Abstract
Generation of optimal humoral immunity to vaccination is essential to protect against devastating infectious agents such as the variola virus that causes smallpox. Vaccinia virus (VV), employed as a vaccine against smallpox, provides an important model of infection. Herein, we evaluated the importance cyclooxygenase-2 (Cox-2) in immunity to VV using Cox-2 deficient mice and Cox-2 selective inhibitory drugs. The effects of Cox-2 inhibition on antibody responses to live viruses such as vaccinia have not been previously described. Here, we used VV infection in Cox-2 deficient mice and in mice chronically treated with Cox-2 selective inhibitors and show that the frequency of VV-specific B cells was reduced, as well as the production of neutralizing IgG. VV titers were approximately 70 times higher in mice treated with a Cox-2 selective inhibitor. Interestingly, Cox-2 inhibition also reduced the frequency of IFN-γ producing CD4+ T helper cells, important for class switching. The significance of these results is that the chronic use of NSAIDs, and other drugs that inhibit Cox-2 activity or expression, blunt the ability of B cells to produce anti-viral antibodies, thereby making vaccines less effective and possibly increasing susceptibility to viral infection. These new findings support an essential role for Cox-2 in regulating humoral immunity.
Keywords: B cells, antibodies, Cox-2, viral infection, T cells
Introduction
Antibodies are essential mediators of antiviral immunity, and important for successful vaccination. Effective vaccines are in demand worldwide, as many fail to strongly stimulate B cell production of protective antibodies [1, 2]. The smallpox vaccine, consisting of live vaccinia virus (VV), is an example of a functional vaccine that elicits a potent immune response, resulting in long lasting B cell memory that can last upwards of 75 years [3–5]. Due to the eradication of smallpox worldwide in 1980, the smallpox vaccine is no longer administered to the general public [6]. However, it is still administered to military personnel, as well as to some health care workers. The threat of bioterrorism has peaked interest in the study of pathogens, such as variola, the causative agent of smallpox, which could be weaponized. It is, therefore, important to understand vaccine-induced immune responses, so that those that are weakly immunogenic, can be improved, and factors that diminish immunity may be avoided.
Non-steroidal anti-inflammatory drugs (NSAIDs) are commonly used to alleviate the side-effects (pain, fever, swelling, edema, etc.) of vaccination and to reduce fever and pain associated with infections. NSAIDs function by inhibiting cyclooxygenases-1 and -2. Cyclooxygenases (Cox) are necessary for the production of prostaglandins, which regulate inflammation [7]. More recently, Cox-2-selective small molecule inhibitors have been developed as treatments for rheumatoid arthritis and for chronic pain. Cox-2 is highly elevated during inflammation and plays an important role in immune regulation [8–10]. B lymphocytes express Cox-2 after activation with stimuli such as CD40 ligand, CpG oligodeoxynucleotides and BCR engagement [11, 12]. Cox-2 expression and activity was also shown to be essential for optimal antibody production in vitro [11, 12]. Cox-2 deficient mice exhibited impaired B cell responses following vaccination with non-infectious human papillomavirus-16 virus-like particles (HPV-16 VLP) [13]. However, whether Cox-2 plays a vital role in the humoral immune response to live virus infection is currently unknown.
Protection against viruses requires both the humoral and cellular arms of the immune system. Antibodies are necessary for viral clearance and prevention of viral replication. Monkeys given anti-CD20 treatment to deplete B cells, died after challenge with monkeypox, but were spared if passive antibody transfer was performed [14, 15]. Xu et al. provide evidence that both B cell deficient mice and mice depleted of CD4+ T cells had highly impaired VV clearance, both due to a lack of antibody production [16]. In the absence of CD4+ T cell help, B cells fail to undergo class switching and somatic hypermutation. These processes are important for generation of highly specific neutralizing antibodies.
The purpose of the present study was to determine, using Cox-2 knockout mice and mice treated with Cox-2 selective inhibitors, whether antibody production would be adversely affected in response to live VV infection. Further, we hypothesized that CD4+ T cell responses, critical for B cell class switching and production of neutralizing antibodies, to VV would also be impaired. Our new results support the concept that chronic use of Cox-2 selective inhibitors during live virus infection will attenuate humoral immunity, possibly making patients more susceptible to infectious agents such as variola.
Materials and Methods
Virus
The Western Reserve strain of vaccinia virus (VV) was grown in 143B fibroblasts.
Cox-2 selective inhibitors
SC-58125 (Cayman Chemical), a celecoxib analogue, and NS-398 (Cayman Chemical, Ann Arbor, MI) were dissolved in DMSO and diluted to 10% in an aqueous solution of hydroxypropyl methyl cellulose (HPMC). Two hundred microliters of the HPMC/Cox-2 inhibitor solution were given to mice via oral gavage two times per week. SC-58125 was administered at 5mg/kg and NS-398 at 10mg/kg. DMSO/HPMC was used as the vehicle control.
Mice and infection protocols
Male C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME), Cox-2 deficient mice (B6.129P2-Ptgs2tm1Unc) and wild-type controls were purchased from Taconic Farms (Hudson, NY). Congenic B6-Ly5.2/Cr mice (NCI, Frederick, MD) were used for antigen presenting cells (APCs) in intracellular cytokine staining (ICS) and IFN-γ ELISPOT assays. Approval of all protocols was obtained from the University of Rochester animal care and use committee. C57BL/6 mice were used in three different infection protocols. All mice were infected i.p. with 1×106 PFU of the Western Reserve strain of VV. Cox-2 deficient mice and wild-type controls were infected on day 0 and sacrificed on day 28. C57BL/6 mice were infected on day 0 and were chronically treated with SC-58125 starting 6 days prior to infection and ending on day 27. C57BL/6 mice were infected with VV on day 0 and were acutely treated with vehicle, NS-398 or SC-58125 starting on day 0 and ending on day 7. Plasma, bone marrow cells and splenocytes were harvested from infected mice at various time points. Four mice were used per treatment in each experiment.
Virus inactivation
Inactivation of VV virus was performed as previously described [5]. VV stocks (4×108 PFU/mL) were incubated with 4′-aminomethyl-trioxsalen (10 µg/mL) (Calbiochem, La Jolla, CA) for 10 minutes at room temperature (RT). VV stocks were then placed in 6-well plates and UV inactivated in a Stratagene 2400 UV Crosslinker (Stratagene, La Jolla, CA) at a setting of 3.0 J/cm2. Inactivation was confirmed with plaque assays. Inactivated VV stocks were stored at −80°C.
VV-specific ELISAs
Ninety six-well ELISA plates were coated and incubated overnight with inactivated VV diluted 1:100 in 1× PBS, 0.1% BSA. Plates were blocked with 1× PBS, 1.0% BSA for 1 hour. Serial dilutions of mouse plasma were diluted in 1× PBS, 0.1% BSA and incubated for 2 hours at RT. Following washing, plates were incubated for 1 hour with alkaline phosphatase conjugated IgM, IgG, IgG1, IgG2a, IgG2b or IgG3 secondary antibodies (1:2000) (Southern Biotech, Birmingham, AL). ELISAs were developed with the p-nitrophenyl phosphatase substrate kit (Pierce/Thermo Fisher Scientific, Rockford, IL) and O.D. 405 nm values were determined on a microplate reader (Bio-Rad, Hercules, CA).
VV-specific ELISPOTs
Ninety six-well ELISPOT plates (Millipore, Billerica, MA) were coated and incubated overnight with inactivated VV diluted 1:20 in 1× PBS, 2.0% BSA, 0.1% Tween-20. Plates were blocked with RPMI 1640 (GIBCO/Invitrogen) supplemented with 5% FBS, 2 mM L-glutamine, 5 × 105 M 2-ME, 10 mM HEPES and 50 µg/mL gentamicin at 37°C for 1 hour prior to addition of cells. Splenocytes and bone marrow harvested from mice were processed into single cell suspensions and incubated in ELISPOT plates for 6 hours (37°C, 5% CO2). Following this incubation, plates were washed and incubated overnight with alkaline phosphatase-conjugated IgM, IgG, IgG1, IgG2a, IgG2b or IgG3 secondary antibodies (1:1000) (Southern Biotech). ELISPOTs were developed with Vector substrate kit (Vector Labs, Burlingame, CA) and counted on a CTL plate reader, using ImmunoSpot software (Cellular Technologies Ltd., Shaker Heights, OH). Protocols were modified based on published protocol by Crotty et al. [17].
Plaque Reduction Assays
143B fibroblasts (6×105/well) cultured in MEM (GIBCO/Invitrogen) supplemented with 8% FBS, 2 mM L-glutamine, 5 × 105 M 2-ME, 10 mM HEPES and 50 µg/mL were plated in 6-well plates and allowed to grow to confluency at 37°C overnight. Western Reserve strain VV was diluted 1 × 10−6 (400 PFU/mL) in MEM, 2.5% FBS. Five hundred microliters of this prep was incubated with dilutions of mouse plasma for 2 hours to allow for neutralization to occur. Cells were then incubated with 500 µL of VV/plasma for 1.5 hours. After this incubation, 1.5 mL of MEM, 2.5% FBS was added to cultures and cells were left at 37°C for 48 hours. Media was removed and cells were stained with a 20% EtOH, 0.1% crystal violet solution. Plaques were counted and are represented as a percent of plaques in the absence of plasma.
Antibody Production Assays
Splenocytes (1×106) were isolated from naive C57BL/6 mice and cultured without stimuli for 48 hours. Cells were treated with DMSO vehicle, SC-58125 or NS-398 at various concentrations. Supernatants were screened for IgM and total IgG production using ELISAs (Bethyl Laboratories, Montgomery, TX) as per manufacturer’s instructions. ELISAs were developed with TMB substrate (KPL, Gaithersburg, MD) and read on a microplate reader.
Prostaglandin Measurement
Splenocytes (5×105) were isolated from mice chronically treated with DMSO vehicle or SC-58125 7 days after infection with VV and cultured for 24 hours in the presence of LPS (10 µg/mL). Supernatants were analyzed for PGE2 production by Enzyme Immunoassay (Cayman Chemical, Ann Arbor, MI).
VV Titers
C57BL/6 mice were chronically treated with either DMSO vehicle or SC-58125 starting 3 days prior to infection. Ovaries harvested from mice 7 days post-infection were homogenized in 1 mL of media and subjected to several rounds of freeze/thaw and sonication. Ovarian viral titers were assessed by plaque assay.
IFN-γ Intracellular Cytokine Staining
C57BL/6 mice were treated with either DMSO vehicle or SC-58125 starting 3 days prior to infection and ending on day 6. Splenocytes harvested on day 7 were stained for intracellular cytokine production. CD4+ and CD8+ cells were enriched with a beading procedure (BD Biosciences) where Class-II MHC+ cells were depleted. Splenocytes from B6-Ly5.2/Cr congenic mice were incubated with or without live VV for 18 hours at 37°C. Class-II depleted splenocytes (1×106) were incubated with uninfected or infected congenic splenocytes (1×106) and with brefeldin A golgi plug (BD Biosciences) for 5 hours at 37°C. Following incubation cells were stained for surface expression of CD45.1, CD19, CD3, CD4, CD8, CD44 and CD62L (BD Biosciences). Cells were fixed and permeabilized using the BD Cytofix/Cytoperm kit and stained for intracellular IFN-γ. Splenocytes were then run on an LSR-II flow cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star Inc., Ashland, OR).
IFN-γ ELISPOTs
C57BL/6 mice were treated with either DMSO vehicle or SC-58125 starting 3 days prior to infection and ending on day 6. Spleens were harvested from VV infected mice sacrificed on day 7. CD4+ T cells were enriched from splenocytes using a beading procedure to deplete Class II MHC+ and CD8+ cells (BD Biosciences). Dilutions of enriched CD4+ T cell from infected C57BL/6 were incubated with B6-Ly5.2/Cr congenic splenocytes (5×105) in ELISPOT plates coated with rat anti-mouse IFN-γ antibody (BD Biosciences). ELISPOT plates were incubated for 24 hours at 37°C. Plates were washed and incubated with a biotinylated rat anti-mouse IFN-γ antibody (BD Biosciences). Following washing, plates were incubated with streptavidin alkaline phosphatase (Jackson ImmunoResearch, West Grove, PA). ELISPOTs were developed and analyzed as previously described.
Statistics
Data are expressed as means ± SEM. Significance was determined by two-tailed Student’s t-test. Probability values of p<0.05 were considered statistically significant.
Results
VV-specific B cell responses are impaired in Cox-2 deficient mice
Cox-2 knockout mice (Cox-2−/−) were infected with VV, Western Reserve strain, in order to determine if Cox-2 deficiency dampened humoral immunity. Plasma and bone marrow cells were harvested from wild-type and Cox-2−/− mice 28 days following infection. Plasma antibody titers were assayed for VV-specific IgG2a, the predominant isotype for viral neutralization and clearance [18, 19]. IgG2a titers were significantly reduced in Cox-2 deficient mice compared to wild-type (Figure 1A). We also observed increased IgM production and reduced production of IgG1, IgG2b and IgG3 in Cox-2−/− mice (data not shown).
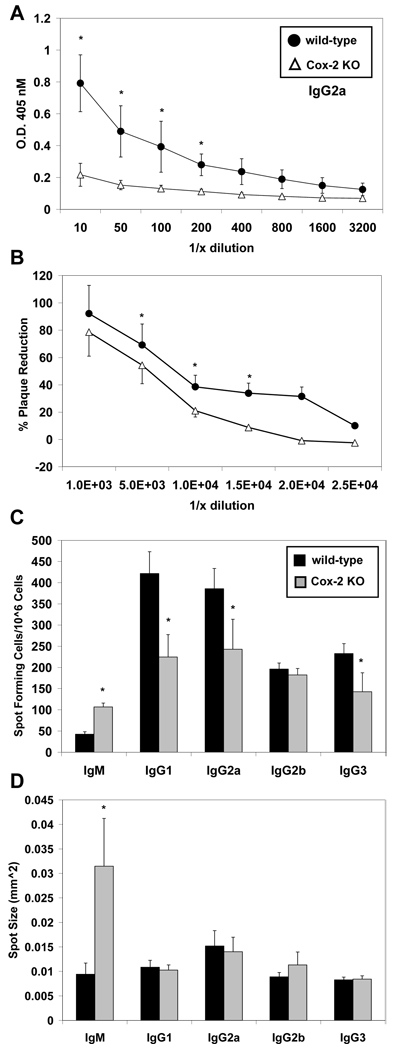
Figure 1.
Cox-2 deficient mice have impaired VV-specific IgG responses. Wild-type and Cox-2−/− mice were infected with 1×106 PFU VV (n = 4). VV-specific IgG2a antibody titers (plasma) were assessed by ELISA 28 days after infection. (A) IgG2a antibody titers were significantly reduced in Cox-2 −/− mice (open triangles) compared to wild-type mice (closed circles). (B) The presence of neutralizing antibody titers in wild-type and Cox-2−/− plasma was assessed by plaque assay. VV was incubated with dilutions (10−2, 5−2, 10−3, 15−3, 20−3, 25−3) of plasma and cultured with 143B fibroblasts for 48 hours. The percent reduction of plaques in the presence of plasma compared to no plasma controls is shown. Bone marrow cells were assessed for VV-specific antibody secreting cells on day 28 by ELISPOT. (C) Numbers of IgM, IgG1, IgG2a, IgG2b or IgG3 secreting cells in wild-type (black) and Cox-−/− (gray) mice are shown. (D) The average spot size for each isotype is also shown. Data are represented as mean ± SEM, * p <0.05.
To determine if Cox-2−/− mice had reduced viral neutralizing capacity, plaque reduction assays were performed. Plasma was incubated with live VV prior to infection of 143B fibroblasts. Wild-type mice had significantly higher neutralizing titers compared to the Cox-2−/− mice (Figure 1B). Based on linear extrapolation, Cox-2−/− plasma neutralized 50% of plaques at a ~5 ×103-fold dilution; while wild-type plasma neutralized 50% of plaques at 8 ×103-fold dilution. Reduced virus neutralization correlates with reduced VV-specific IgG2a titers observed in the Cox-2−/− mice.
We sought to determine if the observed reduction in plasma antibody titers, especially IgG2a, was supported by reduced antibody secreting cell frequencies. To accomplish this IgM, IgG1, IgG2a, IgG2b and IgG3 antibody secreting cells were assessed by ELISPOT (Figure 1C). Interestingly, IgM spot forming bone marrow cells were significantly increased in Cox-2−/− mice and also produced more antibody per cell, as denoted by a significantly larger spot size (Figure 1C & 1D). The frequency of IgG2b spot forming cells was similar in wild-type mice and Cox-2−/−, while the frequency of IgG1, IgG2a and IgG3 antibody secreting cells was significantly lower in the bone marrow of Cox-2−/− mice (Figure 1C). There were no noticeable differences in spot size for the IgG isotypes (Figure 1D). These data indicate that Cox-2 deficiency reduces the frequency of VV-specific antibody secreting cells, and production of IgG2a neutralizing antibodies, generated during VV infection.
Treatment with Cox-2 inhibitors early in the VV infection does not impair VV-specific antibody responses
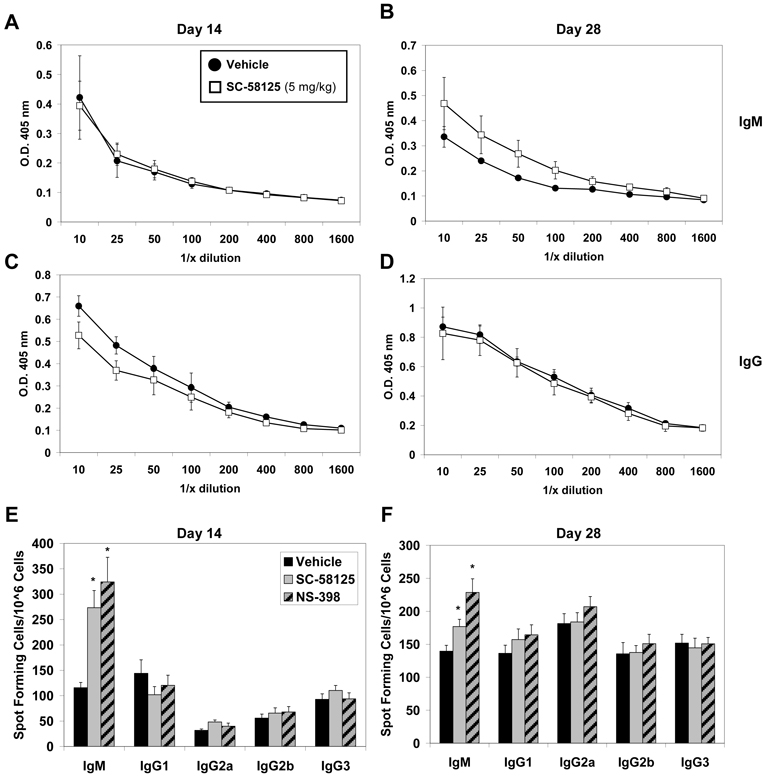
We next investigated whether treatment with Cox-2 selective inhibitors early during infection, representative of an acute dose, would impair humoral immune responses to VV. Mice were orally administered either vehicle, SC-58125 or NS-398, during the first week of infection. We then assessed plasma titers of VV-specific IgM and IgG on days 14 and 28. No differences in IgM titers were observed on day 14, however SC-58125 (Figure 2A & 2C) and NS-398 (data not shown) treated mice had slightly lower VV-specific IgG titers compared to vehicle treated mice. By day 28 VV-specific IgM plasma titers were increased in Cox-2 inhibitor treated mice compared to those that received vehicle (Figure 2B). These results are similar to the heightened IgM responses in Cox-2−/− mice (Figure 1). However, no differences were observed in total IgG titers, as well as IgG1, IgG2a, IgG2b and IgG3, in mice that received acute doses of either vehicle, SC-58125 or NS-398 during the first week of VV infection (Figure 2D and data not shown).
Figure 2.
Treatment with Cox-2 inhibitors early in the VV infection fails to blunt VV-specific B cell antibody production. C57Bl/6 mice (n = 4) were administered vehicle, SC-58125 (5 mg/kg) or NS-398 (10 mg/kg) at the onset of infection and ending on day 6 (3 doses). Plasma titers of VV-specific IgM (A & B) and IgG (C & D) was determined by ELISA on day 14 and 28. The number of VV-specific antibody secreting cells were assessed by ELISPOTs performed on day 14 (E) and day 28 (F) for all antibody isotypes. Data are represented as mean ± SEM, * p <0.05.
To determine if antibody secreting cell frequencies correlated with antibody titers in the plasma, we assessed the number of antibody secreting cells in mice that received Cox-2 selective inhibitors early in the VV infection. VV-specific ELISPOTs showed a significant increase in IgM producing cells on day 14 and 28 in SC-58125 and NS-398 treated mice, compared to those that only received vehicle (Figure 2E & 2F). In contrast, we observed no significant change in IgG secreting cell frequencies (Figure 2E & 2F). Thus, administration of Cox-2 selective inhibitors during the first week of VV infection does not influence the IgG immune response against VV.
Chronic Cox-2 inhibition dampens VV neutralizing antibody responses to VV infection
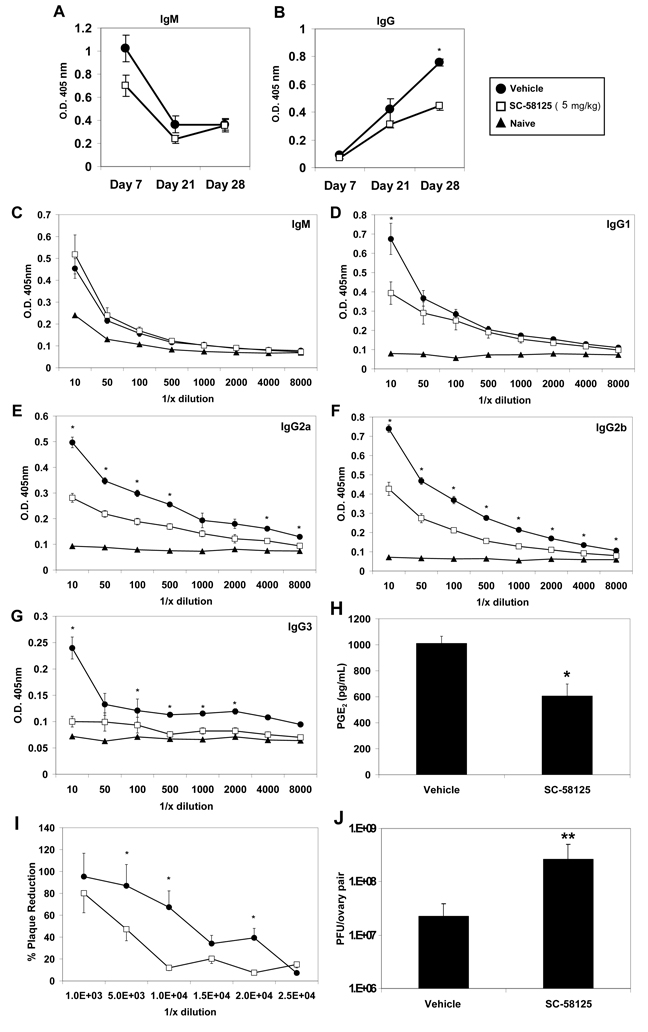
We next determined whether chronic administration of small molecule Cox-2 selective inhibitors prior to and throughout VV infection would negatively influence antibody production. C57BL/6 mice were treated with SC-58125, starting 6 days prior to VV infection and dosed twice a week thereafter, until day 27. A previous report demonstrated that infection with the Western Reserve strain of VV induced IgM responses by day 7 and IgG titers peaked approximately one month after infection [16]. Therefore, plasma was collected from mice on days 7, 21 and 28 following infection to determine if inhibition of Cox-2 dampened VV-specific antibody production (Figure 3A & 3B). No significant differences in plasma IgM titers (1:10 dilution) on day 21, or 28 were observed. Vehicle treated mice demonstrated higher titers on day 7, but these levels were not statistically significant (Figure 2A & 2C). We observed natural VV-specific IgM in the plasma of naive mice which was also reported by Ochsenbein et al.[20]. Plasma IgG titers did not vary significantly on days 7 or 21. However, by day 28, SC-58125 treated mice had significantly lower VV-specific IgG titers (Figure 3B). We further investigated plasma antibody isotype titers 28 days post-infection. IgG2a and IgG2b isotype titers were significantly decreased in mice that received the Cox-2 inhibitor (Figure 3E & 3F). IgG1 and IgG3 levels were also lower in SC-58125 treated mice (Figure 3D & 3G). Cox-2 activity was assessed ex vivo to demonstrate that splenocytes harvested from mice treated with SC-58125 produced significantly less PGE2 compared to vehicle treated controls (Figure 3H). These new data indicate that Cox-2 plays an important role in the production of IgG in response to VV infection.
Figure 3.
Chronic exposure to the Cox-2 inhibitor, SC-58125, impairs production of VV-specific neutralizing IgG. C57BL/6 mice (n = 4) were administered vehicle or SC-58125 (5 mg/kg) starting seven days before infection with VV (1×106 PFU) and ending on day 27. Plasma collected from vehicle treated (closed circles) or SC-58125 treated (open squares) mice on days 7, 21 and 28 was analyzed for VV-specific IgM (A) and IgG (B). VV-specific IgM (C), IgG1 (D), IgG2a (E), IgG2b (F) or IgG3 (G) titers were more extensively characterized by ELISA from day 28 plasma samples. (H) Splenocytes harvested 7 days post-infection were cultured with 10 ug/mL LPS for 24 hours and supernatants were assessed for PGE2 production by EIA. (I) The presence of neutralizing antibody titers in mouse plasma was assessed by plaque assay. VV was incubated with dilutions (10−2, 5−2, 10−3, 15−3, 20−3, 25−3) of vehicle treated or SC-58125 treated mouse plasma and cultured with 143B fibroblasts. The percent reduction of plaques in the presence of plasma compared to no plasma controls is shown. (J) Ovarian viral titers were determined on day 7 post-infection in mice chronically treated with vehicle or SC-58125. Data are represented as mean ± SEM, * p <0.05, **p = 0.06.
Viral plaque reduction assays were performed to evaluate differences in viral neutralization between vehicle and Cox-2 inhibitor treated mice. Plasma from SC-58125 treated mice reduced plaques by 50% at a 4.8 ×103-fold dilution, compared to vehicle treated control plasma, which reduced plaques by 50% at a 1.6 × 104-fold dilution (Figure 3I). These data demonstrate a 3-fold reduction in virus neutralizing capacity of Cox-2 inhibitor treated mice. Therefore, Cox-2 inhibition resulted in a reduced frequency of VV-specific neutralizing antibodies. Viral titers measured in ovaries on day 7 post-infection, were, on average, 70-fold higher in mice treated with SC-58125 (Figure 3J), further indicating a reduction in protective antibodies and overall immunity.
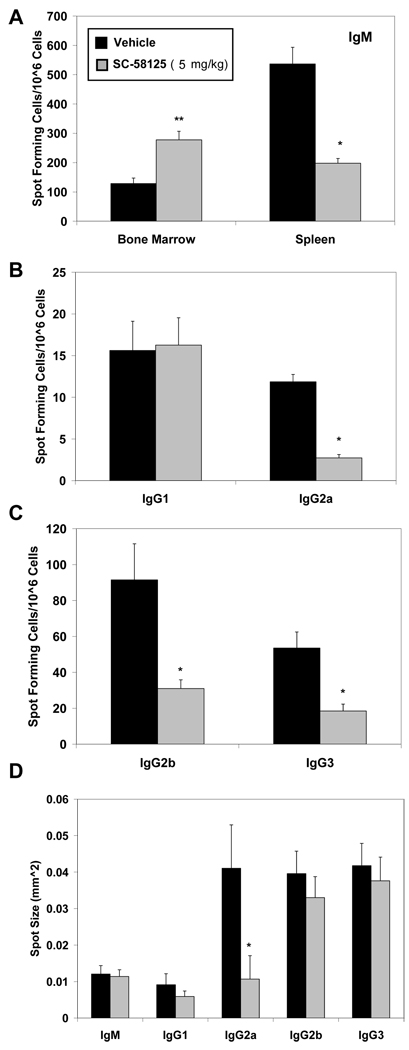
The impaired VV-specific neutralizing antibody production by SC-58125 treated mice prompted us to evaluate whether the frequency of antibody secreting cells was also affected. Bone marrow and spleens were harvested on day 28 following infection and VV-specific antibody secreting cells were quantified by ELISPOT. VV-specific IgM cell numbers in the spleen were significantly reduced in SC-58125 treated mice compared to vehicle treated (Figure 4A). In contrast, IgM secreting cell frequencies were increased in the bone marrow of mice that were administered Cox-2 inhibitors (Figure 4A). VV-specific IgG isotype secreting cell frequencies were also assessed 28 days after infection. IgG2a, IgG2b, IgG3 antibody secreting cell numbers were all significantly decreased in mice treated with SC-58125, whereas IgG1 was unchanged compared to vehicle treated mice (Figure 4B & 4C). IgG2a spot size was significantly decreased, while all other isotypes remained relatively unchanged (Figure 4D), indicating that the output of IgG2a was reduced. These new results demonstrate that chronic administration of Cox-2 selective inhibitors reduces both the frequency of VV-specific antibody secreting cells and titers of neutralizing antibodies.
Figure 4.
Chronic administration of a Cox-2 inhibitor reduces the number of VV-specific antibody secreting cells. C57BL/6 mice were administered either vehicle or SC-58125 (5 mg/kg) starting seven days before infection with VV (1×106 PFU) and continuing on to day 27. (A) Bone marrow cells and splenocytes, harvested on day 28, were assessed by ELISPOT for the preence of VV-specific IgM secreting cells. Splenocytes were further evaluated by ELISPOT for IgG1, Ig2a (B), IgG2b or IgG3 (C) isotype secreting cells. Numbers of spots per 1 × 106 cells are shown. (D) Average spot size is depicted. Data are represented as mean ± SEM, * p <0.05, **p = 0.07.
Cox-2 inhibitors do not significantly influence B cell antibody secretion
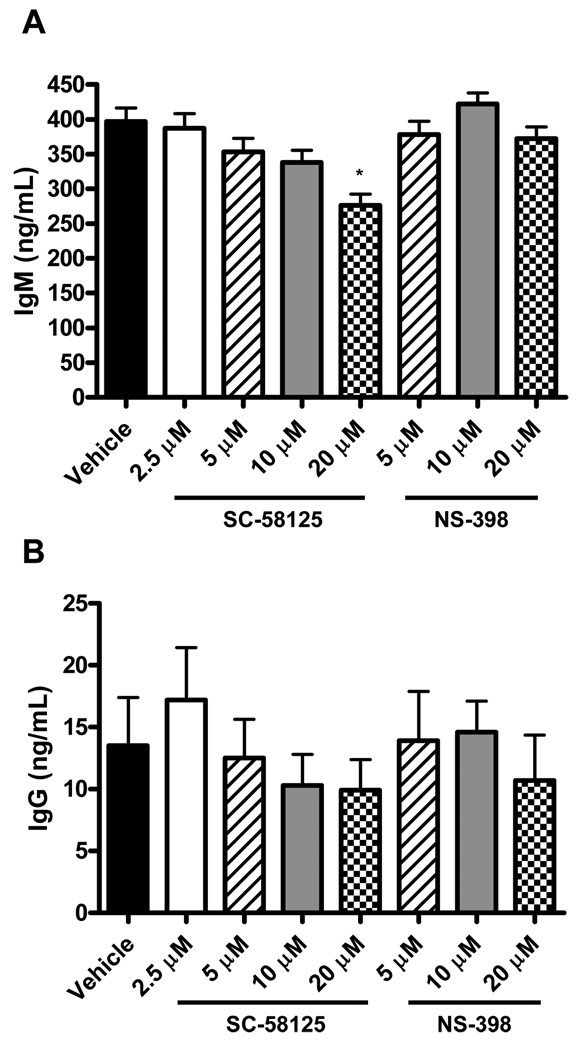
Reduced plasma titers of IgG antibodies could indicate an impaired ability of B cells to secrete antibody in response to VV. We therefore investigated whether inhibition of Cox-2 with either SC-58125 or NS-398 influenced B cells that were already secreting antibody in vitro. Splenocytes (1 × 106/well) that contain antibody secreting cells from naive C57BL/6 mice were cultured with Cox-2 selective inhibitors for 48 hours and assessed for IgM and IgG production via ELISA. Secretion of IgM was slightly reduced by the highest concentration of SC-58125 (Figure 5A). NS-398, on the other hand, had no effect on IgM secretion (Figure 5A). Splenocyte secretion of IgG was not influenced by either SC-58125 or NS-398 (Figure 5B). This demonstrates that Cox-2 does not play a role in the secretion of antibodies, but likely the generation or differentiation of antibody secreting cells.
Figure 5.
Cox-2 inhibitors do not significantly influence B cell antibody secretion. Unstimulated splenocytes (C57BL/6) were cultured for 48 hours in the presence of vehicle, SC-58125 (2.5, 5, 10 or 20 µM) or NS-398 (5, 10 or 20 µM). Supernatant levels of total IgM (A) and IgG (B) were determined by ELISA. Data are represented as mean ± SEM, * p <0.05.
Cox-2 inhibitors reduce the frequency of IFN-γ producing T cells
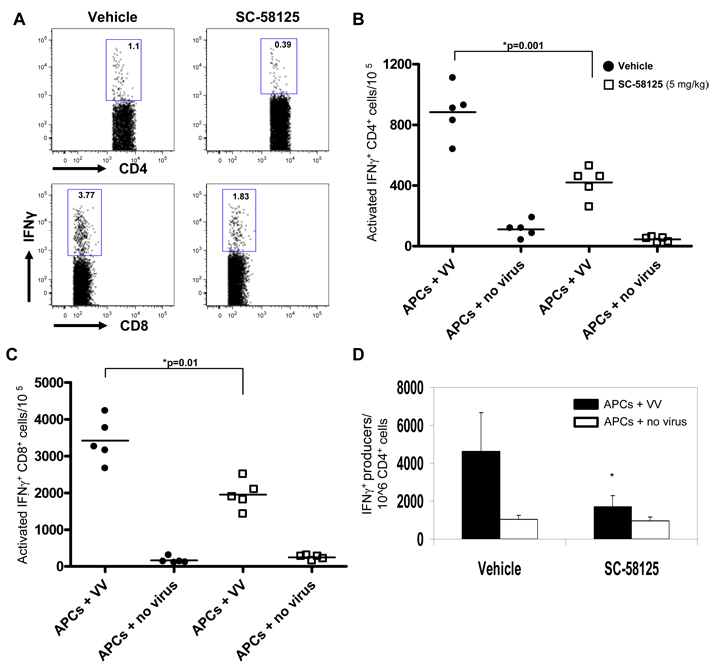
Antibody secreting cell frequency and neutralizing plasma titers were reduced in VV infected mice that were chronically administered Cox-2 inhibitors. CD4+ T cells drive the germinal center reaction in the spleen generating memory B cells and long-lived plasma cells responsible for secreting antibodies [21]. T cell production of IFN-γ in germinal centers generates class-switched B cells that produce Th1 antibodies, IgG2a being the most important for viral neutralization and clearance [18, 19]. Therefore, we hypothesized that mice receiving chronic doses of Cox-2 selective inhibitors would have reduced frequencies of T cells producing IFN-γ. Xu et al. demonstrated that the frequency of CD4+ T cells peaks approximately 7 days following infection with VV [16]. Therefore, VV infected mice were treated with SC-58125 until sacrifice on day 7 to measure CD4 T cell responses. Splenocytes were depleted of Class II MHC+ cells to enrich for CD4 and CD8 effector T cell populations. Enriched T cells from infected mice were cultured with in vitro infected B6-Ly5.2/Cr splenocytes for 5 hours in the presence of brefeldin A, in order to prevent cytokine secretion. Effector cells were also cultured with uninfected B6-Ly5.2/Cr splenocytes to act as a control. Cells were stained for surface expression of CD45.1, CD3, CD4, CD8, CD44 and CD62L, as well as for intracellular IFN-γ. We observed a reduced frequency of activated CD4+ T cells (CD3+CD4+CD44hiCD62Llow) expressing IFN-γ that were present in splenocytes of mice treated with SC-58125 (420 ± 50/105 CD4+ T cells) compared to DMSO vehicle (880 ± 90/105 CD4+ T cells) (Figure 6A & 6B). We observed similar attenuation of CD3+CD8+CD44hiCD62Llow that produced IFN-γ (Figure 6A & 6C), suggesting that cell-mediated immunity might also be impaired in the presence of Cox-2 inhibitors. Culture with uninfected APCs resulted in the generation of very few IFN-γ+ T cells, indicating that IFN-γ production was VV-virus specific.
Figure 6.
Cox-2 selective inhibitors reduce the frequency of splenic IFN-γ secreting CD4+ T cells. C57BL/6 mice (n = 4) infected with VV on day 0 (1×106 PFU) were treated with either DMSO vehicle or SC-58125 (5 mg/kg) starting 3 days prior to infection and ending on day 6. Splenocytes harvested on day 7 were cultured with VV infected B6-Ly5.2/Cr splenocytes for 5 hours in the presence of brefeldin A, to accumulate cytokines. Cells were stained for surface CD45.1, CD3, CD4, CD8, CD44 and CD62L as well as intracellular IFN-γ. B6-Ly5.2/Cr APCs were gated out based on CD45.1 expression. (A) Flow cytometric analysis shows dot plots gated on CD3+CD4+CD44hiCD62Llow cells for intracellular IFN-γ expression. (B) Representative graph depicts the number of CD3+CD4+CD44hiCD62Llow cells per 105 producing IFN-γ from either vehicle treated or SC-58125 treated mice. (C) Representative graph depicts the number of CD3+CD8+CD44hiCD62Llow cells per 105 producing IFN-γ from either vehicle treated or SC-58125 treated mice. (D) ELISPOT analysis of IFN-γ producing enriched CD4+ T cells cultured with uninfected or VV infected B6-Ly5.2/Cr splenocytes. ELISPOT cultures were incubated for 24 hours. Data are represented as mean ± SEM, * p <0.05. Results are representative of 3 separate experiments.
To confirm reduced production of IFN-γ by CD4+ T cells, ELISPOT assays were performed. Splenocytes originating from VV infected mice were depleted of Class II MHC+ and CD8+ cells to enrich for CD4+ T cells. Enriched CD4+ T cells were cultured with either uninfected or VV infected B6-Ly5.2/Cr splenocytes on anti-IFN-γ coated ELISPOT plates. Following 24 hour incubation, ELISPOTs were assessed and showed that significantly fewer cells from SC-58125 treated mice produced IFN-γ compared to vehicle treated mice (Figure 6D). Culture of enriched CD4+ T cells with uninfected APCs demonstrates that IFN-γ was VV-specific (Figure 6D). Overall, intracellular cytokine staining and ELISPOT data demonstrate that the short-term effects of Cox-2 selective inhibitors on CD4+ T cell production of IFN-γ manifests in the long-term in the B cell antibody response.
Discussion
The impact of commonly used inhibitors of Cox-2 on viral infection and anti-viral immunity are largely unknown. We report that chronic administration of Cox-2 selective inhibitors blunts immune responses to VV. Antibody production, especially IgG2a, important for neutralizing virus, was severely attenuated in both Cox-2 deficient mice and mice chronically treated with Cox-2 selective inhibitors, although early acute treatment with Cox-2 inhibitors only had minor effects. IFN-γ production from activated spleen-derived CD4+ T cells was also severely impaired following treatment with SC-58125, indicating that T cell priming of antibody secreting cells could be at least partially responsible for attenuated antibody production. Further, chronic treatment with Cox-2 selective inhibitors resulted in elevated viral titers in vivo, indicating a loss of protective immunity. Overall, our results suggest that people that chronically use NSAIDs or Cox-2 selective drugs may not respond optimally to vaccination and could be more susceptible to viral infection.
Cox-2 selective inhibitors have the potential to dampen immune responses to vaccinations, autoimmune diseases and viral infections. Zhang et al. demonstrated that B cells from a mouse lupus model expressed elevated levels of Cox-2 [22]. Treatment of these mice with Celebrex, a Cox-2 selective inhibitor, decreased the production of autoantibodies and increased survival. Similarly, two models of adjuvant-induced arthritis showed that both Cox-2 deficient mice and rats treated with Cox-2 inhibitors had decreased autoantibody production [23, 24]. In conjunction with our data, these studies demonstrate that Cox-2 plays a critical role in production of antibody and elevated levels in autoimmune disease could contribute to disease progression. Further, Cox-2 selective inhibitors could be used as therapeutics to prevent or delay the onset of autoimmune diseases by reducing the production of pathogenic autoantibodies. Previously, we demonstrated that Cox-2 knockout mice vaccinated with non-infectious HPV-16 VLPs, used to prevent cervical cancer, had an attenuated humoral immune response [13]. Although, the above findings suggest that humoral immune responses against self and to vaccines can be impaired in the absence of Cox-2, live virus infection was not evaluated. VV provides a well-studied and important model of infection, as well as being an efficacious vaccine against smallpox. Here, we showed that using a live VV infection, that Cox-2 deficient mice had a higher frequency of IgM producing cells and a lower frequency of IgG1, IgG2a and IgG3 producing cells. These data correlated with lower titers of VV neutralizing antibody in Cox-2−/− mice. Thus, our new results demonstrate that the humoral immune response to live virus is impaired in mice that lack Cox-2 activity.
Cox-2 selective inhibitors are widely prescribed for chronic pain, arthritis, and even as supplements to cancer chemotherapy. As doses of Cox-2 inhibitors administered early in VV infection did not have a major impact on IgG antibody responses, we asked whether mice receiving chronic doses of Cox-2 selective inhibitors, similar to patient regimens, would have diminished humoral immunity. In our VV infected mice we report that neutralizing antibody titers were severely attenuated following chronic treatment with the Cox-2 selective inhibitor, SC-58125 (Figure 3 & 4). We observed decreased VV-specific IgM in SC-58125 treated mice on day 7 and day 21, but IgM levels recovered by day 28. These data matched well with results in Cox-2 deficient mice, VV-specific IgG titers and antibody secreting cell frequencies were also significantly attenuated in SC-58125 treated mice. IgG2a and IgG2b were the most highly affected isotypes, and these are the most important for viral neutralization. VV neutralizing titers were attenuated in mice administered Cox-2-selective inhibitors, most likely due to decreased IgG2a and IgG2b production. Subsequently, we observed higher viral titers in mice chronically treated with Cox-2 selective inhibitors. Our new results indicate that chronic administration of drugs that diminish Cox-2 activity could impair humoral immunity, making vaccines less efficacious and increasing susceptibility to viral infection.
We observed attenuated antibody production in mice chronically dosed with Cox-2 selective inhibitors and in Cox-2−/− mice. This fully supports the concept that the effect of the Cox-2 selective drugs was Cox-2-dependent. High doses of drug can lead to off target effects [25–27]. However, the doses we used 5 mg/kg, SC-58125, and 10 mg/kg, NS-398, fall within the range used in other studies and result in relatively low pharmacological concentrations [28–30]. We observed some minor differences between Cox-2−/− mice and mice dosed with Cox-2 selective inhibitors. For example, IgG2b levels were attenuated in Cox-2 inhibitor treated mice, but not in Cox-2−/− mice. Although our results do not completely rule out Cox-2-independent effects using Cox-2 selective inhibitors, findings using Cox-2 knockout mice, suggest that few, if any, off target effects were responsible for the attenuated anti-viral humoral response.
Decreased IgG2a neutralizing antibody titers and antibody secreting cell frequencies prompted us to assess T cell responses within spleens of mice chronically treated with Cox-2-selective inhibitors. Production of IFN-γ by Th cells is important for optimal B cell activation and is necessary for class-switching in germinal centers, particularly to IgG2a and IgG2b [31, 32]. Mice lacking B cells, MHC class II or CD40 were more susceptible to secondary infection with a poxvirus, indicating that antibody production is essential for protection [33]. Similarly, both B cell deficient and CD4 depleted mice failed to efficiently clear VV due to lack of neutralizing IgM and IgG antibodies and succumb to virus [16, 34]. Models of experimental autoimmune encephalomyelitis (EAE), a disease mediated by CD4+ Th1 cells, have shown that Cox-2 inhibitors prevent disease symptoms [35, 36]. Rofecoxib and Lumiracoxib, both Cox-2 inhibitors, were shown to reduce IFN-γ production from CD4+ T cells in an EAE model [36]. By flow cytometric analysis we demonstrate the novel finding that VV infected mice treated with SC-58125 had reduced frequencies on day 7 of spleen-derived IFN-γ producing CD3+CD4+CD44hiCD62Llow cells. The CD4+ T cell response, essential for B cell neutralizing antibody production, peaks approximately 7 days post-infection with VV [14, 16, 37]. We further demonstrated the attenuation of CD4+ IFN-γ producing cells in the presence of Cox-2 inhibitors by ELISPOT. These results suggest that reduced frequency of CD4+ IFN-γ+ T cells when Cox-2 is inhibited, at least partially accounts for the attenuated neutralizing antibody response directed against VV through reduced B cell class switching. We also observed a decrease in frequency of activated CD8+ IFN-γ+ T cells which could result in impairment of cellular immunity against VV, as well as protective memory against the virus, though memory was not investigated here.
Chronic treatment of mice with the Cox-2 selective inhibitor, SC-58125, resulted in reduced ability of splenocytes to produce prostaglandins ex vivo. Previously, our lab demonstrated that PGE2 was critical for B cell class switching [38]. This is consistent with our results showing that VV-specific IgM production was enhanced, particularly in Cox-2 deficient mice, while neutralizing IgG titers were reduced in both genetically deficient and mice treated with Cox-2 selective inhibitors, indicating a defect in class switch recombination. More recently, Yao et al. reported that PGE2 promoted the differentiation of Th1 cells and their production of IFN-γ [39]. The data presented herein, showing reduced frequencies of IFN-γ producing CD4 T cells are consistent with these findings. Taken as a whole, our data suggest that the decrease in Cox-2 activity and attenuated PGE2 production impaired both B and T cell function, which resulted in more severe viral infection.
Overall, our results demonstrate that chronic treatment with Cox-2 inhibitors attenuates humoral immunity generated against VV. These new data indicate that Cox-2 is essential for optimal in vivo antibody production in response to viral infection. Chronic use of drugs that inhibit Cox-2, namely NSAIDs and Cox-2 selective inhibitors, could impair viral immunity, as well as vaccine generated immunity. If this were the case in humans, it would be particularly harmful to elderly patients, who commonly take NSAIDs and have difficulty achieving protective immunity to vaccination [40, 41]. Patients suffering from chronic pain, rheumatoid arthritis or osteoarthritis could also be more susceptible to infection and have reduced responses to vaccines, as they are often prescribed Cox-2 inhibitors. Clinical and epidemiological evaluations are necessary to determine whether this class of drugs will blunt human B cell antibody production following viral infection or vaccination. Our data herein clearly demonstrate that Cox-2-selective inhibitors diminish protective antibody responses to viral infection.
Acknowledgements
This work was funded by the National Institutes of Health Grants DE011390, AI071064, ES01247, HHSN266200700008C NYICE contract, N01-AI-50020 CBIM and the Training Program in Oral Sciences DE007202.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflict of interest.
References
- 1.Karlsson Hedestam GB, Fouchier RA, Phogat S, Burton DR, Sodroski J, Wyatt RT. The challenges of eliciting neutralizing antibodies to HIV-1 and to influenza virus. Nat Rev Microbiol. 2008;6(2):143–155. doi: 10.1038/nrmicro1819. [DOI] [PubMed] [Google Scholar]
- 2.Gerhard W, Mozdzanowska K, Zharikova D. Prospects for universal influenza virus vaccine. Emerg Infect Dis. 2006;12(4):569–574. doi: 10.3201/eid1204.051020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9(9):1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 4.Mamani-Matsuda M, Cosma A, Weller S, Faili A, Staib C, Garcon L, et al. The human spleen is a major reservoir for long-lived vaccinia virus-specific memory B cells. Blood. 2008;111(9):4653–4659. doi: 10.1182/blood-2007-11-123844. [DOI] [PubMed] [Google Scholar]
- 5.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171(10):4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 6.Fenner F. Smallpox: emergence, global spread, and eradication. Hist Philos Life Sci. 1993;15(3):397–420. [PubMed] [Google Scholar]
- 7.Rajakariar R, Yaqoob MM, Gilroy DW. COX-2 in inflammation and resolution. Mol Interv. 2006;6(4):199–207. doi: 10.1124/mi.6.4.6. [DOI] [PubMed] [Google Scholar]
- 8.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23(3):144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 9.Iniguez MA, Punzon C, Fresno M. Induction of cyclooxygenase-2 on activated T lymphocytes: regulation of T cell activation by cyclooxygenase-2 inhibitors. J Immunol. 1999;163(1):111–119. [PubMed] [Google Scholar]
- 10.Mongini PK. COX-2 expression in B lymphocytes: links to vaccines, inflammation and malignancy. Clin Immunol. 2007;125(2):117–119. doi: 10.1016/j.clim.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Bernard MP, Phipps RP. CpG oligodeoxynucleotides induce cyclooxygenase-2 in human B lymphocytes: implications for adjuvant activity and antibody production. Clin Immunol. 2007;125(2):138–148. doi: 10.1016/j.clim.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan EP, Pollock SJ, Murant TI, Bernstein SH, Felgar RE, Phipps RP. Activated human B lymphocytes express cyclooxygenase-2 and cyclooxygenase inhibitors attenuate antibody production. J Immunol. 2005;174(5):2619–2626. doi: 10.4049/jimmunol.174.5.2619. [DOI] [PubMed] [Google Scholar]
- 13.Ryan EP, Malboeuf CM, Bernard M, Rose RC, Phipps RP. Cyclooxygenase-2 inhibition attenuates antibody responses against human papillomavirus-like particles. J Immunol. 2006;177(11):7811–7819. doi: 10.4049/jimmunol.177.11.7811. [DOI] [PubMed] [Google Scholar]
- 14.Amanna IJ, Slifka MK, Crotty S. Immunity and immunological memory following smallpox vaccination. Immunol Rev. 2006;211:320–337. doi: 10.1111/j.0105-2896.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- 15.Edghill-Smith Y, Golding H, Manischewitz J, King LR, Scott D, Bray M, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11(7):740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- 16.Xu R, Johnson AJ, Liggitt D, Bevan MJ. Cellular and humoral immunity against vaccinia virus infection of mice. J Immunol. 2004;172(10):6265–6271. doi: 10.4049/jimmunol.172.10.6265. [DOI] [PubMed] [Google Scholar]
- 17.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286(1–2):111–122. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Coutelier JP, van der Logt JT, Heessen FW, Warnier G, Van Snick J. IgG2a restriction of murine antibodies elicited by viral infections. J Exp Med. 1987;165(1):64–69. doi: 10.1084/jem.165.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins JT, Shi J, Burrell BE, Bishop DK, Dunnick WA. Induced expression of murine gamma2a by CD40 ligation independently of IFN-gamma. J Immunol. 2006;177(8):5414–5419. doi: 10.4049/jimmunol.177.8.5414. [DOI] [PubMed] [Google Scholar]
- 20.Ochsenbein AF, Fehr T, Lutz C, Suter M, Brombacher F, Hengartner H, et al. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286(5447):2156–2159. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 21.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Bertucci AM, Smith KA, Xu L, Datta SK. Hyperexpression of cyclooxygenase 2 in the lupus immune system and effect of cyclooxygenase 2 inhibitor diet therapy in a murine model of systemic lupus erythematosus. Arthritis Rheum. 2007;56(12):4132–4141. doi: 10.1002/art.23054. [DOI] [PubMed] [Google Scholar]
- 23.Turull A, Queralt J. Selective cyclooxygenase-2 (COX-2) inhibitors reduce anti-Mycobacterium antibodies in adjuvant arthritic rats. Immunopharmacology. 2000;46(1):71–77. doi: 10.1016/s0162-3109(99)00159-9. [DOI] [PubMed] [Google Scholar]
- 24.Myers LK, Kang AH, Postlethwaite AE, Rosloniec EF, Morham SG, Shlopov BV, et al. The genetic ablation of cyclooxygenase 2 prevents the development of autoimmune arthritis. Arthritis Rheum. 2000;43(12):2687–2693. doi: 10.1002/1529-0131(200012)43:12<2687::AID-ANR8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Ryan EP, Bushnell TP, Friedman AE, Rahman I, Phipps RP. Cyclooxygenase-2 independent effects of cyclooxygenase-2 inhibitors on oxidative stress and intracellular glutathione content in normal and malignant human B-cells. Cancer Immunol Immunother. 2008;57(3):347–358. doi: 10.1007/s00262-007-0374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pyrko P, Kardosh A, Schonthal AH. Celecoxib transiently inhibits cellular protein synthesis. Biochem Pharmacol. 2008;75(2):395–404. doi: 10.1016/j.bcp.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 27.Grosch S, Maier TJ, Schiffmann S, Geisslinger G. Cyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitors. J Natl Cancer Inst. 2006;98(11):736–747. doi: 10.1093/jnci/djj206. [DOI] [PubMed] [Google Scholar]
- 28.Ahmadi M, Emery DC, Morgan DJ. Prevention of both direct and cross-priming of antitumor CD8+ T-cell responses following overproduction of prostaglandin E2 by tumor cells in vivo. Cancer Res. 2008;68(18):7520–7529. doi: 10.1158/0008-5472.CAN-08-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrario A, Fisher AM, Rucker N, Gomer CJ. Celecoxib and NS-398 enhance photodynamic therapy by increasing in vitro apoptosis and decreasing in vivo inflammatory and angiogenic factors. Cancer Res. 2005;65(20):9473–9478. doi: 10.1158/0008-5472.CAN-05-1659. [DOI] [PubMed] [Google Scholar]
- 30.Sheng H, Shao J, Dixon DA, Williams CS, Prescott SM, DuBois RN, et al. Transforming growth factor-beta1 enhances Ha-ras-induced expression of cyclooxygenase-2 in intestinal epithelial cells via stabilization of mRNA. J Biol Chem. 2000;275(9):6628–6635. doi: 10.1074/jbc.275.9.6628. [DOI] [PubMed] [Google Scholar]
- 31.Finkelman FD, Katona IM, Mosmann TR, Coffman RL. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988;140(4):1022–1027. [PubMed] [Google Scholar]
- 32.Bossie A, Vitetta ES. IFN-gamma enhances secretion of IgG2a from IgG2a-committed LPS-stimulated murine B cells: implications for the role of IFN-gamma in class switching. Cell Immunol. 1991;135(1):95–104. doi: 10.1016/0008-8749(91)90257-c. [DOI] [PubMed] [Google Scholar]
- 33.Panchanathan V, Chaudhri G, Karupiah G. Protective immunity against secondary poxvirus infection is dependent on antibody but not on CD4 or CD8 T-cell function. J Virol. 2006;80(13):6333–6338. doi: 10.1128/JVI.00115-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belyakov IM, Earl P, Dzutsev A, Kuznetsov VA, Lemon M, Wyatt LS, et al. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc Natl Acad Sci U S A. 2003;100(16):9458–9463. doi: 10.1073/pnas.1233578100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muthian G, Raikwar HP, Johnson C, Rajasingh J, Kalgutkar A, Marnett LJ, et al. COX-2 inhibitors modulate IL-12 signaling through JAK-STAT pathway leading to Th1 response in experimental allergic encephalomyelitis. J Clin Immunol. 2006;26(1):73–85. doi: 10.1007/s10875-006-8787-y. [DOI] [PubMed] [Google Scholar]
- 36.Ni J, Shu YY, Zhu YN, Fu YF, Tang W, Zhong XG, et al. COX-2 inhibitors ameliorate experimental autoimmune encephalomyelitis through modulating IFN-gamma and IL-10 production by inhibiting T-bet expression. J Neuroimmunol. 2007;186(1–2):94–103. doi: 10.1016/j.jneuroim.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Harrington LE, Most Rv R, Whitton JL, Ahmed R. Recombinant vaccinia virus-induced T-cell immunity: quantitation of the response to the virus vector and the foreign epitope. J Virol. 2002;76(7):3329–3337. doi: 10.1128/JVI.76.7.3329-3337.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roper RL, Graf B, Phipps RP. Prostaglandin E2 and cAMP promote B lymphocyte class switching to IgG1. Immunol Lett. 2002;84(3):191–198. doi: 10.1016/s0165-2478(02)00185-2. [DOI] [PubMed] [Google Scholar]
- 39.Yao C, Sakata D, Esaki Y, Li Y, Matsuoka T, Kuroiwa K, et al. Prostaglandin E2-EP4 signaling promotes immune inflammation through TH1 cell differentiation and TH17 cell expansion. Nat Med. 2009;15(6):633–640. doi: 10.1038/nm.1968. [DOI] [PubMed] [Google Scholar]
- 40.Jackson ML, Nelson JC, Weiss NS, Neuzil KM, Barlow W, Jackson LA. Influenza vaccination and risk of community-acquired pneumonia in immunocompetent elderly people: a population-based, nested case-control study. Lancet. 2008;372(9636):398–405. doi: 10.1016/S0140-6736(08)61160-5. [DOI] [PubMed] [Google Scholar]
- 41.Kumar R, Burns EA. Age-related decline in immunity: implications for vaccine responsiveness. Expert Rev Vaccines. 2008;7(4):467–479. doi: 10.1586/14760584.7.4.467. [DOI] [PubMed] [Google Scholar]