Abstract
Somatosensation is the primary sensory modality employed by rodents in navigating their environments, and mystacial vibrissae on the snout are the primary conveyors of this information to the murine brain. The layout of vibrissae is spatially stereotyped and topographic connections faithfully maintain this layout throughout the neuraxis. Several factors have been shown to influence general vibrissal innervation by trigeminal neurons. Here, the role of a cell surface receptor, EphA4, in directing position-dependent vibrissal innervation is examined. EphA4 is expressed in the ventral region of the presumptive whisker pad and EphA4−/− mice lack the ventroposterior-most vibrissae. Analyses reveal that ventral trigeminal axons are abnormal, failing to innervate emerging vibrissae, and resulting in the absence of a select group of vibrissae in EphA4−/− mice. EphA4’s selective effect on a subset of whiskers implicates cell based signaling in the establishment of position-dependent connectivity and topography in the peripheral somatosensory system.
Keywords: EphA4, Trigeminal ganglion, Axon pathfinding, Maxillary, Vibrissae
Introduction
Nocturnal animals negotiate their worlds primarily through somatosensation, which, in mice, is mainly transmitted via the mystacial vibrissae (whiskers) on the snout. Vibrissae, innervated by specific sensory axons of the maxillary branch of the trigeminal ganglion (Davidson and Hardy, 1952; Dorfl, 1985), are topographically organized into five rows with little variation between individuals (Davidson and Hardy, 1952; Dun and Fraser, 1958; Yamakado and Yohro, 1979; Van Exan and Hardy, 1980). The cell bodies whose axons innervate the vibrissae are also spatially organized within the developing trigeminal ganglion (Erzurumlu and Jhaveri, 1992; Hodge et al., 2007), and these neurons project topographically into the central nervous system such that positional accuracy is maintained in the brainstem, thalamus, and somatosensory cortex (Killackey et al., 1995). Although the reliable organization of vibrissae and neural topography throughout the neuraxis argue for tight spatial regulation during the formation of the somatosensory system, mechanisms responsible for local positioning remain largely unknown.
Mystacial vibrissae emerge during the second half of mouse gestation. Connections between the trigeminal ganglion and the prospective whisker pad are initiated as trigeminal axons extend peripherally around embryonic day (E) 10. At the same time, five horizontal ridges emerge in the target whisker pad whose locations correspond to the position of future whisker rows, A-E (Van Exan and Hardy, 1980). Trigeminal axons begin to invade the target maxillary field between E11.5 and E12.5 and individual follicles become defined within the ridges as they pair with fine axonal endings in the mesenchyme slightly later (E13.5) (Davies and Lumsden, 1984; Davies and Lumsden, 1986; O’Connor and Tessier-Lavigne, 1999). By E14.5, condensations of both mesenchyme and epidermis form the papillae in the locations of mature vibrissae (Hardy, 1949; Hardy, 1951). Coincident with the arrival of trigeminal axons in each row, vibrissal development begins at the caudal-most regions of the whisker pad and progresses rostrally (Davidson and Hardy, 1952). By E15.5, the placement of the follicles is set and whisker growth begins, with whiskers emerging from the follicles a few days later.
Several cues that promote connectivity between trigeminal axons and the whisker pad have been identified. Neurotrophins can act as survival factors for maxillary trigeminal neurons (Pinon et al., 1996; Davies, 1997b; Davies, 1997a): NT-3 and BDNF before and NGF after innervation (Buchman and Davies, 1993; Enokido et al., 1999), with NT-3 and BDNF specifically promoting the extension of maxillary axons over axons of the opthalamic and mandibular trigeminal tracts (O’Connor and Tessier-Lavigne, 1999). Epithelium-derived BMP’s also modify trigeminal axons innervating the maxillary pad (Guha et al., 2004). Moreover, the guidance molecules slit and robo influence trigeminal innervation both peripherally and centrally (Ozdinler and Erzurumlu, 2002; Ma and Tessier-Lavigne, 2007). Vibrissal topography has been addressed in studies of disorganized patterning or failed whisker formation across the entire maxillary pad (Juriloff and Harris, 1983; Juriloff et al., 1987; Harris and Juriloff, 1989; Ohuchi et al., 2003), and strains have been characterized with altered whisker patterning (Van der Loos et al., 1986; Welker and Van der Loos, 1986). In general, these studies apply to whisker pad innervation but do not address region-selective topography. In contrast, a recent, elegant study characterized molecular differences between trigeminal neurons that innervate particular rows of vibrissae, although the focus was mainly on mechanisms responsible for establishing central topography (Hodge et al., 2007). Despite our understanding of general vibrissal growth and central patterning, there is no clear picture of how trigeminal neurons innervate discrete peripheral targets or how the conserved topography of the vibrissae emerges.
In other neural systems, proper matching between axons and their targets relies on a combination of soluble and surface-bound cues (Tessier-Lavigne and Goodman, 1996). Among the latter, Eph/ephrins are a particularly large and versatile set of intercellular signaling molecules. Indeed, Eph/ephrins have been shown to direct spatial topography in sensory systems, most notably the visual system (Frisen et al., 1998; Yates et al., 2001; Cang et al., 2008). The Eph/ephrins were initially defined as axon guidance cues (Cheng et al., 1995; Drescher et al., 1995) but have subsequently been credited with accomplishing a wide range of developmental tasks (Klein, 2004). EphA4 is an example of an Eph receptor with particularly broad functions, serving to inhibit or promote axon guidance in particular contexts (Kullander et al., 2001; Eberhart et al., 2004; Zimmer et al., 2008), to modulate dendritic spine growth (Richter et al., 2007), and to promote proliferation (North et al., 2009). In this first investigation of spatial selectivity during vibrissal pad development, we asked whether EphA4 signaling directs positional connectivity in the murine trigeminal somatosensory system.
Results
Eph expression in the developing maxillary trigeminal system
Within the trigeminal whisker system, EphA4 is the only receptor expressed at appreciable levels by the peripheral whisker pad (Figure 1). EphA4 expression was detected with several methods: reporter gene expression in mice with either ß-galactosidase (LacZ) or Alkaline Phosphatase (AP) inserted into the EphA4 locus via homologous recombination or gene trapping, such that reporter gene expression is present where EphA4 is expressed (Helmbacher et al., 2000; Leighton et al., 2001), immunohistochemistry (IHC) using antibodies specific for EphA4 (North et al., 2009), in situ hybridization (ISH) using a mouse EphA4 probe (Yun et al., 2003a), and the use of a chimeric ligand reagent, capable of detecting Eph binding (Gale et al., 1996). Analysis began at E10.5, prior to arrival of most trigeminal axons to the maxillary pad. At this stage, the whisker pad has thickened and trigeminal neurons have begun to extend sensory axons into the periphery (Figure 1A, top) (Davidson and Hardy, 1952). Both LacZ staining of whole-mount EphA4+/− embryos and IHC on sections revealed high levels of EphA4 expression on the ventral maxillary protrusion at E10.5 (Figure 1B, C). Similarly, both ISH and IHC revealed ventral-high EphA4 expression two days later, at E12.5 (Figure 1D, E). Ventral patterning was apparent at E14.5, with both AP staining and ligand binding (Figure 1F, G). By the time vibrissal development is nearly complete, at E15.5, ventral-high expression remained, visualized with LacZ staining of both a whole EphA4+/− whisker pad and a coronal section (Figure 1H, I). At this latest age, EphA4 was distributed in a ring around each vibrissa, consistent with the location of the outer sheath. This expression analysis, spanning embryonic vibrissal development and using multiple detection methods, revealed that the levels of EphA4 are highest in the ventral whisker pad during the specification and innervation of vibrissae.
Figure 1. EphA4 is ventrally expressed in the developing whisker pad.
A. Developmental schematics with trigeminal ganglia in black and whisker pad in grey, at E10.5 (top), when trigeminal axon extension initiates; at E12.5 (second), when the first axons have reached the maxillary pad and ridges corresponding to the five rows have formed; at E14.5 (third), when innervation by trigeminal neurons is extensive and follicles form along the ridges; and at E15.5 (bottom), when each follicle is innervated and hairs begin to develop. B, C. EphA4 expression at E10.5. Whole-mount LacZ staining of E10.5 EphA4+/− embryo (inset is magnification of maxillary protrusion) (B) and IHC of endogenous E10.5 EphA4 (C). D, E. E12.5 EphA4 expression. ISH (D) and IHC (E) of EphA4 in wild-type maxillary pad. F, G. E14.5 EphA4 expression and trigeminal innervation. Neurofilament (NF) IHC (brown) and PLAP staining (purple) of EphA4+/− (F) and NF IHC (green) and ephrin-A5 ligand body staining (red) of WT maxillary pad (G). H, I. EphA4 expression at E15.5. LacZ expression on sagittal (H) and coronal (I) whisker pad sections. (R, rostral; D, dorsal; L, lateral; tel, telencephalon; np, nasal process; mx, maxillary protrusion; md, mandibular protrusion; op, opthalamic protrusion)
Ligands for EphA4 on innervating trigeminal axons
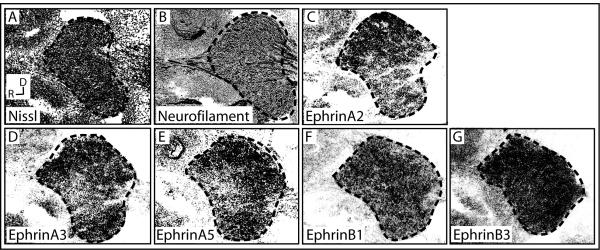
Since Eph receptors are activated by ligands on interacting cells, ephrin expression in the whisker pad’s synaptic partners, trigeminal ganglion cells, was examined. To localize expression of particular ligands, ISH was performed using probes corresponding to all of the ephrins and the topography of expression within the trigeminal ganglion was examined. Ephrin-A1 was present only on blood vessels, consistent with a previous report (Luukko et al., 2005), and expression of ephrin-A4 and -B2 was not detectable in the trigeminal ganglion (data not shown). In contrast, Ephrin-A2, -A3, -A5, -B1, and -B3 were expressed by trigeminal neurons (Figure 2). Of these, ephrin-B1 and -B3 were fairly uniformly expressed within the trigeminal ganglion (Figure 2A, B, F, G), ephrin-A2 was most concentrated dorsally (Figure 2C), and ephrin-A3 and -A5 were well expressed dorsally and ventrally with low levels in the medial domain (Figure 2D, E). Previous studies have demonstrated topography of cell bodies within the ganglion, with cells whose axons innervate the ventral maxillary pad located in the ventral portion of the middle, maxillary lobe (Hodge et al., 2007). This diverse and patterned ligand expression in the trigeminal supports the idea that trigeminal axons, including the maxillary subset, bear a variety of ligands, each of which has been shown to be capable of binding EphA4 (Gale et al., 1996; Kullander et al., 2001; North et al., 2009).
Figure 2. Ephrin expression in the trigeminal ganglion.
E12.5 trigeminal ganglion stained with Nissl (A), IHC for neurofilament (B), or ISH using probes for ephrin-A2 (C), -A3 (D), -A5 (E), -B1 (F), and -B3 (G). (R, rostral; D, dorsal)
Mice lacking EphA4 exhibit perturbed vibrissal patterning
To investigate the role of EphA4 in the organization of the trigeminal whisker system, mutant mice were examined. Comparison of control and EphA4−/− mice revealed that peripheral organization was altered: whisker rows A-D were normal, but low-numbered whiskers in the E row were missing (Figure 3A, B). Since central somatosensory representations reflect peripheral organization (Van der Loos and Woolsey, 1973; Petersen, 2007), patterning throughout the somatosensory pathway was likely to be affected. Indeed, at two levels of the neuraxis: the brainstem (trigeminal nucleus principalis, spinal trigeminal subnucleus interpolaris, and spinal trigeminal subnucleus caudalis) (Figure 4A, B) and cortex (Figure 4C, D), rows A through D were normal, but positions corresponding to caudal E row whiskers were missing both shortly after birth (Postnatal day 3-7, P3-7) and in maturity (P60) (Figure 4). Since caudal vibrissae develop first, tend to be the largest whiskers, receive unique innervation, and rely more heavily on innervation for their presence (Van Exan and Hardy, 1980; Dorfl, 1985; Welker and Van der Loos, 1986), the possibility that the deficit in EphA4−/− mice was concomitant with a defect in the innervation of peripheral whiskers by trigeminal axons was examined.
Figure 3. Vibrissal Patterning is altered in EphA4−/− mice.
Under-side of whisker pads removed from the face and pinned surface-side down, of P14 control (A) and EphA4−/− (B) mice, with photomicrographs on top and tracings of the main rows of whiskers shown as lines on the bottom, with whisker rows A through E labeled and straddler whiskers designated by circles. Position of missing E row vibrissae is indicated by the dashed oval in B. (R, rostral; D, dorsal)
Figure 4. Central whisker representations are perturbed in EphA4−/− mice.
Cytochrome Oxidase staining of three brainstem nuclei- trigeminal nucleus principalis (PrV), left; spinal trigeminal subnucleus interpolaris (SpVi), middle; spinal trigeminal subnucleus caudalis (SpVC), right (A, B) and the primary barrel field in adult somatosensory cortex (C, D) in control (A, C) and EphA4−/− (B, D) mice, with rows A through E labeled. Positions of missing E row vibrissae are indicated by the dashed oval and arrows in B and D.
Aberrant trigeminal innervation of maxillary pads in EphA4 mutant mice
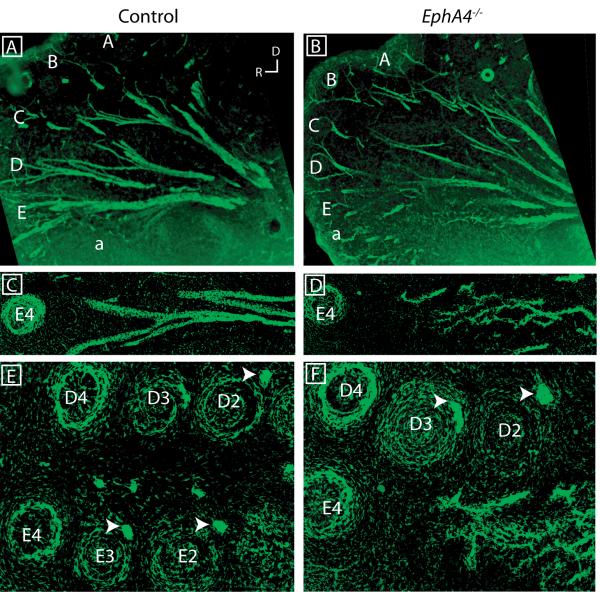
Proper innervation by trigeminal axons corresponds with proper vibrissae formation, particularly for the larger, more caudal whiskers (Van Exan and Hardy, 1980), though the necessity of innervation for formation remains unclear. Because EphA4 guides axonal pathfinding in other developing systems (Helmbacher et al., 2000; Eberhart et al., 2004; Marquardt et al., 2005; Canty et al., 2006) and because the missing vibrissae in EphA4−/− are located in an area that is usually high in EphA4 expression, and are innervated by a different trigeminal fascicle than their caudal counterparts (Dorfl, 1985), we investigated properties of trigeminal axon in control and EphA4−/− whisker pads during vibrissal development. Since the snout is curved, thick sections were used and adjacent sections were examined in order to gain a full sense of trigeminal axon patterning; the images selected are representative of all sections. At E11.5, the trigeminal nerve has invaded the entire dorsoventral extent of the whisker pad in control mice (Figure 5A). In EphA4−/−, dorsal invasion of the nerve was similar to control, while ventral invasion was less evident (Figure 5D). Indeed, dorsal axons were well fasciculated in both control and EphA4−/− mice (Figure 5B, E, E’), but ventral axons appeared less tightly packed and less ordered in the mutant (Figure 5C, F, F’). One day later, at E12.5, dorsal axons were again present in tight accumulations in both genotypes (Figure 5G, H, J, K, K’), while ventral trigeminal axons, which had by now invaded the mutant whisker pad more extensively (Figure 5G, J), still displayed an abnormal and poorly ordered appearance (Figure 5I, L, L’).
Figure 5. Initial trigeminal innervation of the vibrissal pad is perturbed in EphA4−/− mice.
Neurofilament staining of trigeminal axons in the whisker pad at E11.5 (A-F’) and E12.5 (G-L’) mice. Low magnification images of the entire whisker pad (A, D, G, J) and high magnification images of dorsal (B, E, E’, H, K, K’) or ventral (C, F, F’, I, L, L’) axons. (R, rostral; D, dorsal; A and E separated by a dashed line indicate the position of prospective whiskers at E11.5 and E12.5)
By E14.5, when the control whisker pad is innervated throughout its dorsoventral extent, with five rows apparent (Figure 6A), trigeminal axons in the ventral whisker pad of EphA4−/− remained sparse (Figure 6B). In addition, in contrast to the tightly fasciculated E row axons in control whisker pad (Figure 6C), axons in the E row region in EphA4−/− were defasciculated, with a reticular appearance (Figure 6D). Moreover, a caudal deficit was apparent when emerging follicles were examined: follicles ringed by axons were present throughout the rostrocaudal extent of both D and E rows in control animals (Figure 6E), but only D and the rostral E follicles were present in EphA4−/− (Figure 6F). While D row follicles appeared less pronounced at this age, the deficit seems transient since D row whiskers and their representations exist later (Figures 3, 4). Finally, in contrast to the tight axon bundles innervating control caudal E row follicles (arrowheads in Figure 6E), the axons present in the EphA4−/− E row were disorganized and not coupled with a follicle (Figure 6F). Thus, in the absence of EphA4, axonal characteristics were perturbed and follicles did not form. Interestingly, these results parallel data supporting a role for EphA4 mediated signaling between axons and the environment they traverse in the maintenance of tight axon fascicles in the corticospinal system (Canty et al., 2006). Analogous with these results, EphA4 appears to act normally by limiting ventral trigeminal defasciculation, thus promoting the matching of ventral axons with caudal E row targets.
Figure 6. Ventral axons defasciculate abnormally and do not associate with follicles in EphA4−/−.
Neurofilament staining of trigeminal axons in the whisker pad at E14.5, with low magnification images of the entire whisker pad (A, B), high magnification images of the caudal E row tract (C, D), or newly formed D and E row follicles (E, F). (R, rostral; D, dorsal; letters and numbers indicate the position of each vibrissal row at E14.5.)
Discussion
The generation of topography within neural systems by positionally based molecular cues was postulated by Roger Sperry (Sperry, 1963) and his hypothesis was elegantly illustrated by studies characterizing Eph receptor interactions with ephrin ligands in establishing topographic maps in the retinotectal system (Cheng et al., 1995; Drescher et al., 1995). We speculated that Eph/ephrins may play a similar role in positional specification within the developing trigeminal maxillary system since there were high levels of one receptor, EphA4, in the maxillary target field and several ephrin ligands in trigeminal neurons. Our findings implicate EphA4 as influencing in the spatial wiring of peripheral representation. Strikingly, the absence of EphA4 also resulted in a loss of particular sensory structures: a subset of the ventral vibrissae. These results firmly implicate Eph signaling in the establishment of peripheral somatosensory topography.
Because EphA4, a known axon guidance cue, was enriched in the ventral maxillary pad, we investigated whether ventral trigeminal axons were abnormal in EphA4−/− mice. Indeed, E row axons were less substantial, more disorganized, and aberrantly defasciculated in mutant whisker pads. While innervation by trigeminal axons and emergence of peripheral vibrissae are coincident processes, it remains unclear whether the former relies on the latter, or if these processes are merely coincident. Results from several studies suggest that axon innervation is necessary for vibrissal formation, supporting the concept that either a threshold number of endings or a relative difference of nerve endings between locations is required for a follicle to form (Hardy, 1949; Hardy, 1951; Davidson and Hardy, 1952; Welker and Van der Loos, 1986). Our results, that the ventral EphA4−/− maxillary pad is improperly innervated and lacks follicles, is consistent with a model in which the presence of a threshold number of endings induces whisker follicles, a process that for caudal E row vibrissae requires EphA4.
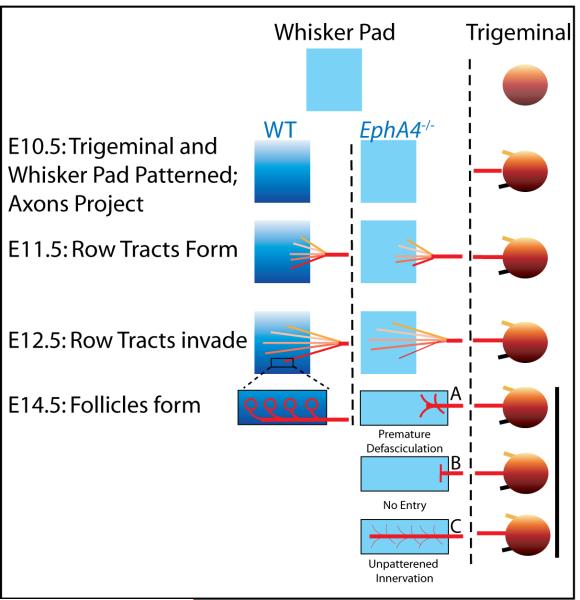
Eph signaling is generally considered a repulsive cue for axon guidance; however, EphA4 can both promote and inhibit axon growth (Eberhart et al., 2004). At first glance, our results- loss of innervation in the absence of EphA4- appear most consistent with an attractive mechanism (Fig. 7, E14.5B). Indeed, the observed delay in ingrowth of ventral axons in EphA4−/− whisker pads may indicate weak attraction. A substantial number of ventral axons, however, do eventually invade the EphA4−/− whisker pad, suggesting that lack of an attractive cue is not the explanation for the observed deficit. Rather, ventral axon targeting appears askew, with improper organization of E row tracts. A recent study of the corticospinal tract demonstrated that EphA4 in the tissue surrounding the descending tract was necessary to repulse the axons, thus preventing early defasciculation and allowing proper target innervation (Canty et al., 2006). Absence of EphA4 in this system led to premature axon defasciculation and degradation of the tract before the target organ was reached. Our data support a similar system in the developing maxillary pad, a classical repulsive mechanism with an interesting twist: ventral axons are repulsed from the surrounding ventral whisker pad in order to prevent E row fascicles from prematurely separating (Figure 7, E14.5A). In this scenario and consistent with our data, the presence of EphA4 in the whisker pad serves to prevent inappropriate branching of E row trigeminal axons so that the proper terminals innervate each vibrissa.
Figure 7. Stages of trigeminal innervation of the forming whisker pad.
Early in development (top), the newly formed whisker pad (left) is largely unspecified, but weak patterning exists in the trigeminal ganglion (right). In control mice (far left), combinations of molecules, including EphA4 and ephrins, positionally pattern the whisker pad and the trigeminal ganglion, respectively, and trigeminal neurons extend axons, including the middle maxillary tract (red) (E10.5). Row tracts emerge as the maxillary nerve reaches the whisker pad (E11.5) and invade the whisker pad topographically (E12.5), eventually ringing whisker follicles (E14.5). In the absence of EphA4 peripheral patterning (middle column), ventral deficits are observed: the ventral-most row tract is slow to arrive (E11.5) and axons invasion is light (E12.5). Premature axon defasciculation is proposed to explain the deficits, with ventral vibrissae not forming (E14.5A). Other possible mechanisms are less consistent with the data: a lack of attraction is predicted to result in axons halting (E14.5B), while lack of peripheral prepatterning is predicted to result in disorganized and nonspecific innervation (E14.5C).
Another possibility is that EphA4 acts to pattern the whisker pad itself (Figure 7, E14.5C). In this case, an improperly patterned maxillary pad may result in nonspecific innervation that might not support vibrissal formation. We examined E10.5 maxillary protrusions for signs of gross morphological differences and found no clear evidence of a prepatterning deficit (data not shown). These observations, together with the arrival of pioneer axons to wild type but not EphA4−/− maxillary protrusion prior to vibrissal formation, suggest a system in which the axons are required for vibrissal growth. The question of whether aberrant axonal guidance causes or is a consequence of missing vibrissae is an important one, however, and future studies must investigate pre-patterning in EphA4−/− on a molecular level to rule peripheral changes as a cause for the E row abnormalities (Figure 7). Until then, our data demonstrate that EphA4 is necessary for the conserved spatial topography of whiskers and support a relationship between axonal innervation and peripheral sensory organ formation.
The establishment and maintenance of spatial maps in the nervous system is governed by a myriad of molecular cues, in both target fields and innervating neurons, that serve to match appropriate synaptic partners, strengthen subsets of synapses, and guide activity-dependent plasticity. Our data contribute new insight into the developmental processes by which such maps are generated by addressing how positionally selective connections are guided, in our case by a surface based Eph receptor. In particular, the selective dependence of the most caudal E row vibrissae on EphA4 for their presence is a striking example of the specification of a subset of sensory structures within a whole. Since at least some vibrissae may require an innervating axon, the EphA4−/− mouse may represent a unique instance in which incomplete innervation leads to a position-dependent deletion within a topographic system. In conclusion, the peripheral somatosensory system seems especially well poised for future investigations of the interplay between attractive and repulsive forces in the definition of spatial maps, intersections between patterning and guidance systems, and the behavioral consequences of altered somatosensory representations.
Brief Methods
Animals
All animal use and care was in accordance with institutional guidelines, Georgetown’s GUACUC protocols #06-022 and 09-020, in compliance with federal policy. Wild-type CD-1 mice were from Charles River Laboratories. LacZ EphA4 mutant mice were generously provided by P. Charnay (INSERM, Paris, France) and were maintained on a C57Bl/6 strain (5-15 generations), bred as heterozygotes (Helmbacher et al., 2000). PLAP EphA4 mutant mice were kindly provided by M. Tessier-Lavigne (Genentech, California, USA) and were maintained on a CDI background (5-15 generations), bred in homozygote/wild type crosses (Leighton et al., 2001). Wild type, heterozygous, and mutant alleles of EphA4 were generated in Mendelian ratios for both lines of EphA4 mutants. The day of the vaginal plug was embryonic day 0.5 (E0.5) and the day of birth, postnatal day 0 (P0). Comparisons between mutant and control mice were performed using littermates.
Brainstem and Cortical Cytochrome Oxidase Staining
Postnatal mice (P3-60) were anesthetized and perfused with saline followed by 4% paraformaldehyde (PFA) in phosphate buffer (0.1M, pH 7.4) (PBS). Brains were sometimes postfixed overnight. For brainstem preparations, the tissue at the base of the skull was embedded in gelatin and sectioned horizontally at 40-100 μm (Henderson et al., 1994). For cortical samples, brains were fixed by immersion in PFA and embedded in 4% suprasieve agarose/ PBS. The cortex was freed from subcortical structures, flattened between glass slides, and sectioned at 200μm (Miller et al., 2006). All sections were pre-treated with 10% sucrose/0.12M phosphate buffer solution at 37° and then incubated in the CO reaction (30 mg cytochrome C, 50 mg diaminobenzidine, 2 mg catalase, and 10g sucrose per 100 ml of 0.12M PB) for 6-24 hours at 37°C. Stained sections were post-treated by immersion in increasingly dilute sucrose solutions (10%, 5%, 0% sucrose in 0.12M PB), mounted, dried, dehydrated through graded ethanols (50%, 70%, 95%, 100% for 5 minutes each), cleared in xylenes, and coverslipped.
Whole-mount Whisker Pad Examination
P10-adult wild-type and EphA4−/− littermates were euthanized, and the skin covering the snout was carefully removed, pinned surface-side down, fixed with 4% PFA for 1 hour, cleared of excess tissue enzymatically using dilute trypsin or manually to reveal vibrissae, and photographed.
Reporter Gene Detection
ß-galactosidase expression was detected using standard staining conditions: sections were post-fixed in 4% PFA, 0.2% glutaraldehyde and then incubated in 4 mM potassium ferrous cyanide, 4 mM potassium ferric cyanide, 0.8 mg/ml Xgal, in PBS containing 2 mM MgCl2 at 30°C for 1 hour to overnight. Following a PBS wash, sections were post-fixed in 4% PFA for 15 minutes, washed in PBS, and either mounted or subjected to immunohistochemistry (below). For alkaline phosphatase staining, sections were incubated in PBS at 65°C for 1 hour to inactivate endogenous phosphatases, pre-treated in Buffer 3 (150 mM NaCl, 100 mM Tris pH 9.5) for 30 minutes, and incubated in reaction buffer (Buffer 3 with 0.35 mg/ml NBT and 0.175 mg/ml BCIP) for 10 minutes to 3 hours, rinsed with TE, fixed in 4% PFA for 10 minutes, washed with water, and mounted.
Immunohistochemistry and Ligand Body Binding
Tissue was incubated in blocking buffer with triton (2.5% goat serum, 2.5% donkey serum, 1% BSA, 1% glycine, 0.1% lysine, 0.4% Triton X-100 in PBS; BB+) for 30 minutes at room temperature (RT) and then incubated in primary antibody or ephrin-A5-fc diluted in BB+ overnight at 4°C. After washing with PBS, tissue was incubated with Alexa-conjugated secondary antibodies (1:800) and either counterstained with bisbenzamide (1:1000) for fluorescent IHC or amplified using an avidin-biotin complex (Vector) and HRP-tagged secondary for non-fluorescent procedures and mounted. The primary antibodies used, their source, and dilutions follow: rabbit α-NF (Sigma, 1:1000), chicken α-NF (Millipore, 1:1000); rabbit α-EphA4 (Zymed, 1:300 or Santa Cruz 1:50); mouse α-EphA4 (Zymed, 1:500). The ligand body was used at 30ug/ul.
In Situ Hybridization
Tissue sections, prepared as described above, were subjected to non-radioactive in situ hybridization. Digoxygenin-labeled antisense probes corresponding to Ephrin ligands were generated from mouse templates (Yun et al., 2003b). Sections were post-fixed in 4% PFA, washed in PBS, acetylated, washed in PBS, incubated in pre-hybridization solution for several hours at room temperature (50% formamide, 5X SSC, 1X Denhardts, 250 μg/ml tRNA, 500 μg/ml fish sperm DNA), hybridized overnight at 65°C in hybridization buffer (50% formamide, 300 mM NaCl, 20 mM Tris-HCl pH 8, 5 mM EDTA, 10 mM Na2HPO4 pH 7.2, 10% Dextran Sulfate, 1X Denhardts, 500 μg/ml tRNA, 200 μg/ml fish sperm DNA) containing 300-500 ng probe/ml, and washed. IHC for the labeled nucleotide was performed using AP detection.
Acknowledgments
We thank Kate Miller for her initial observation of the mutant phenotype described in this study, Fan Wang for her generous training of our lab members in relevant techniques, Dr. Wang and other colleagues in the trigeminal development field for their thoughtful commentary on our manuscript, members of the Donoghue lab for their assistance with the preparation of this document, Patrick Charnay and Marc Tessier-Lavigne for the use of their EphA4 mutant mice, and Joop Arends for his preparation of the brainstem data in Figure 4.
Sponsor information: This work was supported by NIH funds: R01 NS039979 to MJD and P01 NS049048 to MFJ, and start-up funds from Georgetown University to MJD
References
- Buchman VL, Davies AM. Different neurotrophins are expressed and act in a developmental sequence to promote the survival of embryonic sensory neurons. Development. 1993;118:989–1001. doi: 10.1242/dev.118.3.989. [DOI] [PubMed] [Google Scholar]
- Cang J, Niell CM, Liu X, Pfeiffenberger C, Feldheim DA, Stryker MP. Selective disruption of one Cartesian axis of cortical maps and receptive fields by deficiency in ephrin-As and structured activity. Neuron. 2008;57:511–523. doi: 10.1016/j.neuron.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canty AJ, Greferath U, Turnley AM, Murphy M. Eph tyrosine kinase receptor EphA4 is required for the topographic mapping of the corticospinal tract. Proc Natl Acad Sci U S A. 2006;103:15629–15634. doi: 10.1073/pnas.0607350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HJ, Nakamoto M, Bergemann AD, Flanagan JG. Complementary gradients in expression and binding of ELF-1 and Mek4 in development of the topographic retinotectal projection map. Cell. 1995;82:371–381. doi: 10.1016/0092-8674(95)90426-3. [DOI] [PubMed] [Google Scholar]
- Davidson P, Hardy MH. The development of mouse vibrissae in vivo and in vitro. J Anat. 1952;86:342–356. [PMC free article] [PubMed] [Google Scholar]
- Davies A, Lumsden A. Relation of target encounter and neuronal death to nerve growth factor responsiveness in the developing mouse trigeminal ganglion. J Comp Neurol. 1984;223:124–137. doi: 10.1002/cne.902230110. [DOI] [PubMed] [Google Scholar]
- Davies AM. Neurotrophin switching: where does it stand? Curr Opin Neurobiol. 1997a;7:110–118. doi: 10.1016/s0959-4388(97)80128-6. [DOI] [PubMed] [Google Scholar]
- Davies AM. Studies of neurotrophin biology in the developing trigeminal system. J Anat. 1997b;191(Pt 4):483–491. doi: 10.1046/j.1469-7580.1997.19140483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AM, Lumsden AG. Fasciculation in the early mouse trigeminal nerve is not ordered in relation to the emerging pattern of whisker follicles. J Comp Neurol. 1986;253:13–24. doi: 10.1002/cne.902530103. [DOI] [PubMed] [Google Scholar]
- Dorfl J. The innervation of the mystacial region of the white mouse: A topographical study. J Anat. 1985;142:173–184. [PMC free article] [PubMed] [Google Scholar]
- Drescher U, Kremoser C, Handwerker C, Loschinger J, Noda M, Bonhoeffer F. In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell. 1995;82:359–370. doi: 10.1016/0092-8674(95)90425-5. [DOI] [PubMed] [Google Scholar]
- Dun RB, Fraser AS. Selection for an invariant character; vibrissa number in the house mouse. Nature. 1958;181:1018–1019. doi: 10.1038/1811018a0. [DOI] [PubMed] [Google Scholar]
- Eberhart J, Barr J, O’Connell S, Flagg A, Swartz ME, Cramer KS, Tosney KW, Pasquale EB, Krull CE. Ephrin-A5 exerts positive or inhibitory effects on distinct subsets of EphA4-positive motor neurons. J Neurosci. 2004;24:1070–1078. doi: 10.1523/JNEUROSCI.4719-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enokido Y, Wyatt S, Davies AM. Developmental changes in the response of trigeminal neurons to neurotrophins: influence of birthdate and the ganglion environment. Development. 1999;126:4365–4373. doi: 10.1242/dev.126.19.4365. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Jhaveri S. Trigeminal ganglion cell processes are spatially ordered prior to the differentiation of the vibrissa pad. J Neurosci. 1992;12:3946–3955. doi: 10.1523/JNEUROSCI.12-10-03946.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisen J, Yates PA, McLaughlin T, Friedman GC, O’Leary DD, Barbacid M. Ephrin-A5 (AL-1/RAGS) is essential for proper retinal axon guidance and topographic mapping in the mammalian visual system. Neuron. 1998;20:235–243. doi: 10.1016/s0896-6273(00)80452-3. [DOI] [PubMed] [Google Scholar]
- Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE, Henkemeyer M, Strebhardt K, Hirai H, Wilkinson DG, Pawson T, Davis S, Yancopoulos GD. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17:9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- Guha U, Gomes WA, Samanta J, Gupta M, Rice FL, Kessler JA. Target-derived BMP signaling limits sensory neuron number and the extent of peripheral innervation in vivo. Development. 2004;131:1175–1186. doi: 10.1242/dev.01013. [DOI] [PubMed] [Google Scholar]
- Hardy MH. The development of mouse hair in vitro with some observations on pigmentation. J Anat. 1949;83:364–384. 363 pl. [PMC free article] [PubMed] [Google Scholar]
- Hardy MH. The development of pelage hairs and vibrissae from skin in tissue culture. Ann N Y Acad Sci. 1951;53:546–561. doi: 10.1111/j.1749-6632.1951.tb31956.x. [DOI] [PubMed] [Google Scholar]
- Harris MJ, Juriloff DM. Test of the isoallele hypothesis at the mouse first arch (far) locus. J Hered. 1989;80:127–131. doi: 10.1093/oxfordjournals.jhered.a110810. [DOI] [PubMed] [Google Scholar]
- Helmbacher F, Schneider-Maunoury S, Topilko P, Tiret L, Charnay P. Targeting of the EphA4 tyrosine kinase receptor affects dorsal/ventral pathfinding of limb motor axons. Development. 2000;127:3313–3324. doi: 10.1242/dev.127.15.3313. [DOI] [PubMed] [Google Scholar]
- Henderson TA, Johnson EM, Jr., Osborne PA, Jacquin MF. Fetal NGF augmentation preserves excess trigeminal ganglion cells and interrupts whisker-related pattern formation. J Neurosci. 1994;14:3389–3403. doi: 10.1523/JNEUROSCI.14-05-03389.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge LK, Klassen MP, Han BX, Yiu G, Hurrell J, Howell A, Rousseau G, Lemaigre F, Tessier-Lavigne M, Wang F. Retrograde BMP signaling regulates trigeminal sensory neuron identities and the formation of precise face maps. Neuron. 2007;55:572–586. doi: 10.1016/j.neuron.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Juriloff DM, Harris MJ. Abnormal facial development in the mouse mutant first arch. J Craniofac Genet Dev Biol. 1983;3:317–337. [PubMed] [Google Scholar]
- Juriloff DM, Harris MJ, Froster-Iskenius U. Hemifacial deficiency induced by a shift in dominance of the mouse mutation far: a possible genetic model for hemifacial microsomia. J Craniofac Genet Dev Biol. 1987;7:27–44. [PubMed] [Google Scholar]
- Killackey HP, Rhoades RW, Bennett-Clarke CA. The formation of a cortical somatotopic map. Trends Neurosci. 1995;18:402–407. doi: 10.1016/0166-2236(95)93937-s. [DOI] [PubMed] [Google Scholar]
- Klein R. Eph/ephrin signaling in morphogenesis, neural development and plasticity. Curr Opin Cell Biol. 2004;16:580–589. doi: 10.1016/j.ceb.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Kullander K, Croll SD, Zimmer M, Pan L, McClain J, Hughes V, Zabski S, DeChiara TM, Klein R, Yancopoulos GD, Gale NW. Ephrin-B3 is the midline barrier that prevents corticospinal tract axons from recrossing, allowing for unilateral motor control. Genes Dev. 2001;15:877–888. doi: 10.1101/gad.868901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton PA, Mitchell KJ, Goodrich LV, Lu X, Pinson K, Scherz P, Skarnes WC, Tessier-Lavigne M. Defining brain wiring patterns and mechanisms through gene trapping in mice. Nature. 2001;410:174–179. doi: 10.1038/35065539. [DOI] [PubMed] [Google Scholar]
- Luukko K, Loes S, Kvinnsland IH, Kettunen P. Expression of ephrin-A ligands and EphA receptors in the developing mouse tooth and its supporting tissues. Cell Tissue Res. 2005;319:143–152. doi: 10.1007/s00441-004-0951-1. [DOI] [PubMed] [Google Scholar]
- Ma L, Tessier-Lavigne M. Dual branch-promoting and branch-repelling actions of Slit/Robo signaling on peripheral and central branches of developing sensory axons. J Neurosci. 2007;27:6843–6851. doi: 10.1523/JNEUROSCI.1479-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt T, Shirasaki R, Ghosh S, Andrews SE, Carter N, Hunter T, Pfaff SL. Coexpressed EphA receptors and ephrin-A ligands mediate opposing actions on growth cone navigation from distinct membrane domains. Cell. 2005;121:127–139. doi: 10.1016/j.cell.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Miller K, Kolk SM, Donoghue MJ. EphA7-ephrin-A5 signaling in mouse somatosensory cortex: developmental restriction of molecular domains and postnatal maintenance of functional compartments. J Comp Neurol. 2006;496:627–642. doi: 10.1002/cne.20926. [DOI] [PubMed] [Google Scholar]
- North HA, Zhao X, Kolk SM, Clifford MA, Ziskind DM, Donoghue MJ. Promotion of proliferation in the developing cerebral cortex by EphA4 forward signaling. Development. 2009;136:2467–2476. doi: 10.1242/dev.034405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor R, Tessier-Lavigne M. Identification of maxillary factor, a maxillary process-derived chemoattractant for developing trigeminal sensory axons. Neuron. 1999;24:165–178. doi: 10.1016/s0896-6273(00)80830-2. [DOI] [PubMed] [Google Scholar]
- Ohuchi H, Tao H, Ohata K, Itoh N, Kato S, Noji S, Ono K. Fibroblast growth factor 10 is required for proper development of the mouse whiskers. Biochem Biophys Res Commun. 2003;302:562–567. doi: 10.1016/s0006-291x(03)00183-9. [DOI] [PubMed] [Google Scholar]
- Ozdinler PH, Erzurumlu RS. Slit2, a branching-arborization factor for sensory axons in the Mammalian CNS. J Neurosci. 2002;22:4540–4549. doi: 10.1523/JNEUROSCI.22-11-04540.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CC. The functional organization of the barrel cortex. Neuron. 2007;56:339–355. doi: 10.1016/j.neuron.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Pinon LG, Minichiello L, Klein R, Davies AM. Timing of neuronal death in trkA, trkB and trkC mutant embryos reveals developmental changes in sensory neuron dependence on Trk signalling. Development. 1996;122:3255–3261. doi: 10.1242/dev.122.10.3255. [DOI] [PubMed] [Google Scholar]
- Richter M, Murai KK, Bourgin C, Pak DT, Pasquale EB. The EphA4 receptor regulates neuronal morphology through SPAR-mediated inactivation of Rap GTPases. J Neurosci. 2007;27:14205–14215. doi: 10.1523/JNEUROSCI.2746-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry RW. Chemoaffinity in the Orderly Growth of Nerve Fiber Patterns and Connections. Proc Natl Acad Sci U S A. 1963;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Van der Loos H, Welker E, Dorfl J, Rumo G. Selective breeding for variations in patterns of mystacial vibrissae of mice. Bilaterally symmetrical strains derived from ICR stock. J Hered. 1986;77:66–82. doi: 10.1093/oxfordjournals.jhered.a110201. [DOI] [PubMed] [Google Scholar]
- Van der Loos H, Woolsey TA. Somatosensory cortex: structural alterations following early injury to sense organs. Science. 1973;179:395–398. doi: 10.1126/science.179.4071.395. [DOI] [PubMed] [Google Scholar]
- Van Exan RJ, Hardy MH. A spatial relationship between innervation and the early differentiation of vibrissa follicles in the embryonic mouse. J Anat. 1980;131:643–656. [PMC free article] [PubMed] [Google Scholar]
- Welker E, Van der Loos H. Quantitative correlation between barrel-field size and the sensory innervation of the whiskerpad: a comparative study in six strains of mice bred for different patterns of mystacial vibrissae. J Neurosci. 1986;6:3355–3373. doi: 10.1523/JNEUROSCI.06-11-03355.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakado M, Yohro T. Subdivision of mouse vibrissae on an embryological basis, with descriptions of variations in the number and arrangement of sinus hairs and cortical barrels in BALB/c (nu/+; nude, nu/nu) and hairless (hr/hr) strains. Am J Anat. 1979;155:153–173. doi: 10.1002/aja.1001550202. [DOI] [PubMed] [Google Scholar]
- Yates PA, Roskies AL, McLaughlin T, O’Leary DD. Topographic-specific axon branching controlled by ephrin-As is the critical event in retinotectal map development. J Neurosci. 2001;21:8548–8563. doi: 10.1523/JNEUROSCI.21-21-08548.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun ME, Johnson RR, Antic A, Donoghue MJ. EphA family gene expression in the developing mouse neocortex: regional patterns reveal intrinsic programs and extrinsic influence. J Comp Neurol. 2003a;456:203–216. doi: 10.1002/cne.10498. [DOI] [PubMed] [Google Scholar]
- Yun ME, Johnson RR, Antic A, Donoghue MJ. EphA family gene expression in the developing mouse neocortex: regional patterns reveal intrinsic programs and extrinsic influence. Journal of Comparative Neurology. 2003b;456:203–216. doi: 10.1002/cne.10498. [DOI] [PubMed] [Google Scholar]
- Zimmer G, Garcez P, Rudolph J, Niehage R, Weth F, Lent R, Bolz J. Ephrin-A5 acts as a repulsive cue for migrating cortical interneurons. Eur J Neurosci. 2008;28:62–73. doi: 10.1111/j.1460-9568.2008.06320.x. [DOI] [PubMed] [Google Scholar]