Abstract
Planar cell polarity (PCP) signaling polarises cells along tissue axes. Although pathways involved are becoming better understood, outstanding issues include; (i) existence/identity of cues that orchestrate global polarisation in tissues, and (ii) the generality of the link between polarisation of primary cilia and asymmetric localisation of PCP proteins. Mammalian lenses are mainly comprised of epithelial-derived fiber cells. Concentrically arranged fibers are precisely aligned as they elongate along the anterior-posterior axis and orientate towards lens poles where they meet fibers from other segments to form characteristic sutures. We show that lens exhibits PCP, with each fiber cell having a apically situated cilium and in most cases this is polarised towards the anterior pole. Frizzled and other PCP proteins are also asymmetrically localised along the equatorial-anterior axis. Mutations in core PCP genes Van Gogh-like 2 and Celsr1 perturb oriented fiber alignment and suture formation. Suppression of the PCP pathway by overexpressing Sfrp2, shows that whilst local groups of fibers are often similarly oriented, they lack global orientation; consequently when local groups of fibers with different orientations meet they form multiple, small, ectopic suture-like configurations. This indicates that this extracellular inhibitor disrupts a global polarising signal that utilises a PCP-mediated mechanism to coordinate the global alignment and orientation of fibers to lens poles.
Keywords: Growth factors, Lens development, Planar cell polarity, Frizzled, Primary cilia, Secreted frizzled-related protein, Wnt, Loop-tail
INTRODUCTION
It is now becoming generally recognised that cells within tissues often exhibit some degree of coordinated behaviour within the plane so that they move/orient/proliferate in a particular direction or generate polarised structures. This phenomenon, known as planar cell polarity (PCP) was first identified in invertebrates (Lawrence et al., 2007; Seifert & Mlodzik, 2007; Strutt, 2008; Wang & Nathans, 2007). PCP is now also well established in a few vertebrate systems and is becoming recognised as a mechanism that is fundamental to the generation of coordinated behaviour within tissues and organs. Recent studies have also identified a genetic link between the PCP pathway and ciliogenesis in vertebrates (Axelrod, 2008; Park et al., 2006; Ross et al., 2005; Simons & Walz, 2006; Singla & Reiter, 2006). However, in spite of this growing awareness, outside of the well-studied example of sensory hair cells of the ear, the phenomenon of the primary cilium migrating to one side of the cell during establishment of PCP has not been described and consequently it is not clear if this is a general phenomenon in planar-polarised tissues (Axelrod, 2008). There is also a serious gap in knowledge in understanding how PCP signaling coordinates polarised cell behaviour across whole tissues and organs and the existance/identity of putative global directional cues (Seifert & Mlodzik, 2007; Wu & Mlodzik, 2009).
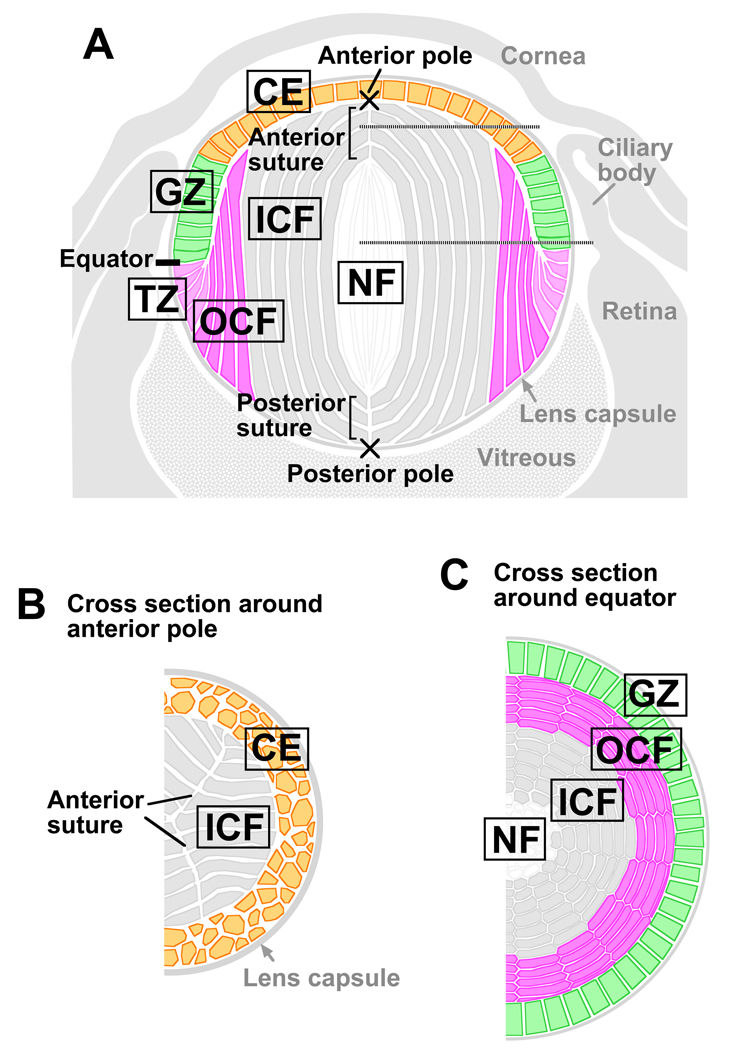
The lens of the eye is an ectodermally-derived tissue, comprised of highly ordered fiber cells that are covered anteriorly by a monolayer of epithelial cells (Fig 1A–C). The proliferative epithelial cells in the germinative zone (GZ) serve as progenitors for secondary fiber cells that initiate differentiation at the lens equator in the transitional zone (TZ) and progressively become added to the fiber mass (McAvoy, 1978a; 1978b). As seen in sagittal sections (Fig 1A), these fiber cells become highly elongated and develop convex curvature as they align along the anterior-posterior lens axis and their ends progressively become orientated towards the lens poles. This process is partially regulated locally by aPKCλ, a apical-basal polarity protein (Sugiyama et al., 2009). Fiber cells are hexagonal in cross-section (Fig 1B,C) and are arranged in a honeycomb-like pattern, forming an array of regularly aligned, concentric rings that comprise the bulk of the lens. As fibers form all around the lens equator, they eventually meet and form end-to-end associations with equivalent fibers from other segments of the lens. Precise alignment and orientation of fibers results in formation of distinct suture lines and in rodents these are characteristically Y-shaped (Kuszak et al., 2004). Any disruption to this organisation impairs light transmission and lens function; however, the mechanism(s) that orchestrates global fiber cell alignment and orientation to generate these characteristic sutures is not well understood.
Fig 1. Diagrammatic representations of the lens.
(A) Sagittal section of eye. (B,C) Horizontal sections from regions indicated by the horizontal dotted lines in (A). Abbreviations: CE, central epithelium; GZ, germinative zone; TZ, transitional zone; OCF, outer cortical fibers; ICF, inner cortical fibers; NF, nuclear fibers.
We previously showed that transgenic mice overexpressing Sfrp2 (Sfrp2+) in lens, a known regulator of Wnt/Frizzled (Fz) signaling, exhibit concave curvature of elongated fibers instead of the normal convex curvature and have defective packing (Chen et al., 2008). Furthermore, components of the Fz/PCP pathway are downregulated/inhibited in the lenses of these mice, indicating that aspects of fiber cell organisation are regulated by the Fz/PCP pathway. However it is still unclear whether the coordinated alignment and orientation of fiber cells is analogous to known PCP models as originally described in Drosophila wing cell (ordered hair alignment/orientation) or mammalian ear cell (sensory cilia polarisation) studies (Seifert & Mlodzik, 2007; Wang & Nathans, 2007). Here we show that lens cells exhibit PCP, with each fiber having a apically situated cilium and in most cases this is polarised to the side of the cell facing towards the anterior pole. Fz and other PCP proteins are also asymmetrically localised along the equatorial-anterior axis. Evidence that PCP has a functional role in lens development comes from identification of defects in cell alignment and suture formation in the lenses of Looptail (Lp) and Crash (Crsh) mice that have mutations in core PCP genes Van Gogh-like 2 (Vangl2) and Celsr1, respectively. Overexpression of Sfrp2 also disrupts fiber cell alignment/orientation in lenses of transgenic mice (Sfrp2+). Interestingly, whilst it is evident that groups of fibers in Sfrp2+ lenses show some coordination of alignment/orientation, this does not operate globally as aberrant suture-like arrangements are common in these lenses. These studies introduce the lens as a new model to study PCP and indicate that polarisation of cilia and PCP proteins may be a general phenomenon in planar-polarised tissues. Furthermore, the results support the existence of a external cue that coordinates global alignment/orientation of cells.
MATERIALS AND METHODS
Animals
The use of animals in this study conformed to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the use of animals in Ophthalmic and Vision Research.
Tissue processing
Adult rats (between postnatal day 21 to 34) were used to provide lenses for preparing whole mounts. Eyeballs were removed and lenses dissected out. Lenses were briefly fixed in methanol (45 seconds) before the capsules with adherent epithelial cells were dissected away from the fiber mass. Confocal microscopy was used to distinguish between epithelial cells and fibers as illustrated in supplementary Fig 1 online.
Primary antibodies used in this study were as follows: mouse monoclonal antibodies against E-cadherin (clone 36, BD) and β-catenin (clone 14, BD); rabbit antibodies against β-catenin (H102, Santa Cruz), pericentrin (Abcam), Prickle1 (Abcam), Dishevelled2 (Biomol), Dishevelled3 (Biomol), Frizzled6 (kindly provided by Jeremy Nathans) and Vangl2 (H-55, Santa Cruz); goat antibodies against Frizzled6 (M-19, Santa Cruz), Vangl2 (N-13, Santa Cruz), Abi2 (P-20, Santa Cruz) and Nephrocystin-2/Inversin (I-19, Santa Cruz). For negative controls, mouse, rabbit and goat whole molecule IgGs were used (Jackson ImmunoResearch Laboratories). Secondary antibodies were Alexa Fluor 488 or 594-conjugated donkey anti-rabbit, mouse or goat IgG (Invitrogen).
Sfrp2+ mice were generated as previously described (Chen et al., 2008). Lenses were isolated from eyeballs and incubated with TRITC-conjugated lectin (Sigma) or Alexa488-conjucated phalloidin (Invitrogen). Optical sectioning, from anterior pole, was used to visualise sutures and alignment/orientation of fibers. Lenses were also sectioned histologically after overnight fixation in NBF and paraffin embedding. Lp mutant mice (Wang et al., 2006b) and wild-type littermates (Embryonic day (E) 17.5 and E18.5 and postnatal day 0) were similarly prepared. Crsh mutant mice (Copp et al., 2003) and wild-type littermates at E18.5 were also prepared for optical and histological sectioning as described above.
Transmission electron microscopy (TEM)
Lenses from postnatal day 1 mice were fixed in 4% paraformaldehye/2% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.4, for 3 hours at room temperature. After washing and postfixation with 1% osmium tetroxide in phosphate buffer, the lenses were stained in 2% uranyl acetate and embedded in Epon-Araldite resin. Sections were stained and viewed on a 7100FA transmission electron microscope (Hitachi Koki, Tokyo, Japan).
RESULTS
PCP is manifested by asymmetric distribution of cilia
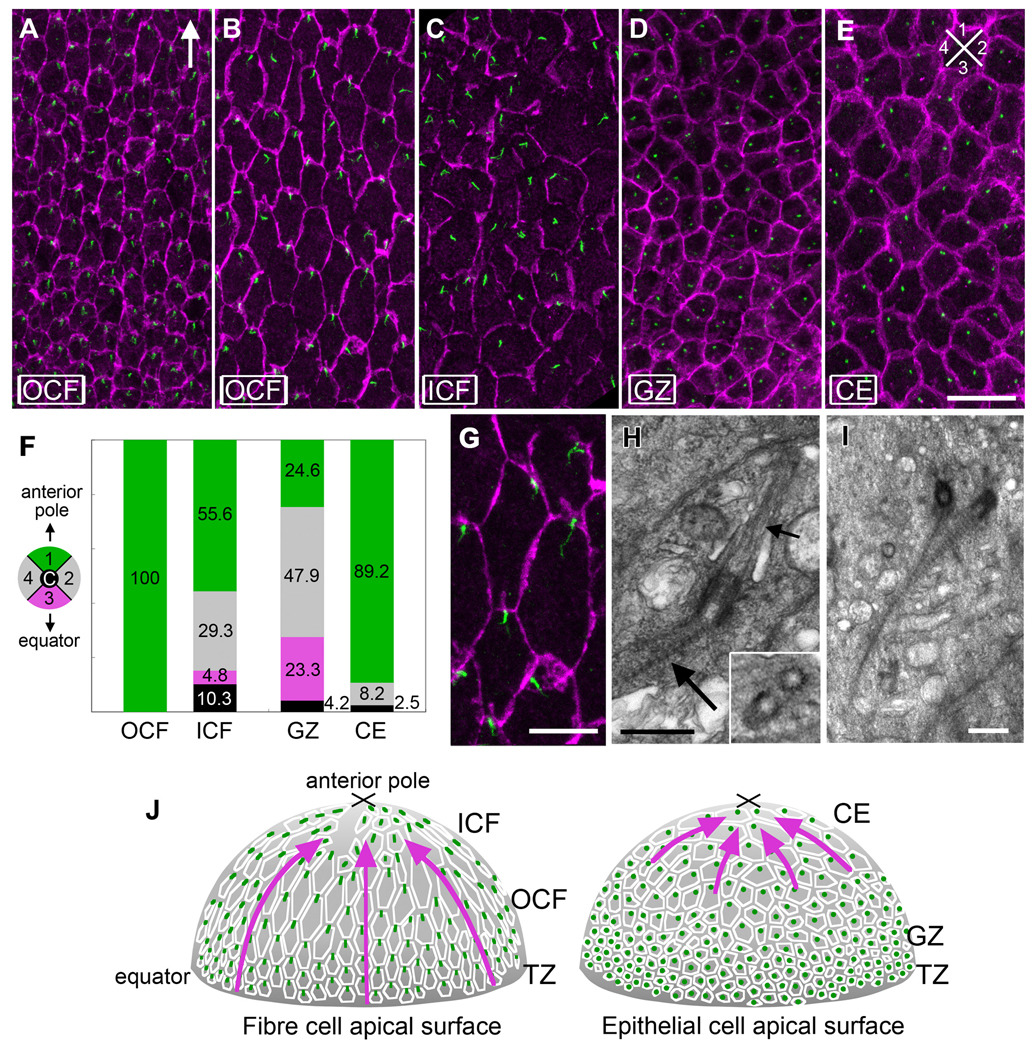
As recent studies have identified a link between polarisation of cilia and PCP, we wanted to ascertain the status of cilia on lens cells. Since the apical ends of lens fiber cells adhere to the overlying epithelial cells, it was difficult to obtain a view of the apical surface of the fibers. However, we found that by using epithelial whole mount preparations that included remnants of the apical ends of fibers (see Methods and supplementary Fig 1 online) we were able to observe the presence and distribution of cilia in lens cells. Pericentrin immuno-reactivity (Salisbury, 2004) shows that virtually all lens cells have a centrosome/cilium (Fig 2). In the outer cortical fibers (OCF), polarisation of the centrosome/cilium is clearly evident (Fig 2 A,B). Particularly in the most mature cells of this region (Fig 2B), cells have a larger apical surface area and tend to be hexagonal in shape, having two short sides and four long sides. In these cells, pericentrin is localised to the short side, proximal to the anterior pole (Fig 2B). In addition, pericentrin reactivity is sometimes branched or Y-shaped and often takes on an elongated form that is frequently aligned along the equatorial-anterior polar axis (see enlarged area of B in G). This polarisation of the centrosome/cilium becomes evident in the TZ where epithelial-fiber differentiation is initiated and the cells begin to elongate and align in regular rows (data not shown). In the inner cortical fibers (ICF) the centrosomes/cilia, although prominent, appear more randomly distributed in this region (Fig 2C). However, this perception may be partly due to cells in the ICF having more variable profiles than the hexagonal-shaped cells in the OCF. When a superimposed grid was used to quantify the location of the centrosome/cilium in each cell this showed more of them in quadrant 1 (55.6%) than in any other quadrant (Fig 2F).
Fig 2. Centrosome/cilium polarisation in lens cells.
(A–E) Pericentrin (green) immuno-reactivity localises the centrosome/cilium and β-catenin (purple) localisation demarcates cell margins in lens whole mounts. In OCF (A, B) and CE (E), the centrosome/cilium is clearly associated with the cell margin proximal to the anterior pole. Arrow in (A) points to anterior pole. Scale bar, 20µm. (F) Histogram showing results from a quantitative analysis of centrosome/cilium polarisation in the different cellular compartments. A superimposed grid was used to determine the position of the centrosome/cilium in the apical region of each cell in relation to four quadrants (colour-coded) and a central region (c). Cells from at least 3 independent adult rat lenses were assessed and the total number of cells counted per compartment were as follows: OCF 247, ICF 331, GZ 639, CE 158. In all compartments, except for GZ, more centrosomes/cilia were in quadrant 1 than any other quadrant. (G) Higher magnification of a region in B. Scale bar, 10µm. (H) TEM shows basal bodies, cilium axoneme (small arrow) and striated rootlet (large arrow) at the apical end of a cortical fiber cell. Central microtubules are absent (inset). (I) TEM shows a pair of basal bodies with associated striated rootlets. Scale bar, 500 nm. (J) Diagrammatic summary with arrows representing regions of lens exhibiting PCP.
In the cobblestone-packed epithelium that overlies the fibers two distinct patterns are evident. In the GZ that lies just above the lens equator where most proliferative activity occurs (McAvoy, 1978a; McAvoy, 1978b), no polarisation of the centrosome/cilium is evident (Fig 2D). This is verified by the quantitative analysis that shows similar numbers of centrosomes/cilia in the different quadrants (Fig 2F). In the central epithelium (CE) that covers most of the anterior lens surface the cells tend to have a larger apical surface area and show polarised distribution of most centrosomes/cilia with 89.2% being in quadrant 1 (Fig 2E). Thus in all regions, except the GZ, lens cells exhibit PCP as in most cases their centrosome/cilium is polarised to the side of the cell that is proximal to the anterior pole of the lens (summarised in Fig 2J with arrows identifying regions exhibiting PCP).
Transmission electron microscopy (TEM) confirms that pericentrin immuno-reactivity is consistent with the presence of basal bodies and associated cilia in the apical regions of lens cells (Fig 2G–I). TEM shows that basal bodies often have a distinct striated rootlet (Fig 2H,I). Moreover, the absence of a central doublet of microtubules (inset in Fig 2H), identifies them as primary, or sensory cilia (Marshall, 2008; Pan & Snell, 2007).
PCP proteins are asymmetrically localised in fibers
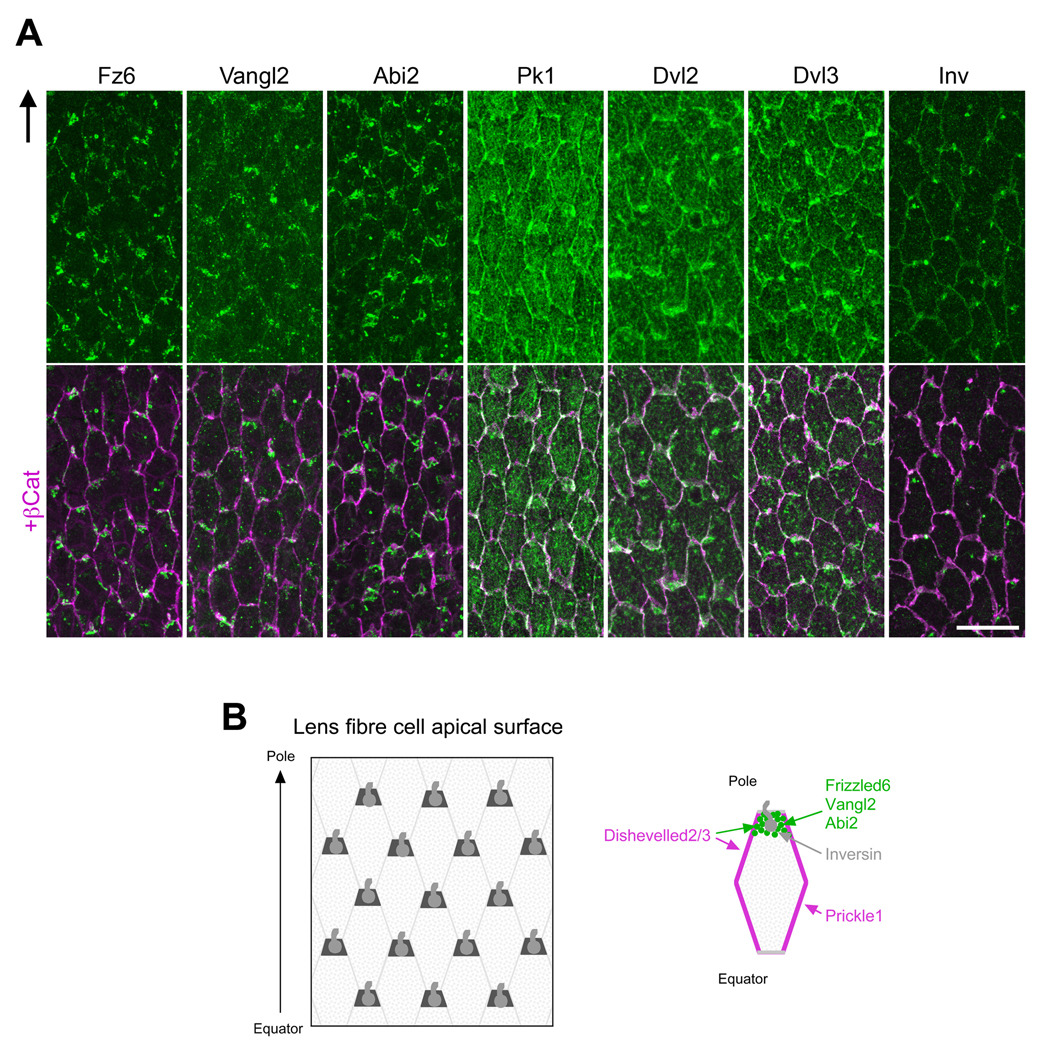
The central theme of PCP is coordination of cellular polarisation within the plane of the cell or tissue layer, and this is partly achieved through asymmetric recruitment of particular PCP proteins to specific cellular domains (Lawrence et al., 2007; Seifert & Mlodzik, 2007; Strutt, 2008; Wang & Nathans, 2007). This asymmetry was first recognised in insects with Drosophila wing being a commonly used example to show that Fz and Dishevelled (Dsh; Dvl in vertebrates) accumulate along the distal side of the hexagonally-shaped cells whereas Van Gogh (Vang; Van Gogh-like, Vangl in vertebrates) and Prickle (Pk) accumulate on the proximal side of cells. To examine if this pathway is involved in lens PCP, we localised core PCP signaling components, Fz6, Dvl2, Dvl3, Pk1, Vangl2 and inversin (a vertebrate orthologue of invertebrate Diego), in lens cells. We also localised Abl-interactor-2 (Abi2) protein as this has been shown to have an important role in regulating cytoskeletal dynamics and the Abi2 knockout mouse (Grove et al., 2004) has a remarkably similar phenotype to the Sfrp2+ mouse in that lens fibers do not develop the convex curvature characteristic of the normal lens (Chen et al., 2008).
Immunolocalisation studies on whole mount preparations show that at the apical tips of the hexagonally shaped OCF, Fz6, Vangl2 and Abi2 are predominately associated with the short side that is proximal to the anterior pole of the lens; whereas Pk1 is predominantly associated with the long sides of each cell with some weak cytoplasmic reactivity also evident (Fig 3). The images shown for Fz6 and Vangl2 were obtained using goat antibodies but were also confirmed using rabbit antibodies (data not shown). Dvl2 and Dvl3 tend to be localised to all sides of each cell with a little more intense reactivity often associated with the short side that is proximal to the anterior pole of the lens. Dvl3 also shows some particulate cytoplasmic localisation. Inversin, like pericentrin, localises to the cell margin that is proximal to the anterior pole of the lens. This is consistent with inversin being a cilium-associated protein (Corbit et al., 2008). Overall, such polarised distribution of core PCP proteins in lens fibers and their reciprocal localisation patterns, especially between Fz6/Vangl2/Abi2 and Pk1, suggests that the Fz/PCP pathway is involved in regulating aspects of fiber cell alignment.
Fig 3. PCP proteins (green) are partitioned to cellular domains.
(A) At apical tips of OCF, Fz6, Vangl2 and Abi2 are predominantly associated with the short side of each cell proximal to anterior pole. In contrast, Pk1 is predominantly localised to the long sides. Dvl2/Dvl3 tend to be localised to all sides of each cell with some cells showing a little more intense reactivity associated with the short side proximal to anterior pole. β-catenin (purple) demarcates cell margins and co-localises with Pk1, Dvl2 and Dvl3. Arrow in (A) points to anterior pole. Scale bar, 20 µm. (B) Representation of the apical tips of fibers (left) and a single fiber (right). Abbreviations: Abi2, Abl-interactor-2; Dvl2/3, Dishevelled 2/3; Fz6, Frizzled 6; Inv, Inversin; OCF, outer cortical fibers; Pk1, Prickle 1; Vangl2, Van Gogh-like 2.
PCP signaling is required for development of normal lens shape and suture formation
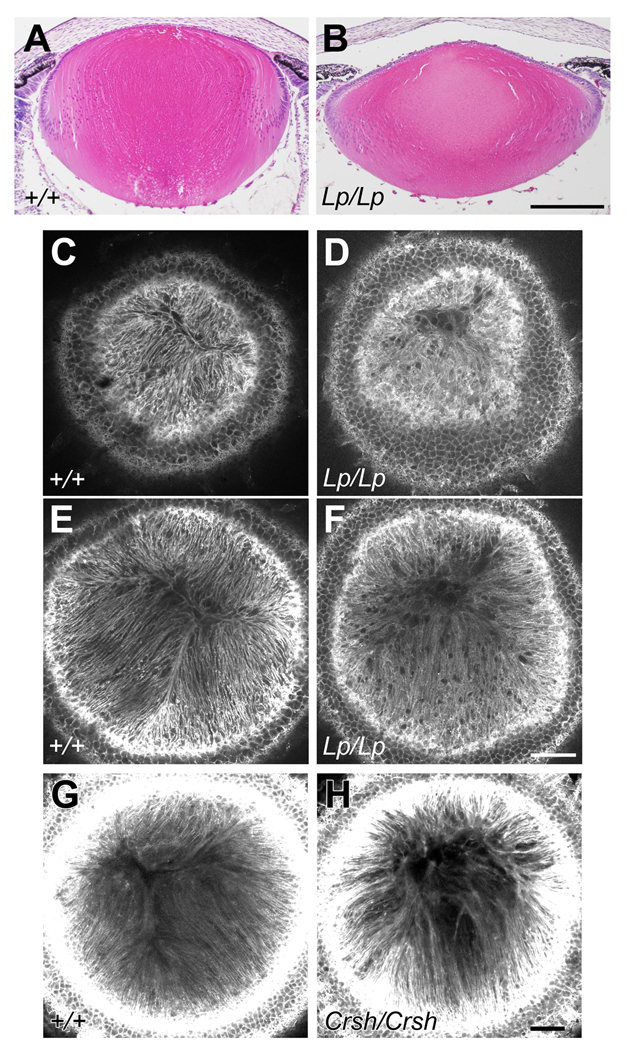
To determine if the PCP pathway has a functional role in lens development we analyzed lenses of mice with a mutation in the Vangl2 gene. These Lp mice display a broad spectrum of PCP defects and have provided major insights into mammalian PCP (Simons & Mlodzik, 2008). Here, for the first time, we show that the lenses of Lp mice have a abnormal shape. In contrast with the spheroidal shape of the P0 wild-types, Lp/Lp lenses tend towards a flatter shape; the anterior/posterior polar axis being reduced and the equatorial axis increased (Fig 4A,B). Similar deviations from wild-type littermate phenotypes are seen in E17.5 and E18.5 Lp/Lp lenses (data not shown). We observed some variation in the severity of the phenotype between Lp/Lp littermates; among six Lp/Lp lenses obtained between ages E17.5 and P0, three lenses clearly showed a flatter shape as indicated in Fig. 4B, but the others maintained a relatively normal overall shape. This variation may reflect incomplete penetration of some Lp phenotypes (Murdoch et al., 2001), and indeed in our sample collection, we observed the open eyelid phenotype in 80% of samples. The variation may also reflect a difference in the progression of the defect, since the three lenses with relatively normal shape did have some disruption in fiber alignment (see below). No defect was detected in nine Lp/+ or wild-type lenses collected as controls.
Fig 4. Lp/Lp and Crsh/Crsh mouse lenses lack the alignment/orientation required to form normal sutures.
(A,B) Sagittal histological sections of P0 lenses stained with haematoxylin and eosin. The Lp/Lp lens clearly has abnormal dimensions being flatter and wider than the lens of the wild-type (WT) littermate (+/+). Scale bar, 200 µm. (C–F) With phalloidin staining confocal microscopy from the anterior polar aspect of P0 lenses shows that fibers in the WT exhibit regular parallel alignment and orientiation so that they form a distinctive Y-shaped suture (C, E). In contrast, fibers in the Lp/Lp lens are not regularly aligned and lack the orientation required to form a normal Y-shaped suture (D, F). Note that images C and D are from horizontal slices closer to the anterior polar surface than images in E and F. (G,H) Phalloidin staining confocal microscopy of E18.5 Crsh/Crsh lens shows fibers are not regularly aligned and lack the orientation required to form a normal Y-shaped suture. Scale bars C-H, 50 µm.
How lens growth is regulated (for example; at different stages of life and in different species) to provide the precise curvature (and other dimensions) that is required for focussing function is not fully understood. However, fundamental to this is the requirement for the coordinated formation of concentric rings of new fibers with appropriate alignment and orientation. Whether this occurs normally, or not, can be assessed by viewing the lens from the anterior pole. In wild-type lenses the normal highly-ordered, parallel alignment and packing of fibers can be seen as they orient towards the poles (Fig 4C,E). As fibers elongate they develop a precise curvature and orientation that determines where they meet equivalent fibers that arise from different segments of the lens. Their subsequent end-to-end associations give rise to characteristic age- and species-specific suture patterns. Rodents (and a number of other mammals) have characteristic Y-shaped sutures (Kuszak et al., 2006). Figure 4D and F shows that Lp/Lp mice that show a pronounced flattened shape also do not form characteristic Y-shaped sutures. Instead the irregularly aligned fibers often extend all the way to the anterior pole. Although the mechanism of suture formation is not understood, this result shows that it is defective in Lp/Lp mice and consequently indicates involvement of a PCP-dependent mechanism. Further support for this comes from the analysis of another mouse model commonly used in PCP studies. Crsh mice have a mutation in the Celsr1 gene and exhibit a wide range of PCP defects (Copp et al., 2003). In these mice we show fiber alignment defects in all six Crsh/Crsh embryos with an open eyelid phenotype, whereas no defects are evident in any of their seven wild-type counterparts at E18.5. (Fig 4G,H).
PCP is disrupted in lenses of Sfrp2+ mice
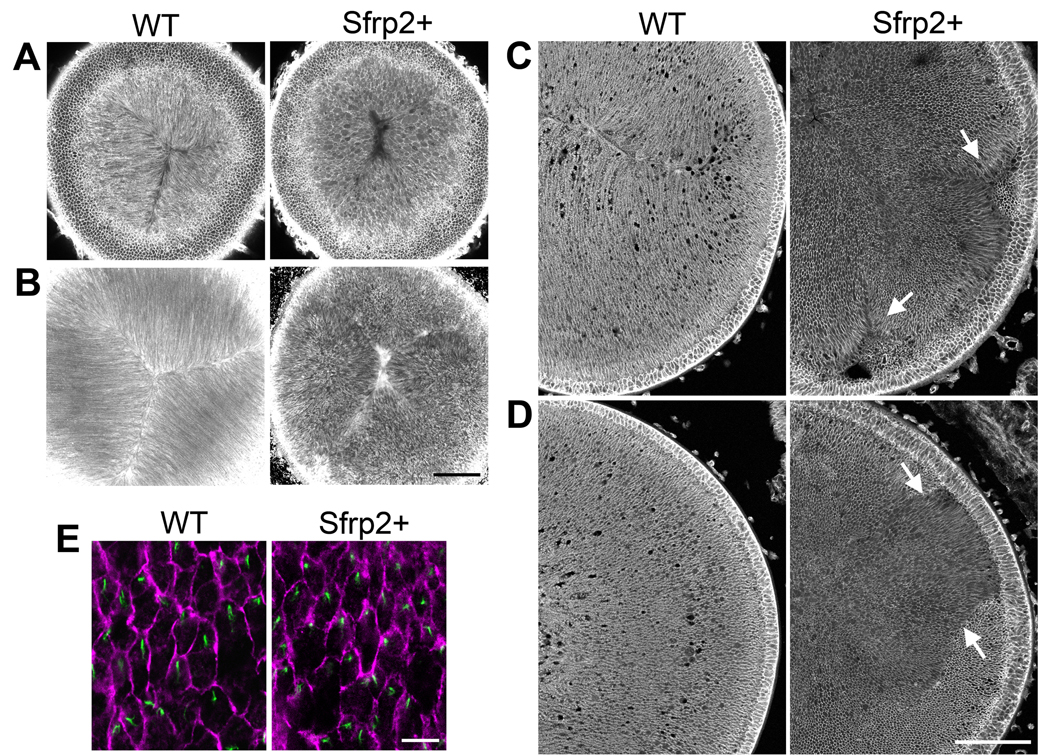
Given the evidence from analysis of Lp/Lp and Crsh/Crsh mice that PCP is required for development of normal alignment/orientation of fibers and formation of characteristic sutures, we carried out similar studies on another mouse model deficient in PCP signaling. Previously we generated mice that overexpress the Wnt/Fz signaling regulator, Sfrp2, specifically in the lens. These Sfrp2+ mice exhibit defective fiber cell curvature and this coincides with downregulation/inhibition of PCP signaling components (Chen et al., 2008). To determine if suture formation is disrupted in these lenses, we observed them from the apical aspect. Compared with wild-type lenses, which show pronounced parallel alignment and orientation of individual fibers to form characteristic Y-shaped sutures, Sfrp2+ lenses show no evidence of regular alignment or orientation (Fig 5A). Interestingly, although the prominent Y-shaped suture does not form, mis-located suture-like arrangements are evident in Sfrp2+ lenses (more pronounced in the stacked image; Fig 5B).
Fig 5. Mice overexpressing Sfrp2 (Sfrp2+) in lens exhibit multiple and disordered suture-like arrangements.
(A,B) With lectin staining confocal microscopy shows that the distinctive Y-shaped suture present in the P3 WT lens is absent from the Sfrp2+ littermate. Scale bar, 100 µm. (C,D) Histological cross sections of P3 lenses stained for β-catenin show that compared with WT lens, fiber orientation in the Sfrp2+ lens appears to be random. However, groups of fibers exhibit some local order and when groups with differing orientations meet, suture-like arrangements (arrows in C) or boundaries (arrows in D) are evident. Scale bar, 100 µm. (E) Pericentrin (green) immuno-reactivity localises the centrosome/cilium and β-catenin (purple) localisation demarcates cell margins in lens whole mounts from WT and Sfrp2+ littermates. Note that in virtually all cells in both WT and sfrp2+ lenses the centrosome/cilium is clearly polarised to one side of the cell. Scale bar, 10 µm.
Histological cross sections of the lens (below the region included in the optical sections shown in Fig 5A, B) provide more detail of the disorganised fiber patterns in Sfrp2+ lenses. Compared with the wild-type lenses, which consistently show that fiber alignment and orientation is highly coordinated, Sfrp2+ lens fibers appear to have much more random alignment and orientation (Fig 5C,D; images are from sequential sections of a Sfrp2+ lens with comparable regions of a wild-type lens - D being further from the anterior pole than C). However, closer examination of Sfrp2+ lenses shows evidence of local order, with the orientations of groups of neighbouring fibers showing a high correlation. When two groups of differently oriented fibers meet, ectopic suture-like structures (Fig 5C arrows) or ectopic boundaries (Fig 5D arrows) are formed. This aberrant pattern of global (but not local) alignment is reminiscent of the phenotypes in the wing hair and body hair patterns in many of the Drosophila and mouse PCP mutants. In these, global patterning is disrupted but there is still evidence of local ordering (Wang et al., 2006a; Wang & Nathans, 2007). This suggests that overexpression of Sfrp2 in the lens disrupts the overall coordination of alignment of fibers by disrupting global planar polarity that is regulated by a Fz/PCP pathway.
Since Sfrp2 acts as a extracellular antagonist that can interfere with interactions between Wnt ligands and Fz receptors (Bovolenta et al., 2008), the defect observed here could be the result of Sfrp2 disrupting a global polarising signal. In this case, cilia/PCP proteins would still be expected to localise asymmetrically and regulate local alignment/orientation. Support for this comes from the observation that individual OCF cells of Sfrp2+ mice exhibit a primary cilium that is similarly polarised towards one side of the cell as in wild-types (Fig 5E). This indicates that in the Sfrp2+ lens, each fiber cell still maintains cellular asymmetry and preserves the potential to react to an external polarising signal. In line with this, we suggest the Sfrp2+ mice do not show the same global orientation towards the anterior pole that is evident in the OCF cells of wild-type lenses, because overexpression of Sfrp2 blocks the extracellular guidance signal.
Hexagonal packing of epithelial cells is inhibited in Lp/Lp and Sfrp2+ lenses
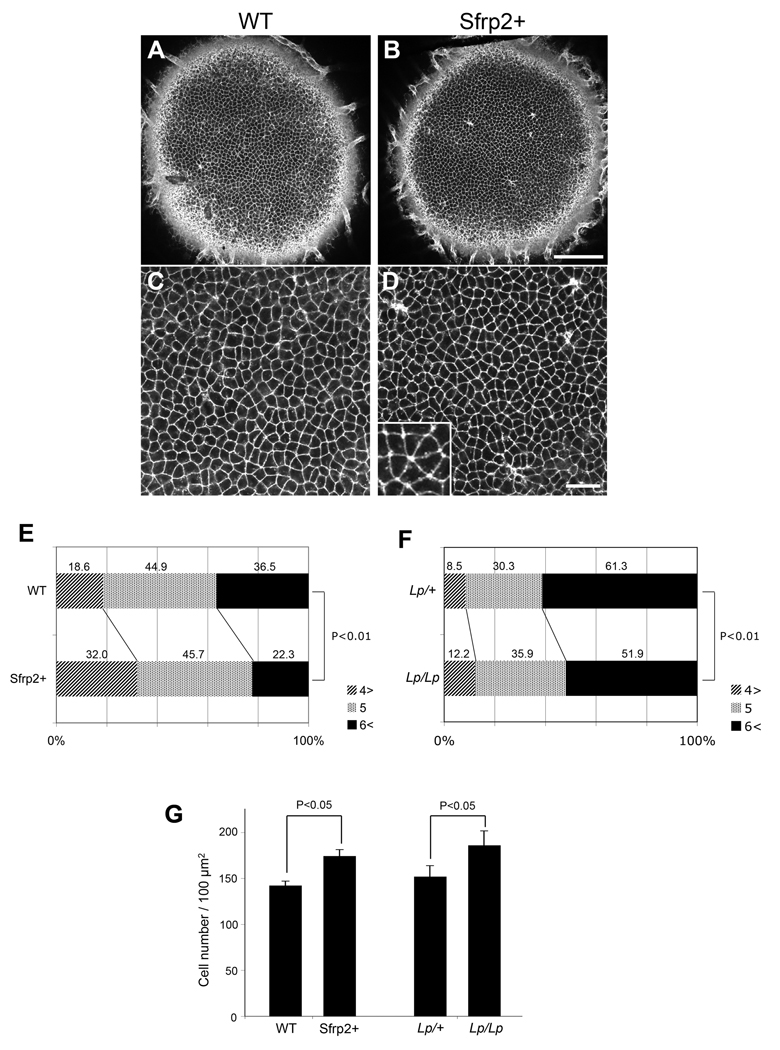
As PCP proteins have also been shown to have an essential role in generating orderly hexagonal packing of epithelial cells in both Drosophila (Classen et al., 2005) and mammals (Narimatsu et al., 2009), we examined the packing of epithelial cells in Sfrp2+ and Lp/Lp mice. Initial observations indicated that central lens epithelial cells of Sfrp2+ lenses are frequently found to be packed in rosettes and show significantly less hexagonal but more square or triangular packing arrangements than in lenses of wild-type littermates (compare Fig 6A,C with Fig 6B,D). Quantitative analysis of the epithelial sheet showed that central epithelial cells in Sfrp2+ mice exhibit significantly less hexagonal (or more sides) and significantly more pentagonal (or fewer sides) packing arrangements than wild-type littermates (Fig 6E). An equivalent analysis of of Lp/Lp mice showed a similar result (Fig 6F). We also observed a slight but significant increase in cell density both in Sfrp2+ and Lp/Lp lenses compared to their wild-type counterparts (Fig. 6G). Overall, this result indicates that epithelial cells, as well as fibers, show evidence of disturbed PCP in these mouse models. Note that since expression of the exogenous Sfrp2 is only clearly detected in fibers (Chen et al., 2008), this also indicates that Sfrp2, secreted from fibers, diffuses sufficiently to interfere with PCP in juxtaposed lens epithelial cells and disturbs their packing.
Fig 6. Lens epithelial packing is disrupted in Sfrp2+ and Lp/Lp lenses.
(A,B) The epithelial layer of lectin-stained whole lenses from WT (A) and Sfrp2+ (B) mice (P4) viewed from the anterior pole. Scale bar, 100 µm. (C,D) A higher magnification view of the epithelial packing arrangement of WT (C) and Sfrp2+ (D) lenses similar to that used for quantitative analysis. Scale bar, 25 µm. Inset in (D) shows a typical rosette-like arrangement frequently observed in the Sfrp2+ lens epithelium. (E) Distribution (%) of cells with 3 or 4 (4>), 5 and 6 or more (6<) cell-cell contacts. This shows a significant decrease in abundance of cells with 6 or more cell-cell contacts (hexagonal-like packing) and a significant increase in cells with 3–4 cell-cell contacts (rosette-like packing) in Sfrp2+ mice compared with WT mice. Cell-cell contacts were counted in pairs of WT and Sfrp2+ lenses from mice of 3 independent litters (P3/P4) and subjected for chi square analysis (Χ2=71.4; n=2,039; P<0.01). (F) A similar analysis conducted with 4 Lp/+ and 3 Lp/Lp lenses (E18.5) also showed a significant reduction in numbers of cell-cell contacts in Lp/Lp lenses (Χ2=14.89; n=1,594; P<0.01). (G) Analysis of cell density in Sfrp2+ and Lp/Lp lenses. Cell numbers in a 100 µm square are shown as means ± S.D (P values by two-tailed Student's t test). Cells counted and numbers of lenses assessed were as follows: WT, 427 (n=3); Sfrp2+ 523 (n=3); Lp/+, 608 (n=4); Lp/Lp, 930 (n=5). This shows a slight, but significant increase in cell density in Sfrp2+ and Lp/Lp lenses compared with their WT and Lp/+ counterparts.
DISCUSSION
How PCP signaling coordinates polarised cell behaviour across whole tissues and organs, and the nature of putative global directional cues is a critically important, but poorly understood, area in PCP biology. Also, whilst studies such as those on sensory hair cells of the mammalian ear show a link between polarisation of cilia and PCP, it is not clear if this a general phenomenon that operates in other PCP organised tissues. In this study we initially show a link between polarization of cilia and PCP in the mammalian eye lens. Evidence that PCP has a functional role in lens development comes from identification of PCP defects in the lenses of Lp/Lp and Crsh/Crsh mice. Finally, using a transgenic mouse model we provide evidence that supports the existence of a external cue that coordinates PCP signaling and generates global alignment of lens cells.
The lens is primarily comprised of highly elongated fiber cells that align along the light path axis and pack in a highly ordered concentric arrangement. The formation of such precise cellular organisation is critical for the optical properties of the lens, but the underlying developmental mechanism(s) remains largely unknown. Evidence that the lens is a Fz/PCP-organised tissue comes from the observation that the centrosome/primary cilium and core PCP proteins in lens cells exhibit a polarised distribution. This is particularly striking in the prominent cilia of the lens fiber cells located in the outer lens cortex. However, although less obvious and with the exception of the GZ, polarization of the centrosome/primary cilium is also evident in the other lens cellular compartments. In general, the orientation of the centrosome/cilium is similar i.e. it lies on the side of the cell that faces away from the lens equator and towards the anterior pole of the lens. Another characteristic manifestation of PCP is the partitioning of PCP components to specific cellular domains (Lawrence et al., 2007; Seifert & Mlodzik, 2007; Strutt, 2008; Wang & Nathans, 2007). Consistent with this, the current study shows that in lens fiber cells, core PCP proteins, notably Fz6, and Vangl2, localize to a different cellular domain than Pk1. The association between Fz6 and Vangl2 in lens appears to be consistent with their reported colocalisation in some studies of sensory ear cells (Montcouquiol et al., 2006; Wang et al., 2006b). This is different from their localisation to opposing sides of the cell in invertebrate models and may indicate differences in intracellular interactions between these proteins in the different phyla (Wang & Nathans, 2007).
The studies of Lp/Lp and Crsh/Crsh mice show that the lens exhibits defects in the alignment/orientation of cells so that characteristic Y-shaped suture lines do not form. As some of these lenses end up being distinctly the wrong shape this result emphasises the importance of coordinating the processes of fiber cell alignment/orientation and suture formation for development of a functionally competent lens. Sutures form where rows of elongated fibers that originate from different segments of the lens, make end-to-end contact. Anteriorly, this is accompanied by their disassociation from the overlying lens epithelium. As these fiber-fiber associations form progressively during development, suture lines can be also be visualised some distance below the lens surface (see Fig 1A). The absence of normal sutures in lp/lp and Crsh/Crsh mice appears to be due to elongating fibers not developing the appropriate alignment/orientation and not making, or maintaining, appropriate contact with equivalent fibers from a different lens segment. As a result, the fibers from the different segments of the lens keep migrating along the overlying epithelium and only meet up when they reach the pole. Even then they do not appear to develop any stable (or transitory) end-to-end association. Thus the absence of normal Vangl-2 and Celsr1 proteins from lens fiber cells results in major disturbances in the three-dimensional cellular architecture of the lens.
Having provided compelling evidence that PCP operates in the lens, this study goes on to provide insights into one of the major questions in PCP biology - the existence of a global polarising signal that coordinates planar polarity across tissues and organs. Such signals have been predicted but so far none have been identified (Seifert & Mlodzik, 2007; Wu & Mlodzik, 2009). Here we show evidence from analysis of transgenic mice that overexpressed Sfrp2 interferes with the coordination of fiber cell planar alignment/orientation. Sfrp2 is known to associate with Wnt ligands and/or Fz receptors (Bovolenta et al., 2008). Moreover, our earlier studies showed that in Sfrp2+ mice, the overexpressed Sfrp2 co-precipitates with Wnt ligand (Chen et al., 2008). Whilst Sfrp2 overexpression and its subsequent interaction with Wnt ligands could potentially disturb all Wnt signaling pathways, given the evidence that the PCP pathway is inhibited in Sfrp2+ mice (Chen et al, 2008) together with the major disturbance evident in the global alignment and orientation of fibers in these mice, this is consistent with a Wnt ligand being the global polarizing cue in a PCP-mediated process. Therefore, although further work is clearly needed to properly identify this cue, this study provides the first evidence for the existence of a extracellular directional signal that underlies the generation of PCP globally across a whole tissue.
Another interesting facet of the Sfrp2+ phenotype is that there is evidence of local alignment and orientation of groups of fibers. This is also shown by the similar polarised distribution of the centromere/primary cilium in these groups of fibers (see Fig 5E). This result is consistent with the observations from various studies that show cell-to-cell interactions can propagate PCP some distance within cell populations (Wu & Mlodzik, 2009). In the event that a Wnt ligand was the polarising cue, the presence of excess Sfrp2 would serve to block this signal but not the PCP that results from cell-cell interactions (also referred to as non-autonomous PCP signaling). The observation that at the anterior pole of the Lp/Lp or Crsh/Crsh mouse lens there is little evidence of local alignment/orientation of fibers is also consistent with the expectation that in these mutants the absence of a functional Vangl2 or Celsr1 protein would result in defective PCP signaling in individual fibers and hence diminish any subsequent propagation of PCP from cell-to-cell.
As in other epithelial tissues, disturbance of PCP influences epithelial packing arrangements. Central lens epithelial cells of Lp/Lp and Sfrp2+ mice both show significantly less hexagonal, but more square or triangular, packing arrangements than wild-type littermates. Whilst we cannot exclude the possibility that the disturbed packing in these mouse models could also be influenced by their cells being slightly more densely packed than in controls, the results are also consistent with the literature that implicates PCP proteins in promoting orderly hexagonal packing of epithelial sheets. For example, similar disruptions to hexagonal packing have recently been reported in Smurf mutant mice that display a range of PCP defects (Narimatsu et al., 2009). Disruptions to orderly hexagonal packing in epithelial cells have also been shown in Drosophila PCP mutants (Classen et al., 2005). How hexagonal packing is disturbed in these mutants is not clear but it has been proposed that it may be a result of inability to remodel tight junctions.
There is now compelling evidence from numerous studies that vitreous-induced FGF signaling initiates fiber differentiation in epithelial cells that shift below the lens equator (McAvoy & Chamberlain, 1989; Schulz et al., 1993; Zhao et al., 2008); however, the mechanism(s) that coordinates fiber cell alignment, orientation and precise suture formation has received scant attention. Clearly these processes are critical for the lens to develop transparency and an appropriate spheroidal shape. Now, for the first time, our current studies implicate PCP signaling as a key regulator of these processes. How FGF signaling influences PCP and how a polarised primary cilium and PCP proteins interact to coordinate alignment and orientation of fibers is unclear at present. Our current model is that, given the role of the primary cilium as a component of the cellular global positioning system (GPS) (Benzing & Walz, 2006), this organelle becomes polarised at the apical tip of each elongating fiber to pick up a signal that emanates from the anterior polar region. This, combined with the intracellular interactions between PCP components and cell-to-cell interactions that promote coordination at the local level, ensures that alignment and orientation of lens cells is globally coordinated. As a result they develop the appropriate curvature and orientation that is required for formation of characteristic lens sutures. Assessment of this model awaits further studies. There are few places where the functional significance of development of precise three-dimensional cellular organisation is better illustrated than in the optical apparatus, in particular the eye lens. Given, its relative simplicity, at least in cellular terms, we predict that the lens will be an excellent model system to help elucidate the role of cilia and PCP components in development of three-dimensional organisation in tissues and organs.
Supplementary Material
Fig. S1. Preparation of lens whole mounts. (A) Diagram showing how the lens capsule is peeled away from the lens fiber cells. Lens epithelial cells remain firmly attached to the lens capsule; however, when the fibers are removed, in some regions, remnants of their apical ends are retained on the apical surface of the epithelial cells. (B) β-catenin (green) is present at the margins of both epithelial and fiber cells but E-cadherin (purple) is specific for epithelial cells. In this whole mount, a large region near the anterior pole of the lens retains remnants of the apical ends of lens fiber cells. Sscale bar, 50 µm. Abbreviations: Ep, epithelial cells; Fb, fiber cells.
ACKNOWLEDGEMENTS
Thanks go to Diana van Driel for her assistance with the electron microscopy. We also thank Michele Madigan and Christina Adler for their assistance. Jeremy Nathans generously provided additional materials for these studies. Y.S. is supported by a Endeavour Research Fellowship, Australia and The Sydney Eye Hospital Foundation. This work was supported by Sydney Foundation for Medical Research, NHMRC (Australia), NIH (USA, R01 EY03177) and ORIA, Australia. This research was undertaken as part of the Vision CRC, New South Wales, Sydney, Australia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Axelrod JD. Basal bodies, kinocilia and planar cell polarity. Nat. Genet. 2008;40:10–11. doi: 10.1038/ng0108-10. [DOI] [PubMed] [Google Scholar]
- Benzing T, Walz G. Cilium-generated signaling: a cellular GPS? Curr. Opin. Nephrol. Hypertens. 2006;15:245–249. doi: 10.1097/01.mnh.0000222690.53970.ca. [DOI] [PubMed] [Google Scholar]
- Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J. Cell Sci. 2008;121:737–746. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- Chen Y, Stump RJ, Lovicu FJ, Shimono A, McAvoy JW. Wnt signaling is required for organization of the lens fiber cell cytoskeleton and development of lens three-dimensional architecture. Dev. Biol. 2008;324:161–176. doi: 10.1016/j.ydbio.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen AK, Anderson KI, Marois E, Eaton S. Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev. Cell. 2005;9:805–817. doi: 10.1016/j.devcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Copp AJ, Greene ND, Murdoch JN. The genetic basis of mammalian neurulation. Nat Rev. Genet. 2003;4:784–793. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, Chuang PT, Reiter JF. Kif3a constrains beta-catenin-dependent Wnt signaling through dual ciliary and non-ciliary mechanisms. Nat. Cell Biol. 2008;10:70–76. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- Grove M, Demyanenko G, Echarri A, Zipfel PA, Quiroz ME, Rodriguiz RM, Playford M, Martensen SA, Robinson MR, Wetsel WC, Maness PF, Pendergast AM. ABI2-deficient mice exhibit defective cell migration, aberrant dendritic spine morphogenesis, and deficits in learning and memory. Mol. Cell Biol. 2004;24:10905–10922. doi: 10.1128/MCB.24.24.10905-10922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuszak JR, Zoltoski RK, Tiedemann CE. Development of lens sutures. Int. J. Dev. Biol. 2004;48:889–902. doi: 10.1387/ijdb.041880jk. [DOI] [PubMed] [Google Scholar]
- Kuszak JR, Mazurkiewicz M, Zoltoski R. Computer modeling of secondary fiber development and growth: I. Nonprimate lenses. Mol. Vis. 2006;12:251–270. [PubMed] [Google Scholar]
- Lawrence PA, Struhl G, Casal J. Planar cell polarity: one or two pathways? Nat. Rev. Genet. 2007;8:555–563. doi: 10.1038/nrg2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WF. The cell biological basis of ciliary disease. J. Cell Biol. 2008;180:17–21. doi: 10.1083/jcb.200710085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy JW. Cell division, cell elongation and distribution of alpha-, beta- and gamma-crystallins in the rat lens. J. Embryol. Exp. Morphol. 1978a;44:149–165. [PubMed] [Google Scholar]
- McAvoy JW. Cell division, cell elongation and the co-ordination of crystallin gene expression during lens morphogenesis in the rat. J. Embryol. Exp. Morphol. 1978b;45:271–281. [PubMed] [Google Scholar]
- McAvoy JW, Chamberlain CG. Fibroblast growth factor (FGF) induces different responses in lens epithelial cells depending on its concentration. Development. 1989;107:221–228. doi: 10.1242/dev.107.2.221. [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, Sans N, Huss D, Kach J, Dickman JD, Forge A, Rachel RA, Copeland NG, Jenkins NA, Bogani D, Murdoch J, Warchol ME, Wenthold RJ, Kelley MW. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J. Neurosci. 2006;26:5265–5275. doi: 10.1523/JNEUROSCI.4680-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch JN, Rachel RA, Shah S, Beermann F, Stanier P, Mason CA, Copp AJ. Circletail, a new mouse mutant with severe neural tube defects: chromosomal localization and interaction with the loop-tail mutation. Genomics. 2001;78:55–63. doi: 10.1006/geno.2001.6638. [DOI] [PubMed] [Google Scholar]
- Narimatsu M, Bose R, Pye M, Zhang L, Miller B, Ching P, Sakuma R, Luga V, Roncari L, Attisano L, Wrana JL. Regulation of planar cell polarity by Smurf ubiquitin ligases. Cell. 2009;137:295–307. doi: 10.1016/j.cell.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Pan J, Snell W. The primary cilium: keeper of the key to cell division. Cell. 2007;129:1255–1257. doi: 10.1016/j.cell.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat. Genet. 2006;38:303–311. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, Tan PL, Phillips HM, Leroux MR, Henderson DJ, Murdoch JN, Copp AJ, Eliot MM, Lupski JR, Kemp DT, Dolfus H, Tada M, Katsanis N, Forge A, Beales PL. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat. Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- Salisbury JL. Primary cilia: putting sensors together. Curr. Biol. 2004;14:R765–R767. doi: 10.1016/j.cub.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Schulz MW, Chamberlain CG, de Iongh RU, McAvoy JW. Acidic and basic FGF in ocular media and lens: implications for lens polarity and growth patterns. Development. 1993;118:117–126. doi: 10.1242/dev.118.1.117. [DOI] [PubMed] [Google Scholar]
- Seifert JR, Mlodzik M. Frizzled/PCP signaling: a conserved mechanism regulating cell polarity and directed motility. Nat. Rev. Genet. 2007;8:126–138. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- Simons M, Mlodzik M. Planar cell polarity signaling: from fly development to human disease. Annu. Rev. Genet. 2008;42:517–540. doi: 10.1146/annurev.genet.42.110807.091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Walz G. Polycystic kidney disease: cell division without a c(l)ue? Kidney Int. 2006;70:854–864. doi: 10.1038/sj.ki.5001534. [DOI] [PubMed] [Google Scholar]
- Singla V, Reiter JF. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- Strutt D. The planar polarity pathway. Curr. Biol. 2008;18:R898–R902. doi: 10.1016/j.cub.2008.07.055. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Akimoto K, Robinson ML, Ohno S, Quinlan RA. A cell polarity protein aPKCλ is required for eye lens form ation and growth. Dev. Biol. 2009;336:246–256. doi: 10.1016/j.ydbio.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Badea T, Nathans J. Order from disorder: Self-organization in mammalian hair patterning. Proc. Natl. Acad. Sci. U S A. 2006a;103:19800–19805. doi: 10.1073/pnas.0609712104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J. Neurosci. 2006b;22:2147–2156. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–658. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- Wu J, Mlodzik M. A quest for the mechanism regulating global planar cell polarity of tissues. Trends Cell Biol. 2009;19:295–305. doi: 10.1016/j.tcb.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Yang T, Madakashira BP, Thiels CA, Bechtle CA, Garcia CM, Zhang H, Yu K, Ornitz DM, Beebe DC, Robinson ML. Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev. Biol. 2008;318:276–288. doi: 10.1016/j.ydbio.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Preparation of lens whole mounts. (A) Diagram showing how the lens capsule is peeled away from the lens fiber cells. Lens epithelial cells remain firmly attached to the lens capsule; however, when the fibers are removed, in some regions, remnants of their apical ends are retained on the apical surface of the epithelial cells. (B) β-catenin (green) is present at the margins of both epithelial and fiber cells but E-cadherin (purple) is specific for epithelial cells. In this whole mount, a large region near the anterior pole of the lens retains remnants of the apical ends of lens fiber cells. Sscale bar, 50 µm. Abbreviations: Ep, epithelial cells; Fb, fiber cells.