Abstract
Tuberoinfundibular peptide of 39 residues (TIP39) is the recently purified endogenous ligand of the previously orphan G-protein coupled parathyroid hormone 2 receptor (PTH2R). The TIP39-PTH2R system is a unique neuropeptide-receptor system whose localization and functions in the central nervous system are different from any other neuropeptides. TIP39 is expressed in 2 brain regions, the subparafascicular area in the posterior thalamus, and the medial paralemniscal nucleus in the lateral pons. Subparafascicular TIP39 neurons seem to divide into a medial and a lateral cell population in the periventricular gray of the thalamus, and in the posterior intralaminar complex of the thalamus, respectively. Periventricular thalamic TIP39 neurons project mostly to limbic brain regions, the posterior intralaminar thalamic TIP39 neurons to neuroendocrine brain areas, and the medial paralemniscal TIP39 neurons to auditory and other brainstem regions, and the spinal cord. The widely distributed axon terminals of TIP39 neurons have a similar distribution as the PTH2R-containing neurons, and their fibers, providing the anatomical basis of a neuromodulatory action of TIP39. Initial functional studies implicated the TIP39-PTH2R system in nociceptive information processing in the spinal cord, in the regulation of different hypophysiotropic neurons in the hypothalamus, and in the modulation of affective behaviors. Recently developed novel experimental tools including mice with targeted mutations of the TIP39-PTH2R system and specific antagonists of the PTH2R will further facilitate the identification of the specific roles of TIP39 and the PTH2R.
Keywords: neuropeptide, subparafascicular, posterior intralaminar thalamic, limbic functions, endocrine hypothalamic regulations, nociceptive action, paralemniscal, auditory brainstem
1 Introduction
Tuberoinfundibular peptide of 39 residues (TIP39) was identified on the basis of its activation of the parathyroid hormone 2 receptor (PTH2R), a seven transmembrane domain G-protein coupled receptor (Usdin et al., 1999b). The distribution of TIP39 containing fibers and terminals is very similar to the distribution of PTH2R-containing neurons and neuronal fibers throughout the brain (Dobolyi et al., 2003b; Faber et al., 2007), and TIP39 is a potent and selective PTH2R agonist (Usdin, 2000). This functional and anatomical match suggests that TIP39 is the endogenous ligand of the PTH2R in the brain, and that they form a neuromodulator system. TIP39 neurons have a highly restricted localization. This pattern of synthesis by cells in a few discrete areas and widespread, but still topographically organized, projections to several distant brain areas resembles several other recently developed neuropeptide systems including, for example, relaxins (Ma and Gundlach, 2007), orexins (Baumann and Bassetti, 2005), calcitonin-gene related peptide (van Rossum et al., 1997), prolactin-releasing peptide (Roland et al., 1999), kisspeptin (Mikkelsen and Simonneaux, 2009), and urocortins (Pan and Kastin, 2008). Based on the available data, however, the TIP39-PTH2R system is a unique neuropeptide-receptor system whose localization and functions in the central nervous system are different from any other neurpeptides.
TIP39 is a member of a small peptide family comprised of parathyroid hormone (PTH), parathyroid hormone-related peptide (PTHrP) and TIP39 (Usdin et al., 1999b). Mature PTH and PTHrP are polypeptides of about 100 residues. They are products of separate genes but they activate the parathyroid hormone 1 receptor (PTH1R) with equal potency (Gensure et al., 2005; Muff et al., 1994). Their first 34 or 36 residues are sufficient for high-affinity binding and full efficacy at the PTH1R, and they share 12 of these amino acids (Gillespie and Martin, 1994; Martin et al., 1991). The primary sequence of TIP39 is quite different. It contains only four of the residues that are common to PTH(1–34) and PTHrP(1–36), and similar residues at several additional positions (Usdin et al., 1999b). However, TIP39 has a backbone structure that can be nearly superimposed on that of PTH (Piserchio et al., 2000). Based on the similarity of TIP39 to PTH and its activation of the PTH2R, TIP39 is referred to as parathyroid hormone 2 in the UniGene database at http://www.ncbi.nlm.nih.gov/sites/entrez (Mm.207078 for the mouse and Hs.339845 for the human gene). However, this name is also used for a second form of PTH found in fish that more closely resembles mammalian PTH than does TIP39 (Gensure et al., 2004). PTH, produced by the parathyroid gland, is the most important regulator of calcium homeostasis. It increases plasma calcium ion concentration via direct actions in the kidney and the skeleton (Hurwitz, 1996; Rizzoli et al., 1992). PTHrP is a paracrine factor that functions in a number of organs and plays a critical role in skeletal development (Law et al., 1994; Martin et al., 1997). TIP39 and the PTH2R are expressed at very low levels in kidney and bone (Usdin et al., 1996; Usdin et al., 1999a). In the periphery, they may play a role in the cardiovascular system (Eichinger et al., 2002; Ross et al., 2005; Ross et al., 2007) and in gonadal function (Usdin et al., 2008). However, TIP39 and the PTH2R are most abundant in the central nervous system (Dobolyi et al., 2002; Usdin et al., 1995).
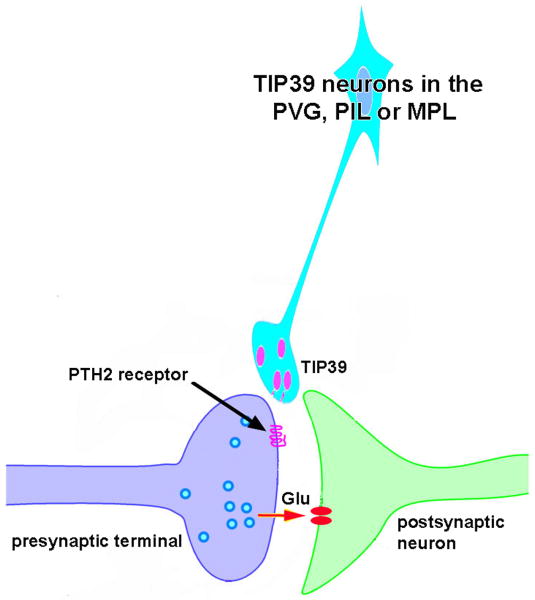
Since the last review on the TIP39-PTH2R system (Usdin et al., 2003), the cell groups expressing TIP39 and the PTH2R as well as their neuronal connections have been identified. TIP39 and the PTH2R have unique expression patterns in the brain, which has facilitated the description of previously unrecognized anatomical structures and connections. TIP39 is expressed in only three sites in the brain, the medial and lateral parts of the subparafascicular area in the caudal thalamus, and the medial paralemnsical nucleus in the lateral pons (Dobolyi et al., 2003b; Faber et al., 2007). Widespread connections of the subparafascicular area implicate this region in the regulation of limbic and endocrine functions (Dobolyi et al., 2003a; Wang et al., 2006b, c). Projections from the medial paralemniscal nucleus to a number of auditory areas, as well as some hypothalamic and viscerosensory regions, suggest that this newly identifed nucleus may play a role in auditory and nociceptive processes (Dobolyi et al., 2003a; Dobolyi et al., 2002; Varga et al., 2008). The distribution of PTH2R containing neurons and fibers (Bagó et al., 2009; Faber et al., 2007; Wang et al., 2000) is consistent with these proposed functions. In fact, the subregional similarities in the locations of TIP39 and PTH2R terminals (Dobolyi et al., 2006a; Faber et al., 2007), together with the demonstration of the glutamatergic nature of PTH2R terminals in the hypothalamus (Bagó et al., 2009; Dobolyi et al., 2006a), lead us to suggest an axo-axonic mechanism of action for the TIP39-PTH2R neuromodulator system. Embryonic (Brenner et al., 2008) and postnatal development (Dobolyi et al., 2006b) of the TIP39-PTH2R system has also been descibed recently. These data support the separation of the medial and lateral subparafascicular TIP39 neurons into separate groups (Brenner et al., 2008), and reveal a decline in TIP39 but not PTH2R levels during the period of sexual maturation (Dobolyi et al., 2006b). Initial functional studies implicate TIP39 in the regulation of the release of pituitary hormones (Sugimura et al., 2003; Ward et al., 2001), affect-related behaviors (Fegley et al., 2008; LaBuda et al., 2004), and the modulation of some aspects of nociceptive signaling (Dobolyi et al., 2002; LaBuda and Usdin, 2004). Furthermore, studies using c-fos to identify activated neurons suggest that TIP39 neurons are involved in sexual function (Wang et al., 2006a) and the audiogenic stress response (Palkovits et al., 2004). Recently developed novel experimental tools including selective antagonists (Kuo and Usdin, 2007; Visegrady et al., 2007) and transgenic animals (Faber et al., 2007; Fegley et al., 2008; Usdin et al., 2008) are likely to further facilitate investigation of the functions of the TIP39-PTH2R system.
2 Peptide and gene structure of tuberoinfundibular peptide of 39 residues (TIP39)
2.1 Purification and sequencing of TIP39
Initial studies of the human PTH2R showed that it is activated by PTH (Usdin et al., 1995). However, Usdin did not detect synthesis of PTH in the rat brain, and found activity that activated the PTH2R and not the PTH1R in bovine hypothalamus (Usdin, 1997). The rat PTH2R is poorly activated by PTH, and bovine hypothalamic extracts contain activity that is more potent and more efficatious at the rat PTH2R than PTH, based on stimulation of cAMP accumulation in cultured cells transfected with the PTH2R (Hoare et al., 1999). This PTH2R-selective ligand, with the sequence SLALADDAAFRERARLLAALERRHWLNSYMHKLLVLDAP, was purified from an acid extract of fifty pounds of bovine hypothalamus (Usdin et al., 1999b). Mass spectrometry provided no evidence for posttranslational modification of any of the amino acids. Chemically synthesized and purified TIP39 had identical molecular weight and the same fragment ions were produced following trypsin digestion (Usdin et al., 1999b).
2.2 Structure of TIP39
The structure of TIP39 in a membrane mimic detergent was elucidated by CD and NMR spectroscopy. Similar to PTH(1–34), TIP39 contains two stable alpha-helices at the N and C termini separated by a region of undefined structure (Piserchio et al., 2000). The N-terminal helix shares a high structural and sequential homology with PTH (Pellegrini et al., 1998), suggesting a similar function in receptor signaling. The structural differences between TIP39 and PTH(1–34) include the lengths and amphipathic character of the helices as well as the location of the flexible region between the helices (Piserchio et al., 2000). A pronounced difference is that TIP39(Trp25) falls in the middle of the structurally unordered region in contrast to a well defined helical portion observed for the PTH(Trp23). The C-terminal helix of TIP39 has some charged residues on the hydrophobic face, reducing the amphipathic nature of the helix in comparison with that of PTH(1–34). This difference could diminish the importance of the interaction of the C terminus of TIP39 with the large extracellular N terminus of the receptor, postulated to be an important step in recognition and binding of PTH to the PTH1R (Mierke et al., 2007). Finally TIP39 places Asp7 in the key position corresponding to PTH(Ile5) and PTHrP(His5), which may contribute to the selectivity of TIP39 toward the PTH2R. NMR spectroscopy also revealed that TIP39 associates with the anionic membrane surface via its positively charges residues but does not insert into the membrane hydrophobic compartment (Mason et al., 2005; Piserchio et al., 2000). However, peptide accumulation at the membrane surface is not significantly increased by this interaction (Mason et al., 2005).
2.3 Structure of the gene encoding TIP39
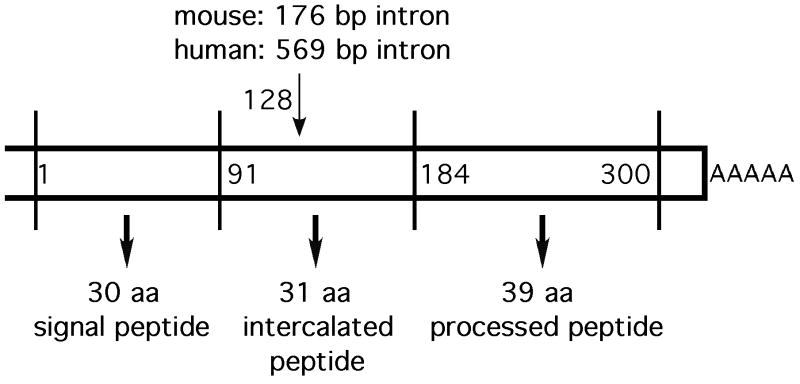
Following the purification of bovine TIP39, the mouse and human genes encoding TIP39 were identified in database searches based on sequence similarities (Dobolyi et al., 2002; Hansen et al., 2002; John et al., 2002). The human TIP39 gene is on the long arm of chromosome 19 at band 19q13.3. Human and murine cDNAs were obtained by RACE-PCR and are available under the GenBank accession numbers: AY037555 and AC073740 (Hansen et al., 2002) and AY048588 and AY048587 (John et al., 2002). On alignment, the nucleotide sequences of the human and murine cDNAs have 80% identity. The cDNAs encoding both TIP39 precursors are rich in guanine and cytosine (GC-content: 74.3% and 69.7%, respectively) compared with a human genome-wide average of 41%. TIP39 cDNA (Fig. 1) consists of a 5′-untranslated sequence of 102 bp, an open reading frame of 300 bp, and 55 bp of 3′-untranslated sequence containing a polyadenylation signal (Hansen et al., 2002; John et al., 2002). The human and mouse genes contain two coding exons separated by an intron at corresponding sites. Exon 1 encodes 43 aa of the TIP39 precursor protein, exon 2 encodes the remaining 57 aa. The first 30 aa are predicted to function as a signal peptide, which is followed by a signal peptidase cleavage site (Dobolyi et al., 2002; Hansen et al., 2002). The TIP39 precursor also contains two possible dibasic cleavage sites (Arg-Arg motif with compatible adjacent residues). The first separates an intercalated peptide from the peptide that was purified, the second is found at position 22/23 within the TIP39 sequence (the 39 residue sequence that was purified is referred to as TIP39 and the predicted primary translation product as pre-pro-TIP39, the putative pro-TIP39 formed following signal peptide cleavage has not been demonstrated). Human and mouse pre-pro-TIP39 share 79% overall identity and there is 89% identity within the TIP39 peptide. Thirty-five out of 39 residues are identical, with changes in amino acid class at positions 24, 27, 31 and a change to a homologous residue at position 35. TIP39 sequences have the highest degree of identity in the N-terminal region, all 23 residues in the N-terminus are identical. There is 100% identity between the sequence of human and bovine TIP39 (Della Penna et al., 2003). The rat TIP39 cDNA was also cloned and found to be very similar to that of the mouse (Della Penna et al., 2003; Dobolyi et al., 2002). The predicted amino acid sequences of mouse and rat TIP39 are identical (Della Penna et al., 2003; Dobolyi et al., 2002).
Fig. 1.
Schematic structure of the mRNA encoding TIP39.
Sequences of peptides homologous to TIP39 are present in teleost species including the Japanese pufferfish fugu (Takifugu rubripes), the zebrafish (Danio rerio), the Nile tilapia (Oreochromis niloticus), and the euryhaline Mozambique tilapia (Oreochromis mossambicus) (Papasani et al., 2004; Shoemaker et al., 2006). The predicted processed peptide of fugu and zebrafish consists of 39 residues (Papasani et al., 2004) while that of tilapia species consists of only 38 residues, corresponding to the first 38 amino acid of other species (Shoemaker et al., 2006). The teleost TIP39 homologues have 81-83% nucleotide sequence identity and 97% amino acid sequence similarity with each other (Papasani et al., 2004; Shoemaker et al., 2006) and 57-63% sequence identity with the mammalian TIP39 homologs (Papasani et al., 2004; Shoemaker et al., 2006). Although teleosts also have an intercalated peptide in the precursor, its sequence is poorly conserved, as is the signal peptide. In addition, the intercalated peptide is much longer in teleosts (fugu TIP39: 72 amino acid residues; zebrafish TIP39: 91 amino acid residues) than the comparable mammalian sequence (31 amino acids). RACE-PCR suggested that there could be variant zebrafish pre-pro-TIP39 sequences (GenBank accession numbers: AY307076 and AY306196), with a short variant initiated at the third Met instead of the first Met and thus lacking 63 nucleotides from the region encoding the poorly conserved signal peptide, but not missing any amino acids of the processed peptide. However, typical splice donor and acceptor sites were not found, thus it is possible that the putative splice variant is an artifact and the zebrafish is not different from other species where no splice variants of the TIP39 gene were identified (Papasani et al., 2004).
3 TIP39 expressing cell groups
3.1 TIP39 expression in different organs
TIP39 expression appears abundant in several rat brain regions and the testis based on non-quantitative RT-PCR, and it is detectable in the eye and the dorsal root ganglia (Dobolyi et al., 2002), kidney and pancreas (Eichinger et al., 2002), and heart (Ross et al., 2005). In human, TIP39 expression was detected by RT-PCR in the brain, trachea, fetal liver, kidney and heart (Hansen et al., 2002). In tilapia species, RT-PCR showed TIP39 expression in kidney, heart, liver and testes (Shoemaker et al., 2006). In the zebrafish, whole-mount in situ hybridization showed TIP39 mRNA in the brain and heart (Papasani et al., 2004). Northern blots in mice showed a prominent message of approximately 4.5 kb in testis, which was also observed, at much lower intensity, in liver, kidney, and possibly heart (John et al., 2002). Additional transcripts of about 1.5 kb and 1.0 kb were detected in these organs as well as in the brain (John et al., 2002). The larger hybridizing RNA may be incompletely processed pre-mRNA.
The distribution of TIP39 expression within individual organs has been investigated by in situ hybridization in mouse testis (John et al., 2002; Usdin et al., 2008), and rat (Dobolyi et al., 2003b; Dobolyi et al., 2002), mouse (Faber et al., 2007; John et al., 2002), and macaque brain (Bagó et al., 2009). In the testis, TIP39 mRNA is expressed in the epithelium of some but not all seminiferous tubules, indicating that it is expressed in a stage-specific manner (John et al., 2002; Usdin et al., 2008). Higher magnification images of TIP39 in situ hybridization suggest that most of the label is present over the middle third of the epithelium, the location of developing spermatogenic cells (Usdin et al., 2008). Immunolabeling of TIP39, and visualizing beta-galactosidase activity in mice containing beta-galactosidase driven by the TIP39 promoter, confirmed the location of TIP39 in developing spermatogenic cells (Usdin et al., 2008). TIP39-expressing neurons have a highly restricted localization in the brain of rodents (Dobolyi et al., 2003b; Dobolyi et al., 2002; Faber et al., 2007; John et al., 2002; Ky and Shughrue, 2002), macaque (Bagó et al., 2008), and zebrafish (Papasani et al., 2004) as described below.
3.2 TIP39 neurons in the posterior diencephalon
3.2.1 The subparafascicular area (SPF)
TIP39-expressing neurons are present in the subparafascicular nucleus and the surrounding subparafascicular area of the thalamus (Dobolyi et al., 2002; John et al., 2002), a relatively little studied area situated in the ventromedial part of the posterior thalamus (Faul and Mehler, 1985). The subparafascicular nucleus was originally described as a large area below the fasciculus retroflexus and the parafascicular nucleus (Rioch, 1929). Subsequently, the subparafascicular nucleus was separated into 2 nuclei. The magnocellular (also called medial) subparafascicular nucleus which has a cytoarchitectonically well-defined circular appearance on coronal sections from bregma levels -3.8 to -4.3 mm (Faul and Mehler, 1985; Paxinos and Watson, 2005). The parvicellular or lateral subparafascicular nucleus (SPFp), situated from bregma levels -4.2 to -4.8 mm has a caudo-laterally extended orientation above the medial lemniscus as far as the ventral part of the medial geniculate body (Ledoux et al., 1987; Papez and Aronson, 1934). The subparafascicular nuclei have been considered thalamic nuclei with unknown function (Faul and Mehler, 1985; Turner and Herkenham, 1991) or members of the posterior intralaminar group of nuclei (LeDoux et al., 1985; Rub et al., 2002). Most authors, however, do not consider them to be midline or intralaminar nuclei (Berendse and Groenewegen, 1991; Van der Werf et al., 2002). The complex shape and the difficulty in defining the area contributed to the introduction of several different terminologies describing brain areas that include the subparafascicular nuclei or parts of them, as well as surrounding brain areas. The term ‘subparafascicular area’ and its ‘compartments’ corresponding to the magnocellular subparafascicular nucleus and the periventricular gray of the thalamus were introduced to describe connectional data (Moriizumi and Hattori, 1991, 1992). The area caudal and dorsal to the subparafascicular nucleus has also been called ‘subfascicular area’ (Peschanski and Mantyh, 1983). The term ‘periventricular gray of the caudal thalamus’ was used to describe the location of the A11 dopaminergic cells (Hökfelt et al., 1984; Hökfelt et al., 1979; Skagerberg and Lindvall, 1985), and to describe sites for stimulus-induced analgesia in the region (Boivie and Meyerson, 1982; Rhodes and Liebeskind, 1978).
TIP39 neurons are situated close to the midline in and around the magnocelllar subparafascicular nucleus within the periventricular gray of the thalamus, as well as in the parvicellular (lateral) subparafascicular nucleus and the surrounding area above the medial lemnsicus and further lateral, as far as the area ventromedial to the medial geniculate body (Fig. 2). Because the medially and laterally positioned TIP39 neurons seemed relatively contiguous on horizontal sections (Fig. 2D) they were initially considered a single cell group and the term subparafascicular area was introduced to describe the distribution of TIP39 neurons. However, recent developmental (Brenner et al., 2008) and functional (Wang et al., 2006a) data, described below, suggest that the periventricular and lateral subparafascicular/posterior intralaminar thalamic TIP39 neurons constitute separate cell groups, which makes the use of the unifying term subparafascicular area less plausible.
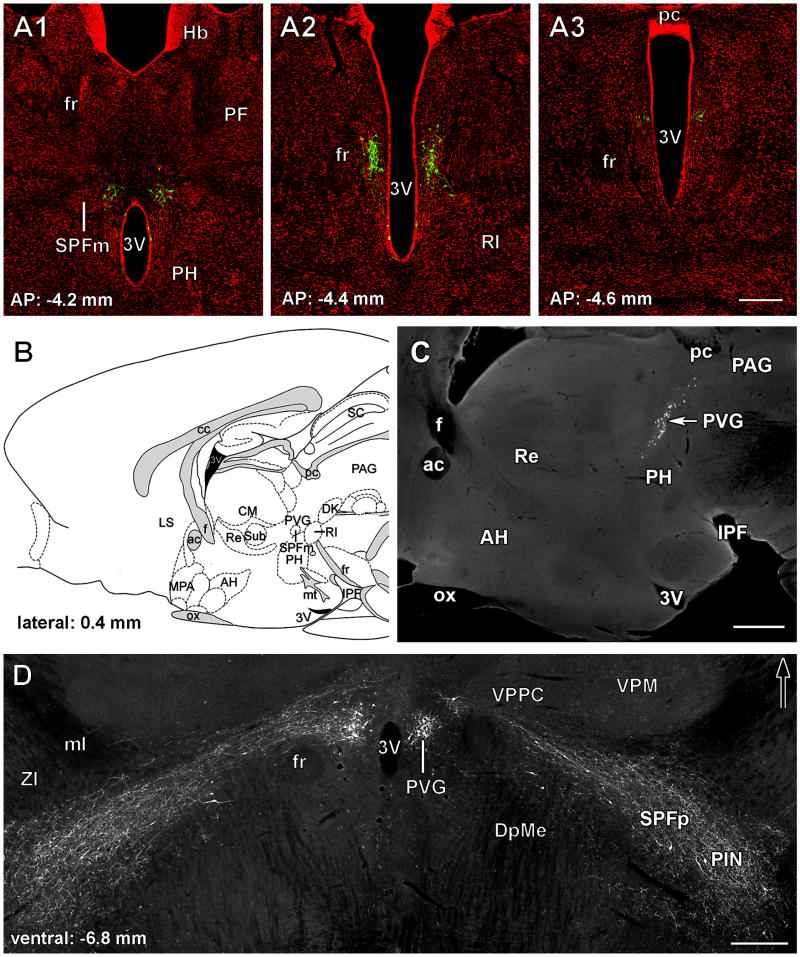
Fig. 2.
TIP39 neurons in the subparafascicular area of the rat posterior thalamus. A: TIP39-ir neurons (green) are shown in the PVG in coronal sections labeled with a fluorescent Nissl dye (red) at antero-posterior (AP) coordinates -4.2, -4.4, and -4.6 mm from the bregma level. B: The position of the PVG is shown in a drawing of a sagittal section of the rat brain at 0.4 mm lateral from the midline (Paxinos and Watson, 1998). C: A photomicrograph of a sagittal section corresponding to panel B shows the location of TIP39-ir neurons in the PVG. D: TIP39-ir neurons and fibers are shown in a horizontal section 6.8 mm ventral to the surface of the brain. The large density of TIP39-ir neurons in the PVG is in contrast to the scattered TIP39-ir neurons in the PIL while TIP39-ir fibers connect the 2 regions of TIP39 expression. Abbreviations: ac, anterior commissure; AH, anterior hypothalamic nucleus; cc, corpus callosum; CM, central median thalamic nucleus; Dk, nucleus of Darkschewitsch; DpMc, deep mesencephalic nucleus; f, fornix; fr, fasciculus retroflexus; IC, inferior colliculus; IPF, interpeduncular fossa; LS, lateral septal nucleus; ml, medial lemniscus; MPA, medial preoptic area; mt, mamillothalamic tract; ox, optic chiasm; PAG, periventricular gray; pc, posterior commissure; PF, parafascicular thalamic nucleus; PH, posterior hypothalamic area; PIN, posterior intralaminar thalamic nucleus; Pn, pontine nuclei; PVG, periventricular gray of the thalamus; py, pyramidal tract; Re, reuniens thalamic nucleus; RI, rostral interstitial nucleus of the medial longitudinal fasciculus; SC, superior colliculus; SPFm, magnocellular subparafascicular thalamic nucleus; SPFp, parvicellular subparafascicular thalamic nucleus; Sub, submedius thalamic nucleus; VPM, ventral posteromedial thalamic nucleus; VPPC, ventral posterior parvicellular thalamic nucleus; ZI, zona incerta; 3V, third ventricle; 4V, fourth ventricle. Scale bar = 500 μm for A and D, and 300 μm for C.
3.2.2 TIP39 neurons in the periventricular gray of the thalamus (PVG)
Periventricular thalamic TIP39 neurons constitute the largest TIP39 cell group in the brain of young adult rats (Dobolyi et al., 2003b) and mice (Faber et al., 2007), with about 600-1000 neurons per side. TIP39 neurons in the PVG appear rostrally first above the third ventricle, close to the midline at bregma level -3.8 mm (Fig. 2A1). These TIP39 neurons are located ventral to the central median nucleus of the thalamus, dorsal to the posterior hypothalamic nucleus and medial to the parvicellular ventral posterior nucleus of the thalamus and mostly medial to the magnocellular subparafascicular nucleus (Dobolyi et al., 2003b; Faber et al., 2007). More caudal TIP39 neurons are located more and more dorsally between the midline and the fasciculus retroflexus (Fig. 2A2). Midway through the rostrocaudal extent of the PVG, a few cells appear ventrally to the main cell group and are aligned immediately next to the caudal end of the third ventricle (Dobolyi et al., 2003b; Faber et al., 2007). Additional TIP39 neurons are situated more laterally, ventral to the fasciculus retroflexus. Caudally, the density of TIP39 cells sharply decreases and the cells disappear as the PVG becomes the periaqueductal gray of the midbrain at the level of the posterior commissure without apparent cytoarchitectonic change (Fig. 2A3). In sagittal sections, the distribution of the periventricular TIP39 neurons has a sigmoid shape with a rostro-ventral to postero-dorsal orientation (John et al., 2002; Wang et al., 2006c) (Fig. 2B,C). These TIP39 neurons in the PVG are intermingled with tyrosine hydroxylase-containing neurons corresponding to the A11 dopaminergic cell group (Hökfelt et al., 1979; Skagerberg and Lindvall, 1985). Although the distributions of TIP39 and dopaminergic cell bodies largely overlap, the dopaminergic cells are situated somewhat more laterally than the TIP39 cells. Furthermore, no TIP39/tyrosine hydroxylase double-labeled cells are present (Dobolyi et al., 2003b; Wang et al., 2006c).
3.2.3 TIP39 neurons in the posterior intralaminar complex of the thalamus (PIL)
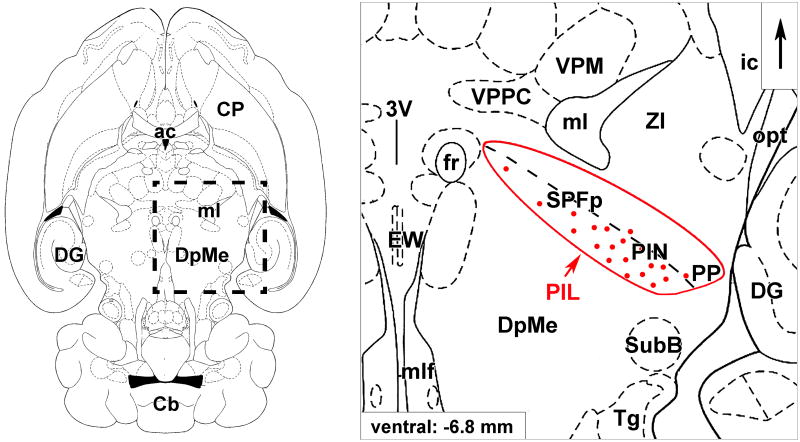
In the adult male rat, only about 200-300 relatively small, fusiform, and horizontally oriented TIP39 neurons are visible per side between bregma levels -4.2 to -6.1 mm in the lateral cell group of the posterior thalamus. However, the number of TIP39 neurons is markedly higher in this area during embryonic (Brenner et al., 2008) and early postnatal development (Dobolyi et al., 2006b) as described below. The laterally positioned TIP39 neurons constitute a horizontal cell line in an area over the medial lemniscus and further laterally as far as the area ventromedial to the medial geniculate body (Figs. 2D, 3). The area over the medial lemniscus corresponds to the parvicellular subparafascicular nucleus (SPFp) while the area ventromedial to the medial geniculate body includes the posterior intralaminar thalamic nucleus (Dobolyi et al., 2003b; Faber et al., 2007). In addition, some TIP39 neurons are situated below the lateral part of the medial lemniscus in the lateral territory of the caudal zona incerta between bregma levels -4.5 to 5.2 mm (Dobolyi et al., 2003b; Faber et al., 2007). A similar caudo-laterally elongated topographical arrangement has been described previously and referred to as the posterior intralaminar complex of the thalamus (PIL) (LeDoux et al., 1985). This term has recently been used to describe the position of the thalamic TIP39 neurons lateral to the fasciculus retroflexus (Brenner et al., 2008; Dobolyi et al., 2006a; Faber et al., 2007). In addition, some studies may have included the brain area where the lateralmost TIP39 neurons are located in a region designated the mesencephalon (Shimura and Shimokochi, 1991) or referred to as the ‘zona incerta/lateral tegmentum continuum’ (Maillard and Edwards, 1991).
Fig. 3.
The posterior intralaminar complex of the thalamus (PIL). Schematic drawings of a horizontal section 6.8 mm ventral to the surface of the brain (Paxinos and Watson, 1998) show the topographical position and the parts of the PIL. The framed area on the left is magnified on the right. Red dots represent the location of TIP39 neurons in the medial part of the PIL. Abbreviations: ac, anterior commissure; ic, internal capsule; Cb, cerebellum; CP, caudate-putamen; DG, dentate gyrus; DpMe, deep mesencephalic nucleus; EW, Edinger-Westphal nucleus; fr, fasciculus retroflexus; ml, medial lemniscus; mlf, medial longitudinal fasciculus; opt, optic tract; PIL, posterior intralaminar complex of the thalamus; PIN, posterior intralaminar thalamic nucleus; PP, peripeduncular nucleus; SPFp, parvicellular subparafascicular thalamic nucleus; SubB, subbrachial nucleus; Tg, tegmental nuclei; VPM, ventral posteromedial thalamic nucleus; VPPC, ventral posterior parvicellular thalamic nucleus; ZI, zona incerta; 3V, third ventricle.
The rostro-medial to caudo-lateral arrangement of TIP39 neurons resembles the distribution of calcitonin-gene related-peptide (CGRP)-containing neurons in the posterior thalamus. CGRP neurons are also situated in the parvicellular part of the subparafascicular nucleus, the posterior intralaminar thalamic nucleus extending as far laterally as the area around the medial geniculate body (Dobolyi et al., 2005; Ishida-Yamamoto and Tohyama, 1989; Kresse et al., 1995; Skofitsch and Jacobowitz, 1985). It can be recognized in horizontal sections that CGRP neurons constitute a contiguous arch of cells in the posterior thalamus (Dobolyi et al., 2005; Kruger et al., 1988), that has been referred to as the ‘CGRP nucleus’ of the thalamus (Kruger et al., 1988). The thalamic brain area corresponding to the region containing CGRP neurons around the medial geniculate body has also been unified by the term ‘posterior paralaminar thalamic nuclei’ due to their location adjacent to the internal medullary lamina, their similar auditory inputs and their pattern of projection to the cerebral cortex (Linke, 1999). In addition to the obvious similarities between the unique caudo-laterally extended distributions of TIP39 and CGRP in the posterior thalamus there are several significant differences. Although a few neurons are double labeled with TIP39 and CGRP in the lateral part of the posterior thalamus (Dobolyi et al., 2003b), TIP39 neurons typically are not labeled by a CGRP antibody and are located medial to the bulk of CGRP neurons in coronal sections (Brenner et al., 2008).
The parvicellular subparafascicular nucleus has been divided into medial and lateral subdivisions based on a dense population of galanin-immunoreactive fibers in the medial subdivision, and the presence of CGRP cells in the lateral subdivision (Coolen et al., 2003a). In addition, the medial subdivision demonstrates c-fos expression following male ejaculation (Coolen et al., 2004; Coolen et al., 2003a) while the lateral subdivision does not (Coolen et al., 2003a; D'Hanis et al., 2007), providing functional evidence for the compartmentalization of the area (Coolen et al., 2003a). Observations that TIP39 neurons are located predominantly medial to CGRP neurons (Brenner et al., 2008) and that they express c-fos in association with ejaculation, as described below (Wang et al., 2006a), suggests that parvicellular subparafascicular TIP39 neurons are located in the medial subdivision of the SPFp (Fig. 3). However, TIP39 and CGRP neurons extend further than the SPFp in the caudolateral direction while maintaining their medio-lateral separation suggesting that not only the SPFp but the whole PIL can be divided into medial and lateral parts, and that the TIP39 neurons are located in the medial (Fig. 3) while the ‘CGRP nucleus’ of the thalamus is in the lateral part of the PIL. Such division is also supported by the projection pattern of the TIP39 neurons in the PIL (discussed below), which is largely different from the striatal, amygdaloid, and perirhinal cortical projections of the thalamic CGRP neurons (Dobolyi et al., 2005; Inagaki et al., 1990; Yasui et al., 1991).
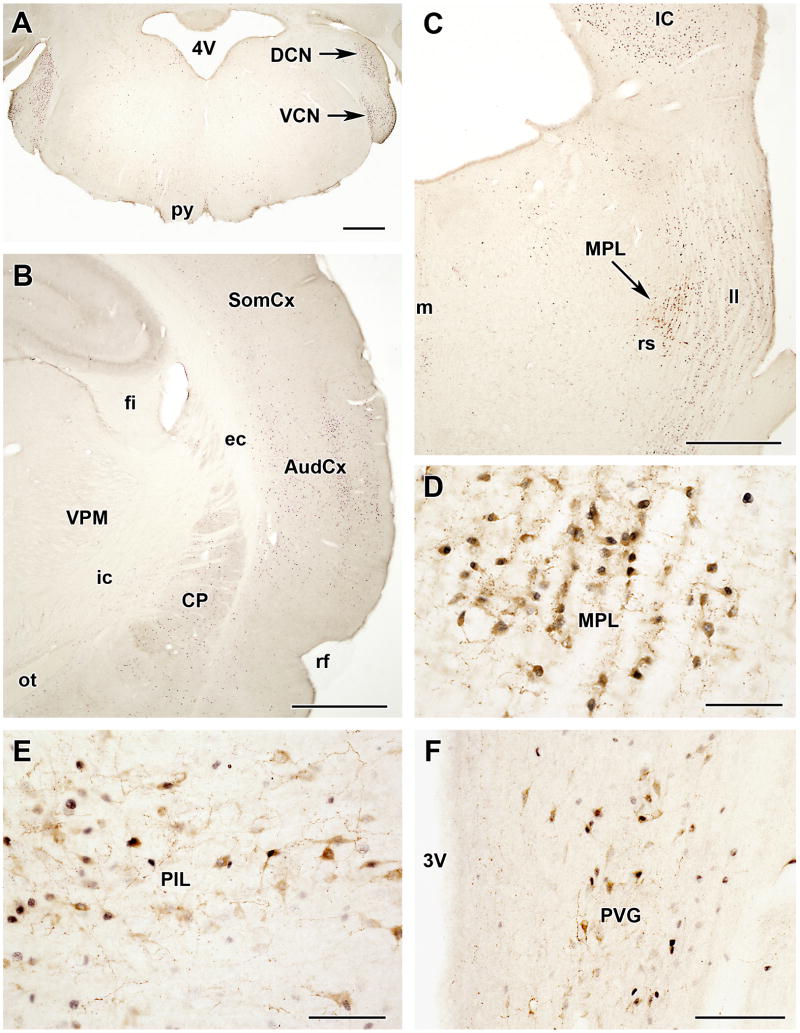
3.3 TIP39 cells in the medial paralemniscal nucleus (MPL)
There is a distinct group of TIP39-containing cell bodies is in the rostral pons between bregma levels -8.0 to 8.5 mm (Dobolyi et al., 2002; Faber et al., 2007; John et al., 2002), medial to the fibers of the lateral lemniscus, immediately dorsal to the rubrospinal tract and rostral to the Kölliker-Fuse nucleus (Fig. 4). When we initially mapped the distribution of TIP39 cell bodies in detail (Dobolyi et al., 2003b), we tentatively named the area of TIP39 expression immediately medial to the auditory relay nuclei of the lateral lemniscus as the medial paralemniscal nucleus (MPL). However, the identification of this nucleus has not been straightforward without labeling TIP39 (Varga et al., 2008). In the literature, often no distinction is made between oral reticular pontine and paralemniscal zones. Areas that include cells that correspond to the MPL have been referred to by a variety of anatomical names with poor topographical characterization, such as “lateral part of the nucleus reticularis pontis oralis” (Papez, 1926), “lateralmost nucleus reticularis pontis oralis” (Leichnetz et al., 1978), “ventrolateral tegmental area” (Herbert et al., 1997), or “dorsolateral pontomesencephalic reticular formation” (Haws et al., 1989). The term “paralemniscal” has also been used without detailed topographical characterization when describing an area in the “paralemniscal zone” whose stimulation elicited pinna movement in cats (Henkel and Edwards, 1978), a group of neurons whose activity changed in response to noxious stimuli in the “paralemniscal reticular formation” (Hardy et al., 1983), a group of neurons expressing c-fos in response to suckling in the “caudal portion of the paralemniscal nucleus” (Li et al., 1999), a group of “audiovocal” neurons in the “paralemniscal tegmentum” (Metzner, 1993), a group of neurons whose stimulation elicited vocalization in the “paralemniscal tegmental area” in bats (Fenzl and Schuller, 2007; Schuller and Radtke-Schuller, 1990), and the “ventral paralemniscal area” in squirrel monkey (Hage and Jurgens, 2006; Hannig and Jurgens, 2006). Furthermore, the existence of a cell group probably corresponding to the MPL described in the present study was mentioned in early studies (Fuse, 1926; Wünscher et al., 1965), and also more recently, as the “caudal part of the paralemniscal nucleus” (Andrezik and Beitz, 1985). However, the MPL is different from the paralemniscal nucleus described in a more rostral and lateral location (Olszewski and Baxter, 1982; Paxinos and Watson, 1998; Taber, 1961). The term “medial paralemniscal nucleus,” introduced originally as the location of TIP39 neurons (Dobolyi et al., 2003b), has been adopted by recent editions of the widely used Paxinos rat brain atlas (Paxinos and Watson, 2005).
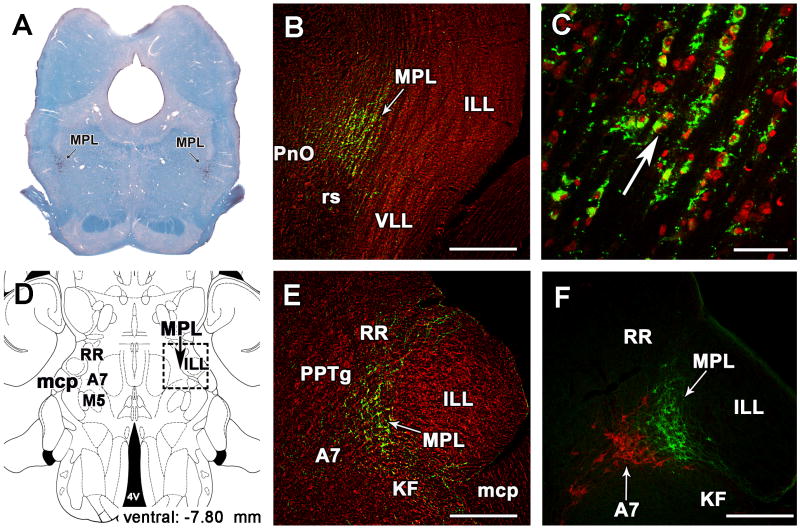
Fig. 4.
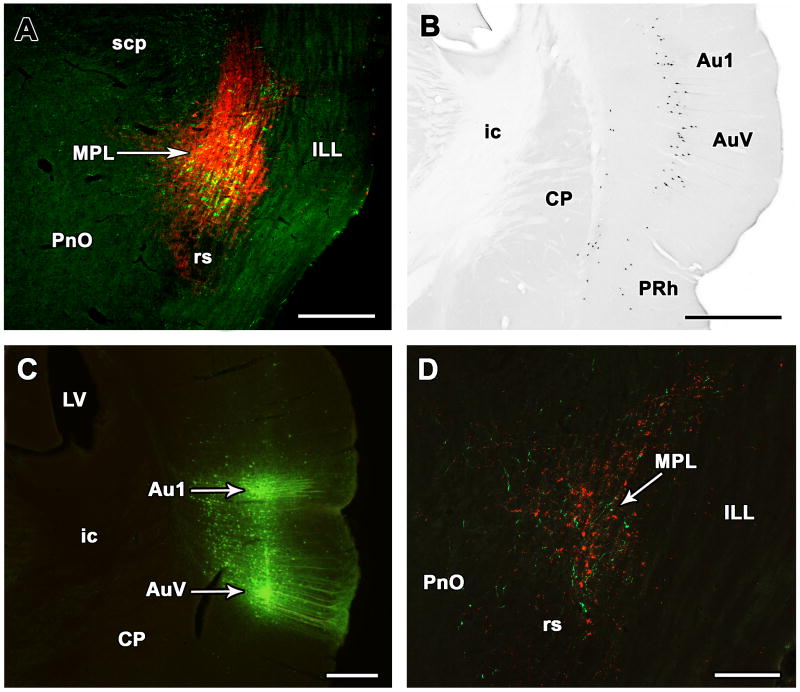
The medial paralemniscal nucleus (MPL) in the rat. A: The position of TIP39 neurons (black arrows) is shown in a coronal section stained with a combination of Luxol dye to visualize myelinated fibers in blue and cresyl-violet to label cell bodies. B: The MPL is delineated by the distribution of TIP39 neurons (green) in a section labeled with fluorescent Nissle dye (red) at 8.3 mm caudal to the bragma level. C: A high magnification photomicrograph demonstrates the dorsolaterally oriented columnar organization of the MPL. D: A schematic drawing of a horizontal section 7.8 mm ventral to the surface of the brain (Paxinos and Watson, 1998) shows the position of the MPL by a black arrow. E: A photomicrograph of a horizontal section corresponding to the position of the framed area in D demonstrated the position of the MPL by TIP39-ir (green). F: The same area in a double immunolabeled section shows the relation of TIP39 neurons (green) in the MPL and TH (red) neurons belonging to the A7 noradrenergic cell group. Panels B, C, and E are modifications of our previously published figures (Varga et al., 2008). Abbreviations: A7, A7 noradrenaline cell group; ILL, intermediate nucleus of the lateral lemniscus; KF, Kölliker-Fuse nucleus; mcp, middle cerebellar peduncle; MPL, medial paralemniscal nucleus; M5, motor nucleus of the trigeminal nerve; PnO, oral part of the pontine reticular nucleus; PPTg, pedunculopontine tegmental nucleus; RR, retrorubral nucleus; rs, rubrospinal tract; VLL, ventral nucleus of the lateral lemniscus; 4V, fourth ventricle. Scale bar = 500 μm for B, E, and F, and 100 μm for C.
Cells of the MPL are distinguished from those in adjacent areas by their organization into dorsolaterally oriented cell columns separated by 20–50-μm wide cell-free zones (Fig. 4C), probably occupied by fibers of the lateral lemniscus that pass through the region (Varga et al., 2008). The ventral border of the MPL is the rubrospinal tract, which is easily distinguished and clearly separated by the abrupt end of the cell columns (Fig. 4B). However, lateral to the rubrospinal tract, the MPL extends somewhat ventrally, which gives the nucleus a triangular shape with ventral, dorsal, and medial angles (Dobolyi et al., 2003b; Paxinos and Watson, 2005). In addition, in the rostral half of the MPL, a small group of large acetylcholinesterase-positive cells of the epirubrospinal nucleus (Paxinos and Butcher, 1985) is located between the medial part of the rubrospinal tract and the medial part of the MPL. The rostral part of the MPL is embedded between the pedunculopontine tegmental and the retrorubral nuclei, from which the MPL is separated by a zone of lower cell density (Fig. 4E). Medially, the MPL borders on the oral part of the pontine reticular formation and the pedunculopontine tegmental nucleus (Fig. 4B,E). The MPL narrows dorsally between the caudal part of the pedunculopontine tegmental nucleus and the dorsal nucleus of the lateral lemniscus, giving the nucleus a cone shape (Fig. 4B). The lateral border of the MPL is the intermediate nucleus of the lateral lemniscus (Fig. 4B). The caudal borders of the MPL are the region of the A7 noradrenaline cell group medially and the Kölliker-Fuse nucleus laterally (Fig. 4E,F). At present, the functional relevance of the topographical relationship between the TIP39 and these noradrenaline neurons is not known.
The MPL is distinguished from adjacent brainstem nuclei both by its cytoarchitecture and by its afferent connections (Varga et al., 2008). Morphologically, three cell populations can be distinguished within the MPL. About one-third of the cells are glial cells. There are about 300-600 TIP39 neurons per side in young adult rats and they account for about 75% of the neuronal population. Non-TIP39 neurons, constituting the third group, are somewhat larger than TIP39 neurons. The distributions of the three cell populations entirely overlap, and all of them participate in the formation of cell columns within the MPL. It is not clear whether the columnar cellular arrangement is an inherent property of the MPL or whether it results from the abundant fiber bundles passing through the MPL (Varga et al., 2008).
3.4 Functional implications derived from the location of TIP39 neurons
The brain areas that contain TIP39 neurons are not very well characterized functionally. However, the literature does suggest some functions, which based on their positions, TIP39 neurons might be involved in. Thus, we briefly outline the most important relevant functions.
3.4.1 Functional associations of the PVG
The PVG is a site of stimulation-induced analgesia (Peschanski and Mantyh, 1983; Rhodes and Liebeskind, 1978). Potent analgesia is obtained in rats following electrical stimulation in the gray matter surrounding the caudal portion of the third ventricle and the midline area of the caudal thalamus that is comparable to that produced by stimulation of the caudal periaqueductal gray. Analgesia outlasts the period of brain stimulation, and is not due to a generalized motor debilitation of the animal (Rhodes and Liebeskind, 1978). In addition, some neurons in the area are activated by noxious stimuli (Dong et al., 1978; Sugiyama et al., 1992). Cold exposure (10 °C), that does not necessarily cause a change in abdominal temperature, induced c-fos in some PVG neurons (Baffi and Palkovits, 2000; Kiyohara et al., 1995; Miyata et al., 1995). In contrast, warm ambient temperature (33 °C) did not induce c-fos expression in the PVG (Kiyohara et al., 1995). It has also been reported that the c-fos expression in the PVG significantly outlasts the cold exposure (Miyata et al., 1995), suggesting that it may have a role in the maintenance of homeostasis during adaptation to cold stress (Baffi and Palkovits, 2000).
3.4.2 Functional associations of the PIL
Studies using electrical stimulation (Shimura and Shimokochi, 1991), lesions (Maillard and Edwards, 1991), and mapping immediate early gene activation patterns (Coolen et al., 1997), implicate the area corresponding to the medial part of the PIL in sexual function. Reportedly, c-fos is expressed in the area following ejaculation, and earlier in the context of mating (Coolen et al., 1997, 1998; Sachs and Meisel, 1988; Veening and Coolen, 1998). Mating-activated neurons project to other regions that show c-fos expression with ejaculation, including the posterodorsal preoptic nucleus, the lateral part of the posterodorsal medial amygdala, and the medial cell group of the sexually dimorphic preoptic area (Coolen et al., 1997; Heeb and Yahr, 2001; Sachs and Meisel, 1988; Veening and Coolen, 1998). In rat, c-fos was induced in some TIP39 neurons following mating behavior suggesting that these cells may be involved in male sexual function (Wang et al., 2006a). Significant c-fos activation in TIP39 neurons took place in the SPFp as well as more lateral parts of the PIL. The number of dual labeled cells and the percentages of TIP39 cells in the PIL that express Fos, as well as the percentages of Fos-positive cells that co-expressed TIP39 significantly increased following one or two ejaculations, compared to animals that displayed only intromissions. Furthermore, males with two ejaculations had higher numbers of dual labeled cells compared to males with one ejaculation.
Based on its connections the posterior intralaminar complex of the thalamus was suggested to process auditory inputs to emotional brain centers, including the amygdala (LeDoux et al., 1990; Linke and Schwegler, 2000; Namura et al., 1997). Indeed, the area ventromedial to the medial geniculate body, which includes the posterior intralaminar thalamic nucleus, contains neurons in which c-fos is induced following high-intensity auditory stimuli (Burow et al., 2005; Campeau and Watson, 1997). Exposure to 30-min of 105 dB white noise induces c-fos in a large portion of the TIP39 neurons in the PIL, but not in the PVG TIP39 neurons (Palkovits et al., 2004, 2009a). Since the hypothalamic paraventricular nucleus receives projections from the PIL (Campeau and Watson, 2000; Palkovits et al., 2004) these findings suggest that TIP39 neurons in the PIL may be involved in mediation of audiogenic stress signals that reach the hypothalamus, and signals that contribute to acoustic fear conditioning that involve the amygdala (Palkovits et al., 2004).
3.4.3 Functional associations of the MPL
As part of the dorsolateral pontomesencephalic tegmentum, the MPL is implicated in brainstem pain-regulatory systems. Stimulation of this region elicits analgesia (Basbaum et al., 1977; Haws et al., 1989) and inhibits the response of spinothalamic tract cells to noxious stimuli (Girardot et al., 1987), and these effects are probably unrelated to the A7 noradrenergic cells (Zhao and Duggan, 1988). Furthermore, the firing rate of some paralemniscal neurons changes in response to noxious stimuli (Hardy et al., 1983).
The paralemniscal vocalization center of bats (Metzner, 1996) and squirrel monkeys (Hage and Jurgens, 2006; Hannig and Jurgens, 2006) occupies a position similar to the paralemniscal TIP39 neurons, medial to the intermediate nucleus of the lateral lemniscus. A group of “audio-vocal neurons” in this area responds to some auditory stimuli and their stimulation evokes vocalization (Fenzl and Schuller, 2007; Hage and Jurgens, 2006; Metzner, 1993; Schuller and Radtke-Schuller, 1990). In cats, stimulation of an area with a description similar to the MPL elicits pinna movement (Henkel and Edwards, 1978), its connections suggests that it conveys information from the superior colliculus to the motor facial nucleus (Henkel, 1981).
Another function in which MPL TIP39 neurons might be involved is the influence of pup exposure and suckling on lactation. Pup exposure and suckling induce c-fos expression in the bed nucleus of the stria terminalis, medial amygdala, lateral parabrachial nucleus, caudal part of the periaqueductal gray, and “caudal part of the paralemniscal nucleus immediately dorsolateral to the A7 cell group” (Li et al., 1999). This pattern would be consistent with activated TIP39 neurons activating other cells in their termination fields following a pup exposure and suckling stimulus.
3.5 Development of TIP39 neurons
There is good agreement on the pattern of TIP39 expression evaluated using in situ hybridization and quantitative RT-PCR for TIP39 mRNA and immunolabeling of TIP39 peptide during embryonic and postnatal development in rat (Brenner et al., 2008; Dobolyi et al., 2006b). As soon as TIP39 labeled neurons appear during development, their localization is similar to that in adult except for the amygdala, where TIP39 containing neurons appear transiently during embryonic development as described below.
In the posterior thalamus, there is a dramatic difference between the developmental expression of TIP39 by medially located periventricular and the laterally located posterior intralaminar neurons. TIP39 appears in and around the posterior intralaminar nucleus at embryonic day (ED)-14.5. The number of TIP39-ir neurons increases markedly by ED-16.5. At this age, TIP39-ir neurons occupy a large area in and around the posterior intralaminar thalamic nucleus including a few cells rostromedially in the parvicellular subparafascicular nucleus. Between ED-16.5 and postnatal day (PND)-5, a gradual decrease in the number of posterior intralaminar TIP39 neurons occurs, and the number of TIP39 neurons in the area remains low after that time point. The distribution of TIP39 neurons and the intensity of their immunolabeling are the same in male and female rats. TIP39 neurons in the posterior intralaminar thalamic nucleus elaborate TIP39-ir fibers by ED-18.5, which can be followed rostrally in the supraoptic decussations towards the hypothalamus. In contrast, TIP39 neurons in the PVG are first visible only on the first postnatal day (Brenner et al., 2008). In this region, the intensity of labeling as well as the number of TIP39 neurons increase substantially thereafter until PND-14. After that, TIP39 levels gradually decrease in the PVG, are markedly reduced by PND-125, and remain very low thereafter (Dobolyi et al., 2006b). The disappearance of TIP39 from cell bodies and fibers only means that TIP39 is not detectable in them, and provides no information regarding whether TIP39 neurons degenerate during development or whether their TIP39 expression decreases. At present, there are no independent markers for TIP39 neurons that would allow testing of these possibilities.
In the medial paralemniscal nucleus, TIP39-ir neurons first appear at ED-14.5 and the number of TIP39-ir neurons as well as the intensity of their labeling increases thereafter (Brenner et al., 2008). At PND-5, TIP39-ir fibers can be observed in the medial paralemniscal nucleus as they leave TIP39-ir cell bodies and project dorsally and ventrally. In contrast to the markedly different embryonic development, the postnatal peak and decrease in the intensity of TIP39 labeling in the MPL is similar to that in the PVG described above (Dobolyi et al., 2006b).
A group of TIP39 neurons appears in the amygdala at ED-16.5 (Brenner et al., 2008). About 100-200 neurons are located in the anterolateral subdivision of the amygdalo-hippocampal transitional zone dorsal to the posterior part of the cortical amygdaloid nucleus and lateral to the posterior part of the medial amygdaloid nucleus, with some cells located in the adjacent posterior subdivision of the basomedial amygdaloid nucleus. The intensity of TIP39 immunolabeling decreases after ED-16.5. By PND-1, TIP39 mRNA expression is low and only faintly immunolabeled TIP39 neurons are visible in the amygdalo-hippocampal transitional zone in both sexes and these neurons are not observed at any later stages of postnatal development, although this area establish moderate density of TIP39-ir fibers during adulthood (Dobolyi et al., 2003b). The posterior part of the amygdala is a relatively little studied brain region. Based on the expression of steroid receptors and its neuronal connections (Canteras et al., 1992), the amygdalo-hippocampal transitional zone has been suggested to play a role in conveying hormonal information toward reproductive brain centers (Simerly, 2002).
3.6 Sexual dimorphism of adult TIP39 expression
In situ hybridization histochemistry and immunocytochemistry reveal the same distribution of TIP39-expressing perikarya in male and female rats and mice (Dobolyi et al., 2006b; Faber et al., 2007) and there is no sex difference in TIP39 mRNA expression in young animals. However, as the level of TIP39 mRNA and peptide decreases with age, it becomes sexually dimorphic in the posterior thalamus and medial paralemniscal nucleus. TIP39 expression is significantly higher in aged female than in aged male rats but even the female levels are markedly decreased as compared to younger animals (Dobolyi et al., 2006b). This sex difference is not due to a particular estrus cycle stage of the female rats. Furthermore, the difference in TIP39 expression between mature adult males and females must not be directly related to their gonadal steroids levels because gonadectomy did not affect TIP39 levels when performed in the adult (Dobolyi et al., 2006b). In turn, gonadectomy performed before PND-24 partially reversed the decrease in TIP39 levels, suggesting that the decrease is related to sexual steroid hormonal effects occurring during the period of sexual maturation (Lamming, 1994). The sex difference in the depletion of TIP39 expression, on the other hand, was still present in gonadectomized animals, suggesting it is set up by events prior to PND-24.
4 Neuronal input to TIP39 neurons
Brain regions with projections to TIP39 neuron containing regions are shown in Fig. 5. These areas were identified following retrograde tracer injection into the regions where TIP39 neurons are concentrated. Double labeling of TIP39 and the anterograde tracer injected into areas that project toward TIP39 neurons, which allows identification of inputs that make close appositions with TIP39 neurons, has been performed so far for only two regions that project to the MPL, as described below.
Fig. 5.
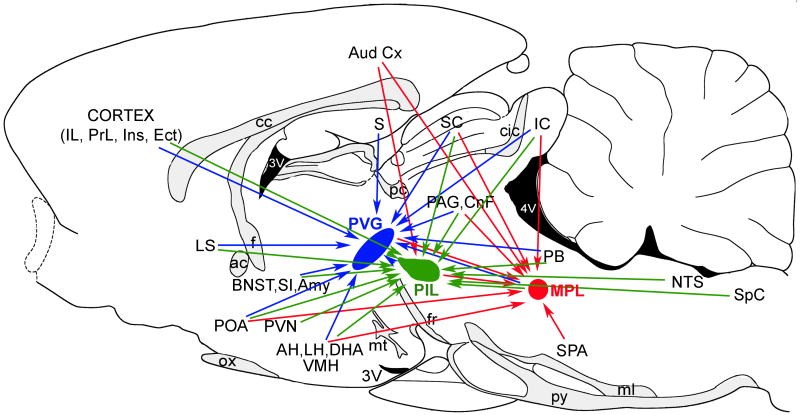
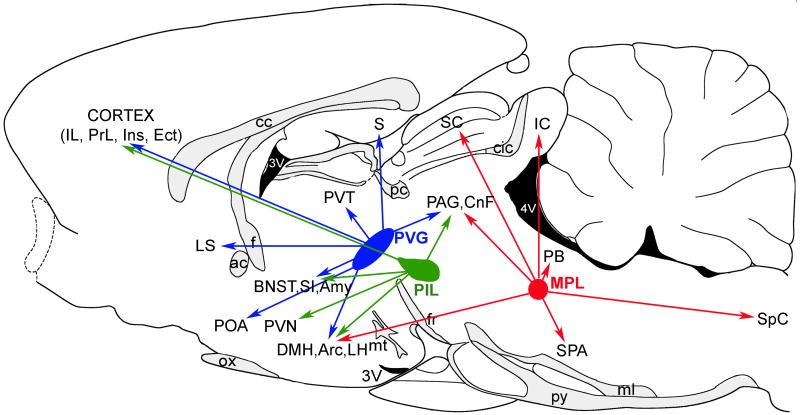
The summary of brain areas providing major neuronal inputs to TIP39-expressing brain regions. The PVG and its neuronal inputs are indicated by blue, the PIL and its inputs by green, and the MPL and its inputs by red arrows. Abbreviations: ac, anterior commissure; AH, anterior hypothalamic nucleus; Amy, amygala; Aud Cx, auditory cortex; BNST, bed nucleus of the stria terminalis; cc, corpus callosum; cic, commissure of the inferior colliculus; CnF, cuneiform nucleus; DHA, dorsal hypothalamic area; Ect, ectorhinal cortex; f, fornix; fr, fasciculus retroflexus; IC, inferior colliculus; IL, infralimbic cortex; Ins, insular cortex; LH, lateral hypothalamic area; LS, lateral septal nucleus; ml, medial lemniscus; MPL, medial paralemniscal nucleus, mt, mamillothalamic tract; NTS, nucleus of the solitary tract; ox, optic chiasm; PAG, periventricular gray; PB, parabrachial nuclei; pc, posterior commissure; PIL, posterior intralaminar complex of the thalamus; POA, preoptic area; PrL, prelimbic cortex; PVG, periventricular gray of the thalamus; PVN, hypothalamic paraventricular nucleus; py, pyramidal tract; S, subiculum; SC, superior colliculus; SI, substantia innominata; SPA, superior periolivary nuclei, Sp5, spinal trigeminal nucleus; VMH, hypothalamic ventromedial nucleus; 3V, third ventricle; 4V, fourth ventricle.
4.1 Neuronal input to TIP39 neurons in the PVG
Because of the elongated shape of the PVG, the inputs towards its rostral, middle and caudal parts have been investigated separately. A large number of brain regions provide significant input to the PVG (Fig. 5), and the projections to the different parts of the PVG are essentially the same (Wang et al., 2006b). Furthermore, the majority of the afferent connections are ipsilateral, with only a small number of retrogradely labeled cells present contralaterally following tracer injections into the PVG (Wang et al., 2006b). The forebrain regions that send significant projections to the PVG are the medial prefrontal, insular, and ectorhinal cortices, the subiculum, the anterior and medial amygdaloid nuclei, and the lateral septum. The rostral portion of the intermediate part of the lateral septal nucleus (also called the lateral septal nucleus, pars anterior) displayed the highest density of retrogradely labeled cells following tracer injections into the PVG. The majority of the projections to the PVG from the diencephalon arise from hypothalamic regions, including the ventromedial nucleus, which contains a very high density of retrogradely labeled cells, the preoptic area, the anteroventral periventricular nucleus, the lateral hypothalamic area, the anterior, posterior and dorsomedial hypothalamic, and the dorsal premamillary nuclei. Other diencephalic regions that project to the PVG include the zona incerta and the area ventromedial to the medial geniculate body where TIP39 expressing neurons of the PIL are located. In the brainstem, the periaqueductal gray projects to the PVG with its dorsolateral division containing a particularly high density of labeled cells throughout its rostro-caudal extent. In addition, deep layers of the superior colliculus, cortical areas of the inferior colliculus, the cuneiform nucleus, the medial paralemniscal nucleus, and the parabrachial nuclei also send significant projection to the PVG (Wang et al., 2006b). The afferent connections of the PVG are consistent with previous anterograde studies on projections to the area from auditory cortices (Arnault and Roger, 1990; Yasui et al., 1990), the anterior hypothalamic nucleus (Risold et al., 1994), the dorsal premamillary nucleus (Canteras et al., 1992), the zona incerta (Watanabe and Kawana, 1982), the area medial to the medial geniculate body (LeDoux et al., 1985), and the inferior colliculus (Arnault and Roger, 1990; Kudo et al., 1984; Yasui et al., 1990). The afferent connections of the PVG are markedly different from those of the midline and intralaminar thalamic nuclei including the parafascicular nucleus (Van der Werf et al., 2002). The extensive hypothalamic but limited thalamic and brainstem inputs imply that the PVG does not belong to this group of thalamic nuclei, as it has been suggested by previous reports (LeDoux et al., 1985; Rub et al., 2002). The PVG has a number of common afferent connections with the periaqueductal gray including insular, ectorhinal, and perirhinal cortical areas, the hypothalamus, the zona incerta, the superior and inferior colliculi, the nucleus cuneiformis, and the parabrachial nuclei (Beitz, 1982; Shipley et al., 1991).
4.2 Neuronal input to TIP39 neurons in the PIL
Although there are no data on the neuronal inputs of the posterior intralaminar thalamic nucleus, the afferent connections of the other part of the PIL, the SPFp have been described (Coolen et al., 2003b). Neurons in the medial prefrontal, insular and somatosensory cortex, the substantia innominata, the medial and lateral preoptic area, the hypothalamic paraventricular nucleus, the anterior amygdaloid area, the medial nucleus of the amygdala, the ventral tegmental area, the parabrachial nuclei, the locus coeruleus, the nucleus of the solitary tract, and the spinal trigeminal nucleus all project predominantly to the medial subdivision of the SPFp while the lateral subdivision receives specific inputs from auditory and visual brain regions (Coolen et al., 2003b). In addition, both subdivisions receive significant projections from the auditory cortex, the lateral septal nucleus, the anterior and hypothalamic ventromedial nuclei, the zona incerta, the periaqueductal gray, the deep layers of the superior colliculus, the cortical layers of the inferior colliculus, the cuneiform nucleus, and the pedunculopontine tegmental nucleus (Coolen et al., 2003b). The afferent connections of the medial subdivision of the SPFp are very similar to those of the PVG (Fig. 5). However, the medial subdivision of the SPFp but not the PVG receives dense projections from the somatosensory cortex, the hypothalamic paraventricular nucleus, the ventral tegmental area, the locus coeruleus, the nucleus of the solitary tract, and the spinal trigeminal nucleus. As described above, c-fos expression is induced in TIP39 neurons of the PIL in male rats following ejaculation but much less so following intromission without ejaculation. It suggests that these TIP39 neurons are part of the afferent circuits that process genital-somatosensory information related to ejaculation that contribute to mating and mating-induced changes in reproductive behaviors. The visceral, somatosensory, proprioceptive, and noxious information that can reach the medial subdivision of the SPFp (Coolen et al., 2003b) support the proposal that TIP39 neurons in the area are in position to integrate and transmit information related to copulation (Wang et al., 2006a). Finally, TIP39 neurons in the PIL can also be activated by high-intensity auditory input (Palkovits et al., 2004, 2009a), although the precise pathway for auditory information has not yet been defined.
4.3 Neuronal input to TIP39 neurons in the MPL
The MPL has afferent neuronal connections distinct from adjacent brain regions including major inputs from the auditory cortex (Fig. 6B), the medial part of the medial geniculate body, superior colliculus, external and dorsal cortices of the inferior colliculus, periolivary area, lateral preoptic area, hypothalamic ventromedial nucleus, lateral and dorsal hypothalamic areas, subparafascicular and posterior intralaminar thalamic nuclei, periaqueductal gray, and the cuneiform nucleus (Varga et al., 2008) (Fig. 5). In addition, injection of anterograde tracer into the auditory cortex and the hypothalamic ventromedial nucleus confirmed distinct projections from these areas to the MPL vs. the adjacent brainstem (Fig. 6C,D), which is in agreement with previous literature on projections of the hypothalamic ventromedial nucleus (Canteras et al., 1994) and the auditory cortex (Perales et al., 2006), respectively. Furthermore, the projections from these two regions appear to terminate on TIP39 neurons based on confocal microscopy of tracer/TIP39 double labeling (Varga et al., 2008).
Fig. 6.
Demonstration of projections from the auditory cortex to the MPL in the rat. A: Injection site of the retrograde tracer cholera toxin beta subunit (red) in the MPL identified by TIP39-ir neurons (green). B: Retrogradely labeled neurons are visible in layer V of the primary auditory cortex and the ventral secondary auditory cortex. A lower density of labeled cells are present in layers Vi and V of the perirhinal cortex and layer VI of the ventral secondary auditory cortex. C: BDA was injected into the primary and secondary auditory cortices along the same pipette tract. E: In the same brain, anterogradely labeled fibers (green) are distributed among TIP39-ir neurons (red) in the MPL. The panels are modifications of our previously published figures (Varga et al., 2008). Abbreviations: Au1, primary auditory cortex; AuV, ventral part of the secondary auditory cortex; CP, caudate-putamen; ic, internal capsule; ILL, intermediate nucleus of the lateral lemniscus; LV, lateral ventricle; MPL, medial paralemniscal nucleus; PnO, oral part of the pontine reticular nucleus; PRh, perirhinal cortex; rs, rubrospinal tract; scp, superior cerebellar peduncle. Scale bar = 500 μm for A, B, and C, and 300 μm for D.
Like the external cortex of the inferior colliculus and the lateral subdivision of the thalamic PIL described above, the MPL receives numerous projections from the auditory cortex (Herbert et al., 1991; Varga et al., 2008; Winer et al., 1998), demonstrates c-fos activation following loud noise (Campeau and Watson, 1997; Palkovits et al., 2004), and does not receive significant direct projections from lower brainstem auditory nuclei (Faye-Lund and Osen, 1985; Saldaña and Merchán, 2005; Varga et al., 2008). The massive collicular and cortical inputs have been demonstrated to contribute to the induction of c-fos in the external cortex of the inferior colliculus and PIL in response to high-intensity auditory stimuli (Burow et al., 2005; Sun et al., 2007). Therefore, collicular and cortical inputs are potentially the anatomical substrate for evoking c-fos activation in TIP39 neurons of the MPL, too (Palkovits et al., 2004). The strong interconnections of the MPL, the PIL and the external cortex of the inferior colliculus as well as their common non-tonotopic organization (Clopton et al., 1974) suggest that they may be involved in related neural functions. Potential functions include the integration of auditory and somatosensory information (Ledoux et al., 1987; Zhou and Shore, 2006), the modulation of vigilance and attention by auditory stimuli (Jane et al., 1965), mediating cortical contributions to filtering (Bregman, 1990) information such as the global location of a sound in space (Middlebrooks et al., 1994), regulating orientation behaviors, e.g. head and pinna movement and audio-vocal interfaces (Thompson, 1997), providing information to a map of auditory space (King et al., 1998), and to the coordination of responses to sound (Schuller et al., 1991), detecting novelty (Perez-Gonzalez et al., 2005) modulating the acoustic startle response (Yeomans et al., 2002), and processing auditory inputs to emotional brain centers e.g. for acoustic fear conditioning (LeDoux et al., 1990).
5 Projections of TIP39 neurons
Widespread TIP39 containing fibers originate from one of the three discrete groups of TIP39 neurons described above, in the PVG, PIL or MPL. Different techniques have been used to establish the projection patterns of individual groups of TIP39 neurons. First, the 3 brain regions containing TIP39 neurons were lesioned and disappearance of TIP39-containing fibers analyzed (Dobolyi et al., 2003a). A combination of targeted bilateral and unilateral lesions provided a great deal of information on the projection of individual TIP39 cell groups (Dobolyi et al., 2003a). Furthermore, anterograde tracers were injected into brain regions containing TIP39 cell bodies, particularly into the PVG (Wang et al., 2006c) and the PIL (Campeau and Watson, 2000). In addition, injections of retrograde tracers into brain regions containing TIP39 fiber terminals can provide information on the origin of projections even if more than one TIP39 cell groups project to the area or if the projections are bilateral. Unfortunately, only a limited amount of work has been performed with this method (Wang et al., 2006c).
5.1 Distribution of TIP39-containing fibers and fiber terminals
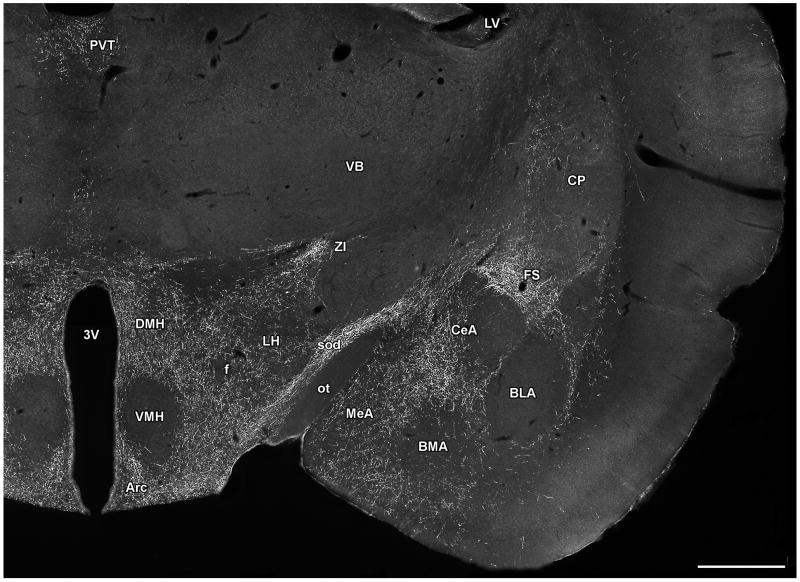
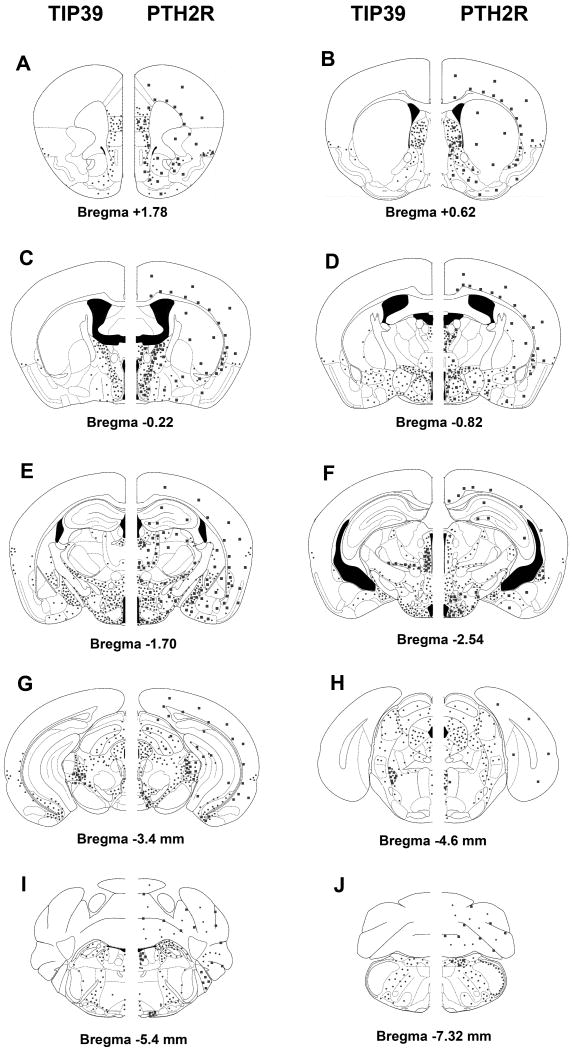
TIP39-imunoreactive fibers have been mapped in and carefully compared between the rat and mouse brain (Dobolyi et al., 2003b; Faber et al., 2007). The only difference observed was in the medial preoptic area, where the intensity of TIP39 fibers was somewhat higher in mice, so the following discussion is applicable to both species. The topographical distribution of TIP39-containing fibers is unique; there is no other neuropeptide or marker known to have a similar distribution. Brain regions containing high densities of TIP39 fibers can be grouped into auditory regions, endocrine/hypothalamic regions, limbic regions, and viscerosensory/nociceptive regions. Auditory regions where TIP39 fibers are abundant include the periolivary area, the medial nucleus of the trapezoid body, the external cortex of the inferior colliculus, deep layers of the superior colliculus, the medial nucleus of the medial geniculate body, and the area ventral to it, and the ectorhinal cortex. Endocrine/hypothalamic brain areas that have a high density of TIP39 fibers are the medial preoptic area, the hypothalamic paraventricular, periventricular, and dorsomedial nuclei (Fig. 7), the arcuate nucleus (Fig. 7), the lateral and dorsal hypothalamic areas (Fig. 7), and the premamillary nuclei. Additional limbic structures that contain a high density of TIP39 fibers include the medial prefrontal cortex (particularly the infralimbic cortex), the shell and cone portions of the nucleus accumbens, the lateral septal nucleus, the bed nucleus of the stria terminalis, the thalamic paraventricular nucleus (Fig. 7), the fundus striati (or amygdalo-striatal transitional zone), the ventral subiculum, and the central and medial amygdaloid nuclei (Fig. 7). Finally, TIP39 fibers are also abundant in some additional brain regions, which can loosely be considered viscerosensory/nociceptive including the lateral parabrachial nucleus, the anterior pretectal nucleus, and the periaqueductal gray. In addition, TIP39 fibers are also present in the dorsolateral funiculus of the spinal cord. Classification of the brain regions containing TIP39 fibers is of course arbitrary because most brain regions participate in multiple functions. In particular, the periaqueductal gray and the premamillary nuclei could easily be considered limbic structures, the central amygdaloid nucleus as viscerosensory, the thalamic paraventricular nucleus as nociceptive, and so on. Despite the obvious problems of grouping brain structures containing TIP39 fibers, it may help with the consideration of functions modulated by TIP39.
Fig. 7.
The localization of TIP39-ir fibers in the diencephalon, and the amygdala. The photomicrograph demonstrates the topographically organized distribution of TIP39-ir fibers in a coronal section of the rat brain at the level of the hypothalamic ventromedial nucleus. Abbreviations: Arc, arcuate nucleus; BLA, basolateral amygdaloid nucleus; BMA, basomedial amygdaloid nucleus; CeA, central amygdaloid nucleus; CP, caudate-putamen; DMH, hypothalamic dosromedial nucleus; f, fornix; FS, fundus striati; LH, lateral hypothalamic area; LV, lateral ventricle; MeA, medial amygdaloid nucleus; ot, optic tract; PVT, paraventricular thalamic nucleus; sod, supraoptic decussations; VB, ventrobasal thalamic nuclei; VMH, hypothalamic ventromedial nucleus; ZI, zona incerta; 3V, third ventricle. Scale bar = 1 mm.
5.2 Projections of PVG TIP39 neurons
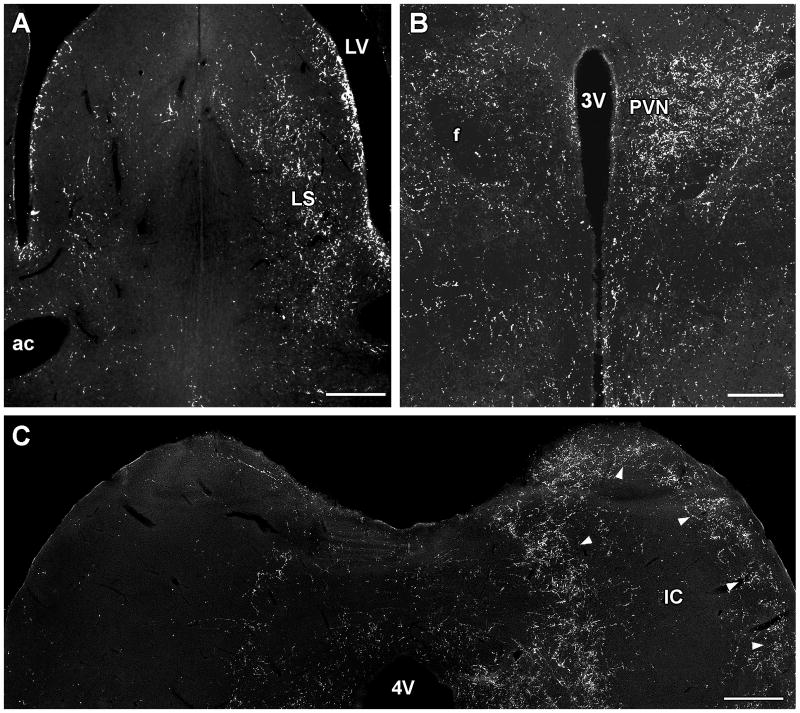
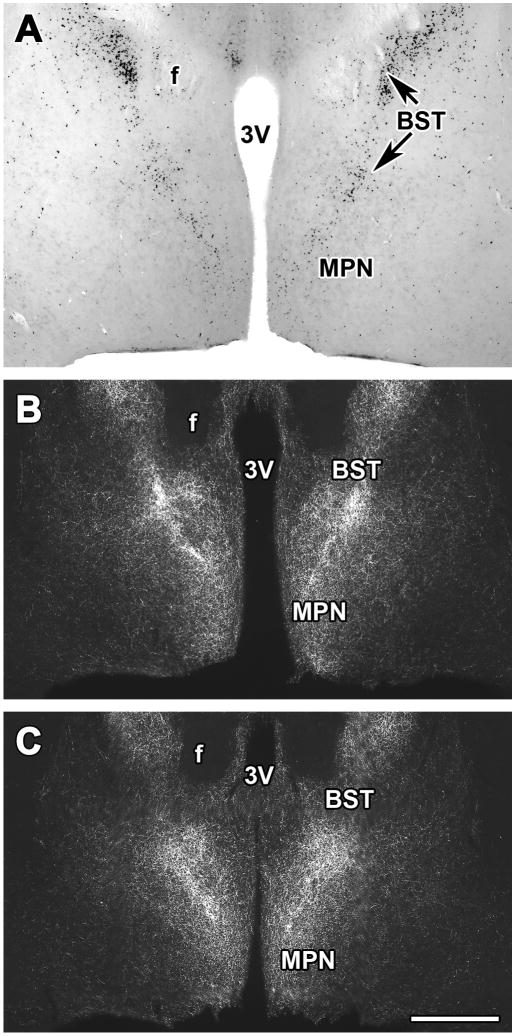
Following electrolytic lesions of the PVG, the density of TIP39 fibers decreased in the medial prefrontal cortex, the shell and cone portions of the nucleus accumbens, the lateral septum (Fig. 8A), the bed nucleus of the stria terminalis, the amygdaloid nuclei, the fundus striati, the ventral subiculum, the thalamic paraventricular nucleus, the hypothalamus, and the periaqueductal gray, suggesting that these regions receive projections from TIP39 neurons in the PVG (Dobolyi et al., 2003a). Unilateral lesions demonstrated that the projections are dominantly ipsilateral (Dobolyi et al., 2003a). Projections to these brain regions from the PVG were supported by anterograde tracer injection (biotinylated dextan amine; BDA) into the PVG (Wang et al., 2006c). Part of the trajectory of anterogradely labeled fibers could be followed from the PVG. A group of fibers leaves the area dorsolaterally, then after a hook-shaped turn they enter the epithalamus and run rostral-wards in the paraventricular nucleus of the thalamus. Some other fibers run rostrally from the nucleus, just dorsal to the medial lemniscus and terminate, most probably in thalamic nuclei. Another group, which comprises the most substantial number of fibers, joins the zona incerta and the supraoptic decussations and enters the basal forebrain/substantia innominata area. From here, the fibers divide into branches to limbic cortical, septal and amygdaloid areas (Wang et al., 2006c). The patterns of anterogradely labeled fibers following injections into rostral as well as caudal part of the PVG were similar to the distribution of TIP39-ir fibers in many areas of the brain, including the medial prefrontal and ectorhinal cortices, the shell part of the accumbens nucleus, the lateral septal nucleus, the bed nucleus of the stria terminalis, the central amygdaloid nucleus, the fundus striati, the paraventricular and posterior intralaminar nuclei of the thalamus, the medial preoptic area, the posterior hypothalamic nucleus, the zona incerta, and the periaqueductal gray (Wang et al., 2006c). Furthermore, these regions all contained fibers double-labeled with BDA and TIP39 supporting the projection of TIP39 fibers to these regions from the PVG. Retrograde tracer injections into the lateral septal nucleus and the fundus striati resulted in labeled TIP39 perikarya in the PVG but not in other regions containing TIP39 neurons (Wang et al., 2006c) suggesting that the PVG provides the only TIP39-containing input to these two brain regions. The percentage of retrogradely labeled TIP39 neurons in the PVG was high indicating that TIP39 neurons are a major PVG output neuron. Double labeled neurons were evenly distributed in the PVG suggesting that TIP39 neurons in the PVG constitute a single cell population (Wang et al., 2006c). In contrast, some of the hypothalamic areas including the anterior, paraventricular, arcuate and dorsomedial nuclei did not contain fibers double-labeled with TIP39 and BDA following PVG BDA injection. Most of these nuclei actually contained a higher density of TIP39-ir than BDA labeled fibers (Wang et al., 2006c). These data suggest that the major source of TIP39 fibers in most hypothalamic areas is not the PVG.
Fig. 8.
The disappearance of TIP39 fibers following lesioning TIP39 neurons in the rat. A: Unilateral lesions of the PVG resulted in the ipsilateral disappearance of TIP39 fibers in the lateral septal nucleus. B: The density of TIP39-ir fibers is markedly reduced in the hypothalamic paraventricular nucleus ipsilateral to a lesion in the PIL. C: The dense network of TIP39 fibers in the external cortex of the inferior colliculus disappears ipsilateral to a lesion of the MPL. Panels B, C, and E are modifications of our previously published figures (Dobolyi et al., 2003a). Abbreviations: ac, anterior commissure; f, fornix; IC, inferior colliculus; LS, lateral septal nucleus; LV, lateral ventricle; PVN, hypothalamic paraventricular nucleus; 3V, third ventricle; 4V, fourth ventricle. Scale bar = 400 μm for A, 200 μm for B, and 500 μm for C.
5.3 Projections of PIL TIP39 neurons
Following lesions of the area medial and ventromedial to the medial geniculate body, including the PIL, TIP39 fibers almost completely disappeared from the ipsilateral amygdala. A sharp reduction was observed in the density of TIP39 fibers in the ipsilateral hypothalamus particularly in the paraventricular (Fig. 8B) and dorsomedial nuclei. In addition, smaller, but visible reductions in the density of TIP39 fibers were observed ipsilateral to the lesion in many other forebrain regions including the medial prefrontal cortex, the nucleus accumbens, and the bed nucleus of the stria terminalis (Dobolyi et al., 2003a). Another study injecting anterograde tracer in an area likely to correspond to the region expressing TIP39 in the PIL described widespread neuronal projections in the rat brain (Campeau and Watson, 2000). The results confirmed previous retrograde studies on projections to the amygdaloid complex (Heeb and Yahr, 2001; LeDoux et al., 1985; Ottersen and Ben-Ari, 1979; Turner and Herkenham, 1991; Yasui et al., 1991), temporal cortex (LeDoux et al., 1985; Turner and Herkenham, 1991), preoptic area (Coolen et al., 1998; Heeb and Yahr, 2001; Simerly and Swanson, 1986), and the ventromedial nucleus of the hypothalamus (LeDoux et al., 1985) and extended these findings by showing fibers and putative terminals in several additional areas, including the lateral ventral and intermediate septal nuclei, several subdivisions of the bed nucleus of the stria terminalis and substantia innominata, and many hypothalamic structures including the preoptic area, the retrochiasmatic area, the paraventricular nucleus, the anterior, lateral, and dorsal hypothalamic areas, the dorsomedial nucleus, the tuber cinereum area (Campeau and Watson, 2000). In addition, retrograde studies confirmed projection from the PIL to the hypothalamic paraventricular nucleus (Campeau and Watson, 2000; Palkovits et al., 2004). Descending fibers from the SPFp have also been reported, including projections to the PVG, the periaqueductal gray, the external cortex of the inferior colliculus, and the superior olivary complex (Yasui et al., 1992). Comparing the studies using neuronal tracers and lesion, it is clear that TIP39 neurons in the PIL project to the amygdala and hypothalamus. They may also provide fibers to the bed nucleus of the stria terminalis and substantia innominata. Furthermore, TIP39 neurons in the PIL may also contribute to TIP39 fibers in the ectorhinal cortex, the periaqueductal gray, the external cortex of the inferior colliculus, and the periolivary area.
5.4 Projections of MPL TIP39 neurons
Following lesion of the MPL, there was an almost complete disappearance of TIP39 fibers from the deep layers of the superior colliculus, the external cortex of the inferior colliculus (Fig. 8C), the cuneiform nucleus, the lateral parabrachial nucleus, the medial nucleus of the trapezoid body, the periolivary area, as well as from the spinal cord, while a moderate density of TIP39-containing fibers remained in the periaqueductal gray (Dobolyi et al., 2003a). Unfortunately, anterograde injections into an area that can be reliably identified as the location of TIP39 neurons in the MPL have not been described. Consequently, the data are scarce regarding the topography of the projection of fibers from the MPL.
5.5 Interconnection of brain regions containing TIP39 neurons
Injection of retrograde tracer into the PVG revealed that cells from the PIL and the MPL, the other two brain regions that contain TIP39 neurons, project to the PVG (Wang et al., 2006b). Although the distribution of retrogradely labeled and TIP39 neurons largely overlapped, no double-labeled cells were observed, suggesting that non-TIP39 neurons project to the other TIP39 expressing brain regions (Wang et al., 2006b). Similarly, following injection of retrograde tracer into the MPL, the PVG and the PIL contained retrogradely labeled cells. The distribution of retrogradely labeled cells in both regions of the posterior thalamus was similar to that of TIP39 neurons in the area. However, cells double-labeled with the retrograde tracer and TIP39 were not observed, suggesting that non-TIP39 PVG and PIL neurons project to the MPL (Varga et al., 2008). In addition, following MPL injection a high density of retrogradely labeled cells was present in the contralateral MPL. These retrogradely labeled cells were also TIP39 negative (Varga et al., 2008). Apart from the mutual interconnection of the TIP39-expressing brain regions by non-TIP39 projections, another strong similarity is the massive input to all three areas from the hypothalamic ventromedial nucleus as well as from the significant inputs from the preoptic area, the periaqueductal gray, the deep layers of the superior colliculus, and the cortical layers of the inferior colliculus (Coolen et al., 2003b; Varga et al., 2008; Wang et al., 2006b). These finding suggest that TIP39 neurons in the three brain regions might participate in some common functions despite their apparently different projections.
5.6 Summary of the origin of TIP39 fibers in different brain regions
The major projections of the three groups of TIP39 neurons are summarized in Figure 9. Brain regions that receive TIP39 fibers predominantly from the PVG include the medial prefrontal cortex, the lateral septal nucleus, the bed nucleus of the stria terminalis, the thalamic paraventricular nucleus, the ventral subiculum, and the fundus striati. Brain regions that receive most of their TIP39 fibers from the PIL are the hypothalamus and the amygdala. TIP39-ir fibers that reach the hypothalamic nuclei from the PIL travel mainly through the supraoptic decussations and the zona incerta, but neurons in the PVG may also project via these two pathways (Palkovits et al., 2009b). Available evidence suggests that all three TIP39-expressing brain regions project to the arcuate nucleus, and that hypothalamic and amygdaloid regions receive a significant number of TIP39 fibers from the PIL as well as the PVG. The MPL provides most TIP39 fibers to brainstem regions including the deep layers of the superior colliculus, the external cortex of the inferior colliculus, the lateral parabrachial nucleus, the periolivary area, the medial nucleus of the trapezoid body, and the spinal cord. However, the external cortex of the inferior colliculus and the spinal cord receive projections from the PVG as well as the PIL, and some of these projections could be TIP39 fibers. Similarly, the superior periolivary area may also receive TIP39 fibers from the PIL. The periaqueductal gray receives a comparable amount of TIP39 fibers from the PVG and MPL, with a smaller potential input from the PIL.
Fig. 9.
The summary of the major projections of TIP39 neurons. The PVG and the projections of its TIP39 neurons are indicated by blue, the PIL and the projections of its TIP39 neurons by green, and the MPL and the projections of its TIP39 neurons by red arrows. Abbreviations: ac, anterior commissure; AH, anterior hypothalamic nucleus; Amy, amygdala; BNST, bed nucleus of the stria terminalis; cc, corpus callosum; cic, commissure of the inferior colliculus; CnF, cuneiform nucleus; DMH, hypothalamic dorsomedial nucleus; Ect, ectorhinal cortex; f, fornix; fr, fasciculus retroflexus; IC, inferior colliculus; IL, infralimbic cortex; Ins, insular cortex; LH, lateral hypothalamic area; LS, lateral septal nucleus; ml, medial lemniscus; MPL, medial paralemniscal nucleus; mt, mamillothalamic tract; ox, optic chiasm; PAG, periventricular gray; PB, parabrachial nuclei; pc, posterior commissure; PIL, posterior intralaminar complex of the thalamus; POA, preoptic area; PrL, prelimbic cortex; PVG, periventricular gray of the thalamus; PVN, hypothalamic paraventricular nucleus; PVT, thalamic paraventricular nucleus; py, pyramidal tract; S, subiculum; SC, superior colliculus; SI, substantia innominata; SPA, superior periolivary nuclei, SpC, spinal cord; VMH, hypothalamic ventromedial nucleus; 3V, third ventricle; 4V, fourth ventricle.
6 The PTH2R as the receptor for TIP39
Since previous reviews have summarized the identification of the PTH2R and compared it to the PTH1R (Hoare and Usdin, 2001; Usdin, 2000; Usdin et al., 2002; Usdin et al., 2000), in the present review, we only briefly describe the pharmacology of PTH receptors with an emphasis on the PTH2R, and focus on recent developments in the field including PTH receptors in zebrafish, signal transduction of PTH receptors, structure-activity studies involving TIP39, and the recent development of antagonists of the PTH2R.
6.1 PTH receptors
The PTH1R was identified by expression cloning in 1991 (Juppner et al., 1991). It is activated with equal potency by PTH and PTHrP and is also referred to as the PTH/PTHrP receptor. The parathyroid hormone 2 receptor (PTH2R) was identified on the basis of its sequence homology with other polypeptide-recognizing receptors following a screen that used PCR with degenerate primers (Usdin et al., 1995). The PTH receptors are seven transmembrane domain receptors belonging to the type II (or family B) class of G protein-coupled receptors (Usdin et al., 2002), membership in which is identified on the basis of sequence similarity, particularly within the transmembrane domains (Harmar, 2001). PTH receptors show the highest degree of sequence similarity to other hormone receptors belonging to the B1 receptor subfamily, which includes receptors for calcitonin and related peptides, corticotropin releasing hormone and related peptides, gastric inhibitory polypeptide, glucagon and related peptides, growth hormone releasing hormone, parathyroid hormone and related peptide, pituitary adenylate cyclase activating peptide, secretin, and vasoactive intestinal peptides. In addition, the PTH receptors have other features that are characteristic of the B1 receptor subfamily including a potential signal sequence and a hydrophilic extracellular domain in the amino terminus that contributes significantly to ligand binding (Harmar, 2001; Usdin et al., 1995). The overall amino acid sequence similarity of the PTH1R and PTH2R is 70% and they share at least 30% amino acid sequence similarity to other hormone receptors belonging to the B1receptor subfamily (Usdin et al., 1995).
Homologs of the mammalian PTH1R and PTH2R as well as a third PTH activated receptor have been identified in zebrafish (Rubin et al., 1999; Rubin and Juppner, 1999). The zebrafish PTH1R is pharmacologically similar to the mammalian PTH1Rs. Rat PTH and PTHrP activate it with very similar potency (Rubin et al., 1999). The zebrafish PTH2R is much like the rat PTH2R (Rubin et al., 1999) as described below. In contrast, the zebrafish PTH3R appears to be somewhat similar to the PTH1R based on its binding affinities and potent activation by rat PTHrP and PTH (Rubin and Juppner, 1999). However, the potency of human PTH is considerably less than that of rat PTH or PTHrP for activation of the zebrafish PTH3R (Hoare et al., 2000b). Thus, the ligand specificity of the zebrafish PTH3R does not clearly match any of PTH receptors that have been pharmacologically characterized in mammals and its signal transduction pathway differs from that of the zebrafish PTH1R (Rubin and Juppner, 1999) as described below. Endogenous ligands for the zebrafish PTH-like receptors have been reported. TIP39 homologues in fish (Papasani et al., 2004; Shoemaker et al., 2006) were described above. In addition, a PTHrP homologue has been identified in fugufish, and this peptide potently activates the zebrafish PTH1 and PTH3Rs (Rubin and Juppner, 1999) suggesting that a zebrafish PTHrP could normally act on these receptors. Two additional natural ligands, zPTH1 and zPTH2, were found by searching the zebrafish genomic database (Gensure et al., 2004). These genes are also present in the Japanese pufferfish genome (Gensure et al., 2004). Fish PTH1 and PTH2 have significant sequence homologies and similar genomic structures to mammalian PTHs (Gensure et al., 2004). In addition, they activate the human and zebrafish PTH1R. zPTH2(1–34) is, however, approximately 30-fold less potent at the zPTH1R than hPTH(1–34), hPTHrP(1–36), and zPTH1(1–34). When tested with zPTH3R, zPTH1(1–34) and hPTHrP(1–36) showed similar potencies, whereas the potency of zPTH2(1–34) was moderately reduced (Gensure et al., 2004). Overall, the PTH system appears more complex in fish than in mammals. However, fish do not have a parathyroid gland, and the functions of PTH and related polypeptides in fish remain to be elucidated (Gensure and Juppner, 2005).
6.2 Signal transduction pathways of the PTH receptors
One interesting functional characteristic of this family of G-protein coupled receptors is their potential to activate more than one second messenger cascade. Dual second messenger signaling has also been demonstrated for a number of receptors that are not within the secretin receptor family (Zhu et al., 1994), but the evidence that dual signaling occurs in native tissues is particularly strong for secretin family receptors and especially for the PTH1R (Chorev, 2002; Gensure et al., 2005). The activated PTH1R initiates a cascade of intracellular processes primarily by signaling through the α-subunit of the stimulatory G-protein, Gs (Schwindinger et al., 1998) which in turn leads to cAMP synthesis and the activation of protein kinase A (Segre et al., 1992). The PTH1R can also signal through Gq, thereby activating phospholipase C (Friedman et al., 1999), increasing intracellular inositol trisphosphate and intracellular calcium ion levels (Tanaka et al., 1995). In addition, PTH1R signaling through the mitogen-activated protein kinase pathway has been shown in several tissues (Chan et al., 2001; Miao et al., 2001). In fact, stimulation of the PTH1R may lead to the activation of the mitogen-activated protein kinase pathway by G protein-independent beta-arrestin signaling as well (Gesty-Palmer et al., 2006). The zebrafish PTH1R also activates multiple signal transduction pathways. However, a potentially significant difference between the zebrafish PTH1R and PTH3R is that activation of the PTH1R stimulates inositol phosphate release in addition to cAMP formation, while the PTH3R does not appear to be coupled to stimulation of phospholipase C (Rubin and Juppner, 1999).
Signal transduction via the PTH2R has also been studied, although in less detail. Activation of the PTH2R in stably transfected HEK-293 cell lines (Behar et al., 1996), and in COS-7 cells (Della Penna et al., 2003; Hoare et al., 1999; Hoare et al., 2000a; Usdin et al., 1995; Usdin et al., 1999b), results in the accumulation of cAMP. In addition, PTH also increases (Behar et al.)i in HEK293 cells (Behar et al., 1996; Della Penna et al., 2003) and in COS-7 cells (Goold et al., 2001) transfected with the hPTH2R. The TIP39 stimulated increase in [Ca2+]i, is typified by an initial rapid onset with a peak at about 20 s, followed by a rapidly declining secondary phase, and return to baseline level within 80 s (Della Penna et al., 2003; Goold et al., 2001). This increase in [Ca2+]i persists in a calcium-free medium suggesting that the increase in [Ca2+]i in response to TIP39 comes largely from intracellular stores. The potency of TIP39 is lower for increases of [Ca2+]i than for increases of cAMP suggesting that the PTH2R is more weakly coupled to increasing [Ca2+]i than to increasing cAMP accumulation (Goold et al., 2001). In addition, the signal transduction pathway of the PTH2R depends on the agonist. Although the human PTH2R can be activated both by TIP39 and PTH (as described below), the resulting cAMP and intracellular Ca signaling is different (Bisello et al., 2004). PTH stimulation of cAMP formation is brief and rapidly resensitizes, the response to TIP39 is sustained and remains partly desensitized for a prolonged period. Furthermore, TIP39 induces beta-arrestin and protein kinase C-beta mobilization, PTH2R desensitization, and receptor internalization whereas PTH does not (Bisello et al., 2004). The effect of PTH on the PTH2R is also different from that on the PTH1R, where PTH promotes phospholipase C/PKC activation, arrestin trafficking, receptor endocytosis, and desensitization of both Gs- and Gq-signaling (Castro et al., 2002; Dicker et al., 1999; Ferrari et al., 1999; Ferrari and Bisello, 2001). These findings suggest that PTH and TIP39 may play distinct physiological activities through the human PTH2R. However, the rat PTH2R is poorly activated by PTH (Hoare et al., 1999) and it is not clear whether PTH reaches brain PTH2Rs, so PTH may not be a physiological agonist of the PTH2R.
6.3 TIP39 interaction with PTH receptors
TIP39 was purified on the basis of its stimulation of cAMP accumulation in HEK293 cells transfected with the PTH2R (Usdin et al., 1999b). Subsequent experiments demonstrated that TIP39 is a high potency specific ligand for the PTH2R. Human (identical to bovine) TIP39 (hTIP39) had essentially no activity at the human or rat PTH1R expressed in COS-7 or HEK293 cells (Hansen et al., 2002; Usdin et al., 1999b) Furthermore, while hTIP39 is a potent agonist of the zebrafish PTH2R, it produced no activation of the zebrafish PTH1R or PTH3R. Nevertheless, hTIP39 was found to bind the human PTH1R receptor with moderate affinity in PTH1R expressing LLCPK1 (Jonsson et al., 2001), and COS-7 cells (Hoare et al., 2000b) as well as in isolated membranes prepared from transfected COS-7 cells (Hoare et al., 2000a). In addition, hTIP39 has a moderate affinity for the zebrafish PTH3R approximately equal to that at the human PTH1R (Hoare et al., 2000b). Based on the binding of TIP39 to the PTH1R, the possibility that it may be an endogenous antagonist has been raised (Jonsson et al., 2001), but this does not seem likely because of the relatively low affinity binding of TIP39 to the PTH1R and their different tissue localizations.
6.4 Agonist pharmacology of the PTH2R
Comparison of the effect of human (identical to bovine) TIP39 (hTIP39), rat (identical to mouse) TIP39 (rTIP39), hPTH(1-34), rPTH(1-34), and hPTHrP on cAMP formation, as well as on intracellular calcium levels in cell lines stably expressing the hPTH2R or the rPTH2R (Goold et al., 2001) is shown in Table 1. hTIP39 at 1 μm concentration evoked a robust response in both assays and both receptors. hPTH(1–34) has a greater EC50 and smaller maximal effect than hTIP39 at the hPTH2R, making it a weak partial agonist, and it has almost no effect at the rPTH2R. rPTH(1–34) was also less potent than hTIP39 but was a near-full agonist at the hPTH2R, while also having almost no effect at the rPTH2R. Human and rat TIP39 have similar potency and efficacy at the rat and human PTH2Rs (Goold et al., 2001). In addition, PTHrP(1–34) did not activate either the human or rat PTH2Rs and PTH displayed a lower efficacy for increasing intracellular calcium levels via the hPTH2R than for the elevation of cAMP accumulation (Della Penna et al., 2003; Goold et al., 2001). Pharmacological data from several studies and several groups of investigators using various combinations of intracellular calcium and cAMP assays, and receptors and cell lines, is consistent (Della Penna et al., 2003; Goold et al., 2001; Hoare et al., 1999; Hoare et al., 2000a; John et al., 2002; Usdin et al., 1999b). Similar to mammalian PTH2Rs, the zebrafish PTH2R (zPTH2R) is potently activated by zTIP39 as well as by hTIP39 while rPTH and hPTH are only partial agonists of the zPTH2R (Hoare et al., 2000b; Papasani et al., 2004).
Table 1.
Agonist pharmacology of human and rat PTH2 receptors expressed in COS-7 cells.
| agonist peptides | hPTH2R | rPTH2R | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| [Ca2+]i | cAMP | binding | [Ca2+]i | cAMP | binding | |||||
| EC50, nM | Emax, % of hTIP39 | EC50, nM | Emax, % of hTIP39 | IC50, nM | EC50, nM | Emax, % of hTIP39 | EC50, nM | Emax, % of hTIP39 | IC50, nM | |
| hTIP39 | 3.8 | 100 | 0.6 | 100 | 26 | 15 | 100 | 1.5 | 100 | 91 |
| mTIP39 | 1.8 | 108±7 | 0.5 | 97±6 | 1.5 | 2.3 | 113±6 | 0.5 | 89±6 | 7.5 |
| hPTH(1-34) | 22 | 44±6 | 1.7 | 99±13 | 18 | 4±8 | 93 | 32±2 | 450 | |
| rPTH(1-34) | 16 | 81±7 | 0.2 | 118±13 | 3.6 | 7±8 | 37 | 38±5 | 160 | |
| PTHrP(1-34) | 5±9 | 850 | 12±2 | 5100 | ||||||
Agonist concentration dependence relationships were measured as the increase in intracellular calcium summed over a 60-s time course, cAMP accumulation measured after 40-min incubations with the ligands. Receptor binding was determined by displacement of 125I-TIP39 binding. The table is a slight modification of our previously published table (Goold et al., 2001).
Ligand binding to human and rat PTH2Rs is consistent with the functional assays. hTIP39 and rTIP39 have slightly higher affinity for displacement of 125I-hTIP39 binding to COS-7 cells expressing the hPTH2R than to cells expressing the rat receptor, and rTIP39 has slightly higher affinity than hTIP39 for both PTH2Rs (Goold et al., 2001). The hPTH2R has somewhat greater selectivity for rPTH(1-34) over hPTH(1–34) and both ligands are significantly weaker at the rPTH2R (Table 1).
6.5 Molecular determinants of ligand-receptor interaction for the PTH2R
Structure–activity studies have generated a model for the interaction of PTH(1–34) and PTHrP(1–34) with the PTH1 receptor: contact of N-terminal residues of either peptide with the juxtamembrane ‘body’ of the PTH1R is responsible for receptor activation, and interactions of C-terminal peptide sequences with the extracellular N-terminus of the receptor contribute substantially to high-affinity binding (Mannstadt et al., 1999). Discrimination between PTH and PTHrP by the hPTH2R has allowed function to be assigned to individual peptide residues. Replacement of residue 23 of PTH with residue 23 of PTHrP prevents binding of PTH to the hPTH2R, whereas transfer of residue 5 from PTHrP to PTH limits the efficacy of PTH (Gardella et al., 1996). Corresponding modifications of PTHrP facilitate its binding to, or activation of, the PTH2R (Gardella et al., 1996). These data support the N-terminal-activation, C-terminal binding model and have extended it to the PTH2R. Consistent with this model, a chimeric receptor composed of the N-terminal extracellular domain of the PTH1R and the remainder (juxtamembrane domain) of the PTH2R was fully activated by TIP39 (Hoare et al., 2000a). This receptor chimera bound TIP39 with an equivalent affinity to the wild-type PTH2R. The reciprocal chimeric receptor was not activated by TIP39 and bound the ligand with an affinity equivalent to that of the PTH1R (Hoare et al., 2000a). Thus, the juxtamembrane receptor domain specifies the signaling and binding selectivity of TIP39 for the PTH2R over the PTH1R. Removing six N-terminal residues of TIP39 eliminated activation of the PTH2R and reduced the binding affinity 70-fold. In contrast, this truncation increased affinity for the PTH1R 10-fold, reversing the PTH2/PTH1R binding selectivity and resulting in a high affinity interaction of TIP(7–39) with the PTH1R (Goold et al., 2001; Hoare et al., 2000a; Jonsson et al., 2001). These findings can be explained by a strong interaction between the N-terminal region of TIP39 and the juxtamembrane domain of the PTH2R, with the corresponding domain of the PTH1R acting as a selectivity barrier against high affinity binding of TIP39. These data are also consistent with the finding that residues that are the same in TIP39, PTH(1–34) and PTHrP(1–36) are within the carboxyl part of these peptides, the region that is responsible for high-affinity receptor binding, as opposed to the N-termini, which are crucial for receptor activation (Hoare and Usdin, 2001; Juppner et al., 1991).
6.6 Antagonists of the PTH2R
Deletion of residues from the amino terminus of PTH or PTHrP reduces their ability to activate the PTH1 receptor while having relatively small effects on their binding affinity. PTH(7-34), PTHrP(7-36), and several analogs of these peptides are PTH1R antagonists (Gardella et al., 1996). Similar modifications of TIP39 were produced in attempts to generate a PTH2R antagonist. Removing amino terminal residues from TIP39 did reduce its ability to activate the PTH2R, but it also reduced its affinity for the PTH2R such that a peptide with no detectable agonist activity, TIP(7-39), had too low an affinity to be useful as a PTH2R antagonist (Hoare et al., 2000a). Surprisingly, removing amino terminus residues from TIP39 increased its affinity for the PTH1R, and TIP(7-39) (Hoare and Usdin, 2000) and TIP(9-39) (Jonsson et al., 2001) are potent PTH1R antagonists.
In a further attempt to develop a PTH2R antagonist, the effect of changing some of the N-terminal residues to ones with different charge or bulk was examined (Kuo and Usdin, 2007). Several potent and selective PTH2R antagonists were developed. One, His4, Tyr5, Trp6, His7-TIP39 (HYWH-TIP39), is particularly promising based on its potency and selectivity. In cultured cells HYWH-TIP39 completely blocks activation of the rPTH2R by TIP39 and has over 30-fold selectivity for the rPTH2R over the rPTH1R (Kuo and Usdin, 2007). The major potential use for HYWH-TIP39 is to facilitate the investigation of TIP39's physiological role by using it within in vivo experiments. Although its refractoriness to peptidase inactivation and its penetration through the blood brain barrier are not known, it is likely to be a useful reagent for investigation of the TIP39-PTH2R system when delivered through intracerebral cannulae.
A novel technology for monitoring the changes in cAMP levels in live cells has recently been applied for screening small-molecule antagonists of the PTH2R (Visegrady et al., 2007). Cyclic nucleotide-gated channels as biosensors were coexpressed with the PTH2R. The technique allowed the measurement of cAMP-dependent calcium influx or membrane depolarization with conventional fluorescent methods in both kinetic and in endpoint modes for high-throughput and subsequent compound screening (Visegrady et al., 2007). This high throughput screen has identified a potential lead compound with potential utility as PTH2R ligands (Visegrady et al., 2007).
7 PTH2R expressing cell groups
The PTH1R is most abundant in kidney and bone where PTH exerts its calcium regulatory functions and it is also present several other organs (Hurwitz, 1996; Rizzoli et al., 1992). In contrast, the PTH2R is not highly expressed in kidney or bone, and has a limited expression pattern in peripheral organs (Usdin et al., 1996; Usdin et al., 1999a). The PTH2R is most abundantly expressed in the central nervous system (Usdin et al., 1995) where it has a more widespread and profoundly different expression pattern (Faber et al., 2007; Wang et al., 2000) than the PTH1R whose mRNA has been demonstrated in the anterodorsal nucleus of the thalamus, the basolateral amygdala, entorhinal cortex, parasubiculum, the mesencephalic nucleus of the trigeminal nerve, the motor nucleus of the trigeminal and the facial nerve, the lateral reticular, pontine and reticulotegmental nuclei, vestibular nuclei, the ventral cochlear nucleus, the hypoglossal nucleus and the area postrema, Purkinje cells, and the Gasserian ganglion (Weaver et al., 1995).
7.1 Peripheral distribution and actions of TIP39 and the PTH2R
As evaluated on Northern blots PTH2R mRNA is most abundant in the brain and is detected in the testis and pancreas (Usdin et al., 1995). In situ hybridization histochemistry (Usdin et al., 1996) and immunohistochemistry (Usdin et al., 1999a) demonstrate expression of the PTH2R in arterial endothelium, which is most clear in large vessels in bronchi and the parenchyma in the lung, in cardiac endothelium, in a small number of cells associated with the vascular pole of renal glomeruli, in the pancreas, in spermatids in the head of the epididymis, in atretic follicles of the ovary, in chondrocytes in thyroid cartilage, in a small number of cells in bone, and in some endocrine cells including thyroid parafollicular cells, and some gastrointestinal peptide synthesizing cells. Since TIP39 has not been reported to be present in the blood, these receptors might be activated by locally produced TIP39. Existing data on the function of PTH2R's in peripheral organs is only briefly summarized, as most of them are likely independent of the neural functions of the TIP39-PTH2R neuromodulator system.
In testis, TIP39 and the PTH2R are expressed in a stage-specific manner within seminiferous tubules (Usdin et al., 2008) and TIP39 expression is greatest in mature testes. Mice with deletion of the gene encoding TIP39 are sterile (Usdin et al., 2008). Testes contained Leydig and Sertoli cells and spermatogonia but no spermatids, because spermatogonia did not complete prophase of meiosis I. In addition, in mice expressing Cre recombinase under control of a spermatid-specific promoter to selectively induce TIP39 expression in the testes of TIP39-/- mice, spermatid production and fertility were rescued. These data demonstrate that TIP39 is essential for germ cell development and suggest that it may act as an autocrine or paracrine agent within the gonads (Usdin et al., 2008).
TIP39 and the PTH2R are also implicated in the regulation of renal hemodynamics. TIP39 and PTH2R mRNA are expressed in rat intrarenal arteries as well as in renovascular smooth muscle cells cultured from these arteries (Eichinger et al., 2002). TIP39 has complex vasoactivity. At 10 nM it constricts and at 1, 100, and 1000 nM it dilates isolated perfused rat kidneys that have been desensitized to the vasodilatory action of PTHrP (1-36).
In addition to the PTH2R expression in the heart described above, TIP39 mRNA is also constitutively expressed in coronary endothelium, isolated cardiomyocytes, in the wall of the ventricles and atria, and in the aorta (Ross et al., 2005). Inotropic actions of TIP39 are observed in Langendorff perfused rat hearts (Ross et al., 2005). TIP39 influenced the contractility of the rat heart via the PTH2R in two different and contrary ways: a positive inotropism mediated by activation of the nitric oxide signaling pathway and a negative inotropism via a yet unknown pathway (Ross et al., 2005). Furthermore, there appears to be an interaction between PTH1R and PTH2R (Ross et al., 2007). Ischemia-dependent endogenously released PTHrP desensitizes the PTH1R, which enables activation of the PTH2R by TIP39. PTH2R activation dilates vessels in a nitrogen oxide-dependent manner (Ross et al., 2007). In contrast to these data, intravenous TIP39 had no effect on blood pressure or heart rate in anesthetized mice and no differences were observed between wild-type and TIP39 knockout mice in a number of measures of cardiovascular function at baseline (Fegley et al., 2008).
A number of peripheral endocrine cells express PTH2Rs (Usdin et al., 1999a). In the thyroid gland PTH2Rs are not observed in the follicular cells but are clearly present on parafollicular C cells. PTH2R labeling partially overlaps somatostatin labeling of C cells demonstrating that only a subpopulation of parafollicular cells express the PTH2R. Expression by these calcitonin synthesizing cells potentially places the PTH2R in a calcium regulating pathway.
All pancreatic islet somatostatin synthesizing D cells are intensely labeled by a PTH2R selective antibody, and none of the other populations of islet cells have detectable immunostaining (Usdin et al., 1999a). This highly selective expression of PTH2Rs on pancreatic islet somatostatin cells suggests that it may modulate somatostatin synthesis or release and thus indirectly affect glucagon or insulin release.
7.2 Distribution of neurons expressing PTH2R in the nervous system
PTH2R expression in the nervous system has been investigated by a great variety of techniques. In situ hybridization histochemistry was used to directly visualize the presence of RNA in the rat nervous system (Wang et al., 2000), in the mouse brain (Faber et al., 2007), and in the macaque brainstem (Bagó et al., 2009) while RT-PCR indicated its expression in several regions of the human brain (Bagó et al., 2009). A mouse line has been developed in which the first coding exon of the PTH2R gene is replaced with the bacterial lacZ coding sequence (Faber et al., 2007). In these knockin mice beta-galactosidase (coded for by the lacZ gene) activity can be detected histochemically in cells in which the PTH2R promoter is active (Faber et al., 2007). The two different methods indicated the same distribution for PTH2R-expressing cells in virtually all brain areas, and this distribution was the same in male and female mice (Faber et al., 2007). In contrast, immunolabeling of PTH2R cell bodies in mouse and human was not reliable. Few and only lightly labeled cell bodies were found, if any at all, in most brain regions (Bagó et al., 2009; Faber et al., 2007). In contrast, the immunolabeling of PTH2R neurons was relatively satisfactory in the rat as demonstrated by the same distribution of the PTH2R in situ hybridization signal and the PTH2R-ir cell bodies in most brain regions (Wang et al., 2000). A potential explanation for this finding may be that the transport of PTH2R protein out of cell bodies and into neuronal fibers is faster in mice and human than in rats. The distribution of PTH2R-expressing neurons in the mouse brain obtained by in situ hybridization as well by beta-galactosidase histochemistry in the PTH2R-lacZ knockin line, and a comparison with the distribution of PTH2R neurons in mice to that in other species is shown in Fig. 10.
Fig. 10.
Schematic diagrams demonstrate the distribution of TIP39 (left side) and the PTH2R (right side) in the brain of mice. The diagrams are modifications from a mouse stereotaxic atlas (Franklin and Paxinos, 1997). Dots represent fibers and fiber terminals, while squares represent cell bodies. The figure is a slight modification of our previously published figure (Faber et al., 2007).
In the cerebral cortex, PTH2R-expressing neurons are widely distributed with a density that varies somewhat between regions. In the limbic system, there are a few PTH2R-expressing neurons in the anterior olfactory nucleus and in the olfactory tubercle. In the septum, only the lateral nucleus contains PTH2R-expressing neurons. This region contains a very high density of PTH2R-expressing neurons, especially in its ventral part. More rostrally, the intermediate part of the lateral septal nucleus is also rich in PTH2R neurons. Most subdivisions of the bed nucleus of the stria terminalis contain a moderate density of PTH2R-expressing neurons, in contrast to the posteromedial part of the medial subdivision where the density is very high. Many PTH2R-expressing neurons are present in the amygdala. The density of PTH2R-expressing neurons is high in the medial nucleus, especially in its posterodorsal subdivision while it is moderate in the anterior amygdaloid area, the central and cortical amygdaloid nuclei, and the amygdala-hippocampal transitional zone. A low density of scattered PTH2R-expressing neurons is present throughout the hippocampal CA areas and a slightly higher in the dentate gyrus and the subiculum. The claustrum and the dorsal endopiriform nucleus contain a fairly large number of PTH2R-expressing neurons, while there is a moderate density of randomly distributed PTH2Rexpressing neurons throughout the accumbens nucleus, the caudate-putamen, the ventral striatum, and in the substantia innominata. The thalamus is relatively poor in PTH2R-expressing neurons except for a few regions. The medial subdivision of the medial geniculate body contains a very high density of PTH2R-expressing neurons, whereas it is moderate in the ventral subdivision. There is a high to moderate density of PTH2R-expressing neurons in some midline and intralaminar thalamic nuclei. In addition, a moderate density of PTH2R-expressing neurons is present in the epithalamic lateral habenular nucleus and the subthalamic zona incerta. Finally, beta-galactosidase histochemical reaction product is present in the dorsal part of the lateral geniculate nucleus of PTH2R knockin mice, but in situ hybridization for the PTH2R does not indicate PTH2R-expressing neurons in this brain area. PTH2R-expressing neurons are abundant in many regions of the hypothalamus. In the preoptic region, a high number of PTH2R-expressing neurons is present in the medial preoptic nucleus, whereas there is a low density of PTH2-expressing neurons in other parts of the medial preoptic area. In the anterior hypothalamic region, a moderate density of PTH2R-expressing neurons is present in the paraventricular and periventricular nuclei, while the anterior hypothalamic nucleus contains a low density of PTH2R-expressing neurons. In the middle portion of the hypothalamus, a high density of PTH2R-expressing neurons is present in the arcuate nucleus, whereas a moderate density is observed in the dorsomedial and perifornical hypothalamic nuclei, and some parts of the lateral hypothalamic area including the so-called far-lateral hypothalamus (Forel's field) immediately next to the internal capsule. In the posterior hypothalamus, a high density of PTH2R-expressing neurons is seen in the medial subdivision of the supramamillary nucleus while its lateral subdivision, and the ventral premamillary, and the tuberomamillary nuclei contain a moderate density of PTH2R-expressing neurons. In contrast, the medial and lateral nuclei of the mamillary body do not contain PTH2R-expressing neurons. There are relatively few PTH2R-expressing neurons in the lower brainstem and cerebellum. The midbrain contains a moderate density of PTH2R-expressing neurons in the lateral interpeduncular, paranigral, and median raphe nuclei, and there are a few scattered PTH2R-expressing neurons in the superior and inferior colliculi. In the pons, there is a moderate to high density of PTH2R-expressing neurons in the sphenoid nucleus of the tegmental area, and in the nucleus of the trapezoid body. In addition, beta-galactosidase (but not in situ hybridization signal) was present in the ventral cochlear nuclei. In the medulla oblongata, the nucleus of the solitary tract contains a moderate density, and the spinal trigeminal nucleus a low density of PTH2R-expressing neurons. A few scattered PTH2R-expressing neurons are present in the cerebellar cortex, with a somewhat higher density in the superficial portion of the molecular layer.
The distribution of PTH2R-expressing cell bodies in mice is similar to that of rats (Wang et al., 2000) in most brain regions, including the cerebral cortex, the caudate-putamen, the claustrum and the endopiriform nucleus, the lateral septum, the hippocampus, the amygdala, and many thalamic, hypothalamic, and brainstem regions, and the cerebellum. In some brain regions, however, marked differences were found in the density of PTH2R-expressing neurons between mice and rats. Most particularly, a strong in situ hybridization signal but no more than a few immunoreactive cell bodies are found in the medial septal, the ventromedial hypothalamic, the parabrachial, and the spinal trigeminal nuclei in rats (Wang et al., 2000). None of these regions contain a significant number of PTH2R-expressing cells in mice. In addition, both in situ hybridization histochemistry and immunocytochemistry reveal a high density of PH2R-expressing neurons in the periventricular hypothalamic nucleus in rat, while this area contains only a moderate number of PTH2R-expressing neurons in mice. In contrast, there are brain regions that are abundant in PTH2R-expressing neurons in mice but not in rats, including the posteromedial part of the medial division of the bed nucleus of the stria terminalis, the medial geniculate body, and the nucleus of the trapezoid body.
The overall distribution of PTH2R mRNA in human and macaque is similar to that in rodents (Bagó et al., 2009). A detailed mapping of PTH2R mRNA expression by in situ hybridization histochemistry has only been performed in the macaque and the regional and subregional distribution is very similar to that of rodents (Bagó et al., 2009). Nevertheless, there are two regions, namely the ventral part of the lateral hypothalamus and the parabigeminal nucleus where PTH2R-expressing cells formed compact cell groups in the macaque while only scattered labeled cells appear in rodents, suggesting a relatively small species difference (Bagó et al., 2009).
PTH2R expression in the spinal cord and sensory ganglia has only been described in the rat. In situ hybridization histochemistry shows numerous PTH2R-positive cells in the marginal layers of the dorsal horn and the lateral cervical nucleus, as well as in dorsal root ganglia, and a few labeled cells in the deep layers of the dorsal horn of the spinal cord (Dobolyi et al., 2002; Wang et al., 2000).
7.3 The distribution of PTH2R fibers and their topographical correlation with TIP39 fibers
PTH2R-immunoreactive fibers are widely distributed in the rat nervous system (Wang et al., 2000), the rat hypothalamus (Dobolyi et al., 2006a), the mouse brain (Faber et al., 2007), and in the human brainstem (Bagó et al., 2009). Strikingly, the distribution of PTH2R-containing fibers is very similar to the distribution of TIP39-containing fibers (see Fig. 10 and Fig. 11B,C, as an example). In addition, no difference was found between males and females in their distribution of PTH2R-containing or TIP39-containing fibers (Faber et al., 2007). The distribution of PTH2R-containing fibers in rat and mouse and, to the extent mapped, in human is almost identical. The few differences may be due to different immunolabeling techniques since no differences were found in the hypothalamus when the same amplification technique was applied (Dobolyi et al., 2006a; Faber et al., 2007). Therefore, we present the distributions of TIP39 and PTH2R fibers only in mice and compare them with each other as well as to the distribution of PTH2R-expressing neurons in Table 2.
Fig. 11.
Comparison of PTH2R-expressing neurons, PTH2R-, and TIP39-immunoreactivities in the preoptic region of mice. A: PTH2R-expressing neurons are present in the posteromedial part of the medial subdivision of the bed nucleus of the stria terminalis (arrows) and the medial preoptic nucleus, as demonstrated by X-gal histochemistry in PTH2R knockin mice. B: The distribution of PTH2R-ir fibers is similar to the distribution of PTH2R-expressing neurons. C: The distribution of TIP39-ir fibers looks exactly the same as the distribution of PTH2R-ir fibers in the preoptic region of mice. The panels are modifications of our previously published figures (Faber et al., 2007). Abbreviations: BST, bed nucleus of the stria terminalis; f, fornix; MPN, medial preoptic nucleus; 3V, third ventricle. Scale bar = 500 μm.
Table 2.
Location of PTH2R perikarya and fibers, as well as TIP39 fibers in mice brain.
| Area | PTH2Rcell bodies | PTH2R-ir fibers | TIP39-ir fibers |
|---|---|---|---|
| Forebrain Cerebral cortex | |||
| Medial prefrontal cx. | + | ++ | ++ |
| Somatomotor cortex | + | 0 | 0 |
| Somatosensory cortex | + | 0 | 0 |
| Insular cortex | ++ | + | + |
| Ectorhinal cortex | + | + | ++ |
| Temporal cortex | + | 0 | 0 |
| Hippocampus | + | 0 | 0 |
| Subiculum | + | + | ++ |
| Septum | |||
| Medial septal nucleus | 0 | 0 | 0 |
| Lateral septal nucleus | +++ | +++ | +++ |
| Bed nu. stria terminalis | + | ++ | +++ |
| Amygdala | |||
| “Fundus striati” | 0 | + | ++ |
| Central nucleus | ++ | ++ | ++ |
| Basal nuclei | + | 0 | 0 |
| Lateral nucleus | + | 0 | 0 |
| Medial nucleus | ++ | ++ | ++ |
| Cortical nucleus | + | + | 0 |
| Amygdalo-hipp. trans. | 0 | 0 | + |
| Basal ganglia | |||
| Caudate nucleus | ++ | 0 | 0 |
| Globus pallidus | + | 0 | 0 |
| Claustrum | ++ | + | + |
| Nucleus accumbens | + | ++ | ++ |
| Substantia innominata | + | + | ++ |
| Diencephalon Thalamus | |||
| Anterior thalamic nu. | 0 | 0 | 0 |
| Midline thalamic nu. | ++ | ++ | ++ |
| Lateral thalamic nuclei | 0 | + | + |
| Ventral thalamic nuclei | 0 | 0 | 0 |
| Reticular nucleus | 0 | 0 | 0 |
| Mediodorsal th. nu. | 0 | 0 | 0 |
| Habenular nuclei | + | + | + |
| Posterior th. nuclei | 0 | + | + |
| Peripeduncular area | + | + | + |
| Suprageniculate th. nu. | + | + | ++ |
| Medial geniculate body | |||
| dorsal nucleus | 0 | 0 | 0 |
| ventral nucleus | ++ | + | 0 |
| medial nucleus | +++ | ++ | + |
| Lateral geniculate body | + | + | 0 |
| Hypothalamus | |||
| Medial preoptic area | +++ | +++ | +++ |
| Lateral preoptic area | + | + | + |
| Supraoptic nucleus | + | ++ | + |
| Supraoptic decuss. | ++ | +++ | |
| Suprachiasmatic nu. | 0 | 0 | 0 |
| Anterior hypoth. nu. | + | + | + |
| Paraventricular nucleus | ++ | +++ | +++ |
| Periventricular nucleus | ++ | ++ | ++ |
| Arcuate nucleus | ++ | +++ | +++ |
| Median eminence | +++ | + | |
| Ventromedial nucleus | + | + | + |
| Dorsomedial nucleus | + | ++ | +++ |
| Lateral hypoth. area | + | ++ | ++ |
| Perifornical nucleus | + | + | + |
| Posterior hypoth. nu. | + | ++ | ++ |
| Tuberomamillary nu. | + | ++ | ++ |
| Premamillary nuclei | + | ++ | ++ |
| Supramamillary nucleus | ++ | ++ | ++ |
| Mamillary body | |||
| Medial mamillary nu. | 0 | 0 | 0 |
| Lateral mamillary nu. | 0 | ++ | ++ |
| Subthalamic nucleus | + | + | + |
| Brainstem Midbrain | |||
| Zona incerta | ++ | +++ | +++ |
| Periaqueductal gray | ++ | +++ | +++ |
| Ventral tegmental area | ++ | +++ | ++ |
| Oculomotor nuclei | 0 | 0 | 0 |
| Substantia nigra | 0 | 0 | 0 |
| Red nucleus | 0 | 0 | 0 |
| Subbrachial nucleus | + | ++ | ++ |
| Praetectal area | + | ++ | ++ |
| Superior colliculus | ++ | ++ | ++ |
| Inferior colliculus | + | ++ | ++ |
| Dorsal raphe nucleus | ++ | +++ | ++ |
| Pons | |||
| Lateral lemniscal nuclei | 0 | 0 | 0 |
| Medial paralemniscal nu. | 0 | + | ++ |
| Pontine tegmentum | ++ | ++ | ++ |
| Parabrachial nuclei | + | ||
| medial | + | + | |
| lateral | +++ | +++ | |
| Pontine nuclei | 0 | 0 | 0 |
| Superior olive | 0 | + | ++ |
| Pontine reticular form. | 0 | + | + |
| Principal trigeminal nu. | + | ++ | ++ |
| Motor trigeminal nu. | 0 | 0 | 0 |
| Pontine raphe nucleus | 0 | ++ | ++ |
| Vestibular nuclei | 0 | + | + |
| Medulla Oblongata | |||
| Cochlear nuclei | 0 | 0 | |
| Spinal trigeminal nu. | + | +++ | + |
| Prepos. hypoglossal nu. | 0 | 0 | 0 |
| Medullary reticular form. | 0 | + | + |
| Inferior olive | 0 | 0 | 0 |
| Dorsal vagal complex | |||
| Nu. of the solitary tract | ++ | ++ | ++ |
| Dorsal motor vagal nu. | 0 | 0 | 0 |
| Area postrema | 0 | 0 | 0 |
| Motor hypoglossal nu. | 0 | 0 | 0 |
| Nucleus ambiguus | 0 | 0 | 0 |
| Medullary raphe nuclei | 0 | 0 | 0 |
| Cerebellum | |||
| Cortex | ++ | + | 0 |
| Nuclei | 0 | 0 | 0 |
| Spinal cord | + | ++ | + |
The average number of labeled cells per section in the nucleus is none (0), 1-10 (+), 11-20 (++), or over 20 (+++). Similarly, the density of PTH2R-ir fibers and fiber terminals is represented as none to low (0), moderate (+), high (++), and very high (+++). The table is a slight modification of our previously published table (Faber et al., 2007).
7.4 The TIP39-PTH2R neuromodulator system
The localization of cell bodies that express TIP39 and those that express the PTH2R are profoundly different. TIP39 expression is confined to the subparafascicular area-posterior intralaminar thalamic complex and the medial paralemniscal nucleus, while the PTH2R is expressed in many brain regions. In general, TIP39-ir neurons have axons that project long distances from the TIP39-expressing perikarya, while PTH2R-ir fibers are often localized in the vicinity of PTH2R-expressing neurons (Fig. 11A,B). Although the origin of PTH2R-ir fibers have not yet been investigated in any brain region, it seems logical that they are of local origin. In contrast to the profoundly different localization of TIP39- and PTH2R-expressing cell bodies, the distributions of TIP39-ir and PTH2R-ir fibers are markedly similar. TIP39-ir and PTH2R-ir fibers are present in the same nuclei and areas throughout the central nervous system except for a few areas described below. Furthermore, TIP39-ir and PTH2R-ir fibers show topographical similarities not only in certain brain regions, but very often also in subdivisions (Fig. 11B,C). In fact, the two distributions are indistinguishable in most brain nuclei and areas. This finding suggests the probable action of TIP39 on the PTH2R in these brain regions. Together with the strong pharmacological evidence that TIP39 is a potent and high-affinity selective ligand for the PTH2R, the anatomical data also support that TIP39 is the endogenous ligand for the PTH2R in the brain. Based on the signal transduction mechanisms used by TIP39 and the PTH2R they may form a neuromodulator system in many brain regions.
In a few areas, including the median eminence, the interpeduncular, and the spinal trigeminal nuclei, as well as the superficial laminae of the spinal cord dorsal horn PTH2R-ir but not TIP39-ir fibers are abundant. This could be explained by the appearance of TIP39 in these areas under specific physiological conditions, although a nonfunctional expression of the PTH2R or the existence of another ligand for the PTH2R in these areas cannot be excluded. It is possible that circulating TIP39 acts on PTH2R's in the median eminence, but a likely source of that TIP39 has not been identified. Mismatches between the distributions of neuropeptides and their putative receptors have also been explained by the ability of peptides that have high affinity for their receptors to act at a very low concentration, potentially after diffusing long distances, as well as by the presence of peptide or receptor in some axonal or dendritic branches in which they do not have functional relevance (Herkenham, 1987; Leng and Ludwig, 2006).
7.5 The glutamatergic nature of PTH2R fibers and a proposed model for the neuromodulatory action of TIP39
Glutamate is the major excitatory neurotransmitter in the central nervous system. Its quantal release depends on its transport into synaptic vesicles. Therefore, glutamatergic terminals contain vesicular glutamate transporters (VGLUT), which are generally used as a marker of glutamatergic terminals (Fremeau et al., 2001). VGLUT-2 is the major VGLUT form in the hypothalamus and the septum (Lin et al., 2003). Most, or all, PTH2-R-containing terminals contain VGLUT-2 in the rat hypothalamus (Dobolyi et al., 2006a) and macaque's septum and hypothalamus (Bagó et al., 2009). The co-localization of PTH2R and VGLUT-2 in fiber terminals suggests that PTH2R-containing neurons in the septum and hypothalamus are excitatory glutamatergic neurons. Glutamic acid decarboxylase, the synthetic enzyme for GABA (Erlander and Tobin, 1991) is also abundant in the hypothalamus (Vincent et al., 1982). Consistent with PTH2R-containing neurons being glutamatergic and not GABAergic, however, no glutamic acid decarboxylase was co-localized with the PTH2R in the hypothalamus (Dobolyi et al., 2006a). TIP39 terminals do not contain either VGLUT-2 or glutamic acid decarboxylase immunoreactivity.
The excellent correlation between PTH2R- and TIP39-ir fibers and fiber terminals together with the finding that PTH2R immunoreactivity is not consistently detectable in cell bodies suggests that TIP39 and PTH2R may be involved in axo-axonal interactions. Both TIP39-ir and PTH2-ir fiber terminals are abundant in the medial preoptic, paraventricular and arcuate nuclei (Dobolyi et al., 2003b; Faber et al., 2007) where the perikarya of the vast majority of the hypothalamic neurosecretory neurons are located (Palkovits, 1992). Neurosecretory cells are likely to be regulated by VGLUT-containing excitatory synapses, as demonstrated for the gonadotropin-releasing hormone-containing cells in the medial preoptic area (Kiss et al., 2003). Assuming that excitatory synapses regulating neurosecretory cells contain PTH2Rs, TIP39 terminals are ideally situated to modulate these synapses. This hypothesis may explain how TIP39 regulates the secretion of hypophysiotropic hormones, i.e. an axo-axonal model (Fig. 12) could represent the general mechanism of actions for the TIP39-PTH2R neuromodulator system.
Fig. 12.
A model on the modulatory action of TIP39. TIP39 neurons located in the PVG, PIL, and MPL project to remote brain areas where their axon terminals approach PTH2R-containing terminals. As demonstrated in the hypothalamus and the septum, PTH2R are present in glutamatergic terminals. It was hypothesized that TIP39 is released from the terminals of axons originating in the PVG, PIL, and MPL, and presynaptically modulates the efficiency of excitatory synaptic transmission by acting on PTH2Rs in the glutamatergic axon terminals.
8 Central regulatory mechanisms affected by TIP39
8.1 Nociceptive information processing
There is intense PTH2R immunoreactivity in superficial layers of the spinal cord dorsal horn where most nociceptive afferents terminate (Wang et al., 2000). This has lead to evaluation of the role of TIP39 in the modulation of spinal nociceptive information transfer. Intrathecal injection of TIP39 stimulates a dose-dependent nocifensive response, caudally directed scratching, biting, and licking (Dobolyi et al., 2002). Intrathecal TIP39 administration also decreased the tail-flick and paw-pressure withdrawal latencies, but did not have a significant effect on withdrawal in response to paw heating. Furthermore, microinjection of TIP39 into the plantar surface of a mouse paw elicited a dose-dependent withdrawal response. These responses to peripheral and central TIP39 administration show that it can activate nociceptive circuits. However, these observations do not address the effects of endogenous TIP39. Because no TIP39 antagonists were available at the time of these experiments, TIP39 was sequestered with an antibody followed by testing nociceptive responses (Dobolyi et al., 2002). Intrathecal injection of the TIP39 antibody increases the response latency in the thermal tail-flick assay. In the paw-pressure test, intrathecal administration of the TIP39 antibody increases the latency to paw withdrawal, corresponding to decreased pressure sensitivity. In contrast, intrathecal delivery of the TIP39 antibody does not significantly affect paw withdrawal latency in response to radiant heat in the Hargreaves' assay (Hargreaves et al., 1988) and it does not affect the response to intraplantar injection of the chemoirritant capsaicin (Dobolyi et al., 2002). Inhibition by the TIP39 antibody of responses in the thermal tail-flick and paw-pressure assays suggests that TIP39 may have a facilitatory role in the underlying circuits. The tail-flick response is thought to be primarily a spinal reflex, whereas paw withdrawal from heat requires supraspinal mediation. The different effects of TIP39 and TIP39 sequestration on these responses may reflect differences in PTH2R expression on neurons in the underlying circuits. However, the intrathecally applied reagents may also reach relevant sites with different efficiency.
To identify the neuronal circuitry responsible for the nociceptive actions of TIP39 in the spinal cord, PTH2R-ir fibers were double labeled with established markers of dorsal horn populations, including CGRP and substance P, as well as isolectin B4, markers of small-diameter primary afferent fibers terminating in the marginal layers,(Molliver et al., 1995; Snider and McMahon, 1998), and protein kinase C gamma, which is synthesized in the spinal cord dorsal horn and labels fibers in lamina III (Malmberg et al., 1997; Polgar et al., 1999). PTH2R labeling showed extensive laminar overlap with isolectin B4, it partially overlapped but was mostly ventral to the area of greatest substance P and CGRP labeling (marginal layers), and dorsal to the layer of labeling by an antibody to protein kinase C gamma (lamina III) placing the PTH2R within lamina II (Dobolyi et al., 2002), which contains most of the central terminals of nociceptive primary afferents. Despite the laminar overlap, PTH2R did not co-localize with isolectin B4 or substance P. There was, however, limited co-localization with CGRP fibers (Dobolyi et al., 2002). The PTH2R is synthesized by a population of dorsal root ganglion (DRG) cells, which seemed to be of the smaller size class (Dobolyi et al., 2002). In addition, TIP39 increased cAMP in F-11 cells (Usdin et al., 1999b), which are a DRG-neuroblastoma hybrid cell line that possesses some of the properties of peptidergic nociceptors (Kusano and Gainer, 1993). Other agents that increase cAMP in DRG neurons potentiate nociception (Taiwo et al., 1989). PTH2R expressing cells are also present within the dorsal horn of the spinal cord (Wang et al., 2000), while relatively little PTH2R synthesis occurs in supraspinal areas that project to the superficial dorsal horn (Wang et al., 2000). Thus, PTH2R fibers in the superficial laminae of the spinal cord dorsal horn may originate in the DRG and/or the spinal cord dorsal horn.
TIP39 mRNA is not detected in the spinal cord by RT-PCR. RT-PCR does suggest that there may be a low level of TIP39 synthesis in DRG, but TIP39 has not been detected in DRG by immunocytochemistry or in situ hybridization histochemistry (Dobolyi et al., 2002). TIP39 acting on PTH2Rs in the superficial laminae may have a supraspinal origin. Neurons within areas expressing TIP39 including the PVG (Björklund and Skagerberg, 1979; Hökfelt et al., 1979; Skagerberg and Lindvall, 1985) and the PIL (Basbaum and Fields, 1979; Carlton et al., 1985; Holstege and Kuypers, 1982) project to the dorsal horn of the spinal cord. Most TIP39 fibers are in the lateral funiculus adjacent to the gray matter, an area that contains both descending and primary afferent fibers. In the spinal cord, fine scattered TIP39-ir fibers are found in the lateral funiculus, the lateral intermediate gray matter, central gray matter, and lamina II and III. As described above, studies using microstimulation and correlation with electrical activity implicate the PVG (Dong et al., 1978; Peschanski and Mantyh, 1983; Rhodes and Liebeskind, 1978; Sugiyama et al., 1992) and the MPL (Basbaum et al., 1977; Girardot et al., 1987; Hardy et al., 1983; Haws et al., 1989; Zhao and Duggan, 1988) in nociception. Thus, it can be hypothesize that descending TIP39 fibers may contribute to modulation of spinal nociceptive information.
TIP39 appears to have another effect on nociception in the brain (LaBuda and Usdin, 2004). In adult male rats i.c.v. TIP39 does not change hot-plate paw withdrawal latency or formalin test behavioral responses. TIP39 partially reverses tactile withdrawal hypersensitivity following carageenan administration. In the place/escape avoidance paradigm, which evaluates affective components of the response to noxious stimuli by presenting a choice between a naturally preferred environment paired with stimulation of a carrageenan sensitized paw and a less preferred environment paired with stimulation of a less sensitive paw, TIP39 decreases the apparent aversiveness of sensitive paw stimulation (LaBuda and Usdin, 2004). Because acute sensory thresholds are unaffected by i.c.v. TIP39, and effects in some nociceptive tests are opposite in direction from effects of intrathecal TIP39, this suggests that TIP39 has distinct brain and spinal roles. It may modulate an affective component of nociception within the brain. The TIP39-PTH2R neuromodulator system is also potentially involved in the processing of nociceptive and viscerosensory input to the limbic system, which is suggested by its presence in brain areas such as the nucleus of the solitary tract, the parabrachial nuclei, the midline thalamic nuclei, the paraventricular hypothalamic nucleus, and the insular and infralimbic cortex that are components of the autonomic-limbic pain-related pathway (Benarroch, 2006).
8.2 Affective processes modulated by TIP39
Several of the forebrain limbic regions that contain TIP39 and the PTH2R are implicated in anxiety and depression-related neuronal circuits (Drevets, 2000; Kent and Rauch, 2003; Manji et al., 2001). Therefore TIP39's effects were evaluated in tests of anxiety- and depression-like behaviors following its i.c.v. administration in rat (LaBuda et al., 2004) and by evaluating behavior changes in mice with targeted null mutation of the TIP39 gene (Tip39(-/-) or TIP39-KO) mice (Fegley et al., 2008). TIP39-KO mice cannot be distinguished from wild-type (WT) littermates on casual observation or in a functional observational battery for general health, reflexes, and basic sensory and motor functions. There were also no significant differences between the genotypes in home cage locomotor activity over 72 hours of monitoring, in motor coordination assessed on an accelerating rotarod or in exploration in a holeboard test. Thus, TIP39-KO mice do not show any gross phenotypic abnormalities that are likely to confound performance on tests for anxiety-like behaviors. TIP39-KO mice did not differ significantly from WT littermates in the open field test (Fegley et al., 2008). Furthermore, i.c.v. TIP39 administration in rat did not significantly modify any of the behaviors evaluated in the open field including total number of partitions entered over a 5 min test, number of rearing incidents, number of fecal boli, or bouts of grooming (LaBuda et al., 2004). At the time of the rat experiments, there were no high-affinity or specific PTH2R antagonists so the effects of several doses of injected TIP39 were compared with those of the truncated analog TIP(7-39) to help evaluate the specificity of the effect of TIP39 injection in rat behavioral experiments. As described above, TIP(7-39) is a very weak PTH2R antagonist (Hoare et al., 2000a). Animals receiving TIP(7-39) did not differ from control animals on any of the tests performed (LaBuda et al., 2004).
8.2.1 Elevated plus maze test
The elevated plus maze is one of the most commonly used tests of anxiety-like behavior in rodents. Animals are allowed to explore a maze that consists of two open and two enclosed arms. The relative amount of time spent in the two conditions is considered an index of the anxiety state of the animal. Anxiolytic agents increase the relative amount of time that an animal spends exploring the open arms.
Following i.c.v. administration of TIP39, rats entered the open arms more frequently and spent more time within the open arms as compared to controls (animals receiving TIP(7-39) or saline) while no differences were observed between groups in the number of closed arm entries or total arm entries suggesting an anxiolytic-like effect of TIP39 (LaBuda et al., 2004). In contrast, under “standard” testing conditions, TIP39-KO mice do not have robust differences from WT controls in several tests of spontaneous anxiety-like behaviors including the novel open field, dark-light emergence and elevated plus maze (Fegley et al., 2008). However, an increase in anxiety-like behavior becomes apparent in TIP39-KO mice that are tested under conditions of increased stress. Either following brief restraint, or under bright illumination, TIP39-KO mice made fewer open arm entries and spent less time in the open arms than WT littermates.
8.2.2 Shock-probe defensive burying test
In the shock-probe burying test animals receive a shock if they contact an electrified rod placed in their cage. This is thought to produce an approach/avoid conflict somewhat like the elevated plus maze, but with a stronger, tangible, aversive stimulus (i.e., shock) than an exploration-based assay (Sluyter et al., 1996; Treit and Fundytus, 1988). Animals typically respond by avoiding the area closest to the rod and by actively pushing bedding on top of it.
TIP39-KO mice spent more time engaged in defensive burying than WT controls in the shock-probe burying test (Fegley et al., 2008). KO mice also spent significantly less time in the zone around the electrified probe. This response is similar to their response in the elevated plus maze in that it demonstrates more anxiety-like behavior by the TIP39-KO mice, and it complements it by showing an active response.
8.2.3 Forced swim test
The forced swim test is commonly used with rodents to evaluate depression-like behavior. Animals are placed in a cylinder of water and the amount of time they spend passively floating vs. actively attempting to escape is measured. Established antidepressant agents decrease the relative amount of time spent passively floating.
I.c.v. administration of TIP39 reduced the duration of immobility, and increased the amount of climbing behavior in the forced swim test (LaBuda et al., 2004). TIP(7-39), administered as a control, had no effect on swimming behavior.
8.2.4 Pavlovian fear conditioning and extinction
Administration of paired tone and shock in a specific context, followed at later times by exposure to either the original context, or to the tone alone in a novel context, is often used to establish and evaluate conditioning in rodents, with freezing used as an index of fear to measure the strength of the conditioning (Blanchard and Blanchard, 1969; Bouton and Bolles, 1979; LeDoux et al., 1984).
Pavlovian fear conditioning and its extinction were evaluated in TIP39-KO mice (Fegley et al., 2008). During conditioning, TIP39-KO mice froze significantly more than WT controls during the second but not first or third tone presentation (Fegley et al., 2008). During a tone test performed 24 hours later in a novel context, TIP39-KO mice froze significantly more than WT controls when the tone was presented, but not during the pre-tone period. TIP39-KO mice also froze significantly more than WT controls when placed in the original training context without tone stimulation (Fegley et al., 2008).
Fear extinction was also assessed in TIP39-KO and WT mice. In this experiment TIP39-KO mice again had greater freezing scores than WT controls during the second (but not first or third) tone-shock pairing during conditioning (Fegley et al., 2008). During extinction training TIP39-KO mice initially froze more than WT controls. With repeated tone presentations WT and TIP39-KO mice had parallel reductions in freezing, such that there was no difference between the groups by the end of the session. Freezing did not differ between the genotypes during extinction recall, and WT and KO mice re-extinguished at the same rate over the remainder of the recall session (Fegley et al., 2008).
8.2.5 The role of TIP39 in affective brain regulatory processes
Antidepressant- and anxiolytic-like effects of TIP39 as well as the increase in anxiety-like behavior in the shock-probe burying test and in the elevated plus maze observed in TIP39-KO mice are consistent with an anxiolytic role of endogenous TIP39. Behavioral effects of TIP39 absence were only apparent in the elevated plus maze when the stress levels of the tests were increased slightly, by prior brief restraint or using bright illumination. This suggests that the role of endogenous TIP39 may involve modulation of the response to stress. In these studies, the stressors that evoked increased anxiety-like behaviors in TIP39-KO mice were in themselves insufficient to produce a significant anxiety-like response in WT controls, suggesting that endogenous TIP39 may play a role in the presence of relatively mild perturbations. The manifestation of a clear phenotype in the shock-probe burying test even without prior stress might be due to the inherently higher level of stress and anxiety-provocation in this shock-based assay (Holmes et al., 2005).
In addition to increased innate anxiety-like behaviors under stress, TIP39-KO mice showed enhanced acquisition and recall of conditioned fear responses. KOs showed more freezing than WT controls after only one tone-shock pairing during conditioning and, subsequently, more freezing during both tone- and context-recall tests. However, based on a similar rate of decline in freezing responses to repeated tone presentation and a similar level of freezing during subsequent tone presentation, there did not appear to be an effect of TIP39 deletion on fear extinction learning or extinction recall, respectively.
The behavioral observations suggest that endogenous TIP39 may modulate fear and stress-induced anxiety and/or reduce the behavioral manifestation of fear and stress following environmental provocation. This is consistent with growing evidence that neuropeptides are preferentially recruited as modulators of fear and anxiety under conditions of stress (Holmes et al., 2003; Hökfelt et al., 2003). Data on TIP39 support the hypothesis that TIP39 modulates anxiety-related behaviors.
8.2.6 Sites of TIP39 effect on affective behaviors
There is a high density and quite restricted distribution of TIP39-positive fibers and PTH2Rs (Dobolyi et al., 2003b; Faber et al., 2007) in regions known to mediate fear and anxiety, including parts of the amygdala, bed nucleus of the stria terminalis, lateral septum, and the medial prefrontal cortex (Drevets, 2000; Kent and Rauch, 2003; Manji et al., 2001). In an attempt to further specify the sites of action of TIP39 within the forebrain, the pattern of c-fos expression was examined following i.c.v. delivery of TIP39. Administration of TIP39 into the lateral ventricle elicited robust increases in the number of c-fos-expressing cells in five brain regions, i.e. in the infralimbic cortex, the lateral hypothalamus, the preoptic area, the lateral septum, and in the paraventricular thalamic nucleus (LaBuda et al., 2004). As shown previously, relatively high PTH2R levels were found in all of these areas. Thus, induction of c-fos expression in these brain areas may indicate that TIP39 directly activates these cells via PTH2Rs. The lack of c-fos activation in other PTH2R expressing cells could be a consequence of different intracellular events following PTH2R activation, or insufficient diffusion of TIP39 to those sites from the lateral ventricle. However, the c-fos activation pattern might be elicited by some more complex events. Anxiogenic drugs with four different mechanisms of action produce a similar c-fos activation pattern, suggesting their influence on a common fear circuitry (Singewald et al., 2003). Neurons in all above listed five TIP39-activated regions also exhibit c-fos expression in response to different stressors. In particular, exposure to the elevated plus maze induces c-fos activation in the infralimbic cortex, in the lateral septum and in the lateral hypothalamus (Campeau et al., 1997; Duncan et al., 1996; Pezzone et al., 1992). Data about c-fos activation together with the neuroanatomical distribution of the PTH2R and TIP39 suggest that limbic regions described above may represent the sites where TIP39 may act to modulate affective or anxiety state.
8.3 Neuroendocrine actions of TIP39 at hypothalamic level
The TIP39-PTH2R neuromodulator system is in optimal position to influence the hypothalamo-pituitary axis through its presence in several hypothalamic areas. Initial functional data suggest that TIP39 may influence the release of adrenocorticotropin (ACTH), luteinizing hormone (LH), growth hormone (GH), and arginine-vasopressin (AVP) from the pituitary.
8.3.1 Hypothalamo-pituitary-adrenal axis
Plasma ACTH increased following i.c.v, administration of TIP39 (Ward et al., 2001). In addition, there was a robust release of corticotropin releasing hormone (CRH) from medial basal hypothalamic explants following incubation with TIP39 (Ward et al., 2001). Therefore, the increased ACTH following i.c.v delivery of TIP39 could be mediated via stimulation of hypothalamic CRH release. Considering the presence of PTH2Rs in the PVN and the external zone of the median eminence (Dobolyi et al., 2006a; Faber et al., 2007; Wang et al., 2000), the projection of TIP39 neurons to the hypothalamic paraventricular nucleus (Dobolyi et al., 2003a; Palkovits et al., 2004) and the in vitro and in vivo effects of TIP39, these data suggest an activating role for TIP39 in the hypothalamo-pituitary-adrenal axis.
8.3.2 Hypothalamo-pituitary-gonadal axis
Plasma LH also increased with i.c.v. administration of TIP39 (Ward et al., 2001). In addition, luteinizing hormone releasing hormone (LHRH) increased in the media of hypothalamic explants following incubation with TIP39 (Ward et al., 2001), suggesting that the increase in plasma LH may be mediated by LHRH. However, TIP39 administered intraperitoneally (ip) also resulted in a significant release of LH 10 min after the injection, compared with saline (Ward et al., 2001). In light of this rapid response of LH to peripheral TIP39, a direct pituitary action is possible. Although PTH2R mRNA was detected in anterior pituitary cells (Usdin et al., 1996), the PTH2R was not demonstrated by immunohistochemistry in the anterior pituitary, probably on account of its low concentration (Usdin et al., 1999a). In addition, TIP39 does not alter the release of any anterior pituitary hormone from monodispersed anterior pituitary cells (Ward et al., 2001). However, the PTH2R is present in the external zone of the median eminence (Dobolyi et al., 2006a; Faber et al., 2007; Wang et al., 2000), where it could mediate the release of hypothalamic LHRH. While further investigation is required to identify the site of action of TIP39 on the hypothalamo-pituitary-gonadal axis, the combined results suggest that TIP39 may contribute to the regulation of the LH secretion, potentially acting both directly and indirectly on the hypothalamo-pituitary-gonadal axis.
8.3.3 Regulation of the secretion of growth hormone
In rat, both in situ hybridization and immunohistochemistry show PTH2R expression by cell bodies in the hypothalamic periventricular nucleus. This area contains somatostatin neurons that project to the median eminence and contribute to the regulation of growth hormone secretion. Double labeling shows a high degree of co-localization of somatostatin and the PTH2R in periventricular neurons (Usdin et al., 1999b), and PTH2R fibers in the median eminence in rats (Dobolyi et al., 2006a), and also in human (Bagó et al., 2009). These anatomical findings suggest that the PTH2R might be involved in the control of GH secretion. In one experiment, TIP39 injection into the lateral ventricle of male rats blocked the appearance of GH almost completely in plasma for the next three hours (Usdin et al., 2003). This is consistent with the suggestion that TIP39 stimulates release of somatostatin, which in turn inhibits GH secretion. However, another study found no alteration in plasma GH following i.c.v. administration of TIP39 and no effect of TIP39 on somatostatin release from hypothalamic explants (Ward et al., 2001).
8.3.4 Arginine-vasopressin release
The effect of centrally administered TIP39 on AVP release was investigated in conscious rats (Sugimura et al., 2003). Plasma AVP levels were significantly lower 5 min following i.c.v. administration of TIP39 as compared with controls (Sugimura et al., 2003). TIP39 did not significantly change plasma Na+ of plasma total protein levels measured for estimation of the change in plasma volume. TIP39 also significantly suppressed the dehydration-induced plasma AVP increase, with its maximum effect occurring 5 min after injection in rats, water deprived for 48 h. Furthermore, the plasma AVP increase in response to either hyperosmolality following i.p. injection of hypertonic saline or hypovolemia following i.p. injection of polyethylene glycol was also significantly attenuated 5 min after i.c.v. TIP39 injection (Sugimura et al., 2003). These inhibitory effects cannot be attributed to a decrease in the level of osmotic or hypovolemic stimulation because plasma Na+ and plasma total protein were not affected by the i.c.v. injection of TIP39. In addition, i.c.v. injection of TIP39 produced a fall in mean arterial blood pressure, which is well known to stimulate AVP secretion suggesting that the inhibitory effect of TIP39 was not based on the change in blood pressure (Sugimura et al., 2003). Taken together, these results suggest that TIP39 inhibits AVP release by central action, without hemodynamic or osmotic alterations. The effect was rapid and did not last long suggesting that TIP39 may play a role in the dynamic regulation of AVP release.
TIP39 and PTH2R are particularly abundant in the hypothalamic periventricular and arcuate nuclei and are scarce in the supraoptic nucleus and the magnocellular part of the hypothalamic paraventricular nucleus where AVP is synthesized. Thus, it seems likely that TIP39 exerts its AVP release-inhibitory effect by acting through other hypothalamic nuclei. The arcuate nucleus is a strong candidate. It contains many opioid neurons (Palkovits, 1988), and a great deal of evidence suggests that opioid systems are involved in the regulation of AVP release (Haaf et al., 1987; Van de Heijning et al., 1991a; Van de Heijning et al., 1991b). Most studies demonstrate that opioids play an inhibitory role in the regulation of AVP release, and that such a release in response to hyperosmolality or hypovolemia is attenuated by opioid agonists (Haaf et al., 1987; Oiso et al., 1988). Because of the massive neural connections between arcuate and the hypothalamic paraventricular nuclei (Baker and Herkenham, 1995; Bell et al., 2000), the possibility that TIP39 suppression of AVP release is mediated by opioids was investigated by co-administration of naloxone. Naloxone significantly reversed the inhibitory effect of TIP39 on dehydration-induced AVP release whereas naloxone alone did not significantly alter the plasma AVP level (Sugimura et al., 2003). Together, these findings suggest that TIP39 plays an inhibitory role in the osmoregulation and baroregulation of AVP release, and that intrinsic opioid systems are involved in the action of TIP39.
8.3.5 The role of TIP39 in the secretion of hypophysiotropic hormones
Experiments investigating the effect of TIP39 on the release of hypothalamic hormones suggest the possibility that TIP39 plays a role in regulating the hypothalamo-pituitary axis, although most of the results must be considered preliminary. First, relatively large doses of peptide were administered in these experiments. These high concentrations might be required because of the need for tissue penetration and the occurrence of proteolysis, as the peptide diffuses through tissue. In addition, dose-response studies and experiments to test the mechanism of action of TIP39's effects on the release of hypothalamic and pituitary hormones were often not performed. It is also clear that manipulation of the effect of endogenous TIP39 is essential to understand its physiological relevance, which requires studies, in which the effects of TIP39 are antagonized. In addition, the effects of a single injection of TIP39 were usually examined, which may not be a good mimic of endogenous TIP39's effect. Little is known about the regulation of TIP39 release or synthesis, and further studies are required to clarify this point, as well.
Based on the pharmacology and anatomy of the TIP39-PTH2R system, it is likely that TIP39 effects are mediated by the PTH2R. However, somatostatin is the only neuroendocrine factor shown yet to co-localize with the PTH2R (Bagó et al., 2009; Dobolyi et al., 2006a), suggesting that TIP39 might directly regulate the secretion of somatostatin but not other hypophysiotropic hormones. As discussed above, there is a high degree of co-localization between VGLUT2 and the PTH2R in the hypothalamus so TIP39 may indirectly regulate other neurosecretory cells via effects at VGLUT2-containing (glutamatergic) excitatory synapses, which contain PTH2R in their terminals.
8.4 Modulatory action of TIP39 on the auditory system
The TIP39-PTH2R neuromodulator system may also be involved in the processing of auditory information because it is present in several auditory brain centers including the auditory cortex, the medial geniculate body and surrounding ‘auditory thalamic areas’, the external cortex of the inferior colliculus, the superior olive, and the nucleus of the trapezoid body (Dobolyi et al., 2003b; Faber et al., 2007). Both neurons and fibers containing TIP39 or PTH2R are located in non-tonotopically organized regions (Clopton et al., 1974). Therefore, we propose that the TIP39-PTH2R neuromodulator system may modulate auditory processes that do not require tonotopic information such as loud noise-induced stress response or acoustic fear-conditioning. Such potential functions of the TIP39-PTH2R neuromodulator system are supported by the loud noise-induced activation in posterior intralaminar and medial paralemniscal TIP39 neurons (Palkovits et al., 2004, 2009a) and their significant neuronal input from the auditory cortex (Varga et al., 2008). Auditory stimulation (30 min 105 dB white noise) elicits c-fos expression in the majority of TIP39-ir neurons in the medial paralemniscal nucleus (Fig. 13C, D), in about half of TIP39-ir neurons in the PIL (Fig. 13E), and about 20% of TIP39-ir neurons in the subparafascicular nucleus (Fig. 13F). The participation of TIP39 neurons in loud noise-induced stress response is also supported by tracer studies demonstrating that parvicellular subparafascicular and medial paralemniscal neurons project to the area of the hypothalamic paraventricular nucleus that contains the CRH synthesizing neurons, and may therefore represent an interconnection between the auditory system and the stress center of the hypothalamus (Palkovits et al., 2004). Similarly, the projection of TIP39 neurons to the amygdala (Dobolyi et al., 2003b) supports their involvement in acoustic fear-conditioning (LeDoux, 2003).
Fig. 13.
Activation of TIP39 neurons in response to acute loud noise in the rat. A: The dorsal as well as the ventral cochlear nuclei demonstrate c-fos activation following exposure to 30-min of 105 dB white noise. B: The density of neurons expressing Fos is high in the auditory cortex but not in the somatosensory cortex. The caudal portion of the caudate-putamen also contains a great number of Fos-expressing neurons. C: Fos-ir neurons are abundant in the inferior colliculus, the nuclei of the lateral lemniscus, as well as the medial paralemniscal nucleus TIP-ir neurons (brown) can be seen at low power. D, E, F: High magnification photomicrographs of double immunolabeled sections demonstrate that a portion of TIP39-ir neurons (brown cell bodies) express Fos (black nuclei) in response to acute loud noise. The percentage of double labeled TIP39 neurons is very high in the MPL (D), somewhat lower in the PIL (E), and relatively modest in the PVG (F). Abbreviations: AudCx, auditory cortex; CP, caudate-putamen; DCN, dorsal cochlear nucleus, ec, external capsule; fi, fimbria hippocampi, ic, internal capsule, IC, inferior colliculus; ll, lateral lemniscus; m, midline; MPL, medial paralemniscal nucleus; ot, optic tract; PIL, posterior intralaminar complex of the thalamus, PVG, periventricular gray of the thalamus, py, pyramidal tract; rf, rhinal fissure; rs, rubrospinal tract; SomCx, somatosensory cortex; VCN, ventral cochlear nucleus; VPM, ventral posteromedial thalamic nucleus, 4V, fourth ventricle. Scale bar = 1 mm for A, B, C, 200 μm for F, and 100 μm for D, and E.
8.5 Involvement of TIP39 neurons in central reproductive regulations
The most established function of TIP39 in peripheral organs is the role in the development of the gonads described above (Usdin et al., 2008). TIP39 and the PTH2R are present in several brain areas involved in some aspect of reproduction, and the developmental expression pattern of TIP39 and the apparent sexual dimorphism in adult TIP39 levels are consistent with a role of the TIP39-PTH2R neuromodulator system in some aspects of reproductive function.
8.5.1 A potential role of TIP39 in male sexual behaviors
The role of the TIP39-PTH2R neuromodulator system in male sexual behaviors is supported by the presence of PTH2R as well as TIP39 fibers in several brain areas that are activated in males following mating (Coolen et al., 1997; Sachs and Meisel, 1988; Veening and Coolen, 1998) including the medial preoptic nucleus, the posteromedial part of the medial subdivision of the bed nucleus of the stria terminalis, and the posterodorsal subdivision of the medial amygdaloid nuclei. In addition, the medial subdivision of the PIL, which has been implicated in sexual functions by stimulation (Shimura and Shimokochi, 1991), lesion (Maillard and Edwards, 1991), and activation studies (Coolen et al., 1997; Holstege et al., 2003) is one of the 3 brain sites that contain TIP39-expressing neurons. Indeed, TIP39 neurons in the PIL have been shown to exhibit c-fos activation following ejaculation, and much less following intromission without ejaculation (Wang et al., 2006a). This suggests that these TIP39 neurons are part of the afferent circuits that process genital-somatosensory information related to ejaculation contributing to mating and mating-induced changes in reproductive behaviors. Based on the visceral, somatosensory, proprioceptive, and noxious information that can reach the PIL (Coolen et al., 2003b), TIP39 neurons in the area are in position to integrate and transmit information related to copulation.
8.5.2 Topography of the TIP39-PTH2R system and changes in TIP39 expression in relation to central reproductive functions
An interconnected network of brain centers regulates behavioral, emotional, and endocrinological influences on reproductive physiology. It has been suggested that limbic, preoptic and hypothalamic areas, including the lateral septum, the bed nucleus of the stria terminalis, the medial preoptic nucleus and area, the anteroventral periventricular, paraventricular and ventromedial hypothalamic nuclei, the arcuate nucleus, the medial and central amygdaloid nuclei, the amygdalo-hippocampal transitional zone, the premamillary nuclei, the ventral subiculum, and the periaqueductal gray, are part of the circuitry of reproductive regulation (Gorski, 1985; Hasen and Gammie, 2005; Holstege et al., 2003; Li et al., 1999; Lin et al., 1998; Lonstein and Stern, 1997; Numan and Insel, 2003; Numan and Sheehan, 1997; Pfaus and Heeb, 1997; Sachs and Meisel, 1988; Simerly, 2002; Veening and Coolen, 1998). The TIP39-PTH2R neuromodulator system is abundant in all of these brain regions (Dobolyi et al., 2003b; Faber et al., 2007) suggesting its involvement in reproductive regulation.
TIP39 levels increase and then decrease during development as described in Chapter 3.7. In the amygdalo-hippocampal transitional zone and in most of the neurons in the PIL, TIP39 disappears during early postnatal development (Brenner et al., 2008; Dobolyi et al., 2006b). As TIP39 can affect the proliferation of some cell types in vitro (Misiano et al., 2003), it is possible that TIP39 might be involved in aspects of neural development including brain sexual development during its transient expression. In addition, TIP39 gradually disappears from neurons in the PVG and the MPL during the period of pubertal development (Dobolyi et al., 2006b), which is consistent with a potential role of TIP39 related to pubertal development.
9 Conclusion and future directions in the research on the TIP39-PTH2R system
TIP39 and the PTH2R form a unique neuropeptide-receptor system. Its signal transduction pathways suggest that it is a neuromodulator and its anatomical distribution suggests that it plays a role in limbic, endocrine, viscerosensory, and auditory functions. Mice with targeted mutations of the TIP39-PTH2R system and specific antagonists have recently become available and these tools should facilitate identification of the specific roles of TIP39 and the PTH2R. The TIP39-PTH2R system may be worth considering as a therapeutic target as it differs neuroanatomically and functionally from other receptor systems that have been proposed as potential drug targets in anxiety, depression, and chronic pain management.
Acknowledgments
Grant support: The work was supported by the Bolyai János Fellowship Grant of the Hungarian Academy of Sciences for AD, the Hungarian Science Foundation NKTH-OTKA K67646 and NFM-OTKA NNF78219 Research Grants for AD, the OTKA NK72929 Research Grant for MP, and the Intramural Research Program of the NIH, National Institute of Mental Health for TBU.
Abbreviations
- ACTH
adrenocorticotropin
- AVP
arginine-vasopressin
- CGRP
calcitonin gene-related peptide
- CRH
corticotropin releasing hormone
- DRG
dorsal root ganglion
- ED
embryonic day
- GH
growth hormone
- i.c.v.
intracerebroventricular
- LH
luteinizing hormone
- LHRH
luteinizing hormone releasing hormone
- MPL
medial paralemniscal nucleus
- PIL
posterior intralaminar compex of the thalamus
- PND
postnatal day
- PTH
parathyroid hormone
- PTHrP
parathyroid hormone-related peptide
- PTH1R
PTH 1 receptor
- PTH2R
PTH 2 receptor
- PTH3R
PTH 3 receptor
- PVG
periventricular gray of the thalamus
- SPF
subparafascicular area
- SPFp
parvicellular subparafascicular thalamic nucleus
- TH
tyrosine hydroxylase
- TIP39
tuberoinfundibular peptide of 39 residues
- TIP39-KO
mice with targeted null mutation of the TIP39 gene
- VGLUT
vesicular glutamate transporter
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrezik JE, Beitz AJ. Reticular formation, central gray and related tegmental nuclei. In: Paxinos G, editor. The Rat Nervous System. Academic Press; New York: 1985. pp. 1–28. [Google Scholar]
- Arnault P, Roger M. Ventral temporal cortex in the rat: connections of secondary auditory areas Te2 and Te3. J Comp Neurol. 1990;302:110–123. doi: 10.1002/cne.903020109. [DOI] [PubMed] [Google Scholar]
- Baffi JS, Palkovits M. Fine topography of brain areas activated by cold stress. A fos immunohistochemical study in rats. Neuroendocrinology. 2000;72:102–113. doi: 10.1159/000054577. [DOI] [PubMed] [Google Scholar]
- Bagó AG, Dimitrov E, Saunders R, Seress L, Palkovits M, Usdin TB, Dobolyi A. Parathyroid hormone 2 receptor and its endogenous ligand tuberoinfundibular peptide of 39 residues are concentrated in endocrine, viscerosensory and auditory brain regions in macaque and human. Neuroscience. 2009;162:128–147. doi: 10.1016/j.neuroscience.2009.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagó AG, Palkovits M, Usdin TB, Seress L, Dobolyi A. Evidence for the expression of parathyroid hormone 2 receptor in the human brainstem. Ideggyogy Sz. 2008;61:123–126. [PMC free article] [PubMed] [Google Scholar]
- Baker RA, Herkenham M. Arcuate nucleus neurons that project to the hypothalamic paraventricular nucleus: neuropeptidergic identity and consequences of adrenalectomy on mRNA levels in the rat. J Comp Neurol. 1995;358:518–530. doi: 10.1002/cne.903580405. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. The origin of descending pathways in the dorsolateral funiculus of the spinal cord of the cat and rat: further studies on the anatomy of pain modulation. J Comp Neurol. 1979;187:513–531. doi: 10.1002/cne.901870304. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Marley NJ, O'Keefe J, Clanton CH. Reversal of morphine and stimulus-produced analgesia by subtotal spinal cord lesions. Pain. 1977;3:43–56. doi: 10.1016/0304-3959(77)90034-3. [DOI] [PubMed] [Google Scholar]
- Baumann CR, Bassetti CL. Hypocretins (orexins): clinical impact of the discovery of a neurotransmitter. Sleep Med Rev. 2005;9:253–268. doi: 10.1016/j.smrv.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Behar V, Pines M, Nakamoto C, Greenberg Z, Bisello A, Stueckle SM, Bessalle R, Usdin TB, Chorev M, Rosenblatt M, Suva LJ. The human PTH2 receptor: binding and signal transduction properties of the stably expressed recombinant receptor. Endocrinology. 1996;137:2748–2757. doi: 10.1210/endo.137.7.8770894. [DOI] [PubMed] [Google Scholar]
- Beitz AJ. The organization of afferent projections to the midbrain periaqueductal gray of the rat. Neuroscience. 1982;7:133–159. doi: 10.1016/0306-4522(82)90157-9. [DOI] [PubMed] [Google Scholar]
- Bell ME, Bhatnagar S, Akana SF, Choi S, Dallman MF. Disruption of arcuate/paraventricular nucleus connections changes body energy balance and response to acute stress. J Neurosci. 2000;20:6707–6713. doi: 10.1523/JNEUROSCI.20-17-06707.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. Pain-autonomic interactions. Neurol Sci. 2006;27 2:S130–133. doi: 10.1007/s10072-006-0587-x. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Groenewegen HJ. Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience. 1991;42:73–102. doi: 10.1016/0306-4522(91)90151-d. [DOI] [PubMed] [Google Scholar]
- Bisello A, Manen D, Pierroz DD, Usdin TB, Rizzoli R, Ferrari SL. Agonist-specific regulation of parathyroid hormone (PTH) receptor type 2 activity: structural and functional analysis of PTH- and tuberoinfundibular peptide (TIP) 39-stimulated desensitization and internalization. Mol Endocrinol. 2004;18:1486–1498. doi: 10.1210/me.2003-0487. [DOI] [PubMed] [Google Scholar]
- Björklund A, Skagerberg G. Evidence for a major spinal cord projection from the diencephalic A11 dopamine cell group in the rat using transmitter-specific fluorescent retrograde tracing. Brain Res. 1979;177:170–175. doi: 10.1016/0006-8993(79)90927-2. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Passive and active reactions to fear-eliciting stimuli. J Comp Physiol Psychol. 1969;68:129–135. doi: 10.1037/h0027676. [DOI] [PubMed] [Google Scholar]
- Boivie J, Meyerson BA. A correlative anatomical and clinical study of pain suppression by deep brain stimulation. Pain. 1982;13:113–126. doi: 10.1016/0304-3959(82)90022-7. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Role of conditioned contextual stimuli in reinstatement of extinguished fear. J Exp Psychol Anim Behav Process. 1979;5:368–378. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- Bregman AS. Auditory Scene Analysis. The Perceptual Organization of Sound. MIT Press; Cambridge: 1990. [Google Scholar]
- Brenner D, Bago AG, Gallatz K, Palkovits M, Usdin TB, Dobolyi A. Tuberoinfundibular peptide of 39 residues in the embryonic and early postnatal rat brain. J Chem Neuroanat. 2008;36:59–68. doi: 10.1016/j.jchemneu.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burow A, Day HE, Campeau S. A detailed characterization of loud noise stress: Intensity analysis of hypothalamo-pituitary-adrenocortical axis and brain activation. Brain Res. 2005;1062:63–73. doi: 10.1016/j.brainres.2005.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Falls WA, Cullinan WE, Helmreich DL, Davis M, Watson SJ. Elicitation and reduction of fear: behavioural and neuroendocrine indices and brain induction of the immediate-early gene c-fos. Neuroscience. 1997;78:1087–1104. doi: 10.1016/s0306-4522(96)00632-x. [DOI] [PubMed] [Google Scholar]
- Campeau S, Watson SJ. Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. J Neuroendocrinol. 1997;9:577–588. doi: 10.1046/j.1365-2826.1997.00593.x. [DOI] [PubMed] [Google Scholar]
- Campeau S, Watson SJ., Jr Connections of some auditory-responsive posterior thalamic nuclei putatively involved in activation of the hypothalamo-pituitary-adrenocortical axis in response to audiogenic stress in rats: an anterograde and retrograde tract tracing study combined with Fos expression. J Comp Neurol. 2000;423:474–491. [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Connections of the posterior nucleus of the amygdala. J Comp Neurol. 1992;324:143–179. doi: 10.1002/cne.903240203. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1994;348:41–79. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Chung JM, Leonard RB, Willis WD. Funicular trajectories of brainstem neurons projecting to the lumbar spinal cord in the monkey (Macaca fascicularis): a retrograde labeling study. J Comp Neurol. 1985;241:382–404. doi: 10.1002/cne.902410310. [DOI] [PubMed] [Google Scholar]
- Castro M, Dicker F, Vilardaga JP, Krasel C, Bernhardt M, Lohse MJ. Dual regulation of the parathyroid hormone (PTH)/PTH-related peptide receptor signaling by protein kinase C and beta-arrestins. Endocrinology. 2002;143:3854–3865. doi: 10.1210/en.2002-220232. [DOI] [PubMed] [Google Scholar]
- Chan GK, Deckelbaum RA, Bolivar I, Goltzman D, Karaplis AC. PTHrP inhibits adipocyte differentiation by down-regulating PPAR gamma activity via a MAPK-dependent pathway. Endocrinology. 2001;142:4900–4909. doi: 10.1210/endo.142.11.8515. [DOI] [PubMed] [Google Scholar]
- Chorev M. Parathyroid hormone 1 receptor: insights into structure and function. Receptors Channels. 2002;8:219–242. [PubMed] [Google Scholar]
- Clopton BM, Winfield JA, Flammino FJ. Tonotopic organization: review and analysis. Brain Res. 1974;76:1–20. doi: 10.1016/0006-8993(74)90509-5. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Allard J, Truitt WA, McKenna KE. Central regulation of ejaculation. Physiol Behav. 2004;83:203–215. doi: 10.1016/j.physbeh.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Peters HJ, Veening JG. Distribution of Fos immunoreactivity following mating versus anogenital investigation in the male rat brain. Neuroscience. 1997;77:1151–1161. doi: 10.1016/s0306-4522(96)00542-8. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Peters HJ, Veening JG. Anatomical interrelationships of the medial preoptic area and other brain regions activated following male sexual behavior: a combined fos and tract-tracing study. J Comp Neurol. 1998;397:421–435. doi: 10.1002/(sici)1096-9861(19980803)397:3<421::aid-cne8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Veening JG, Petersen DW, Shipley MT. Parvocellular subparafascicular thalamic nucleus in the rat: anatomical and functional compartmentalization. J Comp Neurol. 2003a;463:117–131. doi: 10.1002/cne.10740. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Veening JG, Wells AB, Shipley MT. Afferent connections of the parvocellular subparafascicular thalamic nucleus in the rat: evidence for functional subdivisions. J Comp Neurol. 2003b;463:132–156. doi: 10.1002/cne.10739. [DOI] [PubMed] [Google Scholar]
- D'Hanis W, Linke R, Yilmazer-Hanke DM. Topography of thalamic and parabrachial calcitonin gene-related peptide (CGRP) immunoreactive neurons projecting to subnuclei of the amygdala and extended amygdala. J Comp Neurol. 2007;505:268–291. doi: 10.1002/cne.21495. [DOI] [PubMed] [Google Scholar]
- Della Penna K, Kinose F, Sun H, Koblan KS, Wang H. Tuberoinfundibular peptide of 39 residues (TIP39): molecular structure and activity for parathyroid hormone 2 receptor. Neuropharmacology. 2003;44:141–153. doi: 10.1016/s0028-3908(02)00335-0. [DOI] [PubMed] [Google Scholar]
- Dicker F, Quitterer U, Winstel R, Honold K, Lohse MJ. Phosphorylation-independent inhibition of parathyroid hormone receptor signaling by G protein-coupled receptor kinases. Proc Natl Acad Sci U S A. 1999;96:5476–5481. doi: 10.1073/pnas.96.10.5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobolyi A, Irwin S, Makara G, Usdin TB, Palkovits M. Calcitonin gene-related peptide-containing pathways in the rat forebrain. J Comp Neurol. 2005;489:92–119. doi: 10.1002/cne.20618. [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Irwin S, Wang J, Usdin TB. The distribution and neurochemistry of the parathyroid hormone 2 receptor in the rat hypothalamus. Neurochem Res. 2006a;31:227–236. doi: 10.1007/s11064-005-9011-9. [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Palkovits M, Bodnar I, Usdin TB. Neurons containing tuberoinfundibular peptide of 39 residues project to limbic, endocrine, auditory and spinal areas in rat. Neuroscience. 2003a;122:1093–1105. doi: 10.1016/j.neuroscience.2003.08.034. [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Palkovits M, Usdin TB. Expression and distribution of tuberoinfundibular peptide of 39 residues in the rat central nervous system. J Comp Neurol. 2003b;455:547–566. doi: 10.1002/cne.10515. [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Ueda H, Uchida H, Palkovits M, Usdin TB. Anatomical and physiological evidence for involvement of tuberoinfundibular peptide of 39 residues in nociception. Proc Natl Acad Sci U S A. 2002;99:1651–1656. doi: 10.1073/pnas.042416199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobolyi A, Wang J, Irwin S, Usdin TB. Postnatal development and gender-dependent expression of TIP39 in the rat brain. J Comp Neurol. 2006b;498:375–389. doi: 10.1002/cne.21063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong WK, Ryu H, Wagman IH. Nociceptive responses of neurons in medial thalamus and their relationship to spinothalamic pathways. J Neurophysiol. 1978;41:1592–1613. doi: 10.1152/jn.1978.41.6.1592. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Knapp DJ, Breese GR. Neuroanatomical characterization of Fos induction in rat behavioral models of anxiety. Brain Res. 1996;713:79–91. doi: 10.1016/0006-8993(95)01486-1. [DOI] [PubMed] [Google Scholar]
- Eichinger A, Fiaschi-Taesch N, Massfelder T, Fritsch S, Barthelmebs M, Helwig JJ. Transcript expression of the tuberoinfundibular peptide (TIP)39/PTH2 receptor system and non-PTH1 receptor-mediated tonic effects of TIP39 and other PTH2 receptor ligands in renal vessels. Endocrinology. 2002;143:3036–3043. doi: 10.1210/endo.143.8.8960. [DOI] [PubMed] [Google Scholar]
- Erlander MG, Tobin AJ. The structural and functional heterogeneity of glutamic acid decarboxylase: a review. Neurochem Res. 1991;16:215–226. doi: 10.1007/BF00966084. [DOI] [PubMed] [Google Scholar]
- Faber CA, Dobolyi A, Sleeman M, Usdin TB. Distribution of tuberoinfundibular peptide of 39 residues and its receptor, parathyroid hormone 2 receptor, in the mouse brain. J Comp Neurol. 2007;502:563–583. doi: 10.1002/cne.21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul RLM, Mehler WR. Thalamus. In: Paxinos G, editor. The Rat Nervous System. Academic Press; Sydney: 1985. pp. 129–168. [Google Scholar]
- Faye-Lund H, Osen KK. Anatomy of the inferior colliculus in rat. Anat Embryol (Berl) 1985;171:1–20. doi: 10.1007/BF00319050. [DOI] [PubMed] [Google Scholar]
- Fegley DB, Holmes A, Riordan T, Faber CA, Weiss JR, Ma S, Batkai S, Pacher P, Dobolyi A, Murphy A, Sleeman MW, Usdin TB. Increased fear- and stress-related anxiety-like behavior in mice lacking tuberoinfundibular peptide of 39 residues. Genes Brain Behav. 2008;7:933–942. doi: 10.1111/j.1601-183X.2008.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenzl T, Schuller G. Dissimilarities in the vocal control over communication and echolocation calls in bats. Behav Brain Res. 2007;182:173–179. doi: 10.1016/j.bbr.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Ferrari SL, Behar V, Chorev M, Rosenblatt M, Bisello A. Endocytosis of ligand-human parathyroid hormone receptor 1 complexes is protein kinase C-dependent and involves beta-arrestin2. Real-time monitoring by fluorescence microscopy. J Biol Chem. 1999;274:29968–29975. doi: 10.1074/jbc.274.42.29968. [DOI] [PubMed] [Google Scholar]
- Ferrari SL, Bisello A. Cellular distribution of constitutively active mutant parathyroid hormone (PTH)/PTH-related protein receptors and regulation of cyclic adenosine 3′,5′-monophosphate signaling by beta-arrestin2. Mol Endocrinol. 2001;15:149–163. doi: 10.1210/mend.15.1.0587. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- Fremeau RT, Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Friedman PA, Gesek FA, Morley P, Whitfield JF, Willick GE. Cell-specific signaling and structure-activity relations of parathyroid hormone analogs in mouse kidney cells. Endocrinology. 1999;140:301–309. doi: 10.1210/endo.140.1.6462. [DOI] [PubMed] [Google Scholar]
- Fuse G. Vergleichend-anatomische Beobachtungen am Hirnstamme der Saugetiere. VIII.: Eine weitere Bemerkung über den Nucleus ventralis accessorius lemnisci lateralis bei einigen Karnivoren (Katze, Hund, Fuchs, Dachs, Meler anakuma) Arb Anat Inst Sendai. 1926;12:39–44. [Google Scholar]
- Gardella TJ, Luck MD, Jensen GS, Usdin TB, Juppner H. Converting parathyroid hormone-related peptide (PTHrP) into a potent PTH-2 receptor agonist. J Biol Chem. 1996;271:19888–19893. doi: 10.1074/jbc.271.33.19888. [DOI] [PubMed] [Google Scholar]
- Gensure R, Juppner H. Parathyroid hormone without parathyroid glands. Endocrinology. 2005;146:544–546. doi: 10.1210/en.2004-1557. [DOI] [PubMed] [Google Scholar]
- Gensure RC, Gardella TJ, Juppner H. Parathyroid hormone and parathyroid hormone-related peptide, and their receptors. Biochem Biophys Res Commun. 2005;328:666–678. doi: 10.1016/j.bbrc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- Gensure RC, Ponugoti B, Gunes Y, Papasani MR, Lanske B, Bastepe M, Rubin DA, Juppner H. Identification and characterization of two parathyroid hormone-like molecules in zebrafish. Endocrinology. 2004;145:1634–1639. doi: 10.1210/en.2003-0964. [DOI] [PubMed] [Google Scholar]
- Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, Eckhardt AE, Cowan CL, Spurney RF, Luttrell LM, Lefkowitz RJ. Distinct beta-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J Biol Chem. 2006;281:10856–10864. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- Gillespie MT, Martin TJ. The parathyroid hormone-related protein gene and its expression. Mol Cell Endocrinol. 1994;100:143–147. doi: 10.1016/0303-7207(94)90293-3. [DOI] [PubMed] [Google Scholar]
- Girardot MN, Brennan TJ, Martindale ME, Foreman RD. Effects of stimulating the subcoeruleus-parabrachial region on the non-noxious and noxious responses of T1-T5 spinothalamic tract neurons in the primate. Brain Res. 1987;409:19–30. doi: 10.1016/0006-8993(87)90737-2. [DOI] [PubMed] [Google Scholar]
- Goold CP, Usdin TB, Hoare SR. Regions in rat and human parathyroid hormone (PTH) 2 receptors controlling receptor interaction with PTH and with antagonist ligands. J Pharmacol Exp Ther. 2001;299:678–690. [PubMed] [Google Scholar]
- Gorski RA. Sexual dimorphisms of the brain. J Anim Sci. 1985;61 3:38–61. doi: 10.1093/ansci/61.supplement_3.38. [DOI] [PubMed] [Google Scholar]
- Haaf JA, Maigret C, Andringa-Bakker EA, van Wimersma Greidanus TB. Dynorphin-(1-13) is a potent in vivo suppressor of vasopressin levels in the rat. Acta Endocrinol (Copenh) 1987;114:96–101. doi: 10.1530/acta.0.1140096. [DOI] [PubMed] [Google Scholar]
- Hage SR, Jurgens U. On the role of the pontine brainstem in vocal pattern generation: a telemetric single-unit recording study in the squirrel monkey. J Neurosci. 2006;26:7105–7115. doi: 10.1523/JNEUROSCI.1024-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannig S, Jurgens U. Projections of the ventrolateral pontine vocalization area in the squirrel monkey. Exp Brain Res. 2006;169:92–105. doi: 10.1007/s00221-005-0128-5. [DOI] [PubMed] [Google Scholar]
- Hansen IA, Jakob O, Wortmann S, Arzberger T, Allolio B, Blind E. Characterization of the human and mouse genes encoding the tuberoinfundibular peptide of 39 residues, a ligand of the parathyroid hormone receptor family. J Endocrinol. 2002;174:95–102. doi: 10.1677/joe.0.1740095. [DOI] [PubMed] [Google Scholar]
- Hardy SG, Haigler HJ, Leichnetz GR. Paralemniscal reticular formation: response of cells to a noxious stimulus. Brain Res. 1983;267:217–223. doi: 10.1016/0006-8993(83)90873-9. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Harmar AJ. Family-B G-protein-coupled receptors. Genome Biol. 2001;2:30131–30110. doi: 10.1186/gb-2001-2-12-reviews3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasen NS, Gammie SC. Differential fos activation in virgin and lactating mice in response to an intruder. Physiol Behav. 2005;84:681–695. doi: 10.1016/j.physbeh.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Haws CM, Williamson AM, Fields HL. Putative nociceptive modulatory neurons in the dorsolateral pontomesencephalic reticular formation. Brain Res. 1989;483:272–282. doi: 10.1016/0006-8993(89)90171-6. [DOI] [PubMed] [Google Scholar]
- Heeb MM, Yahr P. Anatomical and functional connections among cell groups in the gerbil brain that are activated with ejaculation. J Comp Neurol. 2001;439:248–258. doi: 10.1002/cne.1346. [DOI] [PubMed] [Google Scholar]
- Henkel CK. Afferent sources of a lateral midbrain tegmental zone associated with the pinnae in the cat as mapped by retrograde transport of horseradish peroxidase. J Comp Neurol. 1981;203:213–226. doi: 10.1002/cne.902030205. [DOI] [PubMed] [Google Scholar]
- Henkel CK, Edwards SB. The superior colliculus control of pinna movements in the cat: possible anatomical connections. J Comp Neurol. 1978;182:763–776. doi: 10.1002/cne.901820502. [DOI] [PubMed] [Google Scholar]
- Herbert H, Aschoff A, Ostwald J. Topography of projections from the auditory cortex to the inferior colliculus in the rat. J Comp Neurol. 1991;304:103–122. doi: 10.1002/cne.903040108. [DOI] [PubMed] [Google Scholar]
- Herbert H, Klepper A, Ostwald J. Afferent and efferent connections of the ventrolateral tegmental area in the rat. Anat Embryol (Berl) 1997;196:235–259. doi: 10.1007/s004290050094. [DOI] [PubMed] [Google Scholar]
- Herkenham M. Mismatches between neurotransmitter and receptor localizations in brain: observations and implications. Neuroscience. 1987;23:1–38. doi: 10.1016/0306-4522(87)90268-5. [DOI] [PubMed] [Google Scholar]
- Hoare SR, Bonner TI, Usdin TB. Comparison of rat and human parathyroid hormone 2 (PTH2) receptor activation: PTH is a low potency partial agonist at the rat PTH2 receptor. Endocrinology. 1999;140:4419–4425. doi: 10.1210/endo.140.10.7040. [DOI] [PubMed] [Google Scholar]
- Hoare SR, Clark JA, Usdin TB. Molecular determinants of tuberoinfundibular peptide of 39 residues (TIP39) selectivity for the parathyroid hormone-2 (PTH2) receptor. N-terminal truncation of TIP39 reverses PTH2 receptor/PTH1 receptor binding selectivity. J Biol Chem. 2000a;275:27274–27283. doi: 10.1074/jbc.M003910200. [DOI] [PubMed] [Google Scholar]
- Hoare SR, Rubin DA, Juppner H, Usdin TB. Evaluating the ligand specificity of zebrafish parathyroid hormone (PTH) receptors: comparison of PTH, PTH-related protein, and tuberoinfundibular peptide of 39 residues. Endocrinology. 2000b;141:3080–3086. doi: 10.1210/endo.141.9.7645. [DOI] [PubMed] [Google Scholar]
- Hoare SR, Usdin TB. Tuberoinfundibular peptide (7-39) [TIP(7-39)], a novel, selective, high-affinity antagonist for the parathyroid hormone-1 receptor with no detectable agonist activity. J Pharmacol Exp Ther. 2000;295:761–770. [PubMed] [Google Scholar]
- Hoare SR, Usdin TB. Molecular mechanisms of ligand recognition by parathyroid hormone 1 (PTH1) and PTH2 receptors. Curr Pharm Des. 2001;7:689–713. doi: 10.2174/1381612013397825. [DOI] [PubMed] [Google Scholar]
- Holmes A, Heilig M, Rupniak NM, Steckler T, Griebel G. Neuropeptide systems as novel therapeutic targets for depression and anxiety disorders. Trends Pharmacol Sci. 2003;24:580–588. doi: 10.1016/j.tips.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Holmes A, Li Q, Koenig EA, Gold E, Stephenson D, Yang RJ, Dreiling J, Sullivan T, Crawley JN. Phenotypic assessment of galanin overexpressing and galanin receptor R1 knockout mice in the tail suspension test for depression-related behavior. Psychopharmacology (Berl) 2005;178:276–285. doi: 10.1007/s00213-004-1997-1. [DOI] [PubMed] [Google Scholar]
- Holstege G, Georgiadis JR, Paans AM, Meiners LC, van der Graaf FH, Reinders AA. Brain activation during human male ejaculation. J Neurosci. 2003;23:9185–9193. doi: 10.1523/JNEUROSCI.23-27-09185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege G, Kuypers HG. The anatomy of brain stem pathways to the spinal cord in cat. A labeled amino acid tracing study. Prog Brain Res. 1982;57:145–175. doi: 10.1016/S0079-6123(08)64128-X. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Bartfai T, Bloom F. Neuropeptides: opportunities for drug discovery. Lancet Neurol. 2003;2:463–472. doi: 10.1016/s1474-4422(03)00482-4. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Martensson R, Björklund A, Kleinau S, Goldstein M. Distributional maps of tyrosine hydroxylase immunoreactive neurons in the rat brain. In: Björklund A, Hökfelt T, editors. Handbook of Chemical Neuroanatomy. Elsevier; Amsterdam: 1984. pp. 277–379. [Google Scholar]
- Hökfelt T, Phillipson O, Goldstein M. Evidence for a dopaminergic pathway in the rat descending from the A11 cell group to the spinal cord. Acta Physiol Scand. 1979;107:393–395. doi: 10.1111/j.1748-1716.1979.tb06491.x. [DOI] [PubMed] [Google Scholar]
- Hurwitz S. Homeostatic control of plasma calcium concentration. Crit Rev Biochem Mol Biol. 1996;31:41–100. doi: 10.3109/10409239609110575. [DOI] [PubMed] [Google Scholar]
- Inagaki S, Matsuda Y, Nakai Y, Takagi H. Calcitonin gene-related peptide (CGRP) immunoreactivity in the afferents to the caudate-putamen and perirhinal cortex of rats. Brain Res. 1990;537:263–270. doi: 10.1016/0006-8993(90)90367-k. [DOI] [PubMed] [Google Scholar]
- Ishida-Yamamoto A, Tohyama M. Calcitonin gene-related peptide in the nervous tissue. Prog Neurobiol. 1989;33:335–386. doi: 10.1016/0301-0082(89)90006-3. [DOI] [PubMed] [Google Scholar]
- Jane JA, Masterton RB, Diamond IT. The function of the tectum for attention to auditory stimuli in the cat. J Comp Neurol. 1965;125:165–191. doi: 10.1002/cne.901250203. [DOI] [PubMed] [Google Scholar]
- John MR, Arai M, Rubin DA, Jonsson KB, Juppner H. Identification and characterization of the murine and human gene encoding the tuberoinfundibular peptide of 39 residues. Endocrinology. 2002;143:1047–1057. doi: 10.1210/endo.143.3.8698. [DOI] [PubMed] [Google Scholar]
- Jonsson KB, John MR, Gensure RC, Gardella TJ, Juppner H. Tuberoinfundibular peptide 39 binds to the parathyroid hormone (PTH)/PTH-related peptide receptor, but functions as an antagonist. Endocrinology. 2001;142:704–709. doi: 10.1210/endo.142.2.7945. [DOI] [PubMed] [Google Scholar]
- Juppner H, Abou-Samra AB, Freeman M, Kong XF, Schipani E, Richards J, Kolakowski LF, Jr, Hock J, Potts JT, Jr, Kronenberg HM, et al. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science. 1991;254:1024–1026. doi: 10.1126/science.1658941. [DOI] [PubMed] [Google Scholar]
- Kent JM, Rauch SL. Neurocircuitry of anxiety disorders. Curr Psychiatry Rep. 2003;5:266–273. doi: 10.1007/s11920-003-0055-8. [DOI] [PubMed] [Google Scholar]
- King AJ, Jiang ZD, Moore DR. Auditory brainstem projections to the ferret superior colliculus: anatomical contribution to the neural coding of sound azimuth. J Comp Neurol. 1998;390:342–365. [PubMed] [Google Scholar]
- Kiss J, Kocsis K, Csáki A, Halász B. Evidence for vesicular glutamate transporter synapses onto gonadotropin-releasing hormone and other neurons in the rat medial preoptic area. Eur J Neurosci. 2003;18:3267–3278. doi: 10.1111/j.1460-9568.2003.03085.x. [DOI] [PubMed] [Google Scholar]
- Kiyohara T, Miyata S, Nakamura T, Shido O, Nakashima T, Shibata M. Differences in Fos expression in the rat brains between cold and warm ambient exposures. Brain Res Bull. 1995;38:193–201. doi: 10.1016/0361-9230(95)00093-t. [DOI] [PubMed] [Google Scholar]
- Kresse A, Jacobowitz DM, Skofitsch G. Detailed mapping of CGRP mRNA expression in the rat central nervous system: comparison with previous immunocytochemical findings. Brain Res Bull. 1995;36:261–274. doi: 10.1016/0361-9230(94)00201-b. [DOI] [PubMed] [Google Scholar]
- Kruger L, Sternini C, Brecha NC, Mantyh PW. Distribution of calcitonin gene-related peptide immunoreactivity in relation to the rat central somatosensory projection. J Comp Neurol. 1988;273:149–162. doi: 10.1002/cne.902730203. [DOI] [PubMed] [Google Scholar]
- Kudo M, Tashiro T, Higo S, Matsuyama T, Kawamura S. Ascending projections from the nucleus of the brachium of the inferior colliculus in the cat. Exp Brain Res. 1984;54:203–211. doi: 10.1007/BF00236219. [DOI] [PubMed] [Google Scholar]
- Kuo J, Usdin TB. Development of a rat parathyroid hormone 2 receptor antagonist. Peptides. 2007;28:887–892. doi: 10.1016/j.peptides.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano K, Gainer H. Modulation of voltage-activated Ca currents by pain-inducing agents in a dorsal root ganglion neuronal line, F-11. J Neurosci Res. 1993;34:158–169. doi: 10.1002/jnr.490340203. [DOI] [PubMed] [Google Scholar]
- Ky B, Shughrue PJ. Methods to enhance signal using isotopic in situ hybridization. J Histochem Cytochem. 2002;50:1031–1037. doi: 10.1177/002215540205000805. [DOI] [PubMed] [Google Scholar]
- LaBuda CJ, Dobolyi A, Usdin TB. Tuberoinfundibular peptide of 39 residues produces anxiolytic and antidepressant actions. Neuroreport. 2004;15:881–885. doi: 10.1097/00001756-200404090-00030. [DOI] [PubMed] [Google Scholar]
- LaBuda CJ, Usdin TB. Tuberoinfundibular peptide of 39 residues decreases pain-related affective behavior. Neuroreport. 2004;15:1779–1782. doi: 10.1097/01.wnr.0000134849.63755.15. [DOI] [PubMed] [Google Scholar]
- Lamming GE, editor. Marshall's Physiology of Reproduction. Chapman & Hall; London: 1994. [Google Scholar]
- Law F, Ferrari S, Rizzoli R, Bonjour JP. Parathyroid hormone-related protein: physiology and pathophysiology. Adv Nephrol Necker Hosp. 1994;23:281–294. [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Farb C, Ruggiero DA. Topographic organization of neurons in the acoustic thalamus that project to the amygdala. J Neurosci. 1990;10:1043–1054. doi: 10.1523/JNEUROSCI.10-04-01043.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux JE, Ruggiero DA, Forest R, Stornetta R, Reis DJ. Topographic organization of convergent projections to the thalamus from the inferior colliculus and spinal cord in the rat. J Comp Neurol. 1987;264:123–146. doi: 10.1002/cne.902640110. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Ruggiero DA, Reis DJ. Projections to the subcortical forebrain from anatomically defined regions of the medial geniculate body in the rat. J Comp Neurol. 1985;242:182–213. doi: 10.1002/cne.902420204. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Sakaguchi A, Reis DJ. Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned to acoustic stimuli. J Neurosci. 1984;4:683–698. doi: 10.1523/JNEUROSCI.04-03-00683.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leichnetz GR, Watkins L, Griffin G, Murfin I, Mayer DJ. The projection from the nucleus raphe magnus and other brainstem nuclei to the spinal cord in the rat: a study using the HRP blue-reaction. Neurosci Lett. 1978;8:119–124. [Google Scholar]
- Leng G, Ludwig M. Jacques Benoit Lecture. Information processing in the hypothalamus: peptides and analogue computation. J Neuroendocrinol. 2006;18:379–392. doi: 10.1111/j.1365-2826.2006.01428.x. [DOI] [PubMed] [Google Scholar]
- Li C, Chen P, Smith MS. Neural populations in the rat forebrain and brainstem activated by the suckling stimulus as demonstrated by cFos expression. Neuroscience. 1999;94:117–129. doi: 10.1016/s0306-4522(99)00236-5. [DOI] [PubMed] [Google Scholar]
- Lin SH, Miyata S, Matsunaga W, Kawarabayashi T, Nakashima T, Kiyohara T. Metabolic mapping of the brain in pregnant, parturient and lactating rats using fos immunohistochemistry. Brain Res. 1998;787:226–236. doi: 10.1016/s0006-8993(97)01484-4. [DOI] [PubMed] [Google Scholar]
- Lin W, McKinney K, Liu L, Lakhlani S, Jennes L. Distribution of vesicular glutamate transporter-2 messenger ribonucleic acid and protein in the septum-hypothalamus of the rat. Endocrinology. 2003;144:662–670. doi: 10.1210/en.2002-220908. [DOI] [PubMed] [Google Scholar]
- Linke R. Differential projection patterns of superior and inferior collicular neurons onto posterior paralaminar nuclei of the thalamus surrounding the medial geniculate body in the rat. Eur J Neurosci. 1999;11:187–203. doi: 10.1046/j.1460-9568.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- Linke R, Schwegler H. Convergent and complementary projections of the caudal paralaminar thalamic nuclei to rat temporal and insular cortex. Cereb Cortex. 2000;10:753–771. doi: 10.1093/cercor/10.8.753. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Stern JM. Role of the midbrain periaqueductal gray in maternal nurturance and aggression: c-fos and electrolytic lesion studies in lactating rats. J Neurosci. 1997;17:3364–3378. doi: 10.1523/JNEUROSCI.17-09-03364.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Gundlach AL. Relaxin-family peptide and receptor systems in brain: insights from recent anatomical and functional studies. Adv Exp Med Biol. 2007;612:119–137. doi: 10.1007/978-0-387-74672-2_9. [DOI] [PubMed] [Google Scholar]
- Maillard CA, Edwards DA. Excitotoxin lesions of the zona incerta/lateral tegmentum continuum: effects on male sexual behavior in rats. Behav Brain Res. 1991;46:143–149. doi: 10.1016/s0166-4328(05)80107-x. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Chen C, Tonegawa S, Basbaum AI. Preserved acute pain and reduced neuropathic pain in mice lacking PKCgamma. Science. 1997;278:279–283. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- Mannstadt M, Juppner H, Gardella TJ. Receptors for PTH and PTHrP: their biological importance and functional properties. Am J Physiol. 1999;277:F665–675. doi: 10.1152/ajprenal.1999.277.5.F665. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Moseley JM, Gillespie MT. Parathyroid hormone-related protein: biochemistry and molecular biology. Crit Rev Biochem Mol Biol. 1991;26:377–395. doi: 10.3109/10409239109114073. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Moseley JM, Williams ED. Parathyroid hormone-related protein: hormone and cytokine. J Endocrinol. 1997;154(Suppl):S23–37. [PubMed] [Google Scholar]
- Mason AJ, Lopez JJ, Beyermann M, Glaubitz C. A spectroscopic study of the membrane interaction of tuberoinfundibular peptide of 39 residues (TIP39) Biochim Biophys Acta. 2005;1714:1–10. doi: 10.1016/j.bbamem.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Metzner W. An audio-vocal interface in echolocating horseshoe bats. J Neurosci. 1993;13:1899–1915. doi: 10.1523/JNEUROSCI.13-05-01899.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzner W. Anatomical basis for audio-vocal integration in echolocating horseshoe bats. J Comp Neurol. 1996;368:252–269. doi: 10.1002/(SICI)1096-9861(19960429)368:2<252::AID-CNE6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Miao D, Tong XK, Chan GK, Panda D, McPherson PS, Goltzman D. Parathyroid hormone-related peptide stimulates osteogenic cell proliferation through protein kinase C activation of the Ras/mitogen-activated protein kinase signaling pathway. J Biol Chem. 2001;276:32204–32213. doi: 10.1074/jbc.M101084200. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC, Clock AE, Xu L, Green DM. A panoramic code for sound location by cortical neurons. Science. 1994;264:842–844. doi: 10.1126/science.8171339. [DOI] [PubMed] [Google Scholar]
- Mierke DF, Mao L, Pellegrini M, Piserchio A, Plati J, Tsomaia N. Structural characterization of the parathyroid hormone receptor domains determinant for ligand binding. Biochem Soc Trans. 2007;35:721–723. doi: 10.1042/BST0350721. [DOI] [PubMed] [Google Scholar]
- Mikkelsen JD, Simonneaux V. The neuroanatomy of the kisspeptin system in the mammalian brain. Peptides. 2009;30:26–33. doi: 10.1016/j.peptides.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Misiano P, Scott BB, Scheideler MA, Garnier M. PTH2 receptor-mediated inhibitory effect of parathyroid hormone and TIP39 on cell proliferation. Eur J Pharmacol. 2003;468:159–166. doi: 10.1016/s0014-2999(03)01673-x. [DOI] [PubMed] [Google Scholar]
- Miyata S, Ishiyama M, Shido O, Nakashima T, Shibata M, Kiyohara T. Central mechanism of neural activation with cold acclimation of rats using Fos immunohistochemistry. Neurosci Res. 1995;22:209–218. doi: 10.1016/0168-0102(95)00900-x. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Radeke MJ, Feinstein SC, Snider WD. Presence or absence of TrkA protein distinguishes subsets of small sensory neurons with unique cytochemical characteristics and dorsal horn projections. J Comp Neurol. 1995;361:404–416. doi: 10.1002/cne.903610305. [DOI] [PubMed] [Google Scholar]
- Moriizumi T, Hattori T. Non-dopaminergic projection from the subparafascicular area to the temporal cortex in the rat. Neurosci Lett. 1991;129:127–130. doi: 10.1016/0304-3940(91)90736-d. [DOI] [PubMed] [Google Scholar]
- Moriizumi T, Hattori T. Anatomical and functional compartmentalization of the subparafascicular thalamic nucleus in the rat. Exp Brain Res. 1992;90:175–179. doi: 10.1007/BF00229269. [DOI] [PubMed] [Google Scholar]
- Muff R, Born W, Kaufmann M, Fischer JA. Parathyroid hormone and parathyroid hormone-related protein receptor update. Mol Cell Endocrinol. 1994;100:35–38. doi: 10.1016/0303-7207(94)90275-5. [DOI] [PubMed] [Google Scholar]
- Namura S, Takada M, Kikuchi H, Mizuno N. Collateral projections of single neurons in the posterior thalamic region to both the temporal cortex and the amygdala: a fluorescent retrograde double-labeling study in the rat. J Comp Neurol. 1997;384:59–70. [PubMed] [Google Scholar]
- Numan M, Insel TR. The Neurobiology of Parental Behavior. Springer; New York: 2003. [Google Scholar]
- Numan M, Sheehan TP. Neuroanatomical circuitry for mammalian maternal behavior. Ann N Y Acad Sci. 1997;807:101–125. doi: 10.1111/j.1749-6632.1997.tb51915.x. [DOI] [PubMed] [Google Scholar]
- Oiso Y, Iwasaki Y, Kondo K, Takatsuki K, Tomita A. Effect of the opioid kappa-receptor agonist U50488H on the secretion of arginine vasopressin. Study on the mechanism of U50488H-induced diuresis. Neuroendocrinology. 1988;48:658–662. doi: 10.1159/000125078. [DOI] [PubMed] [Google Scholar]
- Olszewski J, Baxter D. Cytoarchitecture of the Human Brain Stem. Karger; New York: 1982. [Google Scholar]
- Ottersen OP, Ben-Ari Y. Afferent connections to the amygdaloid complex of the rat and cat. I. Projections from the thalamus. J Comp Neurol. 1979;187:401–424. doi: 10.1002/cne.901870209. [DOI] [PubMed] [Google Scholar]
- Palkovits M. Neuropeptides in the brain. Front Neuroendocrinol. 1988;10:1–44. [Google Scholar]
- Palkovits M. Peptidergic neurotransmitters in the endocrine hypothalamus. Ciba Found Symp. 1992;168:3–10. doi: 10.1002/9780470514283.ch2. discussion 10-15. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Dobolyi A, Helfferich F, Usdin TB. Localization and chemical characterization of the audiogenic stress pathway. Ann N Y Acad Sci. 2004;1018:16–24. doi: 10.1196/annals.1296.002. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Dobolyi A, Helfferich F, Usdin TB. Acute loud noise activates TIP39-immunoreactive neurons in the rat brain. Brain Structure and Function. 2009a doi: 10.1007/s00429-009-0233-5. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palkovits M, Usdin T, Dobolyi A. TIP39-immunoreactive input to the hypothalamic nuclei in rats. Neuroscience. 2009b In preparation. [Google Scholar]
- Pan W, Kastin AJ. Urocortin and the brain. Prog Neurobiol. 2008;84:148–156. doi: 10.1016/j.pneurobio.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papasani MR, Gensure RC, Yan YL, Gunes Y, Postlethwait JH, Ponugoti B, John MR, Juppner H, Rubin DA. Identification and characterization of the zebrafish and fugu genes encoding tuberoinfundibular peptide 39. Endocrinology. 2004;145:5294–5304. doi: 10.1210/en.2004-0159. [DOI] [PubMed] [Google Scholar]
- Papez JW. Reticulo-spinal tracts in the cat. Marchi method. J Comp Neurol. 1926;41:365–399. [Google Scholar]
- Papez JW, Aronson LR. Thalamic nuclei of Pithecus (Macacus) rhesus. I. Ventral thalamus. Arch Neurol Psychol. 1934;32:1–26. [Google Scholar]
- Paxinos G, Butcher LL. Organizational principles of the brain as revealed by choline acetyltransferase and acetylcholinesterase distribution and projections. In: Paxinos G, editor. The rat nervous system. Academic Press; Sydney: 1985. pp. 487–521. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Sydney: 1998. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 2005. [Google Scholar]
- Pellegrini M, Royo M, Rosenblatt M, Chorev M, Mierke DF. Addressing the tertiary structure of human parathyroid hormone-(1-34) J Biol Chem. 1998;273:10420–10427. doi: 10.1074/jbc.273.17.10420. [DOI] [PubMed] [Google Scholar]
- Perales M, Winer JA, Prieto JJ. Focal projections of cat auditory cortex to the pontine nuclei. J Comp Neurol. 2006;497:959–980. doi: 10.1002/cne.20988. [DOI] [PubMed] [Google Scholar]
- Perez-Gonzalez D, Malmierca MS, Covey E. Novelty detector neurons in the mammalian auditory midbrain. Eur J Neurosci. 2005;22:2879–2885. doi: 10.1111/j.1460-9568.2005.04472.x. [DOI] [PubMed] [Google Scholar]
- Peschanski M, Mantyh PW. Efferent connections of the subfascicular area of the mesodiencephalic junction and its possible involvement in stimulation-produced analgesia. Brain Res. 1983;263:181–190. doi: 10.1016/0006-8993(83)90311-6. [DOI] [PubMed] [Google Scholar]
- Pezzone MA, Lee WS, Hoffman GE, Rabin BS. Induction of c-Fos immunoreactivity in the rat forebrain by conditioned and unconditioned aversive stimuli. Brain Res. 1992;597:41–50. doi: 10.1016/0006-8993(92)91503-7. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Heeb MM. Implications of immediate-early gene induction in the brain following sexual stimulation of female and male rodents. Brain Res Bull. 1997;44:397–407. doi: 10.1016/s0361-9230(97)00219-0. [DOI] [PubMed] [Google Scholar]
- Piserchio A, Usdin T, Mierke DF. Structure of tuberoinfundibular peptide of 39 residues. J Biol Chem. 2000;275:27284–27290. doi: 10.1074/jbc.M003869200. [DOI] [PubMed] [Google Scholar]
- Polgar E, Fowler JH, McGill MM, Todd AJ. The types of neuron which contain protein kinase C gamma in rat spinal cord. Brain Res. 1999;833:71–80. doi: 10.1016/s0006-8993(99)01500-0. [DOI] [PubMed] [Google Scholar]
- Rhodes DL, Liebeskind JC. Analgesia from rostral brain stem stimulation in the rat. Brain Res. 1978;143:521–532. doi: 10.1016/0006-8993(78)90362-1. [DOI] [PubMed] [Google Scholar]
- Rioch DK. Studies on the diencephalon of carnivora. Part I. The nuclear configuration of the thalamus, epithalamus, and hypothalamus of the dog and cat. J Comp Neurol. 1929;49:1–120. [Google Scholar]
- Risold PY, Canteras NS, Swanson LW. Organization of projections from the anterior hypothalamic nucleus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1994;348:1–40. doi: 10.1002/cne.903480102. [DOI] [PubMed] [Google Scholar]
- Rizzoli R, Ferrari SL, Pizurki L, Caverzasio J, Bonjour JP. Actions of parathyroid hormone and parathyroid hormone-related protein. J Endocrinol Invest. 1992;15:51–56. [PubMed] [Google Scholar]
- Roland BL, Sutton SW, Wilson SJ, Luo L, Pyati J, Huvar R, Erlander MG, Lovenberg TW. Anatomical distribution of prolactin-releasing peptide and its receptor suggests additional functions in the central nervous system and periphery. Endocrinology. 1999;140:5736–5745. doi: 10.1210/endo.140.12.7211. [DOI] [PubMed] [Google Scholar]
- Ross G, Engel P, Abdallah Y, Kummer W, Schluter KD. Tuberoinfundibular peptide of 39 residues: a new mediator of cardiac function via nitric oxide production in the rat heart. Endocrinology. 2005;146:2221–2228. doi: 10.1210/en.2004-1180. [DOI] [PubMed] [Google Scholar]
- Ross G, Heinemann MP, Schluter KD. Vasodilatory effect of tuberoinfundibular peptide (TIP39): requirement of receptor desensitization and its beneficial effect in the post-ischemic heart. Peptides. 2007;28:878–886. doi: 10.1016/j.peptides.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Rub U, Del Tredici K, Del Turco D, Braak H. The intralaminar nuclei assigned to the medial pain system and other components of this system are early and progressively affected by the Alzheimer's disease-related cytoskeletal pathology. J Chem Neuroanat. 2002;23:279–290. doi: 10.1016/s0891-0618(02)00007-8. [DOI] [PubMed] [Google Scholar]
- Rubin DA, Hellman P, Zon LI, Lobb CJ, Bergwitz C, Juppner H. A G protein-coupled receptor from zebrafish is activated by human parathyroid hormone and not by human or teleost parathyroid hormone-related peptide. Implications for the evolutionary conservation of calcium-regulating peptide hormones. J Biol Chem. 1999;274:23035–23042. doi: 10.1074/jbc.274.33.23035. [DOI] [PubMed] [Google Scholar]
- Rubin DA, Juppner H. Zebrafish express the common parathyroid hormone/parathyroid hormone-related peptide receptor (PTH1R) and a novel receptor (PTH3R) that is preferentially activated by mammalian and fugufish parathyroid hormone-related peptide. J Biol Chem. 1999;274:28185–28190. doi: 10.1074/jbc.274.40.28185. [DOI] [PubMed] [Google Scholar]
- Sachs BD, Meisel RL. The Physiology of Male Sexual Behavior. In: Knobil E, Meill J, editors. The Physiology of Reproduction. Raven Press; New York: 1988. [Google Scholar]
- Saldaña E, Merchán MA. Intrinsic and commissural connections of the inferior colliculus. In: Winer JA, Schreiner CE, editors. The Inferior Colliculus. Springer-Verlag; New York: 2005. pp. 155–181. [DOI] [PubMed] [Google Scholar]
- Schuller G, Covey E, Casseday JH. Auditory Pontine Grey: Connections and Response Properties in the Horseshoe Bat. Eur J Neurosci. 1991;3:648–662. doi: 10.1111/j.1460-9568.1991.tb00851.x. [DOI] [PubMed] [Google Scholar]
- Schuller G, Radtke-Schuller S. Neural control of vocalization in bats: mapping of brainstem areas with electrical microstimulation eliciting species-specific echolocation calls in the rufous horseshoe bat. Exp Brain Res. 1990;79:192–206. doi: 10.1007/BF00228889. [DOI] [PubMed] [Google Scholar]
- Schwindinger WF, Fredericks J, Watkins L, Robinson H, Bathon JM, Pines M, Suva LJ, Levine MA. Coupling of the PTH/PTHrP receptor to multiple G-proteins. Direct demonstration of receptor activation of Gs, Gq/11, and Gi(1) by [alpha-32P]GTP-gamma-azidoanilide photoaffinity labeling. Endocrine. 1998;8:201–209. doi: 10.1385/ENDO:8:2:201. [DOI] [PubMed] [Google Scholar]
- Segre GV, Abou-Samra AB, Juppner H, Schipani E, Force T, Urena P, Freeman M, Kong XF, Kolakowski LF, Jr, Hock J, et al. Characterization of cloned PTH/PTHrP receptors. J Endocrinol Invest. 1992;15:11–17. [PubMed] [Google Scholar]
- Shimura T, Shimokochi M. Modification of male rat copulatory behavior by lateral midbrain stimulation. Physiol Behav. 1991;50:989–994. doi: 10.1016/0031-9384(91)90426-o. [DOI] [PubMed] [Google Scholar]
- Shipley MT, Ennis M, Rizvi TA, Behbehani MM. Topographical specificity of forebrain inputs to the midbrain periaqueductal gray: evidence for discrete longitudunally organized input columns. In: Depaulis A, Bandler R, editors. The Midbrain Periaqueductal Gray Matter Functional, Anatomical, and Neurochemical Organization. Plenum Press; New York: 1991. pp. 417–448. [Google Scholar]
- Shoemaker JM, Riley LG, Hirano T, Grau EG, Rubin DA. Differential expression of tuberoinfundibular peptide 38 and glucose-6-phosphatase in tilapia. Gen Comp Endocrinol. 2006;146:186–194. doi: 10.1016/j.ygcen.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. The organization of neural inputs to the medial preoptic nucleus of the rat. J Comp Neurol. 1986;246:312–342. doi: 10.1002/cne.902460304. [DOI] [PubMed] [Google Scholar]
- Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol Psychiatry. 2003;53:275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- Skagerberg G, Lindvall O. Organization of diencephalic dopamine neurones projecting to the spinal cord in the rat. Brain Res. 1985;342:340–351. doi: 10.1016/0006-8993(85)91134-5. [DOI] [PubMed] [Google Scholar]
- Skofitsch G, Jacobowitz DM. Calcitonin gene-related peptide: detailed immunohistochemical distribution in the central nervous system. Peptides. 1985;6:721–745. doi: 10.1016/0196-9781(85)90178-0. [DOI] [PubMed] [Google Scholar]
- Sluyter F, Korte SM, Bohus B, Van Oortmerssen GA. Behavioral stress response of genetically selected aggressive and nonaggressive wild house mice in the shock-probe/defensive burying test. Pharmacol Biochem Behav. 1996;54:113–116. doi: 10.1016/0091-3057(95)02164-7. [DOI] [PubMed] [Google Scholar]
- Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20:629–632. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- Sugimura Y, Murase T, Ishizaki S, Tachikawa K, Arima H, Miura Y, Usdin TB, Oiso Y. Centrally administered tuberoinfundibular peptide of 39 residues inhibits arginine vasopressin release in conscious rats. Endocrinology. 2003;144:2791–2796. doi: 10.1210/en.2002-0017. [DOI] [PubMed] [Google Scholar]
- Sugiyama K, Ryu H, Uemura K. Identification of nociceptive neurons in the medial thalamus: morphological studies of nociceptive neurons with intracellular injection of horseradish peroxidase. Brain Res. 1992;586:36–43. doi: 10.1016/0006-8993(92)91368-o. [DOI] [PubMed] [Google Scholar]
- Sun X, Xia Q, Lai CH, Shum DK, Chan YS, He J. Corticofugal modulation of acoustically induced Fos expression in the rat auditory pathway. J Comp Neurol. 2007;501:509–525. doi: 10.1002/cne.21249. [DOI] [PubMed] [Google Scholar]
- Taber E. The cytoarchitecture of the brain stem of the cat. I. Brain stem nuclei of cat. J Comp Neurol. 1961;116:27–69. doi: 10.1002/cne.901160104. [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Bjerknes LK, Goetzl EJ, Levine JD. Mediation of primary afferent peripheral hyperalgesia by the cAMP second messenger system. Neuroscience. 1989;32:577–580. doi: 10.1016/0306-4522(89)90280-7. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Smogorzewski M, Koss M, Massry SG. Pathways involved in PTH-induced rise in cytosolic Ca2+ concentration of rat renal proximal tubule. Am J Physiol. 1995;268:F330–337. doi: 10.1152/ajprenal.1995.268.2.F330. [DOI] [PubMed] [Google Scholar]
- Thompson AM. Descending Connections of the Auditory Midbrain. In: Ehret G, Romand R, editors. The Central Auditory System. Oxford University Press; Oxford: 1997. pp. 182–199. [Google Scholar]
- Treit D, Fundytus M. A comparison of buspirone and chlordiazepoxide in the shock-probe/burying test for anxiolytics. Pharmacol Biochem Behav. 1988;30:1071–1075. doi: 10.1016/0091-3057(88)90141-4. [DOI] [PubMed] [Google Scholar]
- Turner BH, Herkenham M. Thalamoamygdaloid projections in the rat: a test of the amygdala's role in sensory processing. J Comp Neurol. 1991;313:295–325. doi: 10.1002/cne.903130208. [DOI] [PubMed] [Google Scholar]
- Usdin TB. Evidence for a parathyroid hormone-2 receptor selective ligand in the hypothalamus. Endocrinology. 1997;138:831–834. doi: 10.1210/endo.138.2.5031. [DOI] [PubMed] [Google Scholar]
- Usdin TB. The PTH2 receptor and TIP39: a new peptide-receptor system. Trends Pharmacol Sci. 2000;21:128–130. doi: 10.1016/s0165-6147(00)01455-3. [DOI] [PubMed] [Google Scholar]
- Usdin TB, Bonner TI, Harta G, Mezey E. Distribution of parathyroid hormone-2 receptor messenger ribonucleic acid in rat. Endocrinology. 1996;137:4285–4297. doi: 10.1210/endo.137.10.8828488. [DOI] [PubMed] [Google Scholar]
- Usdin TB, Bonner TI, Hoare SR. The parathyroid hormone 2 (PTH2) receptor. Receptors Channels. 2002;8:211–218. [PubMed] [Google Scholar]
- Usdin TB, Dobolyi A, Ueda H, Palkovits M. Emerging functions for tuberoinfundibular peptide of 39 residues. Trends Endocrinol Metab. 2003;14:14–19. doi: 10.1016/s1043-2760(02)00002-4. [DOI] [PubMed] [Google Scholar]
- Usdin TB, Gruber C, Bonner TI. Identification and functional expression of a receptor selectively recognizing parathyroid hormone, the PTH2 receptor. J Biol Chem. 1995;270:15455–15458. doi: 10.1074/jbc.270.26.15455. [DOI] [PubMed] [Google Scholar]
- Usdin TB, Hilton J, Vertesi T, Harta G, Segre G, Mezey E. Distribution of the parathyroid hormone 2 receptor in rat: immunolocalization reveals expression by several endocrine cells. Endocrinology. 1999a;140:3363–3371. doi: 10.1210/endo.140.7.6855. [DOI] [PubMed] [Google Scholar]
- Usdin TB, Hoare SR, Wang T, Mezey E, Kowalak JA. TIP39: a new neuropeptide and PTH2-receptor agonist from hypothalamus. Nat Neurosci. 1999b;2:941–943. doi: 10.1038/14724. [DOI] [PubMed] [Google Scholar]
- Usdin TB, Paciga M, Riordan T, Kuo J, Parmelee A, Petukova G, Camerini-Otero RD, Mezey E. Tuberoinfundibular Peptide of 39 residues is required for germ cell development. Endocrinology. 2008;149:4292–4300. doi: 10.1210/en.2008-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usdin TB, Wang T, Hoare SR, Mezey E, Palkovits M. New members of the parathyroid hormone/parathyroid hormone receptor family: the parathyroid hormone 2 receptor and tuberoinfundibular peptide of 39 residues. Front Neuroendocrinol. 2000;21:349–383. doi: 10.1006/frne.2000.0203. [DOI] [PubMed] [Google Scholar]
- Van de Heijning BJ, Koekkoek-Van den Herik I, Maigret C, Van Wimersma Greidanus TB. Pharmacological assessment of the site of action of opioids on the release of vasopressin and oxytocin in the rat. Eur J Pharmacol. 1991a;197:175–180. doi: 10.1016/0014-2999(91)90518-u. [DOI] [PubMed] [Google Scholar]
- Van de Heijning BJ, Koekkoek-Van den Herik I, Van Wimersma Greidanus TB. The opioid receptor subtypes mu and kappa, but not delta, are involved in the control of the vasopressin and oxytocin release in the rat. Eur J Pharmacol. 1991b;209:199–206. doi: 10.1016/0014-2999(91)90170-u. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- van Rossum D, Hanisch UK, Quirion R. Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neurosci Biobehav Rev. 1997;21:649–678. doi: 10.1016/s0149-7634(96)00023-1. [DOI] [PubMed] [Google Scholar]
- Varga T, Palkovits M, Usdin TB, Dobolyi A. The medial paralemniscal nucleus and its afferent neuronal connections in rat. J Comp Neurol. 2008;511:221–237. doi: 10.1002/cne.21829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veening JG, Coolen LM. Neural activation following sexual behavior in the male and female rat brain. Behav Brain Res. 1998;92:181–193. doi: 10.1016/s0166-4328(97)00190-3. [DOI] [PubMed] [Google Scholar]
- Vincent SR, Hokfelt T, Wu JY. GABA neuron systems in hypothalamus and the pituitary gland. Immunohistochemical demonstration using antibodies against glutamate decarboxylase. Neuroendocrinology. 1982;34:117–125. doi: 10.1159/000123288. [DOI] [PubMed] [Google Scholar]
- Visegrady A, Boros A, Nemethy Z, Kiss B, Keseru GM. Application of the BD ACTOne technology for the high-throughput screening of Gs-coupled receptor antagonists. J Biomol Screen. 2007;12:1068–1073. doi: 10.1177/1087057107309035. [DOI] [PubMed] [Google Scholar]
- Wang J, Coolen LM, Brown JL, Usdin TB. Neurons containing tuberoinfundibular peptide of 39 residues are activated following male sexual behavior. Neuropeptides. 2006a;40:403–408. doi: 10.1016/j.npep.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Wang J, Palkovits M, Usdin TB, Dobolyi A. Afferent connections of the subparafascicular area in rat. Neuroscience. 2006b;138:197–220. doi: 10.1016/j.neuroscience.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Wang J, Palkovits M, Usdin TB, Dobolyi A. Forebrain projections of tuberoinfundibular peptide of 39 residues (TIP39)-containing subparafascicular neurons. Neuroscience. 2006c;138:1245–1263. doi: 10.1016/j.neuroscience.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Wang T, Palkovits M, Rusnak M, Mezey E, Usdin TB. Distribution of parathyroid hormone-2 receptor-like immunoreactivity and messenger RNA in the rat nervous system. Neuroscience. 2000;100:629–649. doi: 10.1016/s0306-4522(00)00282-7. [DOI] [PubMed] [Google Scholar]
- Ward HL, Small CJ, Murphy KG, Kennedy AR, Ghatei MA, Bloom SR. The actions of tuberoinfundibular peptide on the hypothalamo-pituitary axes. Endocrinology. 2001;142:3451–3456. doi: 10.1210/endo.142.8.8308. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Kawana E. The cells of origin of the incertofugal projections to the tectum, thalamus, tegmentum and spinal cord in the rat: a study using the autoradiographic and horseradish peroxidase methods. Neuroscience. 1982;7:2389–2406. doi: 10.1016/0306-4522(82)90203-2. [DOI] [PubMed] [Google Scholar]
- Weaver DR, Deeds JD, Lee K, Segre GV. Localization of parathyroid hormone-related peptide (PTHrP) and PTH/PTHrP receptor mRNAs in rat brain. Brain Res Mol Brain Res. 1995;28:296–310. doi: 10.1016/0169-328x(94)00222-z. [DOI] [PubMed] [Google Scholar]
- Winer JA, Larue DT, Diehl JJ, Hefti BJ. Auditory cortical projections to the cat inferior colliculus. J Comp Neurol. 1998;400:147–174. [PubMed] [Google Scholar]
- Wünscher W, Shober W, Werner L. Architectonisher Atlas vom Hirnstamm der Ratte. S. Hirzel; Leipzig: 1965. [Google Scholar]
- Yasui Y, Kayahara T, Nakano K, Mizuno N. The subparafascicular thalamic nucleus of the rat receives projection fibers from the inferior colliculus and auditory cortex. Brain Res. 1990;537:323–327. doi: 10.1016/0006-8993(90)90378-o. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Nakano K, Mizuno N. Descending projections from the subparafascicular thalamic nucleus to the lower brain stem in the rat. Exp Brain Res. 1992;90:508–518. doi: 10.1007/BF00230933. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Saper CB, Cechetto DF. Calcitonin gene-related peptide (CGRP) immunoreactive projections from the thalamus to the striatum and amygdala in the rat. J Comp Neurol. 1991;308:293–310. doi: 10.1002/cne.903080212. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Li L, Scott BW, Frankland PW. Tactile, acoustic and vestibular systems sum to elicit the startle reflex. Neurosci Biobehav Rev. 2002;26:1–11. doi: 10.1016/s0149-7634(01)00057-4. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Duggan AW. Idazoxan blocks the action of noradrenaline but not spinal inhibition from electrical stimulation of the locus coeruleus and nucleus Kölliker-Fuse of the cat. Neuroscience. 1988;25:997–1005. doi: 10.1016/0306-4522(88)90052-8. [DOI] [PubMed] [Google Scholar]
- Zhou J, Shore S. Convergence of spinal trigeminal and cochlear nucleus projections in the inferior colliculus of the guinea pig. J Comp Neurol. 2006;495:100–112. doi: 10.1002/cne.20863. [DOI] [PubMed] [Google Scholar]
- Zhu X, Gilbert S, Birnbaumer M, Birnbaumer L. Dual signaling potential is common among Gs-coupled receptors and dependent on receptor density. Mol Pharmacol. 1994;46:460–469. [PubMed] [Google Scholar]