Abstract
Allergic diseases such as asthma result from inappropriate immunologic responses to common environmental allergens in genetically susceptible individuals. Following allergen exposure, interaction of dendritic cells (DC) with CD4+ T cells leads to the production of Th2 cytokines, which induce B cells to synthesize IgE molecules (sensitization phase). These IgE molecules bind to their high affinity receptors (FcεRI) on the surface of mast cells and basophils and their subsequent cross-linking by allergen results in the release of preformed and newly synthesized mediators, which cause bronchoconstriction, lung inflammation and airway hyperresponsiveness (AHR) in asthma (effector phase). The complement components C3a and C5a levels are increased in the lungs of patients with asthma and are likely generated via the actions of both allergen and mast cell proteases. In vivo studies with rodents have shown that while C3a facilitates allergen sensitization in some models C5a inhibits this response. Despite this difference, both anaphylatoxins promote lung inflammation and AHR in vivo indicating that cells other than DC and T cells likely mediate the functional effects of C3a and C5a in asthma. This review focuses on the contribution of C3a and C5a in the pathogenesis of asthma with a particular emphasis on mast cells and basophils. It discusses the mechanisms by which anaphylatoxins activate mast cells and basophils and the associated signaling pathways via which their receptors are regulated by priming and desensitization.
Keywords: Complement, Anaphylatoxin, C3a, C5a, Mast cell, Basophil, G protein, Priming, Desensitization, Signal Transduction, Asthma
1. Role of mast cells in asthma
Allergic diseases such as rhinitis and asthma are the most prevalent respiratory diseases in industrialized societies affecting ~20% and ~7% of the US population, respectively [1,2]. These diseases are caused by an overzealous immune response to allergens in which immunoglobulin E (IgE) and mast cells play critical roles. It is therefore not surprising that tremendous efforts have been directed towards developing therapy based on the modulation of IgE and its receptor, FcεRI. A recent exciting development in mast cell research has been the approval by the U.S. Food and Drug Administration of a humanized monoclonal antibody omalizumab for the treatment of allergic diseases. Omalizumab binds free IgE molecules and the resulting complexes are removed from the circulation. Over time, IgE comes off its receptors on mast cells and they lose their ability to respond to allergen [3,4]. Omalizumab is difficult to manufacture, is expensive, effective on a subset of allergic patients and may not be sufficient alone to prevent hyperresponsiveness [5]. Another approach has been to target the intracellular signaling pathway via which IgE-FcεRI activates mast cells. Given that Syk kinase plays a central role in FcεRI signaling, a number of Syk inhibitors have been developed [6]. One compound, R112, was the first Syk inhibitor to enter clinical studies [7]. These findings suggest that other pathways that also activate mast cells could be targeted for the development of asthma therapeutics.
As discussed in this review, the complement components C3a and C5a are involved in the pathogenesis of asthma and their effects have variously been proposed to involve dendritic cells, T cells, airway epithelial cells and smooth muscle cells [8–16]. Although a number of excellent reviews have recently been published on the roles C3a and C5a in asthma [17–21], the possible involvement of mast cells and basophils have not been discussed in detail. It is noteworthy that murine bone marrow-derived mast cells (BMMC) and rat basophilic leukemia RBL-2H3 cells, which have been extensively used to study FcεRI signaling in mast cells, do not express G protein coupled receptors (GPCRs) for C3a and C5a [22–24]. The purpose of this brief review article is to summarize what is known about the activation and regulation of human mast cells and basophils by C3a and C5a. This review is particularly timely as basophils, which express C3a and C5a receptors, have recently been shown to have previously unrecognized role in the development and maintenance of allergic diseases [25–27]. Thus, understanding the molecular mechanism by which anaphylatoxins activate mast cells and basophils and delineating the signaling pathway via which their functions are regulated may provide a novel therapeutic target for asthma and other allergic diseases.
2. Roles of complement component C3a in the pathogenesis of allergic asthma
The complement system forms an important part of innate immunity against bacteria and other pathogens. As a system of pattern recognition molecules, foreign surface antigens and immune complexes initiate a proteolytic pathway leading to the formation of a lytic membrane attack complex. The anaphylatoxins C3a and C5a are generated as byproducts of complement activation, and they interact with cell surface GPCRs in target cells to mediate a variety of inflammatory responses [28–30]. Recent studies have shown that C3a and C5a levels are elevated in bronchoalveolar lavage (BAL) fluid after segmental allergen challenge in asthmatic but not healthy subjects [9,31,32]. Furthermore, plasma C3a and C5a levels are elevated in acute exacerbations of asthma [31] and C3a receptor is unregulated in subjects who died with asthma compared with subjects who died from other causes [33]. Additionally, single nucleotide polymorphism in C3 or C3a receptor (C3aR) gene increases susceptibility to asthma [34]. In animal models, complement activation modulates both AHR and airway inflammation [35,36]. Furthermore, deletion of C3aR gene or administration of C3aR inhibitors attenuates both AHR and lung inflammation [9,37–40]. Collectively, these findings demonstrate an important role of C3aR in the pathogenesis of asthma.
The mechanism by which C3a regulates AHR and inflammation in asthma is unknown and has been the subject of considerable debate. C3aR is expressed in both antigen-presenting cells (APCs) and activated T cells indicating that C3a may promote asthma by inducing Th2 cytokine production and IgE synthesis [41–44]. Indeed, Drouin et al., [37] reported that in models of Aspergillus fumigatus and ovalbumin-induced pulmonary allergy, C3aR-deficiency in mice on C57BL/6 background results in significant decrease in Th2 cytokine production and IgE synthesis. More recently, Zhang et al., [13] showed that in house dust mite (HDM)-induced allergic asthma C3aR−/− mice produce less Th2 cytokine when compared to wild-type mice. These findings are in contrast with previous reports, which showed that C3aR deficiency in guinea pigs and mice on the BALB/c background are not protected from serum IgE secretion, Th2 cytokine secretion [9,39]. These differences might reflect differences in species and strains of animals, nature of allergen and methods of sensitization used. Despite this, C3aR-deficiency protects animals from allergen-induced AHR and lung inflammation. Furthermore, administration of complement inhibitor in mice after sensitization but before challenge prevented the development of AHR and blocked lung inflammation [36]. Additionally, a small molecule antagonist of C3a receptor, when administered after sensitization but before challenge also significantly inhibited airway inflammation [38]. These findings suggest that although C3a has variable effect on allergen sensitization, its effect on AHR and lung inflammation in animal models of allergic asthma is likely mediated via the activation of C3aR in effector cells such as mast cells and basophils [12,21,36,38].
3. Dual Roles of C5a in the pathogenesis of allergic asthma
As described above, development of allergic asthma in animal models can be modulated either at the level of allergen sensitization or the effector phase. Administration of C5aR monoclonal antibody after sensitization but before allergen challenge leads to substantial improvement of AHR and reduction in airway inflammation [38]. These findings are consistent with the idea that C5a also contributes the pathogenesis of allergic asthma via the modification of the effector phase. However, this contention was challenged by Karp et al., [45], who showed that C5-deficient mice are more susceptible to experimental asthma when compared with C5-sufficient mice indicating that C5a may instead play a protective role in the pathogenesis of asthma. Kohl et al., [15] recently utilized three experimental approaches to resolve this paradox. These included (a) administration of anti-C5a receptor (C5aR) monoclonal antibody to the lung (b) expression of a lung-inducible mutant form of C5a (C5aRA A8Δ71–73) that acts as a C5aR antagonist and (c) C5aR-deficient mice. They found that blocking or deleting C5aR prior to initial allergen sensitization in murine model of allergic asthma either induces or causes a marked enhancement of Th2-polarized immune responses, airway inflammation, and AHR. These effects result from an increase in the number of myeloid dendritic cells and in the production of Th2-selective chemokines. However, when C5aR was blocked during airway allergen challenge in already Th2-sensitized mice, AHR and lung inflammation were attenuated. Based on these findings, it has been proposed that C5a plays a dual role in allergic asthma; protection from the development of maladaptive Th2 immune responses during allergen sensitization at the level of myeloid dendritic and the production of Th2 cytokines but enhancement of airway inflammation and AHR in an established inflammatory environment [15]. This suggests that, as for C3a, the effect of C5a on asthma likely involves the activation of effector cells such as mast cells and basophils.
3.1: Activation of human mast cells by C3a and C5a
Mast cells are important effector cells that orchestrate the development of AHR and inflammation via their close interaction with airway smooth muscle (ASM), T cells and leukocytes [46–50]. In lungs of asthmatic individuals, mast cells are found in different compartments including bronchoalveolar space beneath the basement membrane, adjacent to blood vessels and scattered throughout the ASM bundles [51,52]. The ability of allergen to cross-link FcεRI on mast cells to induce mediator release is well documented [53–55]. In addition to FcεRI, mast cells express C3a and C5a receptors [56–60], which have been implicated in the pathogenesis of asthma.
Two subtypes of human mast cells were initially recognized based on the composition of their secretory granules. Thus, mast cell granules that contain both tryptase and chymase are designated MCTC whereas those that contain only tryptase are known as MCT [61]. Interestingly, MCT cells predominate in the alveolar wall and the epithelium of the lung whereas MCTC cells favor bronchial smooth muscle and glandular regions [62]. Furthermore, MCT cell number in the respiratory epithelium increases during pollen season [63,64] and markedly elevated levels of MCTC cells are found in bronchial smooth muscle cells of patients with asthma [65]. These findings suggest that different mast cell types may play distinct roles in the pathogenesis of asthma.
Studies performed in the 1980 s indicated that while C3a and C5a induce mediator release in human skin mast cells, lung mast cells are unresponsive to these anaphylatoxins [56,66,67]. One possible reason for the discrepancy might reflect the fact that while MCTC cells are the predominant cell type present in the skin they are the minority cell type found in the lung [68,69]. Indeed, Oskeritzian et al, [62] recently showed that MCT cells in the lung do not express C5aR whereas MTC cells do and that this is correlated with substantial C5a-induced degranulation in MCTC cells. It is noteworthy that RBL-2H3 cells and BMMC, which are thought to be counterparts of human MCT mast cells do not express C3aR or C5aR and are unresponsive to anaphylatoxins for mediator release [23,24,70,71].
Although the effects of C3a on human lung MCTC cells are unknown, C3aR are expressed in a human mast cell line, HMC-1 cells [72–74], highly differentiated CD34+-derived primary human mast cells and a newly characterized MCTC type human mast cell line, LAD2 [57,60]. Furthermore, C3a is one of the most potent mast cell chemoattractant known [74,75] and it causes sustained Ca2+ mobilization, degranulation as well as chemokine production in primary human mast cells and LAD2 cells [60,73]. In addition, cell-cell contact between airway smooth muscle (ASM) cells and MCTC cells enhance C3a-induced mast cell mediator release [49]. Given that MCTC cells favor bronchial smooth muscle and glandular locations, it supports the idea that effects of C3a and C5a in the effector phase of asthma are mediated, at least in part, by mast cells.
3.2: Novel pathway for the generation of C3a and C5a requiring mast cells
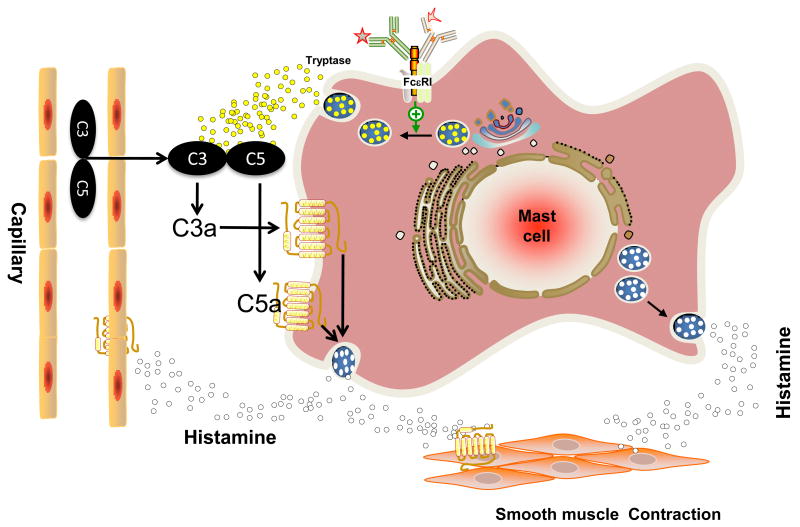
The best known mechanism for the generation of C3a and C5a is the classic IgG/antigen immune-complex pathway but it does not appear to play a major role in the pathogenesis of asthma. The lectin and alternative pathways may participate in the production of these anaphylatoxins but several proteolytic enzymes outside of these complement pathways also can generate anaphylatoxin-like activity, including thrombin, kallikrein, and house dust mite protease [76–80]. Since C3a and C5a are generated in the lung of asthmatic but not in normal individuals, this raises the possibility that mast cells could participate in the generation of these anaphylatoxins. Indeed, Fukuoka et al., [81] recently showed that β-tryptase, the major protease of human mast cells, can directly generate bioactive C3a and C5a in vitro. Furthermore, activation of human MCTC cells via the cross-linking of FcεRI results in the release of tryptase at sufficient concentrations to generate C3a and C5a from C3 and C5, respectively. Mast cell-derived mediators such as histamine can increase vascular permeability and the resulting exudation may serve to recruit C3 and C5 at the site of mast cell activation. The extravascular C3 and C5 are likely to be targets for mast cell-derived tryptase resulting in the generation of C3a and C5a. Thus, initial IgE-mediated release of histamine and tryptase may serve to amplify the allergic reaction through the generation of C3a and C5a, and additional mediator release via the subsequent activation of their GPCRs (see Fig. 1).
Fig. 1. Model for the role of FcεRI and mast cells on C3a and C5a generation and amplification of mediator release.
Mast cell numbers are increased in the lung of allergic individuals, which are likely to be activated via FcεRI. The release of mast-derived mediators such as histamine causes increase in vascular permeability and the resulting exudation likely contains C3 and C5. Tryptase released from activated mast cells act on C3 and C5 to locally generate C3a and C5a. These anaphylatoxins activate MCTC mast cells present in bronchial smooth muscle to further exacerbate symptoms associated with asthma. See text and Fukuoka et al., [81] for further detail. GPCR-independent pathway for the action of C3a is not shown (See Section 3.4).
3.3: GPCR-dependent pathway for the activation of mast cells by C3a and C5a
C3aR and C5aR belong to a family of seven transmembrane domain GPCRs that couple to the Gαi family of heterotrimeric G proteins. Under resting conditions, G proteins exist as heterotrimeric complexes consisting αβγ complex with GDP bound to the α-subunit (Gα). Receptor activation leads to a conformational change in Gα, resulting in an exchange of GTP for GDP. This interaction causes the dissociation of the βγ subunit (Gβγ) from the heterotrimeric complex. Gβ, of which there are many subtypes, plays essential roles in mediating diverse functions of GPCRs. C3a and C5a induce chemotaxis of human mast cell line HMC-1, human cord blood-derived mast cells (CBMC) and cutaneous mast cells in vitro and these responses are inhibited by receptor specific antibodies and pertussis toxin, inhibitor of Gαi family of G proteins [74,75]. Rat basophilic leukemia RBL-2H3 cells have been used extensively study to the molecular details of FcεRI signaling in mast cells. This cell line does not endogenously express receptors for C3a or C5a and does not respond to the anaphylatoxins for degranulation. However, RBL-2H3 cells ectopically expressing C3aR or C5aR are responsive to the anaphylatoxins for signaling and mediator release [22,23,82]. These findings suggest that the effects of C3a and C5a in mast cells are mediated via the activation of their respective GPCRs.
In addition to chemotaxis and degranulation, C3a and C5a also induce chemokine gene expression in mast cells [72,73,83]. The ability of C3a and C5a to induce early degranulation and delayed chemokine production release involves the activation of distinct signaling pathways including phospholipase Cβ (PLCβ)-mediated Ca2+ mobilization and protein kinase C (PKC) as well as phosphoinositide 3 kinase (PI3K) and extracellular signal regulated kinase (ERK) activation. C5a induces degranulation in mast cells via signaling pathways that require PLCβ but not PI3K or ERK [49,56,83,84]. By contrast, C3a promotes cytokine gene expression in mast cells via signaling pathways that require PLCβ, PI3K as well as ERK [72,83,85].
3.4: GPCR-independent pathway for the activation of mast cells by C3a
Basic peptides such as compound 48/80, substance P and mastoparan have been known for many decades to cause degranulation in rat peritoneal mast cells and human skin mast cells [56,86,87]. High concentrations of these peptides (micromolar range) are required for mast cell degranulation and their effects are blocked by neuraminidase, which hydrolyzes sialic acid residues on the cell surface, decreasing it’s negative charge. These peptides also activate purified Gαi proteins and treatment of mast cells with benzalkonium chloride, an inhibitor of Gαi, blocks degranulation. Based on these findings, it has been proposed that basic peptides utilize negatively charged residue on the surface of mast cells to induce degranulation by directly activating G proteins. It is noteworthy that C3a is a basic protein and is one of the few plasma proteins that can be generated at micromolar levels [88]. Furthermore, C3a (1 – 30 μM) causes degranulation of rat peritoneal mast cells and this response is inhibited by neuraminidase, pertussis toxin and benzalkonium chloride [88,89]. These findings suggest that C3a activates mast cell by two pathways; one at low concentration via the activation of cell surface GPCR and the other at high concentration involving the direct activation of G proteins.
4: Role of basophils in allergic diseases
Tissue mast cells and blood basophils share several features including surface expression of FcεRI, the presence of basophilic granules in the cytoplasm and the release of shared important chemical mediators. While the availability of genetically mast cell-deficient mice have provided a valuable tool to study the role of mast cells in allergic diseases, no mutant mice have been reported that selectively lack basophils. This, together with the fact that basophils represent a minor component of circulating blood leukocytes (<1%) and their similarities with mast cells, they have been neglected as minor and possibly redundant “circulating mast cells”. However, the recent development of two monoclonal antibodies that selectively deplete murine basophils have been instrumental in identifying novel roles for basophils in promoting allergen-induced Th2 cell differentiation, enhancing humoral memory immune responses [26,90,91], mediating IgG-mediated systemic anaphylaxis and IgE-mediated chronic allergic inflammation [92–94]. In most of these situations, complements are likely activated generating both C3a and C5a. These anaphylatoxins, particularly C5a, have the capability to release histamine, leukotriene C4 (LTC4) and the Th2 cytokines IL-4 and IL-13 comparable in magnitude to those induced via FcεRI cross-linking [95–98]. In the sections below, I discuss the roles of C3a and C5a in mediator release in human basophils and the signal transduction pathways involved in their activation and regulation.
4.1: C3a and C5a-induced histamine and LTC4 release in basophils; priming by IL-3
Whether or not leukocytes express C3aR has been the subject of considerable debate. Zwirner et al., [11] recently utilized monoclonal antibodies against two different epitopes on the third extracellular domain of the human C3aR to show that human basophils express ~8100 receptors/cells. However, C3a, at concentrations that activate RBL-2H3 cells stably expressing human C3aR [70,99], do not induce mediator release in human basophils [12]. The reason for this difference is unknown but could reflect differences in the expression levels of C3aR in human basophils and transfected RBL-2H3 cells.
Hematopoietic growth factors such as interleukin-5 (IL-5), granulocyte/macrophage colony stimulating factor (GM-CSF) and in particular interleukin-3 (IL-3) profoundly modify the effector function of mature human basophils. IL-3 is generated in large amounts from T cells and antigen/IgE-activated human basophils [100]. Interestingly, pre-incubation of human basophils with concentrations of IL-3 that are ~100-fold lower than those required for colony formation render them responsive to extremely low concentration of C3a (1 nM) for histamine release and the generation of LTC4 [12]. The release of these mediators in IL-3 primed cells is very rapid, being complete within 0.5 – 2 min. It has been proposed that IL-3 may induce priming of C3aR-mediated response via the induction of a high affinity state of the C3aR. However, this contention has yet to be verified experimentally. Using C3aR−/− mice and a monoclonal antibody to selectively deplete basophils in mice in vivo, it has been shown that C3aR expressed in basophils greatly contribute to peanut extract-induced anaphylaxis [101]. It is, however, unknown whether C3a induces mediator release in mouse basophils via the activation of C3aR and if this effect requires priming by IL-3 and other cytokines.
Human basophils express approximately twice as many C5aR on their surface as C3aR [11]. Unlike C3a, C5a causes rapid histamine release in the absence of IL-3 [12,102] (Table 1). Neither C3a nor C5a causes LTC4 production in human basophils [12,102]. However, basophils preincubated with low concentrations of IL-3 profoundly synergize with C3a and C5a to induce large quantities of LTC4. [103]. The magnitude of IL-3/C5a-induced LTC4 synthesis is much larger than that induced by IL3 and C3a combination [8,12,97]. The effect of IL-3 on priming has been investigated in some detail for C5a, which occurs in two phases. The first phase occurs very rapidly after exposure to IL-3, starting at 1 min, with optimal effects at 5 – 15 min but reduced at 2 h [8,96,102]. The second phase is observed after 18 – 24h of IL-3 treatment, and the magnitude of the C5a response is often greater than that observed after acute IL-3 pretreatment [104]. It is noteworthy that basophils are known to participate in the chronic phase of allergic diseases and lipid-derived mediators accumulate at these sites at amounts exceeding those found during immediate reaction [97]. It is likely that both C5a and IL-3 are generated continuously and concomitantly at the site of inflammation to induce LTC4 generation [97]. It is noteworthy that leukotriene antagonists have been used in the treatment of chronic allergic inflammation such as asthma [105]. It is therefore possible that C5a contributes to allergic diseases via the production of LTC4 in cytokine-primed basophils.
Table 1.
Regulation of C3a and C5a Receptor function in human basophils by IL-3
| Histamine Release | LTC4 | IL-4 | IL-13 | |
|---|---|---|---|---|
| C3a | − | − | − | − |
| IL-3 | − | − | − | − |
| IL-3 +C3a | + | + | − | − |
| C5a | ++ | − | − | − |
| IL-3 + C5a | ++++ | ++++ | +++ | ++++ |
Human basophils express ~8100 C3aR and ~13,500 C5aR on their surface. (−) indicates no or little mediator release. (+) to (++++) low to very high mediator release. See text for detail.
4.2: C5a, but not C3a, induces IL-4 and IL-13 in IL-3-primed human basophils
IL-4 and IL-13 are key immunoregulatory cytokines, which induce and amplify Th2-type immune responses and promote IgE synthesis [106,107]. Receptors for IL-4 and IL-13 are also expressed on airway smooth muscle cells and IL-13 causes smooth muscle contraction, promotes mucous secretion and lung remodeling [108–110]. Not surprisingly, these cytokines have been targeted for asthma therapeutics [111,112].
Basophils are a prominent source of IL-4 and IL-13, which are rapidly produced upon FcεRI cross-linking [98,100,113]. It is noteworthy that neither C3a nor C5a induce IL-4 or IL-13 in human basophils. However, in the presence of IL-3 co-stimulation, C5a but not C3a, induces large quantities of these cytokines [95,103,113,114] (Table 1). The magnitude and duration of the IL-4/IL-13 induction in response to IL-3/C5a are often greater than that induced via IgE cross-linking. The priming effect of IL-3 on C5a-induced Th2 cytokine generation does not depend on the sequence of their addition but requires their sustained presence. Furthermore, monoclonal C5aR antibody, and pertussis toxin block IL-3/C5a-induced Th2 cytokine production [95]. In addition, the effect of C5a on this response can be mimicked by C5a-derived peptides that are known to activate C5aR [95]. These findings, in total, suggest that basophil-specific modulation of C5aR or its signaling pathways may modulate both the Th2 response and the effector phase of asthma.
4.3: Signaling pathways for C3a and C5a-induced mediator release and priming by IL-3
The abilities of C3a (+IL-3) and C5a to induce histamine and LTC4 release in human basophils are likely mediated via the activation of their respective cell surface GPCRs [11]. Although the mechanism by which IL-3 primes C3a-induced LTC4 generation remains unknown, the signaling pathway via which IL-3 mediates both early and late phases of C5aR priming for the LTC4 generation has been studied in some detail. Elegant work by Miura et al., [115,116] supports the view that synchronized regulation of cytosolic phospholipase A2 (cPLA2) activity is required for the generation of arachidonic acid, which acts as a substrate for LTC4 synthesis. For optimal action, cPLA2 requires Ca2+ for phospholipid binding and it’s phosphorylation by extracellular signal regulated kinase (ERK). It is interesting to note that in the absence of IL-3, C5a causes a transient increase cytosolic Ca2+ that lasts for about 30 – 45 sec and induces cPLA2 phosphorylation that is not apparent until after the Ca2+ response returns to basal level [96]. Thus, Ca2+-mediated translocation of cPLA2 may be dissociated from the membrane before phosphorylation and activation of the enzyme can occur. However, short term pretreatment of human basophils with IL-3 causes rapid cPLA2 phosphorylation but does not alter the characteristics of C5a-induce Ca2+ response. Thus, it has been proposed that the ability of IL-3 to allow C5a to promote LTC4 release results from the preconditioning of cPLA2 due to its phosphorylation. Under this condition, brief transient Ca2+ mobilization that occurs following C5a stimulation overlaps to allow its full enzymatic with the pre-existing phosphorylated cPLA2 activity.
The ability of IL-3 to prime C5a-induced LTC4 generation after 18 h preincubation also involves synchronization of cPLA2 phosphorylation and Ca2+ mobilization but by different mechanism [8]. In this situation, exposure of basophils to IL-3 has no effect on the delayed cPLA2 activation by C5a but it converts a transient C5a-induced Ca2+ response to a sustained one, thus facilitating overlap of two synergistic signals; cPLA2 phosphorylation and Ca2+ mobilization, which are required for optimal cPLA2 activity and LTC4 generation. An important factor that distinguishes between early and late priming by IL-3 involves new protein synthesis. Thus, while treatment of basophils with cycloheximide inhibits both the sustained phase of the Ca2+ response to C5a and late priming effect of IL-3, it has no effect on early priming [8]. It is therefore possible that chronic exposure of basophils to IL-3 increases the expression of C5aR to induce priming. However, this explanation is unlikely as IL-3 has a global effect in modulating LTC4 generation in response to basophil stimulation by other receptor-mediated pathways [96,103,117,118].
5: Role of Receptor phosphorylation on C3aR and C5aR desensitization
Most, if not all GPCRs undergo desensitization that dampens cellular responses in the presence of continued stimulation. Importantly, desensitization regulates mediator release and thus prevents tissue damage [119]. This process involves agonist-induced receptor phosphorylation and β-arrestin recruitment [120]. The carboxyl terminus of GPCRs expressed in mast cells and basophils display low sequence conservation except for a large number of clustered phosphorylation sites [121]. C3aR possess ten potential phosphorylation sites in two distinct clusters. C3a causes rapid phosphorylation of its receptors in RBL-2H3 cells stably expressing human C3aR or HMC-1 cells natively expressing the receptor [70,122]. Phosphopeptide mapping analysis showed that C3a causes phosphorylation of the receptor at both serine and threonine residues. Replacing all ten serine and threonine residues with alanine leads to more robust G protein activation and greater degranulation when compared to wild-type receptors [123–128]. These findings are consistent with the notion that, as in many other cell types, receptor phosphorylation desensitizes C3aR function in mast cells.
In addition to mast cells and basophils, C5aR is expressed in human neutrophils. Boulay and colleagues [129–131] have utilized neutrophil-like HL60 cells and transfected COS-7 cells to show that although C5aR possesses six serine and 5 threonine residues at its carboxyl terminus, C5a causes phosphorylation of the receptor only at the serine residues with a maximal stoichiometry of 6 mol of PO4/mol of receptor at Ser314, Ser317, Ser327, Ser332, Ser334, and Ser338. Using a mutagenesis approach they have shown that C5aR undergoes sequential phosphorylation with Ser334 as the major initial site followed by residues at positions Ser332 and Ser338 playing significant roles. Christophe et al., [130], demonstrated that phosphorylation of either of two serine pairs, namely Ser332 and Ser334 or Ser334 and Ser338, is critical for the phosphorylation of C5aR and its subsequent desensitization. Replacement of Ser residues at these sites with Ala and their transfection in undifferentiated HL-60 cells results in a more sustained calcium mobilization, enhanced ERK phosphorylation and greater superoxide generation when compared to cells expressing wild-type receptors [130]. C5a also causes phosphorylation of its receptor in HMC-1 cells natively expressing C5aR and RBL-2H3 cells stably expressing the human receptor [23,132]. Pollok-Kopp et al., [133] recently showed that when compared to RBL-2H3 cells expressing wild-type receptor, C5a causes enhanced Ca2+ mobilization in RBL-2H3 cells expressing a mutant C5aR in which all six serine residues were replaced with alanine. This enhanced Ca2+ mobilization was partially reversed in cells expressing C5aR that had intact residues at positions Ser327, Ser334 and Ser338. This indicates that C5aR phosphorylation at multiple sites regulates signaling in mast cells and presumably in basophils.
5.1. Role of GRKs on C3aR and C5aR phosphorylation and Desensitization
GPCRs are phosphorylated by a family of protein kinases, collectively known as GRKs (G protein coupled receptor kinases). Of the seven known GRKs, four (GRK2, GRK3, GRK5 and GRK6) are expressed in peripheral blood leukocytes, myeloid cell lines [134–138] and human mast cells (Guo, Q and Ali, H; unpublished data). All GRKs (60–80kDa) possess a similar structural organization consisting of an amino terminal domain (185 amino acids) a catalytic domain (270 amino acids) and a carboxyl terminal domain (105 to 230 amino acids). There are, however, important differences in the mechanism via which GRK2/GRK3 vs. GRK5/GRK6 are localized to the proximity of the receptor to induce receptor phosphorylation [139]. GRK2 and GRK3 are found primarily in the cytoplasm and undergo translocation to the plasma membrane upon G protein activation, via their interaction with Gβγ subunit and membrane phospholipids. By contrast, GRK5 and GRK6 do not associate with Gβγ but interact with phospholipids or require lipid modification for their association with receptors. Overexpression of GRK2, GRK3, GRK5 and GRK6 with C3aR in COS cells enhanced agonist-induced receptor phosphorylation [122]. However, only GRK2 and GRK3 caused significant inhibition C3a-induced G-protein activation. Furthermore, introduction of monoclonal antibodies to GRK2 and GRK3 inhibited agonist-induced C3aR phosphorylation but antibodies to GRK5 or GRK6 had no effect. These findings suggest that recruitment of GRK2 or GRK3 following C3aR activation leads to receptor phosphorylation and desensitization. The role of C3aR phosphorylation by GRK5 and GRK6 has yet to be determined.
Langkabel et al., [132] showed that as for C3aR, overexpression of GRK2, GRK3, GRK5 or GRK6 augmented C5a-induced phosphorylation of its receptors in transfected COS-7 cells. By contrast, Milcent et al., [140] demonstrated that overexpression of GRK2 or GRK6 has no effect on agonist-induced C5aR phosphorylation. Despite this difference, GRK6−/− mice have elevated serum IL-6 in an in vivo K/BxN model of inflammatory arthritis and enhanced granulocyte migration toward C5a in vitro [141]. These findings suggest that GRK6 may desensitize inflammatory responses by regulating granulocyte trafficking and reducing cytokine generation in response to C5a in vivo. It remains to be determined which GRK regulates C5aR phosphorylation in mast cells and basophils to modulate allergic asthma.
5.2: Role of β-arrestin on desensitization, internalization and modulation of downstream signaling
One of the most intensely studied proteins that interact with phosphorylated GPCRs is β-arrestin. Two isoforms of β-arrestins (β-arrestin 1 and 2) are expressed in many cell types including mast cells [142,143]. In transfected RBL-2H3 cells, β-arrestin associates with wild-type but not phosphorylation-deficient C3aR [70]. Furthermore, overexpression of β-arrestin with C3aR enhances receptor internalization [20]. These findings are consistent with the notion that agonist-induced receptor phosphorylation leads to β-arrestin recruitment which promotes desensitization and internalization. However, the specific phosphorylation site in the carboxyl terminus of C3aR that interacts with β-arrestin and the GRK which mediates these responses remains unknown. Boulay and colleagues showed that agonist-induced C5a phosphorylation in neutrophil-like HL-60 cells, caused β-arrestin recruitment resulting in desensitization and internalization [129,130].
In addition to its role in receptor desensitization, β-arrestin acts as an adapter molecule to regulate diverse cellular function independent of desensitization [144–146]. For example, β-arrestins directly interact with several Src family kinases, ubiquitin ligases, protein phosphatases, microtubules, etc., and serve as scaffolds facilitating signaling in two MAP kinase cascades, leading to the activation of ERK1/2 and JNK3 [147–149]. Although the role of β-arrestin on the activation of downstream signaling pathways has not been studied in detail in mast cells and basophils, it does not appear to be required for C3a and C5a-mediated ERK phosphorylation. For example phosphorylation deficient C3aR and C5aR, which do not associate with β-arrestin support greater ERK phosphorylation [22,130]. This suggests that unlike many GPCRs, β-arrestin plays inhibitory rather than stimulatory role in C3a and C5a-induced ERK phosphorylation in mast cells and basophils (Fig. 3).
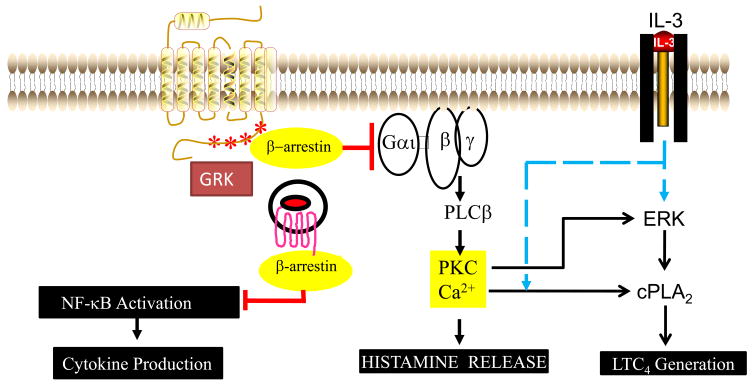
Fig. 3. Model for the role of GRKs and β-arrestin on the regulation of C3a and C5a receptors.
C3a and C5a bind to their GPCRs on mast cells and basophils to activate PLCβ, cPLA2 and NF-κB to induce release of different mediators. IL-3 enhances C3a and C5a-induced mediator release in human basophils. Effects of IL-3 on enhanced C5a-induced LTC4 generation involves ERK phosphorylation (early) and greater C5a-induced Ca2+ mobilization (late) (Blue dotted lines). Receptor phosphorylation by GRKs serves to recruit β-arrestin and this complex interacts with G protein to desensitize degranulation and LTC4 generation. Internalized β-arrestin-associated receptor inhibits NF-κB activation to block delayed cytokine gene expression (red lines).
NF-κB is a transcription factor that regulates the expression of a variety genes leading to the formation of chemokine and cytokines. In resting cells, most of the NF-κB is bound to a potent inhibitor IκB, thus retaining this complex in the cytoplasm [150]. Upon cell activation IκB is phosphorylated by IκB kinase (IKK) leading to its proteosomal degradation. NF-κB, once dissociated from IκB, rapidly translocates to the nucleus where it binds to specific promoters of the target genes. Although several IκB isoforms are known, Gao et al., [151] made the surprising observation that β-arrestin 2 directly interacts with IκBα to inhibit GPCR-mediated NF-κB activity. Witherow et al [152], showed that although both β-arrestin 1 and β-arrestin 2 associate with IκBα as well as upstream kinases such as IKKα, IKKβ and NIK, only β-arrestin 1 inhibits NF-κB activity and cytokine production. Our recent studies with platelet activating factor (PAF) β-arrestin inhibits NF-κB activity and chemokine induction in mast cells [20,153]. Furthermore, overexpression of β-arrestin enhances agonist-induced C3aR internalization and blocks chemokine CCL2 generation [20]. Also, similar to the situation with PAFR [153], expression of C3aR in mouse embryonic fibroblasts deficient in both β-arrestin-1 and β-arrestin-2, inhibits agonist-induced C3aR internalization but enhances NF-κB activity (Ali, H., unpublished data). These findings, in total suggest that unlike the situation with many GPCRs, β-arrestin plays a critical role in inhibition of C3a and C5a-induced degranulation, ERK phosphorylation, NF-κB activation, LTC4 generation and cytokine synthesis (Fig. 3).
6. Summary and Conclusions
This review discusses the role of anaphylatoxins in the pathogenesis of allergic asthma. Studies with C3aR−/−, C5a−/− mice as well as receptor-specific antibodies and inhibitors have shown that although C3a and C5a have opposing effects on allergen sensitization, they promote two important features of asthma, AHR and lung inflammation. These findings indicate that effects of C3a and C5a in allergic asthma involve the activation of effector cells. Given that mast cells are important effector cells in asthma and that basophils play a critical role in chronic allergy, this review has focused mainly on the activation of these cells by C3a and C5a and the regulation of their receptors by priming and desensitization.
Emerging evidence suggests that tryptase released from FcεRI-activated mast cells generate C3a and C5a from C3 and C5, respectively, and that these anaphylatoxins act on MCTC mast cells found in bronchial smooth muscles to induce mediator release causing smooth muscle contraction (Fig. 1). GPCRs for C3a and C5a are expressed on the surface of human basophils but there are important differences in the magnitude and diversity of mediators induced by these anaphylatoxins and their synergy with IL-3 (Table 1 and Fig. 2). It appears that, as for other GPCRs, agonist-induced receptor phosphorylation plays a critical role in the desensitization of C3aR and C5aR. In many cell types, β-arrestin acts as an adapter molecule to activate ERK and other intracellular signaling pathways. However, studies with transfected cell lines indicate that β-arrestin is not only involved in the desensitization of C3a and C5a-induced degranulation, it inhibits both ERK phosphorylation and NF-κB activation (Fig. 3). This suggests that β-arrestin could be targeted in mast cells and basophils for the modulation of allergic diseases. It must be pointed out that although most of mediator release and signaling studies discussed in this review were performed with primary human mast cells and basophils, phosphorylation and desensitization studies utilized cell lines such as RBL-2H3 cells, neutrophil-like HL-60 cells and COS7 cells. Future studies are therefore required to confirm these findings in primary mast cells and basophils and ultimately in animal models to evaluate the potential for the development of novel asthma therapeutics by targeting anaphylatoxin signaling in these FcεRI-bearing immune cells.
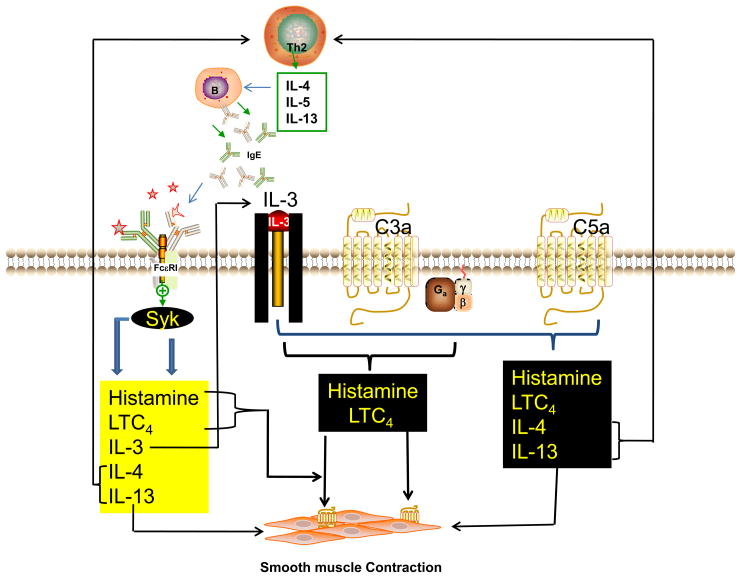
Fig. 2. Possible role of basophil-derived IL-3 on the priming of C3a and C5a-induced mediator release.
IL-3 generated via the activation of FcεRI in basophils interacts with it s cell surface receptors on basophils to prime both C3a-induced histamine release and LTC4 generation. IL-3 also enhances C5a-induced histamine release, LTC4 generation (early phase and later phase) as well as Th2 cytokines IL-4 and IL-13. These basophil-derived mediators are likely to have profound influence on allergen sensitization, bronchial smooth contraction and delayed inflammation.
Acknowledgments
Studies performed in the author’s laboratory described in this review were supported by National Institutes of Health grants HL063372 and HL085774.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beaven MA, Hundley TR. Mast cell related diseases: genetics, signaling pathways, and novel therapies. In: Finkel TH, Gutkind JS, editors. Signal Transduction and Human Disease. Hoboken, NJ: 2003. pp. 307–355. [Google Scholar]
- 2.US CDC. Forecasted state-specific estimates of self reported asthma prevalence-United States. MMWR Morb Mortal Wkly Rep. 1998;47:1022–1025. [PubMed] [Google Scholar]
- 3.Tarantini F, Baiardini I, Passalacqua G, Braido F, Canonica GW. Asthma treatment: ‘magic bullets which seek their own targets’. Allergy. 2007;62:605–610. doi: 10.1111/j.1398-9995.2007.01390.x. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn R. Immunoglobulin E blockade in the treatment of asthma. Pharmacotherapy. 2007;27:1412–1424. doi: 10.1592/phco.27.10.1412. [DOI] [PubMed] [Google Scholar]
- 5.Djukanovic R, Wilson SJ, Kraft M, Jarjour NN, Steel M, Chung KF, et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med. 2004;170:583–593. doi: 10.1164/rccm.200312-1651OC. [DOI] [PubMed] [Google Scholar]
- 6.Masuda ES, Schmitz J. Syk inhibitors as treatment for allergic rhinitis. Pulm Pharmacol Ther. 2008;21:461–467. doi: 10.1016/j.pupt.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Meltzer EO, Berkowitz RB, Grossbard EB. An intranasal Syk-kinase inhibitor (R112) improves the symptoms of seasonal allergic rhinitis in a park environment. J Allergy Clin Immunol. 2005;115:791–796. doi: 10.1016/j.jaci.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 8.Miura K, MacGlashan DW., Jr Dual phase priming by IL-3 for leukotriene C4 generation in human basophils: difference in characteristics between acute and late priming effects. J Immunol. 2000;164:3026–3034. doi: 10.4049/jimmunol.164.6.3026. [DOI] [PubMed] [Google Scholar]
- 9.Humbles AA, Lu B, Nilsson CA, Lilly C, Israel E, Fujiwara Y, et al. A role for the C3a anaphylatoxin receptor in the effector phase of asthma. Nature. 2000;406:998–1001. doi: 10.1038/35023175. [DOI] [PubMed] [Google Scholar]
- 10.Drouin SM, Kildsgaard J, Haviland J, Zabner J, Jia HP, McCray PB, Jr, et al. Expression of the complement anaphylatoxin C3a and C5a receptors on bronchial epithelial and smooth muscle cells in models of sepsis and asthma. J Immunol. 2001;166:2025–2032. doi: 10.4049/jimmunol.166.3.2025. [DOI] [PubMed] [Google Scholar]
- 11.Zwirner J, Gotze O, Begemann G, Kapp A, Kirchhoff K, Werfel T. Evaluation of C3a receptor expression on human leucocytes by the use of novel monoclonal antibodies. Immunology. 1999;97:166–172. doi: 10.1046/j.1365-2567.1999.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bischoff SC, de Weck AL, Dahinden CA. Interleukin 3 and granulocyte/macrophage-colony-stimulating factor render human basophils responsive to low concentrations of complement component C3a. Proc Natl Acad Sci U S A. 1990;87:6813–6817. doi: 10.1073/pnas.87.17.6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Lewkowich IP, Kohl G, Clark JR, Wills-Karp M, Kohl J. A protective role for C5a in the development of allergic asthma associated with altered levels of B7-H1 and B7-DC on plasmacytoid dendritic cells. J Immunol. 2009;182:5123–5130. doi: 10.4049/jimmunol.0804276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lambrecht BN. An unexpected role for the anaphylatoxin C5a receptor in allergic sensitization. J Clin Invest. 2006;116:628–632. doi: 10.1172/JCI27876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohl J, Baelder R, Lewkowich IP, Pandey MK, Hawlisch H, Wang L, et al. A regulatory role for the C5a anaphylatoxin in type 2 immunity in asthma. J Clin Invest. 2006;116:783–796. doi: 10.1172/JCI26582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dillard P, Wetsel RA, Drouin SM. Complement C3a regulates Muc5ac expression by airway clara cells independently of Th2 responses. Am J Respir Crit Care Med. 2007;175:1250–1258. doi: 10.1164/rccm.200701-049OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerard NP, Gerard C. Complement in allergy and asthma. Curr Opin Immunol. 2002;14:705–708. doi: 10.1016/s0952-7915(02)00410-7. [DOI] [PubMed] [Google Scholar]
- 18.Wust SK, Blumenthal MN, Corazalla EO, Benson BA, Dalmasso AP. Complement in asthma: sensitivity to activation and generation of C3a and C5a via the different complement pathways. Transl Res. 2006;148:157–163. doi: 10.1016/j.trsl.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Kohl J. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009;49:2753–2766. doi: 10.1016/j.molimm.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaidi AK, Ali H. C3a receptors signaling in mast cells. Adv Exp Med Biol. 2007;598:126–140. doi: 10.1007/978-0-387-71767-8_10. [DOI] [PubMed] [Google Scholar]
- 21.Ali H, Panettieri RA., Jr Anaphylatoxin C3a receptors in asthma. Respir Res. 2005;6:19. doi: 10.1186/1465-9921-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahamed J, Ali H. Distinct roles of receptor phosphorylation, G protein usage, and mitogen-activated protein kinase activation on platelet activating factor-induced leukotriene C4 generation and chemokine production. J Biol Chem. 2002;277:22685–22691. doi: 10.1074/jbc.M110210200. [DOI] [PubMed] [Google Scholar]
- 23.Ali H, Richardson RM, Tomhave ED, Didsbury JR, Snyderman R. Differences in phosphorylation of formylpeptide and C5a chemoattractant receptors correlate with differences in desensitization. J Biol Chem. 1993;268:24247–24254. [PubMed] [Google Scholar]
- 24.Erdei A, Kerekes K, Pecht I. Role of C3a and C5a in the activation of mast cells. Exp Clin Immunogenet. 1997;14:16–18. [PubMed] [Google Scholar]
- 25.Karasuyama H, Mukai K, Tsujimura Y, Obata K. Newly discovered roles for basophils: a neglected minority gains new respect. Nat Rev Immunol. 2009;9:9–13. doi: 10.1038/nri2458. [DOI] [PubMed] [Google Scholar]
- 26.Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, et al. Basophils contribute to Th2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 28.Boos L, Szalai AJ, Barnum SR. C3a expressed in the central nervous system protects against LPS-induced shock. Neurosci Lett. 2005;387:68–71. doi: 10.1016/j.neulet.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Kildsgaard J, Hollmann TJ, Matthews KW, Bian K, Murad F, Wetsel RA. Cutting edge: targeted disruption of the C3a receptor gene demonstrates a novel protective anti-inflammatory role for C3a in endotoxin-shock. J Immunol. 2000;165:5406–5409. doi: 10.4049/jimmunol.165.10.5406. [DOI] [PubMed] [Google Scholar]
- 30.Bao L, Osawe I, Haas M, Quigg RJ. Signaling through up-regulated C3a receptor is key to the development of experimental lupus nephritis. J Immunol. 2005;175:1947–1955. doi: 10.4049/jimmunol.175.3.1947. [DOI] [PubMed] [Google Scholar]
- 31.Nakano Y, Morita S, Kawamoto A, Suda T, Chida K, Nakamura H. Elevated complement C3a in plasma from patients with severe acute asthma. J Allergy Clin Immunol. 2003;112:525–530. doi: 10.1016/s0091-6749(03)01862-1. [DOI] [PubMed] [Google Scholar]
- 32.Castro FF, Schmitz-Schumann M, Rother U, Kirschfink M. Complement activation by house dust: reduced reactivity of serum complement in patients with bronchial asthma. Int Arch Allergy Appl Immunol. 1991;96:305–310. doi: 10.1159/000235513. [DOI] [PubMed] [Google Scholar]
- 33.Fregonese L, Swan FJ, Van Schadewijk A, Dolhnikoff M, Santos MA, Daha MR, et al. Expression of the anaphylatoxin receptors C3aR and C5aR is increased in fatal asthma. J Allergy Clin Immunol. 2005;115:1148–1154. doi: 10.1016/j.jaci.2005.01.068. [DOI] [PubMed] [Google Scholar]
- 34.Hasegawa K, Tamari M, Shao C, Shimizu M, Takahashi N, Mao XQ, et al. Variations in the C3, C3a receptor, and C5 genes affect susceptibility to bronchial asthma. Hum Genet. 2004;115:295–301. doi: 10.1007/s00439-004-1157-z. [DOI] [PubMed] [Google Scholar]
- 35.Drouin SM, Corry DB, Kildsgaard J, Wetsel RA. Cutting edge: the absence of C3 demonstrates a role for complement in Th2 effector functions in a murine model of pulmonary allergy. J Immunol. 2001;167:4141–4145. doi: 10.4049/jimmunol.167.8.4141. [DOI] [PubMed] [Google Scholar]
- 36.Taube C, Rha YH, Takeda K, Park JW, Joetham A, Balhorn A, et al. Inhibition of complement activation decreases airway inflammation and hyperresponsiveness. Am J Respir Crit Care Med. 2003;168:1333–1341. doi: 10.1164/rccm.200306-739OC. [DOI] [PubMed] [Google Scholar]
- 37.Drouin SM, Corry DB, Hollman TJ, Kildsgaard J, Wetsel RA. Absence of the complement anaphylatoxin C3a receptor suppresses Th2 effector functions in a murine model of pulmonary allergy. J Immunol. 2002;169:5926–5933. doi: 10.4049/jimmunol.169.10.5926. [DOI] [PubMed] [Google Scholar]
- 38.Baelder R, Fuchs B, Bautsch W, Zwirner J, Kohl J, Hoymann HG, et al. Pharmacological targeting of anaphylatoxin receptors during the effector phase of allergic asthma suppresses airway hyperresponsiveness and airway Inflammation. J Immunol. 2005;174:783–789. doi: 10.4049/jimmunol.174.2.783. [DOI] [PubMed] [Google Scholar]
- 39.Bautsch W, Hoymann HG, Zhang Q, Meier-Wiedenbach I, Raschke U, Ames RS, et al. Cutting edge: guinea pigs with a natural C3a-receptor defect exhibit decreased bronchoconstriction in allergic airway disease: evidence for an involvement of the C3a anaphylatoxin in the pathogenesis of asthma. J Immunol. 2000;165:5401–5405. doi: 10.4049/jimmunol.165.10.5401. [DOI] [PubMed] [Google Scholar]
- 40.Wills-Karp M, Koehl J. New insights into the role of the complement pathway in allergy and asthma. Curr Allergy Asthma Rep. 2005;5:362–369. doi: 10.1007/s11882-005-0007-y. [DOI] [PubMed] [Google Scholar]
- 41.Gutzmer R, Lisewski M, Zwirner J, Mommert S, Diesel C, Wittmann M, et al. Human monocyte-derived dendritic cells are chemoattracted to C3a after up-regulation of the C3a receptor with interferons. Immunology. 2004;111:435–443. doi: 10.1111/j.1365-2567.2004.01829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirchhoff K, Weinmann O, Zwirner J, Begemann G, Gotze O, Kapp A, Werfel T. Detection of anaphylatoxin receptors on CD83+ dendritic cells derived from human skin. Immunology. 2001;103:210–217. doi: 10.1046/j.1365-2567.2001.01197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soruri A, Kiafard Z, Dettmer C, Riggert J, Kohl J, Zwirner J. IL-4 down-regulates anaphylatoxin receptors in monocytes and dendritic cells and impairs anaphylatoxin-induced migration in vivo. J Immunol. 2003;170:3306–3314. doi: 10.4049/jimmunol.170.6.3306. [DOI] [PubMed] [Google Scholar]
- 44.Werfel T, Kirchhoff K, Wittmann M, Begemann G, Kapp A, Heidenreich F, et al. Activated human T lymphocytes express a functional C3a receptor. J Immunol. 2000;165:6599–6605. doi: 10.4049/jimmunol.165.11.6599. [DOI] [PubMed] [Google Scholar]
- 45.Karp CL, Grupe A, Schadt E, Ewart SL, Keane-Moore M, Cuomo PJ, et al. Identification of complement factor 5 as a susceptibility locus for experimental allergic asthma. Nat Immunol. 2000;1:221–226. doi: 10.1038/79759. [DOI] [PubMed] [Google Scholar]
- 46.Taube C, Wei X, Swasey CH, Joetham A, Zarini S, Lively T, et al. Mast cells, FcεRI, and IL-13 are required for development of airway hyperresponsiveness after aerosolized allergen exposure in the absence of adjuvant. J Immunol. 2004;172:6398–6406. doi: 10.4049/jimmunol.172.10.6398. [DOI] [PubMed] [Google Scholar]
- 47.Brightling CE, Bradding P. The Re-emergence of the mast cell as a pivotal cell in asthma pathogenesis. Curr Allergy Asthma Rep. 2005;5:130–135. doi: 10.1007/s11882-005-0086-9. [DOI] [PubMed] [Google Scholar]
- 48.Page S, Ammit AJ, Black JL, Armour CL. Human mast cell and airway smooth muscle cell interactions: implications for asthma. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1313–1323. doi: 10.1152/ajplung.2001.281.6.L1313. [DOI] [PubMed] [Google Scholar]
- 49.Thangam EB, Venkatesha RT, Zaidi AK, Jordan-Sciutto KL, Goncharov DA, Krymskaya VP, et al. Airway smooth muscle cells enhance C3a-induced mast cell degranulation following cell-cell contact. FASEB J. 2005;19:798–800. doi: 10.1096/fj.04-2797fje. [DOI] [PubMed] [Google Scholar]
- 50.Robinson DS. The role of the mast cell in asthma: induction of airway hyperresponsiveness by interaction with smooth muscle? J Allergy Clin Immunol. 2004;114:58–65. doi: 10.1016/j.jaci.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 51.Casolaro V, Galeone D, Giacummo A, Sanduzzi A, Melillo G, Marone G. Human basophil/mast cell releasability. V. Functional comparisons of cells obtained from peripheral blood, lung parenchyma, and bronchoalveolar lavage in asthmatics. Am Rev Respir Dis. 1989;139:1375–1382. doi: 10.1164/ajrccm/139.6.1375. [DOI] [PubMed] [Google Scholar]
- 52.Marone G, Triggiani M, de Paulis A. Mast cells and basophils: friends as well as foes in bronchial asthma? Trends Immunol. 2005;26:25–31. doi: 10.1016/j.it.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 53.Cai Y, Bjermer L, Halstensen TS. Bronchial mast cells are the dominating LTC4S-expressing cells in aspirin-tolerant asthma. Am J Respir Cell Mol Biol. 2003;29:683–693. doi: 10.1165/rcmb.2002-0174OC. [DOI] [PubMed] [Google Scholar]
- 54.Venkatachalam TK, Qazi S, Samuel P, Uckun FM. Inhibition of mast cell leukotriene release by thiourea derivatives. Bioorg Med Chem Lett. 2003;13:485–488. doi: 10.1016/s0960-894x(02)00992-7. [DOI] [PubMed] [Google Scholar]
- 55.Andrade MV, Hiragun T, Beaven MA. Dexamethasone suppresses antigen-induced activation of phosphatidylinositol 3-kinase and downstream responses in mast cells. J Immunol. 2004;172:7254–7262. doi: 10.4049/jimmunol.172.12.7254. [DOI] [PubMed] [Google Scholar]
- 56.el-Lati SG, Dahinden CA, Church MK. Complement peptides C3a- and C5a-induced mediator release from dissociated human skin mast cells. J Invest Dermatol. 1994;102:803–806. doi: 10.1111/1523-1747.ep12378589. [DOI] [PubMed] [Google Scholar]
- 57.Woolhiser MR, Brockow K, Metcalfe DD. Activation of human mast cells by aggregated IgG through FcεRI: additive effects of C3a. Clin Immunol. 2004;110:172–180. doi: 10.1016/j.clim.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 58.Thangam EB, Venkatesha RT, Zaidi AK, Jordan-Sciutto KL, Goncharov DA, Krymskaya VP, et al. Airway smooth muscle cells enhance C3a-induced mast cell degranulation following cell-cell contact. FASEB J. 2005;19:798–800. doi: 10.1096/fj.04-2797fje. [DOI] [PubMed] [Google Scholar]
- 59.Ali H, Panettieri RA., Jr Anaphylatoxin C3a receptors in asthma. Respir Res. 2005;6:19. doi: 10.1186/1465-9921-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Venkatesha RT, Thangam EB, Zaidi AK, Ali H. Distinct regulation of C3a-induced MCP-1/CCL2 and RANTES/CCL5 production in human mast cells by extracellular signal regulated kinase and PI3 kinase. Mol Immunol. 2005;42:581–587. doi: 10.1016/j.molimm.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 61.Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci U S A. 1986;83:4464–4468. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oskeritzian CA, Zhao W, Min HK, Xia HZ, Pozez A, Kiev J, et al. Surface CD88 functionally distinguishes the MCTC from the MCT type of human lung mast cell. J Allergy Clin Immunol. 2005;115:1162–1168. doi: 10.1016/j.jaci.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gibson PG, Allen CJ, Yang JP, Wong BJ, Dolovich J, Denburg J, et al. Intraepithelial mast cells in allergic and nonallergic asthma. Assessment using bronchial brushings. Am Rev Respir Dis. 1993;148:80–86. doi: 10.1164/ajrccm/148.1.80. [DOI] [PubMed] [Google Scholar]
- 64.Juliusson S, Pipkorn U, Karlsson G, Enerback L. Mast cells and eosinophils in the allergic mucosal response to allergen challenge: changes in distribution and signs of activation in relation to symptoms. J Allergy Clin Immunol. 1992;90:898–909. doi: 10.1016/0091-6749(92)90462-b. [DOI] [PubMed] [Google Scholar]
- 65.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 66.Schulman ES, Post TJ, Henson PM, Giclas PC. Differential effects of the complement peptides, C5a and C5a des Arg on human basophil and lung mast cell histamine release. J Clin Invest. 1988;81:918–923. doi: 10.1172/JCI113403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fureder W, Agis H, Willheim M, Bankl HC, Maier U, Kishi, et al. Differential expression of complement receptors on human basophils and mast cells. Evidence for mast cell heterogeneity and CD88/C5aR expression on skin mast cells. J Immunol. 1995;155:3152–3160. [PubMed] [Google Scholar]
- 68.Xia HZ, Kepley CL, Sakai K, Chelliah J, Irani AM, Schwartz LB. Quantitation of tryptase, chymase, FcεRIα, and FcεRγ mRNAs in human mast cells and basophils by competitive reverse transcription-polymerase chain reaction. J Immunol. 1995;154:5472–5480. [PubMed] [Google Scholar]
- 69.Irani AM, Bradford TR, Kepley CL, Schechter NM, Schwartz LB. Detection of MCT and MCTC types of human mast cells by immunohistochemistry using new monoclonal anti-tryptase and anti-chymase antibodies. J Histochem Cytochem. 1989;37:1509–1515. doi: 10.1177/37.10.2674273. [DOI] [PubMed] [Google Scholar]
- 70.Ahamed J, Haribabu B, Ali H. Cutting edge: Differential regulation of chemoattractant receptor-induced degranulation and chemokine production by receptor phosphorylation. J Immunol. 2001;167:3559–3563. doi: 10.4049/jimmunol.167.7.3559. [DOI] [PubMed] [Google Scholar]
- 71.Erdei A, Andreev S, Pecht I. Complement peptide C3a inhibits IgE-mediated triggering of rat mucosal mast cells. Int Immunol. 1995;7:1433–1439. doi: 10.1093/intimm/7.9.1433. [DOI] [PubMed] [Google Scholar]
- 72.Ahamed J, Venkatesha RT, Thangam EB, Ali H. C3a enhances nerve growth factor-induced NFAT activation and chemokine production in a human mast cell line, HMC-1. J Immunol. 2004;172:6961–6968. doi: 10.4049/jimmunol.172.11.6961. [DOI] [PubMed] [Google Scholar]
- 73.Ali H, Ahamed J, Hernandez-Munain C, Baron JL, Krangel MS, Patel DD. Chemokine production by G protein-coupled receptor activation in a human mast cell line: roles of extracellular signal-regulated kinase and NFAT. J Immunol. 2000;165:7215–7223. doi: 10.4049/jimmunol.165.12.7215. [DOI] [PubMed] [Google Scholar]
- 74.Nilsson G, Johnell M, Hammer CH, Tiffany HL, Nilsson K, Metcalfe DD, et al. C3a and C5a are chemotaxins for human mast cells and act through distinct receptors via a pertussis toxin-sensitive signal transduction pathway. J Immunol. 1996;157:1693–1698. [PubMed] [Google Scholar]
- 75.Hartmann K, Henz BM, Kruger-Krasagakes S, Kohl J, Burger R, Guhl S, Haase I, et al. C3a and C5a stimulate chemotaxis of human mast cells. Blood. 1997;89:2863–2870. [PubMed] [Google Scholar]
- 76.Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12:682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 77.Huber-Lang M, Younkin EM, Sarma JV, Riedemann N, McGuire SR, Lu KT, et al. Generation of C5a by phagocytic cells. Am J Pathol. 2002;161:1849–1859. doi: 10.1016/S0002-9440(10)64461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wetsel RA, Kolb WP. Complement-independent activation of the fifth component (C5) of human complement: limited trypsin digestion resulting in the expression of biological activity. J Immunol. 1982;128:2209–2216. [PubMed] [Google Scholar]
- 79.Maruo K, Akaike T, Ono T, Okamoto T, Maeda H. Generation of anaphylatoxins through proteolytic processing of C3 and C5 by house dust mite protease. J Allergy Clin Immunol. 1997;100:253–260. doi: 10.1016/s0091-6749(97)70233-1. [DOI] [PubMed] [Google Scholar]
- 80.Wiggins RC, Giclas PC, Henson PM. Chemotactic activity generated from the fifth component of complement by plasma kallikrein of the rabbit. J Exp Med. 1981;153:1391–1404. doi: 10.1084/jem.153.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fukuoka Y, Xia HZ, Sanchez-Munoz LB, Dellinger AL, Escribano L, Schwartz LB. Generation of anaphylatoxins by human β-tryptase from C3, C4, and C5. J Immunol. 2008;180:6307–6316. doi: 10.4049/jimmunol.180.9.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ames RS, Tornetta MA, Foley JJ, Hugli TE, Sarau HM. Evidence that the receptor for C4a is distinct from the C3a receptor. Immunopharmacology. 1997;38:87–92. doi: 10.1016/s0162-3109(97)00079-9. [DOI] [PubMed] [Google Scholar]
- 83.Venkatesha RT, Berla Thangam E, Zaidi AK, Ali H. Distinct regulation of C3a-induced MCP-1/CCL2 and RANTES/CCL5 production in human mast cells by extracellular signal regulated kinase and PI3 kinase. Mol Immunol. 2005;42:581–587. doi: 10.1016/j.molimm.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 84.ter Laan B, Molenaar JL, Feltkamp-Vroom TM, Pondman KW. Interaction of human anaphylatoxin C3a with rat mast cells demonstrated by immunofluorescence. Eur J Immunol. 1974;4:393–395. doi: 10.1002/eji.1830040517. [DOI] [PubMed] [Google Scholar]
- 85.Ali H, Ahamed J, Hernandez-Munain C, Baron JL, Krangel MS, Patel DD. Chemokine production by G protein-coupled receptor activation in a human mast cell line: roles of extracellular signal-regulated kinase and NFAT. J Immunol. 2000;165:7215–7223. doi: 10.4049/jimmunol.165.12.7215. [DOI] [PubMed] [Google Scholar]
- 86.Ferry X, Landry Y. Agmatine: a mastoparan-like activity related to direct activation of heterotrimeric G proteins. Eur J Pharmacol. 2002;435:19–26. doi: 10.1016/s0014-2999(01)01561-8. [DOI] [PubMed] [Google Scholar]
- 87.Aridor M, Rajmilevich G, Beaven MA, Sagi-Eisenberg R. Activation of exocytosis by the heterotrimeric G protein Gi3. Science. 1993;262:1569–1572. doi: 10.1126/science.7504324. [DOI] [PubMed] [Google Scholar]
- 88.Mousli M, Hugli TE, Landry Y, Bronner C. Peptidergic pathway in human skin and rat peritoneal mast cell activation. Immunopharmacology. 1994;27:1–11. doi: 10.1016/0162-3109(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 89.Mousli M, Hugli TE, Landry Y, Bronner C. A mechanism of action for anaphylatoxin C3a stimulation of mast cells. J Immunol. 1992;148:2456–2461. [PubMed] [Google Scholar]
- 90.Denzel A, Maus UA, Rodriguez Gomez M, Moll C, Niedermeier M, Winter C, et al. Basophils enhance immunological memory responses. Nat Immunol. 2008;9:733–742. doi: 10.1038/ni.1621. [DOI] [PubMed] [Google Scholar]
- 91.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mukai K, Matsuoka K, Taya C, Suzuki H, Yokozeki H, Nishioka K, et al. Basophils play a critical role in the development of IgE-mediated chronic allergic inflammation independently of T cells and mast cells. Immunity. 2005;23:191–202. doi: 10.1016/j.immuni.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 93.Mukai K, Obata K, Tsujimura Y, Karasuyama H. New insights into the roles for basophils in acute and chronic allergy. Allergol Int. 2009;58:11–19. doi: 10.2332/allergolint.08-RAI-0059. [DOI] [PubMed] [Google Scholar]
- 94.Tsujimura Y, Obata K, Mukai K, Shindou H, Yoshida M, Nishikado H, et al. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity. 2008;28:581–589. doi: 10.1016/j.immuni.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 95.Eglite S, Pluss K, Dahinden CA. Requirements for C5a receptor-mediated IL-4 and IL-13 production and leukotriene C4 generation in human basophils. J Immunol. 2000;165:2183–2189. doi: 10.4049/jimmunol.165.4.2183. [DOI] [PubMed] [Google Scholar]
- 96.MacGlashan DW, Jr, Hubbard WC. IL-3 alters free arachidonic acid generation in C5a-stimulated human basophils. J Immunol. 1993;151:6358–6369. [PubMed] [Google Scholar]
- 97.Ochensberger B, Rihs S, Brunner T, Dahinden CA. IgE-independent interleukin-4 expression and induction of a late phase of leukotriene C4 formation in human blood basophils. Blood. 1995;86:4039–4049. [PubMed] [Google Scholar]
- 98.Schroeder JT, MacGlashan DW, Jr, Kagey-Sobotka A, White JM, Lichtenstein LM. IgE-dependent IL-4 secretion by human basophils. The relationship between cytokine production and histamine release in mixed leukocyte cultures. J Immunol. 1994;153:1808–1817. [PubMed] [Google Scholar]
- 99.Ames RS, Lee D, Foley JJ, Jurewicz AJ, Tornetta MA, Bautsch W, et al. Identification of a selective nonpeptide antagonist of the anaphylatoxin C3a receptor that demonstrates antiinflammatory activity in animal models. J Immunol. 2001;166:6341–6348. doi: 10.4049/jimmunol.166.10.6341. [DOI] [PubMed] [Google Scholar]
- 100.Schroeder JT, Chichester KL, Bieneman AP. Human basophils secrete IL-3: evidence of autocrine priming for phenotypic and functional responses in allergic disease. J Immunol. 2009;182:2432–2438. doi: 10.4049/jimmunol.0801782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Khodoun M, Strait R, Orekov T, Hogan S, Karasuyama H, Herbert DR, et al. Peanuts can contribute to anaphylactic shock by activating complement. J Allergy Clin Immunol. 2009;123:342–351. doi: 10.1016/j.jaci.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kurimoto Y, de Weck AL, Dahinden CA. Interleukin 3-dependent mediator release in basophils triggered by C5a. J Exp Med. 1989;170:467–479. doi: 10.1084/jem.170.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ochensberger B, Tassera L, Bifrare D, Rihs S, Dahinden CA. Regulation of cytokine expression and leukotriene formation in human basophils by growth factors, chemokines and chemotactic agonists. Eur J Immunol. 1999;29:11–22. doi: 10.1002/(SICI)1521-4141(199901)29:01<11::AID-IMMU11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 104.MacGlashan D, Jr, Warner J. Stimulus-dependent leukotriene release from human basophils: a comparative study of C5a and Fmet-leu-phe. J Leukoc Biol. 1991;49:29–40. doi: 10.1002/jlb.49.1.29. [DOI] [PubMed] [Google Scholar]
- 105.Riccioni G, Bucciarelli T, Mancini B, Di Ilio C, D’Orazio N. Antileukotriene drugs: clinical application, effectiveness and safety. Curr Med Chem. 2007;14:1966–1977. doi: 10.2174/092986707781368522. [DOI] [PubMed] [Google Scholar]
- 106.Redrup AC, Howard BP, MacGlashan DW, Jr, Kagey-Sobotka A, Lichtenstein LM, Schroeder JT. Differential regulation of IL-4 and IL-13 secretion by human basophils: their relationship to histamine release in mixed leukocyte cultures. J Immunol. 1998;160:1957–1964. [PubMed] [Google Scholar]
- 107.Schroeder JT. Basophils beyond effector cells of allergic inflammation. Adv Immunol. 2009;101:123–161. doi: 10.1016/S0065-2776(08)01004-3. [DOI] [PubMed] [Google Scholar]
- 108.Faffe DS, Whitehead T, Moore PE, Baraldo S, Flynt L, Bourgeois K, et al. IL-13 and IL-4 promote TARC release in human airway smooth muscle cells: role of IL-4 receptor genotype. Am J Physiol Lung Cell Mol Physiol. 2003;285:L907–914. doi: 10.1152/ajplung.00120.2003. [DOI] [PubMed] [Google Scholar]
- 109.Laporte JC, Moore PE, Baraldo S, Jouvin MH, Church TL, Schwartzman IN, et al. Direct effects of interleukin-13 on signaling pathways for physiological responses in cultured human airway smooth muscle cells. Am J Respir Crit Care Med. 2001;164:141–148. doi: 10.1164/ajrccm.164.1.2008060. [DOI] [PubMed] [Google Scholar]
- 110.Peng Q, Matsuda T, Hirst SJ. Signaling pathways regulating interleukin-13-stimulated chemokine release from airway smooth muscle. Am J Respir Crit Care Med. 2004;169:596–603. doi: 10.1164/rccm.200307-888OC. [DOI] [PubMed] [Google Scholar]
- 111.Blease K. Therapeutics targeting IL-13 for the treatment of pulmonary inflammation and airway remodeling. Curr Opin Investig Drugs. 2008;9:1180–1184. [PubMed] [Google Scholar]
- 112.Blease K, Lewis A, Raymon HK. Emerging treatments for asthma. Expert Opin Emerg Drugs. 2003;8:71–81. doi: 10.1517/14728214.8.1.71. [DOI] [PubMed] [Google Scholar]
- 113.Dahinden CA, Rihs S, Ochsensberger B. Regulation of cytokine expression by human blood basophils. Int Arch Allergy Immunol. 1997;113:134–137. doi: 10.1159/000237527. [DOI] [PubMed] [Google Scholar]
- 114.Ochensberger B, Daepp GC, Rihs S, Dahinden CA. Human blood basophils produce interleukin-13 in response to IgE-receptor-dependent and -independent activation. Blood. 1996;88:3028–3037. [PubMed] [Google Scholar]
- 115.Miura K, Hubbard WC, MacGlashan DW., Jr Phosphorylation of cytosolic phospholipase A2 by IL-3 is associated with increased free arachidonic acid generation and leukotriene C4 release in human basophils. J Allergy Clin Immunol. 1998;102:512–520. doi: 10.1016/s0091-6749(98)70142-3. [DOI] [PubMed] [Google Scholar]
- 116.Miura K, Schroeder JT, Hubbard WC, MacGlashan DW., Jr Extracellular signal-regulated kinases regulate leukotriene C4 generation, but not histamine release or IL-4 production from human basophils. J Immunol. 1999;162:4198–4206. [PubMed] [Google Scholar]
- 117.Vilarino N, Miura K, MacGlashan DW., Jr Acute IL-3 priming up-regulates the stimulus-induced Raf-1-MEK-ERK cascade independently of IL-3-induced activation of ERK. J Immunol. 2005;175:3006–3014. doi: 10.4049/jimmunol.175.5.3006. [DOI] [PubMed] [Google Scholar]
- 118.Miadonna A, Salmaso C, Dimarco MP, Pugni L, Milazzo N, Tedeschi A, et al. Priming and inducing effects of interleukin-3 on histamine release from cord-blood basophils. Allergy. 1997;52:992–998. doi: 10.1111/j.1398-9995.1997.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 119.Ali H, Haribabu B, Richardson RM, Snyderman R. Mechanisms of inflammation and leukocyte activation. Med Clin North Am. 1997;81:1–28. doi: 10.1016/s0025-7125(05)70503-4. [DOI] [PubMed] [Google Scholar]
- 120.Freedman NJ, Lefkowitz RJ. Desensitization of G protein-coupled receptors. Recent Prog Horm Res. 1996;51:319–351. discussion 352–313. [PubMed] [Google Scholar]
- 121.Ali H, Richardson RM, Haribabu B, Snyderman R. Chemoattractant receptor cross-desensitization. J Biol Chem. 1999;274:6027–6030. doi: 10.1074/jbc.274.10.6027. [DOI] [PubMed] [Google Scholar]
- 122.Langkabel P, Zwirner J, Oppermann M. Ligand-induced phosphorylation of anaphylatoxin receptors C3aR and C5aR is mediated by “G protein-coupled receptor kinases. Eur J Immunol. 1999;29:3035–3046. doi: 10.1002/(SICI)1521-4141(199909)29:09<3035::AID-IMMU3035>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 123.Ahamed J, Haribabu B, Ali H. Cutting edge: differential regulation of chemoattractant receptor- induced degranulation and chemokine production by receptor phosphorylation. J Immunol. 2001;167:3559–3563. doi: 10.4049/jimmunol.167.7.3559. [DOI] [PubMed] [Google Scholar]
- 124.Ahamed J, Ali H. Distinct roles of receptor phosphorylation, G protein usage, and mitogen-activated protein kinase activation on platelet activating factor-induced leukotriene C4 generation and chemokine production. J Biol Chem. 2002;277:22685–22691. doi: 10.1074/jbc.M110210200. [DOI] [PubMed] [Google Scholar]
- 125.Richardson RM, DuBose RA, Ali H, Tomhave ED, Haribabu B, Snyderman R. Regulation of human interleukin-8 receptor A: identification of a phosphorylation site involved in modulating receptor functions. Biochemistry. 1995;34:14193–14201. doi: 10.1021/bi00043a025. [DOI] [PubMed] [Google Scholar]
- 126.Richardson RM, Haribabu B, Ali H, Snyderman R. Cross-desensitization among receptors for platelet activating factor and peptide chemoattractants. Evidence for independent regulatory pathways. J Biol Chem. 1996;271:28717–28724. doi: 10.1074/jbc.271.45.28717. [DOI] [PubMed] [Google Scholar]
- 127.Haribabu B, Richardson RM, Fisher I, Sozzani S, Peiper SC, Horuk R, et al. Regulation of human chemokine receptors CXCR4. Role of phosphorylation in desensitization and internalization. J Biol Chem. 1997;272:28726–28731. doi: 10.1074/jbc.272.45.28726. [DOI] [PubMed] [Google Scholar]
- 128.Vines CM, Xue M, Maestas DC, Cimino DF, Prossnitz ER. Regulation of N-formyl peptide-mediated degranulation by receptor phosphorylation. J Immunol. 2002;169:6760–6766. doi: 10.4049/jimmunol.169.12.6760. [DOI] [PubMed] [Google Scholar]
- 129.Braun L, Christophe T, Boulay F. Phosphorylation of key serine residues is required for internalization of the complement 5a (C5a) anaphylatoxin receptor via a β-arrestin, dynamin, and clathrin-dependent pathway. J Biol Chem. 2003;278:4277–4285. doi: 10.1074/jbc.M210120200. [DOI] [PubMed] [Google Scholar]
- 130.Christophe T, Rabiet MJ, Tardif M, Milcent MD, Boulay F. Human complement 5a (C5a) anaphylatoxin receptor (CD88) phosphorylation sites and their specific role in receptor phosphorylation and attenuation of G protein-mediated responses. Desensitization of C5a receptor controls superoxide production but not receptor sequestration in HL-60 cells. J Biol Chem. 2000;275:1656–1664. doi: 10.1074/jbc.275.3.1656. [DOI] [PubMed] [Google Scholar]
- 131.Giannini E, Brouchon L, Boulay F. Identification of the major phosphorylation sites in human C5a anaphylatoxin receptor in vivo. J Biol Chem. 1995;270:19166–19172. doi: 10.1074/jbc.270.32.19166. [DOI] [PubMed] [Google Scholar]
- 132.Langkabel P, Zwirner J, Oppermann M. Ligand-induced phosphorylation of anaphylatoxin receptors C3aR and C5aR is mediated by “G protein-coupled receptor kinases. Eur J Immunol. 1999;29:3035–3046. doi: 10.1002/(SICI)1521-4141(199909)29:09<3035::AID-IMMU3035>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 133.Pollok-Kopp B, Huttenrauch F, Rethorn S, Oppermann M. Dynamics of protein kinase C mediated phosphorylation of the complement C5a receptor on serine-334. J Biol Chem. 2006 doi: 10.1074/jbc.M601317200. [DOI] [PubMed] [Google Scholar]
- 134.Chuang TT, Sallese M, Ambrosini G, Parruti G, De Blasi A. High expression of β-adrenergic receptor kinase in human peripheral blood leukocytes. Isoproterenol and platelet activating factor can induce kinase translocation. J Biol Chem. 1992;267:6886–6892. [PubMed] [Google Scholar]
- 135.Haribabu B, Snyderman R. Identification of additional members of human G-protein-coupled receptor kinase multigene family. Proc Natl Acad Sci U S A. 1993;90:9398–9402. doi: 10.1073/pnas.90.20.9398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Loudon RP, Perussia B, Benovic JL. Differentially regulated expression of the G-protein-coupled receptor kinases, β-Ark and GRK6, during myelomonocytic cell development in vitro. Blood. 1996;88:4547–4557. [PubMed] [Google Scholar]
- 137.Lombardi MS, Kavelaars A, Schedlowski M, Bijlsma JW, Okihara KL, et al. Decreased expression and activity of G-protein-coupled receptor kinases in peripheral blood mononuclear cells of patients with rheumatoid arthritis. FASEB J. 1999;13:715–725. doi: 10.1096/fasebj.13.6.715. [DOI] [PubMed] [Google Scholar]
- 138.Oppermann M, Mack M, Proudfoot AE, Olbrich H. Differential effects of CC chemokines on CC chemokine receptor 5 (CCR5) phosphorylation and identification of phosphorylation sites on the CCR5 carboxyl terminus. J Biol Chem. 1999;274:8875–8885. doi: 10.1074/jbc.274.13.8875. [DOI] [PubMed] [Google Scholar]
- 139.Penn RB, Pronin AN, Benovic JL. Regulation of G protein-coupled receptor kinases. Trends Cardiovasc Med. 2000;10:81–89. doi: 10.1016/s1050-1738(00)00053-0. [DOI] [PubMed] [Google Scholar]
- 140.Milcent MD, Christophe T, Rabiet MJ, Tardif M, Boulay F. Overexpression of wild-type and catalytically inactive forms of GRK2 and GRK6 fails to alter the agonist-induced phosphorylation of the C5a receptor (CD88): evidence that GRK6 is autophosphorylated in COS-7 cells. Biochem Biophys Res Commun. 1999;259:224–229. doi: 10.1006/bbrc.1999.0758. [DOI] [PubMed] [Google Scholar]
- 141.Tarrant TK, Rampersad RR, Esserman D, Rothlein LR, Liu P, Premont RT, et al. Granulocyte chemotaxis and disease expression are differentially regulated by GRK subtype in an acute inflammatory arthritis model (K/BxN) Clin Immunol. 2008;129:115–122. doi: 10.1016/j.clim.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Santini F, Penn RB, Gagnon AW, Benovic JL, Keen JH. Selective recruitment of arrestin-3 to clathrin coated pits upon stimulation of G protein-coupled receptors. J Cell Sci. 2000;113:2463–2470. doi: 10.1242/jcs.113.13.2463. [DOI] [PubMed] [Google Scholar]
- 143.Miller WE, Lefkowitz RJ. Expanding roles for β-arrestins as scaffolds and adapters in GPCR signaling and trafficking. Curr Opin Cell Biol. 2001;13:139–145. doi: 10.1016/s0955-0674(00)00190-3. [DOI] [PubMed] [Google Scholar]
- 144.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. β-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 145.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by β-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 146.Lefkowitz RJ, Whalen EJ. β-arrestins: traffic cops of cell signaling. Curr Opin Cell Biol. 2004;16:162–168. doi: 10.1016/j.ceb.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 147.Wang Q, Lu R, Zhao J, Limbird LE. Arrestin serves as a molecular switch, linking endogenous α2-adrenergic receptor to SRC-dependent, but not SRC-independent, ERK activation. J Biol Chem. 2006;281:25948–25955. doi: 10.1074/jbc.M605415200. [DOI] [PubMed] [Google Scholar]
- 148.Luttrell LM, Roudabush FL, Choy EW, Miller WE, Field ME, Pierce KL, et al. Activation and targeting of extracellular signal-regulated kinases by β-arrestin scaffolds. Proc Natl Acad Sci U S A. 2001;98:2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Luttrell LM, Lefkowitz RJ. The role of β-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- 150.Ghosh S, Karin M. Missing pieces in the NF-κB puzzle. Cell. 2002;109 (Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 151.Gao H, Sun Y, Wu Y, Luan B, Wang Y, Qu B, Pei G. Identification of β-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-κB pathways. Mol Cell. 2004;14:303–317. doi: 10.1016/s1097-2765(04)00216-3. [DOI] [PubMed] [Google Scholar]
- 152.Witherow DS, Garrison TR, Miller WE, Lefkowitz RJ. β-Arrestin inhibits NF-κB activity by means of its interaction with the NF-κB inhibitor IκBα. Proc Natl Acad Sci U S A. 2004 doi: 10.1073/pnas.0402851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Venkatesha RT, Ahamed J, Nuesch C, Zaidi AK, Ali H. Platelet-activating factor-induced chemokine gene expression requires NF-κB activation and Ca2+/calcineurin signaling pathways: inhibition by receptor phosphorylation and β-arrestin recruitment. J Biol Chem. 2004;279:44606–44612. doi: 10.1074/jbc.M408035200. [DOI] [PubMed] [Google Scholar]