Abstract
There is a well-documented disruption of the neural network associated with reward evaluation in schizophrenia. This same system is involved in coding the incentive value of food in healthy individuals, however no study to date has examined anhedonia and its relation to food in schizophrenia. Relative preference and hedonic food ratings were examined in schizophrenia patients and healthy controls. In the relative preference task, subjects viewed photographs of food items and selected the one that they most preferred. Hedonic ratings were obtained by asking subjects how much they liked the food stimulus on a scale of 1–5. There were no overall response time differences between the two groups in the relative preference task but schizophrenic patients showed subtle differences in their hedonic ratings of foods compared with control subjects. Schizophrenic patients gave more positive hedonic ratings for food than controls and the use of fewer positive ratings was associated with increased anhedonia, particularly with loss of sexual interest. These results suggest that while making relative preference judgments may be intact, hedonic values attached to food may be altered in schizophrenia, and they may be related to dysfunction in more basic vegetative systems. These findings may help to elucidate a more general model of food preference and judgment in human subjects based on documenting characteristics associated with a known dysfunctional system in schizophrenia.
Keywords: relative reward, anhedonia, consummatory, volitional, wanting, liking
1. Introduction
Experiencing and consuming food is hedonic (Kringelbach, 2004; McCreadie et al., 1998), and anhedonia (dysfunction in experiencing pleasure) along with poor dietary choices are associated with schizophrenia (Kraepelin et al., 1919). However, experiencing pleasure is not universally disrupted in schizophrenia (Schurhoff et al., 2003), and it is unclear how real-life food choices are related to clinical anhedonia in this population. Psychologically, general food reward mechanisms may be mediated by either consummatory “liking” (pleasure and positive affect; involving temporal and frontal cortices) or volitional “wanting” (incentive and motivation; involving mesolimbic dopamine) mechanisms (Berridge, 1996) subsumed by related but functionally and anatomically dissociable neural systems (Finlayson et al., 2006) that are also disrupted in schizophrenia (Elman et al., 2006). Dysfunction of the volitional system in schizophrenia has been identified as a salient aspect of anhedonia (Wolf, 2006), but there is little available information about the specific characteristics of consummatory pleasure in schizophrenia, including decisions involving relative reward (preference) and hedonic judgment (liking). Investigating food preference and liking may help to further characterize the experience of pleasure and the phenomenology of consummatory anhedonia in schizophrenia and elucidate the pathophysiology associated with these decisions.
The prefrontal cortex is a likely neuroanatomical substrate of making food reward and judgment decisions. Patients with lesions in the ventral region of the PFC display anhedonia (Blumer and Benson, 1975), and human food preference is altered with PFC lesions and dysfunction (Ikeda et al., 2002; Kim and Choi, 2002; Regard and Landis, 1997). Food preference alterations, including incentive value reversal, occur in response to primate ventral PFC damage (Butter et al., 1969), and there are ventral PFC cells that respond to anticipated food reward and to the changing motivational value of food (Watanabe, 1999). Tremblay and Schultz (1999) demonstrated primate ventral PFC cells that responded to the changing reward value of different foods as they were paired with increasingly preferred ones. General PFC and more specific ventral region anomalies have been documented in schizophrenia (Crespo-Facorro et al., 2000; Goldstein et al., 1999), arguing for functional and physical overlap of food reward systems and schizophrenic pathophysiology.
Neurochemically, the endogenous opioid system contributes to the consummatory value and relative preference of food (Taha et al., 2006), while the dopaminergic system is important in volitional food reward and in establishing food reward contingencies (Rolls, 1999; Schultz, 2001; Wise et al., 1978; Wise et al., 1978). Recent evidence has also implicated serotonergic influence in human food preference (Prado-Lima et al., 2006). Dysfunction of these neurotransmitter systems has been observed in schizophrenia (Schmauss and Emrich, 1985; Tamminga, 2006). Although the relationships among dopamine, reward, and schizophrenia have received much attention (Chau et al., 2004), far less is known about serotonin and opioid-specific modulation of choice reward in schizophrenia and its relationship with anhedonia. Thus behavioral models of consummatory decision-making in schizophrenia would serve as a basis for further neurobiological investigations.
Food choice and consumption are complex processes based on several integrated factors including content, context, experience, culture, and availability (Mela, 1999). The present study examined two related hedonic decision making processes: (1) relative reward decisions made when selecting the most preferred food from several competing choices, and (2) liking judgments, which invoke affective discriminations (how much a food is liked). Both of these processes involve hierarchical information likely derived from implicit learning rather than being hard-wired (Greene et al., 1975). There are several reasons to suggest that these systems may be disrupted in schizophrenia. Nutritious food choices in schizophrenic patients tend to be poor (McCreadie et al., 1998; Peet, 2004) and there is evidence for continued disrupted food choice learning following nutrition training (McCreadie et al., 2005). In addition to loss of food-derived satisfaction (Kovess-Masfety et al., 2006) in schizophrenia, examining food preference and reward decisions may elucidate some of the processes that contribute to overall poor physical health and obesity.
In this investigation, we asked whether individuals with schizophrenia (SZ) make relative reward decisions similarly to healthy controls (CO), and if people with schizophrenia rate the hedonic value of food differently compared to healthy controls. For the relative reward decision, we examined response times for making preference decisions for food choices. In the hedonicity ratings task, schizophrenic and control subjects rated the hedonic value of food on a scale of 1–5. We expected subjects with schizophrenia to have more difficulty making preference decisions (reflected by slower response times) than healthy controls and to give lower hedonic ratings.
2. Method
2.1. Participants
18 outpatient individuals (6 females; Mean age = 40.5 years, Range 21 – 58 years) who met the DSM-IV criteria for schizophrenia were recruited from a local clinic and18 healthy control subjects (8 females; Mean age = 38.9 years, Range 20 – 52 years) were recruited from the community. Exclusion criteria included current substance use, neurological disorders, brain injury, and mental retardation. Controls were excluded if they had a past or present DSM-IV Axis I or Axis II disorder or a family history of psychosis. Groups were matched for years of education (SZ: M = 12.4, SD = 2.6 years, CO: M = 13.4, SD = 1.9 years, P = 0.21) and Wechsler Abbreviated Scale of Intelligence (WASI) FSIQ (SZ: M = 88.6, SD = 14.2; CO: M = 96.6, SD = 14.3, P = 0.12). For subjects with schizophrenia, clinical symptoms were assessed with the Brief Psychiatric Rating Scale (BPRS: M = 27.4, SD = 16.9) (Lukoff et al., 1986) and the Scales for the Assessment of Positive (SAPS: M = 32.4, SD = 24.4) and Negative (SANS: M = 29.1, SD = 24.3) Symptoms (Andreasen, 1982; Andreasen and Olsen, 1982). All schizophrenia patients were clinically stable and taking second generation antipsychotic medications at the time of testing, and their mean chlorpromazine dose equivalent (mg./day) (Davis, 1974; Woods, 2003) was (M=93.6, SD=159.7). The mean illness duration was 16.4 years (SD = 9.6) for individuals with schizophrenia. Controls completed the Schizotypal Personality Questionnaire (SPQ; (Raine, 1991)), a self-report measure with 74 true-false items (M = 17.6, SD = 7.7). All subjects were screened for food habits and were excluded if they reported major restrictions (vegetarianism, strict dietary rules) that would affect reactions to food items. The study was approved by the Vanderbilt University Institutional Review Board, and informed consent was obtained from all participants, who were compensated.
2.2. Apparatus and procedure
Tasks were programmed and presented using E-Prime (Psychology Software Tools, Pittsburgh, PA). Participants viewed images presented 50 cm. away on a 17” computer screen and responded using the keyboard. Colored images were food from one of four categories (desserts, meat, pasta, and vegetables) selected for appetitive quality. All subjects were right-handed, completing both tasks in a single testing session prior to eating lunch to provide some control over food satiety.
2.2.1. Food preference decisions
Participants made decisions about food presented in two conditions (preference or control, Figure 1a). For each condition, three food images from the same category were presented horizontally on a black background, numbered 1 to 3 beneath each image. In the preference condition, participants selected the one that they most preferred. In the control condition, participants were asked to select the item made with the most ingredients. The stimulus screen stayed on until subjects pressed a key or 6 seconds have elapsed. An instruction screen presented before each block indicated which decision to make, and the corresponding decision words “prefer” or “ingredients” appeared at the top of each stimulus screen to remind subjects of the current task. There were 14 preference and control blocks, each containing five trials. The presentation of preference and control blocks were counterbalanced.
Figure 1.
The relative reward (A) and rating (B) paradigms. The relative reward paradigm (A) asked subjects to make 2 decisions about food: to select which of 3 foods they preferred the most (experimental condition), or to select the food made with the most ingredients (control condition) in alternating blocks. In the rating paradigm (B), subjects viewed sequential food images and were asked to rate each on a 1–5 scale according to how much they liked the food shown.
2.2.2. Food Ratings task
After instruction and practice trials, participants were asked to rate 120 food items chosen equally from the four categories (meat, vegetable, pasta, dessert). Each trial consisted of the presentation of a color photograph of food item and subjects were asked to rate the food item on a Likert-type scale (Figure 1b). A trial ended when subjects pressed a key to indicate their ratings or 6 seconds have elapsed. The scale instructed subjects to rate food according to: 1- do not like the food at all, 2- do not really like the food, 3- neither like nor dislike the food, 4- like the food, 5- like the food very much. The rating scale was presented visually beneath each food image. Individual ratings and response times were recorded.
3. Results
3.1. Preference decisions
Using response time as the dependent variable with experimental condition (preference vs. ingredients task) as the within subjects factor and diagnosis (SZ, CO) as the between subjects factor, the main effect for experimental condition was highly significant (F(1, 34)= 215.64, P< 0.001; r = 0.96). Overall, subjects made preference decisions more quickly (M=3208.1 ms., SD=575.6 ms.) than number of ingredients decisions (M=3241.8 ms., SD=580.5 ms.). The main effect of group was not significant (F(1,34)=2.83, P=0.10).The interaction between group and condition was not significant (F(1,34)=0.52, P=0.48).
3.2. Food ratings
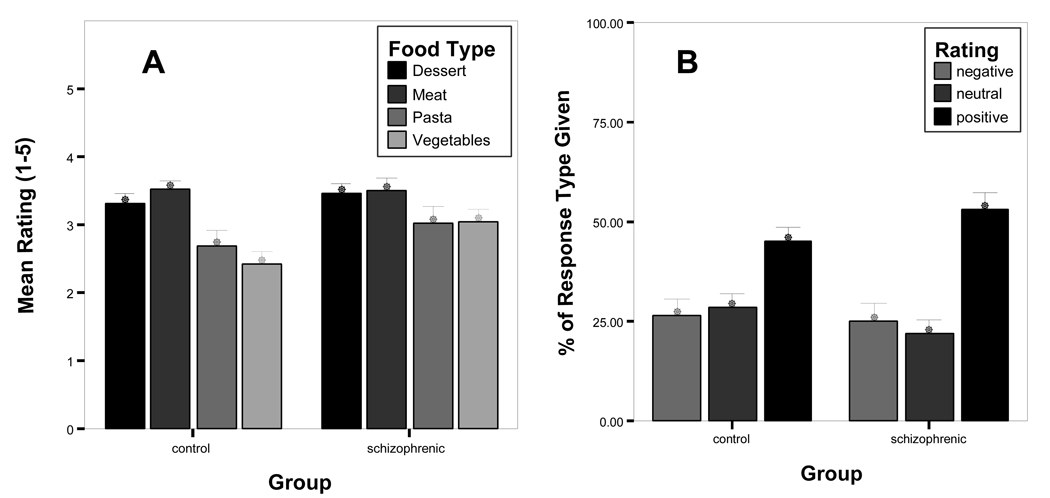
Using the rating choice as the dependent variable with food category (vegetables, pasta, desserts, meat) as the within subjects factor and group (SZ, CO) as the between subjects factor, the main effect for food category was significant (F(2,68)= 22.12, P< 0.001; r = 0.73) (Figure 2a). Overall, subjects preferred meat (M=3.5, SD=0.66) to vegetables (M=2.7, SD=0.85) (P<0.01) and pasta (M=2.9, SD=1.02) (P<0.01). They also preferred desserts (M=3.4, SD=0.59) to vegetables (P<0.001) and pasta (P<0.01). Pasta and vegetables were rated similarly, as were desserts and meat. The main effect of group was not significant, (F(1,34)=1.55, P=0.22). However, the interaction between group and food category was significant (F(2,68)=2.94, P=0.45; r=0.31) indicating a medium effect. Subjects with schizophrenia (M=3.1, SD=0.79) gave higher ratings to the vegetable pictures compared to controls (M=2.4, SD=0.79) (P<0.05).
Figure 2.
Food rating responses given by subjects with schizophrenia and healthy controls. From Graph A, subjects with schizophrenia used the more positive ratings (4 or 5) to judge food images overall. From Graph B, subjects with schizophrenia rated the vegetable food images as being more liked compared to healthy controls.
We then examined hedonic ratings for food. Individuals with schizophrenia (M =3.3, SD=0.65) and controls (M=3.1, SD=0.53) did not differ in their overall average rating (F(1,34)=1.22, P=0.28). We then grouped ratings into negative and positive categories. Ratings of “1” and “2” were combined to form a negative rating category, the ratings of “4” and “5” were combined to form a positive rating category and the rating of 3 to form a neutral category. There was a main effect for rating category (F(2,68)=15.87, P<0.001; r=0.67) such that there were more positive ratings (M=49.1%, SD=16.5%) compared to the neutral (M=25.1%, SD=15.5%) or negative (M=25.8%, SD=18.5%) ratings in both groups. The main effect for group was not significant (F(1,34)= 0.0, P=1.0). The interaction between group and rating was not significant (F(2,68)=1.15, P=0.32), thus individuals with schizophrenia and healthy controls seem to apply preference ratings to food in a similar manner.
3.3. Relationships with Symptoms
Total SANS and SAPS scores were not associated with food ratings or preference decisions. We examined relationships between anhedonia and food preference/rating more specifically using the SANS Anhedonia/Asociality factor ratings (including lack of recreational interests (RI), lack of sexual interests and activity (SI), inability to feel intimate and close (IC), relationships with friends and peers (FP), and the global anhedonia-asociality rating), and the BPRS blunted affect (BA) and emotional withdrawal (EW) items. Please see Table 1 for correlations. Relationships between negative symptoms and food ratings clustered around the SANS SI item. An increase in reported problems with sexual interest and activity was associated with lower positive food ratings in the following areas: average overall rating applied to food images (rho= −0.59, P=0.02), ratings of meat dishes (rho= −0.58, P=0.02), and the percent of positive (using a “4” or “5”) ratings applied to food images overall (rho= −0.58, P=0.02). There were no significant correlations between SPQ total and Constricted Affect items and food ratings for healthy controls. There were no associations between relative preference and liking dependent variables and the medication dose as indicated by the chlorpromazine binding equivalents.
Table 1.
The relationships between hedonic food ratings from different categories and symptom ratings.
| BPRS | SANS | SAPS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | BA | EW | Total | RI | SI | IC | FP | GAA | Total | |
| Vegetables | 0.06 | 0.3 | 0.14 | 0 | −0.1 | −0.09 | 0.18 | 0.29 | 0.22 | 0.1 |
| Pasta | −0.18 | −0.17 | −0.2 | −0.25 | −0.24 | −0.19 | 0.03 | −0.01 | −0.17 | −0.24 |
| Desserts | 0.36 | 0.13 | 0.14 | 0.38 | −0.21 | −0.37 | 0.08 | 0.06 | 0.04 | −0.06 |
| Meat | 0.21 | −0.19 | 0.04 | 0.27 | −0.39 | * −0.58 | −0.11 | −0.32 | −0.28 | −0.45 |
| Overall | 0.15 | −0.24 | −0.15 | 0.21 | −0.38 | * −0.59 | −0.18 | −0.37 | −0.25 | −0.4 |
Correlation is significant at the 0.05 level.
BPRS (Brief Psychiatric Rating Scale); BA (Blunted Affect); EW (Emotional Withdrawal)
SANS (Scale for the Assessment of Negative Symptoms); RI (Lack of recreational interests); SI (Lack of sexual interests and activity); IC (inability to feel intimate and close); FP (relationships with friends and peers); GAA (Global Anhedonia-Asociality)
SAPS (Scale for the Assessment of Positive Symptoms)
4. Discussion
Based on previous findings suggesting anhedonia, decreased incentive reward valuation, and functional pathology in schizophrenia in brain regions involved in making preference decisions, we expected subjects with schizophrenia to display slower response times to preference decisions compared to a control task and to rate foods with less hedonic value compared to healthy controls. The relative preference paradigm did not reveal differences between schizophrenic and control subjects in decision-making response times overall. However, measuring only response time may not capture subtleties in the food preference decision-making process.
For food hedonic ratings, all subjects tended to use the positive ratings overall. This reiterates the overall hedonic nature of the food stimuli and the premise that in the relative reward paradigm, subjects were indeed selecting from a range of preferred stimuli. Subjects with schizophrenia did not exhibit overall decreased hedonic judgments for food, but there were subtle differences in schizophrenia subjects’ food rating that may be related to particular facets of anhedonia including sexual disinterest. This may speak to the underlying biological mechanisms that subsume similar vegetative processes such as appetite, sexual desire, and motivation to achieve pleasure.
In a similar study using the University of Pennsylvania Smell Identification Test, Doop and Park (2006) found that subjects with schizophrenia judged all odorants to be more pleasant overall and found that schizophrenic subjects used a restricted range of positive ratings. We found a similar phenomenon in schizophrenia where higher hedonic values are given to stimuli that are less appetitive to healthy controls, for example vegetables. The higher ratings given to vegetables by subjects with schizophrenia probably do not represent an increased preference for vegetables in particular (McCreadie et al., 1998), but the use of higher ratings for all food substances. In other words, individuals with schizophrenia seem to be more positive towards hedonic stimuli.
Our results do suggest that data showing poor dietary habits and choices in schizophrenia may be due to decreased availability of healthy food rather than to active disinterest in eating healthier foods. Our results suggest that patients with schizophrenia would be less likely to discriminate between food choices, and that they would enjoy eating healthier vegetable dishes that may not be readily available in institutional settings. Thus, programs directed at offering healthy choices may be met with success and appreciation by patients. Our results suggest that patients enjoy different, and even healthier foods, than they may eat on average. This disconnection between their internal preference state and their behavioral choices may be at least partially explained by Frith’s (1992) conceptualization of schizophrenia as a disorder of “willed action”, or having difficulty converting internal states to actions when the desire is internally driven rather than imposed form the environment (Langdon et al., 2007).
Kapur (2003) has suggested that dopamine mediates the motivational salience of stimuli. Our results did not indicate a relationship between chlorpromazine dose equivalencies (which index the blockade of dopamine D2 receptors) and the preference or liking measures, but all subjects with schizophrenia were taking atypical antipsychotic drugs which have differential effects on the serotonergic system in addition to the dopamine receptors.
One methodological caveat is that we presented subjects with pictures rather than actual food items; however cognitive and emotional reactions to food cues have been previously studied using pictures. These studies have shown that food pictures can elicit increased physiological responses and subjective measures of preference (Drobes et al., 2001), and they can be powerful motivational probes in fMRI paradigms, eliciting emotional responses and corresponding limbic activation (LaBar et al., 2001) depending on baseline satiety levels. In this study, all subjects were tested before receiving a meal in an attempt to minimize potential differences in satiety levels, and they were screened for subjective food restrictions (i.e. no vegetarians).
We investigated food preference and liking in schizophrenia, a mental disorder that may represent an ideal population for studying the underlying neurobiology of food valuation and preference based on overlapping brain circuitry of the hedonic system and neuropathology in schizophrenia. Although the relative preference paradigm did not reveal quantitative differences in preference decision-making in schizophrenia, this type of paradigm may be a useful approach to functional neuroimaging studies in the future in order to uncover potential qualitative differences in the underlying neural circuitry that is related to reward function. Our results indicate that there are subtle differences in food liking in schizophrenia, possibly based on a lower hedonic threshold for food, and that these differences may be related to the same processes involved in other vegetative mechanisms in schizophrenia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Archives of General Psychiatry. 1982;39:784–788. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Olsen S. Negative v. positive schizophrenia. Definition and validation. Archives of General Psychiatry. 1982;39:789–794. doi: 10.1001/archpsyc.1982.04290070025006. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neuroscience and Biobehavioral Reviews. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Blumer D, Benson DF. Personality changes with frontal and temporal lobe lesions. In: Blumer D, Benson DF, editors. Psychiatric Aspects of Neurological Disease. New York: Grune & Stratton; 1975. pp. 151–170. [Google Scholar]
- Butter CM, McDonald JA, Snyder DR. Orality, preference behavior, and reinforcement value of nonfood object in monkeys with orbital frontal lesions. Science. 1969;164:1306–1307. doi: 10.1126/science.164.3885.1306. [DOI] [PubMed] [Google Scholar]
- Chau DT, Roth RM, Green AI. The neural circuitry of reward and its relevance to psychiatric disorders. Curr Psychiatry Rep. 2004;6:391–399. doi: 10.1007/s11920-004-0026-8. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Kim J, Andreasen NC, O'Leary DS, Magnotta V. Regional frontal abnormalities in schizophrenia: a quantitative gray matter volume and cortical surface size study. Biological Psychiatry. 2000;48:110–119. doi: 10.1016/s0006-2332(00)00238-9. [DOI] [PubMed] [Google Scholar]
- Davis JM. Dose equivalence of the antipsychotic drugs. Journal of Psychiatric Research. 1974;11:65–69. doi: 10.1016/0022-3956(74)90071-5. [DOI] [PubMed] [Google Scholar]
- Doop ML, Park S. On knowing and judging smells: identification and hedonic judgment of odors in schizophrenia. Schizophrenia Research. 2006;81:317–319. doi: 10.1016/j.schres.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Elman I, Borsook D, Lukas SE. Food intake and reward mechanisms in patients with schizophrenia: implications for metabolic disturbances and treatment with second-generation antipsychotic agents. Neuropsychopharmacology. 2006;31:2091–2120. doi: 10.1038/sj.npp.1301051. [DOI] [PubMed] [Google Scholar]
- Finlayson G, King N, Blundell JE. Is it possible to dissociate 'liking' and 'wanting' for foods in humans? A novel experimental procedure. Physiology and Behavior. 2007;90:36–42. doi: 10.1016/j.physbeh.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Frith C. The Cognitive Neuropsychology of Schizophrenia. Hove UK: Lawrence Erlbaum; 1992. [Google Scholar]
- Goldstein JM, Goodman JM, Seidman LJ, Kennedy DN, Makris N, Lee H, Tourville J, Caviness VV, Jr, Faraone SV, Tsuang MT. Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Archives of General Psychiatry. 1999;56:537–547. doi: 10.1001/archpsyc.56.6.537. [DOI] [PubMed] [Google Scholar]
- Greene LS, Desor JA, Maller O. Heredity and experience: their relative importance in the development of taste preference in man. Journal of Comparative & Physiological Psychology. 1975;89:279–284. doi: 10.1037/h0076802. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Brown J, Holland AJ, Fukuhara R, Hodges JR. Changes in appetite, food preference, and eating habits in frontotemporal dementia and Alzheimer's disease. Journal of Neurology Neurosurgery and Psychiatry. 2002;73:371–376. doi: 10.1136/jnnp.73.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. American Journal of Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kim JS, Choi S. Altered food preference after cortical infarction: Korean style. Cerebrovascular Diseases. 2002;13:187–191. doi: 10.1159/000047774. [DOI] [PubMed] [Google Scholar]
- Kovess-Masfety V, Xavier M, Moreno Kustner B, Suchocka A, Sevilla-Dedieu C, Dubuis J, Lacalmontie E, Pellet J, Roelandt JL, Walsh D. Schizophrenia and quality of life: a one-year follow-up in four EU countries. BMC Psychiatry. 2006;6:39. doi: 10.1186/1471-244X-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML. Food for thought: hedonic experience beyond homeostasis in the human brain. Neuroscience. 2004;126:807–819. doi: 10.1016/j.neuroscience.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Langdon R, McLaren J, Polito V, Coltheart M, Ward PB. Willed action in schizophrenia. Psychiatry Research. 2007;150:193–197. doi: 10.1016/j.psychres.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Lukoff D, Nuechterlein KH, Ventura J. Manual for the Expanded Brief Psychiatric Rating Scale. Schizophrenia Bulletin. 1986;12:594–560. [Google Scholar]
- McCreadie RG, Kelly C, Connolly M, Williams S, Baxter G, Lean M, Paterson JR. Dietary improvement in people with schizophrenia: randomised controlled trial. British Journal of Psychiatry. 2005;187:346–351. doi: 10.1192/bjp.187.4.346. [DOI] [PubMed] [Google Scholar]
- McCreadie RG, Macdonald E, Blacklock C, Tilak-Singh D, Wiles D, Halliday J, Paterson J. Dietary intake of schizophrenic patients in Nithsdale, Scotland: case-control study. British Medical Journal. 1998;317:784–785. doi: 10.1136/bmj.317.7161.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mela DJ. Food choice and intake: the human factor. Proceedings of the Nutrition Society. 1999;58:513–521. doi: 10.1017/s0029665199000683. [DOI] [PubMed] [Google Scholar]
- Peet M. Diet, diabetes and schizophrenia: review and hypothesis. British Journal of Psychiatry. 2004;47:S102–S105. doi: 10.1192/bjp.184.47.s102. [DOI] [PubMed] [Google Scholar]
- Prado-Lima PS, Cruz IB, Schwanke CH, Netto CA, Licinio J. Human food preferences are associated with a 5-HT(2A) serotonergic receptor polymorphism. Molecular Psychiatry. 2006;11:889–891. doi: 10.1038/sj.mp.4001872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A. The SPQ: A scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophrenia Bulletin. 1991;17:555–564. doi: 10.1093/schbul/17.4.555. [DOI] [PubMed] [Google Scholar]
- Regard M, Landis T. "Gourmand syndrome": eating passion associated with right anterior lesions. Neurology. 1997;48:1185–1190. doi: 10.1212/wnl.48.5.1185. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Oxford. New York: Oxford University Press; 1999. The brain and emotion. [Google Scholar]
- Schmauss C, Emrich HM. Dopamine and the action of opiates: a reevaluation of the dopamine hypothesis of schizophrenia. With special consideration of the role of endogenous opioids in the pathogenesis of schizophrenia. Biological Psychiatry. 1985;20:1211–1231. doi: 10.1016/0006-3223(85)90179-9. [DOI] [PubMed] [Google Scholar]
- Schultz W. Reward signaling by dopamine neurons. Neuroscientist. 2001;7:293–302. doi: 10.1177/107385840100700406. [DOI] [PubMed] [Google Scholar]
- Schurhoff F, Szoke A, Bellivier F, Turcas C, Villemur M, Tignol J, Rouillon F, Leboyer M. Anhedonia in schizophrenia: a distinct familial subtype? Schizophrenia Research. 2003;61:59–66. doi: 10.1016/s0920-9964(02)00237-2. [DOI] [PubMed] [Google Scholar]
- Taha SA, Norsted E, Lee LS, Lang PD, Lee BS, Woolley JD, Fields HL. Endogenous opioids encode relative taste preference. European Journal of Neuroscience. 2006;24:1220–1226. doi: 10.1111/j.1460-9568.2006.04987.x. [DOI] [PubMed] [Google Scholar]
- Tamminga CA. The neurobiology of cognition in schizophrenia. Journal of Clinical Psychiatry. 2006;67:e11. [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Watanabe M. Neurobiology. Attraction is relative not absolute. Nature. 1999;398:661–663. doi: 10.1038/19414. [DOI] [PubMed] [Google Scholar]
- Wise RA, Spindler J, deWit H, Gerberg GJ. Neuroleptic-induced "anhedonia" in rats: pimozide blocks reward quality of food. Science. 1978;201:262–264. doi: 10.1126/science.566469. [DOI] [PubMed] [Google Scholar]
- Wise RA, Spindler J, Legault L. Major attenuation of food reward with performance-sparing doses of pimozide in the rat. Canadian Journal of Psychology. 1978;32:77–85. doi: 10.1037/h0081678. [DOI] [PubMed] [Google Scholar]
- Wolf DH. Anhedonia in schizophrenia. Current Psychiatry Reports. 2006;8:322–328. doi: 10.1007/s11920-006-0069-0. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. Journal of Clinical Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]