Abstract
Since many people with chronic fatigue present with pain and many people with chronic pain present with fatigue, we tested if fatigue would enhance the response to pain in male and female mice. We further tested for activation of brainstem nuclei by the fatigue task using c-fos as a marker. Fatigue was induced by having mice spontaneously run in running wheel for 2 hours. Carrageenan (0.03%) was injected into the gastrocnemius muscle either 2h before or 2h after the fatigue task. The mechanical sensitivity of the paw (von Frey filaments), muscle (tweezers), grip force and running wheel activity were assessed before and 24h after injection of carrageenan. Both male and female mice that performed the fatigue task, either before or after intramuscular injection of carrageenan, showed an enhanced mechanical sensitivity of the paw, but not the muscle. Ovariectomized mice showed a similar response to male mice. There was a decrease in running wheel activity after carrageenan injection, but no change in grip force suggesting that mice had no deficit in motor performance induced by the carrageenan. C-fos expression was observed in the nucleus raphe pallidus, obscurus, and magnus after the fatigue task suggesting increased activity in the raphe nuclei in response to the fatigue task. Therefore, widespread hyperalgesia is enhanced by the fatigue response but not hyperalgesia at the site of insult. We suggest this effect is sex-dependent and involves mechanisms in the brainstem to result in an enhanced hyperalgesia.
Keywords: pain, fatigue, sex, hyperalgesia, exercise
1. Introduction
Fatigue is associated with a number of clinical diseases, which include symptoms of chronic pain conditions such as fibromyalgia (FM), rheumatoid arthritis and osteoarthritis [2;6;17;20;21;31;37;40;41;46]. Conversely, patients with chronic fatigue syndrome (CFS) report a significant amount of musculoskeletal pain that interferes with activities of daily living [21]. Patients with arthritis, CFS or FM exhibit an enhanced fatiguing response to exercise [11;29] as well as muscle weakness and decreased endurance [15;16;19;38]. Further, these conditions have a female predominance. Together these data suggest potential interactions between fatigue and pain that are sex-dependent.
In chronic pain conditions, fatigue is generally described as a whole body feeling of muscle fatigue and loss of energy. Fatigue is experimentally defined as a temporary decrease in muscle force in response to exercise [8]. Davis and Bailey [5] define fatigue as ‘an acute impairment of exercise performance that includes both an increase in the perceived effort necessary to exert a desired force and the eventual inability to produce that force”. They further propose that central fatigue is a type of fatigue associated with alterations in central nervous system function that cannot be explained by dysfunction in the muscle itself. For people with chronic conditions such as arthritis, FM, or CFS, this definition describes the fatigue they experience.
Our laboratory recently demonstrated that exercise-induced whole body fatigue results in an enhanced nociceptive response of the paw (i.e. secondary hyperalgesia) to repeated intramuscular injections of acidic saline [45]. Furthermore, there are no changes in peripheral markers of fatigue in the muscle: histology, pH, lactate, phosphate, and creatinine kinase [45]. These data suggests, central, rather than peripheral, mechanisms underlie the enhanced pain response to fatigue.
Interestingly, caudal raphe nuclei, nucleus reticularis obscurus (NRO)/nucleus reticularis pallidus (NRP), mediate not only motor responses but can also play a role in modulating nociceptive stimuli [14]. An enhancement of the tail flick reflex, i.e. facilitation, occurs after low intensity electrical stimulation or low doses of glutamate in the NRO/NRP, as well as the nucleus raphe magnus (NRM)[48]. Neurons in the NRO/NRP show an increase in c-fos expression and are excited by noxious stimuli [25];[4], and additionally are excited by motor activity, i.e. treadmill running [36]. Thus, we propose that since the NRO/NRP responds to both motor activity and to noxious stimuli that the interaction between fatigue and pain occurs in these nuclei.
We therefore, hypothesized that changes in the central nervous system account for the enhanced nociceptive responsiveness to fatigue-conditioning stimuli; and that the fatigue task will activate neurons in the NRO/NRP and possibly the NRM. We further hypothesize that that there will be a difference for sex with females showing a greater enhanced nociceptive responsiveness to the fatigue-conditioning stimulus.
2. Materials and Methods
2.1. Fatigue Model
C57BL/6 mice, male and female, mice were used for these experiments (n=56 female; n=36 male). Fatigue was induced by 2h of spontaneous running in a running wheel. Mice were acclimated for 2 days prior to the fatigue task, 3 times per day for 10 min at each time in the running wheel. Mice were encouraged to run by tapping the cage when they stopped running. On average mice ran 2.28 +/− 0.133 m during the 2h run. This produces an 8.1 +/− 4.% in hindpaw grip force in males [45] and an 8.5 +/− 4.5% decrease in hindpaw grip force for females (n=4) immediately after the fatigue task. To confirm that there was no damage to the skin after the fatigue task we examined the skin (n=2) with hemotoxylin and eosin (H&E) staining. After perfusion with 4% paraformaldeyde, the skin was removed and postfixed in 2% paraformaldehyde with 15% sucrose for 3 days. The skin was then cut at 40 µm on a cryostat and stained with H&E for subsequent analysis by light microscopy.
2.2. Inflammatory muscle pain model
0.03% carrageenan, 20 µl, was injected intramuscularly while the animal was anesthetized with isoflurane (2–4%). This dose of carrageenan does not normally produce an increased mechanical sensitivity of the paw. To confirm inflammation in response to this low dose of carrageenan, we examined the muscle 24h after injection by H&E staining (n=2). The muscle was snap fozen in isopentane and cut at 20 µm on a cryostat, stained with H&E, and examined by light microscopy.
2.3. Ovariectomy
Female mice were ovariectomized according to previously published procedures [3;28]. Mice were anesthetized with isoflurane (3%), a small incision was made in the abdomen, and the uterine horns were lifted out of the body cavity. The ovaries were snipped off of the uterine horns along with attached connective tissue. The incision was closed by suturing with 5-0 silk. The acclimation training and testing of the mice was performed 1 wk after surgery.
2.4. Measurement of muscle withdrawal thresholds
Deep tissue hyperalgesia of the injected and contralateral muscle was tested with a pair of calibrated forceps as previously described [13;30;33]. Mice were acclimated in a restraining device 2 times a day for 2 days, each session consisting of 5 min. The forceps are equipped with two strain gauges to measure force. To measure muscle withdrawal threshold, animals were placed in the restrainer, and the experimenter compressed the gastrocnemius muscle with the tip of the forceps while the hindlimb was extended. Compression continued until the animal withdrew the leg. The maximum force applied at withdrawal was recorded as the muscle withdrawal threshold. Three trials 5 min apart at each time period were performed and averaged to obtain one reading per time period. We previously showed that application of EMLA cream to the skin does not change the decrease in withdrawal threshold. However, application of lidocaine gel to the muscle reverses the hyperalgesia. Therefore, we interpret this as a selective test for deep tissue sensitivity [30]. A decreased withdrawal threshold is interpreted as primary muscle hyperalgesia.
2.5. Measurement of paw sensitivity
Cutaneous hyperalgesia of the paw ipsilateral and contralateral to the injected muscle was tested with calibrated von Frey filaments [45]. Mice were placed in Lucite cubicles on a screen platform. Five von Frey filaments (Force: 0.08, 0.2, 0.3, 0.7, 1.5 mN) were applied to the plantar surfaces of both hindpaws 10 times. Two trials of 10 were averaged at each time period. Mice showed an increasing number of responses to the increasing forces of von Frey filaments. An increased number of responses was interpreted as secondary cutaneous hyperalgesia.
2.6. Muscle Grip Force
We have previously measured grip force after the 2h running wheel fatigue task and show an 8.1% +/− 4.2 decrease in hindpaw grip force in males [45] immediately after the task. We measured grip force and showed a similar decrease in female mice (n=4) with an 8.5% +/− 4.5 decrease in hindpaw grip force immediately after the fatigue task, and returned to normal 2h later, at the time of muscle injection. In addition, we measured grip force before and after induction of inflammation as a measure of maximal contraction ability. Mice were familiarized with the testing procedure twice per day for 2 days prior to data collection to the grip force device. Mice were pulled by the tail to read grip force on the forelimb and then pulled by the tail again to read grip force on hindlimb. Grip force was analyzed on both the forelimbs and hindlimbs and an average of 3 trials recorded.
2.7. Running Cage Activity
Mice were placed in individual cages with cage tops that had a running wheel on one side to measure voluntary running activity. Mice were acclimated in these wheels for 6 days. On the 6th day the timers on the running wheels were set at 3:00 p.m. and were read at 9:00 a.m. the following day, i.e. overnight activity was measured for a total of 18 hours. Sensors on the cages measured the number of revolutions the wheel made during the 18 h time period. After baseline readings we injected carrageenan into the muscle and then measured activity for the evening following the injection.
2.8. Behavioral Protocol
Two different experimental protocols were used: 1) fatigue 2h before muscle insult (n=8 male; n=8 female); 2) fatigue 2h after muscle insult (n=6 male; n=6 female). In each a control group that did not perform the fatigue task was used for comparison: 1) non-runners for fatigue before muscle insult (n=8 male; n=8 female), 2) non-runners for fatigue after muscle insult (n=6 male; n=6 female). Since there were statistically significant differences between males and females we tested a separate group of animals that received ovariectomy 1 week before the experiment (n=8 fatigue before muscle insult; n=8 fatigue after muscle insult). We measured paw, muscle mechanical sensitivity, and grip force before and 24 h after muscle insult in each group. In a separate group of mice we measured cage running wheel activity after injection of carrageenan into the muscle insult in a group that performed the fatigue task (n=4 males; n=3 females) and a group that did not perform the fatigue (n=4 males; n=4 females). As controls we measured paw withdrawal threshold (n=4), muscle withdrawal threshold (n=4) and grip force (n=4) before and after the fatigue task in animals without injection of carrageenan.
2.9. c-fos immunoreactivity
Male mice were deeply anesthetized with sodium pentobarbital (60 mg/kg, i.p.) and transcardially perfused with heparanized saline followed by 4% paraformaldehyde. The brainstem was removed and placed in 30% sucrose overnight prior to processing. Tissue sections were cut on a cryostat at 40 µm and sections stained free-floating using standard immunohistochemistry techniques. Briefly sections were blocked with 3% normal goat serum and the Avidin-Blocking Kit (Vector Laboratories) prior to overnight incubation in the primary antibody overnight at room temperature (rabbit-anti-c-fos; 1:5000; Santa Cruz). On Day 2, sections were incubated in a biotinylated goat anti-rabbit-IgG (1:1000) followed by Streptavidin conjugated with horseradish peroxidase (1:1000) and reaction with diaminobenzidine, hydrogen peroxide, and nickel enhancement. The following groups were used: controls (n=4), immediately after a 2h fatigue task (n=4), 1h after the fatigue task (n=4). Non-specific staining was evaluated by running tissue without the primary antibody, and by performing a dilution series with the primary antibody. No nuclei were observed if the primary antibody was not included in the stain. Use of more concentrated dilutions resulted in greater background staining (non-specific staining) while use of less concentrated dilutions resulted in light to no staining of nuclei. Sections were imaged on a Olympus BX-51 and 5 sections per animal that included the raphe nuclei were counted and added to give one number per animal. The individuals who did the counting were blind to the treatments that each animal received.
2.10. Statistical analysis
A repeated measures ANOVA analyzed data across time (before and 24h after carrageenan) and force (5 forces) for effects of sex (male and female) and fatigue (fatigue and no-fatigue)for the mechanical sensitivity of the paw. A repeated measures ANOVA analyzed differences across time (before and 24h after carrageenan) for sex (male and female) and fatigue (fatigue and ano-faitigue) for the muscle withdrawal threshold. Ovariectomized mice were analyzed separately for the mechanical withdrawal thresholds of the paw by comparing to female mice with a repeated measures ANOVA. The ipsilateral and the contralateral sides were analyzed separately A one-way ANOVA compared differences between groups for number of c-fos positive cells in the NRO, NRP and NRM followed by post-hoc comparisons with a Tukey’s test.
3. Results
3.1. Mechanical sensitivity
3.1.1. Fatigue prior to carrageenan injection
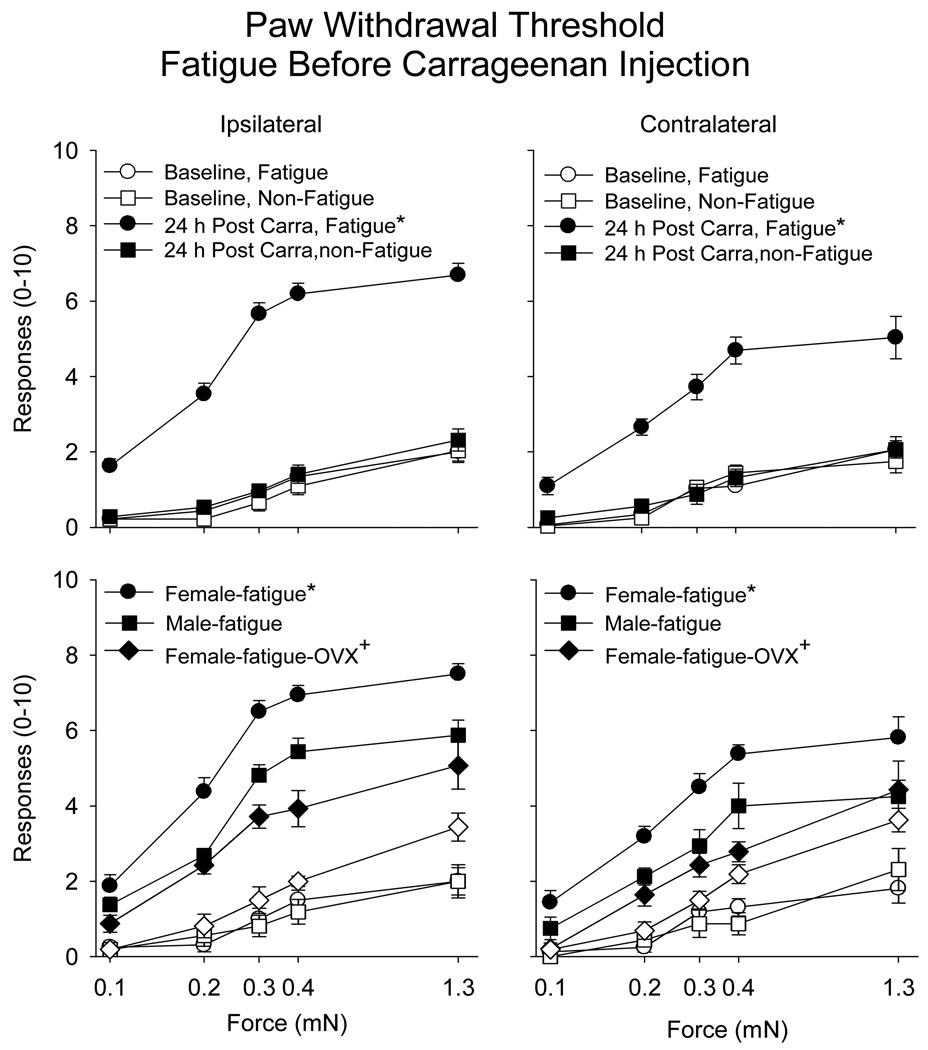
There was a significantly increased number of withdrawals to repeated application of von Frey filaments applied to the hind paw in the animals that performed the fatigue task 2 h prior to injection of 0.03 % carrageenan when compared to those that did not perform the fatigue task (see Table 1). This enhanced effect occurred both ipsilaterally and contralaterally (Figure 1). Furthermore, the enhanced responsiveness to mechanical stimuli was greater in female mice when compared to male mice, both ipsilaterally and contralaterally (Figure 1)(Table 1). No changes in mechanical responsiveness of the paw occurred in animals immediately or 24h after the fatigue task alone, without carrageenan injection (Table 2). Similarly, no difference in muscle withdrawal thresholds occurred immediately after or 24h after the fatigue task alone, without carrageenan injection (Table 2). The fatigue task alone had no effect on skin histology immediately after the 2h running task. Inflammation was confirmed in the animals injected with 0.03% carrageenan. There was infiltration of neutrophils into the ipsilateral gastrocnemius muscle 24h after injection as assessed with H&E staining of the muscle.
Table 1.
Within- and between-subjects statistical results for the paw withdrawal threshold analyzed with a repeated measures (time and force) ANOVA with two factors (sex, runner).
| Fatigue Before Carrageenan | Fatigue After Carrageenan | ||||||
|---|---|---|---|---|---|---|---|
| Source | Limb | df | F | p | df | F | p |
| Time | Ipsilateral | 1,28 | 454.3 | .0001 | 1,20 | 294.2 | .0001 |
| Contralateral | 1,28 | 65.8 | .0001 | 1,20 | 79.2 | .0001 | |
| Time * sex | Ipsilateral | 1,28 | 15.8 | .0001 | 1,20 | 2.63 | .120 |
| Contralateral | 1,28 | 4.6 | .039 | 1,20 | 1.35 | .326 | |
| Time * fatigue | Ipsilateral | 1,28 | 345.7 | .0001 | 1,20 | 104.0 | .0001 |
| Contralateral | 1,28 | 55.6 | .0001 | 1,20 | 7.5 | .013 | |
| Time * sex * fatigue | Ipsilateral | 1,28 | 9.7 | .004 | 1,20 | .006 | .939 |
| Contralateral | 1,28 | 2.5 | .124 | 1,20 | 1.07 | .381 | |
| Force | Ipsilateral | 4,112 | 130.0 | .0001 | 4,80 | 35.2 | .0001 |
| Contralateral | 4,112 | 85.2 | .0001 | 4,80 | 8.8 | .0001 | |
| Force * sex | Ipsilateral | 4,112 | .381 | .822 | 4,80 | 2.9 | .061 |
| Contralateral | 4,112 | .458 | .766 | 4,80 | .217 | .928 | |
| Force * fatigue | Ipsilateral | 4,112 | 17.1 | .0001 | 4,80 | 18.9 | .0001 |
| Contralateral | 4,112 | 5.6 | .0001 | 4,80 | 2.6 | .043 | |
| Force * sex * fatigue | Ipsilateral | 4,112 | 1.0 | .403 | 4,80 | 1.2 | .293 |
| Contralateral | 4,112 | .446 | .775 | 4,80 | .386 | .818 | |
|
Force * time Force * time * sex |
Ipsilateral | 4,112 | 23.6 | .0001 | 4,80 | 62.3 | .0001 |
| Contralateral | 4,112 | 5.6 | .0001 | 4,80 | 7.9 | .0001 | |
| Ipsilateral | 4,112 | 1.2 | .304 | 4,80 | 6.4 | .0001 | |
| Contralateral | 4,112 | .959 | .433 | 4,80 | 1.05 | .385 | |
| Force * time * fatigue | Ipsilateral | 4,112 | 18.4 | .0001 | 4,80 | 70.4 | .0001 |
| Contralateral | 4,112 | 8.7 | .0001 | 4,80 | 11.3 | .0001 | |
| Force * time * sex * fatigue | Ipsilateral | 4,112 | 1.4 | .213 | 4,80 | 3.5 | .022 |
| Contralateral | 4,112 | 1.2 | .294 | 4,80 | .812 | .378 | |
| aIntercept | Ipsilateral | 1,28 | 595.4 | .0001 | 1,20 | 280.9 | .0001 |
| Contralateral | 1,28 | 321.1 | .0001 | 1,20 | 76.9 | .0001 | |
| aSex | Ipsilateral | 1,28 | 8.6 | .006 | 1,20 | 6.2 | .021 |
| Contralateral | 1,28 | 6.9 | .013 | 1,20 | .902 | .354 | |
| aFatigue | Ipsilateral | 1,28 | 144.5 | .0001 | 1,20 | 73.1 | .0001 |
| Contralateral | 1,28 | 48.4 | .0001 | 1,20 | 4.5 | .047 | |
| aSex * fatigue | Ipsilateral | 1,28 | 2.9 | .098 | 1,20 | .948 | .342 |
| Contralateral | 1,28 | 1.0 | .309 | 1,20 | .401 | .534 | |
Note: bold represents significant results.
represents between subjects effects
Figure 1.
Number of responses to repeated application of 5 von Frey filaments applied to the paw before and after muscle inflammation in the group that fatigued before injection of carrageenan (circles) compared to those that did not perform the fatigue task (squares) for the ipsilateral and the contralateral hindpaw (male and female mice combined). Bilateral increases in the number of responses to mechanical stimuli applied to the paw were observed in the group that fatigued after injection of carrageenan when compared to the nonfatigued group (*)(top panels). Bottom panels show responses in female mice, male mice, and female mice with ovariectomy (open symbols before insult, closed symbols before insult). Female mice had a greater increase in the number of responses to mechanical stimuli applied to the paw when compared to male mice (*). Ovariectomy resulted in a reduced number of responses compared to intact female mice (+), and was similar to that observed in males Data are the mean ± S.E.M.
Table 2.
Mechanical sensitivity of the paw and muscle to repeated application of von Frey filaments before and after the fatigue task remained unchanged. Data for paw are the number of withdrawals out of 10 to repeated apploication of 5 different forces and for muscle are withdrawal threshold in mN to squeezing the muscle.
| Site | Baseline | Immediately after fatigue task | 24h after fatigue task | |||
|---|---|---|---|---|---|---|
| Ipsilateral | Contralateral | Ipsilateral | Contralateral | Ipsilateral | Contralateral | |
| Paw, 0.1 mN | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.17 ± 0.17 | 0.17 ± 0.17 |
| Paw, 0.2 mN | 0.12 ± 0.12 | 0.12 ± 0.12 | 0.12 ± 0.12 | 0 ± 0 | 0.33 ± 0.17 | 0.33 ± 0.17 |
| Paw, 0.3 mN | 0.12 ± 0.12 | 0.38 ± 0.24 | 0.62 ± 0.38 | 0.25 ± 0.25 | 0.67 ± 0.44 | 1.0 ± 0.28 |
| Paw, 0.4 mN | 0.62 ± 0.24 | 0.62 ± 0.31 | 0.88 ± 0.43 | 0.75 ± 0.43 | 1.7 ± 0.33 | 0.67 ± 0.33 |
| Paw, 1.3 mN | 1.75 ± 0.25 | 2.25 ± 0.43 | 0.12 ± 0.59 | 0.88 ± 0.55 | 1.8 ± 0.60 | 1.7 ± 0.44 |
| Muscle | 1826 ± 22 mN | 1913 ± 35 mN | 1935 ± 24 mN | 1934 ± 57 mN | 1943 ± 43 mN | 1857 ± 56 mN |
Data are the mean ± S.E.M.
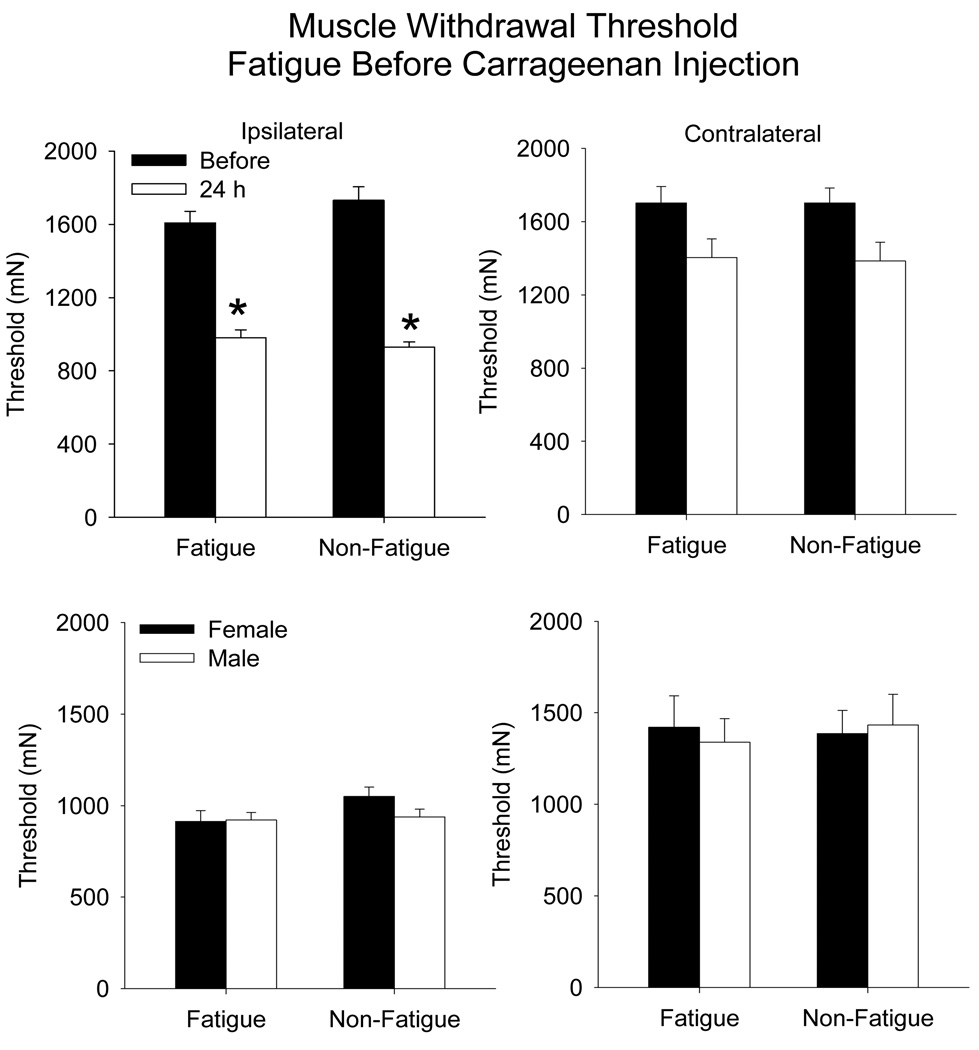
Muscle withdrawal thresholds were decreased 24 h after carrageenan injection similarly between the fatigued and the non-fatigued animals, and there was no difference between males and females (Figure 2) (time: ipsilateral, F1,20 = 375, p=0.0001; contralateral F1,20 = 45.4 p=0.0001). Decreases occurred bilaterally for the muscle withdrawal thresholds with the greatest decrease occurring for the inflamed muscle.
Figure 2.
Bar graphs representing the mean withdrawal threshold to mechanical stimulation applied to the muscle before (closed bars) and after (open bars) muscle inflammation in animals (males and females combined) that performed the fatiguing exercise prior to the carrageenan injection compared to controls that did not exercise (top panels). A decrease in withdrawal threshold occurred for the inflamed muscle in both groups on the ipsilateral side. There was no difference between the withdrawal threshold for female and male mice (bottom panels). Data are the mean ± S.E.M.
3.1.2. Fatigue after carrageenan injection
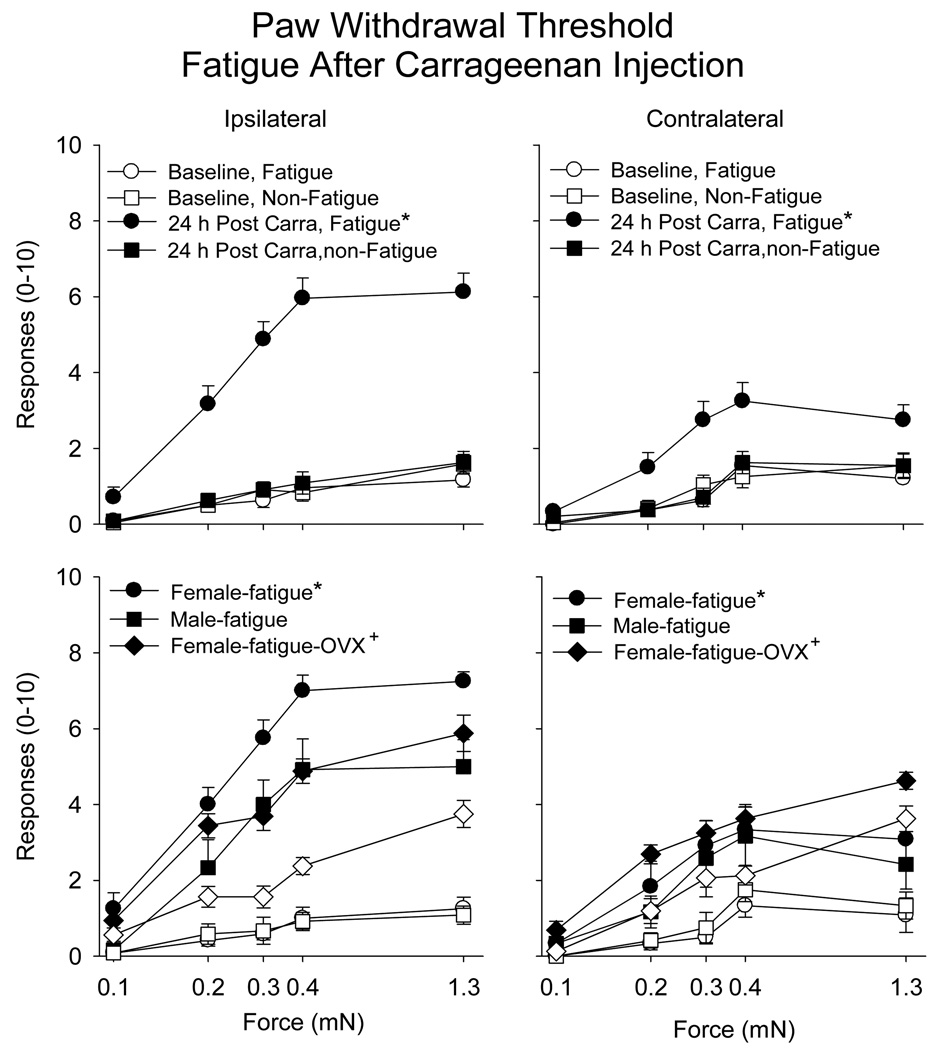
When the fatigue task was performed 2 h after the carrageenan injection there was a significantly increased number of withdrawals to repeated application of von Frey filaments applied to the hind paw (Table 1). This effect occurred bilaterally, but was greater for the ipsilateral than the contralateral hindlimb (Figure 3). Furthermore, for the ipsilateral side, there was an enhanced responsiveness in female mice when compared to male mice (Figure 3)(Table 1).
Figure 3.
Number of responses to repeated application of 5 von Frey filaments applied to the paw before and after muscle inflammation in the group that fatigued after injection of carrageenan (circles) compared to those that did not perform the fatigue task (squares) for the ipsilateral and the contralateral hindpaw (male and female mice combined). Bilateral increases in the number of responses to mechanical stimuli applied to the paw were observed in the group that fatigued after injection of carrageenan when compared to the nonfatigued group (*). Bottom panels show data presented in top panels separated into male and female mice, and with ovariectomized mice (open symbols before insult; closed symbols after insult). Female mice had a greater increase in the number of responses to mechanical stimuli applied to the paw when compared to male mice (*). Ovariectomy resulted in a reduced number of responses compared to intact female mice (+), and was similar to that observed in males. Data are the mean ± S.E.M.
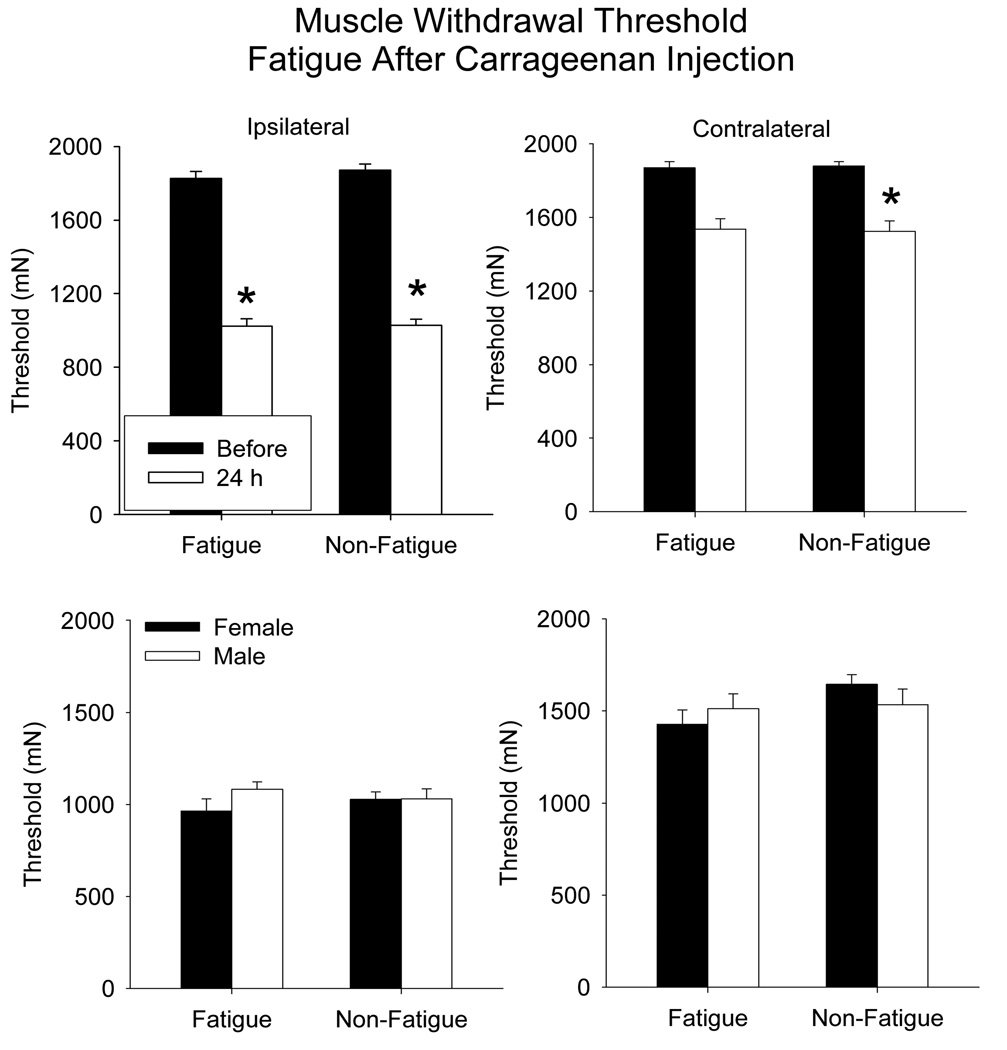
Muscle withdrawal thresholds were decreased 24 h after carrageenan injection similarly between the fatigued and the non-fatigued animals, and there was no difference between males and females (Figure 4) (ipsilateral, F1,20=54.9, p=0.0001; contralateral F1,20=81, p=0.0001). Decreases occurred bilaterally for the muscle withdrawal thresholds with the greatest decrease occurring for the inflamed muscle.
Figure 4.
Bar graphs representing the mean withdrawal threshold to mechanical stimulation applied to the muscle before (closed bars) and after (open bars) muscle inflammation in animals (male and female mice combined) that performed the fatiguing exercise after carrageenan injection compared to controls that did not exercise (top panels). A decrease in withdrawal threshold occurred for the inflamed muscle in both groups on the ipsilateral side. There was no difference between the withdrawal threshold for female and male mice (bottom panels). Data are the mean ± S.E.M.
3.2. Effect of ovariectomy
Prior ovariectomies in female mice showed a similar development of mechanical sensitivity of the paw, i.e. secondary hyperalgesia, when compared to males. The number of withdrawals to von Frey Filaments was significantly less in ovariectomized mice compared to non-ovariectomized female mice who were run either before (ipsilateral: F1,29=5.5, p=0.008; contralateral F1,29=5.4, p=0.008) or after (ipsilateral: F1,29=21.7, p=0.0001; contralateral F1,29=11.4, p=0.0001) injection of carrageenan (Figure 1,Figure 3).
3.3. Evaluation of muscle performance
Grip force 24 h after the carrageenan injection remained unchanged in all groups of animals when compared to values prior to injection of carrageenan suggesting the animals still had normal strength after injection of carrageenan (see Table 3 for values). Animals performed an average of 15,550 ± 1823 revolutions per 18 h that was reduced to 10,077 ± 1,446 revolutions per 18 h. Running cage activity was similarly (p<0.05) reduced from baseline 24 h after carrageenan injection to 74 ± 10 % (n=4 males; n=4 females) in the animals that performed the fatigue task with carrageenan injection and to 77 ± 10 % (n=4 males; n=3 females) in the animals that did not perform the fatigue task but received carrageenan injection suggesting that carrageenan injection reduced the animal’s willingness to be physically active.
Table 3.
Grip force of the hindpaws and forepaws before and 24h after carrageenan in the animals that performed the fatigue task either before or after injection of carrageenan.
| Fatigue Task | Baseline | 24h post carrageenan | |
|---|---|---|---|
|
Fatigue before carrageenan |
hindpaw | 61.8 ± 2.3 g | 64.8 ± 2.5 g |
| forepaw | 95 ± 1.0 g | 93 ± 1.5 g | |
|
Fatigue after carrageenan |
hindpaw | 60.6 ± 1.8 g | 62.1 ± 2.3 g |
| forepaw | 93.9 ± 1.3 g | 95.3 ± 1.5 g | |
Data are the mean + S.E.M.
The distance ran during the fatigue task was similar between all groups whether or not they received a prior injection of carrageenan. The average distance ran was 2.28 m +/− 0.13. Thus, with minimal prodding (tapping on the top of the wheel) the mice were able to perform the activity.
3.4. C-fos immunoreactivity
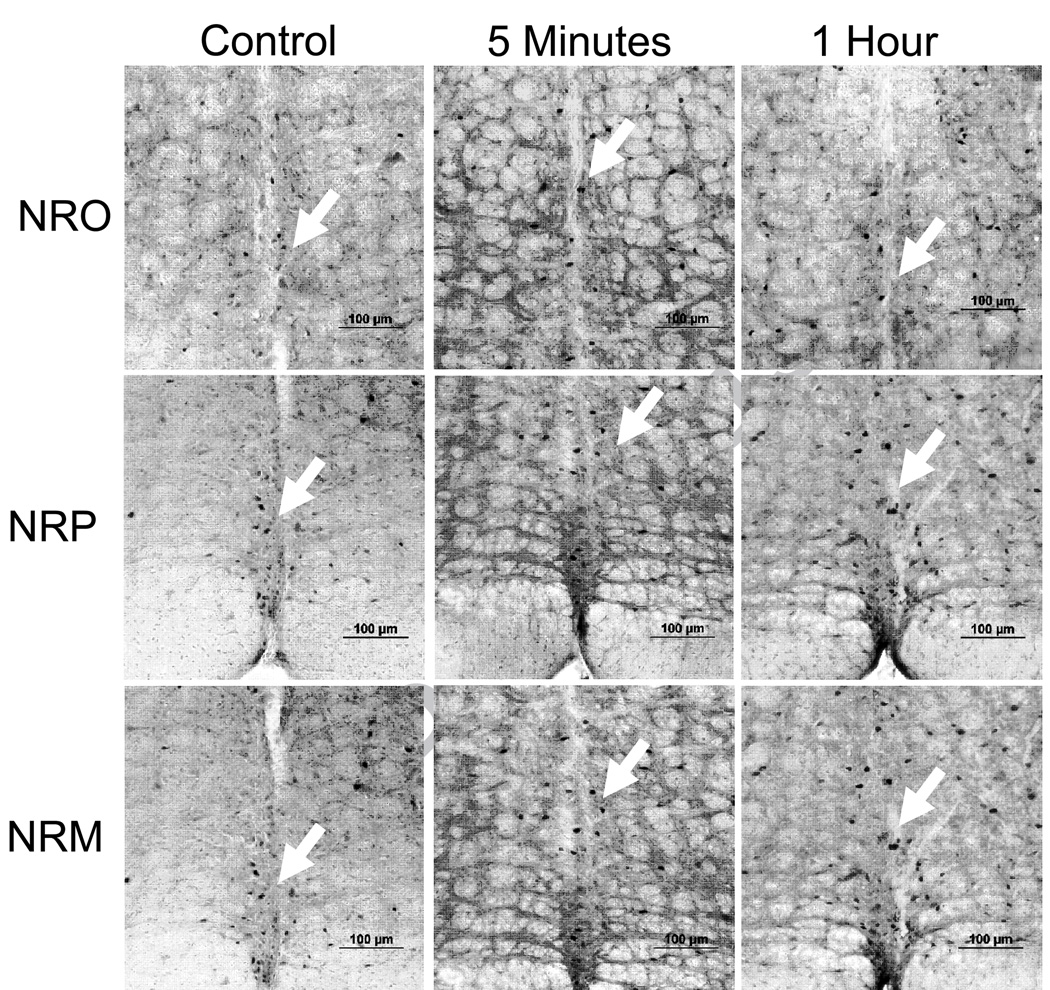
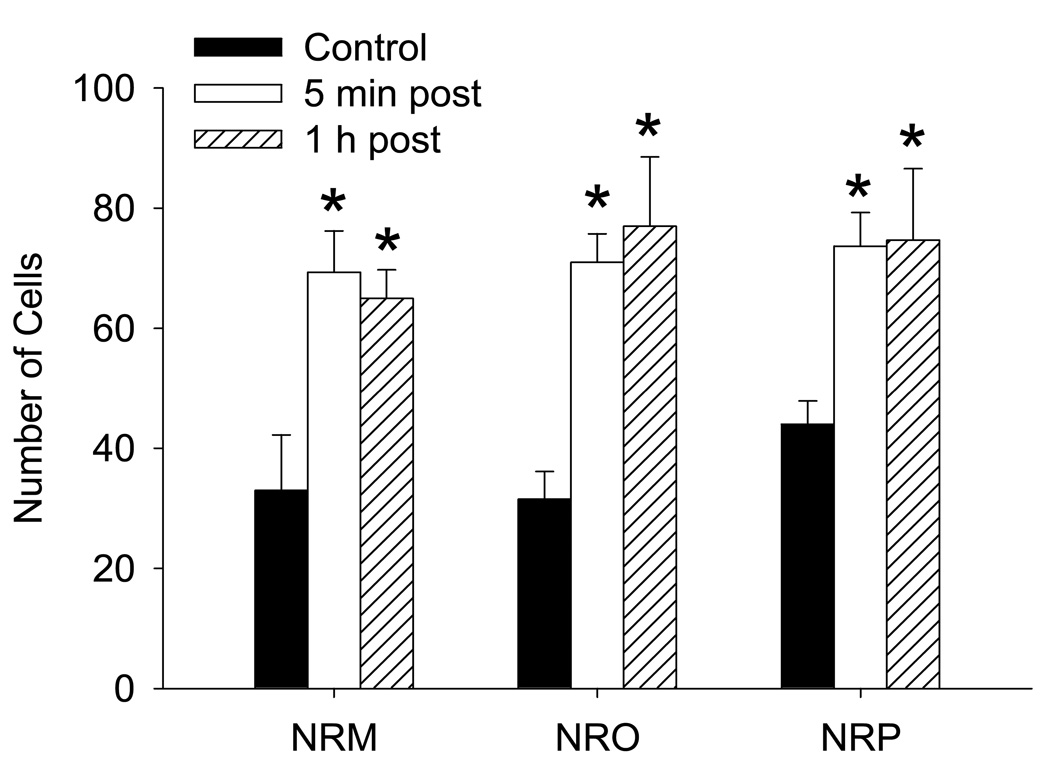
Since the RVM is involved in development of secondary hyperalgesia, and the NRO/NRP are involved in fatigue responses, as well as nociception, we tested if fatigue would result in increased activation of neurons in these nuclei. Figure 5 shows representative examples of c-fos expression in each nucleus for controls that did not run, and for animals either immediately or 1 h after the fatigue task. Quantitative analysis shows an increased number of c-fos-positive cells in the NRM (F2,11=7.4, p=0.01), NRO (F2,11=10.6, p=0.004) and the NRP (F2,11=5.1,p=0.03) after the fatigue task (Figure 6). The number of cells in the NRM, NRO and NRP were significantly increased immediately and 1 h after the fatigue task.
Figure 5.
Photomicrographs of c-fos immunoreactivity (arrows) in the nucleus raphe obscurus (NRO), nucleus raphe pallidus (NRP) and the nucleus raphe magnus (NRM) from control mice that did not perform the fatigue task and from mice 5 minutes and 1 hour after the completion of the fatigue task. Bar = 100 µm.
Figure 6.
The mean number of total c-fos positive cells in each region of the NRM, NRO and NRP for each group of animals. Significant increases in the number of cells occurred both 5 minutes and 1 h after completion of the 2h fatigue task when compared to control mice that did not run. *, significantly increase from controls
4. Discussion
4.1. Fatigue enhances secondary hyperalgesia through central mechanisms
The current study shows an enhanced nociceptive response in animals that performed a whole-body muscle fatigue task either before or after the injection of a low-dose of carrageenan. This dose of carrageenan when given alone results in a local reduction in withdrawal threshold of the muscle, i.e. primary hyperalgesia, without increased responsiveness to mechanical stimulation of the paw, i.e. secondary hyperalgesia. The fatigue-conditioning stimulation resulted in the development of secondary hyperalgesia in response to this low dose carrageenan but did not enhance the primary hyperalgesia. We propose that this enhancement is due to changes in the central nervous system as 1) prior data shows that the fatigue-conditioning task does not result in changes within the muscle [45], 2) the enhancement of hyperalgesia occurs for secondary hyperalgesia measures and not primary hyperalgesia measures, and 3) prior work shows that secondary hyperalgesia is facilitated by brainstem nuclei but that primary hyperalgesia is unaffected by lesions of the RVM [24;26;27;34].
The enhanced responsiveness to mechanical stimulation of the paw occurs bilaterally if the fatigue stimulus is given either before or after injection of carrageenan. Further, the response on the contralateral side was not as great as the ipsilateral hindlimb. The bilateral nature of the enhanced hyperalgesia further supports a role for central mechanisms, primarily supraspinal sites. Specifically, the raphe nuclei send projections bilaterallty to the spinal cord [1;47]. Electrical or chemical stimulation of the NRM results in bilateral effects [12;32] and local anesthetic applied to the NRM produces a bilateral reduction in hyperalgesia [33]. Facilitation from the RVM after paw inflammation is widespread affecting not only for the inflamed paw but also the uninflamed paw and tail [32]. Further, contralateral hyperalgesia, once developed, is unaffected by removal of afferent input, by lesion or local anesthetic, to the spinal cord [18;42–44] suggesting central mechanisms maintain the spread of hyperalgesia. Thus, the present results, together with prior literature suggest that bilateral cutaneous secondary hyperalgesia, as observed after muscle insult, likely involves facilitatory influences descending from the central nervous system and in particular the medullary raphe nuclei.
In the RVM, two types of cells are capable of modulating nociception: ON-cells in the RVM facilitate pain, and OFF-cells inhibit pain [7]. A third type of cell, neutral cell, does not respond to nociceptive stimuli. In animals with neuropathic pain, the removal of ON-cells, with dermorphin-saporin, prevents the secondary hyperalgesia associated with nerve injury [26]. Further, after paw inflammation, neutral cells show a phenotypic switch to either ON-cells or OFF-cells in the RVM; and population recordings show a greater percentage of ON-cells and OFF-cells after inflammation [22]. These data support that there is both an increase in facilitation and inhibition after inflammation, which has been confirmed experimentally [27;35].
The current study showed increases in c-fos expression in the brainstem raphe nuclei, NRM, NRO, and NRP, after the 2 h fatigue-conditioning stimuli. Historically the NRM has been thought to process nociceptive information and can both facilitate and inhibit nociceptive stimuli [7;14;36](see above discussion). On the other hand, the NRO and NRP have typically been thought to mediate motor activity, including fatigue [14]. Central fatigue may arise from an inability of the brain to maintain adequate excitatory drive to muscles [10]. One of the primary functions of central raphe nuclei is to increase excitability of brainstem and spinal motor neurons to facilitate motor output [39]. Jacobs and colleagues suggest that the NRO/NRP is particularly suited to modulate fatigue [9;14]. Specifically, in awake cats, recordings from neurons in the NRO/NRP decrease their discharge rates during a fatigue task [9] and recovery of neuronal activity increases gradually over 45 minutes correlating with recovery from fatigue. In addition low intensity electrical stimulation or low doses of glutamate microinjected into the NRO/NRP enhances the tail flick reflex and activity of nociceptive dorsal horn neurons [48]. In the current study, we show increases in c-fos expression in the raphe nuclei (NRO/NRM/NRP) in response to fatigue-conditioning stimuli, and prior studies show increases in c-fos expression in response to noxious stimuli [25]. Neurons in the NRO/NRP are excited by noxious stimuli [4] as well as by spontaneous treadmill running [36]. Thus, we propose that the raphe nuclei are uniquely designed to integrate motor and sensory information, and are particularly important for the fatigue-enhanced response to pain.
4.2. Role of gender in nociception and fatigue
In the current study, female mice showed a greater fatigue-enhanced hyperalgesia than male mice and the ovariectomy of female mice reduced this enhanced effect. Previously, we were unable to show sex differences after a higher dose of intramuscular carrageenan, 3% (Sluka et al., 2007), suggesting that carrageenan does not result in the observed sex differences. However, Tall and Crisp (2004) show an enhanced mechanical sensitivity after carrageenan inflammation (0.15ml of 4%, paw) in female rats when compared to males; and several studies show enhanced responsiveness to other inflammatory irritants such as prostaglandin E2 and complete Fruend’s adjuvant in females (Dina et al., 2001; Cook and Nickerson, 2005). In some cases, there is an enhanced effect during the estrous cycle after inflammation (Cook and Nickerson, 2005). The sex differences observed in the current study may be unrelated to the carrageenan, but rather related to the fatigue-enhanced nociceptive response to the carrageenan. Previously, using a Rota-Rod to force mice to run in a controlled manner, we showed greater fatigue that was task-dependent, in female mice when compared to male mice [3]. The differences between males and females in this fatigue task required both ovariectomy and testosterone [3]. These data support the notion that fatigue tasks, and potentially fatigue-enhanced hyperalgesia may be sex- dependent.
Human female subjects show a greater temporal summation effect when compared to males (Fillingem et al., 1998; Sarlani et al., 2002; Sarton et al., 2000) and there is enhanced temporal summation in females with chronic pain such as fibromyalgia and temporomandibular disorder (Staud et al., 2003; 2001; 2004; Maixner et al., 1998; Sarlani, 2007). Furthermore, females are more likely to develop referred pain than males after intramuscular infusion of acidic saline (Frey Law et al., 2008). These data suggest that central sensitivity to nociceptive stimuli is enhanced in females when compared to males supporting the enhanced secondary hyperalgesia response observed in the current study in females when compared to male mice.
4.3. Limitations
One limitation in interpretation of the data from the current study involved the use of a fatigue task to induce whole-body muscle fatigue. This may not be an exact replica of the type of fatigue observed in patients with fibromyalgia, chronic fatigue syndrome, or inflammatory arthritis. However, we suggest a whole body, low-level, centrally mediated fatigue task mimics that observed in people with fibromyalgia who generally have a whole body feeling of fatigue but can still present with normal fatiguing response to muscle contractions. As many as 76% of people with chronic musculoskeletal pain condition report fatigue and as many as 94% of people with chronic fatigue syndrome report musculoskeletal pain. Most people can distinguish easily between physical fatigue and mental fatigue and tend to report physical fatigue [23;46]. We previously showed an enhanced hyperalgesic response to pH 5.0 saline, a non-inflammatory pain stimuli, after a similar fatigue task that results in physical muscle fatigue [45]. Although that study was not exhaustive, we were unable to show changes in the muscle in terms of histological changes, changes in creatinine kinase, lactate, pH, oxygen, carbon dioxide, or phosphate [45]. Thus, while not conclusive, a central fatigue is likely contributing to the enhancement and spread of hyperalgesia in this model. Understanding interactions between fatigue and muscle pain in a simplified model will lead to better insight into potential mechanisms underlying chronic musculoskeletal pain conditions and potentially improve treatment of these conditions.
4.4. Summary
In summary, the current study shows increased activity in raphe nuclei of the medulla in response to fatigue, and that fatigue enhances the response to low doses of carrageenan. In particular, the spread of pain is enhanced by fatigue, but not the pain at the site of insult. The effect is greater in female mice and depends on intact ovaries. This sex-dependent enhancement of the development of secondary hyperalgesia may be critical to understanding the female predominance of widespread pain, and suggests that females are more likely to develop referred or widespread pain.
Acknowledgments
Supported by AR052316 and AR053509. We thank Jing Danielson for technical assistance with histology, and Herbert K. Proudfit for technical advice and critically reading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interest.
References
- 1.Antal M, Petko M, Polgar E, Heizmann CW, StormMathisen J. Direct evidence of an extensive GABAergic innervation of the spinal dorsal horn by fibres descending from the rostral ventromedial medulla. N S. 1996;73:509–518. doi: 10.1016/0306-4522(96)00063-2. [DOI] [PubMed] [Google Scholar]
- 2.Bazelmans E, Bleijenberg G, Voeten MJ, van der Meer JW, Folgering H. Impact of a maximal exercise test on symptoms and activity in chronic fatigue syndrome. J Psychosom Res. 2005;59:201–208. doi: 10.1016/j.jpsychores.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Burnes LA, Kolker SJ, Danielson JF, Walder RY, Sluka KA. Enhanced muscle fatigue occurs in male but not female ASIC3−/− mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1347–R1355. doi: 10.1152/ajpregu.00687.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dantas MA, Co W, Futuro-Neto HA. Responses of neurons of the nucleus raphe obscurus to noxious stimuli. Braz J Med Biol Res. 1990;23:923–926. [PubMed] [Google Scholar]
- 5.Davis JM, Bailey SP. Possible mechanisms of central nervous system fatigue during exercise. Med Sci Sports Exerc. 1997;29:45–57. doi: 10.1097/00005768-199701000-00008. [DOI] [PubMed] [Google Scholar]
- 6.De Becker P, Roeykens J, Reynders M, McGregor N, De Meirleir K. Exercise capacity in chronic fatigue syndrome. Arch Intern Med. 2000;160:3270–3277. doi: 10.1001/archinte.160.21.3270. [DOI] [PubMed] [Google Scholar]
- 7.Fields HL, Basbaum AI, Heinricher MM. Central Nervous System Mechanisms of Pain Modulation. In: McMahon SB, Koltzenburg M, editors. Textbook of Pain. Philadelphia: Elsevier; 2006. pp. 125–142. [Google Scholar]
- 8.Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- 9.Fornal CA, Martin-Cora FJ, Jacobs BL. "Fatigue" of medullary but not mesencephalic raphe serotonergic neurons during locomotion in cats. Brain Res. 2006;1072:55–61. doi: 10.1016/j.brainres.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 11.Gogia PP, Sabbahi MA. Electromyographic analysis of neck muscle fatigue in patients with osteoarthritis of the cervical spine. Spine. 1994;19:502–506. doi: 10.1097/00007632-199403000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Hurley RW, Hammond DL. The analgesic effects of supraspinal mu and delta opioid receptor agonists are potentiated during persistent inflammation. J Neurosci. 2000;20:1249–1259. doi: 10.1523/JNEUROSCI.20-03-01249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikeuchi M, Kolker SJ, Burnes LA, Walder RY, Sluka KA. Role of ASIC3 in the primary and secondary hyperalgesia produced by joint inflammation in mice. Pain. 2008;137:662–669. doi: 10.1016/j.pain.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs BL, Martin-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Brain Res Rev. 2002;40:45–52. doi: 10.1016/s0165-0173(02)00187-x. [DOI] [PubMed] [Google Scholar]
- 15.Jacobsen S, Danneskiold-Samsoe B. Isometric and isokinetic muscle strength in patients with fibrositis syndrome. New characteristics for a difficult definable category of patients. Scand J Rheumatol. 1987;16:61–65. [PubMed] [Google Scholar]
- 16.Jacobsen S, Danneskiold-Samsoe B. Dynamic muscular endurance in primary fibromyalgia compared with chronic myofascial pain syndrome. Arch Phys Med Rehabil. 1992;73:170–173. [PubMed] [Google Scholar]
- 17.Kadetoff D, Kosek E. The effects of static muscular contraction on blood pressure, heart rate, pain ratings and pressure pain thresholds in healthy individuals and patients with fibromyalgia. Eur J Pain. 2007;11:39–47. doi: 10.1016/j.ejpain.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Kalra A, Urban MO, Sluka KA. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS) J Pharmacol Exp Ther. 2001;298:257–263. [PubMed] [Google Scholar]
- 19.Liikavainio T, Lyytinen T, Tyrvainen E, Sipila S, Arokoski JP. Physical function and properties of quadriceps femoris muscle in men with knee osteoarthritis. Arch Phys Med Rehabil. 2008;89:2185–2194. doi: 10.1016/j.apmr.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Maquet D, Croisier JL, Renard C, Crielaard JM. Muscle performance in patients with fibromyalgia. Joint Bone Spine. 2002;69:293–299. doi: 10.1016/s1297-319x(02)00373-1. [DOI] [PubMed] [Google Scholar]
- 21.Meeus M, Nijs J, Meirleir KD. Chronic musculoskeletal pain in patients with the chronic fatigue syndrome: A systematic review. Eur J Pain. 2006;11:377–386. doi: 10.1016/j.ejpain.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Miki K, Zhou QQ, Guo W, Guan Y, Terayama R, Dubner R, Ren K. Changes in gene expression and neuronal phenotype in brain stem pain modulatory circuitry after inflammation. J Neurophysiol. 2002;87:750–760. doi: 10.1152/jn.00534.2001. [DOI] [PubMed] [Google Scholar]
- 23.Parrish BP, Zautra AJ, Davis MC. The role of positive and negative interpersonal events on daily fatigue in women with fibromyalgia, rheumatoid arthritis, and osteoarthritis. Health Psychol. 2008;27:694–702. doi: 10.1037/0278-6133.27.6.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pertovaara A, Wei H, Hamalainen MM. Lidocaine in the rostroventromedial medulla and the periaqueductal gray attenuates allodynia in neuropathic rats. Neurosci Lett. 1996;218:127–130. doi: 10.1016/s0304-3940(96)13136-0. [DOI] [PubMed] [Google Scholar]
- 25.Pinto M, Lima D, Castro-Lopes J, Tavares I. Noxious-evoked c-fos expression in brainstem neurons immunoreactive for GABAB, mu-opioid and NK-1 receptors. Eur J Neurosci. 2003;17:1393–1402. doi: 10.1046/j.1460-9568.2003.02586.x. [DOI] [PubMed] [Google Scholar]
- 26.Porreca F, Burgess SE, Gardell LR, Vanderah TW, Malan TP, Ossipov MH, Lappi DA, Lai J. Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the muopioid receptor. J Neurosci. 2001;21:5281–5288. doi: 10.1523/JNEUROSCI.21-14-05281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. TRENDS NEUROSCI. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 28.Riffo-Vasquez Y, Ligeiro de Oliveira AP, Page CP, Spina D, Tavares-de-Lima W. Role of sex hormones in allergic inflammation in mice. Clin Exp Allergy. 2007;37:459–470. doi: 10.1111/j.1365-2222.2007.02670.x. [DOI] [PubMed] [Google Scholar]
- 29.Schillings ML, Kalkman JS, van der Werf SP, van Engelen BG, Bleijenberg G, Zwarts MJ. Diminished central activation during maximal voluntary contraction in chronic fatigue syndrome. Clin Neurophysiol. 2004;115:2518–2524. doi: 10.1016/j.clinph.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Skyba DA, Radhakrishnan R, Sluka KA. Characterization of a method for measuring primary hyperalgesia of deep somatic tissue. Journal of Pain. 2005;6:41–47. doi: 10.1016/j.jpain.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Staud R, Robinson ME, Price DD. Isometric exercise has opposite effects on central pain mechanisms in fibromyalgia patients compared to normal controls. Pain. 2005;118:176–184. doi: 10.1016/j.pain.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Terayama R, Dubner R, Ren K. The roles of NMDA receptor activation and nucleus reticularis gigantocellularis in the time-dependent changes in descending inhibition after inflammation. Pain. 2002;97:171–181. doi: 10.1016/s0304-3959(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 33.Tillu DV, Gebhart GF, Sluka KA. Descending facilitatory pathways from the RVM initiate and maintain bilateral hyperalgesia after muscle insult. Pain. 2007 doi: 10.1016/j.pain.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urban MO, Zahn PK, Gebhart GF. Descending facilitatory influences from the rostral medial medulla mediate secondary, but not primary hyperalgesia in the rat. N S. 1999;90:349–352. doi: 10.1016/s0306-4522(99)00002-0. [DOI] [PubMed] [Google Scholar]
- 35.Vanegas H, Schaible HG. Descending control of persistent pain: inhibitory or facilitatory? Brain Res Brain Res Rev. 2004;46:295–309. doi: 10.1016/j.brainresrev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci. 1995;15:5346–5359. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vierck CJ, Jr, Staud R, Price DD, Cannon RL, Mauderli AP, Martin AD. The effect of maximal exercise on temporal summation of second pain (windup) in patients with fibromyalgia syndrome. J Pain. 2001;2:334–344. doi: 10.1054/jpai.2001.25533. [DOI] [PubMed] [Google Scholar]
- 38.Wallman KE, Sacco P. Sense of effort during a fatiguing exercise protocol in chronic fatigue syndrome. Res Sports Med. 2007;15:47–59. doi: 10.1080/15438620601184331. [DOI] [PubMed] [Google Scholar]
- 39.White SR, Fung SJ, Jackson DA, Imel KM. Serotonin, norepinephrine and associated neuropeptides: effects on somatic motoneuron excitability. Prog Brain Res. 1996;107:183–199. doi: 10.1016/s0079-6123(08)61865-8. [DOI] [PubMed] [Google Scholar]
- 40.Whiteside A, Hansen S, Chaudhuri A. Exercise lowers pain threshold in chronic fatigue syndrome. Pain. 2004;109:497–499. doi: 10.1016/j.pain.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 41.Wolfe F, Hawley DJ, Wilson K. The prevalence and meaning of fatigue in rheumatic disease. J Rheumatol. 1996;23:1407–1417. [PubMed] [Google Scholar]
- 42.Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- 43.Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post injury pain hypersensitivity. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 44.Woolf CJ, Wall PD. Relative effectiveness of C primary afferent fibers of different origins in evoking a prolonged facilitation of the flexor reflex in the rat. J Neurosci. 1986;6:1433–1442. doi: 10.1523/JNEUROSCI.06-05-01433.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yokoyama T, Lisi TL, Moore SA, Sluka KA. Muscle Fatigue Increases the Probability of Developing Hyperalgesia in Mice. J Pain. 2007;8:692–699. doi: 10.1016/j.jpain.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zautra AJ, Fasman R, Parish BP, Davis MC. Daily fatigue in women with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Pain. 2007;128:128–135. doi: 10.1016/j.pain.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Zemlan FP, Behbehani MM, Beckstead RM. Ascending and Descending Projections from Nucleus Reticularis Magnocellularis and Nucleus Reticularis Gigantocellularis - An Autoradiographic and Horseradish-Peroxidase Study in the Rat. Brain Res. 1984;292:207–220. doi: 10.1016/0006-8993(84)90757-1. [DOI] [PubMed] [Google Scholar]
- 48.Zhuo M, Gebhart GF. Biphasic modulation of spinal nociceptive transmission from the medullary raphe nuclei in the rat. J Neurophysiol. 1997;78:746–758. doi: 10.1152/jn.1997.78.2.746. [DOI] [PubMed] [Google Scholar]