Abstract
Gyrification is the process by which the brain undergoes changes in surface morphology to create sulcal and gyral regions. The period of greatest development of brain gyrification is during the third trimester of pregnancy, a period of time in which the brain undergoes considerable growth. Little is known about changes in gyrification during childhood and adolescence, although considering the changes in gray matter volume and thickness during this time period, it is conceivable that alterations in the brain surface morphology could also occur during this period of development. The formation of gyri and sulci in the brain allows for compact wiring that promotes and enhances efficient neural processing. If cerebral function and form are linked through the organization of neural connectivity, then alterations in neural connectivity, i.e., synaptic pruning, may also alter the gyral and sulcal patterns of the brain. This paper reviews developmental theories of gyrification, computational techniques for measuring gyrification, and the potential interaction between gyrification and neuronal connectivity. We also present recent findings involving alterations in gyrification during childhood and adolescence.
Keywords: Gyrification, Adolescence, Development, Connectivity, Cortical Morphology
Introduction
Gyrification is a fascinating and poorly understood developmental process that refers to the development of the folding surface patterns on the brain (Welker, 1990; Zilles, Armstrong, Moser, Schleicher, & Stephan, 1989), many of which readily distinguish the human brain from that of other organisms. Since gray matter (GM) forms an external layer around the brain, gyrification results in a dramatic increase in the cortical surface area and thus, in the volume of cortical GM. The ratio of brain cortical GM to body size is the highest in humans compared to all animals, with dolphins and porpoises being relatively close (Macphail, 1982). However, unlike dolphins and porpoises, humans utilize their entire brains, rather than alternately putting one of their hemispheres to sleep while the other remains active (Ridgway et al., 2006).
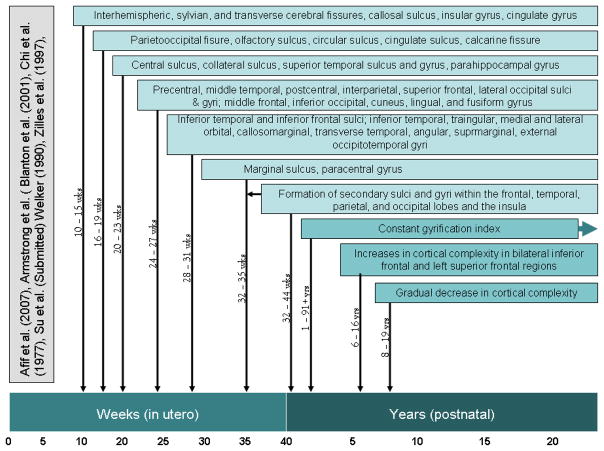
The development of gyrification begins prior to birth (see Figure 1), with the early stages of gyral and sulcal formation taking place between 10 to 15 weeks of human fetal life (Chi, Dooling, & Gilles, 1977; Zilles et al., 1997). During the third trimester of fetal life, when the brain is undergoing considerable growth (Chi et al., 1977), the brain develops from a relatively smooth, lissencephalic structure to a brain that more closely resembles the morphology of the adult brain (Armstrong, Schleicher, Omran, Curtis, & Zilles, 1995; Naidich et al., 1994; Retzius, 1891; Welker, 1990). In 1988 Zilles et al. (1988) described a quantitative approach to measure gyrification, known as the ‘gyrification index’ (GI). Brains that have a higher degree of cortical folding yield larger values of the GI. This measure was applied to quantify the comparative anatomy of gyrification (Zilles et al., 1989; Zilles, Armstrong, Schleicher, & Kretschmann, 1988) as well as the developmental trajectory of gyrification in humans (Armstrong et al., 1995). They found that the GI, which is defined as the ratio between the lengths of coronal outlines for the brain including and excluding the sulcal regions, increases dramatically during the third trimester, and then remains relatively constant throughout development (Armstrong et al., 1995; Dareste, 1862). Since the brain nearly triples its volume from birth to adulthood, the process of gyrification continues through this developmental period, maintaining this constant ratio.
Figure 1.
Developmental Timeline for Gyrification.
This constant GI ratio through birth is interesting and may allow for dating specific events that affect gyrification in the late prenatal period (Dubois et al., 2008). However, this constant GI ratio finding was based on 97 brains, which although a substantial number of postmortem brains, is a relatively small number considering they range from 11 weeks of gestational life to 95 years of age. Considering the pronounced decrease in GM that occurs during adolescent development (Gogtay et al., 2004; Sowell et al., 2003), it is somewhat surprising that the GI would remain constant. There have been no MRI studies to date that have specifically addressed the constancy of the GI during typical development.
Recently, new methods have been developed to study cortical foldings without computing gyrification indexes, e.g., shape analysis based on high dimensional spherical basis such as SPHAM (Shen et al., 2004) or spherical wavelets (Yu et al., 2007). These methods provide different ways to characterize shape features.
One motive for studying gyrification is to better understand the neurobiology associated with the development of cortical folding patters. However, an alternate motive for studying gyrification is to provide a better understanding of the structural variability between brains (Mangin et al., 2004; White, 2001; Zilles et al., 1997). For example, the central sulcus can vary in location by up to 2 cm between individuals (Talairach & Tournoux, 1988). This variability results in significant challenges for spatial normalization, which is a common practice in evaluating structural and functional brain images in neuroimaging studies (Mangin et al., 2004).
The goal of this paper is to provide a review of the development of gyrification with a focus on changes that lead up to and through adolescence. We will include a review of the developmental precursors that contribute to gyrification, primary theories of gyrification, and also a description of current computational algorithms used to numerically measure gyrification. We will discuss genetic and environmental contributions to gyrification. Finally, we will present work in-progress on age-related differences in gyrification in typically developing adolescents. Our findings support a changing surface morphology that is associated with underlying neurodevelopmental changes during adolescence.
Precursors to Gyrification
The early antecedents of gyrification occur during the first months of fetal life, when neurons that have formed via mitosis at the ventricular zone migrate outward along radial glial guide cells to the outer layers of the brain. This migration begins at approximately six weeks of fetal life and its description is known as the radial unit hypothesis of cortical formation (Rakic, 1988, 1995, 2000). This migration of neurons outward from the ventricular zone forms the basis for the cortical gray matter (GM).
Prior to six weeks of fetal life, the neural progenitor cells located in the ventricular zone begin symmetric cell division, with each stem cell producing two identical stem cells with each mitotic cycle (Rajkowska & Goldman-Rakic, 1995). Thus, this period results in an exponential growth in neuronal progenitor cells. Then, at approximately six weeks of gestational age, the progenitor cells make a gradual shift to asymmetric division. During asymmetric cell division, one daughter cell remains as an undifferentiated stem cell which undergoes further replication, while the other daughter cell matures into a neuron that migrates outward to the cortex. The migration forms an inside-out pattern, with later generations passing through the previously developed cells before reaching their ultimate positions in the cortex (Sidman & Rakic, 1973). The completed cortical GM consists of six layers of cells that have migrated in this inside-out pattern.
During these two phases of symmetric (before six weeks) and asymmetric (six weeks to 12 weeks) cell division, small perturbations can influence either the thickness or surface area of the cortex. In turn, these events can influence gyrification. For example, before six weeks of age, one additional mitotic cycle could potentially double the number of neural progenitor cells, which could have a profound influence on the number of migrating cells.
The surface area of the brain is closely associated with the number of radial units formed by symmetrical division along the ventricular zone (Rakic, 1988, 1995). A larger number of radial units implies that there will be a larger number of lined projections to the cortical plate, and thus a greater surface area of the brain (Rakic, 1974; Sidman & Rakic, 1973). Since each round of mitosis results in an exponential increase in the number of progenitor cells, small changes affecting the duration of symmetric growth will have a dramatic impact on surface area (Barondes et al., 1997). This developmental principle has been called ‘late equals large’, as neurons migrating into late-developing brain structures undergo a longer period of symmetrical division, resulting in a larger size of these structures. This principle has been verified for the developmental time table and corresponding sizes of brain structures in many different species (Finlay & Darlington, 1995; Striedter, 2005).
When the embryos of monkeys are irradiated during the symmetric phase of progenitor cell division, there is a decrease in the total surface area of the brain. However, when radiation is applied after six weeks, during the phase of asymmetric cell division, it results in a deletion of cortical cell layers, and in turn, a decrement in cortical thickness (Barondes et al., 1997). It also disrupts the development of gyrification (Stewart, Richman, & Caviness, 1975). Thus, the thickness of the six-layered cortex is influenced by events that occur during the asymmetric period of cell division.
Recent studies have found that the migration of neurons into the cortex is not as straightforward as initially thought, with several neuronal subpopulations showing different migratory patterns, e.g., (Nadarajah, Alifragis, Wong, & Parnavelas, 2003). In particular, the radial migration pattern described above is characteristic of pyramidal cells, while different types of cortical interneurons pursue a more tangential migratory path to their target layers (Kriegstein & Noctor, 2004). The effect of the tangential mode of migration on cortical morphology is likely small or has only a local influence on the development of the cortical layers. The vast majority of cortical neurons are pyramidal cells that follow the predominant radial path; interneurons form connections only in their immediate vicinity.
An additional factor that may affect the surface morphology of the brain is apoptosis, or programmed cell death (Haydar, Kuan, Flavell, & Rakic, 1999). Apoptosis can result in the elimination of up to 50% of the neurons that are generated early in development (Cowan, Fawcett, O’Leary, & Stanfield, 1984; Levitt, 2003). Since apoptosis contributes to total neuronal number, and thus to both total brain volume and neuronal connectivity, apoptosis also likely contributes to brain surface morphology in ways that are as yet poorly understood.
To summarize, these prenatal and early postnatal events provide a critical foundation for subsequent changes in gyrification that may occur during childhood, adolescence, and into adulthood.
Phylogeny of Gyrification
A fascinating feature of the human brain is the disproportionally large surface area of the cerebral cortex in relation to its volume, which is due to an extension of its developmental period as compared to other brain regions (Finlay & Darlington, 1995; T. White & Hilgetag, 2008). Greater surface area implies a larger amount of cortical gray matter and thus, a greater potential for computational abilities. The phylogenetic increase in the surface area of the human brain has far exceeded the growth in cortical thickness (Welker, 1990). For example, in humans the surface area of the brain is 1,700 times larger than in shrews, yet the thickness of the cortex is only six times greater (Hofman, 1989). Compared to macaque monkeys, the surface area of the human brain is approximately ten times greater, whereas the thickness of the human cortex is only two fold greater (Barondes et al., 1997). This patterning implies that during evolution, the cortex expanded laterally rather than vertically (Chenn & Walsh, 2002), resulting in a convoluted human cortical sheet that is about three times as large as the inner surface of the skull (Hilgetag & Barbas, 2005, 2006; Richman, Stewart, Hutchinson, & Caviness, 1975; Toro & Burnod, 2005; Van Essen, 1997; Welker, 1990).
In theory, a greater number of neurons in the cortex could also be obtained by increasing the cortical thickness, rather than increasing cortical surface area. Tripling cortical thickness, from about 5 to 15mm, would allow for a lissencephalic human cortex with only a minor increase in brain volume. However, computer modeling studies (Murre & Sturdy, 1995; Ruppin, Schwartz, & Yeshurun, 1993) suggest that this mechanism would be ill fated. Given the formidable degree of connectivity among cortical neurons (each forming, on average, a thousand or more connections with other neurons (Braitenberg & Schüz, 1998)), the volume of connections grows exponentially with the number of neurons. Thus, the extra projections required to link neurons in the additional cortical layers would lead to a situation of highly inefficient wiring in the thickened cortex. In order to fit all the connections, neurons within the cortex would need to take detours, resulting in highly inefficient processing (Chklovskii, Schikorski, & Stevens, 2002). These theoretical studies support the idea that the segregation of brain tissue into components of cell bodies within the GM and connections within the WM, in concert with the volume-saving folding of the cortical sheet, reflects an efficiently designed wiring and volume arrangement for the very dense connectivity found in the cerebral cortex (Murre & Sturdy, 1995; Ruppin et al., 1993).
Theories of Gyrification
A number of theories have surfaced that describe the developmental processes underlying the gyrification of the cerebral cortex. The first theory emerged over a century ago and postulated that higher and lower growth rates of different brain regions separate gyral regions as a result of tension and local tissue deformation (His, 1874). This theory can be referred to as a “mechanical theory” because it advocates that tensile forces promote gyral development. Several additional mechanical theories emerged over the course of the subsequent 50 years. These theories were similar and postulated that there was differential growth between the gyri and sulci and that regions destined to be become gyri are established by active, localized growth (Retzius, 1891) in combination with friction of the different brain structures against each other and with the surrounding skull (Le Gross Clark, 1945). Subsequent analyses, however, have suggested that gyrification is dependent on mechanisms within the cortical regions, as opposed to restraints of the skull or connections with subcortical regions (Barron, 1950). During the period of rapid brain growth, differential expansion of the individual cortical layers may lead to a buckling of the laminar cortical sheet (Hilgetag & Barbas, 2005, 2006; Richman et al., 1975; Toro & Burnod, 2005; Van Essen, 1997; Welker, 1990). However, theories of cortical morphogenesis need to explain why convolutions are modified even after the destruction of connections (Goldman-Rakic, 1980; Goldman-Rakic & Rakic, 1984).
Thus, a third theory emerged a decade ago postulating that viscoelastic tension exerted by cortical fibers contributes to the shaping of cortical convolutions (Van Essen, 1997). This theory proposes that neuronal connections that develop during the second trimester produce localized fiber tension which draws densely interconnected regions closer together. As regions of greater connectivity move closer together in an enclosed and rapidly growing brain, they form outward bulging gyri. Alternatively, more sparsely connected regions drift apart and form the sulci. The tension, although very small for an individual axon (Heidemann, Lamoureux, & Buxbaum, 1995), is summed by the very large number of neurons, thus creating differential forces that interact within the rapidly growing brain to form the gyri and sulci. Accordingly, the characteristic pattern of the convolutions is explained by the highly specific organization of the underlying connectivity (Sporns, Chialvo, Kaiser, & Hilgetag, 2004). In drawing regions of greater connectivity closer together, the transit time of the action potentials is decreased, thus enhancing the overall efficiency of brain function. If tension produced by the neuronal connections is involved in the mechanisms of gyrification, then changes in the patterning of the gyri and sulci are expected outcomes of synaptic pruning. Such a theory could link brain surface morphology with regional neuronal connectivity within a developmental framework (T. White & Hilgetag, 2008).
Meshing the age-related differences in the morphology of the cerebral cortex to changes in neural connectivity is intriguing. Age-related synaptic and dendritic arborization may result in decreasing the tensile forces that form the gyral and sulcal regions (Van Essen, 1997; T. White & Hilgetag, 2008). Histological studies of the neuronal pathways have found that the neural fibers tend to course horizontal to the surface in the sulci, whereas in the gyri fiber pathways tend to be oriented tangential to the cortical surface (Welker, 1990). A release of tension would occur along the line horizontal to the predominance of pruned connections. Theoretically, this would result in a widening of the sulci and a greater, or more peaked, curvature of the gyri (T. White, Andreasen, Nopoulos, & Magnotta, 2003). These features have been found in a group of healthy adults (Magnotta et al., 1999) using techniques to measure the Gaussian-weighted average curvature, or the concavity and convexity of the gyri and sulci.
Measures of Gyrification
Utilizing coronal sections of postmortem brains, Zilles et al. (1988) (Armstrong et al., 1991; Zilles et al., 1988) manually measured the ratio between the length of the outer folded surface of the brain (including sulci) and the length of the outer surface excluding sulci (see Figure 2-a). While the two-dimensional approach was applied to coronal brain sections, sections obtained at 45° oblique angles obtained GI’s that were within 8% of the coronal GI measures. GI has been applied to study both the phylogeny (Armstrong et al., 1991; Zilles et al., 1988) and ontogeny (Armstrong et al., 1995) of cortical gyrification. Brains that have a higher degree of cortical folding yield larger values of the GI. Anterior-to-posterior maps of human GI measures have shown greater gyrification in the prefrontal and temporal/parietal association regions of the brain as compared to other regions (Zilles et al., 1988). During development, the GI begins to increase prior to the third trimester and plateaus at birth.
Figure 2.
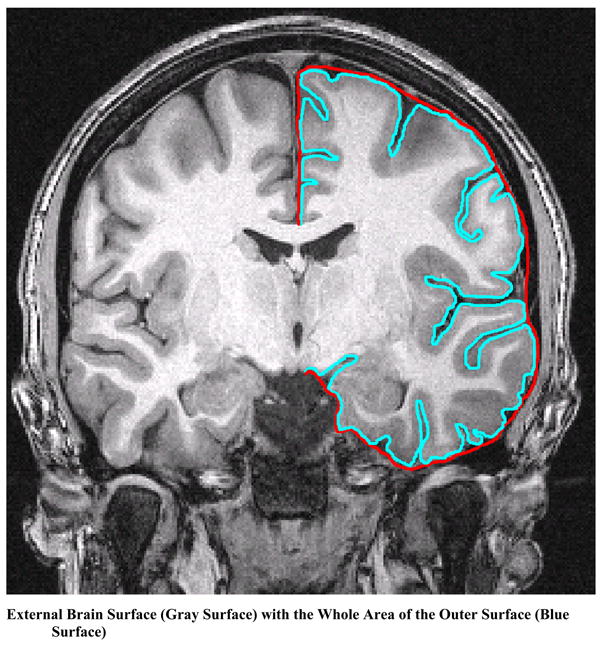
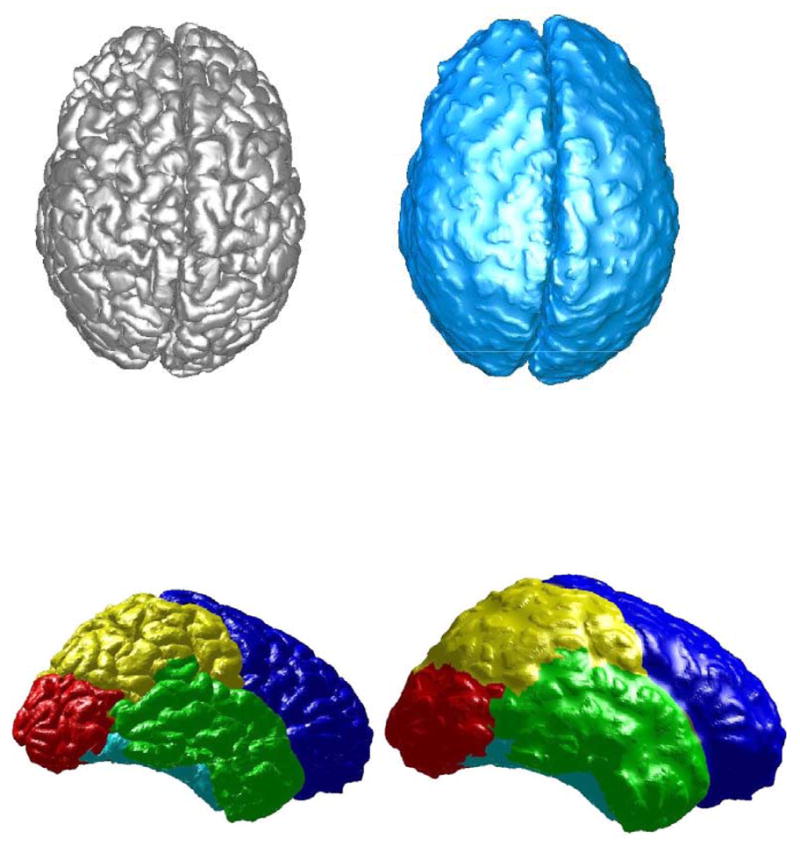
Figure 2a. The Standard Gyrification Index (GI) is a Ratio of the External Brain Surface (Turquoise Line) with the outer Surface excluding the Sulci (Red Line).
Figure 2b. Local 3D Gyrification Index (LGI) is the Area Ratio of the Selected Region on the External Brain Surface with the Corresponding Region on the Outer Surface (The Corresponding Regions are Marked as the Same Color)
Interestingly, this plateau appears to remain constant across the lifespan, in spite of the rapid brain development and changes associated with aging (Armstrong et al., 1995). However, since developmental studies typically require large samples, and since postmortem samples in younger individuals are difficult to obtain, it would be beneficial for the study of Armstrong et al. (Armstrong et al., 1995), which had an sample size of 97 individuals, to be replicated/validated with a larger sample size to confirm this finding.
Alternative, computational methods for measuring the surface morphology of the brain involve regional measurements of curvature (i.e., convexity and concavity) (Van Essen & Drury, 1997) and sulcal depth of the cortical surface from magnetic resonance (MR) images (Koenderink & van Doorn, 1992; Luders et al., 2006; Magnotta et al., 1999). For measurements of curvature, these methods first define a triangular surface covering a layer within the cortical rim. Once the surface is constructed, (for example) the angles between vectors normal to the triangular isosurfaces are used to calculate the regional curvature of the cortex. These regional measures can be utilized to study regional curvature between groups (Luders et al., 2004; Luders et al., 2006), or averaged to study global differences (Magnotta et al., 1999; T. White, Andreasen, & Nopoulos, 2002).
For depth measurements of the sulci, approaches have been utilized that are based on either Euclidean distance (Lohmann, 1998), geodesic surface distance (Rettmann, Han, Xu, & Prince, 2002), or geodesic distance marked by the path within the cerebrospinal fluid (CSF) (Kao et al., 2007). Different distance measurements provide different measures of sulcal depth that may also influence measures of cortical complexity. Gyrification is one measure of cortical complexity. Among these depth measurements, geodesic distance (the path length through the sulcus traveling only through the sulcal CSF and not through tissue) provides a better understanding of the true cortical depth, as it takes into account the circuitous nature of the sulci (see in Figure 2-a).
Since the original gyrification index was defined on coronal sections, unlike curvature and sulcal depth measurements, it does not take into account the 3-D nature of the cortical surface. The GI may be altered if the slice orientation is different, thus it is important to introduce a 3D gyrification index to eliminate the shortcomings of the coronal 2D GI. A number of neuroimaging software packages are currently available to generate 3D reconstructions of human brains, e.g., Freesurfer (Dale et al. 1999)1, SurfRelax (Larsson, 2001)2, and BrainVisa (Mangin et al. 2004)3. These software packages provide the early preprocessing stages, which are the essential first steps in deriving a 3D gyrification index.
A simple extension of the 2D gyrification index to 3D is to use the area ratio between the outer hull surface, which is a surface wrapped around the brain, and the cortical surface. A localized 3-D gyrification index has been developed and applied to a group of children affected by 22q11 Deletion Syndrome (Schaer et al., 2008). This approach uses 3D triangulated mesh reconstructions of both cortical surfaces and outer hull surfaces and measures the amount of cortical surface buried in the sulci (region of interest) by constructing a non-intrinsic sphere with different radii. The index defined for each point on the cortical surface is obtained through a depth-weighted sum of neighboring points on the outer hull surface. Compared to previous measures of the sulcus index (Dubois et al., 2007), this approach is fully localized and thus helps to better define the characteristics of any region of interest in the brain, not restricted to any one sulcus or gyrus.
Another new approach uses the ratio between the surface area of the cortical surface included within a small sphere placed over the cortex with the surface area of a disc corresponding to the radius of the sphere (Toro et al., 2008). This method turns out to be less sensitive, since it is dependent on choosing a specific radius for the sphere, and different radii may give different results. This technique has been applied to a sample of 314 subjects, 164 females and 150 males, and shows a disproportionate ratio of cortical surface area to brain size, similar to the earlier observations across species (Prothero & Sundsten, 1984). In addition, there is an increase in cortical folding in the prefrontal cortex for larger brains. Since this approach does not require the construction of an outer hull surface, it results in a simple and efficient algorithm in comparison to alternative methods (Schaer et al., 2008). Meanwhile, these new methodologies raise important methodological questions in comparision to the previously defined measures (Van Essen & Drury, 1997; Zilles et al., 1988), namely, that the computation should be independent of brain size, since altered brain surface morphology does not necessarily follow the same pattern as alterations in brain size.
Recently, we proposed several new intrinsic and geometric techniques to compute global and local gyrification indices. The simple extension from 3D global GI (see Figure 2-b) to 3D local GI is to find the corresponding regions on the outer hull surface for any selected region of interest on the external brain surface (see Figure 2-c). Furthermore, 3D GI can also be weighted by local quantities, i.e., curvature and sulcal depth, and is fully intrinsic and different than the method proposed by Schaer et al. (Schaer et al. 2008; Toro et al., 2008) in that it does not depend on a chosen radius nor on a corresponding non-intrinsic sphere to determine the region used to calculate the local GI. The incorporation of the robust sulcal depth computation developed in (Kao et al. 2007) as part of the GI measurement is important to characterize different levels of convolutions in human brains. By applying our method to a population of typically developing children, the proposed measurement of depth-weighted local gyrification turns out to be more robust in finding the developmental differences between children and adolescents, e.g., we observe significantly increased gyrification in the right parietal lobe and right cingulate cortex, as well as age-rated differences in the left frontal, right parietal and the right cingulate cortex between the ages of 8 to 19 years. These findings provide some references for future study of the relationship between gyrification and neurological and psychiatric conditions, in addition to the development of other more advanced techniques to quantify the gyrification of the human brain.
Heritability in Gyrification
Even though the development of the sulcal and gyral patterns in the brain is strongly influenced by genetic processes (Piao et al., 2004), studies of monozygotic twins (MZ), who share the same genetic complement, show considerable differences in their surface morphology (Thompson et al., 2001; T. White et al., 2002). For example, correlations in volume measurements in MZ twins are on average greater than 0.95, whereas measures of gyral and sulcal curvature are significantly less correlated and on the order of 0.5 (Chow, Zipursky, Mikulis, & Bassett, 2002). It is plausible that the greater non-shared environmental influences that are present postnatally for twins, coupled with the pronounced cortical plasticity of early development, bring about differences in MZ twins in the patterns of cortical surface morphology.
In a study of both MZ and dizygotic twins (DZ), Bartley et al. demonstrated that the development of cortical patterns is determined primarily by random environmental factors (Bartley, Jones, & Weinberger, 1997). In addition, evaluating the gyral patterns in monozygotic twins, Lohmann et al. (Lohmann, von Cramon, & Steinmetz, 1999) found that the deeper and developmentally earlier sulci of the brain (i.e., the central sulcus or the sylvian fissure) are more highly correlated than the superficial, or tertiary sulci (i.e., the caudomedial lobule). The tertiary sulci, which develop mainly after birth, appear to be more affected by non-genetic influences. To summarize the genetic contribution to gyrification, it is thought that while genetic processes play a large role in gyrification, especially early in development, non-shared environmental factors have a major contribution to the surface morphology of the brain.
Gyrification in Development
If tension produced by neuronal connections is involved in the mechanisms of gyrification, then synaptic pruning or dendritic arborization later in development could conceivably alter brain surface morphology. Indeed, local and remote changes in gyrification have been observed after experimental white-matter lesions in the developing primate brain (Goldman-Rakic, 1980; Goldman-Rakic & Rakic, 1984). The link between gyrification and axonal tension has also been supported by recent experimental findings in the primate brain showing a strong correlation between the densities and trajectories of fiber projections (Hilgetag & Barbas, 2006; Toro & Burnod, 2005).
It is known from postmortem studies that while there is little loss of neurons between childhood and adulthood (Peters, Morrison, Rosene, & Hyman, 1998), there is a considerable amount of synaptic pruning that occurs during childhood and adolescence (Huttenlocher, 1979; Huttenlocher & Dabholkar, 1997; Huttenlocher & de Courten, 1987). In fact, synaptic pruning is thought to underlie the developmental changes (Gogtay et al., 2004; Sowell et al., 2004) and differences (Sowell et al., 2003; Sowell, Trauner, Gamst, & Jernigan, 2002) seen in GM volume between children, adolescents, and adults. After the age of 18, it has been demonstrated that changes in surface curvature occur, affecting both the gyri and the sulci (Magnotta et al., 1999). The sulci tend to develop less curvature, becoming broader, while the gyri develop greater curvature, becoming more peaked. In addition, there is thinning of the cortical mantel, and these differences are consistent with an increased amount of CSF associated with aging.
While there have been a number of studies that evaluate gyrification in developmental and psychiatric disorders in children and adolescents (Bearden et al., 2009; Kesler et al., 2006; Thompson et al., 2005; T. White et al., 2003), the only study to date that has evaluated surface complexity associated with typical development found increased cortical complexity in prefrontal regions between the ages of six to sixteen years (Blanton et al., 2001). Thus, there appears to be increased cortical molding as children progress into mid-adolescence in regions that have been both functionally and structurally associated with more protracted brain maturation (Conklin, Curtis, Katsanis, & Iacono, 2000; Huttenlocher & Dabholkar, 1997; Huttenlocher, De Courten, Garey, & van der Loos, 1982; Karatekin, Marcus, & White, 2007; Luciana & Nelson, 2000; Sowell et al., 2002).
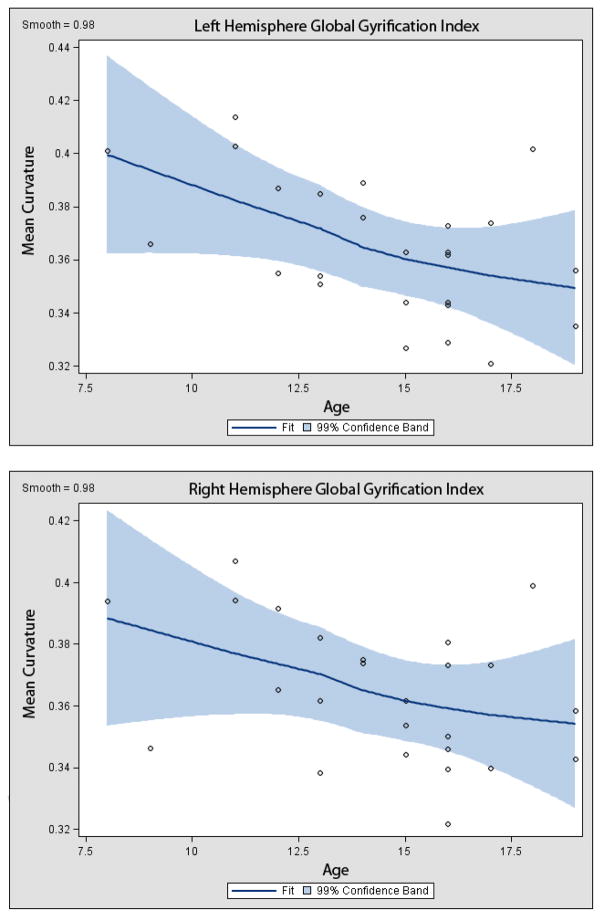
We have preliminary work (Su et al., submitted) that demonstrates a gradual decrease in the amount of cortical complexity during adolescence in both the right and left hemispheres (Figure 3). We speculate that as synaptic pruning and dendritic arborization proceeds, there are resulting alterations in the surface morphology of the brain. We are currently exploring how these differences in global GI relate to the underlying curvature, thickness, and depth of the sulci (Su et al., in progress) as these will likely reflect age-related differences in underlying brain connectivity.
Figure 3.
Localized Regression Curves Demonstrating Age-Related Differences in Global Gyrification Indices in the Left and Right Hemispheres during Adolescence. Images acquired using SAS (Cary, NC).
Alterations in brain connectivity associated with development would likely have functional outcomes. Thus, exploring the relationship between the developmental trajectories of regional brain gyrification and performance on specific neuropsychological tasks may shed light on the functional consequences of developmental changes in gyrification. In addition, coupling functional connectivity measures using high-resolution functional MRI (i.e. spin-echo techniques at high field) with measures of gyrification will also provide information on this relationship. Finally, little is known about whether changes in the surface morphology are associated with other neurodevelopmental changes during this age range, such as myelination. With higher resolution structural and diffusion tensor imaging techniques, it may be possible to assess these relationships, keeping in mind that although processes may have similar developmental trajectories, this does not necessarily mean that they directly influence each other.
Gender Differences in Gyrification
There are mixed studies regarding gender differences in gyrification, with a postmortem study of GI finding no differences (Zilles et al., 1988), and an MR study showing that females have greater cortical complexity (Luders et al., 2004). Since females have on average smaller brain volumes (Nopoulos, Flaum, O’Leary, & Andreasen, 2000), the greater cortical complexity, or gyrification, may produce a brain with equal functional abilities (Luders et al., 2004). More work in this area is needed.
Discussion
The phylogeny of the human nervous system has resulted in a highly complex brain that is associated with a high degree of cortical folding. Humans, dolphins, and porpoises stand apart from other species in having a disproportionately large cerebral cortex-to-body size ratio (Allmann, 2000). The cortical folding increases the surface area of the cortical gray matter and enhances the overall compactness of the brain. While an increase in cortical gray matter could also be achieved by increasing cortical thickness, it turns out that such morphology is ill fated. As an example, a slightly larger lisencephalic, or smoothed surfaced human brain is theoretically possible by increasing the cortical thickness three fold, from approximately 5 to 15 mm. This increase in cortical thickness would preserve the total volume of cortical gray matter, however, computational models have shown that the necessary packing of connections within the cortex would preclude such an evolutionary change (Murre & Sturdy, 1995; Ruppin et al., 1993).
Since each neuron has, on average, more than a thousand connections with other neurons (Braitenberg & Schüz, 1998), the volume of space required to allow for these connections grows exponentially with the number of neurons. The result would be highly congested and inefficient neuronal pathways, with some neurons taking circuitous paths in order to reach their final destinations (Chklovskii et al., 2002). Thus, the development of gyral and sulcal folds allows for an optimized compaction of neuronal fibers with an efficient transit time for neuronal signaling. The parcellation of brain tissue into the computationally powerful cortical layers and efficient signal transmission through myelinated fibers, coupled with a compacted gyrification pattern, have resulted in an efficient wiring and volume arrangement for the very dense connectivity that exists within the human brain (Murre & Sturdy, 1995; Ruppin et al., 1993; Wen & Chklovskii, 2005).
The ‘form fitting function’ design raises the question as to whether alterations in function also result in alterations of form, mediated by neural connectivity. Neurons within the sulci tend to orient horizontal to the cortical surface, due partially to U-fibers that connect gyri via the sulci (Welker, 1990). Thus, synaptic pruning, or a release of tension that occurs along these neuronal fibers could potentially create a broadening or widening of the sulci (T. White et al., 2003). Alternatively, the neurons within the gyri tend to be more numerous and lie on average more tangential to the cortical surface (Hilgetag & Barbas, 2005, 2006; Richman et al., 1975; Toro & Burnod, 2005; Van Essen, 1997; Welker, 1990). Alternatively, releasing tension in these fibers could cause the gyri to develop greater curvature, or to become more peaked. Interestingly, the sulcal and gyral brain regions that demonstrated the greatest differences in surface morphology in adolescents with schizophrenia, also were those regions that had the greatest decrease in cortical thickness (T. White et al., 2003). These gyrification abnormalities may be related to the pronounced cognitive deficits seen in adolescents with schizophrenia (T. White, Ho, Ward, O’Leary, & Andreasen, 2006).
Finally, there is a direct relationship between disorders of neuronal migration (i.e., lissencephaly and polymicrogyri) and aberrant neuronal connectivity (Stewart et al., 1975). These disorders of neuronal migration have profound effects on the gyral and sulcal patterns in the brain and are associated with significant cognitive deficits. The timing of the pathology for these developmental disorders occurs before 24 weeks gestational age, a time when neuronal migration is laying the foundation for gyrification (Neal et al., 2007). These disorders support the connection between altered form and altered function in the human brain.
While the tension based morphogenesis theory has been gaining recent support (Hilgetag & Barbas, 2006; Van Essen, 1997), other theories also exist. Alternate theories include those that involve differential growth or gyrogenesis (Le Gross Clark, 1945), mechanical factors, such as abutting cortical plates (Richman et al., 1975), or a combination of the two (Todd, 1982). There are several important aspects to consider when evaluating the different theories of gyrification. Primarily, while there is variability in the spatial location between gyri and sucli between individuals, there are actually more similarities than differences. The primary gyri and sulci are readily identified in different brains, and although they may have some differences in shape and contours, they do follow a common trajectory (Thompson, Schwartz, Lin, Khan, & Toga, 1996). In addition, the primary and secondary sulci are under greater genetic control than the tertiary sulci (Lohmann et al., 1999), and twin brains have significantly greater similarities in their surface morphology (Bartley et al., 1997; T. White et al., 2002). Thus early stages of gyrification involve genetically-mediated processes intrinsic to growth (Neal et al., 2007), not dependent on external forces of the skull, (Barron, 1950), and are intrinsic to the development of the cortex and thus not dependent on connections with subcortical structures (Barron, 1950; Welker, 1990).
In summary, during the third trimester of fetal life, the brain evolves from a relatively smooth surfaced structure to a morphology of ‘fissures and folds’ that resembles the adult human brain (Welker, 1990). The mechanisms behind the process of gyrification are largely unknown, although one recent hypothesis links brain connectivity with gyrification (Van Essen, 1997). This hypothesis postulates that regions with greater neural connectivity are associated with greater tension that allows these brain regions to remain in closer proximity during brain growth, thus forming gyri. Alterations in connections, such as that which occurs during synaptic pruning and dendritic arborization, could conceivably also alter the morphology of the gyri and sulci.
Our current work demonstrates evidence that such differences in brain surface morphology are occurring during adolescence, and we suspect that these morphological differences relate to the underlying connections in the developing brain. Adolescence is a time of particular interest, as higher-order cognitive functions are continuing to develop (Conklin, Luciana, Hooper, & Yarger, 2007; Karatekin et al., 2007; Luciana & Nelson, 2000) and brain structure continues to mature into early adulthood (Giedd, 2004; Sowell et al., 2003). As these changes take place, it is possible that measures of gyrification may provide more localized measures of changes in the underlying connectivity. Identifying local changes or differences in connectivity will assist in pinpointing structure/function relationships associated with typical adolescents development as well as changes associated with emerging psychiatric disorders. The latter is of particular interest, since adolescence and early adulthood is a period where the incidence of several major psychiatric disorders, including schizophrenia, major depressive disorder, and bipolar affective disorder, dramatically increases.
Future work should include longitudinal studies of children, adolescents, and young adults to measure the trajectory of gyrification patterns associated with development. Coupling these studies with trajectories of neuropsychological development may help to understand the functional relationship between performance on these tasks and the regional changes in gyrification. In addition, longitudinal studies in high-risk populations will allow for the detection of the timing and location of regional changes in gyrification. Coupling high-resolution structural imaging with other imaging techniques, such as diffusion tensor imaging, functional MRI, or optical imaging will provide important information on local and regional connectivity associated with the developmental trajectory of surface morphology. While the study of gyrification has received less attention than other aspects of neuroscience to date, it is quite likely that interesting secrets of neurodevelopment are hidden within the processes involved in the ‘fissuring and folding’ of the human brain.
Acknowledgments
Support for this work is through NIMH grant MH068540, NIH CON000000004051-3014, a junior investigator award through the Essel Foundation and the Blowitz-Ridgeway Foundation through the National Alliance for Research in Schizophrenia and Affective Disorders (NARSAD), and the MIND Research Network. Chiu-Yen Kao is partially supported by NSF DMS-0811003 and acknowledges the support and hosting from Mathematical Biosciences Institute at The Ohio State University. We would also like to acknowledge grants that support the infrastructure for the Siemen’s TRIO scanner at the University of Minnesota: P30 NS057091 & P41 RR00807. Finally, we would like to thank the anonymous reviewers for their helpful comments and suggestions.
Footnotes
FreeSurfer, see http://surfer.nmr.mgh.harvard.edu/
SurRelax, see http://www.cns.nyu.edu/~jonas/software.html
BrainVisa, see http://brainvisa.info/
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allmann J. Evolving brains. W H Freeman & Co; 2000. [Google Scholar]
- Armstrong E, Curtis M, Buxhoeveden DP, Fregoe C, Zilles K, Casanova MF, et al. Cortical gyrification in the rhesus monkey: a test of the mechanical folding hypothesis. Cereb Cortex. 1991;1(5):426–432. doi: 10.1093/cercor/1.5.426. [DOI] [PubMed] [Google Scholar]
- Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K. The ontogeny of human gyrification. Cereb Cortex. 1995;5(1):56–63. doi: 10.1093/cercor/5.1.56. [DOI] [PubMed] [Google Scholar]
- Barondes SH, Alberts BM, Andreasen NC, Bargmann C, Benes F, Goldman-Rakic P, et al. Workshop on schizophrenia. Proc Natl Acad Sci U S A. 1997;94(5):1612–1614. doi: 10.1073/pnas.94.5.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron DH. An experimental analysis of some factors involved in the development of the fissure pattern of the cerebral cortex. J Exp Zool. 1950;113:553–581. [Google Scholar]
- Bartley AJ, Jones DW, Weinberger DR. Genetic variability of human brain size and cortical gyral patterns. Brain. 1997;120(Pt 2):257–269. doi: 10.1093/brain/120.2.257. [DOI] [PubMed] [Google Scholar]
- Bearden CE, van Erp TG, Dutton RA, Lee AD, Simon TJ, Cannon TD, et al. Alterations in midline cortical thickness and gyrification patterns mapped in children with 22q11.2 deletions. Cereb Cortex. 2009;19(1):115–126. doi: 10.1093/cercor/bhn064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton RE, Levitt JG, Thompson PM, Narr KL, Capetillo-Cunliffe L, Nobel A, et al. Mapping cortical asymmetry and complexity patterns in normal children. Psychiatry Res. 2001;107(1):29–43. doi: 10.1016/s0925-4927(01)00091-9. [DOI] [PubMed] [Google Scholar]
- Braitenberg V, Schüz A. Cortex: statistics and geometry of neuronal connectivity. 2. Berlin: Springer; 1998. [Google Scholar]
- Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297(5580):365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- Chi JG, Dooling EC, Gilles FH. Gyral development of the human brain. Ann Neurol. 1977;1(1):86–93. doi: 10.1002/ana.410010109. [DOI] [PubMed] [Google Scholar]
- Chklovskii DB, Schikorski T, Stevens CF. Wiring optimization in cortical circuits. Neuron. 2002;34(3):341–347. doi: 10.1016/s0896-6273(02)00679-7. [DOI] [PubMed] [Google Scholar]
- Chow EW, Zipursky RB, Mikulis DJ, Bassett AS. Structural brain abnormalities in patients with schizophrenia and 22q11 deletion syndrome. Biol Psychiatry. 2002;51(3):208–215. doi: 10.1016/s0006-3223(01)01246-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin HM, Curtis CE, Katsanis J, Iacono WG. Verbal working memory impairment in schizophrenia patients and their first-degree relatives: evidence from the digit span task. Am J Psychiatry. 2000;157(2):275–277. doi: 10.1176/appi.ajp.157.2.275. [DOI] [PubMed] [Google Scholar]
- Conklin HM, Luciana M, Hooper CJ, Yarger RS. Working memory performance in typically developing children and adolescents: behavioral evidence of protracted frontal lobe development. Dev Neuropsychol. 2007;31(1):103–128. doi: 10.1207/s15326942dn3101_6. [DOI] [PubMed] [Google Scholar]
- Cowan WM, Fawcett JW, O’Leary DD, Stanfield BB. Regressive events in neurogenesis. Science. 1984;225(4668):1258–1265. doi: 10.1126/science.6474175. [DOI] [PubMed] [Google Scholar]
- Dareste MC. Sur les rapports de la masse encéphalique avec le développment de l’intelligence. Bull Soc Anthropol. 1862;3:26–54. [Google Scholar]
- Dubois J, Benders M, Borradori-Tolsa C, Cachia A, Lazeyras F, Ha-Vinh Leuchter R, et al. Primary cortical folding in the human newborn: an early marker of later functional development. Brain. 2008;131(Pt 8):2028–2041. doi: 10.1093/brain/awn137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay BL, Darlington RB. Linked Regularities in the Development and Evolution of Mammalian Brains. Science. 1995;268:1578–1584. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Morphological consequences of prenatal injury to the primate brain. Prog Brain Res. 1980;53:1–19. [PubMed] [Google Scholar]
- Goldman-Rakic PS, Rakic P. Experimental modification of gyral patterns. In: Geschwind N, Galaburda A, editors. Cerebral dominance. Cambridge, MA: Harvard University Press; 1984. pp. 179–192. [Google Scholar]
- Haydar TF, Kuan CY, Flavell RA, Rakic P. The role of cell death in regulating the size and shape of the mammalian forebrain. Cereb Cortex. 1999;9(6):621–626. doi: 10.1093/cercor/9.6.621. [DOI] [PubMed] [Google Scholar]
- Heidemann SR, Lamoureux P, Buxbaum RE. Cytomechanics of axonal development. Cell Biochem Biophys. 1995;27(3):135–155. doi: 10.1007/BF02738107. [DOI] [PubMed] [Google Scholar]
- Hilgetag CC, Barbas H. Developmental mechanics of the primate cerebral cortex. Anat Embryol (Berl) 2005;210(5–6):411–417. doi: 10.1007/s00429-005-0041-5. [DOI] [PubMed] [Google Scholar]
- Hilgetag CC, Barbas H. Role of mechanical factors in the morphology of the primate cerebral cortex. PLoS Comput Biol. 2006;2(3):e22. doi: 10.1371/journal.pcbi.0020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- His W. Unsere Körperform und das physiologische Problem ihrer Entstehung. Leipzig: F. C. W. Vogel; 1874. [Google Scholar]
- Hofman MA. On the evolution and geometry of the brain in mammals. Prog Neurobiol. 1989;32(2):137–158. doi: 10.1016/0301-0082(89)90013-0. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387(2):167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, de Courten C. The development of synapses in striate cortex of man. Hum Neurobiol. 1987;6(1):1–9. [PubMed] [Google Scholar]
- Huttenlocher PR, De Courten C, Garey LJ, van der Loos H. Synaptic development in human cerebral cortex. Int J Neurol. 1982;17:144–154. [PubMed] [Google Scholar]
- Kao CY, Hofer M, Sapiro G, Stem J, Rehm K, Rottenberg DA. A geometric method for automatic extraction of sulcal fundi. IEEE Trans Med Imaging. 2007;26(4):530–540. doi: 10.1109/TMI.2006.886810. [DOI] [PubMed] [Google Scholar]
- Karatekin C, Marcus DJ, White T. Oculomotor and manual indexes of incidental and intentional spatial sequence learning during middle childhood and adolescence. J Exp Child Psychol. 2007;96(2):107–130. doi: 10.1016/j.jecp.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Kesler SR, Vohr B, Schneider KC, Katz KH, Makuch RW, Reiss AL, et al. Increased temporal lobe gyrification in preterm children. Neuropsychologia. 2006;44(3):445–453. doi: 10.1016/j.neuropsychologia.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Koenderink JJ, van Doorn AJ. Surface shape and curvature scales. Image and Vision Computing. 1992;10(8):557–564. [Google Scholar]
- Kriegstein AR, Noctor SC. Patterns of neuronal migration in the embryonic cortex. Trends Neurosci. 2004;27(7):392–399. doi: 10.1016/j.tins.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Le Gross Clark WE. Deformation patterns on the cerebral cortex. In: LeGross Clark WE, Medawar PB, editors. Essays on Growth and Form. Oxford: Oxford University PRess; 1945. pp. 1–23. [Google Scholar]
- Levitt P. Structural and functional maturation of the developing primate brain. J Pediatr. 2003;143(4 Suppl):S35–45. doi: 10.1067/s0022-3476(03)00400-1. [DOI] [PubMed] [Google Scholar]
- Lohmann G. Extracting line representations of sulcal and gyral patterns in MR images of the human brain. IEEE Trans Med Imaging. 1998;17(6):1040–1048. doi: 10.1109/42.746714. [DOI] [PubMed] [Google Scholar]
- Lohmann G, von Cramon DY, Steinmetz H. Sulcal variability of twins. Cereb Cortex. 1999;9(7):754–763. doi: 10.1093/cercor/9.7.754. [DOI] [PubMed] [Google Scholar]
- Luciana M, Nelson CA. Neurodevelopmental assessment of cognitive function using CANTAB: Validation and future goals. In: Ernst s, Rumsey, editors. Functional Neuroimaging in Child Psychiatry. Cambridge: Cambridge University Press; 2000. pp. 379–397. [Google Scholar]
- Luders E, Narr KL, Thompson PM, Rex DE, Jancke L, Steinmetz H, et al. Gender differences in cortical complexity. Nat Neurosci. 2004;7(8):799–800. doi: 10.1038/nn1277. [DOI] [PubMed] [Google Scholar]
- Luders E, Thompson PM, Narr KL, Toga AW, Jancke L, Gaser C. A curvature-based approach to estimate local gyrification on the cortical surface. Neuroimage. 2006;29(4):1224–1230. doi: 10.1016/j.neuroimage.2005.08.049. [DOI] [PubMed] [Google Scholar]
- Macphail E. Brain and Intelligence in Vertebrates. Oxford, England: Clarendon Press; 1982. [Google Scholar]
- Magnotta VA, Andreasen NC, Schultz SK, Harris G, Cizadlo T, Heckel D, et al. Quantitative in vivo measurement of gyrification in the human brain: changes associated with aging. Cereb Cortex. 1999;9(2):151–160. doi: 10.1093/cercor/9.2.151. [DOI] [PubMed] [Google Scholar]
- Mangin JF, Riviere D, Cachia A, Duchesnay E, Cointepas Y, Papadopoulos-Orfanos D, et al. A framework to study the cortical folding patterns. Neuroimage, 23 Suppl. 2004;1:S129–138. doi: 10.1016/j.neuroimage.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Murre JM, Sturdy DP. The connectivity of the brain: multi-level quantitative analysis. Biol Cybern. 1995;73(6):529–545. doi: 10.1007/BF00199545. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Alifragis P, Wong R, Parnavelas J. Neuronal migration in the developing cerebral cortex: observations based on real-time imaging. Cereb Cortex. 2003;13:607–611. doi: 10.1093/cercor/13.6.607. [DOI] [PubMed] [Google Scholar]
- Naidich TP, Grant JL, Altman N, Zimmerman RA, Birchansky SB, Braffman B, et al. The developing cerebral surface. Preliminary report on the patterns of sulcal and gyral maturation--anatomy, ultrasound, and magnetic resonance imaging. Neuroimaging Clin N Am. 1994;4(2):201–240. [PubMed] [Google Scholar]
- Neal J, Takahashi M, Silva M, Tiao G, Walsh CA, Sheen VL. Insights into the gyrification of developing ferret brain by magnetic resonance imaging. J Anat. 2007;210(1):66–77. doi: 10.1111/j.1469-7580.2006.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopoulos P, Flaum M, O’Leary D, Andreasen NC. Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Res. 2000;98(1):1–13. doi: 10.1016/s0925-4927(99)00044-x. [DOI] [PubMed] [Google Scholar]
- Peters A, Morrison JH, Rosene DL, Hyman BT. Feature article: are neurons lost from the primate cerebral cortex during normal aging? Cereb Cortex. 1998;8(4):295–300. doi: 10.1093/cercor/8.4.295. [DOI] [PubMed] [Google Scholar]
- Piao X, Hill RS, Bodell A, Chang BS, Basel-Vanagaite L, Straussberg R, et al. G protein-coupled receptor-dependent development of human frontal cortex. Science. 2004;303(5666):2033–2036. doi: 10.1126/science.1092780. [DOI] [PubMed] [Google Scholar]
- Prothero JW, Sundsten JW. Folding of the cerebral cortex in mammals. A scaling model. Brain Behav Evol. 1984;24:152–167. doi: 10.1159/000121313. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cereb Cortex. 1995;5(4):307–322. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974;183(123):425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241(4862):170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18(9):383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- Rakic P. Radial unit hypothesis of neocortical expansion. Novartis Found Symp. 2000;228:30–42. doi: 10.1002/0470846631.ch3. [DOI] [PubMed] [Google Scholar]
- Rettmann ME, Han X, Xu C, Prince JL. Automated sulcal segmentation using watersheds on the cortical surface. Neuroimage. 2002;15(2):329–344. doi: 10.1006/nimg.2001.0975. [DOI] [PubMed] [Google Scholar]
- Retzius A. Ueber den Bau der Oberflachenschicht der Grosshirnrinde beim Menschen und bei den Saugethieren. Verh Biol Ver. 1891;3:90–103. [Google Scholar]
- Richman DP, Stewart RM, Hutchinson JW, Caviness VS., Jr Mechanical model of brain convolutional development. Science. 1975;189(4196):18–21. doi: 10.1126/science.1135626. [DOI] [PubMed] [Google Scholar]
- Ridgway S, Houser D, Finneran J, Carder D, Keogh M, Van Bonn W, et al. Functional imaging of dolphin brain metabolism and blood flow. J Exp Biol. 2006;209(Pt 15):2902–2910. doi: 10.1242/jeb.02348. [DOI] [PubMed] [Google Scholar]
- Ruppin E, Schwartz EL, Yeshurun Y. Examining the volume efficiency of the cortical architecture in a multi-processor network model. Biol Cybern. 1993;70(1):89–94. doi: 10.1007/BF00202570. [DOI] [PubMed] [Google Scholar]
- Schaer M, Cuadra MB, Tamarit L, Lazeyras F, Eliez S, Thiran JP. A surface-based approach to quantify local cortical gyrification. IEEE Trans Med Imaging. 2008;27(2):161–170. doi: 10.1109/TMI.2007.903576. [DOI] [PubMed] [Google Scholar]
- Sidman RL, Rakic P. Neuronal migration, with special reference to developing human brain: a review. Brain Res. 1973;62(1):1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6(3):309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol. 2002;44(1):4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Sporns O, Chialvo DR, Kaiser M, Hilgetag CC. Organization, development and function of complex brain networks. Trends Cogn Sci. 2004;8(9):418–425. doi: 10.1016/j.tics.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Stewart RM, Richman DP, Caviness VS., Jr Lissencephaly and Pachygyria: an architectonic and topographical analysis. Acta Neuropathol (Berl) 1975;31(1):1–12. doi: 10.1007/BF00696881. [DOI] [PubMed] [Google Scholar]
- Striedter G. Principles of Brain Evolution. Sinauer; 2005. [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotactic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Stuttgart/New York: Thieme Verlag; 1988. [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen VP, Huttunen M, et al. Genetic influences on brain structure. Nat Neurosci. 2001;4(12):1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Lee AD, Dutton RA, Geaga JA, Hayashi KM, Eckert MA, et al. Abnormal cortical complexity and thickness profiles mapped in Williams syndrome. J Neurosci. 2005;25(16):4146–4158. doi: 10.1523/JNEUROSCI.0165-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Schwartz C, Lin RT, Khan AA, Toga AW. Three-dimensional statistical analysis of sulcal variability in the human brain. J Neurosci. 1996;16(13):4261–4274. doi: 10.1523/JNEUROSCI.16-13-04261.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd PH. A geometric model for the cortical folding pattern of simple folded brains. J Theor Biol. 1982;97(3):529–538. doi: 10.1016/0022-5193(82)90380-0. [DOI] [PubMed] [Google Scholar]
- Toro R, Perron M, Pike B, Richer L, Veillette S, Pausova Z, Paus T. Brain size and folding of the human cerebral cortex. Cerebral Cortex. 2008;18:2352–2357. doi: 10.1093/cercor/bhm261. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385(6614):313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- Welker W. Why does cerebral cortex fissure and fold. In: Jones EG, Peters A, editors. Cerebral Cortex. New York: Plenum Press; 1990. pp. 3–136. [Google Scholar]
- Wen Q, Chklovskii DB. Segregation of the brain into gray and white matter: a design minimizing conduction delays. PLoS Comput Biol. 2005;1(7):e78. doi: 10.1371/journal.pcbi.0010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White Anatomic and functional variability: The effects of filter size in group fMRI data analysis. NeuroImage. 2001;13:577–588. doi: 10.1006/nimg.2000.0716. [DOI] [PubMed] [Google Scholar]
- White T, Andreasen NC, Nopoulos P. Brain Volumes and Surface Morphology in Monozygotic Twins. Cereb Cortex. 2002;12(5):486–493. doi: 10.1093/cercor/12.5.486. [DOI] [PubMed] [Google Scholar]
- White T, Andreasen NC, Nopoulos P, Magnotta V. Gyrification abnormalities in childhood- and adolescent-onset schizophrenia. Biol Psychiatry. 2003;54(4):418–426. doi: 10.1016/s0006-3223(03)00065-9. [DOI] [PubMed] [Google Scholar]
- White T, Hilgetag CC. Gyrification of the Human Brain. In: Nelson CA, Luciana M, editors. Developmental Cognitive Neuroscience. Cambridge, MA: MIT Press; 2008. [Google Scholar]
- White T, Ho BC, Ward J, O’Leary D, Andreasen NC. Neuropsychological performance in first-episode adolescents with schizophrenia: a comparison with first-episode adults and adolescent control subjects. Biol Psychiatry. 2006;60(5):463–471. doi: 10.1016/j.biopsych.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Zilles K, Armstrong E, Moser KH, Schleicher A, Stephan H. Gyrification in the cerebral cortex of primates. Brain Behav Evol. 1989;34(3):143–150. doi: 10.1159/000116500. [DOI] [PubMed] [Google Scholar]
- Zilles K, Armstrong E, Schleicher A, Kretschmann HJ. The human pattern of gyrification in the cerebral cortex. Anat Embryol. 1988;179(2):173–179. doi: 10.1007/BF00304699. [DOI] [PubMed] [Google Scholar]
- Zilles K, Schleicher A, Langemann C, Amunts K, Morosan P, Palomero-Gallagher N, et al. Quantitative analysis of sulci in the human cerebral cortex: Development, regional heterogeneity, gender difference, asymmetry, intersubject variability, and cortical architecture. Hum Brain Mapp. 1997;5:218–221. doi: 10.1002/(SICI)1097-0193(1997)5:4<218::AID-HBM2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]