Abstract
Background
We undertook this study to characterize the relationship between survival of patients with stage IIIB/IV Non-Small Cell Lung Cancer (NSCLC) and pack years of cigarette smoking.
Methods
We analyzed data from patients with stage IIIB/IV NSCLC who had completed a prospective smoking questionnaire. We evaluated the impact of pack years of cigarette smoking, age, sex, Karnofsky Performance Status (KPS), and presence of weight loss >5% on overall survival using univariate and multivariate analyses.
Results
Smoking history and clinical data were available for 2,010 patients with stage IIIB/IV NSCLC (1004 women, 1006 men). Seventy percent (1409) smoked >15 pack years, 13% (270) were former and current smokers who had smoked ≤ 15 pack years, and 16% (331) were never smokers (<100 lifetime cigarettes). Never smokers had a longer median survival relative to former or current smokers (17.8 months vs 11.3 months, log rank p<0.001). Among smokers, patients with ≤ 15 pack year history of smoking had a longer median survival than patients who had smoked > 15 pack years (14.6 months vs 10.8 months, log rank p =0.03). As the number of pack years increased, the median overall survival decreased (log rank p <0.001). Multivariate analysis showed that history of smoking was an independent prognostic factor (Hazard Ratio 1.36; p<0.001).
Conclusions
More cigarette smoking, measured in pack years, was associated with decreased survival after diagnosis of stage IIIB/IV NSCLC. Trials assessing survival in stage IIIB/IV NSCLC should report detailed cigarette smoking history for all patients.
Introduction
Although cigarette smoking causes the majority of new cases of lung cancer in the United States, over 30,000 patients diagnosed with NSCLC each year have never smoked cigarettes. For patients with NSCLC, a history of smoking cigarettes is a negative prognostic factor. 1–16 However, among “smokers,” a history of cigarette smoking can range from patients who smoked a few cigarettes a day for a few years to patients who smoked packs of cigarettes daily for decades.
The importance of amount of cigarette smoking history, as measured by pack years, is clear from our understanding of the epidemiology of epidermal growth factor receptor (EGFR) gene mutations. While somatic EGFR mutations are widely known to be more common in patients with NSCLC who never smoked cigarettes, patients with more limited smoking history are more likely to have EGFR mutations than those with heavy smoking history. 17 In fact, the frequency of EGFR mutations is not significantly different between patients with NSCLC who never smoked and those who smoked cigarettes up to 15 pack years. 17
Patients with stage IV lung adenocarcinoma whose tumors harbor EGFR mutations in exon 19 or 21 have rates of response >70% and prolonged progression free survival after treatment with the EGFR tyrosine kinase inhibitors (TKIs) gefitinib or erlotinib. 18–22 The presence of EGFR mutations predicts response to EGFR TKI therapy better than smoking status and may be a positive prognostic factor in advanced lung adenocarcinoma irrespective of therapy. 18 23
Identification and characterization of prognostic factors for patients with NSCLC is important to allow comparison of patient populations in clinical trials and to help guide therapies for some patients. The best prognostic factor for patients with NSCLC is stage. 24 Among patients with stage IIIB/IV NSCLC, positive prognostic factors include Karnofsky Performance Status (KPS) ≥ 80%, absence of significant weight loss (>5%) and female sex. 25,26 To characterize the relationship between survival and pack years of cigarette smoking, we reviewed prospectively collected smoking data, clinical characteristics, and outcome data for patients with stage IIIB/IV NSCLC.
Patients and Methods
Study Design and Patients
All patients evaluated by the Thoracic Oncology Service at Memorial Sloan – Kettering Cancer Center (MSKCC) complete a prospectively administered smoking questionnaire as part of the standard clinical assessment. Using the smoking questionnaire the number of pack years was determined for patients with stage IIIB/IV NSCLC. This cohort includes patients with Stage IIIB/IV disease at initial diagnosis and patients diagnosed with Stage IV NSCLC at the time of recurrent disease after previous surgery or radiation. From the medical record, we also obtained data for sex, race/ethnicity, age, KPS and presence of weight loss >5% within 6 months of the initial visit. This review of records was done under a waiver of authorization approved by the MSKCC Institutional Review Board and Privacy Board.
Patients were categorized as never smokers if they smoked less than 100 cigarettes. Former smokers had quit at least 1 year prior to the visit. Current smokers continued to smoke or quit less than one year prior to the visit. Race and ethnicity were reported by the patient.
Statistical Analysis
Differences in clinical characteristics among smoking groups (never, ≤ 15 pack year, and >15 pack year) were tested using Chi-square test for categorical variable and ANOVA for continuous variable. Overall survival time was measured from the date of diagnosis of stage IIIB/IV NSCLC until the date of death. Living patients were censored at the date of last evaluation at the institution. Survival data were obtained using the medical record and the Social Security death index. Survival status was updated in March 2008. Survival probabilities were calculated by the Kaplan-Meier method and compared among different groups using the log-rank test. Univariate and multivariate Cox regression analyses were performed to identify variables with independent prognostic significance. Statistical analyses were done using SAS (SAS Institute, Inc., Cary, NC) software.
Results
Patient Characteristics
We evaluated 2010 patients with stage IIIB/IV NSCLC between June 2003 and March 2006. The demographic characteristics of the patients are summarized in Table 1. Never smokers comprised 16% of our cohort, with current and former smokers representing 29% and 54% of the patients respectively. Former and current smokers were subdivided into patients who had smoked ≤ 15 pack years and >15 pack years. 17 Never smokers and former or current smokers with ≤ 15 pack years of smoking were younger than patients with >15 pack year smoking history (median ages 59, 60 and 65 respectively; p<0.001) and more likely to be women (66%, 60% and 44% respectively; p<0.001). The majority of the patients were white. The largest proportion of Asian patients was among never smokers (15%) compared to ≤ 15 pack year (6%) and >15 pack year (1%) groups.
Table 1.
Patients Characteristics
| Never n (%) |
≤15 pack years n (%) |
>15 pack years n (%) |
p | |
|---|---|---|---|---|
| 331 (16) | 270 (13) | 1409 (70) | ||
| Age (years) | <0.001 | |||
| Median | 59 | 60 | 65 | |
| Range | 24–93 | 31–87 | 33–90 | |
| Sex | <0.001 | |||
| Male | 114 (34) | 109 (40) | 783 (56) | |
| Female | 217 (66) | 161 (60) | 626 (44) | |
| Race | ||||
| White | 254 (77) | 229 (85) | 1316 (93) | <0.001 |
| Asian | 48 (15) | 16 (6) | 17 (1) | |
| Black | 21 (6) | 21 (8) | 63 (4) | |
| Other/unknown | 8 (2) | 4 (1) | 13 (1) | |
| Histologic Subtype | <0.001 | |||
| Adenocarcinoma | 227(69) | 166(61) | 695 (49) | |
| Squamous cell carcinoma | 13 (4) | 13 (5) | 171 (12) | |
| NSCLC | 91(27) | 91(34) | 543 (39) | |
| Karnofsky Performance Status | 0.02 | |||
| ≥80% | 203 (61) | 166 (61) | 779 (55) | |
| 70% | 61 (18) | 34 (13) | 279 (20) | |
| <70% | 29 (9) | 21 (8) | 153 (11) | |
| Not recorded | 38 (11) | 49 (18) | 198 (14) | |
| Stage | 0.76 | |||
| IIIB | 67 (20) | 51 (19) | 296 (21) | |
| IV | 264 (80) | 219 (81) | 1113 (79) | |
| Weight loss | 0.10 | |||
| >5% | 89 (27) | 88 (33) | 481(34) | |
| ≤5% | 188 (57) | 146 (54) | 750 (53) | |
| Not recorded | 54 (16) | 36 (13) | 178 (13) | |
Survival Analysis
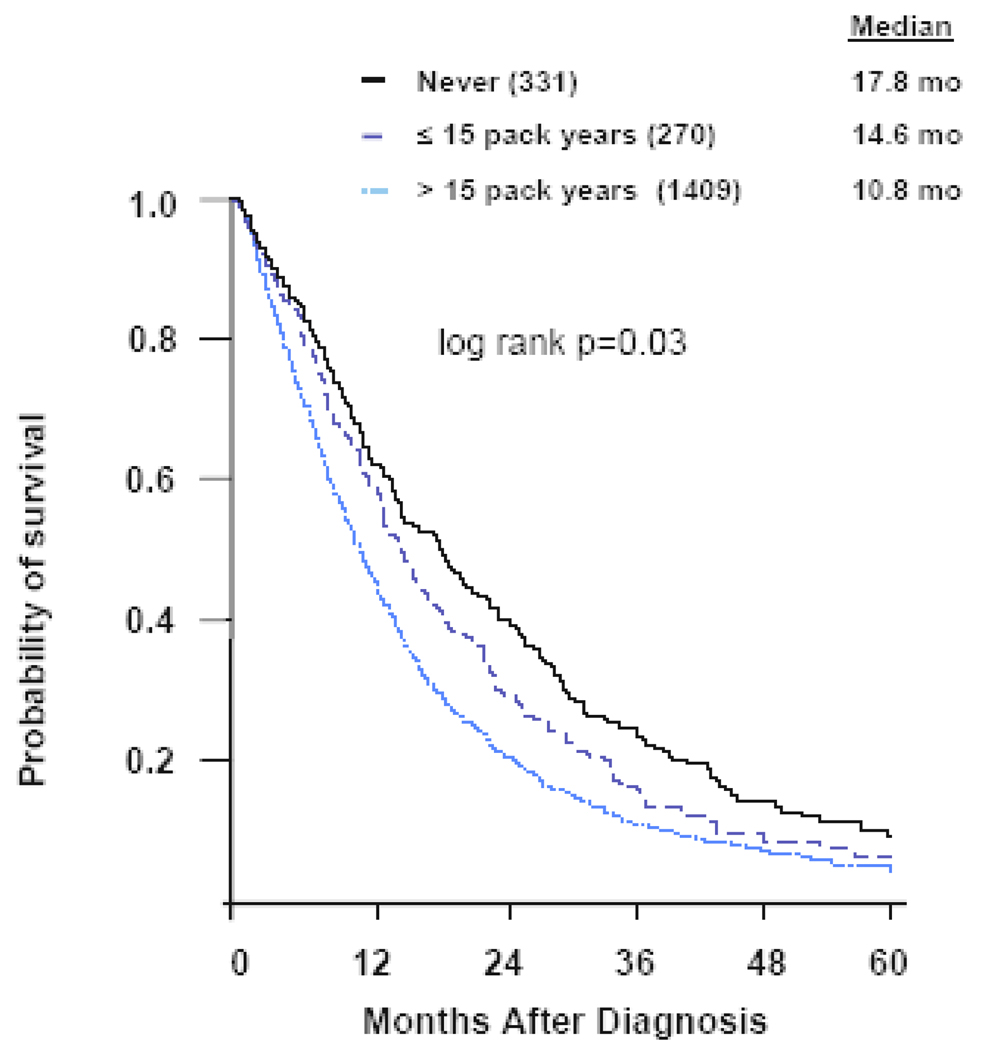
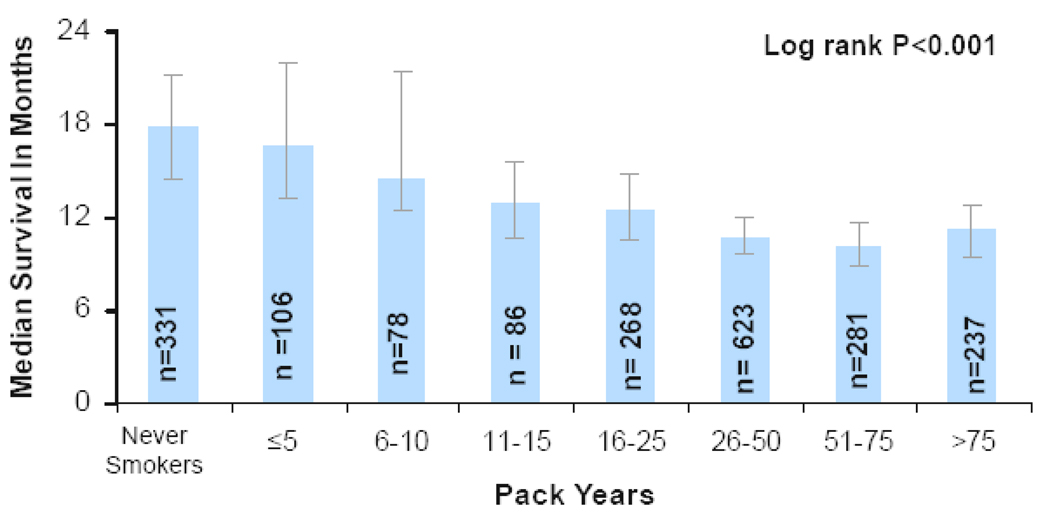
A total of 1568 deaths (78%) had occurred at the time of analysis. Never smokers had a longer median survival than former and current smokers (17.8 months vs 11.3 months; log rank p<0.001; Figure 1). Never smokers had a 2-year survival rate of 39% compared to 21% for former and current smokers (log rank p<0.001; Figure 1). The effect on survival of the amount of cigarette smoking was apparent when we subdivided the former and current smokers into ≤ 15 pack year and >15 pack year groups. Never smokers had a 3 month longer median survival than smokers who smoked ≤ 15 pack years, and 7 month longer survival than those who smoked >15 pack years (17.8 months vs 14.6 months vs 10.8 months; log rank p=0.03; Figure 2). Similarly, the 2-year survival rate was greatest for never smokers, as compared to ≤ 15 pack year and >15 pack year smokers (39% vs 29% vs 20%; log rank p=0.03). We further subdivided former and current smokers into groups by smaller increments of pack years smoked. Due to the large number of subgroups the survival data is presented as median survivals (Figure 3). As the number of pack years increased, the median survival decreased. In univariate analysis, including weight loss, sex, age, KPS, and smoking history, smoking history predicted overall survival with a hazard ratio similar to other known prognostic factors (Table 2). In a multivariate analysis (Table 3), including sex, age, KPS, and weight loss, cigarette smoking history was an independent prognostic factor.
Figure 1.
Overall survival for never vs former and current smokers
Figure 2.
Overall survival by pack years of cigarettes smoked
Figure 3.
Median Survival by Pack Years of Cigarette Smoking
Table 2.
Univariate Survival Analysis
| Variable | Unadjusted HR (95%CI) |
p |
|---|---|---|
| Weight loss >5% vs ≤ 5% |
1.64 (1.47 to 1.83) |
<0.001 |
| Male vs. Female |
1.31 (1.19 to 1.45) |
<0.001 |
| Age (10 year increments) | 1.10 (1.05 to 1.15) |
<0.001 |
| Karnofsky Performance Status (10% increments) |
0.46 (0.34 to 0.64) |
<0.001 |
| Smoking Status | ||
| Current + Former vs. Never |
1.50 (1.30 to 1.72) |
<0.001 |
| ≤ 15 pack year vs. Never |
1.21 (1.00 to 1.46) |
0.05 |
| > 15 pack year vs. <15 pack year |
1.30 (1.12 to 1.51) |
< 0.001 |
| > 15 pack year vs. Never |
1.57 (1.36 to 1.80) |
<0.001 |
HR, hazard ratio. HR >1.00 indicates worse survival.
Table 3.
Multivariate Survival Analysis
| Smoking Status | Adjusted HR* (95%CI) |
p |
|---|---|---|
| Current + Former vs. Never | 1.36 (1.16 to 1.60) | <0.001 |
| ≤ 15 pack year vs. Never | 1.15 (0.92 to 1.43) | 0.22 |
| >15 pack years vs. ≤15 pack years | 1.23 (1.03 to 1.48) | 0.02 |
| >15 pack year vs. Never | 1.42 (1.20 to 1.67) | <0.001 |
HR, hazard ratio
Adjusted for age, sex, Karnofsky Performance Status, and weight loss > 5%. HR >1.00 indicates worse survival.
Discussion
NSCLC in never smokers has a distinct biology, natural history, and responsiveness to EGFR tyrosine kinase inhibitor (EGFR TKI) therapy. 27 Although multiple previous studies have demonstrated the negative effects of smoking on patients with NSCLC, these results are confounded by several factors. 1–16 In some reports, never smokers accounted for a small proportion of patients. 6,10 While most reports defined never smokers as individuals who smoked <100 cigarettes, some previous studies have used inconsistent definitions of never smokers. 5–8,15 In other studies, retrospective chart reviews did not include a detailed dose quantification by pack years of cigarettes smoked.3,6–8,11,13,15,16 Most studies include a heterogeneous population that includes all stages and types of lung cancer. 1,3,5,6,8,10,11,15 Small-cell lung cancer, a disease with a different natural history from NSCLC, has even been included in some analyses. 6,10
In contrast, our cohort consists exclusively of patients with stage IIIB/IV NSCLC. We obtained detailed smoking history data by using a prospectively administered questionnaire. With this large cohort of North American patients with advanced NSCLC, we demonstrated an inverse relationship between the amount of cigarette smoking and survival. Our analysis is unique because it demonstrates that the amount of cigarette smoking is important.
While the large number of patients with clearly documented cigarette smoking history and a uniform stage of disease make this data powerful, there are limitations to our analysis. Current and former smokers are grouped together in this analysis. While a number of other studies have shown that lung cancer patients who continue to smoke have a poorer outcome 6,8–10,14, our analysis is the first to highlight a significant dose-response relationship between smoking and survival. Patients in this cohort received a wide variety of therapies, raising the possibility that the outcomes could have been affected by the particular therapies administered. Scagliotti et al noted that never smokers with NSCLC who received one of two cisplatin-based doublet chemotherapies had a five-month better median survival in comparison to former or current smokers, supporting a survival difference based on smoking status and independent of treatment type. 28 Since patients with a minimal smoking history are more likely to carry sensitizing EGFR mutations and therefore respond to erlotinib or gefitinib, there is the possibility that the effect on overall survival is a result of EGFR tyrosine kinase inhibitors. However, earlier patient cohorts studied by Nordquist et al and Marks et al that demonstrated survival differences based on cigarette use were not treated with EGFR TKIs. 8,29 Finally, we have not carried out a systematic evaluation of the medical co-morbidities for these patients. We have accounted for these issues by adjusting for performance status, age, and weight loss through the multivariate analysis. The uniformly short overall survival for patients with advanced non-small cell lung cancer makes it unlikely that medical co-morbidities alone could account for the findings observed here. Toh et al showed that there was no significant association between presence of comorbidities and survival on univariate and multivariate analyses. 11 Finally, in the Nordquist study, the improvements in overall survival seen with never smokers were the same as the improvements in cancer-specific survival. 8
These results, together with published studies, emphasize the negative prognostic effects of cigarette smoking in patients with non-small cell lung cancer. 1–16 We have demonstrated for the first time that an inverse relationship exists between the extent of cigarette smoking and survival. These data suggest that NSCLC in never smokers is one end of a spectrum, demonstrating the negative prognostic impact of cigarette smoking. Based on these findings, we recommend that in clinical trials, when an imbalance could exist in the amount of cigarettes smoked by patients in the comparison arms, investigators should stratify patients by smoking status. All clinical trials assessing survival in patients with stage IIIB/IV NSCLC should report the cigarette smoking history for all patients.
Acknowledgments
Supported, in part, by NIH 5T32CA009207
Footnotes
Presented in part at the ASCO Annual Meeting, Chicago, May 30-June 3, 2008.
References
- 1.Sobue T, Suzuki T, Fujimoto I, et al. Prognostic factors for surgically treated lung adenocarcinoma patients, with special reference to smoking habit. Jpn J Cancer Res. 1991;82:33–39. doi: 10.1111/j.1349-7006.1991.tb01742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujisawa T, Iizasa T, Saitoh Y, et al. Smoking before surgery predicts poor long-term survival in patients with stage I non-small-cell lung carcinomas. J Clin Oncol. 1999;17:2086–2091. doi: 10.1200/JCO.1999.17.7.2086. [DOI] [PubMed] [Google Scholar]
- 3.Martins SJ, Pereira JR. Clinical factors and prognosis in non-small cell lung cancer. Am J Clin Oncol. 1999;22:453–457. doi: 10.1097/00000421-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Myrdal G, Lambe M, Gustafsson G, et al. Survival in primary lung cancer potentially cured by operation: influence of tumor stage and clinical characteristics. Ann Thorac Surg. 2003;75:356–363. doi: 10.1016/s0003-4975(02)04321-7. [DOI] [PubMed] [Google Scholar]
- 5.Tan YK, Wee TC, Koh WP, et al. Survival among Chinese women with lung cancer in Singapore: a comparison by stage, histology and smoking status. Lung Cancer. 2003;40:237–246. doi: 10.1016/s0169-5002(03)00038-2. [DOI] [PubMed] [Google Scholar]
- 6.Tammemagi CM, Neslund-Dudas C, Simoff M, et al. Smoking and lung cancer survival: the role of comorbidity and treatment. Chest. 2004;125:27–37. doi: 10.1378/chest.125.1.27. [DOI] [PubMed] [Google Scholar]
- 7.Toh CK, Wong EH, Lim WT, et al. The impact of smoking status on the behavior and survival outcome of patients with advanced non-small cell lung cancer: a retrospective analysis. Chest. 2004;126:1750–1756. doi: 10.1378/chest.126.6.1750. [DOI] [PubMed] [Google Scholar]
- 8.Nordquist LT, Simon GR, Cantor A, et al. Improved survival in never-smokers vs current smokers with primary adenocarcinoma of the lung. Chest. 2004;126:347–351. doi: 10.1378/chest.126.2.347. [DOI] [PubMed] [Google Scholar]
- 9.Sardari Nia P, Weyler J, Colpaert C, et al. Prognostic value of smoking status in operated non-small cell lung cancer. Lung Cancer. 2005;47:351–359. doi: 10.1016/j.lungcan.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Ebbert JO, Williams BA, Sun Z, et al. Duration of smoking abstinence as a predictor for non-small-cell lung cancer survival in women. Lung Cancer. 2005;47:165–172. doi: 10.1016/j.lungcan.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 11.Toh C-K, Gao F, Lim W-T, et al. Never-Smokers With Lung Cancer: Epidemiologic Evidence of a Distinct Disease Entity. J Clin Oncol. 2006;24:2245–2251. doi: 10.1200/JCO.2005.04.8033. [DOI] [PubMed] [Google Scholar]
- 12.Yoshino I, Kawano D, Oba T, et al. Smoking status as a prognostic factor in patients with stage I pulmonary adenocarcinoma. Ann Thorac Surg. 2006;81:1189–1193. doi: 10.1016/j.athoracsur.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 13.Tsao AS, Liu D, Lee JJ, et al. Smoking affects treatment outcome in patients with advanced nonsmall cell lung cancer. Cancer. 2006;106:2428–2436. doi: 10.1002/cncr.21884. [DOI] [PubMed] [Google Scholar]
- 14.Sawabata N, Miyoshi S, Matsumura A, et al. Prognosis of smokers following resection of pathological stage I non-small-cell lung carcinoma. Gen Thorac Cardiovasc Surg. 2007;55:420–424. doi: 10.1007/s11748-007-0159-x. [DOI] [PubMed] [Google Scholar]
- 15.Bryant A, Cerfolio RJ. Differences in epidemiology, histology, and survival between cigarette smokers and never-smokers who develop non-small cell lung cancer. Chest. 2007;132:185–192. doi: 10.1378/chest.07-0442. [DOI] [PubMed] [Google Scholar]
- 16.Yano T, Miura N, Takenaka T, et al. Never-smoking nonsmall cell lung cancer as a separate entity: clinicopathologic features and survival. Cancer. 2008;113:1012–1018. doi: 10.1002/cncr.23679. [DOI] [PubMed] [Google Scholar]
- 17.Pham D, Kris MG, Riely GJ, et al. Use of cigarette-smoking history to estimate the likelihood of mutations in epidermal growth factor receptor gene exons 19 and 21 in lung adenocarcinomas. J Clin Oncol. 2006;24:1700–1704. doi: 10.1200/JCO.2005.04.3224. [DOI] [PubMed] [Google Scholar]
- 18.Mok T, Wu Y, Thongprasert S, et al. Phase III, randomised, open-label, first-line study of gefitinib vs carboplatin / paclitaxel in clinically selected patients with advanced non-small-cell lung cancer (IPASS). European Society of Medical Oncology (ESMO) meeting Stockholm; 2008. [DOI] [PubMed] [Google Scholar]
- 19.Paz-Ares L, Sanchez JM, Garcia-Velasco A. A prospective phase II trial of erlotinib in advanced non-small cell lung cancer (NSCLC) patients (p) with mutations in the tyrosine kinase (TK) domain of the epidermal growth factor receptor (EGFR). In: Grunberg SM, et al., editors. Am Soc Clin Oncol; 2006 ASCO Annual Meeting; Atlanta,GA. 2006. p. 369s. [Google Scholar]
- 20.Pignon JP, Tribodet H, Scagliotti GV, et al. Lung Adjuvant Cisplatin Evaluation (LACE): A pooled analysis of five randomized clinical trials including 4,584 patients. J Clin Oncol (Meeting Abstracts) 2006;24:7008. [Google Scholar]
- 21.Sunaga N, Yanagitani N, Kaira K, Tomizawa Y, Iijima H, Otani Y, Tanaka S, Suga T, Dobashi K, Mori M. Phase II study of the efficacy of gefitinib in patients with non-small cell lung cancer with the EGFR mutations. J Clin Oncol. 2006;24 [Google Scholar]
- 22.Sutani A, Nagai Y, Udagawa K, Uchida Y, Murayama Y, Tanaka T, Miyazawa H, Kanazawa M, Hagiwara K, Kobayashi K. Phase II study of gefitinib for non-small cell lung cancer (NSCLC) patients with epidermal growth factor receptor (EGFR) gene mutations detected by PNA-LNA PCR clamp. J Clin Oncol. 2006;24 doi: 10.1038/sj.bjc.6603466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the Epidermal Growth Factor Receptor and in KRAS Are Predictive and Prognostic Indicators in Patients With Non-Small-Cell Lung Cancer Treated With Chemotherapy Alone and in Combination With Erlotinib. J Clin Oncol:JCO. 2005 doi: 10.1200/JCO.2005.02.857. 2005.02.857. [DOI] [PubMed] [Google Scholar]
- 24.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 25.Finkelstein D, Ettinger D, Ruckdeschel J. Long-term survivors in metastatic non-small cell lung cancer: An eastern cooperative oncology group study. J Clin Oncol. 1986;4:702–709. doi: 10.1200/JCO.1986.4.5.702. [DOI] [PubMed] [Google Scholar]
- 26.O'Connell JP, Kris MG, Gralla RJ, et al. Frequency and prognostic importance of pretreatment clinical characteristics in patients with advanced nonsmall-cell lung cancer treated with combination chemotherapy. J Clin Oncol. 1986;4:1604–1614. doi: 10.1200/JCO.1986.4.11.1604. [DOI] [PubMed] [Google Scholar]
- 27.Miller VA, Kris MG, Shah N, et al. Bronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced nonsmall-cell lung cancer. J Clin Oncol. 2004;22:1103–1109. doi: 10.1200/JCO.2004.08.158. [DOI] [PubMed] [Google Scholar]
- 28.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 29.Marks JL, Broderick S, Zhou Q, et al. Prognostic and therapeutic implications of EGFR and KRAS mutations in resected lung adenocarcinoma. J Thorac Oncol. 2008;3:111–116. doi: 10.1097/JTO.0b013e318160c607. [DOI] [PubMed] [Google Scholar]