Abstract
With an ever growing population of aged individuals who are at risk of developing Alzheimer disease (AD), there is an urgent need for a sensitive, specific, and preferably non-invasive diagnostic standard of disease progression. Mainstream thinking suggests that early intervention is key to maximizing the opportunity for a successful treatment regimen in AD and, as such, an early and accurate means of diagnosis is essential. In this study, we applied a recently described antibody-antigen dissociation technique to samples obtained as part of a population-based analysis of the prevalence of AD. Stratified sampling and random selection strategies were combined to obtain a representative population for screening of individuals older than 55 years. Serum antibodies to amyloid-β (Aβ)1–42 were measured before and after antigen dissociation. The difference between the two measurements was indicated as the dissociation delta (Δ). Our analyses showed that the levels of dissociated antibody in AD patients were always significantly different from controls and that levels of Aβ antibody after dissociation, but not non-dissociated levels, correlated negatively (p<0.05) with both duration of the disease and age in the AD patients. Moreover, the change in concentration of Aβ antibody from pre- to post-dissociation (i.e., the dissociation Δ) directly reflected the progression of AD in terms of both time since diagnosis and age of the patients, with a lower dissociation Δ indicating a more advanced stage of AD. Ultimately, these data suggest that dissociated Aβ antibody levels are of significant diagnostic value at the onset of the neurodegenerative process and, thereafter, may be a useful biomarker for disease progression.
Keywords: Alzheimer disease, amyloid-β, antigen-antibody complexes, biomarker, diagnosis
Introduction
Amyloid-β (Aβ) is the major protein component of senile plaques that accumulate in specific brain regions of patients with Alzheimer disease (AD), and its presence is used for a definitive postmortem diagnosis of the disorder (Andreasen et al., 1999; Andreasen et al., 2001; Castellani et al., 2006; McKeel et al., 2004; Murayama and Saito, 2004). Unfortunately, current treatments provide only symptomatic relief that provides extremely limited impact on disease progression (Marlatt et al., 2008). Further, disease modifying clinical trials are often confounded by using patients at more advanced stages of disease. Therefore, it is currently thought that early treatment may provide greater opportunity for therapeutic efficacy. Unfortunately, such early diagnosis has been hampered by a lack of a diagnostic standard. Similarly, treatment efficacy in clinical trials relies on subjective changes since there are no current biomarkers of disease progression.
Given these voids, considerable efforts over the last decade have been expended on developing tools for the early diagnostic of disease and/or biomarkers of disease progression. Most efforts in this regard have focused on serum or cerebrospinal fluid (CSF) levels of amyloid-β (Aβ) or antibodies to Aβ (Kester et al., 2009; Pauwels et al., 2009; Verbeek et al., 2009; Verwey et al., 2009) and have been unequivocal with conflicting data with little diagnostic predictive value (Blennow, 2004). Unfortunately, ignored in most studies is the fact that, in biological fluids, antibodies and antigens are in a state of dynamic equilibrium between bound and unbound forms that is concentration dependent. Although the amount of antibody against a particular antigen in a given disease state may be strongly elevated, only a fraction of the total amount is likely detectable by ELISA (enzyme linked immunosorbentassay) due to interference by these antigen-antibody complexes. In this regard, previously we demonstrated the importance of measuring total amounts of antibody following dissociation of the antigen-antibody complexes and, by using both in vitro and in vivo approaches, we provided an explanation of the discrepancy in existing data and provided a novel diagnostic strategy (Gustaw et al., 2008a). Similar approaches were employed in a transgenic animal model of AD (Li et al., 2004).
In this study, we expand upon the use of this technique for the assessment of Aβ antibody levels in sera collected from a population-based series of newly diagnosed AD subjects and controls. Our findings show that dissociated Aβ antibody levels and the dissociation Δ (dissociated minus non-dissociated) may be useful tools for the reliable and early diagnosis of disease as well as for providing an assessment of disease progression. As such, this technique may be useful in monitoring therapeutic efficacy in clinical trials.
Patients and Methods
Population based sampling design
The current study is a part of a large population-based study named BERCAL (Badanie Epidemiologiczne Rozpowszechniena Choroby Alzheimera i innych form demencji w województwie Lubelskim).
The participants of the BERCAL study were randomly selected from the population-based sample within the Lublin Region of Poland composed of 2,182,191 inhabitants in the year 2005 when the population was sampled. As a result of the project, the prevalence of AD in Lublin’s Region Poland was calculated as 1634.6/100,000 inhabitants (Gustaw et al., 2008b). The BERCAL study design is described in more detail elsewhere (Gustaw et al., 2008b), yet it is important to mention that dementia was diagnosed by the Diagnostic and Statistical Manual of Mental Disorders 4th edition (DSM-IV) criteria (American Psychiatric Association, 2000) and AD was diagnosed in accordance to the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association (NINCDS - ADRDA) criteria (McKhann et al., 1984).
Normal control subjects were recruited from the same population. Control participants had normal cognition excluding mild cognitive impairment (MCI).
The study was carried out in accordance with the local IRB agreement. Written informed consent was obtained from the patient (if possible), the caregiver, and/or the patient's representative (if applicable) before beginning detailed screening activities.
In vivo dissociation of Aβ antibody in human sera
The technique for the in vitro dissociation of Aβ antibodies has been previously described in detail and shown to be reliable and reproducible (Gustaw et al., 2008a). Briefly, sera (AD, n=48 and healthy, age- and gender-matched controls, n=20) were diluted 1:100 with dissociation buffer (PBS buffer with 1.5% BSA and 0.2 M glycine-acetate pH 2.5) to a 500 µl final volume and incubated for 20 min at room temperature (RT). The sera were then pipetted into the sample reservoir of Microcon centrifugal filter device, YM-10 (10,000 MW cut-off; Millipore) and centrifuged at 14,100 rpm for 20 min at RT. The sample reservoir was then separated from the flowthrough, which was inverted into a second tube and centrifuged at 5000 rpm for 3 min at RT. The collected solution containing the antibody dissociated from the Aβ peptide immediately was adjusted to pH 7.0 with 1 M Tris buffer, pH 9.0. The retentate volume was reconstituted to the initial volume (500 µl) with ELISA dilution buffer (PBS with 1.5% BSA and 0.1% Tween-20). The collected sera were then added to an ELISA plate at several dilutions to determine the antibody titer. As a control, the same serum was treated in an identical process except that the sera were diluted into buffer at pH 7.0 instead of dissociation buffer, pH 2.5. The difference between the dissociated sera and the corresponding non-dissociated sera was analyzed as the dissociation Δ.
Measurements of antibody titers by ELISA
NUNC Maxisorp 96 well ELISA plates were coated with 50 µl/well Aβ1–42 5µg/ml in PBS, pH 7 and incubated overnight at 4°C. Plates were washed 5 times with washing buffer (0.45% BSA + 0.05% Tween-20), and then blocked (300 µl/well) for one hour at 37°C with 1.5% BSA + 0.05% Tween 20 in PBS. Following blocking, the plates were washed 4 times with washing buffer and samples applied (50 µl/well) in duplicate or triplicate and incubated at 37°C for one hour. The plates were then washed 10 times with washing buffer. Anti-human IgG (H+L) antibody (Southern Biotechnology Associates Inc.) was diluted 1:2500 and added at 50 µl/well.
Samples in quadruplicate were incubated for 1 hour at 37°C. After incubation, the plates were washed 10 times, developed with TMB (Pierce 1-step Ultra, Sigma), the reaction stopped with 2M sulfuric acid (50 µl) and the plates were analyzed spectrophotometrically at 450 nm.
Statistical Analysis
Quadruplicates of each sample were analyzed using the student’s t-test to obtain mean values and standard deviation for further analysis. Mann-Whitney test and Pearson Product Moment Correlation were used to compare and correlate outcome parameters respectively.
Statistical significance was marked when Power of the test was > 0.8 for alpha=0.05. Sigma Stat 3.0 for Windows was used for statistical analyses. All p values <0.05 were regarded as statistically significant.
Results
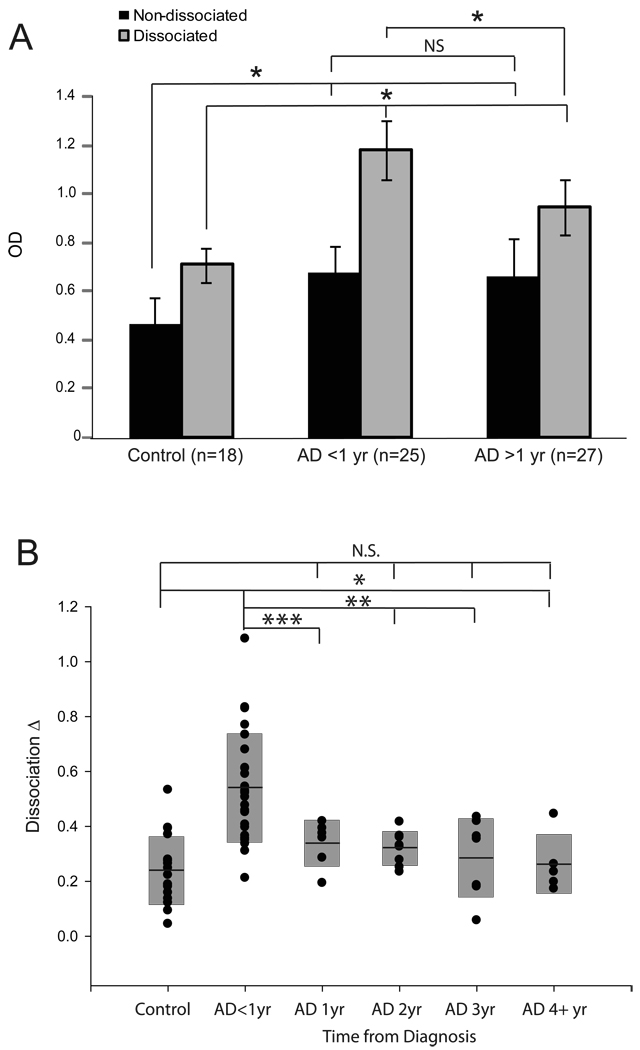
Sera collected from newly diagnosed AD patients and age-matched controls were analyzed for antibodies against Aβ both before and after dissociation of Aβ. Statistical analysis shows significantly greater antibody levels both before and after dissociation in AD groups when compared to control (Figure 1A,B). As expected, within the control group, differences in antibody level were statistically greater after dissociation, however, within the AD group, these differences were even larger (median O.D. 1.07 vs 0.7 respectively, P<0.001) (Figure 1B). In fact, the dissociation Δ, the difference between antibody levels after and before dissociation, was much greater in AD than in controls (median difference 0.4 vs 0.2, P<0.001) (Figure 1B) and also was related to disease progression with newly diagnosed cases.
Figure 1.
Serum Aβ antibodỵ levels were measured in control cases (n=18), cases having an AD diagnosis of less than 1 year (n=25), and AD cases with an AD diagnosis greater than 1 year (n=27) before and after antibody dissociation. A. Both groups of AD patients had significantly higher levels of serum Aβ antibodies compared to controls both before and after dissociation. Before dissociation, the levels were unchanged between the AD groups with a diagnosis of either <1 year or >1 year, yet, following dissociation, the levels in cases with a diagnosis of less than 1 year were significantly higher compared to the other AD cases. B. Closer examination of the antibody levels before and after dissociation within each case (the Dissociation Δ), revealed only the AD cases with a diagnosis of <1 year were significantly different from control. All other AD patients divided according to years since diagnosis showed no differences from control based on the Dissociation Δ, yet were different from the AD cases diagnosed <1 year. (NS= not significant; *= p<0.001; **=p<0.01; ***p<0.05).
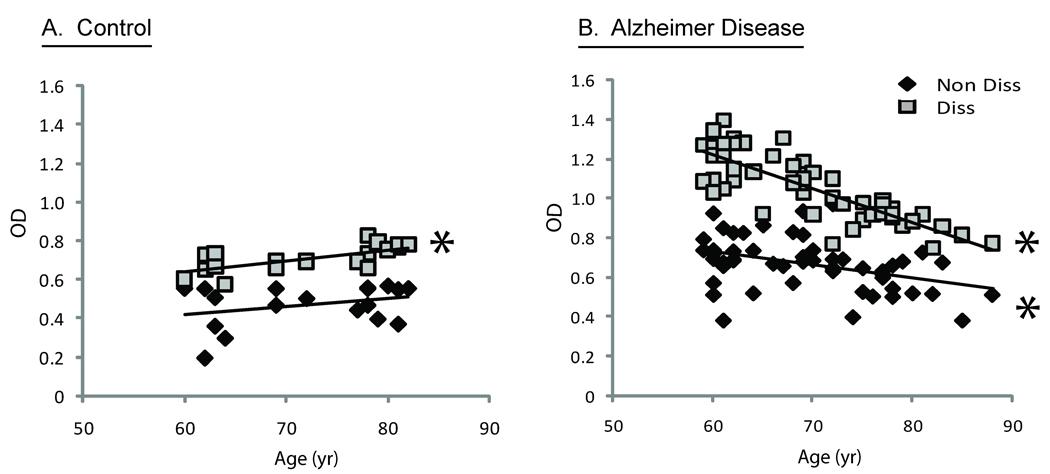
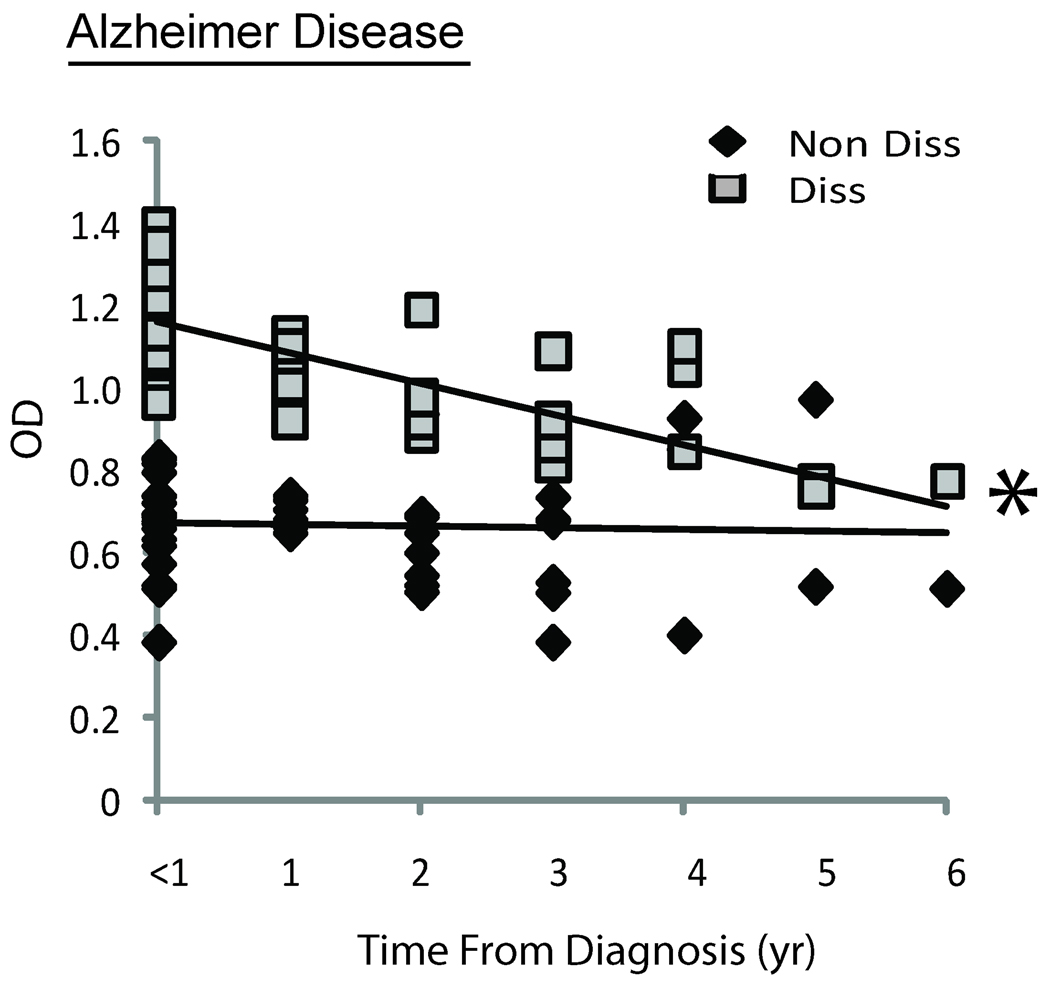
The level of Aβ antibodies was correlated with other patient and disease parameters. Both the age of the patients examined (Figure 3B) and the time from when the first clinical symptoms were noticed (Figure 2), which approximately reflected the duration of the disease, were negatively correlated with levels of Aβ1–42 after dissociation in the AD patients (correlation coefficient: −0.7 and −0.5, p<0.05). Moreover, the difference in antibody levels after dissociation (i.e., dissociation Δ) was the greatest in patients with less than one year following their clinical diagnosis (Figure 1B). The level of antibody detected after dissociation, however, did not correlate with age in the controls (Figure 3A), and the levels of antibody both before and after dissociation in both AD and control groups were not statistically correlated to Mini-Mental Status Examination (Figure 4A,B).
Figure 3.
Serum Aβ antibodies in control patients were plotted versus age. Following dissociation, there is a significant positive correlation (p<0.XX) (A). Serum Aβ antibodies in AD patients both before and after dissociation reveal significant negative correlation with age (B).
Figure 2.
Serum Aβ antibodies before and after dissociation in AD patients with a diagnosis of less than 1 year to 6 years. Notably, only the dissociated antibody levels are significantly correlated with time since diagnosis (p<0.01).
Figure 4.
Serum Aβ antibodies measure both before and after dissociation are not correlated with Mini-Mental Status Examination scores in either control (A) or AD (B) patients.
Discussion
To date, there has been considerable variation, with often contradictory data, in studies probing the potential for AD diagnosis using serum Aβ antibody levels. On the one hand, a number of reports describe that patients with AD have lower levels of Aβ serum antibodies as compared to age-matched controls (Du et al., 2001; Weksler et al., 2002), while other studies indicate the opposite: the level of anti- Aβ antibodies may be much higher in AD as compared to control subjects (Mruthinti et al., 2004). Similarly, although our study did not show, like many others, Aβ antibody titers have been previously reported to be negatively correlated to cognitive status, with more cognitively-impaired individuals exhibiting higher anti-Aβ titers (Mruthinti et al., 2004). Such discrepancies in data attest to the fact that the immunochemical measurement of plasma Aβ is complex and requires sufficient and particular analysis. Specifically, variables such as the binding of Aβ to other proteins (Biere et al., 1996; Sagare et al., 2007), its tendency to aggregate (Fullwood et al., 2006), and its strong affinity for Aβ antibodies (Gustaw et al., 2008a; Oh et al., 2008) must be taken into account, and in this study, our dissociation technique does just that.
In our previous in vitro experiments (Gustaw et al., 2008a), Aβ1–42 antigen effectively blocked antibody detection, yet following dissociation of the complex with low pH buffer, detection of the Aβ antibody was completely restored. This relatively simple method involved passing the Aβ peptide through a filter after its dissociation from the antibody, separating the antibody from its antigen, and preventing any rebinding, thus allowing it to be detectable at greater levels after dissociation in vitro. Ultimately, we developed the dissociation technique using human plasma samples from AD patients and age-matched controls (Gustaw et al., 2008a) and found the assay to be simple, fast, reliable, and reproducible.
It has been proposed that during the long preclinical, non-symptomatic phase of AD, the levels of Aβ42 are on average higher than normal (Ida et al., 1996). The formation of Aβ in the brain, as well as the progressive aggregation of Aβ42, then leads to the decline of plasma levels to within normal limits-not significantly different from the population with no AD risk. At the MCI stage, the levels of Aβ42 are high (Quesada et al., 1997), and later in the course of the disease, Aβ42 in plasma decreases, as shown by several studies that reported no difference of Aβ plasma levels between AD patients and controls (Ida et al., 1996). At the MCI stage, the levels of Aβ42 are still high presumably because the amyloid load is generally limited to the mesial temporal lobe (Petersen et al., 2001). A number of studies, however, reported a large variability of Aβ values in plasma that lead to a large overlap between normal controls and MCI, and longitudinal studies in amnestic MCI cases yielded conflicting results on the value of Aβ plasma levels as being predictive of AD (Blasko et al., 2008; Mayeux et al., 2003). According to these results, plasma Aβ levels reflect Aβ metabolism in the brain not only in the early phase of AD, but also in MCI. Bearing in mind the above mentioned data, the level of antibodies to Aβ, before and after disassociation, in MCI cases will be assessed in future studies.
The same process of disease progression, then, may in fact present a low, apparently basal level of Aβ in sera of the patient with advanced AD mainly because of the long process of antibody production and amyloid binding to antigen.
Measurement of Aβ in sera is complicated by the fact that peripheral tissues might contribute to both the circulating amyloid pool and AD pathology within the brain and its vasculature. The wide spread of plasma Aβ values is also due in part to the ability of Aβ to bind to a variety of plasma and membrane proteins. Sources outside the central nervous system (CNS) should therefore be taken into consideration, because any intervention to reduce cerebral Aβ is assessed by monitoring Aβ plasma levels.
Aβ, which accumulates in the cerebral microvessels in an age-dependent manner, plays a key role in the pathogenesis of cerebral amyloid angiopathy (Zhu et al., 2007). Platelets are an important cellular element in vasculopathy of various causes. Aβ may activate or potentiate platelet aggregation. Exogenous Aβ potentiates platelet aggregation caused by collagen and other agonists. At higher concentrations, Aβ induces platelet aggregation, which is accompanied by an increase in thromboxane A2 formation. These actions of Aβ on platelets are causally related to Aβ activation of p38 mitogen-activated protein kinase (MAPK) (Cattabeni et al., 2004).
Roher et al. (2009) evaluated the amounts of Aβ peptides in the CNS and in reservoirs outside the CNS and their potential impact on Aβ plasma levels and AD pathology. Aβ levels were measured in the plasma of AD and non-demented controls in a longitudinal study, the plasma of a cohort of AD patients receiving a cholinesterase inhibitor, and the skeletal muscle, liver, aorta, platelets, leptomeningeal arteries, and in gray and white matter of AD and control subjects. Plasma Aβ levels fluctuated over time and among individuals, suggesting continuous contributions from brain and peripheral tissues and associations with reactive circulating proteins. Arteries with atherosclerosis had larger amounts of Aβ1–40 than disease-free vessels. Inactivated platelets contained more Aβ peptides than activated ones. All the measurements show, AD brain and skeletal muscle contained increased levels of Aβ (Roher et al., 2009).
In this current work, the dissociation assay was performed on newly diagnosed AD and control plasma samples obtained as a part of a BERCAL study protocol, where patients were recruited from the sample population. The experimental group was selected from those patients making initial visits following first signs of cognitive loss. The phenomenon of increasing antibody titers after dissociation was confirmed (Figure 1). Using this methodology, we previously reported that only following dissociation were the Aβ1–42 antibody levels consistently reliable for distinguishing AD and control. In the present study, this is confirmed, and additional data is presented to show the validity of this assay to scrutinize even newly diagnosed cases, a major step in consideration of early diagnosis.
In accordance with the proposed hypothesis, it was indeed found that the longer the cognitive decline had been present, the lower the levels of Aβ in the human sera (Figure 2). Additionally, older people had lower levels of amyloid detected in sera (Figure 3). These correlations became significant after the process of amyloid dissociation (Figure 1). Thus, the dissociated Aβ1–42 antibody levels are of compelling diagnostic value in the beginning of the neurodegenerative process in AD patients. One conclusion is that early in the AD process, an increase in Aβ production elicits a strong immunological response that could be responsible for the negative correlation of Aβ1–42 antibodies and duration of symptoms.
Thus, evaluation of samples in our study demonstrated the basic premise of the aforementioned hypothesis: Aβ-antigen effectively masks the detection of Aβ auto-antibody, but its dissociation can increase the sensitivity of measurement, most notably when compared to a non-dissociated sample of the same sera. The longer the process lasts, the lower non-dissociated level of binding compounds. Significantly, as predicted by our model (Gustaw et al., 2008a), the increase in Aβ auto-antibody levels in AD cases following dissociation is much greater than that in controls (Figure 1A,B). This study provides an explanation for the variability reported in previous studies using non-dissociated samples as well as providing strong support for the dissociation method. The fact that the effect is dependent on Aβ level and more apparent in affected AD individuals may be of exceptional value in the search for a reliable biomarker.
Acknowledgments
Work in the authors’ laboratories is supported by the National Institutes of Health (AG026151) and the Alzheimer’s Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen N, Hesse C, Davidsson P, Minthon L, Wallin A, Winblad B, Vanderstichele H, Vanmechelen E, Blennow K. Cerebrospinal fluid beta-amyloid(1–42) in Alzheimer disease: differences between early- and late-onset Alzheimer disease and stability during the course of disease. Arch Neurol. 1999;56:673–680. doi: 10.1001/archneur.56.6.673. [DOI] [PubMed] [Google Scholar]
- Andreasen N, Minthon L, Davidsson P, Vanmechelen E, Vanderstichele H, Winblad B, Blennow K. Evaluation of CSF-tau and CSF-Abeta42 as diagnostic markers for Alzheimer disease in clinical practice. Arch Neurol. 2001;58:373–379. doi: 10.1001/archneur.58.3.373. [DOI] [PubMed] [Google Scholar]
- Castellani RJ, Lee HG, Zhu X, Nunomura A, Perry G, Smith MA. Neuropathology of Alzheimer disease: pathognomonic but not pathogenic. Acta Neuropathol (Berl) 2006;111:503–509. doi: 10.1007/s00401-006-0071-y. [DOI] [PubMed] [Google Scholar]
- McKeel DW, Jr, Price JL, Miller JP, Grant EA, Xiong C, Berg L, Morris JC. Neuropathologic criteria for diagnosing Alzheimer disease in persons with pure dementia of Alzheimer type. J Neuropathol Exp Neurol. 2004;63:1028–1037. doi: 10.1093/jnen/63.10.1028. [DOI] [PubMed] [Google Scholar]
- Murayama S, Saito Y. Neuropathological diagnostic criteria for Alzheimer's disease. Neuropathology. 2004;24:254–260. doi: 10.1111/j.1440-1789.2004.00571.x. [DOI] [PubMed] [Google Scholar]
- Marlatt MW, Lucassen PJ, Perry G, Smith MA, Zhu X. Alzheimer's disease: cerebrovascular dysfunction, oxidative stress, and advanced clinical therapies. J Alzheimers Dis. 2008;15:199–210. doi: 10.3233/jad-2008-15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kester MI, Blankenstein MA, Bouwman FH, van Elk EJ, Scheltens P, van der Flier WM. CSF biomarkers in Alzheimer's disease and controls: associations with APOE genotype are modified by age. J Alzheimers Dis. 2009;16:601–607. doi: 10.3233/JAD-2009-0999. [DOI] [PubMed] [Google Scholar]
- Pauwels EK, Volterrani D, Mariani G. Biomarkers for Alzheimer's disease. Drug News Perspect. 2009;22:151–160. doi: 10.1358/dnp.2009.22.3.1354128. [DOI] [PubMed] [Google Scholar]
- Verbeek MM, Kremer BP, Rikkert MO, Van Domburg PH, Skehan ME, Greenberg SM. Cerebrospinal fluid amyloid beta(40) is decreased in cerebral amyloid angiopathy. Ann Neurol. 2009;66:245–249. doi: 10.1002/ana.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwey NA, Veerhuis R, Twaalfhoven HA, Wouters D, Hoozemans JJ, Bollen YJ, Killestein J, Bibl M, Wiltfang J, Hack CE, Scheltens P, Blankenstein MA. Quantification of amyloid-beta 40 in cerebrospinal fluid. J Immunol Methods. 2009;348:57–66. doi: 10.1016/j.jim.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer's disease. NeuroRx. 2004;1:213–225. doi: 10.1602/neurorx.1.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustaw KA, Garrett MR, Lee HG, Castellani RJ, Zagorski MG, Prakasam A, Siedlak SL, Zhu X, Perry G, Petersen RB, Friedland RP, Smith MA. Antigen-antibody dissociation in Alzheimer disease: a novel approach to diagnosis. J Neurochem. 2008a;106:1350–1356. doi: 10.1111/j.1471-4159.2008.05477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Cao C, Chackerian B, Schiller J, Gordon M, Ugen KE, Morgan D. Overcoming antigen masking of anti-amyloidbeta antibodies reveals breaking of B cell tolerance by virus-like particles in amyloidbeta immunized amyloid precursor protein transgenic mice. BMC Neurosci. 2004;5:21. doi: 10.1186/1471-2202-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustaw K, Woznica I, Bylina J. Rozpowszechnienie zespolow otepiennych w tym choroby Alzheimera w populacji mieszkancow wojewodztwa lubelskiego. Medycyna Ogolna. 2008b;4:381–394. [Google Scholar]
- American Psychiatric Association. The diagnostic and statistical manual of mental disorders, text revision (DSM-IV-TR) 4th edition ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Du Y, Dodel R, Hampel H, Buerger K, Lin S, Eastwood B, Bales K, Gao F, Moeller HJ, Oertel W, Farlow M, Paul S. Reduced levels of amyloid beta-peptide antibody in Alzheimer disease. Neurology. 2001;57:801–805. doi: 10.1212/wnl.57.5.801. [DOI] [PubMed] [Google Scholar]
- Weksler ME, Relkin N, Turkenich R, LaRusse S, Zhou L, Szabo P. Patients with Alzheimer disease have lower levels of serum anti-amyloid peptide antibodies than healthy elderly individuals. Exp Gerontol. 2002;37:943–948. doi: 10.1016/s0531-5565(02)00029-3. [DOI] [PubMed] [Google Scholar]
- Mruthinti S, Buccafusco JJ, Hill WD, Waller JL, Jackson TW, Zamrini EY, Schade RF. Autoimmunity in Alzheimer's disease: increased levels of circulating IgGs binding Abeta and RAGE peptides. Neurobiol Aging. 2004;25:1023–1032. doi: 10.1016/j.neurobiolaging.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Biere AL, Ostaszewski B, Stimson ER, Hyman BT, Maggio JE, Selkoe DJ. Amyloid beta-peptide is transported on lipoproteins and albumin in human plasma. J Biol Chem. 1996;271:32916–32922. doi: 10.1074/jbc.271.51.32916. [DOI] [PubMed] [Google Scholar]
- Sagare A, Deane R, Bell RD, Johnson B, Hamm K, Pendu R, Marky A, Lenting PJ, Wu Z, Zarcone T, Goate A, Mayo K, Perlmutter D, Coma M, Zhong Z, Zlokovic BV. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat Med. 2007;13:1029–1031. doi: 10.1038/nm1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullwood NJ, Hayashi Y, Allsop D. Plasma amyloid-beta concentrations in Alzheimer's disease: an alternative hypothesis. Lancet Neurol. 2006;5:1000–1001. doi: 10.1016/S1474-4422(06)70611-1. author reply 1002-3. [DOI] [PubMed] [Google Scholar]
- Oh ES, Troncoso JC, Fangmark Tucker SM. Maximizing the potential of plasma amyloid-beta as a diagnostic biomarker for Alzheimer's disease. Neuromolecular Med. 2008;10:195–207. doi: 10.1007/s12017-008-8035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ida N, Hartmann T, Pantel J, Schroder J, Zerfass R, Forstl H, Sandbrink R, Masters CL, Beyreuther K. Analysis of heterogeneous A4 peptides in human cerebrospinal fluid and blood by a newly developed sensitive Western blot assay. J Biol Chem. 1996;271:22908–22914. doi: 10.1074/jbc.271.37.22908. [DOI] [PubMed] [Google Scholar]
- Quesada JJ, Ferrucci L, Calvani D, Valente C, Salani B, Bavazzano A. Formal education as an effect modifier of the relationship between Mini-Mental State Examination score and IADLs disability in the older population. Aging (Milano) 1997;9:175–179. doi: 10.1007/BF03340146. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Blasko I, Jellinger K, Kemmler G, Krampla W, Jungwirth S, Wichart I, Tragl KH, Fischer P. Conversion from cognitive health to mild cognitive impairment and Alzheimer's disease: prediction by plasma amyloid beta 42, medial temporal lobe atrophy and homocysteine. Neurobiol Aging. 2008;29:1–11. doi: 10.1016/j.neurobiolaging.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Mayeux R, Honig LS, Tang MX, Manly J, Stern Y, Schupf N, Mehta PD. Plasma A[beta]40 and A[beta]42 and Alzheimer's disease: relation to age, mortality, and risk. Neurology. 2003;61:1185–1190. doi: 10.1212/01.wnl.0000091890.32140.8f. [DOI] [PubMed] [Google Scholar]
- Zhu X, Smith MA, Honda K, Aliev G, Moreira PI, Nunomura A, Casadesus G, Harris PL, Siedlak SL, Perry G. Vascular oxidative stress in Alzheimer disease. J Neurol Sci. 2007;257:240–246. doi: 10.1016/j.jns.2007.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattabeni F, Colciaghi F, Di Luca M. Platelets provide human tissue to unravel pathogenic mechanisms of Alzheimer disease. Prog europsychopharmacol Biol Psychiatry. 2004;28:763–770. doi: 10.1016/j.pnpbp.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Roher AE, Esh CL, Kokjohn TA, Castano EM, Van Vickle GD, Kalback WM, Patton RL, Luehrs DC, Daugs ID, Kuo YM, Emmerling MR, Soares H, Quinn JF, Kaye J, Connor DJ, Silverberg NB, Adler CH, Seward JD, Beach TG, Sabbagh MN. Amyloid beta peptides in human plasma and tissues and their significance for Alzheimer's disease. Alzheimers Dement. 2009;5:18–29. doi: 10.1016/j.jalz.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]