Abstract
Alcohol consumption during pregnancy can lead to a variety of cognitive and other birth defects, collectively termed fetal alcohol spectrum disorders (FASD), which includes the Fetal Alcohol Syndrome (FAS). This study examined the impact of gestational alcohol exposure on the morphology of the cingulate gyrus, given this region’s role in cognitive control, attention, and emotional regulation, all of which are affected in children with FASD. Thirty-one youth (ages 8–16) with histories of heavy prenatal alcohol exposure (n = 21) and demographically-matched comparison subjects (n = 10) underwent structural magnetic resonance imaging. The cingulate gyrus was manually delineated, and parcellated volumes of grey and white matter were compared across groups. Alcohol-exposed individuals had significantly smaller raw cingulate grey matter, white matter, and tissue volumes, compared to controls. After adjusting for respective cranial tissue constituents, only white matter volumes remained significantly reduced, and this held regardless of whether or not the child qualified for a diagnosis of FAS. A correlation between posterior cingulate grey matter volume and the WISC-III Freedom from Distractibility Index was also observed in alcohol-exposed children. These data suggest that cingulate white matter is compromised beyond global white matter hypoplasia in alcohol-exposed individuals, regardless of FAS diagnosis. The observed volumetric reductions in the cingulate gyrus may contribute to the disruptive and emotionally dysregulated behavioral profile commonly observed in this population.
Keywords: Prenatal alcohol exposure, Fetal alcohol syndrome, MRI, White matter, Cognitive control, Attention deficits
1. Introduction
Fetal Alcohol Syndrome (FAS) is a developmental disorder characterized by physical growth retardation, facial dysmorphology, and central nervous system dysfunction (Lemoine et al., 1968; Jones and Smith, 1973). The syndrome occurs in roughly 0.5 to 2.0 live births per 1000 (May and Gossage, 2001), however, many alcohol-exposed individuals exhibit cognitive and behavioral abnormalities without the characteristic facial features or growth deficiency required for a diagnosis of FAS (Mattson et al., 1998). In recognition of this phenomenon, the non-diagnostic term fetal alcohol spectrum disorders (FASD) is now used to describe the range of effects that are associated with gestational alcohol exposure (Bertrand et al., 2005). Individuals with FASD present with a variable profile of neuropsychological deficits, which may include impaired general intelligence, attention, learning and memory, psychomotor and visuospatial abilities, and adaptive and executive functioning (Mattson and Riley, 1998; Streissguth et al., 2004; Kodituwakku, 2007).
The cingulate gyrus is of interest to the investigation of brain-behavior relationships relating to prenatal alcohol exposure, in part, because of the region’s putative role in mediating attention and cognitive control. The anterior cingulate has been associated with executive attention (Posner and Rothbart, 1998), conflict monitoring and decision making (Botvinick, 2007). Attention deficits are a hallmark feature of FASD, and attention-deficit/hyperactivity disorder is frequently diagnosed in this population (Steinhausen and Spohr, 1998; Bhatara et al., 2006; Fryer et al., 2007a). Accordingly, previous research has revealed deficits in frontal lobe functions in individuals with prenatal alcohol exposure including impaired cognitive flexibility, planning, and inhibition (for review, see Rasmussen, 2005). Impairment in executive functions such as these has been linked to poor social skills in children with FASD (Schonfeld et al., 2006), and the functional role of the anterior cingulate in governing aspects of social cognition and reward-based learning is increasingly appreciated (Rushworth et al., 2007).
In addition to being involved in cognitive control and attention, the cingulate gyrus is well connected with the limbic system, and both anterior (Devinsky et al., 1995) and posterior (Maddock, 1999) regions of the gyrus have been associated with affective processing. Children with FASD commonly show emotional dysregulation. Increased rates of psychopathology, including disruptive behavioral and depressive disorders, have been identified in children prenatally exposed to alcohol (Fryer et al., 2007a) as well as increased negative affect, attachment insecurity (O’Connor et al., 2002), and depressive symptoms (O’Connor and Kasari, 2000). Damage to the cingulate may contribute to dysregulated affect and behavior commonly observed in individuals with FASD.
Prenatal alcohol exposure is associated with alterations of several brain regions in addition to global cranial hypoplasia. Specifically, neuroimaging studies have identified volumetric reductions and shape abnormalities in the corpus callosum and regions of the cerebellum, as well as reduced basal ganglia volume (for review, see Guerri et al., 2009). Though the cingulate has been relatively unexamined in neuroimaging studies of individuals with prenatal alcohol exposure histories, several regions that share functional and anatomical connections to the cingulate have been implicated in the literature on FASD. Structural magnetic resonance imaging (MRI) has revealed volumetric reductions of frontal and especially parietal lobes (Archibald et al., 2001), along with shape alterations in orbitofrontal and inferior parietal cortical regions (Sowell et al., 2002). Metabolic abnormalities, driven by increased absolute intensity of glial markers creatine and choline, have also been noted in several brain regions, including anterior cingulate, lateral parietal cortices, and frontal white matter (Fagerlund et al., 2006). Furthermore, alcohol-exposed individuals have shown altered patterns of functional activation in prefrontal regions including areas of the middle frontal gyrus, a region found to function in concert with the anterior cingulate (Koski and Paus, 2000), during measures of response inhibition (Fryer et al., 2007b), working memory (Malisza et al., 2005), and verbal learning (Sowell et al., 2007).
Structural, metabolic, and functional brain alterations in areas associated with the cingulate that occur in individuals with FASD argue for the relevance of detailed examination of the gyrus in this population. Therefore, the present study used a previously validated, sensitive approach to evaluate cingulate volume in youth with and without prenatal alcohol exposure. Given that functions associated with the cingulate are impaired in FASD (i.e., cognitive control, attention, emotion regulation), we hypothesized that the cingulate gyrus would be affected by alcohol teratogenesis, as evidenced by regional volume reductions in children with prenatal alcohol exposure compared to control youth. In addition, we expected that cingulate white matter would be more affected than grey based on a previous study of this cohort showing that cerebral white matter volumes were reduced beyond grey matter hypoplasia, in individuals with FASD (Archibald et al., 2001).
2. Methods
2.1 Subjects
Study participants included children and adolescents (ages 8–16 years) with heavy prenatal alcohol exposure (ALC; n = 21) and typically developing peers (CON; n = 10). ALC subjects were selected from a retrospective cohort of children with histories of heavy prenatal alcohol exposure who are enrolled in an ongoing study at the Center for Behavioral Teratology (CBT), San Diego State University (SDSU). All alcohol-exposed participants had documented histories of heavy prenatal alcohol exposure and were evaluated by a dysmorphologist with expertise in alcohol teratogenesis (Dr. Kenneth Lyons Jones). Ten of the children in the ALC group met criteria for FAS (Lemoine et al., 1968; Jones and Smith, 1973). Subjects with FASD were originally referred to the CBT by medical providers, or were self-referred. CON subjects were recruited via community outreach or were self-referred, and were matched to ALC subjects on the basis of age, race, socioeconomic status, and sex. Additionally, CON subjects were excluded from participation for psychiatric and neurological conditions, assessed at initial enrollment and supervised by a licensed clinical psychologist (S.N.M.). Groups were not matched on IQ, as heavy prenatal alcohol exposure is associated with impaired intelligence (for review, see Mattson and Riley, 1998), and IQ matching would likely result in a nonrepresentative sample. Likewise, ALC subjects were not excluded on the basis of psychiatric illness status, which occur at higher rates than in the general population (Fryer et al., 2007a). All participants completed the Wechsler Intelligence Scale for Children, III (WISC-III; Wechsler, 1991), and IQ scores across the entire sample ranged from 45 to 127. In addition, caregivers completed the Child Behavior Checklist (CBCL; Achenbach, 1991), a self-report measure of child behavioral problems, as perceived by the caregiver. See Table 1 for sample demographics.
Table 1.
Sample demographics for children in the alcohol-exposed (ALC) or non-exposed control (CON) groups
| ALC [M±SD] | CON [M±SD] | P | |
|---|---|---|---|
| N | 21 | 10 | |
| Age (years) | 10.87±2.17 | 11.86±1.68 | n.s.* |
| Sex (% female) | 52 | 50 | n.s. |
| Race (% Caucasian) | 75 | 60 | n.s. |
| Socioeconomic status (SES)§ | 41.00±12.34 | 49.90±10.29 | n.s. |
| Home placement (% living with one or more biological parents) |
38 | 100 | 0.001 |
| Caregiver education (years of formal schooling)¶ |
13.62±1.60 | 14.00±1.69 | n.s. |
| CBCL- Total Problems T score | 67.71±10.37 | 43.90±9.07 | < 0.001 |
| WISC-III Full Scale IQ | 82.62±15.43 | 108.10±13.10 | < 0.001 |
| WISC-III Freedom from Distractibility Index |
81.48±15.79 | 111.60±11.47 | < 0.001 |
n.s. = non-significant (p > 0.05)
SES: Socioeconomic status as determined by Hollingshead’s Four Factor Index.
Education information was ascertained from current caregivers and reflects biological parent education only in cases of biological home placements. Caregiver education was unavailable for 2 CON subjects.
Before the neuroimaging session, written informed parental consent and child assent were obtained via protocols approved by the SDSU Institutional Review Board. Subjects received a financial incentive for participation in the study. General exclusionary criteria included a history of head trauma, contraindication for MRI scanning (e.g., metallic implants in the body), claustrophobia, or serious medical conditions (e.g., epilepsy).
2.2 Neuroimaging procedures
High resolution anatomical brain scans were collected for each participant with a 1.5T magnet (Signa: General Electric, Milwaukee, USA). The acquisition protocol was a gradient-echo (SPGR) T1-weighted series with TR = 24ms, TE = 5ms, NEX = 2, flip angle = 45 degrees, 24 cm field of view, with contiguous 1.2mm sections, for 124 slices. Scans were acquired at the Scripps Green Hospital in La Jolla, California.
2.3 Image analysis
Image data were imported into BrainImage 5.2.5 (Reiss, 2002) for blinded semi-automatic image processing and quantification (Kates et al., 1999). These procedures have been previously described and validated (Kaplan et al., 1997; Reiss et al., 1998). The protocol for delineation of the cingulate gyrus was developed by Reiss and colleagues (cf., Reiss et al., 2004), and involves manual isolation of cingulate gyri for each subject. The protocol requires outlining the cingulate in the sagittal plane, at a predetermined distance from the sagittal midline. The image is then rotated into coronal orientation, and regions of interest (ROIs) are drawn for the right and left cingulate separately using the sagittal outline as a boundary marker (Figure 1). ROIs were applied to individual grey and white matter segmented images, and cingulate grey matter and white matter volumes were automatically calculated. The cingulate gyri were then divided into two sections via a vertical line drawn halfway between the anterior and posterior commissures on a midsagittal slice, enabling calculation of anterior and posterior subdivision volumes. ROIs were drawn by trained analysts, blind to subject classification, who achieved reliable interrater agreement (interclass correlation values were 0.90 and 0.92 for the right and left cingulate, respectively).
Figure 1.
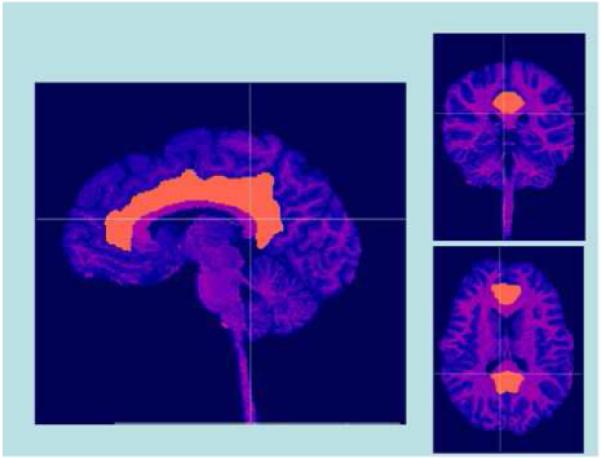
3-dimensional view of manually delineated cingulate gyrus in one subject. Crosshairs depict planar location.
2.4 Statistical analyses
Analysis of variance (ANOVA) techniques were used to examine raw cingulate volumes separately for grey matter, white matter, and total tissue (grey + white matter). These ANOVAs used a 2×2×2 repeated measures design to examine the effects of hemisphere (right vs. left), subdivision (anterior vs. posterior), and group (ALC vs. CON) on cingulate volume. Because FASD is associated with microcephaly, analysis of covariance (ANCOVA) techniques were also employed, to examine the effects of cingulate volume independent of cranial volume. The ANCOVAs employed the same 2×2×2 design described for the ANOVAs, with the addition of one covariate representing that tissue constituent’s cranial volume (e.g., cranial grey matter was entered as a covariate for the ANCOVA examining cingulate grey matter volumes, etc.). The alpha level for ANOVA and ANCOVA analyses was set at P < 0.05. In an effort to constrain type one error, contrasts of interest relating to between-group effects were specified a priori, whereas effects of hemisphere or subdivision, collapsed across group, were not of theoretical interest and thus were not examined. Two hierarchical regression models were then computed to further explore the hypothesis that cingulate white matter would be more affected in FASD than grey matter. Total cranial volume (grey or white) was entered into the first step of the regression model predicting cingulate volume (grey or white), followed by group (ALC vs. CON), to determine whether group status still accounted for significant variance in cingulate volume, after partialing out the variance associated with total brain size. Lastly, within each group, Pearson’s correlation coefficients were used to quantify the association between cingulate subregional volumes (anterior, posterior) and WISC-III IQ measures: 1) Full Scale IQ and 2) the Freedom from Distractibility (FD) index, which reflects working memory and attention abilities.
3. Results
Assumptions of normality and equal variances were evaluated to ensure that use of parametric statistics was appropriate. In many cases it is ill advised to use analysis of covariance (ANCOVA) in the case of between-group differences on the covariate (cf. Lord, 1969; Miller and Chapman, 2001), as the technique cannot truly “control for” or statistically adjust the dependent variable in light of the covariate. However, in the case of the present study, unadjusted (raw) analyses cannot answer a substantiative question of interest to this study, as to whether hypothesized reductions of cingulate volumes are commensurate with or are above and beyond total reductions in brain volume. Proportional measures are a general alternative to ANCOVA. However, it has been argued that ratio and proportion measures of imaged brain morphology are unreliable (Arndt et al., 1991). Given these methodological considerations, both raw and covariate-adjusted analyses are presented (see Table 2).
Table 2.
Volumes of total brain, cingulate, and hemispheric and regional cingulate subdivisions in alcohol-exposed (ALC) or non-exposed control (CON) groups
| Total Cranial and Cingulate Volumes | ||||
|---|---|---|---|---|
|
Mean Raw Volume (cc) |
||||
| Region | ALC [M±SD] | CON [M±SD] | P | Cohen’s d |
| Cingulate Grey Matter | 25.47±3.64 | 29.45±2.22 | 0.002 | 1.32 |
| Cingulate White Matter | 9.33±1.62 | 12.34±1.29 | < 0.001 | 2.06 |
| Cranial Grey Matter | 717.41±72.23 | 815.97±57.22 | 0.001 | 1.51 |
| Cranial White Matter | 456.73±68.64 | 567.58±73.82 | < 0.001 | 1.56 |
| Cingulate Regional Volumes, Raw and Adjusted | ||||||
|---|---|---|---|---|---|---|
| Mean Raw Volume (cc) | Mean Adjusted Volume (cc) | |||||
|
ALC [M±SD] |
CON [M±SD] |
Cohen’s d |
ALC [M±SD] |
CON [M±SD] |
Cohen’s d |
|
| Cingulate Grey Matter | ||||||
| Left Hemisphere |
12.10±2.28 |
13.85±0.15 |
1.08 |
13.11±1.56 |
13.93±1.68 |
0.51 |
| Right Hemisphere |
13.37±2.38 |
15.61±1.73 |
1.08 |
11.33±1.33 |
12.26±1.42 |
0.68 |
| Anterior Subdivision |
9.11±2.20 |
10.98±2.26 |
0.84 |
10.71±1.97 |
12.20±2.12 |
0.73 |
| Posterior Subdivision |
12.96±2.20 |
14.74±2.43 |
0.77 |
13.73±1.56 |
13.99±1.68 |
0.16 |
| Cingulate White Matter | ||||||
| Left Hemisphere |
4.70±0.91 |
6.04±0.33 |
1.96 |
4.96±0.55 |
5.56±0.60 |
1.04 |
| Right Hemisphere |
4.63±0.93 |
6.27±1.16 |
1.56 |
4.71±0.55 |
5.27±0.60 |
0.97 |
| Anterior Subdivision |
2.81±0.82 |
3.66±1.03 |
0.91 |
3.92±0.64 |
4.50±0.70 |
0.86 |
| Posterior Subdivision |
6.05±1.36 |
8.05±1.14 |
1.59 |
5.74±0.78 |
6.33±0.82 |
0.74 |
3.1 Raw cingulate volume analyses
Results of the ANOVAs indicate decreased cingulate grey matter (F (1, 29) = 11.23; P = 0.002; partial η2 = 0.279) and total tissue (F (1, 29) = 19.40; P < 0.001; partial η2 = 0.401) volumes in ALC subjects compared to CON. There were no interaction effects (group x hemisphere or group x subdivision) for either the cingulate grey matter or tissue ANOVAs (0.269 < P’s < 0.805). The main effect of group was also significant in the ANOVA examining raw cingulate white matter (F (1, 29) = 25.84; P < 0.001; partial η2 = 0.471); however these group differences are best interpreted in context of the interaction effects, which were observed for both group x hemisphere (F (1, 29) = 6.69; P = 0.015; partial η2 = 0.188), and group x subdivision (F (1, 29) = 4.41; P = 0.045; partial η2 = 0.132). Examination of marginal means indicated that these interaction effects were driven by greater ALC cingulate white matter reductions in the right hemisphere and the posterior subsection respectively, compared to CON.
3.2 Cranial volume-adjusted cingulate volume analyses
ANCOVAs examining grey matter (F (1, 28) = 1.99; P = 0.169; partial η2 = 0.066) and total tissue (F (1, 28) = 3.59; P = 0.069; partial η2 = 0.114) were non-significant for between-group effects, suggesting that cingulate grey matter and total tissue volumes were not reduced in the ALC group, above and beyond cranial volume. However, there was a main effect of group for cingulate white matter volume (F (1, 28) = 5.63; P = 0.025; partial η2 = 0.167), indicating that the reduction in cingulate white matter volume observed in the ALC group was greater than that seen in cranial white matter volume. To determine the impact of FAS diagnosis (FAS versus ALC subjects without FAS) on this white matter finding, a post hoc ANCOVA analysis was conducted using only the ALC subjects (n = 21). No significant effect of dysmorphic grouping was noted (F (1,18) = 0.009; P = 0.926; partial η2 < 0.001). This analysis suggests that white matter reductions of the cingulate gyrus occur in the ALC group, irrespective of whether or not the individual qualified for a diagnosis of FAS. No interaction effects were observed in the cingulate grey matter, white matter, total tissue, or FAS diagnosis covariate analyses (0.084 < P’s < 0.975).
3.3 Hierarchical regression models
Because prenatal alcohol exposure is associated with microcephaly, the specificity of regional volume effects must be evaluated in light of overall reductions in brain size. In order to further test our hypothesis of cingulate white matter vulnerability, we computed two hierarchical regression analyses: the first regressed total cingulate grey matter volume on total cranial grey matter volume (first step entered into the model), and group status (second step). The second analysis regressed total cingulate white matter volume on total cranial white matter volume (first step), and group status (second step). The results of the grey matter regression indicated that the one-step model was more parsimonious, because while cranial grey matter volume accounted for significant variance in cingulate grey matter volume (F (1, 29) = 20.34; P < 0.001; Adjusted R2 = 0.39), group status did not explain any additional variance in the two-step model (P = 0.239; ΔR2 = 0.03). However, results of the white matter regression showed that the two-step model should be retained (F (2, 28) = 47.52; P < 0.001; Adjusted R2 = 0.76; ΔR2 = 0.05), as group status was a significant predictor (P = 0.022), above and beyond cranial white matter volume (P < 0.001). Results of the regression analyses support our ANCOVA findings, by indicating that group status predicted white, but not grey matter, cingulate volumes, after controlling for the effects of respective cranial volume.
Lastly, an effort was made to examine the regional specificity of the observed cingulate white matter hypoplasia. Pearson’s correlation coefficients were computed separately within each group to quantify the relationship between cingulate subregion (anterior, posterior) and respective lobar white matter volume (frontal, parietal). In the CON group, non-significant positive trends were observed between anterior cingulate and frontal lobe volume (r = 0.62, P = 0.056), and posterior cingulate and parietal lobe volume (r = 0.56, P = 0.093). In the ALC group, posterior cingulate and parietal lobe white matter volume were positively correlated (r = 0.64; P = 0.002), while no association was observed between anterior cingulate and frontal lobe white matter volume (r = 0.18; P = 0.438).
3.4 Correlation of subregional cingulate volumes with neurobehavioral performance
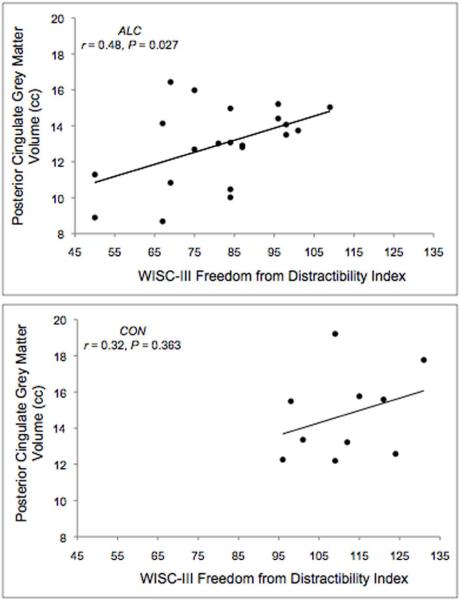
Pearson’s correlation coefficients characterized the relationship of cingulate volumes with WISC-III IQ and FD measures. A significant positive association between FD and posterior cingulate grey matter volume (r = 0.48; P = 0.027) was observed in the ALC group, suggesting that reduced cingulate volumes were associated with poorer performance on tasks tapping working memory and attention (see Figure 2). All other correlations examined were not significant (P’s > 0.05). See Table 3 for presentation of two-tailed within-group correlation coefficients.
Figure 2.
Scatterplots depicting correlations between posterior cingulate grey matter volume and the WISC-III Freedom from Distractibility Index for alcohol-exposed (ALC) or non-exposed control (CON) children.
Table 3.
Pearson 2-tailed correlations between cingulate subregional volumes and neuropsychological measures for alcohol-exposed (ALC) or control (CON) children
| Correlation with Cingulate Regional Volumes | ||||
|---|---|---|---|---|
| ALC | CON | |||
| Cingulate Subregion | FSIQ | FD | FSIQ | FD |
| Anterior Grey Matter | −0.05 | −0.03 | 0.21 | −0.58 |
| Anterior White Matter | −0.24 | −0.07 | 0.34 | −0.38 |
| Posterior Grey Matter | 0.37 | 0.48* (0.027) | −0.07 | 0.32 |
| Posterior White Matter | 0.16 | 0.29 | −0.01 | 0.44 |
FSIQ: Full Scale IQ; FD: WISC-III Freedom from Distractibility Index
Significant correlations (P < 0.05) are indicated in parentheses; all other correlations are non-significant (P > 0.05).
4. Discussion
Upon examining raw cingulate volumetric measurements, we observed significantly smaller cingulate grey matter, white matter, and tissue (grey + white) volumes in alcohol-exposed youth, compared to typically developing peers. In addition, interaction effects were observed in the white matter analysis, demonstrating that the white matter reduction in raw cingulate volume was driven by the right hemisphere (for the group x hemisphere interaction) and posterior subregion (for the group x subdivision interaction) of the cingulate. However, ANCOVA analyses adjusted for respective cranial tissue volumes indicated that only the main effect of white matter remained significant once overall brain size was taken into account. The hypothesis that cingulate white matter would be more affected than grey matter was further supported by hierarchical regression analyses, which showed that group status (ALC vs. CON) accounted for significant variance in cingulate white, but not grey matter volume, after first accounting for respective tissue cranial volume. Therefore, our results suggest that smaller cingulate grey matter can be explained by overall brain size reductions, but that reductions in cingulate white matter occur above and beyond global white matter hypoplasia. Correlation analyses suggest that while posterior cingulate white matter reductions are in accordance with the regional parietal hypoplasia previously described (Archibald et al., 2001; Sowell et al., 2001b), the reduction of the anterior cingulate was not associated with frontal lobe volume, suggesting regional specificity of white matter reductions in the anterior portion of the gyrus.
With regard to FAS diagnosis, a post hoc analysis indicated that the cingulate white matter reduction occurred in the alcohol-exposed individuals in our sample, regardless of a diagnosis of FAS. Given the small effect size of this finding, the lack of statistical significance does not appear to be driven by insufficient power. The lack of a FAS diagnostic effect within the alcohol-exposed group is in concordance with, and may help explain, the highly similar cognitive and behavioral functioning exhibited by alcohol-exposed individuals with and without FAS (Mattson et al., 1998).
A significant association was observed between posterior cingulate grey matter volume and the WISC-III Freedom from Distractibility Index (FD) in alcohol-exposed individuals, but not in controls; however, these findings are not specific to the cingulate, as parietal lobe grey matter volume was similarly correlated with FD (results not shown). Posterior cingulate grey matter volume did not correlate with Full Scale IQ; thus, it appears that posterior cortical volume specifically relates to attention and working memory abilities. FD performance appears to be a meaningful marker of FASD, as FD was part of a classifying algorithm that was able to accurately distinguish between alcohol-exposed and non-exposed children (Lee et al., 2004). Children with FASD show deficits in aspects of executive functions including attention and working memory (Rasmussen, 2005), and our findings suggest that these impairments may be related to posterior cortical hypoplasia.
By providing a detailed account of cingulate morphology in this population, the present study expands on previous neuroimaging studies of FASD, which have shown structural, functional, and metabolic abnormalities in areas related to cognitive control. Prior studies of this cohort have noted global hypoplasia, most prominently in white matter (Archibald et al., 2001; Sowell et al., 2002), and aberrant shape (Sowell et al., 2002) in the frontal and parietal lobes in FASD. Disruption in frontal systems is important to note in the context of the present study, since anterior cingulate and other prefrontal regions are thought to be functionally associated in the mediation of cognitive control (Cohen et al., 2005; Cole and Schneider, 2007). Thus, multiple components of the networks responsible for cognitive control appear to be targets of alcohol teratogenesis, which may help explain some of the behavioral impairments in individuals with FASD. Given that the anterior cingulate is implicated in avoidance learning (Botvinick, 2007), dysfunction of this region may play a role in impaired adaptive functioning, social cognition, and development of psychopathology in individuals with FASD.
The white matter findings observed in the present study build upon existing literature examining white matter morphology in the context of prenatal alcohol exposure. In addition to global white matter disruption, anomalies in the corpus callosum have also been documented in the literature, including hypoplasia (Riley et al., 1995; Swayze et al., 1997; Sowell et al., 2001a; Autti-Rämö et al., 2002), hypervariability in shape (Bookstein et al., 2001, 2002), and posterior subregion displacements (Sowell et al., 2001a). More recently, diffusion tensor imaging studies have described compromised fiber integrity in regions of the corpus callosum in individuals with FASD (Ma et al., 2005; Wozniak et al., 2006). Together, these studies provide convergent evidence of significant white matter disruption following prenatal alcohol exposure. Callosal abnormalities may relate to the findings of the present study, as cingulate axons appear to play a role in the formation of the corpus callosum during development (Koester and O’Leary, 1994; Rash and Richards, 2001) and may pioneer a pathway for other cortical callosal-forming axons (Rash and Richards, 2001). It is thus possible that there is a relationship between the cingulate and corpus callosum anomalies induced by gestational alcohol exposure.
Studies of other populations with attention deficits have reported structural and functional abnormalities in the cingulate gyrus. For example, cingulate volume is reduced in individuals with attention-deficit/hyperactivity disorder (ADHD) (Overmeyer et al., 2001; Carmona et al., 2005; Seidman et al., 2006). Functional alterations in the dorsal anterior cingulate have also been identified across various measures of cognitive control in ADHD, and it has been suggested that dysfunction in this region could lead to the classic symptoms of inattention, impulsivity, and hyperactivity (see Bush et al., 2005, for review). A resting state functional connectivity analysis revealed decreased coherence between the anterior and posterior cingulate in individuals with ADHD, compared to controls (Castellanos et al., 2008). The authors speculated that abnormal anterior-posterior cingulate connectivity may be related to lapses in attention and serve as a locus of dysfunction in ADHD. Studies relating cingulate abnormalities to impaired executive function in ADHD provide further support for our speculation that cingulate hypoplasia may contribute to cognitive control deficits in FASD. In addition, a recent structural MRI study of children with prenatal poly-substance exposures, including alcohol, revealed thinner right anterior cingulate cortex that was related to social problems and marginally related to attention problems in exposed children compared to controls (Walhovd et al., 2007).
Although these findings contribute to our understanding of alcohol’s teratogenic effects on the brain, it is important to interpret results in light of the following limitations. Given the large age range of our sample (8 to 16 years), ongoing brain maturation is expected. Due to the cross-sectional design of our study, we are unable to evaluate maturational trends related to cingulate development. Future studies might employ a longitudinal design to address this issue. Also, due to the retrospective nature of our study recruitment, we are unable to control for early environmental variables (such as home placement, trauma history) and other fetal and maternal characteristics that might also be expected to affect brain development.
In summary, results of this study provide evidence of cingulate hypoplasia, especially in white matter, associated with FASD. Structural damage to the cingulate may contribute to the profile of neuropsychological deficits observed in individuals with FASD, most likely in the areas of cognitive control, attention, and emotion regulation. Future studies relating aspects of cingulate morphology to executive control in alcohol-exposed individuals are warranted to identify the neurobehavioral effects of cingulate hypoplasia in this population. Fiber-tracking based DTI studies would also be useful to characterize cingulate fiber integrity and connectivity in the context of the hypoplasia observed herein.
Acknowledgement
This research was supported by NIAAA: R01 AA010417, T32 AA013525 to EPR, R01 AA010820, U01 AA014834 to SNM, and F31 AA016051 to SLF. The authors thank Dr. David Sobel and the laboratory of Dr. Terry Jernigan for scan acquisition and radiological review, and Dr. Shelli Kesler for image analysis consultation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. University of Vermont, Department of Psychiatry; Burlington, Vermont: 1991. [Google Scholar]
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Developmental Medicine and Child Neurology. 2001;43:148–154. [PubMed] [Google Scholar]
- Arndt S, Cohen G, Alliger RJ, Swayze VW, II, Andreasen NC. Problems with ratio and proportion measures of imaged cerebral structures. Psychiatry Research: Neuroimaging. 1991;40:79–89. doi: 10.1016/0925-4927(91)90031-k. [DOI] [PubMed] [Google Scholar]
- Autti-Rämö I, Autti T, Korkman M, Kettunen S, Salonen O, Valanne L. MRI findings in children with school problems who had been exposed prenatally to alcohol. Developmental Medicine and Child Neurology. 2002;44:98–106. doi: 10.1017/s0012162201001748. [DOI] [PubMed] [Google Scholar]
- Bertrand J, Floyd RL, Weber MK. Guidelines for identifying and referring persons with fetal alcohol syndrome. Morbidity and Mortality Weekly Report. 2005;54:1–14. [PubMed] [Google Scholar]
- Bhatara V, Loudenberg R, Ellis R. Association of attention deficit hyperactivity disorder and gestational alcohol exposure: An exploratory study. Journal of Attention Disorders. 2006;9:515–522. doi: 10.1177/1087054705283880. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Sampson PD, Streissguth AP, Connor PD. Geometric morphometrics of corpus callosum and subcortical structures in the fetal-alcohol-affected brain. Teratology. 2001;64:4–32. doi: 10.1002/tera.1044. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Streissguth AP, Sampson PD, Connor PD, Barr HM. Corpus callosum shape and neuropsychological deficits in adult males with heavy fetal alcohol exposure. NeuroImage. 2002;15:233–251. doi: 10.1006/nimg.2001.0977. [DOI] [PubMed] [Google Scholar]
- Botvinick MM. Conflict monitoring and decision making: Reconciling two perspectives on anterior cingulate function. Cognitive, Affective, and Behavioral Neuroscience. 2007;7:356–366. doi: 10.3758/cabn.7.4.356. [DOI] [PubMed] [Google Scholar]
- Bush G, Valera EM, Seidman LJ. Functional neuroimaging of attention-deficit/hyperactivity disorder: A review and suggested future directions. Biological Psychiatry. 2005;57:1273–1284. doi: 10.1016/j.biopsych.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Carmona S, Vilarroya O, Bielsa A, Trèmols V, Soliva JC, Rovira M, Tomàs J, Raheb C, Gispert JD, Batlle S, Bulbena A. Global and regional gray matter reductions in ADHD: A voxel-based morphometric study. Neuroscience Letters. 2005;389:88–93. doi: 10.1016/j.neulet.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal B, Sonuga-Barke EJS, Rotrosen J, Adler LA, Milham MP. Cingulate-precuneus interactions: A new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biological Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Heller AS, Ranganath C. Functional connectivity with anterior cingulate and orbitofrontal cortices during decision-making. Cognitive Brain Research. 2005;23:61–70. doi: 10.1016/j.cogbrainres.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. NeuroImage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Fagerlund Å, Heikkinen S, Autti-Rämö I, Korkman M, Timonen M, Kuusi T, Riley EP, Lundbom N. Brain metabolic alterations in adolescents and young adults with fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research. 2006;30:2097–2104. doi: 10.1111/j.1530-0277.2006.00257.x. [DOI] [PubMed] [Google Scholar]
- Fryer SL, McGee CL, Matt GE, Riley EP, Mattson SN. Evaluation of psychopathological conditions in children with heavy prenatal alcohol exposure. Pediatrics. 2007a;119:e733–e741. doi: 10.1542/peds.2006-1606. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Tapert SF, Mattson SN, Paulus MP, Spadoni AD, Riley EP. Prenatal alcohol exposure affects frontal-striatal BOLD response during inhibitory control. Alcoholism: Clinical and Experimental Research. 2007b;31:1415–1424. doi: 10.1111/j.1530-0277.2007.00443.x. [DOI] [PubMed] [Google Scholar]
- Guerri C, Bazinet A, Riley EP. Foetal alcohol spectrum disorders and alterations in brain and behaviour. Alcohol and Alcoholism. 2009;44:108–114. doi: 10.1093/alcalc/agn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Kaplan DM, Liu AMC, Abrams MT, Warsofsky IS, Kates WR, White CD, Kaufmann WE, Reiss AL. Application of an automated parcellation method to the analysis of pediatric brain volumes. Psychiatry Research: Neuroimaging. 1997;76:15–27. doi: 10.1016/s0925-4927(97)00055-3. [DOI] [PubMed] [Google Scholar]
- Kates WR, Warsofsky IS, Patwardhan A, Abrams MT, Liu AMC, Naidu S, Kaufmann WE, Reiss AL. Automated Talairach atlas-based parcellation and measurement of cerebral lobes in children. Psychiatry Research: Neuroimaging. 1999;91:11–30. doi: 10.1016/s0925-4927(99)00011-6. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW. Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: A review. Neuroscience and Biobehavioral Reviews. 2007;31:192–201. doi: 10.1016/j.neubiorev.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Koester SE, O’Leary DD. Axons of early generated neurons in cingulate cortex pioneer the corpus callosum. The Journal of Neuroscience. 1994;14:6608–6620. doi: 10.1523/JNEUROSCI.14-11-06608.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski L, Paus T. Functional connectivity of the anterior cingulate cortex within the human frontal lobe: A brain-mapping meta-analysis. Experimental Brain Research. 2000;133:55–65. doi: 10.1007/s002210000400. [DOI] [PubMed] [Google Scholar]
- Lee KT, Mattson SN, Riley EP. Classifying children with heavy prenatal alcohol exposure using measures of attention. Journal of the International Neuropsychological Society. 2004;10:271–277. doi: 10.1017/S1355617704102142. [DOI] [PubMed] [Google Scholar]
- Lemoine P, Harousseau H, Borteyru J-P, Menuet J-C. Les enfants de parents alcooliques. Anomalies observees. A propos de 127 cas [Children of alcoholic parents. Abnormalities observed in 127 cases] Ouest Medical. 1968;21:476–482. [Google Scholar]
- Lord FM. Statistical adjustments when comparing preexisting groups. Psychological Bulletin. 1969;72:336–337. [Google Scholar]
- Ma X, Coles CD, Lynch ME, LaConte SM, Zurkiya O, Wang D, Hu X. Evaluation of corpus callosum anisotropy in young adults with fetal alcohol syndrome according to diffusion tensor imaging. Alcoholism: Clinical and Experimental Research. 2005;29:1214–1222. doi: 10.1097/01.alc.0000171934.22755.6d. [DOI] [PubMed] [Google Scholar]
- Maddock RJ. The retrosplenial cortex and emotion: New insights from functional neuroimaging of the human brain. Trends in Neuroscience. 1999;22:310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Malisza KL, Allman A-A, Shiloff D, Jakobson L, Longstaffe S, Chudley AE. Evaluation of spatial working memory function in children and adults with fetal alcohol spectrum disorders: A functional magnetic resonance imaging study. Pediatric Research. 2005;58:1150–1157. doi: 10.1203/01.pdr.0000185479.92484.a1. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcoholism: Clinical and Experimental Research. 1998;22:279–294. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling LJ, Delis DC, Jones KL. Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology. 1998;12:146–153. doi: 10.1037//0894-4105.12.1.146. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome: A summary. Alcohol Research and Health. 2001;25:159–167. [PMC free article] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- O’Connor MJ, Kasari C. Prenatal alcohol exposure and depressive features in children. Alcoholism: Clinical and Experimental Research. 2000;24:1084–1092. [PubMed] [Google Scholar]
- O’Connor MJ, Kogan N, Findlay R. Prenatal alcohol exposure and attachment behavior in children. Alcoholism: Clinical and Experimental Research. 2002;26:1592–1602. doi: 10.1097/01.ALC.0000034665.79909.F0. [DOI] [PubMed] [Google Scholar]
- Overmeyer S, Bullmore ET, Suckling J, Simmons A, Williams SCR, Santosh PJ, Taylor E. Distributed grey and white matter deficits in hyperkinetic disorder: MRI evidence for anatomical abnormality in an attentional network. Psychological Medicine. 2001;31:1425–1435. doi: 10.1017/s0033291701004706. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Attention, self-regulation and consciousness. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 1998;353:1915–1927. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash BG, Richards LJ. A role for cingulate pioneering axons in the development of the corpus callosum. The Journal of Comparative Neurology. 2001;434:147–157. doi: 10.1002/cne.1170. [DOI] [PubMed] [Google Scholar]
- Rasmussen C. Executive functioning and working memory in fetal alcohol spectrum disorder. Alcoholism: Clinical and Experimental Research. 2005;29:1359–1367. doi: 10.1097/01.alc.0000175040.91007.d0. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Eckert MA, Rose FE, Karchemskiy A, Kesler S, Chang M, Reynolds MF, Kwon H, Galaburda A. An experiment of nature: Brain anatomy parallels cognition and behavior in Williams syndrome. The Journal of Neuroscience. 2004;24:5009–5015. doi: 10.1523/JNEUROSCI.5272-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Hennessey JG, Rubin M, Beach L, Abrams MT, Warsofsky IS, Liu AM, Links JM. Reliability and validity of an algorithm for fuzzy tissue segmentation of MRI. Journal of Computer Assisted Tomography. 1998;22:471–479. doi: 10.1097/00004728-199805000-00021. [DOI] [PubMed] [Google Scholar]
- Riley EP, Mattson SN, Sowell ER, Jernigan TL, Sobel DF, Jones KL. Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcoholism: Clinical and Experimental Research. 1995;19:1198–1202. doi: 10.1111/j.1530-0277.1995.tb01600.x. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Buckley MJ, Behrens TEJ, Walton ME, Bannerman DM. Functional organization of the medial frontal cortex. Current Opinion in Neurobiology. 2007;17:220–227. doi: 10.1016/j.conb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Schonfeld AM, Paley B, Frankel F, O’Connor MJ. Executive functioning predicts social skills following prenatal alcohol exposure. Child Neuropsychology. 2006;12:439–452. doi: 10.1080/09297040600611338. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N, Monuteaux MC, Boriel DL, Kelkar K, Kennedy DN, Caviness VS, Bush G, Aleardi M, Faraone SV, Biederman J. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biological Psychiatry. 2006;60:1071–1080. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Lu LH, O’Hare ED, McCourt ST, Mattson SN, O’Connor MJ, Bookheimer SY. Functional magnetic resonance imaging of verbal learning in children with heavy prenatal alcohol exposure. NeuroReport. 2007;18:635–639. doi: 10.1097/WNR.0b013e3280bad8dc. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Thompson PM, Jernigan TL, Riley EP, Toga AW. Mapping callosal morphology and cognitive correlates: Effects of heavy prenatal alcohol exposure. Neurology. 2001a;57:235–244. doi: 10.1212/wnl.57.2.235. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. Voxel-based morphometric analyses of the brain in children and adolescents prenatally exposed to alcohol. NeuroReport. 2001b;12:515–523. doi: 10.1097/00001756-200103050-00018. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cerebral Cortex. 2002;12:856–865. doi: 10.1093/cercor/12.8.856. [DOI] [PubMed] [Google Scholar]
- Steinhausen H-C, Spohr H-L. Long-term outcome of children with fetal alcohol syndrome: Psychopathology, behavior and intelligence. Alcoholism: Clinical and Experimental Research. 1998;22:334–338. doi: 10.1111/j.1530-0277.1998.tb03657.x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. Journal of Developmental and Behavioral Pediatrics. 2004;25:228–238. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- Swayze VW, II, Johnson VP, Hanson JW, Piven J, Sato Y, Giedd JN, Mosnik D, Andreasen NC. Magnetic resonance imaging of brain anomalies in fetal alcohol syndrome. Pediatrics. 1997;99:232–240. doi: 10.1542/peds.99.2.232. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Moe V, Slinning K, Due-Tonnessen P, Bjornerud A, Dale AM, van der Kouwe A, Quinn BT, Kosofsky B, Greve D, Fischl B. Volumetric cerebral characteristics of children exposed to opiates and other substances in utero. NeuroImage. 2007;36:1331–1344. doi: 10.1016/j.neuroimage.2007.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children. 3rd ed. The Psychological Corporation; San Antonio, Texas: 1991. [Google Scholar]
- Wozniak JR, Mueller BA, Chang P-N, Muetzel RL, Caros L, Lim KO. Diffusion tensor imaging in children with fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research. 2006;30:1799–1806. doi: 10.1111/j.1530-0277.2006.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]