Abstract
Here we present a 24-week fetus with Smith-Lemli-Opitz syndrome (SLOS), alobar holoprosencephaly (HPE) and cyclopia (synophthalmia). Following birth, we suspected SLOS in this fetus due to the additional findings of ambiguous genitalia and bilateral 2–3 toe syndactyly. The diagnosis of SLOS was confirmed by finding an elevated amniotic fluid 7-dehydrocholesterol level (9890 ng/mL; normal range = 3–9 ng/mL), and molecularly by detecting two different mutations in the DHCR7 gene, the gene causing SLOS. The first mutation was an IVS8-1G>T change and the second was a deletion of exons 3 and 4; this latter mutation has not been reported previously. The mother carries the deletion, while the father carries the splice-site mutation. Also of note, the father has an abnormally low total plasma cholesterol level (104–109 mg/dL). This is the most severe case of HPE described in any patient with SLOS. We postulate that the HPE in this case resulted from severe impairment of Sonic Hedgehog signaling secondary to abnormal cholesterol metabolism; however, the unique combination of mutations in the fetus functionally appears to be no different from other homozygous null mutations reported in DHCR7. Therefore, there must be other yet to be identified factors that contributed to the severity of HPE in SLOS.
Keywords: Cyclopia, synophthalmia, Smith-Lemli-Opitz syndrome, birth defects, cholesterol, 7-dehydrocholesterol, holoprosencephaly
INTRODUCTION
Holoprosencephaly (HPE) occurs in 5–6% of individuals with Smith-Lemli-Opitz syndrome (SLOS) [Kelley and Hennekam, 2000; Caruso et al., 2004] and represents the most severe form of this syndrome. The precise mechanism by which HPE is produced in SLOS is not proven [Kelley et al., 1996]. Further, there is no specific genotype-phenotype correlation for the occurrence of HPE in SLOS, but most cases known to us have carried two known or predicted null mutations. This observation is consonant with the reported inverse correlation between the predicted severity of a DHCR7 mutation on enzymatic activity [Witsch-Baumgartner et al., 2000; Ciara et al., 2004] and the plasma cholesterol level [Cunniff et al., 1997] and the general severity of SLOS. Here we present a fetus who had SLOS, alobar HPE, and cyclopia (synophthalmia), the first reported individual with cyclopia in SLOS. The fetus possessed a splice-site mutation (IVS8-1G>T) and a deletion of the 3rd and 4th exons of the DHCR7 gene. The latter mutation has not been reported previously in this syndrome, and both mutations are predicted to preclude synthesis of functional enzyme. We discuss this unique case and factors that may have contributed to the unusually severe phenotype.
CLINICAL REPORT
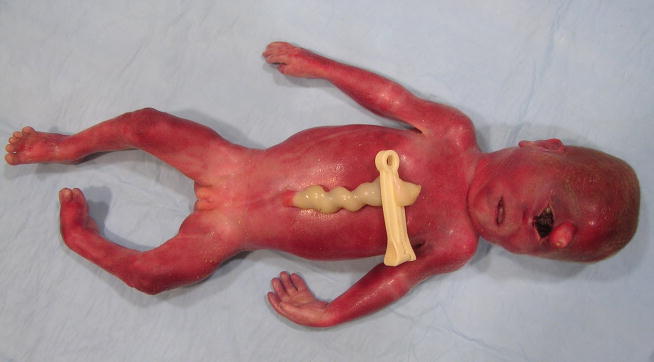
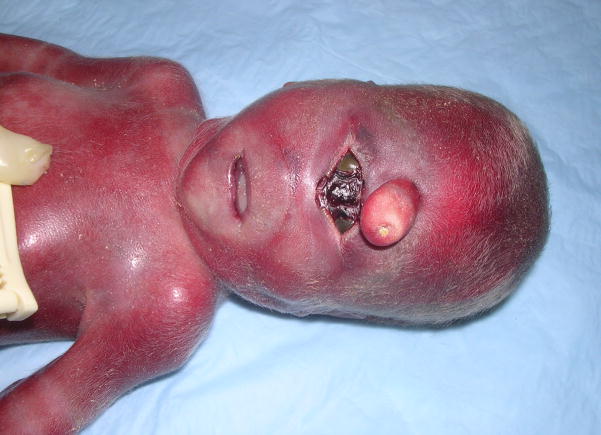
The pregnancy of the fetus reported here was to a 17-year-old, gravida 3, para 1, abortion 1 woman and was complicated by the finding of a low maternal serum estriol level and ultrasonic abnormalities including hydranencephaly vs. HPE, clubfoot deformity, and a 2-vessel umbilical cord. Fetal growth was normal. An amniotic fluid cell karyotype was normal, 46,XY. The only medications taken by the mother were prenatal vitamins and over-the-counter allergy medication. Delivery was induced at 24 weeks. Birth length was 33 cm (75th centile), birth weight 581 g (~60th centile), and occipitofrontal circumference (OFC) 18.5 cm (−2 to −3 SD). The fetus had microcephaly, acrocephaly, a one cm midline proboscis above a single palpebral fissure, two fused globes containing two separate corneae (synophthalmia), no nose or philtrum, an intact upper lip and palate, a small phallus with partial labioscrotal fusion, hypospadias, and left talipes equinovarus (Figs. 1, 2). In the center of the proboscis was a non-patent pit with a scaly attached plug (Fig. 2). At the lateral aspects of the palpebral fissure there were eyelashes. The ears were normal in structure and position. Mildly redundant nuchal skin was also noted. Limb abnormalities included short proximal metacarpals of the thumbs, clinodactyly of the right 2nd digit and both 5th digits, hypoplastic flexion creases of the left 3rd and 4th digits, the foot deformity, and bilateral partial 2–3 toe syndactyly, but no polydactyly. Additional autopsy findings included a fetus of appropriate-size for gestational age with absence of the interhemispheric fissure; one holosphere consistent with alobar HPE; absence of the Sylvian fissures; a fluid-filled, sac-like structure in the posterior aspect of the cerebrum; absent olfactory bulbs and tracts; hypoplastic bilobed right and unilobed left lungs; hepatosplenomegaly; short small intestine (26 cm); distal colonic aganglionosis; and hypoplastic right umbilical artery. The posterior fossa structures and spinal cord were normal. Other than being enlarged, the liver and spleen were grossly normal and histologically showed only congestion. The placenta was small and weighed 116 g (<10th centile – expected weight for 24 weeks = 189 g), and there also was patchy mild chronic lymphoplasmocytic deciduitis. The postnatally determined amniotic fluid 7-dehydrocholesterol (7DHC) level was 9890 ng/mL (normal range = 3–9 ng/mL), confirming the clinical diagnosis of SLOS. The family history is remarkable for Prader-Willi syndrome in a maternal cousin. The parents are phenotypically normal and deny consanguinity. They also have a normal daughter born prior to this fetus. The first pregnancy of the mother ended in miscarriage at 8 weeks of gestation.
Figure 1.
Frontal view of 24-week-old fetus. Note cyclopia (synophthalmia), ambiguous genitalia and partial syndactyly of 2nd and 3rd toes on the right. Note also hypercoiled u. cord.
Figure 2.
Facial view of fetus. Observe the proboscis with central pit and scaly plug; centrally located, single palpebral fissure with two fused globes; separate corneas; absent nose; smooth philtrum and intact lip.
Molecular analysis of the DHCR7 gene of the fetus found an IVS8-1G>T mutation in one gene and complete deletion of exons 3 and 4 in the other. Microarray analysis was normal. Furthermore, molecular analysis of the four most common genes (SHH, ZIC2, SIX3 and TGIF) associated with HPE showed no disease causing mutations. No plasma 7DHC or total cholesterol levels were done on the fetus. However, the 7DHC/cholesterol ratios in liver, spleen and lung tissue were 0.24, 0.26 and 0.46, respectively (normal 0.01–0.11), and on liver for patients with SLOS, 0.50 [Kelley RI, unpublished data, 2009]. Total fasting plasma cholesterol levels were 150 and 104 mg/dL (normal = 125–200 mg/dL) for the mother and the father, respectively. Repeat total fasting plasma cholesterol levels of the parents two months later were 137 and 109 mg/dL, respectively. Plasma triglyceride and HDL cholesterol levels in the mother were 101 mg/dL (normal 53–104 mg/dL) and 43 mg/dL (normal 35–135 mg/dL), respectively, and in the father, 136 mg/dL and 37 mg/dL, respectively.
DISCUSSION
We report here on a 24-week gestation fetus with cyclopia (synophthalmia), alobar HPE, and Smith-Lemli-Opitz syndrome. To our knowledge, this is the most severe case of HPE and the first case of cyclopia reported in SLOS. On molecular analysis, the fetus has two different mutations the DHCR7 gene, a splice-site mutation (IVS8-1G>T) and a deletion of the entire 3rd and 4th exons. This latter deletion encompassed all of DHCR7’s exons 3 and 4 and has not been previously reported. According to the Human Gene Mutation Database (https://portal.biobase-international.com/hgmd/pro/start.php), there have been no deletions over 100 nucleotides reported in DHCR7. A similar mutation to IVS8-1G>T, IVS8-1G>C, has been detected in about 29% of SLOS patients [Kelley and Hennekam, 2000; Witsch-Baumgartner et al., 2000], and is predicted to be a null mutation [Witsch-Baumgartner et al., 2000]. That the father had an abnormally low plasma total cholesterol level is not surprising in view of the lower mean cholesterol level of heterozygote parents of SLOS patients [Cunniff et al., 1997]. We found no mutations in the four common genes that are associated with HPE in the fetal DNA. Also, the pregnancy history was unremarkable for any known teratogen causing HPE.
There have been eight other reported cases of SLOS with HPE [McKeever and Young, 1990; Muenke et al., 1994; Kelley et al., 1996; Cunniff et al., 1997; Kratz and Kelley, 1999; Nowaczyk et al., 2001]. Of these eight cases, only one has had molecular analysis of DHCR7 and this case was homozygous for IVS8-1G>C mutation [Nowaczyk et al., 2001], but other unpublished SLOS fetuses with HPE and two null mutations are known to one of the authors [RIK]. Although the genotype in these cases and the current case would predict severe SLOS and might be expected to explain the presence of HPE, there also have been nine other homozygous IVS8-1G>C cases of SLOS reported [Waterham et al., 1998; Witsch-Baumgartner et al., 2000; Nowaczyk et al., 2001; Ciara et al., 2004]. Other cases with other homozygous null mutations in DHCR7 have also been reported but none has had HPE either [Loffler et al., 2000; Ciara et al., 2004].
Several theories have been advanced to explain the occurrence of HPE in SLOS [Kelley and Hennekam, 2000]. Kelley et al. [1996] proposed that HPE in SLOS is related to abnormal sterol metabolism and the function of Sonic Hedgehog protein. Stone et al. [1996] have proposed that there is interaction between low cholesterol and/or elevated 7DHC and signaling proteins such as SHH, PTCH-1 and PTCH-2, or their receptors, although later studies by Cooper et al. [1998] showed strong evidence that the PATCH receptor, Smoothened, is the signaling protein affected by the low sterol levels in SLOS. Concomitant mutations in DHCR7 and one of the genes causing HPE, such as SHH, could be another mechanism. However, there is as yet no published case supporting this reasonable possibility [Nowaczyk et al., 2001]. Silve et al. [1998] suggested impaired signaling of SHH by interference in the lamin B receptor, which has a similar sterol binding domain to DHCR7, although sterol levels may have no role in the function of Lamin B. Mutations in megalin, the LDL receptor in embryonic neuroepithelium involved in the transport of maternal placental LDL, have also been shown to produce HPE in rodents [Willnow et al., 1996]. Deficiency of cholesterol could also interfere with embryonic plasma membrane function and cell-to-cell interaction [Dehart et al., 1997]. Other mechanisms could include impaired transplacental transport of cholesterol, environmental factors, other maternal or fetal genes [Nowaczyk et al., 2001], cholesterol levels in the affected fetus and/or mother, or a combination of these factors [Kelley and Hennekam, 2002; Witsch-Baumgartner et al., 2004].
This case provides evidence that elevated levels of 7DHC are not involved in the formation of HPE and the severity of the HPE facial phenotypes in individuals with SLOS. This appears to be such since reported levels of amniotic fluid 7DHC in cases of SLOS have a mean value of 7,300 ng/mL with a range of 1,800–12,800 ng/mL [Kratz and Kelley, 1999]. The amniotic fluid 7DHC level in our case was well within the range of other SLOS cases, and thus would not explain the presence of HPE and the severity of the facial findings in our case. However, Kratz and Kelley [1999] did find a strong correlation between levels of amniotic fluid 7DHC and clinical severity of affected fetuses, but not a correlation between levels of amniotic fluid cholesterol levels and clinical severity. In part this is because there is a more than 10-fold range of cholesterol levels in amniotic fluid of apparently normal pregnancies, which indicates a poor correlation between the fetal and amniotic fluid cholesterol levels [Kratz, LE and Kelley RI, unpublished data].
From a molecular stand point, the second mutation in this fetus, deletion of exons 3 and 4 in DHCR7, would be predicted to block the function of this gene, resulting in no cholesterol production determined by this gene. The father’s abnormally low levels of cholesterol (104 and 109 mg/dL) likely is not related to the severity of the HPE in the case here since other individuals with SLOS have been reported with null mutations in DHCR7 with and without HPE [Waterham et al., 1998; Witsch-Baumgartner et al., 2000; Nowaczyk et al., 2001; Ciara et al., 2004]. Likely the HPE in our case resulted from severe impairment of Sonic Hedgehog signaling secondary to abnormal cholesterol metabolism. The unique combination of mutations in this fetus functionally appears to be no different from other homozygous null mutations reported in DHCR7. Therefore, there must be other unidentified factors that contributed to the severity of HPE in SLOS.
Acknowledgments
The cooperation of the parents of the fetus reported here in graciously recognized. Without their support this study could not have been completed. Dr. Steve Dlouhy assisted in the storage and preparation of DNA samples and we recognize his efforts. We also thank Dr. Erich Roessler for his valuable input to the Discussion. This work was supported in part by the Division of Intramural Research of the National Human Genome Research Institute, National Institutes of Health.
Biographies
David D. Weaver is a clinical geneticist in the Department of Medical and Molecular Genetics, Indiana University, with primary interest in dysmorphology.
Benjamin D. Solomon is a clinical genetics fellow at the National Human Genome Research Institute, National Institutes of Health, Bethesda, Maryland. His research interests include conditions resulting from early errors in embryogenesis.
Kelly Akin-Samson was a genetic counselor in the Maternal and Fetal Medicine Section of the Department of Obstetrics and Gynecology, Indiana University. She now lives in New Jersey.
Richard I. Kelley is the Director of the Clinical Mass Spectrometry Laboratory at the Kennedy Krieger Institute and Professor of Pediatrics at the Johns Hopkins University in Baltimore, Maryland. His primary interest is in elucidating the biochemical basis of genetic disorders with focus on Smith-Lemli-Opitz syndrome.
Maximilian Muenke is Chief and Senior Investigator of Medical Genetics Branch, National Human Genome Research Institute, National Institutes of Health, Bethesda, Maryland. He has had a long-term interest in holoprosencephaly, craniofacial syndromes and attention deficit hyperactivity disorder.
References
- Caruso PA, Poussaint TY, Tzika AA, Zurakowski D, Astrakas LG, Elias ER, Bay C, Irons MB. MRI and 1H MRS findings in Smith-Lemli-Opitz syndrome. Neuroradiology. 2004;46:3–14. doi: 10.1007/s00234-003-1110-1. [DOI] [PubMed] [Google Scholar]
- Ciara E, Nowaczyk MJM, Witsch-Baumgartner M, Malunowicz E, Popowska E, Jezela-Stanek A, Piotrowicz M, Waye JS, Utermann G, Krajewska-Walasek M. DHCR7 mutations and genotype-phenotype correlation in 37 Polish patients with Smith-Lemli-Opitz syndrome. Clin Genet. 2004;66:517–524. doi: 10.1111/j.1399-0004.2004.00350.x. [DOI] [PubMed] [Google Scholar]
- Cooper MK, Porter JA, Young KE, Beachy PA. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science. 1998;280:1603–1607. doi: 10.1126/science.280.5369.1603. [DOI] [PubMed] [Google Scholar]
- Cunniff C, Kratz LE, Moser A, Natowicz MR, Kelley RI. Clinical and biochemical spectrum of patients with RSH/Smith-Lemli-Opitz syndrome and abnormal cholesterol metabolism. Am J Med Genet. 1997;68:263–269. [PubMed] [Google Scholar]
- Dehart DB, Lanoue L, Tint GS, Sulik KK. Pathogenesis of malformations in a rodent modal for Smith-Lemli-Opitz syndrome. Am J Med Genet. 1997;68:328–337. doi: 10.1002/(sici)1096-8628(19970131)68:3<328::aid-ajmg15>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Kelley RI, Hennekam RCM. The Smith-Lemli-Opitz syndrome. J Med Genet. 2000;37:321–335. doi: 10.1136/jmg.37.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley RI, Roessler E, Hennekam RCM, Feldman GL, Kosaki K, Jones MC, Palumbos JC, Muenke M. Holoprosencephaly in RSH/Smith-Lemli-Opitz syndrome: Does abnormal cholesterol metabolism affect the function of Sonic Hedgehog? Am J Med Genet. 1996;66:478–484. doi: 10.1002/(SICI)1096-8628(19961230)66:4<478::AID-AJMG22>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Kratz LE, Kelley RI. Prenatal diagnosis of RSH/Smith-Lemli-Opitz syndrome. Am J Med Genet. 1999;82:376–381. [PubMed] [Google Scholar]
- Loffler J, Trojovsky A, Casati B, Kroisel PM, Utermann G. Homozygosity for the W151X stop mutation in the Δ7-sterol reductase gene (DHCR7) causing a lethal form of Smith-Lemli-Opitz syndrome: Retrospective molecular diagnosis. Am J Med Genet. 2000;95:174–177. doi: 10.1002/1096-8628(20001113)95:2<174::aid-ajmg16>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- McKeever PA, Young ID. Smith-Lemli-Opitz syndrome II: a disorder of the fetal adrenals? J Med Genet. 1990;27:465–466. doi: 10.1136/jmg.27.7.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muenke M, Henneham RCM, Kelley RI. Holoprosencephaly as a manifestation of Smith-Lemli-Opitz syndrome. Am J Hum Genet. 1994;55:36A. [Google Scholar]
- Nowaczyk MJM, Farrell SA, Sirkin WL, Velsher L, Krakowiak PA, Waye JS, Porter FD. Smith-Lemli-Opitz (RHS) syndrome: Holoprosencephaly and homozygous IVS8–1G→C genotype. Am J Med Genet. 2001;103:75–80. doi: 10.1002/1096-8628(20010915)103:1<75::aid-ajmg1502>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Silve S, Dupuy PH, Ferrara P, Loison G. Human lamin B receptor exhibits sterol C14-reductase activity in Saccharomyces cervisiae. Biochim Biophys Acta. 1998;1392:233–244. doi: 10.1016/s0005-2760(98)00041-1. [DOI] [PubMed] [Google Scholar]
- Stone DM, Hynes M, Armanini M, Swanson TA, Gu Q, Johnson RL, Scott MP, Pennica D, Goddard A, Phillips H, Noll M, Hooper JE, de Sauvage F, Rosenthal A. The tumour-suppressor gene patched encodes for candidate receptor for Sonic hedgehog. Nature. 1996;384:129–133. doi: 10.1038/384129a0. [DOI] [PubMed] [Google Scholar]
- Waterham HR, Wijburg FA, Hennekam RCM, Vreken P, Poll-The BT, Dorland L, Duran M, Jira PE, Smeitink JAM, Wevers RA, Wanders RJA. Smith-Lemli-Opitz syndrome is caused by mutations in the 7-dehydrocholesterol reductase gene. Am J Hum Genet. 1998;63:329–338. doi: 10.1086/301982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willnow TE, Hilpert J, Armstrong SA, Rohlmann A, Hammer RE, Burns DK, Herz J. Defective forebrain development in mice lacking gp330/megalin. Proc Natl Acad Sci USA. 1996;93:8460–8464. doi: 10.1073/pnas.93.16.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witsch-Baumgartner M, Fitzky BU, Ogorelkova M, Kraft HG, Moebius FF, Glossmann H, Seedorf U, Gillessen-Kaesbach G, Hoffmann GF, Clayton P, Kelley RI, Utermann G. Mutational spectrum in the Δ7-sterol reductase and genotype-phenotype correlation in 84 patients with Smith-Lemli-Opitz syndrome. Am J Hum Genet. 2000;66:402–412. doi: 10.1086/302760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witsch-Baumgartner M, Gruber M, Kraft HG, Rossi M, Clayton P, Giros M, Haas D, Kelley RI, Krajewska-Walasek M, Utermann G. Maternal apo E genotype is a modifier of the Smith-Lemli-Opitz syndrome. J Med Genet. 2004;41:577–584. doi: 10.1136/jmg.2004.018085. [DOI] [PMC free article] [PubMed] [Google Scholar]