Abstract
Adolescence is characterized by increased risk-taking, novelty seeking, and locomotor activity, all of which suggest a heightened appetitive drive. The neurotransmitter dopamine is typically associated with behavioral activation and heightened forms of appetitive behavior in mammalian species, and this pattern of activation has been described in terms of a neurobehavioral system that underlies incentive motivated behavior. Adolescence may be a time of elevated activity within this system. This review provides a summary of changes within cortical and subcortical dopaminergic systems that may account for changes in cognition and affect that characterize adolescent behavior. Because there is a dearth of information regarding neurochemical changes in human adolescents, models for assessing links between neurochemical activity and behavior in human adolescents will be described using molecular genetic techniques. Furthermore, we will suggest how these techniques can be combined with other methods such as pharmacology to measure the impact of dopamine activity on behavior and how this relation changes through the lifespan.
I. Introduction
As reviewed by other papers within this issue and in the literature as a whole, adolescence is characterized by widespread neurobiological changes such as shifts in brain matter composition (see papers by Paus, Gogtay & Thompson, and Schmithorst, this issue), modifications of neural synchrony (Uhlhaas et al., 2009), increased hormonal release (Styne, 1994), and neurochemical alterations (Doremus-Fitzwater et al., this issue; Spear et al., 2000). Much of this work has focused on changes in brain structure as well as attempts to define adolescent-unique patterns of functional brain activity in the context of cognitive and emotional behaviors (see papers by Luna et al. and Somerville et al., this issue). This latter set of findings has identified brain regions where activation patterns are distinct in adolescents versus children and adults as they perform cognitive and emotional tasks, leading to renewed conceptualizations of brain systems that operate in a distinctive manner during this period of the lifespan (Bjork et al., 2004; Bunge et al., 2002; May et al., 2004; Ernst et al., 2005; Galvan et al., 2006; Luna et al., 2004). Moreover, it is clear that adolescents differ from adults on behavioral measures of decision-making, planning, working memory, and inhibitory control (Asato et al., 2006; Crone et al., 2004; Hooper et al., 2004; Luciana et al., 2005; Luciana et al., 2009; Luna et al., 2004). That said, it has been a challenge to definitively associate the changes in neuroarchitecture that have been described across adolescence with changing patterns of behavior during this period of the lifespan, particularly with respect to risk-taking and aspects of behavioral regulation. Age-related sources of variation in structure-function relations are relatively small in magnitude (Olson et al., 2009; Schmithorst, 2005; Shaw et al., 2006), and some structure-function relations are not easily attributable to maturational processes (Olesen, Nagy, Westerberg, & Klingberg, 2003). Given that adolescence is a period in the lifespan characterized by alarming increases in risk-taking behaviors and that these behavioral patterns are relatively impervious to educational interventions (Steinberg, 2008), it has become commonplace to assert that they have a basis in brain development. Synaptic structure is becoming refined during adolescence, and the prefrontal cortex (PFC) may be among the last regions to attain a maturational plateau. Recent formulations have emphasized that adolescent patterns of frontal-limbic integration are different from what has been observed in adults and children (Fareri, Martin & Delgado, 2008; Galvan et al., 2006). However, none of these brain substrates definitively underlies adolescents’ tendencies to select risky alternatives when faced with options that are probabilistically risky versus safe or their tendencies to seek out situations where there is an increased opportunity for risk-taking. Thus, perhaps there are other brain-behavior associations, in combination with existing approaches, which should receive greater scrutiny within empirical studies. For instance, it is unknown whether age-related changes in neurochemistry would better account for the changing behavioral patterns that are observed during adolescence. The direct study of neurochemistry in human developmental samples has remained elusive, primarily because of difficulties in measuring chemical substrates using non-invasive procedures. The overarching purpose of this review is to outline a rationale for the direct examination, in humans, of neurochemical substrates that may underlie individual differences in adolescent risk-seeking and subsequent risk-taking. One exemplar within the monoaminergic transmitter system will be highlighted.
At the outset, it should be emphasized that, in our view, a particularly salient feature of behavior that is viewed as problematic in adolescents concerns the failure to choose the less risky versus more risky alternative when faced with a situation where there are signals regarding the positive and negative outcomes associated with each potential choice. Perhaps more than one alternative cannot be simultaneously appreciated, and there is an information processing failure. Or perhaps the weighting of outcomes between alternatives is skewed such that there is an imbalance across motivational systems that might otherwise moderate behavior. It may be that positive outcomes are extremely salient, that negative outcomes are less salient than at other points in the lifespan, or that negative outcomes are not appreciated at all. Alternatively, perhaps all potential outcomes are equally salient and weighed but for some reason, risky alternatives are selected such that there is a response bias. In deciphering these possibilities, it is important to consider, then, how multiple potential behavioral choices gain access to information processing networks, how each choice is evaluated in terms of positive and negative outcome contingencies, and how response selection takes place.
In addressing these issues, the monoamines have been of specific interest because of extensive data linking their activity to multiple domains of affect, cognition, and motor behaviors (Depue & Spoont, 1986; Mattay et al., 2002; Sawaguchi & Goldman-Rakic, 1994; Wise, 2004), as well as psychiatric disorders such as depression, schizophrenia, and substance abuse (Eison & Eison, 1994; Weinberger, 1987; Wise, 1998). These associations may be especially relevant to adolescence, given the broad changes in cognition and affect that occur during this time as well as increased rates of psychiatric disorders (Costello et al., 1999; Kessler et al., 2005; Paus, Keshaven & Giedd, 2008; Shedler & Block, 1990, Szymanski et al., 1995). One perspective is that discrete monoaminergic transmitter systems promote integrated and systematized behaviors that are adaptive to the species as a whole but are problematic for some individuals.
II. A Neurobehavioral Systems Framework to Describe Adolescent Behavior
Adolescence represents a unique period in the human (and animal) lifespan. On the one hand, human adolescents are highly dependent upon their parents and educators for support. On the other hand, the major life task during this period is to move toward a state of relative autonomy in support of independent living, individuation, and the selection and maintenance of relationships outside of the family. Ethological perspectives have argued that this transition from caregiver dependence to independent survival necessarily involves increases in social affiliation, risk taking, and skill acquisition (Spear, 2000). Empirical evidence supports this claim. Human adolescents spend significantly more time with peers compared to children (Csikszentmihalyi, Larson & Prescott, 1977), facilitating the acquisition of new skills and broad social support (Harris, 1995). In addition, while risk-taking may be associated with obvious negative outcomes (Irwin Jr. & Millstein, 1992), it also promotes the exploration of adult roles (Silbereisen & Reitzle, 1992), increases self-esteem (Silbereisen & Reitzle, 1992; but see McCarthy & Hoge, 1984), and promotes reproductive success (Spear 2003; Wilson & Daly, 1985). Increased exploration and novelty-seeking decrease the likelihood of inbreeding and encourage the exploration of remote sources of food, shelter, potential mates and other resources that promote survival. Thus, these behaviors facilitate appetitive drives that are extremely appropriate to the life tasks inherent to this developmental period and, to the extent that these drives are regulated, they facilitate goal attainment and long-term survival. They can be viewed as part of a cohesive brain-based system that is dedicated to the translation of positive motivation to adaptive action.
In the psychology, ethology, and behavioral neuroscience literatures, there are numerous descriptors for the behavioral system that underlies appetitive motivation, including Schneirla’s (1959) approach system, Gray’s (1987) behavioral activation system, MacLean’s (1986) search system, Panksepp’s (1992, 1998) foraging/expectancy and seeking systems and Depue’s behavioral facilitation system (Depue & Iacono, 1989; Depue & Collins, 1999). Despite nominal distinctions, there is general agreement that this system provides positive activation that motivates an individual to approach distal sources of reward. Activity in this system promotes subjective feelings of excitation, positive engagement, and a desire for exploration. It may be reflected by facets of the personality trait dimension of extraversion (Depue & Collins, 1999) and by temperamental descriptions such as surgency, (Derryberry & Rothbart, 1988). An essential feature of this system’s activation is that the individual is motivated to want something that is not immediately present. Accordingly, this state can be conceptualized as one of psychological wanting or craving (Nesse & Berridge, 1997), and specific targets of desire can vary depending on an individual’s reinforcement history, present state of deprivation, and/or future goals. Thus, a target’s reward salience, as well as its spatial and temporal proximity to the individual, are critical determinants of the system’s activation of incentive motivation (Depue & Collins, 1999). Importantly, one does not need a strong level of incentive motivation to obtain what is present and attainable. Rather, this type of motivation propels individuals to move toward positive reinforcements that are removed from the current context. Accordingly, frontal brain systems are necessary to structure approach behavior so that it is adaptive for future goal attainment.
Activation of this motivational system will not only propel an individual to seek novel experiences, but in the absence of other influences, the activation will structure how the individual responds to novelty when unexpectedly encountered. Novelty inherently implies risk or uncertainty. Instead of reacting with neophobia or caution as might be the case in the context of fear or anxiety (Depue & Collins, 1999), novel or uncertain situations will be greeted with approach and engagement. Within a neurobehavioral systems framework, novelty is a unique stimulus. It not only serves as an innate cue for potential reward but also as a cue for potential threat (Depue & Collins, 1999; Gray, 1973; Panksepp, 1992). Thus, individual differences in responses to novelty can be indicative of the underlying motivational state that is guiding behavior. If an individual is motivated by reward-seeking as opposed to risk-aversion, then s/he will be inclined to approach novelty, which we suggest is the case for most individuals during adolescence. In this manner, the activation of this system biases behavioral responding not only toward risk-seeking (generalized approach toward rewards) but also toward risk-taking (positive engagement in the context of uncertainty).
Consistent with an emotional systems framework for understanding this and other basic motivations, this is a system that has an evolutionary link in the development of modern mammalian behavior, it has an identifiable set of neural substrates, and it is characterized by a well-defined pattern of neurochemical modulation (Panksepp, 1998). Dopamine is hypothesized to be the primary transmitter that acts within and across limbic, striatal, and frontal circuitry to promote incentive-guided behavior and its regulation (Depue & Collins, 1999). Given that approach behavior critically relies on motor functions, it is not surprising that the neural underpinnings of this system rely on an integration of the motor system and limbic and cortical structures that promote and structure positive incentive-guided behavior. This system can also mediate some negative emotional states, particularly those associated with frustrative non-reward (sadness and anger associated with reward that is expected but not attained).
How this system changes over the course of human development has not been comprehensively studied (Luciana, 2001). We will review evidence to suggest that the coherent set of behaviors that comprise this motivational system is neurobiologically grounded in activity within the ascending mesocorticolimbic dopamine system, which innervates ventral striatal, core limbic, and prefrontal regions, and that activity within this system changes over the course of the human lifespan, reaching a relative high point during human adolescence/emerging adulthood and declining thereafter.
A peak in activity during adolescence predicts that there will be increases in exploratory behavior, novelty seeking, in reward salience, and in reinforcement learning such that learning will be biased toward responses to positive versus negative feedback. Viewing adolescence from this perspective holds the promise of explaining two important aspects of adolescent behavior: why risk-seeking increases in this developmental group as a whole, but importantly, why some individuals are more vulnerable to the potentially negative consequences of this neurobehavioral shift than others. Many theories of adolescent brain development that rely primarily on brain structural changes, which can be considered highly normative in scope, do not adequately explain this latter aspect of adolescent behavior, that is, that not all teens are equally vulnerable during this period of the lifespan (although for a recent empirical exception, see work by Shaw et al., 2006).
Adolescence has been described as a period of heightened affective reactivity characterized by increased sensitivity to both rewarding and aversive stimuli (see Somerville, Jones, & Casey in this special issue). It is characterized by transient changes in personality traits such as increased rates of sensation-seeking and extraversion (McCrae et al., 2000; Zuckerman et al., 1978). Conversely, conscientiousness increases after adolescence and into middle adulthood (McCrae et al., 2000). Adolescents engage in heightened levels of risk-taking behaviors in their daily lives, and they are prone to engage in riskier behaviors as measured by laboratory paradigms, particularly under conditions of peer influence (Gardener & Steinberg, 2005; Steinberg, 2008). Finally, adolescents appear especially sensitive to rewarding cues, as evidenced by exaggerated neural responses when exposed to them, specifically within structures innervated by mesolimbic dopamine (Ernst et al., 2005; Galvan et al., 2006). In animal models, periadolescent rodents exhibit heightened novelty seeking, risk-taking, locomotor activity, and grooming as compared to adults (Adriani, Chiarotti, & Laviola, 1998; Laviola et al., 1995; Macri, Adriani, Chiarotti, & Laviola, 2002; Spear & Brake, 1983), all of which are argued to be roughly analogous to approach system constructs mentioned above.
III. This system is grounded in activity within the dopamine system
Dopamine activity in core limbic regions has been implicated as a primary substrate for incentive-motivational behavior. Mesolimbic dopamine projections originate in the midbrain ventral tegmental area (VTA)/substantia nigra (SN) complex and terminate in limbic structures including the olfactory tubercle, nucleus accumbens, amygdala, hippocampus, and septum, as well as the medial prefrontal cortex (Bjorklund & Dunnett, 2007; Dahlstrom & Fuxe, 1964). Lesions halt exploration and approach behaviors (Koob, Riley, Smith, & Robbins, 1978). The administration of dopamine or dopamine agonists into these regions initiates novelty-seeking and goal-directed locomotor behaviors, whereas the administration of dopamine antagonists results in opposite effects (Fouriezos, Hansson, & Wise, 1978; Le Moal & Simon, 1991; Pijnenburget al., 1976). Furthermore, adult rats exhibiting higher rates of locomotor activity and novelty seeking demonstrate higher rates of drug self-administration as well as elevated dopamine concentrations as compared to rats exhibiting low baseline levels of activity (Piazza et al., 1991). The link between dopamine and incentive-guided motivational behavior is robust in the animal literature with locomotor processes serving as the primary indicator of an incentive state. These behaviors in the context of drug administration are also moderated by animals’ abilities to tolerate delays. Animals who have difficulty waiting before executing a response, and who tend to make high numbers of anticipatory responses before rewarding stimuli are presented, escalate their drug self-administration dramatically over time after the behavior is acquired. These animals are also more vulnerable to drug reinstatement after extinction (Economidou et al., 2009; Robinson et al., 2009).
In humans, there has been interest in linking dopamine to the modulation of personality traits that are related to the approach system construct. For example, the personality trait of Cloninger’s novelty-seeking was associated in a recent positron emission tomography (PET) study with a decreased availability of D2 autoreceptors in the midbrain (Zald et al., 2008), suggesting high levels of this trait in individuals with relatively greater amounts of synaptic dopamine. Eysenck’s extraversion modulates the impact of dopamine antagonists on cognitive performance in humans (Wacker, Chavanon, & Stemmler, 2006), and levels of dopamine in cerebrospinal fluid are positively correlated with trait levels of extraversion (King et al., 1986). Similar associations have been demonstrated with respect to Tellegen’s positive emotionality, such that individuals with high levels of this trait show increased neuroendocrine responses to a dopamine receptor agonist (Depue et al., 1994). Finally, individuals whose genetic predispositions result in higher levels of dopamine in neural synapses, particularly in circuits involved in reward processing, demonstrate increased levels of brain activation in response to rewards (Dreher et al., 2009; Hariri, 2009). Thus, though these data represent but a sampling of the literature, there is a wealth of available evidence linking high levels of functionally available dopamine to appetitive behavior, with data converging on the idea that increases in mesolimbic/ventral striatal dopamine activity propel an organism to engage in incentive-motivated appetitive behaviors and impact the individual’s ability to profit from positive and negative feedback experiences in the context of reinforcement-based learning (Frank, 2005; Holroyd & Coles, 2002; Schultz et al., 1997). The structures that comprise the mesolimbic dopamine projections are generally regulated by monoamine oxidase (MAO) activity in neural synapses, by sustained regulatory activity of the dopamine transporter, and by high numbers of receptors (including terminal autoreceptors) in both the D1 and D2 receptor families (Bannon & Roth, 1983; Tam & Roth, 1997).
Dopamine also innervates cortical regions. In rodents, the areas that are innervated primarily include the frontal, cingulate, and entorhinal cortices; in primates, innervation extends throughout the cortex (Bjorklund & Dunnett, 2007). Prefrontal synapses have received a maximal amount of empirical assessment and are characterized by low activity of the dopamine transporter, by relatively low numbers of DA autoreceptors, by catechol-O-methyltransferase (COMT) versus monoamine oxidase (MAO) activity in DA catabolism, and by a relative abundance of D1 relative to D2 receptors (Bannon & Roth, 1983; Meador-Woodruff et al., 1994). Prefrontal dopamine activity is important for aspects of cognition that encompass flexible responses to changing environmental circumstances (Floresco & Magyar, 2006; Goldman-Rakic, 1987; Seamans & Yang, 2004). It is important to emphasize that dopamine does not necessarily modulate all prefrontal functions. We have previously outlined a rationale for the notion that the cognitive functions modulated by dopamine are those that serve incentive-motivated behaviors (Luciana, 2001). Spatial working memory, for example, serves to bring an organism into contact with spatial locations that have been targeted as motivationally salient, presumably because of the presence of primary rewards, across temporal delays.
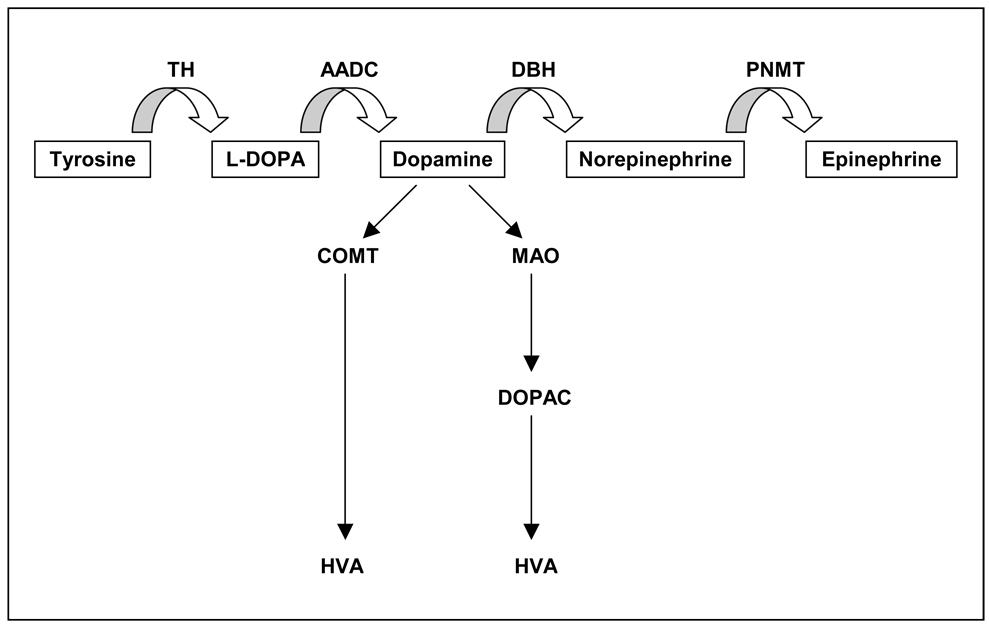
There are several aspects of dopamine neurotransmission that are important to appreciate in evaluating this literature. The first concerns dopamine’s synthesis pathway and metabolism. Dopamine synthesis begins with the amino acid tyrosine. Tyrosine is converted to dopamine through the actions of several enzymes, the most important of which is tyrosine hydroxylase. The overwhelming majority of dopamine cell bodies originate in the midbrain ventral tegmental area (VTA) and substantia nigra (SN). Dopamine that is released from the VTA and SN reaches its targets through segregated axonal pathways. The net level of functional dopamine activity within a region will depend on (a) how much dopamine has been released; (b) the collateralization of terminals from the presynaptic cell; (c) catabolic events in the synapse that impact how much dopamine will reach postsynaptic targets; (d) the activity of the dopamine transporter, which also regulates how much dopamine reaches postsynaptic receptors; and (e) the number and types of postsynaptic receptors that are present in a target region (see Figure 1). Moreover, recent formulations suggest that the dopamine system as a whole is compartmentalized into two systems, one that tonically releases dopamine into extrasynaptic space and one that phasically releases dopamine in response to behaviorally-salient events. The two subsystems interact to modulate adaptive response selection (Grace et al., 2007).
Figure 1.
Dopamine Synthesis Pathway: Dopamine’s synthesis pathway begins with the amino acid tyrosine and is regulated by the activity of tyrosine hydroyxylase. As indicated in the figure, dopamine in neural synapses is regulated by monoamine oxydase (MAO) and catechol-O-methyltransferase (COMT) activity.
Biologically-based individual differences impact these processes. The synthesis of tyrosine hydryxylase is under genetic control (Vadasz et al., 2007), and genes also regulate the activity of catabolic enzymes (MAO, COMT: Lachman et al., 1996; Sabol et al., 1998). DA cell number is genetically influenced (Fink & Reis, 1981). As noted by Depue & Collins (1999), individual differences in cell numbers may have dramatic effects on the availability of synaptic dopamine in a region due to the high degree of axon collateralization near terminal sites. For instance, a single DA neuron can establish close to one million synaptic contacts within the striatum (Grace 1991; Groves et al. 1995). Moreover, each medium spiny striatal neuron receives input from thousands of DA terminals (Grace 1991). Animal studies have revealed plasticity in the coupling that emerges over time between neurons that synthesize dopamine and receptors in target regions, such that higher cell numbers are associated with higher numbers of receptors in target regions as well as the increased expression of behaviors that are DA-modulated (Fink & Reis, 1981; Sved et al., 1985). Thus, individuals may be biologically primed to experience a certain level of activity in the dopamine system, and this level of activity may impact the individual’s propensity to engage in approach behavior. High levels of activity confer a tendency toward high levels of appetitive drive, a perspective that contradicts reward deficiency explanations for excesses in appetitive behavior (Comings & Bloom, 2000). Indeed, based on biologically-based constraints, each person could be considered to have a “reaction range” that determines how high or low that person’s incentive motivated behavior could be as a direct consequence of the reactivity of that person’s dopamine system. Adolescence may represent a critical period in the human lifespan for dopamine system activity as well as reactivity and the associated expression of behavioral traits that facilitate novelty-seeking, exploration, and incentive motivation. Indeed, it seems clear that on both group and individual levels, novelty-seeking and risk-taking are at relative highs during adolescence, while risk aversion is at a relative low. Thus, there are individual differences in the nature of appetitive activity, and these individual difference factors will determine how vulnerable a given person is to difficulties associated with the more general age-related increases in activity within this system as they occur.
This formulation considers dopamine’s modulation of behavior at a “macro” level, where dopamine activity serves a broad overarching behavioral function. This view is appealing at the psychological level of analysis but may be viewed as simplistic within cellular and molecular neuroscience. However, dopamine’s activity as a modulator of processes that promote goal-directed activity is inherent to other theoretical frameworks, which are not inconsistent with the primary view expressed here.
For instance, in a now-classic paper, Alexander, deLong, and Strick (1986) outlined the existence of five parallel segregated information processing loops, each of which involved discrete regions of the striatum, neocortex, and thalamus. Each loop was dedicated to a specific behavioral function or domain of function. One was involved in primary sensorimotor responding; one was involved in general oculomotor control. The remaining three were directed by distinct regions of the frontal lobe, including the dorsolateral prefrontal cortex (involved in planning and other higher order executive functions), the lateral orbitofrontal cortex (involved in affective processing), and the anterior cingulate/medial orbitofrontal cortices (involved in attention and rule-based learning). These circuits enabled the basal ganglia to influence a broad range of behavior via interactions with the thalamus and cortex. Two of the loops discussed by Alexander and colleagues provide a heuristic that is relevant: the dorsolateral prefrontal and the orbitofrontal processing loops. These segregated processing pathways subserve higher-order planning, working memory and behavioral control processes (dorsolateral loop) and affective responses to reinforcement cues from the environment (orbitofrontal processing loop). Activity in each of these processing streams is impacted by dopaminergic neuromodulation to facilitate cognitive or emotive aspects of successful approach behavior, respectively, primarily via interactions with the dorsal striatum, ventral striatum, and thalamus. The existence of these segregated networks has been supported by recent anatomical tracing and tractography studies (Cohen et al., 2009;), and this framework has served as a basis for recent modeling of dopamine’s role as a modulator of striatal and prefrontal function (Frank, 2005; O’Reilly & Frank, 2006).
These latter models, based on computational frameworks, advocate that each cortico-striatal loop is comprised of competing pathways, each of which is primarily modulated by a specific subtype of dopamine receptor activity. One pathway, termed the direct pathway because of its influence over striatal output pathways from the internal segment of the globus pallidus to the thalamus, is involved in positive feedback and is hypothesized to be D1 modulated. The second pathway, the indirect pathway (so-named because it impacts thalamo-cortical processing through a more complex series of synapses involving the external and internal segments of the globus pallidus as well as the thalamus), is involved in negative feedback and is hypothesized to be D2 modulated. Relative activation of the direct pathway biases processing toward a “go” state, such that there will be a facilitation of whatever motor commands are currently represented in the cortex and an inhibition of potential responses that might otherwise compete with them. In this manner, DA acts a modulator by sharpening the contrast between signals from neurons that are in a facilitated depolarized “up-state” and inhibiting those from neurons that are below the resting state potential from firing unexpectedly. This modulation is achieved by molecular interactions among sodium, potassium, and calcium molecules that mediate the opening of voltage-gated membrane channels. The net result is an increase in the neural signal-to-noise ratio, a model that is consistent with Oades’ (1985) classic formulation of dopamine activity in neural circuits. Thus, within this modulatory framework, “go” does not simply mean “act” in the behavioral sense but refers, instead, to the nature of neural signals that will be amplified in the presence of dopamine activation. Theoretical accounts of dopamine’s functioning within neural networks assume that the “go” state activity will be linked to behaviors that are appropriate to a given context, helping the individual learn which of several potential responses should be selected in the midst of the patterning of positive and negative feedback that is associated with a given event.
What has not been discussed in these models is the state of affairs that is present if the “go” state activity involves potentiation of a neural input or output selection that is inappropriate to a given context but nevertheless salient to the individual. These models also do not address how inputs are selected and how selection of a given input is made in the face of competing alternatives or distractors or when “go state activity” is weak. That may be the situation in adolescence when hedonic signals of reward are amplified due to high activation levels in limbic circuits while regulatory signals are weak or inconsistently activated due to prefrontal immaturity.
Dopamine is also involved in reinforcement learning via striatal mechanisms. Dopamine neurons have been identified in the striatum on the basis of their firing properties. These neurons exhibit phasic activation after liquid and food rewards are delivered to deprived animals. In the course of learning instrumental responses that will yield positive reinforcements (reward delivery), these neurons fire in response to conditioned cues that signal the presence of the rewards. Moreover, when rewards are expected but not delivered, these neurons depress their firing rates (Schultz, et al., 2000). Thus, it has been hypothesized that striatal DA neurons respond to primary rewards outside of the learning context, code reward prediction in response to cues that signal reward delivery, and provide an alerting function when rewards are expected but not delivered (when reward prediction fails). Once cues reliably predict the presence of rewards, dopamine neurons fail to respond to their occurrence, indicating that learning is complete. In this regard, these responses resemble teaching signals that have been postulated by reinforcement learning theories. These signals inform other brain regions that a reward is predicted based on probabilistic contingencies between the occurrence of environmental cues and the past delivery of rewards. On a broader behavioral level, this patterning is sensible in that dopamine potentiates exploratory processes in response to unpredicted or novel stimuli. Once exploration is complete (when the environment is mapped and stimuli are predictable), dopamine’s activation as an effector should cease. Human fMRI activations reflecting the distinct processes of reward magnitude, probability, and expected value have been observed to occur in separate striatal regions (Tobler, O’Doherty, Dolan & Schulz, 2007). Other brain regions also participate in the reward’s evaluation and subsequent decision-making. In contrast, the ventromedial PFC encodes reward probability, and is therefore sensitive to the uncertainty of potential reinforcing contexts (Tobler et al., 2007). Prior to reward delivery, potential negative outcomes must also be weighed. The ventrolateral frontal cortex evaluates potential reinforcements on the basis of their punishment contingencies, weighing the reward prediction signal against the judgment of its expected value. Accordingly, the lateral frontal cortex would represent a critical processing node through which reward-relevant information is integrated and then, via connections with the striatum, response selection takes place. Deficits in this processing stream could stem from two mechanisms, one of which would be the relative absence of the general enabling function established by tonic levels of dopamine acting upon postsynaptic cells. The second mechanism would be due to a defective reward signal due to dysregulations in the phasic transmission of reward information in the context of ongoing behavior. Importantly, as the above review suggests, the striatum provides input to the prefrontal cortex (as well as other brain regions) to enable the brain’s ultimate calculation of the overall expected utility associated with a given response. Moreover, these various components of reinforcement learning rely on dopaminergic signals that span cortical and subcortical regions to accomplish salient behavioral objectives. An important implication of this analysis as applied to adolescence is that “dual systems” models (see Fareri et al., 2008 for review) invoked to explain adolescent behavior can be expanded given that the activity in a single neurochemical system can account for the pattern of findings attributed to two functional anatomical systems.
Thus, regardless of whether dopamine activity is considered in relation to behavioral activation patterns at a macro level, to cellular events that impact biasing in neural pathways, or in relation to the modulation of segregated neural circuits devoted to cognition and emotion, it may be important to consider the state of the system in adolescence as compared to other points in the lifespan with the hypothesis that tonic dopamine levels are elevated during this time and that this tonic elevation impacts the efficiency with which phasic signaling can adaptively regulate behavior.
IV. Dopamine activity in adolescence
The dopamine system undergoes significant alterations during adolescence, though there is considerable cross-species variation with respect to these changes. Several aspects of dopamine system activity will be described: dopamine concentrations, innervation, and receptor density. Dopamine concentrations and receptor densities have been measured in brain slices in sacrificed animals and in the context of human autopsy studies. Innervation patterns and receptor density have been assessed via tracing studies.
In non-human primates, concentrations of dopamine shift gradually toward anterior regions until adolescence, when dopamine concentrations are highest in the prefrontal cortex (Goldman-Rakic & Brown, 1982; Irwin et al., 1994). In contrast, concentrations in posterior regions such as the parietal lobe peak earlier in childhood (or, in the case of the occipital lobe, exhibit no change after birth) (Brown & Goldman, 1977; Goldman-Rakic & Brown, 1982). Studies in human cortex are unavailable, and results regarding changes in subcortical structures are mixed, with data suggesting that dopamine concentrations in the striatum either decrease with age or undergo no developmental alterations (Haycock et al., 2003; Kalaria et al., 1993).
Given increases in prefrontal DA concentrations during adolescence, it is little surprise that dopaminergic innervation of this area increases during this time period as well (Rosenberg & Lewis, 1994; 1995). These findings have been demonstrated in primary motor cortex, dorsomedial cortex, and the principal sulcus, with projections to cortical layer III undergoing the most pronounced change. While region-wide innervation of prefrontal cells by dopamine neurons appears to increase in layer III, the density of connections between dopaminergic cells and individual axons in this area undergoes a protracted linear development (Lambe, Krimer, & Goldman-Rakic, 2000). The fact that layer III specifically undergoes developmental changes is important, as dopamine projections to this area form synapses with pyramidal neurons that are implicated in representational memory processes (Goldman-Rakic, 1998) and serve as the primary source of afferent projections to other cortices (Jones, 1984).
The functional significance of these findings is unclear. Dopamine decreases the voltage threshold required for cell firing in these pyramidal cells (Henze et al., 2000); thus, adolescence may by characterized by increased excitability of the neural networks underlying prefrontally modulated cognition. On the surface, it may seem as though increased excitability of these networks may help to facilitate prefrontally-modulated cognitive functions such as working memory. However, evidence suggests that dopamine transmission occurs within a small window of optimal functioning, whereby both excessive and deficient levels of dopamine impair behavioral performance (Goldman-Rakic, 1998). For example, low doses of amphetamine (a dopamine agonist) increase locomotor activity in rodents, whereas higher doses result in stereotypies (Laviola & Adriani, 1998). Similarly, Williams and Goldman-Rakic (1995) demonstrated that low doses of a D1 receptor antagonist increased task-related neuronal activity in a working memory paradigm, though higher doses of that same antagonist inhibited task-related activity. As might be expected given these findings, high doses of both dopamine agonists and antagonists impair spatial working memory performance (Seamans, Floresco, & Phillips, 1995; Zahrt, Taylor, Mathew, & Arnsten, 1997). In humans, administration of the D2 agonist bromocriptine facilitates cognitive performance in individuals who exhibit poor performance at baseline, whereas those with higher levels of performance at baseline do not experience similar improvements (Cools, Sheridan, Jacobs, & D'Esposito, 2007; Kimberg, D’Esposito, & Farah, 1997). Thus, both animal and human data indicate that the relationship between dopamine availability and cognitive performance is characterized by an inverted-U shaped function. Accordingly, in adolescence, the prefrontal peak in dopamine activity that is observed may “overdose” this region given that presynaptic receptor and dopamine transporter activity are less salient here as compared to subcortical and striatal brain regions, as described above.
In primate cortex, the density of D1 and D2 dopamine receptors peaks between 2 and 4 postnatal months, equivalent to the early childhood period in humans (Lidow, Goldman-Rakic, & Rakic, 1991; Lidow & Rakic, 1992). These changes occur simultaneously across prefrontal, somatosensory, and visual cortices, with densities of both D1 and D2 receptors stabilizing to adult levels at 60 months. Few studies have assessed the ontogeny of dopamine receptor density in humans, but those that have indicate that densities decrease between childhood and adulthood (Montague et al., 1999; Seeman et al., 1987). Unfortunately, these studies are based on convenience morbidity samples, and as a result there are very few data points available for adolescence specifically. Thus, findings with respect to humans should be considered tentative. The course of DA receptor development mirrors that of synaptic pruning, indicating that these processes may be related.
Unlike primates, rodents lack significant cortical variation in dopaminergic concentrations (Kehr, Lindqvist, & Carlsson, 1976). Several groups have demonstrated that DA tissue concentrations increase postnatally until adulthood (Giorgi et al., 1987; Ungethüm et al., 1996), though other data indicate that dopamine synthesis peaks during adolescence relative to both childhood and adulthood (Andersen, Dumont, & Teicher, 1997). Similarly, dopaminergic innervation of the PFC and anterior cingulate cortex (ACC) increases until postnatal day (P) 60, which is equivalent to early adulthood (Berger et al., 1985; Kalsbeek et al., 1988). Thus, a majority of the evidence indicates that DA concentrations and dopaminergic innervation of the cortex in rats increases until adulthood and does not exhibit adolescent-limited peaks, with the exception, perhaps, of dopamine synthesis (Anderson et al., 1997).
While information regarding dopaminergic innervation in rodents is sparse, there is a large literature documenting the ontogeny of dopamine receptors in this species. D1 and D2 receptors follow similar developmental trajectories, exhibiting a peak between P28 and P42 in the caudate and putamen and decreasing thereafter (Andersen et al., 1997; Tarazi, Tomasini, & Baldessarini, 1998a; 1999; Teicher, Andersen, & Hostetter Jr., 1995). Others have indicated that dopamine receptor density stabilizes beginning in periadolescence, but these findings might relate to a lack of measurement in the time points defining that period (as opposed to directly prior to and following periadolescence) (Leslie et al., 1991; Rao et al., 1991). Findings regarding the development of dopamine D1 receptors in the ventral striatum are less clear, with data revealing periadolescent peaks (Tarazi et al., 1991), linear increases into adulthood (Leslie et al., 1991), and peaks in both periadolescence and adulthood (Andersen et al., 1997; Teicher et al., 1995). D2 receptors have not been found to exhibit these density peaks later in adulthood (Teicher et al., 1995). The trajectory of receptor density in the rodent cortex is less clear, with one line of evidence suggesting that cortical receptors either peak prior to periadolescence or exhibit linear increases between childhood and adulthood (Leslie et al., 1991; Murrin & Zeng, 1990; Tarazi & Baldessarini, 2000), a pattern similar to that of cortical innervation in rodents. However, others have demonstrated that both D1 and D2 densities peak in the frontal cortex during adolescence (Andersen et al., 2000), as does D1 receptor density on afferent projections from the PFC to the ventral striatum (Brenhouse, Sonntag, & Andersen, 2008), indicating that this might region may be characterized by peaks in dopamine receptor density as well.
Although D1 and D2 receptors exhibit similar developmental trajectories, age by receptor-type interactions have been demonstrated whereby peaks in D1 density are more pronounced compared to D2 density (though both decline during later stages of adolescence and plateau at similar points in time; Gelbard et al., 1989). Furthermore, analyses of gender differences have demonstrated that only male rodents exhibit periadolescent-limited overproduction of D1 and D2 receptors in the striatum, though males and females are characterized by similar densities in adulthood (Andersen et al., 1997). Similar findings were revealed with respect to D2 receptors in the nucleus accumbens, with males exhibiting a peak in receptors at P100 that was absent in females. Gender differences in receptor overproduction do not appear to be caused by developmental changes in gonadal hormones, as evidenced by the fact that neither castration nor ovariectomy alter the trajectory of dopamine receptor ontogeny (Andersen, Thompson, Krenzel, & Teicher, 2002). Other evidence suggests that changes in dopamine receptors are similar in males and females (Lieb et al., 1996); however, rates of change during adolescence were not directly compared.
In summary, the available evidence suggests that both primates and rodents exhibit increases in functionally available dopamine during adolescence, though differences exist with respect to the regions and aspects of the dopamine system affected. In primates, cortical and subcortical tissue concentrations of dopamine are increased during adolescence (Goldman-Rakic & Brown, 1982; Irwin et al., 1994). In addition, dopaminergic innervation of the frontal cortex also peaks during adolescence relative to childhood and adulthood, specifically in cortical layer III, which contains pyramidal cells responsible for cortico-cortical information processing (Rosenberg & Lewis, 1995). D1 and D2 receptor densities appear to be heightened during adolescence compared to adulthood in both cortical and subcortical regions, though peaks in receptor density occur in childhood (Lidow & Rakic, 1992; Seeman et al., 1987). Conversely, rodents exhibit monotonic increases in dopaminergic innervation of the cortex lasting until early adulthood (Kalsbeek et al., 1988). Additionally, some evidence suggests that cortical dopamine synthesis, as well as synaptic dopamine availability in the striatum, peak during adolescence (Andersen, Dumont, & Teicher, 1997; Stamford, 1989). Similar to innervation patterns, receptor densities in the cortex increase throughout adolescence (Tarazi & Baldessarini, 2000). However, D1 and D2 densities do appear to peak in subcortical structures such as the striatum and nucleus accumbens, as do the densities of D2-like D3 and D4 receptors (Andersen et al., 2002; Tarazi, Tomasini, & Baldessarini, 1998b). Thus, it appears that in rodents, adolescence is characterized by a subcortical increase in dopamine compared to childhood and adulthood. Conversely, dopamine availability in the cortex is lower compared to adulthood (but see Andersen, Dumont, & Teicher, 1997).
V. Implications for Cortical and Subcortically-Mediated Behavioral Processes
In primates, cortical and subcortical tissue concentrations of dopamine are increased during adolescence; DA innervation of the frontal cortex also peaks especially in cortical layer III; D1 and D2 receptor densities are heightened in both cortical and subcortical regions. Accordingly, there is no clear evidence for a cortical/subcortical differentiation in terms of the overall level of dopamine activity. It is high in both regions. What this means in terms of behavioral outcomes is that dopamine-modulated behaviors that are mediated by each region might be enhanced, behaviors might be “disabled” if the respective systems are overdosed, or there may be an imbalance if the threshold for overdose is different between frontal and subcortical regions. This latter interpretation seems possible given that the PFC dopamine system is less rigorously regulated in terms of autoreceptor and dopamine transporter activity (Bannon & Roth, 1983; Sesack et al., 1998). In addition, the prefrontal cortex is still in a state of flux regarding its structural development given that synaptic pruning is ongoing during adolescence. Pruning does not occur equivalently across all types of synapses. Most pruned synapses are excitatory, involving glutamate receptors (Smiley & Goldman-Rakic, 1993). When glutamate levels fall, a state of activity is created that results in an enhancement of phasic dopamine signaling (Grace, 1991; O’Donnell & Grace, 1998). Thus, in the context of synaptic pruning in the PFC, we might expect an exuberance of dopamine neurotransmission. Dopamine synapses within the PFC on pyramidal cells and on GABA-ergic non-pyramidal cells. The pruning process may result in a summated state of activity that upsets the excitatory-inhibitory balance such that the PFC is overdosed. In subcortical (limbic and striatal) regions, the overdose threshold may be higher. Thus, during adolescence, dopamine levels are at a functional high, leading to elevated patterns of exploration, novelty-seeking, incentive salience, and locomotor activity, all of which serve to bring the individual into contact with biologically salient incentives.
In rodents, in contrast, the evidence suggests that there is a decreased level of cortical dopamine in adolescence, which would negatively impact prefrontally-guided behaviors. At the same time, dopamine levels in limbic and striatal regions are elevated, yielding an overall behavioral profile that is similar to that which characterizes primates.
These patterns could explain adolescents’ cognitive failures or immaturities with respect to prefrontally-mediated functions that are DA-modulated in the context of the pruning process and could also explain heightened incentive-seeking. Motivation is at a high, but without a tightly-honed regulatory system to keep it in check and to guide that motivation toward goals that are spatially or temporally removed from the current context. The nature of the non-optimal PFC regulation could stem from structural immaturity given that synapses are still being pruned. Thus, it is our perspective that the dopamine system is in a relative state of over-drive during adolescence and that this overactivity brings incentive-motivational systems to a maximally-heightened (but not overdosed) state and overdoses prefrontal systems which are then taxed for two reasons: the slower pace of structural development and high levels of circulating dopamine without regulatory control. How the former process within the PFC interacts with the latter one remains to be specified, but it seems clear that some aspects of the PFC’s structural immaturity could be linked to the PFC’s deficient functioning when dopamine levels are high. It could be that behavioral inhibition is weaker when DA facilitates PFC synaptic connections already lacking in optimal connection specificity. In this case, strong “go” signals (arising from striatal/limbic DA) would interact with under-synchronized (due to immature pruning) “no-go”signals, and particularly in contexts containing cues for both potential reward and potential punishment. What has yet to be demonstrated is convincing evidence that pruning impacts neural synchrony in a manner that would contribute to interactions between the immature brain structure and neurochemical activity. Should these hypothesized associations have merit, we emphasize that these general patterns would operate within the context of individual differences as summarized above such that the adolescent surge in dopamine availability impacts some individuals more than others depending on baseline levels.
VI. Measurement Issues: Molecular Genetics and Benefits of Pharmacological Challenge
The evidence provided here suggests that the dopaminergic system undergoes extensive remodeling during adolescence, and we have speculated as to how these changes might be related to typical adolescent behaviors based on neurochemical-behavior associations in adult samples. Direct assessment of neurotransmission in human samples is difficult to conduct due to obvious human subject concerns, but it is evident that a complete understanding of adolescent development depends upon knowledge of how these systems interact to influence behavior. Thus, until less invasive techniques for accessing neurochemical modulation are developed, creative study designs that utilize current methodologies will be necessary to further our understanding of how neurochemical systems drive normative patterns of behavioral development.
A number of researchers have made initial forays into this type of work. For example, numerous groups have used functional MRI techniques to document developmental differences in neural systems during the completion of reward-related tasks, which implicate differences in regions innervated by dopamine such as the OFC and ventral striatum (Van Leijenhorst, Zanolie1, Van Meel, Westenberg, Rombouts & Crone, 2009; Ernst et al., 2005; Galvan et al., 2006; May et al., 2004). Similar studies have found that adolescents exhibit a unique pattern of amygdaloid activation in response to emotional facial expressions (Baird et al., 2001; Thomas et al., 2001). It is tempting to speculate how activational changes in these regions might be related to the underlying neurochemical and neuromodulatory systems given that these systems modulate reward and affective processing (Chambers et al., 2003); however, MRI techniques alone cannot resolve the role that these chemicals might play in the behaviors studied. This is especially so given that theories of both hypoactive and hyperactive neurotransmission may be used to explain observed changes in brain activity (Spear, 2000); it is unclear how results obtained in neuroimaging can provide insight into directional changes in dopamine activity during adolescence. That said, they are suggestive. For instance, the Galvan et al. (2006) study, though based on a small sample and not replicated, indicates that adolescents exhibit hyper-responsivity of the ventral striatum in response to reward compared to adults, which could reflect hyperactivity of the modulatory dopamine system or of other neurochemicals involved in reward processing, such as opiates or oxytocin (Guastella et al., 2008; Vargas-Perez et al., 2009).
Our group has attempted to obtain insight into dopamine function during adolescence using molecular genetic techniques. We have pursued the idea that gene-behavior associations may be different at different points in the lifespan depending on the underlying state of the chemical system that is regulated by a given gene. For example, we recently assessed how the relationship between a single nucleotide polymorphism (SNP) on the gene that codes for catechol O-methyltransferase (COMT) enzyme activity (the Val158-Met SNP) and cognition differs during adolescence as compared to adulthood; we have interpreted these findings within the context of differential baseline levels of dopamine during this developmental period. This work will be described as a model to illustrate the utility of this approach, although we emphasize at the outset that other genes that regulate different aspects of dopamine neurotransmission could be similarly studied.
COMT is an enzyme that degrades synaptic dopamine. A functional polymorphism has been identified, which consists of a guanine (G) to adenine (A) mutation and results in an amino acid substitution of methionine (Met) for valine (Val) in enzyme synthesis. Within this SNP, the Met allele is associated with lower levels of COMT activity as compared to the Val allele, which is associated with higher levels of COMT activity. Accordingly, Val-Val individuals have lower levels of dopamine available in neural synapses where COMT is active as compared to Met-Met individuals (Lachman et al., 1996). As might be predicted from this relationship, the Met allele has been linked to relatively better performance on cognitive tasks such as working memory and set-shifting in both healthy adults and adults with schizophrenia (Goldberg et al., 2003; Malhotra, 2002). Interestingly, COMT activity has been hypothesized to predict an individual’s place on the inverted-U shaped function describing the relationship between dopaminergic signaling and cognitive performance (Goldman-Rakic, 1998; Mattay et al., 2003). Mattay et al. (2003) genotyped adults who performed a working memory task in the context of a functional MRI paradigm. Participants completed two sessions, one under the influence of a placebo and the other under the influence of amphetamine, a dopamine agonist. Met-Met individuals outperformed their Val-Val counterparts on a test of working memory at baseline; however, after the administration of d-amphetamine, their performance suffered. Their functional neuroimaging data also suggested prefrontal inefficiency on amphetamine relative to placebo. In contrast, the Val-Val individuals improved their performance on amphetamine. These findings were interpreted to suggest that Met-Met individuals performed poorly after amphetamine administration due to levels of dopamine that were too high (i.e. falling to the right of the inverted-U), whereas Val-Val individuals moved closer to the apex of the curve.
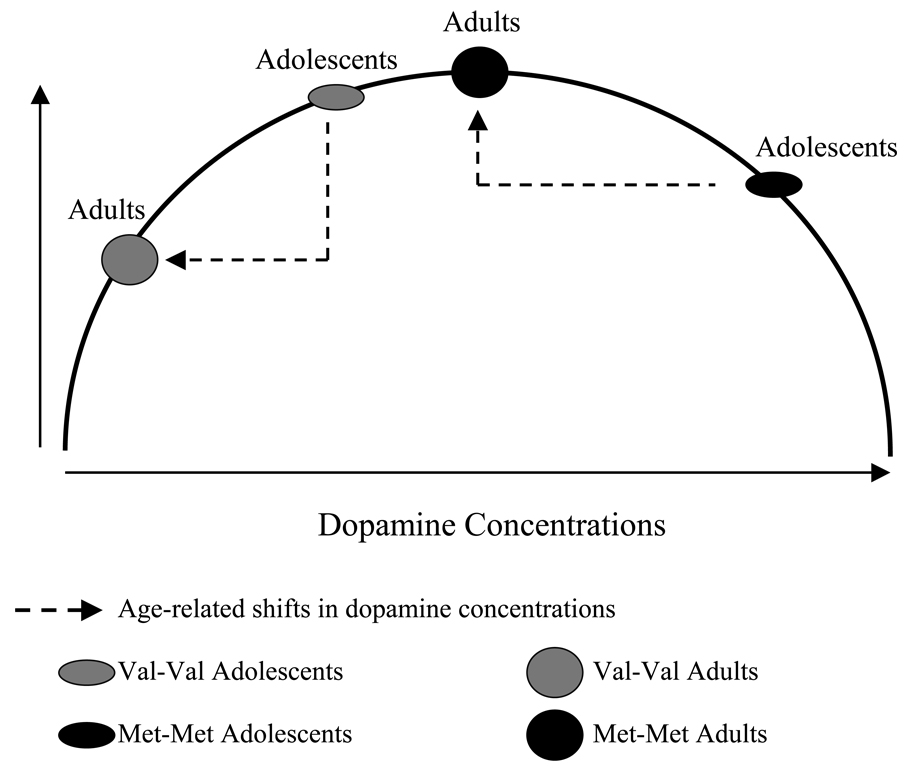
We have recently proposed that similar processes may occur in adolescence. That is, adolescence may be a period in the lifespan that simulates the effects of amphetamine administration as described by Mattay et al. Developmental increases in dopamine availability may shift the relative relationship between COMT activity and behavior in accord with an inverted-U shaped function (see Figure 2, adapted from Wahlstrom et al., 2007). Prior to adolescence, Met-Met individuals may perform relatively well on measures of prefrontal cognition. During adolescence, when circulating levels of dopamine in the prefrontal cortex are high, their performance may suffer. In adulthood, they would perform relatively better once again as dopamine levels stabilize. In contrast, Val-Val individuals would be expected to perform relatively worse on prefrontally-mediated tasks prior to adolescence but to perform somewhat better (though still worse than other groups) during adolescence. Heterozygotes would fall in between these two groups in their performance levels, reaching an optimal level of function in adolescence and an optimal level of performance relative to the other groups.
Figure 2.
Dopamine’s Inverted-U-Shaped Performance Function and Lifespan Development: Dopamine regulates behavior according to an inverted U-shaped performance function. As discussed in the text, the nature of this function may change during the human lifespan. Higher levels of available dopamine in adolescence versus adulthood may push some individuals toward a more optimal position on the curve, while some individuals may be disadvantaged during this time due to levels that are too high.
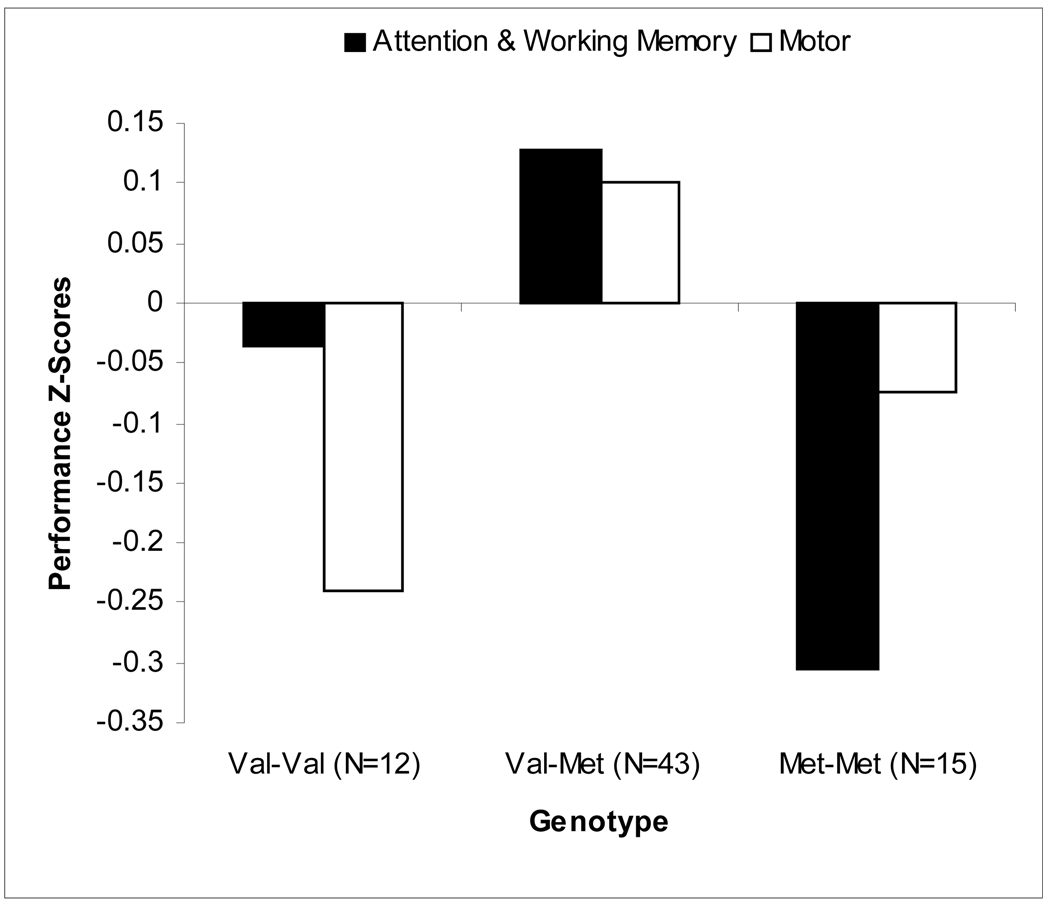
We obtained genetic samples from 205 participants in the 9-to-23 year-old age range, all of whom completed a battery of neuropsychological tests that have been shown to be modulated by prefrontal dopamine, including a visuomanual delayed response task modeled after the work of Patricia Goldman-Rakic in primates (Goldman-Rakic, 1987). In an initial analysis of 9–17 year-olds, we reported that the heterozygous genotype (Val-Met) was associated with the best performance on measures of attention and working memory (Wahlstrom et al., 2007). Met-Met and Val-Val individuals both performed relatively poorly (see Figure 3). The genotype groups did not differ in other demographic or cognitive parameters that might have impacted performance. Interpreted within the context of developmental changes in dopamine, it is possible that these findings reflect the interaction of adolescent-limited increases in dopamine availability and COMT genotype. Specifically the interaction between the age-dependent dopaminergic increases and the Met-Met configuration produced excessive levels of dopamine that translated to inefficient cognitive functioning. Likewise, when combined with developmental increases in dopamine, the heterozygotes may rest towards the apex of the curve, and Val-Val individuals might receive similar (or greater, based on the fact that baseline functioning was worse) benefits.
Figure 3.
Illustration of the COMT genotype-by-behavior association in an adolescent sample: Adolescents, ages 9 to 17, who varied in COMT genotype completed a battery of neurocognitive tasks that reflect dopamine activity. COMT Val/Met heterzygotes performed better than homozygotes on measures of working memory, attention, and motor function in accord with the model illustrated in Figure 2, suggesting that they experience relatively optimal levels of dopamine activity during this period of the lifespan. (From Wahlstrom et al., 2007, with permission).
Similar models have been proposed to account for decrements in working memory and other fluid abilities that are associated with normal aging (Lindenberger et al., 2008; Backman et al., 2006; Nagel et al., 2008). Using the COMT genotype as an example, Val-Val individuals may be relatively vulnerable to the effects of aging on frontal dopamine concentrations, since the aging process leads to lower levels of available dopamine.
Longitudinal analyses in support of this model would be ideal, and data collection to that end is in progress in our laboratory. However, it would also provide supportive evidence to demonstrate that the optimal genotype for cognitive performance changes with age using cross-sectional data. After expanding the sample described above, we attempted to characterize Age-by-Genotype interactions on prefrontal cognition by dividing our sample into three age groups (9–12 year-olds (n=54), 13–17 year-olds (n=85), and 18–25 year-olds (n=62)). These groupings represent early, mid, and late adolescence. The three groups are demographically similar, and genotypic frequencies adhere to the Hardy-Weinberg equilibrium in the sample. Two cognitive tasks were the focus of analysis, the digit span task (forward and backward versions) as well as a spatial delayed response task (performance under 500 millisecond and 8-second delay conditions: Luciana & Collins, 1997). A repeated measures ANOVA was implemented entering z-scores for these four variables (digit span forward, digit span backward, spatial delayed response error 500 milliseconds and spatial delayed response error 8 seconds, all scored so that a high score represents good performance) to represent two levels of Task (digit span versus delayed response) and, within the task groupings, two levels of Process (attention versus working memory). Age group and COMT genotype were entered as between-groups variables. There was a task by age group interaction, but otherwise, there were no interactions between task or process and age group and genotype. There was a main effect of age group, F(2,192)=28.55, p<.001, ηp2=.22, as well as an age group by COMT interaction, F(4,192)=2.45, p<.05, ηp2= .05. The main effect of age group was due to worse performance in 9–12 year-olds relative to each of the other groups, who did not differ from one another. The task by age group interaction is due to stronger age effects for the digit span task than for the delayed response task. The digit span task continues to show developmental improvement through this age range, whereas performance on the delayed response task asymptotes earlier.
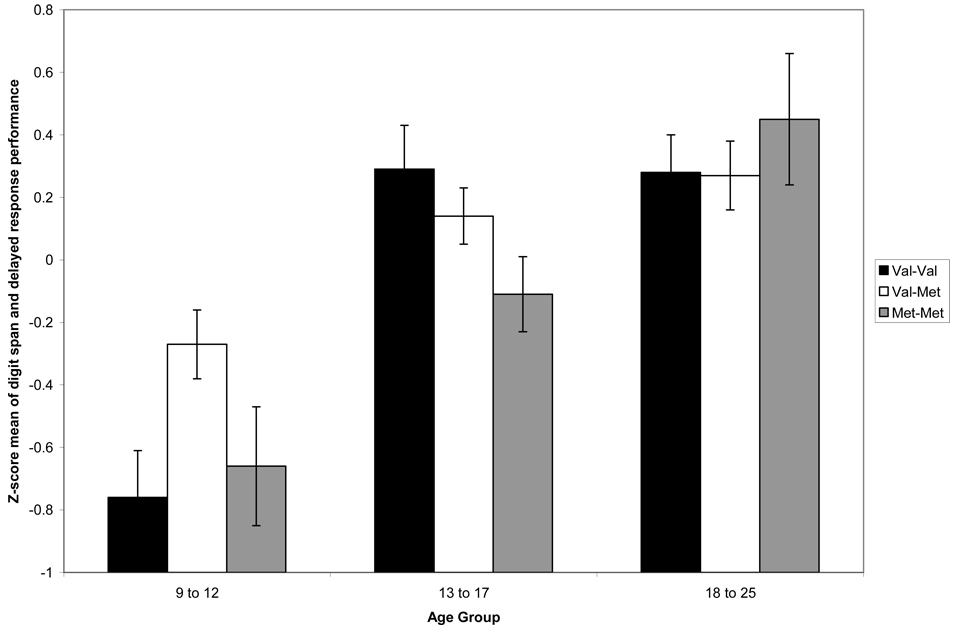
In terms of the age group by COMT interaction (see Figure 4), there were no reliable effects of COMT observed within discrete age groups. However, when age effects were examined within each COMT genotype, different developmental trends appeared evident. For Val-Val individuals, there was an overall age group effect, F(2,54)= 13.76, p<.01, ηp2=.34, with 9–12 year-olds performing worse than 13–17 year-old and 18–25 year-olds. For Val-Met individuals, there was also a main effect of age group that was somewhat lower in magnitude, F(2,98)=7.76, p<.01, ηp2= .14. Again, 9–12 year-olds performed worse than the older two groups who did not differ from one another. For Met-Met individuals, the age group effect was pronounced, F(2,40)=7.92, p<.01, ηp2=.28, but with a different pattern from the other groups. The 9–12 year-olds performed worse than the older two groups, but the 13–17 year-olds also performed worse (at a trend level of p=.06) than 18–25 year-olds. Thus, there is evidence of a more protracted course of development of dopamine-modulated executive functions in Met homozygotes. Figure 4 suggests that this distinct developmental course may lead to superior performance, relative to the other genotypic groups, by adulthood. Longitudinal assessment of the sample would clarify these trends. Of course, these conclusions are tentative given the small sample sizes, but we are intrigued by the suggestion of differences in neuroplasticity through adolescence based on genotype. Our model suggests that Met-Met individuals might be vulnerable to high circulating levels of dopamine in PFC circuits during adolescence but that this vulnerability may resolve with increasing age. It is important to note here that other groups have demonstrated support for the notion that adolescent development modulates the relationship between COMT and cognition. For example, Gothelf and colleagues (2005) assessed intellectual performance in a sample of adolescents with velo-cardio-facial-syndrome (VCFS) at ages 13 and 18 years, and the results of their longitudinal design indicated that the optimal COMT genotype at these two time points differed.
Figure 4.
COMT by Age Group Interaction: An expansion of the study described in Figure 3 allowed age-by-genotype interactions to be characterized. An age-by-genotype interaction was observed for attention and working memory function when comparing 9–12, 13–17 and 18–25 year-olds. As described in the text, individuals who carry the Val allele level off in their development of these skills prior to those who are Met homozygotes. Met-Met homozygotes show a developmental acceleration of performance from ages 13–17 to 18–25 that is not present in the other groups. Values represent estimated marginal means plus/minus one standard error.
Though not a definitive set of findings, this work indicates a role for molecular genetics in the study of adolescent-limited changes in neurochemical development, using genetic polymorphisms as probes for neurotransmitter availability. This approach should be particularly useful provided that a theoretical framework exists describing clear relationships between behavior, age, the neurochemical system of interest, and the specified genetic probe. Although our interest in this realm of assessment ultimately focuses on individual differences in adolescent risk-taking, it may not be appropriate to study COMT in relation to reward salience or other incentive-motivated behaviors given that it is hypothesized to be most active in dorsal prefrontal versus limbic or striatal regions (Beyer & Stekee, 2000; Carr & White, 1987). It is likely that there are other candidate genes that control for processes that are expressed more strongly in these regions and that could be examined for age-by-genotype interactions. For instance, the Taq1 A allele, associated with D2 dopamine receptor mechanisms (Neville et al., 2004), has been associated with novelty seeking tendencies in adults and in adolescents as well as with reinforcement learning (Berman et al., 2002; Frank & Hutchinson, 2009). The D4 dopamine receptor and the dopamine transporter genes are also likely candidates for analysis, because individual differences in SNPs associated with these genes have been linked to variations in personality traits, externalizing behaviors, and higher-order cognition (DeYoung et al., 2006; Durston et al., 2008; Munafo et al., 2008). In summary, the field of molecular genetics has been inspired by recent suggestions of genotype-by-context interactions in shaping the quality of observed behavior (Caspi, 2003). In this case, age serves as the contextual variable.
Though promising, this model of measuring developmental changes in neurochemistry is indirect and relies on large samples for reliable conclusions to be drawn. In addition, a number of methodological considerations must be addressed given the interpretive leaps required (for example, how age is treated as an independent variable, and/or how age groups are stratified given that the expected changes in the neurochemical system of interest may have a dramatic effect on findings). Moreover, although we did not consider sex differences in our analyses due to low power, there may be sexually dimorphic associations between genetic indices of dopamine activity and behavior (see DeYoung et al., in press for an example). It could readily be argued that from an evolutionary standpoint, males receive a higher benefit for exploration and status seeking in the transition from adolescence to adulthood. Accordingly, we might expect stronger associations between dopamine activity and relevant behaviors in males or between gene-behavior associations.
It would be ideal to measure neurochemical responses and/or baseline activity more directly. Positron emission tomography (PET) provides one avenue through which to conduct such studies (Backman et al., 2006), although these studies are not feasible in healthy children and adolescents due to the radioactive ligands that are used during scanning. More direct conclusions could also be drawn through the use of acute psychopharmacological challenge paradigms, which have provided important and useful information regarding the impact of dopamine manipulations on adult cognition (Cools et al., 2007; Floresco & Magyar, 2006; Luciana & Collins, 1997). Animal studies using pharmacological probes indicate, though, that this approach as applied to humans would be complex and that different phenotypic responses might be evident for different drugs, for different doses of the same drug, and for individuals of different ages and clinical states (see Spear, 2000 for review). Nonetheless, we argue that attempts to reconcile these issues would greatly expand the assessment of adolescent brain development so that theories involving neurochemical function could be empirically validated.
VIII. Conclusions
The surge in incentive-reward motivation that is observed during adolescence coheres with models of behavior that advocate for the existence of a dopamine-modulated neurobehavioral system that underlies incentive-driven behavior. This system has been thoroughly described (Depue & Collins, 1999; Panksepp, 1998) but has not been considered from a developmental perspective. Whether developmental changes in this system’s activation are beneficial or not depends on one’s perspective. On the one hand, adolescence is characterized by a number of positive behavioral changes as one moves toward increasing independence. One the other hand, these changes include increases in risk-taking and novelty seeking, as well as a heightened risk for the development of psychiatric disorders such as depression, schizophrenia, and substance abuse (Eison & Eison, 1994; Weinberger, 1987; Wise, 1998). Recent methodological advances have allowed for a number of important insights into how brain development impacts adolescent behavior. A notable omission, however, concerns changes in neurotransmitter functions and their links to cognition and affect during this period, which is a result of the difficulty in measuring these systems non-invasively. We contend that this is a critical component in understanding human adolescent development considering the well-established links between neurotransmission and behavior in adult samples. Moreover, it is the case that neurochemistry can be altered. If adolescents experience over-or-underactivity in neurochemical systems relative to other points in the lifespan and if this transient dysregulation is extremely maladaptive for an individual, it can be treated. The promise of intervention for those at greatest risk is important given the mortality associated with adolescent risk-taking behaviors.
We have suggested models for measuring these associations, which include molecular genetics (Wahlstrom et al., 2007) and pharmacological challenge (Rosa-Neto et al., 2005; Sallee et al., 1998; Soloff et al., 2000). These techniques have been implemented in humans only to a limited extent to answer developmental questions (i.e., change over time). Specific areas of investigation would include the use of genotyping in large samples to infer whether adolescence is a distinct period in development from the standpoint of gene-behavior associations, whether links between neurochemical activity and behavior in adolescent samples are best explained by trait versus state models of functional association, and the extent to which neurochemical activity in distinct brain regions (striatum versus prefrontal cortex, for example) contributes to the weighing of alternatives as adolescents make decisions in times of uncertainty. A focus on these questions is important for our understanding of normative aspects of adolescent development, particularly the accelerated development of incentive motivation that permits adolescents to strive toward adult roles and future goals. These are positive changes for the majority of individuals. However, the study of neurochemistry can also inform us regarding individual differences in the emergence of problematic risk-taking and psychopathology and why this vulnerability emerges during adolescence.
Acknowledgments
Preparation of this manuscript was supported by grant DA017843 awarded to Monica Luciana by the National Institute on Drug Abuse. The genotyping studies reported here were supported by a seed grant from the University of Minnesota’s Biomedical Genomics Center awarded to Tonya White, M.D.. Dustin Wahlstrom was supported by T32 grant MH017069. The support of the University of Minnesota’s General Clinical Research Center (M01-RR00400 National Center for Research Resources, National Institutes of Health) and Center for Neurobehavioral Development are also gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behavioral Neuroscience. 1998;112(5):1152–1166. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Dumont NL, Teicher MH. Developmental differences in dopamine synthesis inhibition by (+)-7-OH-DPAT. Naunyn-Schmiedeberg's Archives of Pharmacology. 1997;356:173–181. doi: 10.1007/pl00005038. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AP, Krenzel E, Teicher MH. Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology. 2002;27:683–691. doi: 10.1016/s0306-4530(01)00069-5. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Asato MR, Sweeney JA, Luna B. Cognitive processes in the development of TOL performance. Neuropsychologia. 2006;44(12):2259–2269. doi: 10.1016/j.neuropsychologia.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neuroscience and Biobehavioral Reviews. 2006;30:791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Baird AA, Gruber SA, Fein DA, Mass LC, Steingard RJ, Renshaw PF, et al. Functional magnetic resonance imaging of facial affect recognition in children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38(2):195–199. doi: 10.1097/00004583-199902000-00019. [DOI] [PubMed] [Google Scholar]
- Bannon MJ, Roth RH. Pharmacology of mesocortical dopamine neurons. Pharmacological Reviews. 1983;35(1):53–68. [PubMed] [Google Scholar]
- Beckstead RM, Domesick VB, Nauta WJH. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Research. 1979;175:191–217. doi: 10.1016/0006-8993(79)91001-1. [DOI] [PubMed] [Google Scholar]
- Berger B, Verney C, Febvret A, Vigny A, Helle K. Postnatal ontogenesis of the dopaminergic innervation in the rat anterior cingulate cortex (area 24). Immunocytochemical and catecholamine fluorescence histochemical analysis. Developmental Brain Research. 1985;21:31–47. doi: 10.1016/0165-3806(85)90021-5. [DOI] [PubMed] [Google Scholar]
- Berman S, Ozkaragoz T, Young RM, Noble EP. D2 dopamine receptor gene polymorphism discriminates two kinds of novelty seeking. Personality and Individual Differences. 2002;33(6):867–882. [Google Scholar]
- Beyer CE, Steketee JD. Intra-medial prefrontal cortex injection of quinpirole, but not SKF 38393, blocks the acute motor-stimulant response to cocaine in the rat. Psychopharmacology (Berlin) 2000;151(2–3):211–218. doi: 10.1007/s002139900345. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: Similarities and differences from young adults. J Neuroscience. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends in Neuroscience. 2007;30(5):194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Bourgeois J, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cerebral Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. The Journal of Neuroscience. 2008;28(10):2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Goldman PS. Catecholamines in neocortex of rhesus monkeys: regional distribution and ontogenetic development. Brain Research. 1977;124:576–580. doi: 10.1016/0006-8993(77)90960-x. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JDE. Neurochemical Development 34 Development offrontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr GD, White NM. Effects of systemic and intracranial amphetamine injections on behavior in the open field: a detailed analysis. Pharmacology Biochemistry and Behavior. 1987;27(1):113–122. doi: 10.1016/0091-3057(87)90485-0. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, et al. Influence of Life Stress on Depression: Moderation by a Polymorphism in the 5-HTT Gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. American Journal of Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Schoene-Bake JC, Elger CE, Weber B. Connectivity-based segregation of the human striatum predicts personality characteristics. Nature Neuroscience. 2009;12:32–34. doi: 10.1038/nn.2228. [DOI] [PubMed] [Google Scholar]
- Comings DE, Blum K. Reward deficiency syndrome: genetic aspects of behavioral disorders. Progress in Brain Research. 2000;126:325–341. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- Cools R, Sheridan M, Jacobs E, D'Esposito M. Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. Journal of Neuroscience. 2007;27:5506–5514. doi: 10.1523/JNEUROSCI.0601-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Erkanli A, Federman E, Angold A. Development of psychiatric comorbidity with substance abuse in adolescents: effects of timing and sex. Journal of Clinical Child Psychology. 1999;28(3):298–311. doi: 10.1207/S15374424jccp280302. [DOI] [PubMed] [Google Scholar]
- Crofts HS, Dalley JW, Collins P, Van Denderen JCM, Everitt BJ, Robbins TW, Roberts AC. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire attentional set. Cerebral Cortex. 2001;11:1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- Crone EA, van der Molen MW. Developmental changes in real-life decision-making performance on a gambling task previously shown to depend on the ventromedial prefrontal Neurochemical Development 35 cortex. Developmental Neuropsychology. 2004;25:251–279. doi: 10.1207/s15326942dn2503_2. [DOI] [PubMed] [Google Scholar]
- Csikszentmihalyi M, Larson R, Prescott S. The ecology of adolescent activity and experience. Journal of Youth and Adolescence. 1977;6:281–294. doi: 10.1007/BF02138940. [DOI] [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brainstem neurons. Acta Physiologica Scandinavica. 1964;62 Suppl. 232:1–55. [PubMed] [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences. 1999;22:491–517. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- Depue RA, Iacono WG. Neurobehavioral aspects of affective disorders. Annual Review of Psychology. 1989;40:457–492. doi: 10.1146/annurev.ps.40.020189.002325. [DOI] [PubMed] [Google Scholar]
- Depue RA, Luciana M, Arbisi P, Collins P, Leon A. Dopamine and the structure of personality: Relation of agonist-induced dopamine D2 activity to positive emotionality. Journal of Personality and Social Psychology. 1994;67:485–498. doi: 10.1037//0022-3514.67.3.485. [DOI] [PubMed] [Google Scholar]
- Depue RA, Spoont MR. Conceptualizing a serotonin trait: A behavioral dimension of constraint. Annals of the New York Academy of Sciences. 1986;487:47–62. doi: 10.1111/j.1749-6632.1986.tb27885.x. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Rothbart MK. Arousal, affect, and attention as components of temperament. Journal of personality and social psychology. 1988;55(6):958–966. doi: 10.1037//0022-3514.55.6.958. [DOI] [PubMed] [Google Scholar]
- DeYoung CG, Getchell M, Koposov RA, Yrigollen CM, Haeffel GJ, Klinteberg B, Oreland L, Ruchkin VV, Pakstis AJ, Grigorenko EL. Variation in the catechol-O-methyltransferase Val158Met polymorphism associated with conduct disorder and ADHD symptoms among adolescent male delinquents. Psychiatric Genetics. doi: 10.1097/YPG.0b013e32833511e4. (in press) in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung CG, Peterson JB, Séguin JR, Mejia JM, Pihl RO, Beitchman JH, Jain U, Tremblay RE, Kennedy JL, Palmour RM. The dopamine D4 receptor gene and moderation of the association between externalizing behavior and IQ. Archives of General Psychiatry. 2006;63:1410–1416. doi: 10.1001/archpsyc.63.12.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Kohn P, Kolachana P, Weinberger DR, Berman KF. Variation in dopamine genes influences responsivity of the human reward system. Proceedings of the National Academy of Sciences. 2009;106(2):617–622. doi: 10.1073/pnas.0805517106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Fossella JA, Mulder MJ, Casey BJ, Ziermans TB, Vessaz MN, Van Engeland H. Dopamine transporter genotype conveys familial risk of attention deficit/hyperactivity disorder through striatal activation. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47(1):61–67. doi: 10.1097/chi.0b013e31815a5f17. [DOI] [PubMed] [Google Scholar]
- Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ. High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biological Psychiatry. 2009;65(10):851–856. doi: 10.1016/j.biopsych.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Eison AS, Eison MS. Serotonergic mechanisms in anxiety. Progress in Neuropsychopharmacology and Biological Psychiatry. 1994;18:47–62. doi: 10.1016/0278-5846(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec SP, McClure EB, Monk CS, Leibenluft, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. NeuroImage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ. A Model for Personality. New York: Springer; 1981. [Google Scholar]
- Fareri DS, Martin LN, Delgado MR. Reward-related processing in the human brain: developmental considerations. Developmental Psychopathoogy. 2008;20(4):1191–1211. doi: 10.1017/S0954579408000576. [DOI] [PubMed] [Google Scholar]
- Fink JS, Reis DJ. Genetic variations in midbrain dopamine cell number: parallel with differences in responses to dopaminergic agonists and in naturalistic behaviors mediated by dopaminergic systems. Brain Research. 1981;222:335–349. doi: 10.1016/0006-8993(81)91037-4. [DOI] [PubMed] [Google Scholar]
- Fink JS, Smith GP. Mesolimbic and mesocortical dopaminergic neurons are necessary for normal exploratory behavior in rats. Neuroscience Letters. 1980;17:61–65. doi: 10.1016/0304-3940(80)90062-2. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology. 2006;188(4):567–585. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- Foote SL, Morrison JH. Postnatal development of laminar innervation patterns by monoaminergic fibers in monkey (Macaca Fascicularis) primary visual cortex. The Journal of Neuroscience. 1984;4(11):2667–2680. doi: 10.1523/JNEUROSCI.04-11-02667.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouriezos G, Hansonn P, Wise RA. Neuroleptic-induced attenuation of brain stimulation reward in rats. Journal of Comparative Physiology and Psychology. 1978;92:661–671. doi: 10.1037/h0077500. [DOI] [PubMed] [Google Scholar]
- Frank MJ. Dynamic dopamine modulation in the basal ganglia: a neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. Journal of Cognitive Neuroscience. 2005;17(1):51–72. doi: 10.1162/0898929052880093. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Hutchinson K. Genetic contributions to avoidance-based decisions: Striatal D2 receptor polymorphisms. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.04.048. (in press)doi: 10,1016/j.neuroscience.2009,04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. The Journal of Neuroscience. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardener M, Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: an experimental study. Developmental Psychology. 2005;41:625–635. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- Gelbard HA, Teicher MH, Faedda G, Baldessarini RJ. Postnatal development of dopamine D1 and D2 receptor sites in rat striatum. Brain Research. 1989;49:123–130. doi: 10.1016/0165-3806(89)90065-5. [DOI] [PubMed] [Google Scholar]
- Giorgi O, De Montis G, Porceddu ML, Mele S, Calderini G, Toffano G, Biggio G. Developmental and age-related changes in D1-dopamine receptors and dopamine content in the rat striatum. Developmental Brain Research. 1987;35:283–290. doi: 10.1016/0165-3806(87)90053-8. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Goldman D, Weinberger DR. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Archives of General Psychiatry. 2003;60:889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of the prefrontal cortex and the regulation of behavior by representational knowledge. In: Plum F, Mountcastle V, editors. Handbook of Physiology. Vol 5. Bethesda, MD: American Physiological Society; 1987. p. 373. [Google Scholar]
- Goldman-Rakic PS. The cortical dopamine system: Role in memory and cognition. Advancements in Pharmacology. 1998;42:707–711. doi: 10.1016/s1054-3589(08)60846-7. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Brown RM. Postnatal development of monoamine content and synthesis in the cerebral cortex of rhesus monkeys. Developmental Brain Research. 1982;4:339–349. doi: 10.1016/0165-3806(82)90146-8. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Lidow MS, Gallager DW. Overlap of dopaminergic, adrenergic, and serotoninergic receptors and complementarity of their subtypes in primate prefrontal cortex. The Journal of Neuroscience. 1990;10:2125–2138. doi: 10.1523/JNEUROSCI.10-07-02125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D, Eliez S, Thompson T, Hinard T, Penniman L, Feinstein C, et al. COMT genotype predicts longitudinal cognitive decline and psychosis in 22q11.2 deletion syndrome. Nature Neuroscience. 2005;8:1500–1502. doi: 10.1038/nn1572. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behavior. Trends in Neuroscience. 2007;30(5):220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Gray JA. Causal theories of personality and how to test them. In: Royce JR, editor. Multivariate analysis and psychological theory. Academic Press; 1973. [Google Scholar]
- Gray J. The neuropsychology of emotion and personality. In: Stahl SM, Iversen S, Goodman E, editors. Cognitive Neurochemistry. Oxford University Press; 1987. [Google Scholar]
- Gray JA. Framework for a taxonomy of psychiatric disorder. In: van Goozen SHM, van de Poll N, Sargeant JA, editors. Emotions: Essays on Emotional Theory. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1994. pp. 29–59. [Google Scholar]
- Groves P, Garcia-Munoz M, Linder J, Manley M, Martone M, Young S. Elements of the intrinsic organization and information processing in the neostriatum. In: Houk J, Davis J, Beiser D, editors. Models of information processing in basal ganglia. Boston: MIT; 1995. [Google Scholar]
- Guastella AJ, Mitchell PB, Matthews F. Oxytocin enhances the encoding of positive social memories in humans. Biological Psychiatry. 2008;64(3):256–258. doi: 10.1016/j.biopsych.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Hariri AR. The neurobiology of individual differences in complex behavioral traits. Annual Reviews of Neuroscience. 2009;32:255–247. doi: 10.1146/annurev.neuro.051508.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JR. Where is the child's environment? A group socialization theory of development. Psychological Review. 1995;102:458–489. [Google Scholar]
- Haycock JW, Becker L, Ang L, Furukawa Y, Hornykiewicz O, Kish SJ. Marked disparity between age-related changes in dopamine and other presynaptic dopaminergic markers in human striatum. Journal of Neurochemistry. 2003;87:574–585. doi: 10.1046/j.1471-4159.2003.02017.x. [DOI] [PubMed] [Google Scholar]
- Henze DA, Gonzales-Burgos GR, Urban NN, Lewis DA, Barrionuevo G. Dopamine increases excitability of pyramidal neurons in primate prefrontal cortex. Journal of Neurophysiology. 2000;84:2799–2809. doi: 10.1152/jn.2000.84.6.2799. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Hooper CJ, Luciana M, Conklin HM, Yarger RS. Adolescents’ performance on the Iowa Gambling Task: implications for the development of decision making and ventromedial prefrontal cortex. Developmental Psychology. 2004;40:1148–1158. doi: 10.1037/0012-1649.40.6.1148. [DOI] [PubMed] [Google Scholar]
- Irwin I, DeLanney LE, McNeil T, Chan P, Forno LS, Murphy GM, Jr., et al. Aging and the nigrostriatal dopamine system: a non-human primate study. Neurodegeneration. 1994;3:251–265. [PubMed] [Google Scholar]
- Irwin CE, Jr., Millstein SG. Correlates and predictors of risk-taking behavior during adolescence. In: Lipsitt LP, Mitnick LL, editors. Self-regulatory Behavior and Risk Taking: Causes and Consequences. Norwood, NJ: Ablex Publishing; 1992. pp. 3–21. [Google Scholar]
- Jones EG. Laminar distribution of cortical efferent cells. In: Peters A, Jones EG, editors. Cerebral Cortex. Vol. 1. New York: Plenum Press; 1984. pp. 521–553. [Google Scholar]
- Kalaria RN, Fiedler C, Hunsaker C, III, Sparks DL. Synaptic neurochemistry of human striatum during development: changes in sudden infant death syndrome. Journal of Neurochemistry. 1993;60:2098–2105. doi: 10.1111/j.1471-4159.1993.tb03494.x. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HBM. Development of the dopaminergic innervation in the prefrontal cortex of the rat. The Journal of Comparative Neurology. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Kehr W, Lindqvist M, Carlsson A. Distribution of dopamine in the rat cerebral cortex. Journal of Neurological Transmission. 1976;38:173–180. doi: 10.1007/BF01249437. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Life-time prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of Geeral. Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, D’Esposito M, Farah MJ. Effects of bromocriptine on human subjects depends on working memory capacity. Neuroreport. 1997;8:3581–3585. doi: 10.1097/00001756-199711100-00032. [DOI] [PubMed] [Google Scholar]
- King RJ, Mefford IN, Wang C, Murchison A, Caligari EJ, Berger PA. CSF dopamine levels correlate with extraversion in depressed patients. Psychiatry Research. 1986;19(4):305–310. doi: 10.1016/0165-1781(86)90123-x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Riley SJ, Smith SC, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi and olfactory tubercle on feeding, locomotor activity, and amphetamine anorexia in the rat. Journal of Comparative and Computational Psychology. 1978;92(5):917–927. doi: 10.1037/h0077542. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski C, Weinshilboum R. Human catechol O-methyltransferase pharmacogenetics: Description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Krimer LS, Goldman-Rakic PS. Differential postnatal development of catecholamine and serotonin inputs to identified neurons in prefrontal cortex of rhesus monkeys. The Journal of Neuroscience. 2000;20:8780–8787. doi: 10.1523/JNEUROSCI.20-23-08780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G, Adriani W. Evaluation of unconditioned novelty seeking and d-amphetamine conditioned motivation in rats. Pharmacology Biochemistry and Behavior. 1998;59:1011–1020. doi: 10.1016/s0091-3057(97)00531-5. [DOI] [PubMed] [Google Scholar]
- Laviola G, Wood RD, Kuhn C, Francis R, Spear LP. Cocaine sensitization in periadolescent and adult rats. The Journal of Pharmacology and Experimental Therapeutics. 1995;275:345–357. [PubMed] [Google Scholar]
- Leslie CA, Robertson MW, Cutler AJ, Bennett JP., Jr. Postnatal development of D1 dopamine receptors in the medial prefrontal cortex, striatum, and nucleus accumbens of normal and neonatal 6-hydroxydopamine treated rats: A quantitative autoradiographic analysis. Developmental Brain Research. 1991;62:109–114. doi: 10.1016/0165-3806(91)90195-o. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Rakic P. Synchronized overproduction of neurotransmitter receptors in diverse regions of the primate cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:10218–10221. doi: 10.1073/pnas.88.22.10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow MS, Rakic P. Scheduling of monoaminergic neurotransmitter receptor expression in the primate neocortex during postnatal development. Cerebral Cortex. 1992;2:401–416. doi: 10.1093/cercor/2.5.401. [DOI] [PubMed] [Google Scholar]
- Lieb K, Andersen C, Lazarov N, Zienecker R, Urban I, Reisert I, Pilgrim C. Pre-and postnatal development of dopaminergic neuron numbers in the male and female mouse midbrain. Developmental Brain Research. 1996;94:37–43. doi: 10.1016/0165-3806(96)00063-6. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Nagel IE, Chicherio C, Li S-C, Heekeren HR, Bäckman L. Age-related decline in brain resources modulates genetic effects on cognitive functioning. Frontiers in Neuroscience. 2008;2:234–244. doi: 10.3389/neuro.01.039.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moal M, Simon H. Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiological Reviews. 1991;71:155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- Luciana M, Collins PF, Depue RA. Opposing roles for dopamine and serotonin in the modulation of human spatial working memory functions. Cerebral Cortex. 1998;8:216–226. doi: 10.1093/cercor/8.3.218. [DOI] [PubMed] [Google Scholar]
- Luciana M, Collins PF, Olson EA, Schissel AM. Tower of London performance in healthy adolescents: The development of planning skills and associations with self-reported inattention and impulsivity. Developmental Neuropsychology. 2009;34(4):461–475. doi: 10.1080/87565640902964540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Conklin HM, Hooper CJ, Yarger RS. The development of nonverbal working memory and executive control processes in adolescents. Child Development. 2005;76:697–712. doi: 10.1111/j.1467-8624.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- Luciana M, Depue RA, Arbisi P, Leon A. Facilitation of working memory in humans by a D2 dopamine receptor agonist. Journal of Cognitive Neuroscience. 1992;4:58–68. doi: 10.1162/jocn.1992.4.1.58. [DOI] [PubMed] [Google Scholar]
- Luciana M. Dopamine-opiate modulations of reward-seeking behavior: Implications for the functional assessment of prefrontal development. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. MIT Press; 2001. pp. 647–662. [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Development. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Macri S, Adriani W, Chiarotti F, Laviola G. Risk taking during exploration of a plus-maze is greater in adolescent than in juvenile or adult mice. Animal Behaviour. 2002;64:541–546. [Google Scholar]
- MacLean PD. Ictal symptoms relating to the nature of affects and their cerebral substrate. In: Plutchik E, Kellerman H, editors. Emotion: Theory, research, and experience. Volume 3, Biological Foundations of Emotion. New York: Academic Press; 1986. pp. 61–90. [Google Scholar]
- Malhotra AK, Kestler LJ, Mazzanti C, Bates JA, Goldberg T, Goldman D. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. American Journal of Psychiatry. 2002;159:652–654. doi: 10.1176/appi.ajp.159.4.652. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Tessitore A, Callicott JH, Bertolino A, Goldberg TE, Chase TN, et al. Dopaminergic modulation of cortical function in patients with Parkinson’s disease. Annals of Neurology. 2002;51(2):156–164. doi: 10.1002/ana.10078. [DOI] [PubMed] [Google Scholar]
- May JC, Delgado MR, Dahl RE, Stenger VA, Ryan ND, Fiez JA, Carter CS. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biological Psychiatry. 2004;55:359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- McCarthy JD, Hoge DR. The dynamics of self-esteem and delinquency. American Journal of Sociology. 1984;90:396–410. [Google Scholar]
- McCrae RR, Costa PT, Jr., Ostendorf F, Angleitner A, Hrebickova M, Avia MD, et al. Nature over Nurture: Temperament, personality, and life span development. Journal of Personality and Social Psychology. 2000;78(1):173–186. doi: 10.1037//0022-3514.78.1.173. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Damask SP, Watson SJ. Differential expression of autoreceptors in the ascending dopamine systems of the human brain. Proceedings of the National Academy of Sciences. 1994;91(17):8297–8301. doi: 10.1073/pnas.91.17.8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague DM, Lawler CP, Mailman RB, Gilmore JH. Developmental regulation of the dopamine D1 receptor in human caudate and putamen. Neuropsychopharmacology. 1999;21:641–649. doi: 10.1016/S0893-133X(99)00062-7. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Yalcin B, Willis-Owen SA, Flint J. Association of the dopamine D4 receptor (DRD4) gene and approach-related personality traits: Meta-analysis and new data. Biological Psychiatry. 2008;63(2):197–206. doi: 10.1016/j.biopsych.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Murrin LC, Zeng W. Ontogeny of dopamine D1 receptors in rat forebrain: a quantitative autoradiographic study. Developmental Brain Research. 1990;57:7–13. doi: 10.1016/0165-3806(90)90178-2. [DOI] [PubMed] [Google Scholar]
- Nagel IE, Chicherio C, Li S, von Oertzen T, Sander T, Villringer A, Heekeren HR, Bäckman L, Lindenberger U. Human aging magnifies genetic effects on executive functioning and working memory. Frontiers of Human Neuroscience. 2008;2(1) doi: 10.3389/neuro.09.001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesse RM, Berridge KC. Psychoactive drug use in evolutionary perspective. Science. 1997;278(3):63–66. doi: 10.1126/science.278.5335.63. [DOI] [PubMed] [Google Scholar]
- Neville MJ, Johnstone EC, Walton RT. Identification and characterization of ANKK1: a novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Hum Mutat. 2004;23:540–545. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- Oades R. The role of noradrenaline in tuning and dopamine in switching between signals in the CNS. Neuroscience and Biobehavioral Reviews. 1985;9:261–282. doi: 10.1016/0149-7634(85)90050-8. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J. Neurosci. 1995;15:3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Dysfunction in multiple interrelated systems as the neurobiological bases of schizophrenic symptom clusters. Schizophrenia Bulletin. 1998;24:267–283. doi: 10.1093/oxfordjournals.schbul.a033325. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, Frank MJ. Making working memory work: A computational model of learning in the frontal cortex and basal ganglia. Neural Computation. 2006;18:283–328. doi: 10.1162/089976606775093909. [DOI] [PubMed] [Google Scholar]
- Olson EA, Collins PF, Hooper CJ, Muetzel R, Lim KO, Luciana M. White matter integrity predicts delay discounting behavior in adolescents: a diffusion tensor imaging study. Journal of Cognitive Neuroscience, Posted on-line 3 September 2008. 2008 doi: 10.1162/jocn.2009.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen PJ, Nagy Z, Westerberg H, Klingberg T. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Brain Research Cognitive Brain Research. 2003;18(1):48–57. doi: 10.1016/j.cogbrainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Affective Neuroscience: The Foundations of Human and Animal Emotions. Oxford: Oxford University Press; 1998. [Google Scholar]
- Panksepp J. A critical role for “affective neuroscience” in resolving what is basic about basic emotions. Psychological Review. 1992;99(3):554–560. doi: 10.1037/0033-295X.99.3.554. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Sciences. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Maccari S, Deminière J, Le Moal M, Mormède P, Simon H. Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:2088–2092. doi: 10.1073/pnas.88.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijnenburg AJ, Honig WM, Van der Heyden JA, Van Rossum JM. Effects of chemical stimulation of the mesolimbic dopamine system upon locomotor activity. European Journal of Pharmacology. 1976;35:45–58. doi: 10.1016/0014-2999(76)90299-5. [DOI] [PubMed] [Google Scholar]
- Rao PA, Molinoff PB, Joyce JN. Ontogeny of dopamine D1 and D2 receptor subtypes in rat basal ganglia: a quantitative autoradiographic study. Developmental Brain Research. 1991;60:161–177. doi: 10.1016/0165-3806(91)90045-k. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Eagle DM, Economidou D, Theobald DE, Mar AC, Murphy ER, Robbins TW, Dalley JW. Behavioural characterisation of high impulsivity on the 5-choice serial reaction time task: specific deficits in 'waiting' versus 'stopping'. Behavioral Brain Research. 2008;196(2):310–316. doi: 10.1016/j.bbr.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Changes in the dopaminergic innervation of monkey prefrontal cortex during late postnatal development: A tyrosine hydroxylase immunohistochemical study. Biological Psychiatry. 1994;36:272–277. doi: 10.1016/0006-3223(94)90610-6. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: A tyrosine hydroxylase immunohistochemical analysis. The Journal of Comparative Neurology. 1995;358:383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Human Genetics. 1998;103:273–279. doi: 10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. The role of D1-dopaminie receptor in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. Journal of Neurophysiology. 1994;71:515–528. doi: 10.1152/jn.1994.71.2.515. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Matsumura M, Kubota K. Dopamine enhances the neuronal activity related to a delayed response task in monkey prefrontal cortex. Journal of Neurophysiology. 1990;63:1401–1412. doi: 10.1152/jn.1990.63.6.1401. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive functions correlate with white matter architecture in a normal pediatric population: A diffusion tensor MRI study. Human Brain Mapping. 2005;26:139–147. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneirla T. An evolutionary and developmental theory of biphasic processes inderlying approach and withdrawal. In: Jones M, editor. Nebraska Symposium on Motivation. University of Nebraska Press; 1959. [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:15–93. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex. 2000;10(3):272–283. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Progress in Neurobiology. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. Selective impairment on a delayed radial arm task following local administration of a selective D1, but not a D2, antagonist into the prefrontal cortex. Abstracts of the Society for Neuroscience. 1995;21:19–42. [Google Scholar]
- Seeman P, Bzowej NH, Guan HC, Bergeron C, Becker LE, Reynolds GP, et al. Human brain dopamine receptors in children and aging adults. Synapse. 1987;1:399–404. doi: 10.1002/syn.890010503. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Guido MA, Levey AI. Cellular and subcellular localization of the dopamine transporter in rat cortex. Advances in Pharmacoogy. 1998;42:171–174. doi: 10.1016/s1054-3589(08)60720-6. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shedler J, Block J. Adolescent drug use and psychological health: a longitudinal inquiry. American Psychologist. 1990;45:612–630. doi: 10.1037//0003-066x.45.5.612. [DOI] [PubMed] [Google Scholar]
- Silbereisen RK, Reitzle M. On the constructive role of problem behavior in adolescence: further evidence on alcohol use. In: Lipsitt LP, Mitnick LL, editors. Self-regulatory Behavior and Risk Taking: Causes and Consequences. Norwood, NJ: Ablex Publishing; 1992. pp. 199–217. [Google Scholar]
- Smiley JF, Goldman-Rakic PS. Heterogeneous targets of dopamine synapses in monkey prefrontal cortex demonstrated by serial section electron microscopy: a laminar analysis using the silver-enhanced diaminobenzidine sulfide (SEDS) immunolabeling technique. Cereral. Cortex. 1993;3:223–238. doi: 10.1093/cercor/3.3.223. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Neurodevelopment during adolescence. In: Cicchetti D, Walker EF, editors. Neurodevelopmental Mechanisms in Psychopathology. Cambridge: Cambridge University Press; 2003. pp. 62–83. [Google Scholar]
- Spear LP, Brake SC. Behavior and psychopharmacological responsivity in rats. Developmental Psychobiology. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Stamford JA. Development and aging of the rat nigrostriatal system dopamine system studied with fast cyclic voltammetry. Journal of Neurochemistry. 1989;52:1582–1589. doi: 10.1111/j.1471-4159.1989.tb09212.x. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styne DM. Physiology of puberty. Hormone Research. 1994;41(2):3–6. doi: 10.1159/000183949. [DOI] [PubMed] [Google Scholar]
- Sved AF, Baker HA, Reis DJ. Number of dopamine neurons predicts prolactin levels in two inbred mouse strains. Experientia. 1985;41:644–646. doi: 10.1007/BF02007700. [DOI] [PubMed] [Google Scholar]
- Szymanski S, Lieberman JA, Alvi JM, Mayerhoff D, Loebel A, Geisler A, et al. Gender differences in onset of illness, treatment response, course, and biological indexes in first-episode schizophrenic patients. American Journal of Psychiatry. 1995;152:698–703. doi: 10.1176/ajp.152.5.698. [DOI] [PubMed] [Google Scholar]
- Tam SY, Roth RH. Mesoprefrontal dopaminergic neurons: can tyrosine availability influence their functions? Biochemical Pharmacology. 1997;53(4):441–453. doi: 10.1016/s0006-2952(96)00774-5. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D1, D2, and D4 receptors in rat forebrain. International Journal of Developmental Neuroscience. 2000;18:29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D4-like receptors in rat forebrain regions: comparisons with D2-like receptors. Developmental Brain Research. 1998a;110:227–233. doi: 10.1016/s0165-3806(98)00111-4. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine and serotonin transporters in rat caudate-putamen and nucleus accumbens septi. Neuroscience Letters. 1998b;254:21–24. doi: 10.1016/s0304-3940(98)00644-2. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D1-like receptors in rat cortical and striatolimbic brain regions: An autoradiographic study. Developmental Neuroscience. 1999;21:43–49. doi: 10.1159/000017365. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC., Jr. Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Developmental Brain Research. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Tellegen A, Waller NG. Exploring personality through test construction: development of the multidimensional personality questionnaire. In: Briggs SR, Cheek JM, editors. Personality Measures: Development and Evaluation. Greenwich, CN: JAI Press; 1992. [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, Casey BJ. Amygdala response to facial expressions in children and adults. Biological Psychiatry. 2001;49(4):309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Tobler PN, O’Doherty JP, Dolan RJ, Schulz W. Reward value coding distinct from risk attitude-related uncertainty coding in human reward systems. Journal of Neurophysiology. 2007;97:1621–1632. doi: 10.1152/jn.00745.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge EM, Weickert CS, Kleinman JE, Herman MM, Chen J, Kolachana BS, et al. Catechol-o-methyltransferase enzyme activity and protein expression in human prefrontal cortex across the postnatal lifespan. Cerebral Cortex. 2007;17(5):1206–1212. doi: 10.1093/cercor/bhl032. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Singer W, Haenschel C, Sireteanu R, Rodriguez E. The development of neural synchrony reflects later maturation and restructuring of functional networks in humans. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(24):9866–9871. doi: 10.1073/pnas.0900390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungethüm U, Chen Y, Gross J, Bjelke B, Bolme P, Eneroth P. Effects of perinatal asphyxia on the mesocortical/mesolimbic dopamine system of neonatal and 4-week-old male rats. Experimental Brain Research. 1996;112:403–410. doi: 10.1007/BF00227946. [DOI] [PubMed] [Google Scholar]
- Vadasz C, Smiley JF, Figarsky K, Saito M, Toth R, Gyetvai BM, Oros M, Kovacs KK, Mohan P, Wang R. Mesencephalic dopamine neuron number and tyrosine hydroxylase content: Genetic control and candidate genes. Neuroscience. 2007;149(3):561–572. doi: 10.1016/j.neuroscience.2007.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SARB, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex. 2009 doi: 10.1093/cercor/bhp078. Epub ahead of print: doi:10.1093. [DOI] [PubMed] [Google Scholar]
- Vargas-Perez H, Kee RT, Walton CH, Hansen DM, Razavi R, Clarke L, et al. Ventral tegmental area BDNF induces an opiate-dependent-like reward state in naïve rats. Science. 2009;324(5935):1732–1734. doi: 10.1126/science.1168501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker J, Chavanon M, Stemmler G. Investigating the dopaminergic basis of extraversion in humans: A multilevel approach. Journal of Personality and Social Psychology. 2006;91(1):171–187. doi: 10.1037/0022-3514.91.1.171. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Hooper CJ, Vrshek-Schallhorn S, Oetting WS, Brott MJ, Luciana M. Association of the Catechol-O-methyltransferase (COMT) gene to prefrontally-mediated cognitions in adolescents. Biological Psychiatry. 2007;61(5):626–632. doi: 10.1016/j.biopsych.2006.05.045. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Archives of General Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Blockade of dopamine D1 receptors enhances memory fields of prefrontal neurons in primate cerebral cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Wilson M, Daly M. Competitiveness, risk taking, and violence: The young male syndrome. Ethology and Sociobiology. 1985;6:59–73. [Google Scholar]
- Wise RA. Drug-activation of brain reward pathways. Drug and Alcohol Dependence. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning, and motivation. Nature Reviews Neuroscience. 2004;5(6):483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre P. Brain dopamine and reward. Annual Reviews of Psychology. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Wise RA, Spindler J, deWit H, Gerber GJ. Neuroleptic-induced ‘anhedonia’ in rats: pimozide blocks reward quality of food. Science. 1978;201:262–264. doi: 10.1126/science.566469. [DOI] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AFT. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. The Journal of Neuroscience. 1997;17(21):8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, Cowan RL, Riccardi P, Baldwin RM, Ansari MS, Li R, Shelby ES, Smith CE, McHugo M, Kessler RM. Midbrain Dopamine Receptor Availability Is Inversely Associated with Novelty-Seeking Traits in Humans. The Journal of Neuroscience. 2008;28(53):14372–14378. doi: 10.1523/JNEUROSCI.2423-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M, Eysenck S, Eysenck HJ. Sensation seeking in England and America: cross-cultural, age, and sex comparisons. Journal of Consulting and Clinical Psychology. 1978;46:139–149. doi: 10.1037//0022-006x.46.1.139. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Kuhlman DM. Personality and risk-taking: Common biosocial factors. Journal of Personality. 2000;68:999–1029. doi: 10.1111/1467-6494.00124. [DOI] [PubMed] [Google Scholar]