Clin Immunol. 2005;115:93–101. doi: 10.1016/j.clim.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 52.Wilson J. Angiogenesis and increased airway vascularity in airway diseases. Arch Physiol Biochem. 2003;111:361–363. doi: 10.3109/13813450312331337603. [DOI] [PubMed] [Google Scholar]
- 53.McDonald DM. Angiogenesis and remodeling of airway vasculature in chronic inflammation. Am J Resp Crit Care Med. 2001;164:S39–45. doi: 10.1164/ajrccm.164.supplement_2.2106065. [DOI] [PubMed] [Google Scholar]
- 54.Howell K, Preston RJ, McLoughlin P. Chronic hypoxia causes angiogenesis in addition to remodelling in the adult rat pulmonary circulation. J Physiol. 2003;547:133–145. doi: 10.1113/jphysiol.2002.030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park JW, Kim HP, Lee SJ, Wang X, Wang Y, Ifedigbo E, Watkins SC, Ohba M, Ryter SW, Vyas YM, Choi AM. Protein kinase C alpha and zeta differentially regulate death-inducing signaling complex formation in cigarette smoke extract-induced apoptosis. J Immunol. 2008;180:4668–4678. doi: 10.4049/jimmunol.180.7.4668. [DOI] [PubMed] [Google Scholar]
- 56.Siflinger-Birnboim A, Johnson A. Protein kinase C modulates pulmonary endothelial permeability: a paradigm for acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2003;284:L435–451. doi: 10.1152/ajplung.00106.2002. [DOI] [PubMed] [Google Scholar]