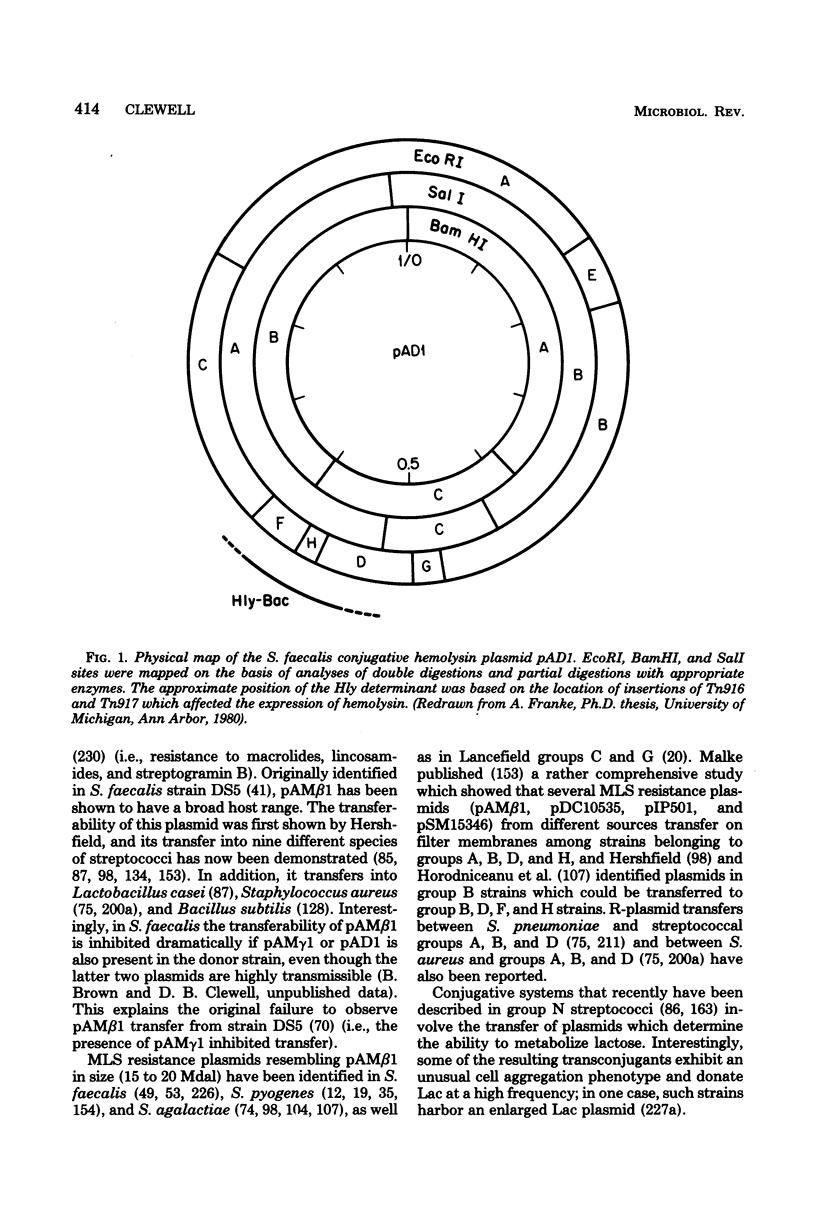
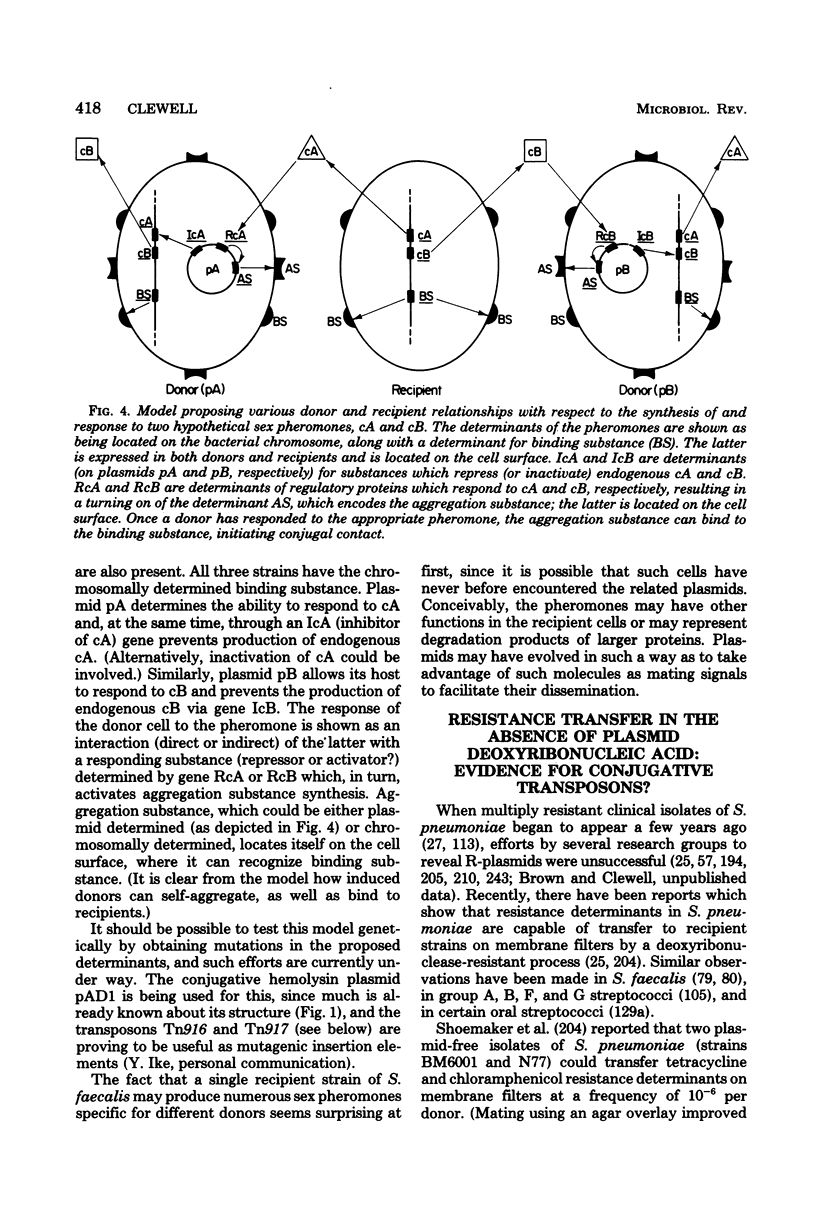
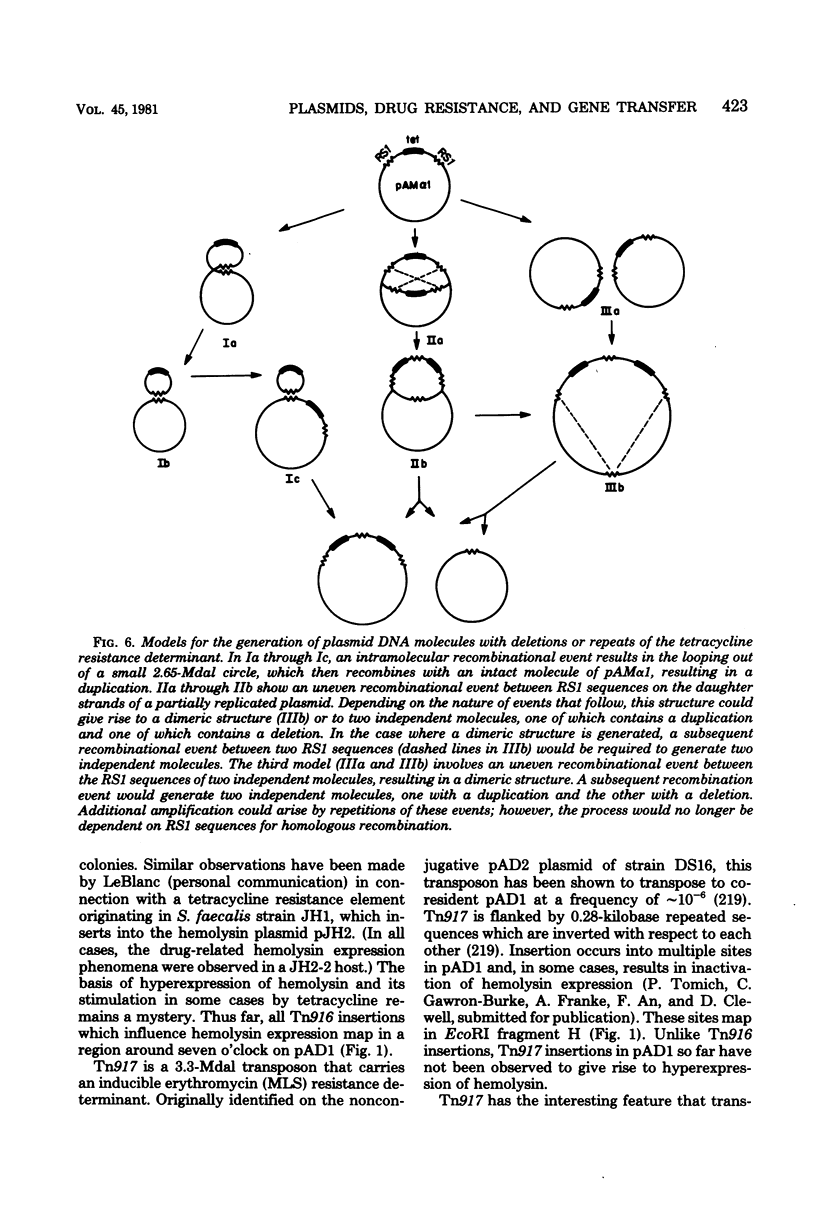
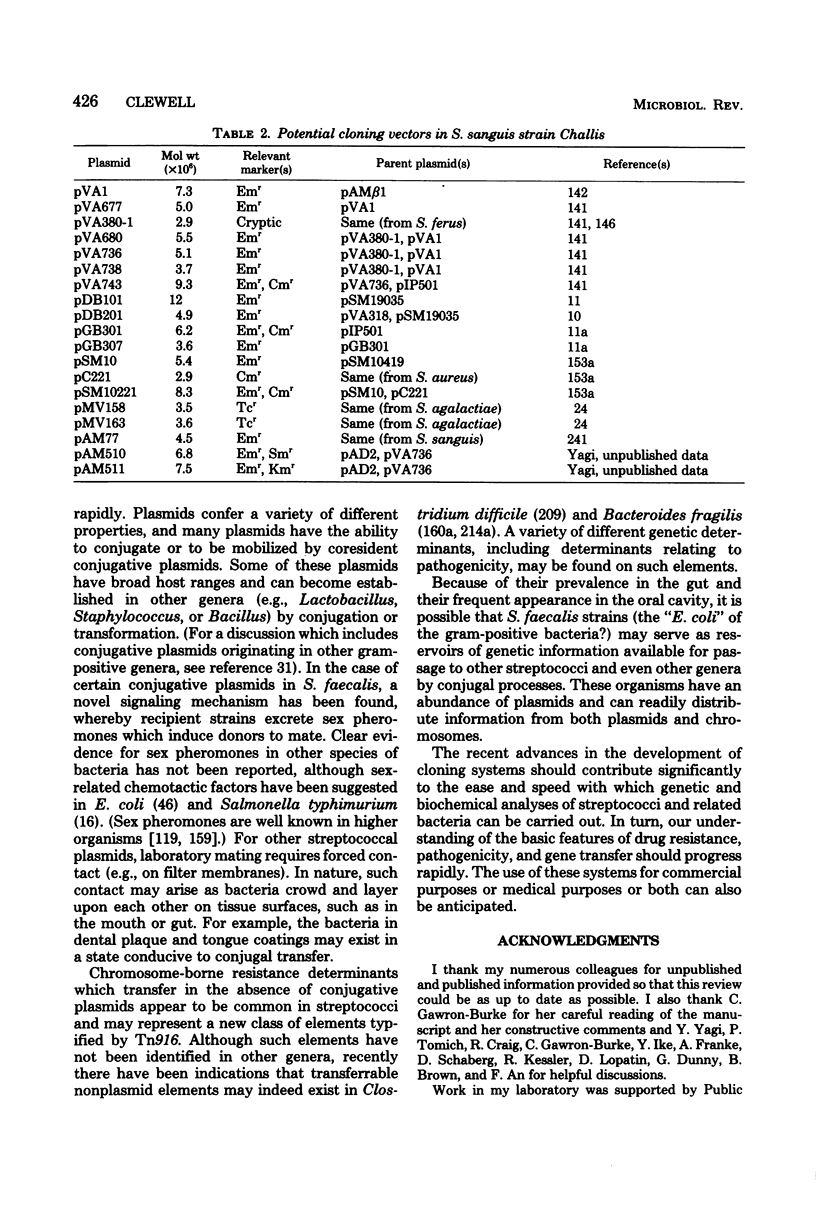
Full text
PDF























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., McKay L. L. Plasmids, loss of lactose metabolism, and appearance of partial and full lactose-fermenting revertants in Streptococcus cremoris B1. J Bacteriol. 1977 Jan;129(1):367–377. doi: 10.1128/jb.129.1.367-377.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony B. F., Concepcion N. F. Group B streptococcus in a general hospital. J Infect Dis. 1975 Nov;132(5):561–567. doi: 10.1093/infdis/132.5.561. [DOI] [PubMed] [Google Scholar]
- Arthur A., Sherratt D. Dissection of the transposition process: a transposon-encoded site-specific recombination system. Mol Gen Genet. 1979 Oct 1;175(3):267–274. doi: 10.1007/BF00397226. [DOI] [PubMed] [Google Scholar]
- BRACCO R. M., KRAUSS M. R., ROE A. S., MACLEOD C. M. Transformation reactions between Pneumococcus and three strains of Streptococci. J Exp Med. 1957 Aug 1;106(2):247–259. doi: 10.1084/jem.106.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK T. D., DAVIE J. M. PROBABLE IDENTITY OF A GROUP D HEMOLYSIN WITH A BACTERIOCINE. J Bacteriol. 1963 Oct;86:708–712. doi: 10.1128/jb.86.4.708-712.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. J., Barrett F. F., Gordon R. C., Yow M. D. Suppurative meningitis due to streptococci of Lancefield group B: a study of 33 infants. J Pediatr. 1973 Apr;82(4):724–729. doi: 10.1016/s0022-3476(73)80606-7. [DOI] [PubMed] [Google Scholar]
- Baker C. J., Webb B. J., Barrett F. F. Antimicrobial susceptibility of group B streptococci isolated from a variety of clinical sources. Antimicrob Agents Chemother. 1976 Jul;10(1):128–131. doi: 10.1128/aac.10.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barany F., Tomasz A. Genetic transformation of Streptococcus pneumoniae by heterologous plasmid deoxyribonucleic acid. J Bacteriol. 1980 Nov;144(2):698–709. doi: 10.1128/jb.144.2.698-709.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basinger S. F., Jackson R. W. Bacteriocin (hemolysin) of Streptococcus zymogenes. J Bacteriol. 1968 Dec;96(6):1895–1902. doi: 10.1128/jb.96.6.1895-1902.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke D., Ferretti J. J. Molecular cloning of an erythromycin resistance determinant in streptococci. J Bacteriol. 1980 Nov;144(2):806–813. doi: 10.1128/jb.144.2.806-813.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke D., Ferretti J. J. Physical mapping of plasmid pDB101: a potential vector plasmid for molecular cloning in streptococci. Plasmid. 1980 Sep;4(2):130–138. doi: 10.1016/0147-619x(80)90002-5. [DOI] [PubMed] [Google Scholar]
- Behnke D., Malke H., Hartmann M., Walter F. Post-transformational rearrangement of an in vitro reconstructed group-A streptococcal erythromycin resistance plasmid. Plasmid. 1979 Oct;2(4):605–616. doi: 10.1016/0147-619x(79)90058-1. [DOI] [PubMed] [Google Scholar]
- Behnke D., Tomich P. K., Clewell D. B. Electron microscopic mapping of deletions on a streptococcal plasmid carrying extraordinarily long inverted repeats. Plasmid. 1980 Sep;4(2):139–147. doi: 10.1016/0147-619x(80)90003-7. [DOI] [PubMed] [Google Scholar]
- Bergner-Rabinowitz S., Davies A. M. Sensitivity of Streptococcus pyogenes types to tetracycline and other antibiotics. Isr J Med Sci. 1970 May-Jun;6(3):393–398. [PubMed] [Google Scholar]
- Bezdek M., Soska J. Sex-determined chemotaxis in Salmonella typhimurium LT2. Folia Microbiol (Praha) 1972;17(5):366–369. doi: 10.1007/BF02884104. [DOI] [PubMed] [Google Scholar]
- Biswas G. D., Ravin A. W. Heterospecific transformation of Pneumococcus and Streptococcus. IV. Variations in hybrid DNA produced by recombination. Mol Gen Genet. 1971;110(1):1–22. doi: 10.1007/BF00276040. [DOI] [PubMed] [Google Scholar]
- Bougueleret L., Bieth G., Horodniceanu T. Conjugative R plasmids in group C and G streptococci. J Bacteriol. 1981 Feb;145(2):1102–1105. doi: 10.1128/jb.145.2.1102-1105.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgault A. M., Wilson W. R., Washington J. A., 2nd Antimicrobial susceptibilities of species of viridans streptococci. J Infect Dis. 1979 Sep;140(3):316–321. doi: 10.1093/infdis/140.3.316. [DOI] [PubMed] [Google Scholar]
- Burdett V. Identification of tetracycline-resistant R-plasmids in Streptococcus agalactiae (group B). Antimicrob Agents Chemother. 1980 Nov;18(5):753–760. doi: 10.1128/aac.18.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buu-Hoï A., Horodniceanu T. Conjugative transfer of multiple antibiotic resistance markers in Streptococcus pneumoniae. J Bacteriol. 1980 Jul;143(1):313–320. doi: 10.1128/jb.143.1.313-320.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canosi U., Morelli G., Trautner T. A. The relationship between molecular structure and transformation efficiency of some S. aureus plasmids isolated from B. subtilis. Mol Gen Genet. 1978 Nov 9;166(3):259–267. doi: 10.1007/BF00267617. [DOI] [PubMed] [Google Scholar]
- Chassy B. M. A gentle method for the lysis of oral streptococci. Biochem Biophys Res Commun. 1976 Jan 26;68(2):603–608. doi: 10.1016/0006-291x(76)91188-8. [DOI] [PubMed] [Google Scholar]
- Cleary P. P., Johnson Z., Wannamaker L. Genetic instability of M protein and serum opacity factor of group A streptocci: evidence suggesting extrachromosomal control. Infect Immun. 1975 Jul;12(1):109–118. doi: 10.1128/iai.12.1.109-118.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Brown B. L. Sex pheromone cAD1 in Streptococcus faecalis: induction of a function related to plasmid transfer. J Bacteriol. 1980 Aug;143(2):1063–1065. doi: 10.1128/jb.143.2.1063-1065.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Franke A. E. Characterization of a plasmid determining resistance to erythromycin, lincomycin, and vernamycin Balpha in a strain Streptococcus pyogenes. Antimicrob Agents Chemother. 1974 May;5(5):534–537. doi: 10.1128/aac.5.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Bauer B. Plasmid-determined tetracycline resistance in Streptococcus faecalis: evidence for gene amplification during growth in presence of tetracycline. Proc Natl Acad Sci U S A. 1975 May;72(5):1720–1724. doi: 10.1073/pnas.72.5.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Dunny G. M., Schultz S. K. Characterization of three plasmid deoxyribonucleic acid molecules in a strain of Streptococcus faecalis: identification of a plasmid determining erythromycin resistance. J Bacteriol. 1974 Jan;117(1):283–289. doi: 10.1128/jb.117.1.283-289.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. M., Calandra G. B., Huff E., Nugent K. M. Attributes of potential utility in differentiating among "group H" streptococci or Streptococcus sanguis. J Dent Res. 1976 Jan;55:A142–A153. doi: 10.1177/002203457605500106011. [DOI] [PubMed] [Google Scholar]
- Collins J. F., Broda P. Motility, diffusion and cell concentration affect pair formation in Escherichia coli. Nature. 1975 Dec 25;258(5537):722–723. doi: 10.1038/258722a0. [DOI] [PubMed] [Google Scholar]
- Colón A. E., Cole R. M., Leonard C. G. Intergroup lysis and transduction by streptococcal bacteriophages. J Virol. 1972 Mar;9(3):551–553. doi: 10.1128/jvi.9.3.551-553.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contente S., Dubnau D. Marker rescue transformation by linear plasmid DNA in Bacillus subtilis. Plasmid. 1979 Oct;2(4):555–571. doi: 10.1016/0147-619x(79)90054-4. [DOI] [PubMed] [Google Scholar]
- Cooke E. M., Ewins S. P. Properties of strains of Escherichia coli isolated from a variety of sources. J Med Microbiol. 1975 Feb;8(1):107–111. doi: 10.1099/00222615-8-1-107. [DOI] [PubMed] [Google Scholar]
- Cords B. R., McKay L. L., Guerry P. Extrachromosomal elements in group N streptococci. J Bacteriol. 1974 Mar;117(3):1149–1152. doi: 10.1128/jb.117.3.1149-1152.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P. M., Carlier C., Chabbert Y. A. Plasmid-linked tetracycline and erythromycin resistance in group D "streptococcus". Ann Inst Pasteur (Paris) 1972 Dec;123(6):755–759. [PubMed] [Google Scholar]
- Courvalin P. M., Carlier C., Croissant O., Blangy D. Identification of two plasmids determining resistance to tetracycline and to erythromycin in group D streptococcus. Mol Gen Genet. 1974;132(3):181–192. doi: 10.1007/BF00269391. [DOI] [PubMed] [Google Scholar]
- Courvalin P. M., Shaw W. V., Jacob A. E. Plasmid-mediated mechanisms of resistance to aminoglycoside-aminocyclitol antibiotics and to chloramphenicol in group D streptococci. Antimicrob Agents Chemother. 1978 May;13(5):716–725. doi: 10.1128/aac.13.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P., Carlier C., Collatz E. Plasmid-mediated resistance to aminocyclitol antibiotics in group D streptococci. J Bacteriol. 1980 Aug;143(2):541–551. doi: 10.1128/jb.143.2.541-551.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Craig R. A., Carron R. L., Clewell D. B. Plasmid transfer in Streptococcus faecalis: production of multiple sex pheromones by recipients. Plasmid. 1979 Jul;2(3):454–465. doi: 10.1016/0147-619x(79)90029-5. [DOI] [PubMed] [Google Scholar]
- Finch R. G., French G. L., Phillips I. Group B streptococci in the female genital tract. Br Med J. 1976 May 22;1(6020):1245–1247. doi: 10.1136/bmj.1.6020.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finland M. Emergence of antibiotic resistance in hospitals, 1935-1975. Rev Infect Dis. 1979 Jan-Feb;1(1):4–22. doi: 10.1093/clinids/1.1.4. [DOI] [PubMed] [Google Scholar]
- Franke A. E., Clewell D. B. Evidence for a chromosome-borne resistance transposon (Tn916) in Streptococcus faecalis that is capable of "conjugal" transfer in the absence of a conjugative plasmid. J Bacteriol. 1981 Jan;145(1):494–502. doi: 10.1128/jb.145.1.494-502.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier M. L., Zimmerman L. N. Genetic loci of hemolysin production in Streptococcus faecalis subsp. zymogenes. J Bacteriol. 1977 Jun;130(3):1064–1071. doi: 10.1128/jb.130.3.1064-1071.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier M. L., Zimmerman L. N. Plasmid-borne resistance to ultraviolet light and phage in Streptococcus faecalis ssp. zymogens. Can J Microbiol. 1980 Oct;26(10):1253–1255. doi: 10.1139/m80-208. [DOI] [PubMed] [Google Scholar]
- Fuchs P. G., Zajdel J., Dobrzański W. T. Possible plasmid nature of the determinant for production of the antibiotic nisin in some strains of Streptococcus lactis. J Gen Microbiol. 1975 May;88(1):189–192. doi: 10.1099/00221287-88-1-189. [DOI] [PubMed] [Google Scholar]
- Gasson M. J., Davies F. L. High-frequency conjugation associated with Streptococcus lactis donor cell aggregation. J Bacteriol. 1980 Sep;143(3):1260–1264. doi: 10.1128/jb.143.3.1260-1264.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson E. M., Chace N. M., London S. B., London J. Transfer of plasmid-mediated antibiotic resistance from streptococci to lactobacilli. J Bacteriol. 1979 Jan;137(1):614–619. doi: 10.1128/jb.137.1.614-619.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold O. G., Jordan H. V., van Houte J. The prevalence of enterococci in the human mouth and their pathogenicity in animal models. Arch Oral Biol. 1975 Jul;20(7):473–477. doi: 10.1016/0003-9969(75)90236-8. [DOI] [PubMed] [Google Scholar]
- Granato P. A., Jackson R. W. Bicomponent nature of lysin from Streptococcus zymogenes. J Bacteriol. 1969 Nov;100(2):865–868. doi: 10.1128/jb.100.2.865-868.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. B., Abiko Y., Curtiss R., 3rd Characterization of the Streptococcus mutans plasmid pva318 cloned into Escherichia coli. Infect Immun. 1981 Mar;31(3):1034–1043. doi: 10.1128/iai.31.3.1034-1043.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., Bedinger P., Champoux J. J., Falkow S. Deletions affecting the transposition of an antibiotic resistance gene. Proc Natl Acad Sci U S A. 1977 Feb;74(2):702–706. doi: 10.1073/pnas.74.2.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen S. D., Henrichsen J. Twitching motility and possession of polar fimbriae in spreading Streptococcus sanguis isolates from the human throat. Acta Pathol Microbiol Scand B. 1975 Apr;83(2):133–140. doi: 10.1111/j.1699-0463.1975.tb00083.x. [DOI] [PubMed] [Google Scholar]
- Hershfield V. Plasmids mediating multiple drug resistance in group B streptococcus: transferability and molecular properties. Plasmid. 1979 Jan;2(1):137–149. doi: 10.1016/0147-619x(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Higuchi M., Araya S., Higuchi M. Plasmid DNA satellite bands seen in lysates of Streptococcus mutans that form insoluble extracellular polysaccharides. J Dent Res. 1976 Mar-Apr;55(2):266–271. doi: 10.1177/00220345760550021801. [DOI] [PubMed] [Google Scholar]
- Higuchi M., Endo K., Hoshino E., Araya S. Preferential induction of rough variants in Streptococcus mutans by ethidium bromide. J Dent Res. 1973 Sep-Oct;52(5):1070–1075. doi: 10.1177/00220345730520051401. [DOI] [PubMed] [Google Scholar]
- Higuchi M., Rhee G. H., Araya S., Higuchi M. Bacteriophage deoxyribonucleic acid-induced mutation of Streptococcus mutans. Infect Immun. 1977 Mar;15(3):938–944. doi: 10.1128/iai.15.3.938-944.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M., Rhee G. H., Araya S., Higuchi M. Transfection of Streptococcus sanguis by phage deoxyribonucleic acid isolated from Streptococcus mutans. Infect Immun. 1977 Mar;15(3):945–949. doi: 10.1128/iai.15.3.945-949.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Posttranscriptional modification of mRNA conformation: mechanism that regulates erythromycin-induced resistance. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7079–7083. doi: 10.1073/pnas.77.12.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horodniceanu T., Bouanchaud D. H., Bieth G., Chabbert Y. A. R plasmids in Streptococcus agalactiae (group B). Antimicrob Agents Chemother. 1976 Nov;10(5):795–801. doi: 10.1128/aac.10.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horodniceanu T., Bougueleret L., Bieth G. Conjugative transfer of multiple-antibiotic resistance markers in beta-hemolytic group A, B, F, and G streptococci in the absence of extrachromosomal deoxyribonucleic acid. Plasmid. 1981 Mar;5(2):127–137. doi: 10.1016/0147-619x(81)90014-7. [DOI] [PubMed] [Google Scholar]
- Horodniceanu T., Bougueleret L., El-Solh N., Bieth G., Delbos F. High-level, plasmid-borne resistance to gentamicin in Streptococcus faecalis subsp. zymogenes. Antimicrob Agents Chemother. 1979 Nov;16(5):686–689. doi: 10.1128/aac.16.5.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horodniceanu T., Bougueleret L., El-Solh N., Bouanchaud D. H., Chabbert Y. A. Conjugative R plasmids in Streptococcus agalactiae (group B). Plasmid. 1979 Apr;2(2):197–206. doi: 10.1016/0147-619x(79)90038-6. [DOI] [PubMed] [Google Scholar]
- Hu S., Otsubo E., Davidson N., Saedler H. Electron microscope heteroduplex studies of sequence relations among bacterial plasmids: identification and mapping of the insertion sequences IS1 and IS2 in F and R plasmids. J Bacteriol. 1975 May;122(2):764–775. doi: 10.1128/jb.122.2.764-775.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder S. L., Streitfeld M. M. Inducible and constitutive resistance to macrolide antibiotics and lincomycin in clinically isolated strains of Streptococcus pyogenes. Antimicrob Agents Chemother. 1973 Sep;4(3):327–331. doi: 10.1128/aac.4.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder S. L., Streitfeld M. M. Transfer of erythromycin resistance from clinically isolated lysogenic strains of Streptococcus pyogenes via their endogenous phage. J Infect Dis. 1978 Sep;138(3):281–286. doi: 10.1093/infdis/138.3.281. [DOI] [PubMed] [Google Scholar]
- Jacob A. E., Douglas G. J., Hobbs S. J. Self-transferable plasmids determining the hemolysin and bacteriocin of Streptococcus faecalis var. zymogenes. J Bacteriol. 1975 Mar;121(3):863–872. doi: 10.1128/jb.121.3.863-872.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M. R., Koornhof H. J., Robins-Browne R. M., Stevenson C. M., Vermaak Z. A., Freiman I., Miller G. B., Witcomb M. A., Isaäcson M., Ward J. I. Emergence of multiply resistant pneumococci. N Engl J Med. 1978 Oct 5;299(14):735–740. doi: 10.1056/NEJM197810052991402. [DOI] [PubMed] [Google Scholar]
- Jokipii A. M., Jokipii L. Presumptive identification and antibiotic susceptibility of group B streptococci. J Clin Pathol. 1976 Aug;29(8):736–739. doi: 10.1136/jcp.29.8.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama A., Ishikawa E., Ando T., Arai T. Isolation of plasmid DNA from naturally-occurring strains of Streptococcus mutans. Arch Oral Biol. 1978;23(12):1099–1103. doi: 10.1016/0003-9969(78)90115-2. [DOI] [PubMed] [Google Scholar]
- Kempler G. M., McKay L. L. Characterization of Plasmid Deoxyribonucleic Acid in Streptococcus lactis subsp. diacetylactis: Evidence for Plasmid-Linked Citrate Utilization. Appl Environ Microbiol. 1979 Feb;37(2):316–323. doi: 10.1128/aem.37.2.316-323.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R., McKay L. L., Baldwin K. A. Improved lysis of group N streptococci for isolation and rapid characterization of plasmid deoxyribonucleic acid. Appl Environ Microbiol. 1978 Mar;35(3):592–600. doi: 10.1128/aem.35.3.592-600.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer F. J., Chang A. C., Cohen S. N. Indirect selection of bacterial plasmids lacking identifiable phenotypic properties. J Bacteriol. 1975 Oct;124(1):225–231. doi: 10.1128/jb.124.1.225-231.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogstad D. J., Korfhagen T. R., Moellering R. C., Jr, Wennersten C., Swartz M. N. Aminoglycoside-inactivating enzymes in clinical isolates of Streptococcus faecalis. An explanation for resistance to antibiotic synergism. J Clin Invest. 1978 Aug;62(2):480–486. doi: 10.1172/JCI109149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogstad D. J., Korfhagen T. R., Moellering R. C., Jr, Wennersten C., Swartz M. N. Plasmid-mediated resistance to antibiotic synergism in enterococci. J Clin Invest. 1978 Jun;61(6):1645–1653. doi: 10.1172/JCI109085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogstad D. J., Smith R. M., Moellering R. C., Jr, Parquette A. R. Visualization of cell-cell contact during conjugation in Streptococcus faecalis. J Bacteriol. 1980 Feb;141(2):963–967. doi: 10.1128/jb.141.2.963-967.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl S. A., Larsen L. D., McKay L. L. Plasmid Profiles of Lactose-Negative and Proteinase-Deficient Mutants of Streptococcus lactis C10, ML(3), and M18. Appl Environ Microbiol. 1979 Jun;37(6):1193–1195. doi: 10.1128/aem.37.6.1193-1195.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S. Uptake of circular deoxyribonucleic acid and mechanism of deoxyribonucleic acid transport in genetic transformation of Streptococcus pneumoniae. J Bacteriol. 1979 May;138(2):404–409. doi: 10.1128/jb.138.2.404-409.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Weisblum B. Altered methylation of ribosomal RNA in an erythromycin-resistant strain of Staphylococcus aureus. Proc Natl Acad Sci U S A. 1971 Apr;68(4):856–860. doi: 10.1073/pnas.68.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen L. D., McKay L. L. Isolation and characterization of plasmid DNA in Streptococcus cremoris. Appl Environ Microbiol. 1978 Dec;36(6):944–952. doi: 10.1128/aem.36.6.944-952.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Crow V. L., Lee L. N., Garon C. F. Influence of the lactose plasmid on the metabolism of galactose by Streptococcus lactis. J Bacteriol. 1979 Feb;137(2):878–884. doi: 10.1128/jb.137.2.878-884.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Hassell F. P. Transformation of Streptococcus sanguis Challis by plasmid deoxyribonucleic acid from Streptococcus faecalis. J Bacteriol. 1976 Oct;128(1):347–355. doi: 10.1128/jb.128.1.347-355.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Hawley R. J., Lee L. N., St Martin E. J. "Conjugal" transfer of plasmid DNA among oral streptococci. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3484–3487. doi: 10.1073/pnas.75.7.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc D. J., Cohen L., Jensen L. Transformation of group F streptococci by plasmid DNA. J Gen Microbiol. 1978 May;106(1):49–54. doi: 10.1099/00221287-106-1-49. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Jones K. R., Wood P. H. Chimeric streptococcal plasmids and their use as molecular cloning vehicles in Streptococcus sanguis (Challis). J Bacteriol. 1980 Sep;143(3):1425–1435. doi: 10.1128/jb.143.3.1425-1435.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Keeler C. L., Jr, Jones K. R., Wood P. H. Molecular characterization of unique deletion mutants of the streptococcal plasmid, pAM beta 1. Plasmid. 1980 Jul;4(1):8–16. doi: 10.1016/0147-619x(80)90079-7. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Reider J. L., Virgili S. S., Kopecko D. J. Survey of the extrachromosomal gene pool of Streptococcus mutans. Infect Immun. 1977 Jul;17(1):215–226. doi: 10.1128/iai.17.1.215-226.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Scott C. L. Evidence for a disseminated plasmid in Streptococcus mutans. Infect Immun. 1978 Apr;20(1):296–302. doi: 10.1128/iai.20.1.296-302.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Virgili S. S., Scott C. L. Extrachromosomal gene systems in Streptococcus mutans. Adv Exp Med Biol. 1978;107:859–868. doi: 10.1007/978-1-4684-3369-2_96. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Wood P. H., Jones K. R. Genetic transformation of Streptococcus sanguis (Challis) with cryptic plasmids from Streptococcus ferus. Infect Immun. 1980 Jun;28(3):692–699. doi: 10.1128/iai.28.3.692-699.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Wood P. H., Jones K. R. Simple method for demonstrating small plasmid deoxyribonucleic acid molecules in oral streptococci. Appl Environ Microbiol. 1980 May;39(5):1070–1073. doi: 10.1128/aem.39.5.1070-1073.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malke H., Burman L. G., Holm S. E. Molecular cloning in streptococci: physical mapping of the vehicle plasmid pSM10 and demonstration of intergroup DNA transfer. Mol Gen Genet. 1981;181(2):259–267. doi: 10.1007/BF00268435. [DOI] [PubMed] [Google Scholar]
- Malke H. Genetics of resistance to macrolide antibiotics and lincomycin in natural isolates of Streptococcus pyogenes. Mol Gen Genet. 1974;135(4):349–367. doi: 10.1007/BF00271149. [DOI] [PubMed] [Google Scholar]
- Malke H., Jacob H. E., Störl K. Characterization of the antibiotic resistance plasmid ERL1 from Streptococcus pyogenes. Mol Gen Genet. 1976 Mar 30;144(3):333–338. doi: 10.1007/BF00341732. [DOI] [PubMed] [Google Scholar]
- Malke H., Reichardt W., Hartmann M., Walter F. Genetic study of plasmid-associated zonal resistance to lincomycin in Streptococcus pyogenes. Antimicrob Agents Chemother. 1981 Jan;19(1):91–100. doi: 10.1128/aac.19.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malke H., Starke R., Köhler W., Kolesnichenko G., Totolian A. A. Bacteriophage P13234mo-mediated intra- and intergroup transduction of antibiotic resistance among streptococci. Zentralbl Bakteriol Orig A. 1975 Sep;233(1):24–34. [PubMed] [Google Scholar]
- Malke H. Transfer of a plasmid mediating antibiotic resistance between strains of Streptococcus pyogenes in mixed cultures. Z Allg Mikrobiol. 1975;15(8):645–649. doi: 10.1002/jobm.3630150810. [DOI] [PubMed] [Google Scholar]
- Marder H. P., Kayser F. H. Transferable plasmids mediating multiple-antibiotic resistance in Streptococcus faecalis subsp. liquefaciens. Antimicrob Agents Chemother. 1977 Aug;12(2):261–269. doi: 10.1128/aac.12.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes R., Burkardt H. J., Schmitt R. Repetition of tetracycline resistance determinant genes on R plasmid pRSD1 in Escherichia coli. Mol Gen Genet. 1979 Jan 10;168(2):173–184. doi: 10.1007/BF00431443. [DOI] [PubMed] [Google Scholar]
- McKay L. L., Cords B. R., Baldwin K. A. Transduction of lactose metabolism in Streptococcus lactis C2. J Bacteriol. 1973 Sep;115(3):810–815. doi: 10.1128/jb.115.3.810-815.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miehl R., Miller M., Yasbin R. E. Plasmid mediated enhancement of uv resistance in Streptococcus faecalis. Plasmid. 1980 Mar;3(2):128–134. doi: 10.1016/0147-619x(80)90104-3. [DOI] [PubMed] [Google Scholar]
- Morrison D. A., Baker M. F. Competence for genetic transformation in pneumococcus depends on synthesis of a small set of proteins. Nature. 1979 Nov 8;282(5735):215–217. doi: 10.1038/282215a0. [DOI] [PubMed] [Google Scholar]
- Mottes M., Grandi G., Sgaramella V., Canosi U., Morelli G., Trautner T. A. Different specific activities of the monomeric and oligomeric forms of plasmid DNA in transformation of B. subtilis and E. coli. Mol Gen Genet. 1979 Jul 24;174(3):281–286. doi: 10.1007/BF00267800. [DOI] [PubMed] [Google Scholar]
- Nakae M., Murai T., Kaneko Y., Mitsuhashi S. Drug resistance in Streptococcus pyogenes isolated in Japan. Antimicrob Agents Chemother. 1977 Sep;12(3):427–428. doi: 10.1128/aac.12.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORLAND F. J., BLAYNEY J. R., HARRISON R. W., REYNIERS J. A., TREXLER P. C., ERVIN R. F., GORDON H. A., WAGNER M. Experimental caries in germfree rats inoculated with enterococci. J Am Dent Assoc. 1955 Mar;50(3):259–272. doi: 10.14219/jada.archive.1955.0061. [DOI] [PubMed] [Google Scholar]
- Odakura Y., Tanaka T., Hashimoto H., Mitsuhashi S. Mutation of R factors capable of specifying hypersynthesis of penicillinase. Antimicrob Agents Chemother. 1973 Mar;3(3):315–324. doi: 10.1128/aac.3.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. R., Brown B. L., Clewell D. B. Analysis of plasmid deoxyribonucleic acid in a cariogenic strain of Streptococcus faecalis: an approach to identifying genetic determinants on cryptic plasmids. J Bacteriol. 1977 May;130(2):759–765. doi: 10.1128/jb.130.2.759-765.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. R., Brown B. L., Clewell D. B. Characterization of plasmids determining hemolysin and bacteriocin production in Streptococcus faecalis 5952. J Bacteriol. 1977 May;130(2):948–950. doi: 10.1128/jb.130.2.948-950.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAKULA R., HULANICKA E., WALCZAK W. Transformation reactions between different species of streptococci and between streptococci, pneumococci and staphylococci. Schweiz Z Pathol Bakteriol. 1959;22(2):202–214. doi: 10.1159/000160592. [DOI] [PubMed] [Google Scholar]
- PAKULA R., WALCZAK W. On the nature of competence of transformable streptococci. J Gen Microbiol. 1963 Apr;31:125–133. doi: 10.1099/00221287-31-1-125. [DOI] [PubMed] [Google Scholar]
- PERRY D., SLADE H. D. Transformation of streptococci to streptomycin resistance. J Bacteriol. 1962 Mar;83:443–449. doi: 10.1128/jb.83.3.443-449.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattee P. A. Distribution of Tn551 insertion sites responsible for auxotrophy on the Staphylococcus aureus chromosome. J Bacteriol. 1981 Jan;145(1):479–488. doi: 10.1128/jb.145.1.479-488.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattee P. A., Thompson N. E., Haubrich D., Novick R. P. Chromosomal map locations of integrated plasmids and related elements in Staphylococcus aureus. Plasmid. 1977 Nov;1(1):38–51. doi: 10.1016/0147-619x(77)90007-5. [DOI] [PubMed] [Google Scholar]
- Perry D., Kuramitsu H. K. Genetic transformation of Streptococcus mutans. Infect Immun. 1981 Jun;32(3):1295–1297. doi: 10.1128/iai.32.3.1295-1297.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S., Novick R. P. Tn554--a site-specific repressor-controlled transposon in Staphylococcus aureus. Nature. 1979 Mar 29;278(5703):476–478. doi: 10.1038/278476a0. [DOI] [PubMed] [Google Scholar]
- Prakash K., Ravindran P. C., Sharma K. B. Increasing incidence of group B beta hemolytic streptococci from human sources. Indian J Med Res. 1973 Apr;61(4):506–513. [PubMed] [Google Scholar]
- Ptashne K., Cohen S. N. Occurrence of insertion sequence (IS) regions on plasmid deoxyribonucleic acid as direct and inverted nucleotide sequence duplications. J Bacteriol. 1975 May;122(2):776–781. doi: 10.1128/jb.122.2.776-781.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAYCROFT R. E., ZIMMERMAN L. N. NEW MODE OF GENETIC TRANSFER IN STREPTOCOCCUS FAECALIS VAR. LIQUEFACIENS. J Bacteriol. 1964 Apr;87:799–801. doi: 10.1128/jb.87.4.799-801.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina J. L., Ravin A. W. Switches in macromolecular synthesis during induction of competence for transformation of Streptococcus sanguis. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6062–6066. doi: 10.1073/pnas.77.10.6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins-Brown R. M., Gaspar M. N., Ward J. I., Wachsmuth I. K., Koornhof H. J., Jacobs M. R., Thornsberry C. Resistance mechanisms of multiply resistant pneumococci: antibiotic degradation studies. Antimicrob Agents Chemother. 1979 Mar;15(3):470–474. doi: 10.1128/aac.15.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rownd R., Mickel S. Dissociation and reassociation of RTF and r-determinants of the R-factor NR1 in Proteus mirabilis. Nat New Biol. 1971 Nov 10;234(45):40–43. doi: 10.1038/newbio234040a0. [DOI] [PubMed] [Google Scholar]
- Saunders C. W., Guild W. R. Monomer plasmid DNA transforms Streptococcus pneumoniae. Mol Gen Genet. 1981;181(1):57–62. doi: 10.1007/BF00339005. [DOI] [PubMed] [Google Scholar]
- Saunders C. W., Guild W. R. Pathway of plasmid transformation in Pneumococcus: open circular and linear molecules are active. J Bacteriol. 1981 May;146(2):517–526. doi: 10.1128/jb.146.2.517-526.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders C. W., Guild W. R. Properties and transforming activities of two plasmids in Streptococcus pneumoniae. Mol Gen Genet. 1980;180(3):573–578. doi: 10.1007/BF00268062. [DOI] [PubMed] [Google Scholar]
- Severin M. J., Wiley J. L. Change in susceptibility of group B streptococci to penicillin G from 1968 through 1975. Antimicrob Agents Chemother. 1976 Aug;10(2):380–381. doi: 10.1128/aac.10.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J. A. Molecular model for the transposition and replication of bacteriophage Mu and other transposable elements. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1933–1937. doi: 10.1073/pnas.76.4.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivakumar A. G., Hahn J., Grandi G., Kozlov Y., Dubnau D. Posttranscriptional regulation of an erythromycin resistance protein specified by plasmic pE194. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3903–3907. doi: 10.1073/pnas.77.7.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Smith M. D., Guild W. R. DNase-resistant transfer of chromosomal cat and tet insertions by filter mating in Pneumococcus. Plasmid. 1980 Jan;3(1):80–87. doi: 10.1016/s0147-619x(80)90036-0. [DOI] [PubMed] [Google Scholar]
- Shoemaker N. B., Smith M. D., Guild W. R. Organization and transfer of heterologous chloramphenicol and tetracycline resistance genes in pneumococcus. J Bacteriol. 1979 Aug;139(2):432–441. doi: 10.1128/jb.139.2.432-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J. L., Hurst S. F., Liberman E. S., Coleman S. E., Bleiweis A. S. Mutanolysin-induced spheroplasts of Streptococcus mutants are true protoplasts. Infect Immun. 1981 Feb;31(2):808–815. doi: 10.1128/iai.31.2.808-815.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J., Markowitz S. M., Macrina F. L. Transferable tetracycline resistance in Clostridium difficile. Antimicrob Agents Chemother. 1981 Jun;19(6):997–1003. doi: 10.1128/aac.19.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. D., Guild W. R. A plasmid in Streptococcus pneumoniae. J Bacteriol. 1979 Feb;137(2):735–739. doi: 10.1128/jb.137.2.735-739.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. D., Guild W. R. Improved method for conjugative transfer by filter mating of Streptococcus pneumoniae. J Bacteriol. 1980 Oct;144(1):457–459. doi: 10.1128/jb.144.1.457-459.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. D., Shoemaker N. B., Burdett V., Guild W. R. Transfer of plasmids by conjugation in Streptococcus pneumonias. Plasmid. 1980 Jan;3(1):70–79. doi: 10.1016/s0147-619x(80)90035-9. [DOI] [PubMed] [Google Scholar]
- Stuart J. G., Ferretti J. J. Genetic analysis of antibiotic resistance in Streptococcus pyogenes. J Bacteriol. 1978 Feb;133(2):852–859. doi: 10.1128/jb.133.2.852-859.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart J. G., Ferretti J. J. Transduction of rifampin resistance in group A streptococci. J Bacteriol. 1973 Aug;115(2):709–710. doi: 10.1128/jb.115.2.709-710.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMASZ A., HOTCHKISS R. D. REGULATION OF THE TRANSFORMABILITY OF PHEUMOCOCCAL CULTURES BY MACROMOLECULAR CELL PRODUCTS. Proc Natl Acad Sci U S A. 1964 Mar;51:480–487. doi: 10.1073/pnas.51.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagg J. R., Wannamaker L. W. Genetic basis of streptococcin A-FF22 production. Antimicrob Agents Chemother. 1976 Aug;10(2):299–306. doi: 10.1128/aac.10.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Control of the competent state in Pneumococcus by a hormone-like cell product: an example for a new type of regulatory mechanism in bacteria. Nature. 1965 Oct 9;208(5006):155–159. doi: 10.1038/208155a0. [DOI] [PubMed] [Google Scholar]
- Tomich P. K., An F. Y., Clewell D. B. Properties of erythromycin-inducible transposon Tn917 in Streptococcus faecalis. J Bacteriol. 1980 Mar;141(3):1366–1374. doi: 10.1128/jb.141.3.1366-1374.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomich P. K., An F. Y., Damle S. P., Clewell D. B. Plasmid-related transmissibility and multiple drug resistance in Streptococcus faecalis subsp. zymogenes strain DS16. Antimicrob Agents Chemother. 1979 Jun;15(6):828–830. doi: 10.1128/aac.15.6.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomura T., Hirano T., Ito T., Yoshioka M. Transmission of bacteriocinogenicity by conjugation in group D streptococci. Jpn J Microbiol. 1973 Nov;17(6):445–452. doi: 10.1111/j.1348-0421.1973.tb00930.x. [DOI] [PubMed] [Google Scholar]
- Ubukata K., Konno M., Fujii R. Transduction of drug resistance to tetracycline, chloramphenicol, macrolides, lincomycin and clindamycin with phages induced from Streptococcus pyogenes. J Antibiot (Tokyo) 1975 Sep;28(9):681–688. doi: 10.7164/antibiotics.28.681. [DOI] [PubMed] [Google Scholar]
- WINKLER K. C., VAN AMERONGEN J. Bacteriologic results from 4,000 root canal cultures. Oral Surg Oral Med Oral Pathol. 1959 Jul;12(7):857–875. doi: 10.1016/0030-4220(59)90036-2. [DOI] [PubMed] [Google Scholar]
- Walsh P. M., McKay L. L. Recombinant plasmid associated cell aggregation and high-frequency conjugation of Streptococcus lactis ML3. J Bacteriol. 1981 Jun;146(3):937–944. doi: 10.1128/jb.146.3.937-944.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannamaker L. W., Almquist S., Skjold S. Intergroup phage reactions and transduction between group C and group A streptococci. J Exp Med. 1973 Jun 1;137(6):1338–1353. doi: 10.1084/jem.137.6.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B., Holder S. B., Halling S. M. Deoxyribonucleic acid sequence common to staphylococcal and streptococcal plasmids which specify erythromycin resistance. J Bacteriol. 1979 Jun;138(3):990–998. doi: 10.1128/jb.138.3.990-998.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergren G., Emilson C. G. Transformation of streptococci to streptomycin resistance by oral streptococcal DNA. Arch Oral Biol. 1977;22(8-9):533–537. doi: 10.1016/0003-9969(77)90051-6. [DOI] [PubMed] [Google Scholar]
- Westergren G. Transformation of Streptococcus sanguis to a rough colonial morphology with an increased ability to adhere. Arch Oral Biol. 1978;23(10):887–891. doi: 10.1016/0003-9969(78)90292-3. [DOI] [PubMed] [Google Scholar]
- Willetts N., Skurray R. The conjugation system of F-like plasmids. Annu Rev Genet. 1980;14:41–76. doi: 10.1146/annurev.ge.14.120180.000353. [DOI] [PubMed] [Google Scholar]
- Yagi Y., Clewell D. B. Amplification of the tetracycline resistance determinant of plasmid pAM alpha 1 in Streptococcus faecalis: dependence on host recombination machinery. J Bacteriol. 1980 Aug;143(2):1070–1072. doi: 10.1128/jb.143.2.1070-1072.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi Y., Clewell D. B. Identification and characterization of a small sequence located at two sites on the amplifiable tetracycline resistance plasmid pAMalpha1 in Streptococcus faecalis. J Bacteriol. 1977 Jan;129(1):400–406. doi: 10.1128/jb.129.1.400-406.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Embden J. D., Engel H. W., van Klingeren B. Drug resistance in group D streptococci of clinical and nonclinical origin: prevalence, transferability, and plasmid properties. Antimicrob Agents Chemother. 1977 Jun;11(6):925–932. doi: 10.1128/aac.11.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Embden J. D., Soedirman N., Engel H. W. Transferable drug resistance to group A and group B streptococci. Lancet. 1978 Mar 25;1(8065):655–656. doi: 10.1016/s0140-6736(78)91153-4. [DOI] [PubMed] [Google Scholar]



