Abstract
Cocaine has high abuse liability but only a subset of individuals who experiment with it develop dependence. The DSM-IV (APA, 2000) provides criteria for diagnosing cocaine abuse and cocaine dependence as distinct disorders- the latter characterized by additional symptoms related to loss of control over drug use. In this study, two groups of cocaine users (n=8/group), matched on demographic factors and length of cocaine use history and meeting criteria for either cocaine abuse (CocAb) or cocaine dependence (CocDep), were compared on 1) measures related to impulsivity and sensation seeking, 2) response to experimenter-administered cocaine (0, 12.5, 25 and 50 mg/70 kg, iv), and 3) cocaine self-administration using a Relapse Choice and a Progressive Ratio Procedure (0, 12.5 and 25 mg/70 kg, iv). Groups did not differ on impulsivity or sensation seeking scores. After experimenter-administered cocaine, the CocAb group reported feeling more suspicious and observers rated them significantly higher on unpleasant effects (e.g., irritability, difficulty concentrating). In contrast, the CocDep group reported significantly greater desire for cocaine, which was sustained over the course of the study, and gave higher street value estimates for cocaine (p< .05). While cocaine self-administration was dose-related and generally comparable across the two procedures, the CocDep users chose to take significantly more cocaine than the CocAb users. These data suggest that, while regular long-term users of cocaine with cocaine abuse or dependence diagnoses cannot be distinguished by trait measures related to impulsivity, they do exhibit significant differences with regard to cocaine-directed behavior and response to cocaine administration.
Keywords: cocaine, self-administration, DSM-IV diagnosis, abuse, dependence, humans
1. Introduction
Epidemiological data show that the susceptibility to drug dependence is not a uniform risk — as individuals who have access to drugs do not always experiment, individuals who experiment do not always proceed to regular use, and individuals who use regularly do not always advance to compulsive use, even with a drug like cocaine, which is associated with very high abuse liability (e.g. Brady and Griffiths, 1976; Stafford et al., 1998). In a retrospective twin-registry study on the transition from drug use to dependence, cocaine was identified as possessing the highest probability of leading from regular use to abuse or dependence among the range of illicit drugs examined (Tsuang et al., 1999). Cocaine has also been characterized as having a more “explosive” risk for the development of drug dependence, that is, the risk of advancing to dependence occurs faster after initial experimentation compared to other drugs such as marijuana and alcohol for which the transition to dependence occurs more slowly (Wagner and Anthony, 2002).
Numerous factors are believed to contribute to the relative vulnerability to the development of drug dependence, including genetic predisposition (Kendler et al., 2000; Nestler, 2000), biological responses to drug exposure (Robinson and Berridge, 1993; Koob and Le Moal, 2000; Lyvers, 2000), and a wide range of environmental, developmental and social factors (e.g. Dusenbury et al., 1992; Kendler et al., 1999; Brook et al., 2000; Friedman and Glassman, 2000). The concept of this transition from drug abuse to drug dependence was described by a former Director of the National Institute on Drug Abuse as turning on the “switch” (Leshner, 1997). Popularization of the metaphorical “switch” provided a useful conceptual framework particularly for the lay audience, and, importantly spurred efforts to elucidate neuroadaptive changes that may mediate compulsive drug-taking behaviors.
While non-human models lend themselves readily to explore directly causative factors in the development of drug dependence (e.g., quantification of the relative rate of developing tolerance or sensitization), parallel controlled examination of similar phenomena in humans are more challenging. Studies requiring purposeful induction of the transition to addiction or dependence are precluded by ethical constraints. Alternatively, longitudinal studies examining the trajectory of drug experimentation to dependence require years to complete, and retrospective analyses require large groups of subjects and employ correlational techniques. An alternative strategy is to conduct controlled evaluations in which subjects, who are susceptible and presently drug dependent, are compared to others who, despite a comparable duration of drug use, have not developed drug dependence.
One defining feature that differentiates dependent individuals from abusers without dependence is their apparent “loss of control” over their drug use. That is, drug-dependent individuals whose use is frequent and compulsive, thus leading to negative consequences, are considered to have a loss of control, while sporadic users who maintain a regular use pattern without escalation are categorized dissimilarly. Indeed, “loss of control” has been used interchangeably with the term addiction and applied to conditions of excessive drug use (Jaffe, 1990), and even more widely investigated as a defining construct in alcoholism (Jellinek, 1960; Kahler et al., 1995). This concept has made its way into the treatment and recovery community on a broad scale in self-help groups based upon the 12-step ideology in which participants are encouraged to admit that they are helpless or powerless over their drug use. This construct has been operationalized and incorporated into widely used diagnostic criteria for drug dependence. Specifically, the “loss of control” construct is reflected in the DSM-IV dependence criteria (APA, 2000), whereby 1) “the substance is often taken in larger amounts or over a longer period than was intended,” and 2) “there is a persistent desire or unsuccessful efforts to cut down or control substance use.” In contrast to this clinical viewpoint, a behavior analyst might argue that the problem of compulsive drug use or drug dependence is not due to the loss of control, but rather due to the exquisite control that the drug (as a reinforcer) or the environmental context associated with drug use (as a conditioned reinforcer or discriminative stimulus) has come to exert over the individual's behavior and the concomitant relative decline in the value of alternative reinforcers (e.g., food, activities, social experiences), see for example (Johanson et al., 1976). The dependence criterion from the DSM-IV “important social, occupational, or recreational activities are given up or reduced because of substance use” is a diagnostic parallel that reflects this relative decline in reinforcing value of alternative reinforcers.
Despite intensive clinical research efforts directed toward understanding cocaine abuse and dependence and identifying effective treatments over the past two decades, surprisingly few studies have sought to evaluate directly factors that may differentiate individuals who intensify their use to a state of dependence compared to those who sustain regular, but controlled, patterns of use and remain in the diagnostic category of “abuse.” To examine differences between cocaine abusers with and without cocaine dependence with respect to their response to cocaine, drug-directed behaviors, or factors that may confer risk or resiliency to drug-taking and relapse in the laboratory, this study capitalized upon paradigms designed to assess drug-seeking behavior that have been developed and widely explored with non-human and, to a lesser extent, human subjects. Human self-administration studies have examined the influence of pharmacological factors (e.g. Fischman et al., 1990; Foltin and Fischman, 1994) and environmental factors (Griffiths et al., 1974; Silverman et al., 1994) on drug self-administration. To our knowledge, this paradigm has not been applied to study individual differences in the vulnerability to cocaine dependence, differences between groups who vary in their drug use characteristics, or the influence of environmental and pharmacological factors on cocaine-seeking behavior in dependent and non-dependent drug users.
The present study compared individuals meeting DSM-IV criteria for cocaine dependence to those meeting diagnostic criteria for cocaine abuse, but not dependence. As dependence can occur soon after initiation of drug use or after extended experimentation, it was important that the diagnosis of individuals meeting criteria only for cocaine abuse not be attributable to recent initiation of cocaine use (i.e., a lack of opportunity to develop dependence because of a short history of drug use). Thus, in addition to matching on basic demographic characteristics, groups were also matched on the duration of cocaine exposure (e.g., years of use) as a proxy for the opportunity to develop dependence. This controlled laboratory evaluation sought to compare individuals who report a loss of control over their cocaine use in the natural environment to those who exhibit regular but controlled use (as distinguished by frequency of reported cocaine use and DSM-IV diagnosis) on trait characteristics (e.g., impulsivity and sensation seeking), pharmacodynamic response to cocaine and on cocaine self-administration behaviors in the presence and absence of alternative reinforcers.
2. Methods
2.1 Subjects
Subjects were healthy adult male and female volunteers recruited through local newspaper advertisements and word-of-mouth. All subjects were determined to be in good health following medical history, physical examination, electrocardiogram (ECG), laboratory tests and were without psychiatric disorders other than their drug abuse according to a structured psychiatric interview (SCID, Structured Clinical Interview for Diagnosis, DSM-IV; Spitzer and Williams, 1986). Subjects were excluded if they were seeking treatment for cocaine abuse, had a history of seizures, cardiovascular disease, or diabetes, were pregnant, or were physically dependent on any other drug other than nicotine and caffeine. To qualify, all volunteers were required to report cocaine use for at least a 6-month duration, report current use within the last 30 days, and report either injecting or smoking cocaine.
Subjects were recruited to meet criteria for one of two groups: CocAb or CocDep users. CocAb users were required to 1) report cocaine use no more than twice weekly on average for two months prior to study admission, 2) meet DSM-IV criteria for cocaine abuse, but 3) not meet diagnostic criteria for cocaine dependence according to the SCID. CocDep users were required to 1) report cocaine use an average of six or more days per week for two months prior to study admission, and 2) meet diagnostic criteria for cocaine dependence according to the SCID, endorsing at least one of the criteria related to “loss of control.” Specifically, the “loss of control” construct is reflected in the DSM-IV dependence criteria (APA, 2000) whereby 1) “the substance is often taken in larger amounts or over a longer period than was intended,” or 2) “there is a persistent desire or unsuccessful efforts to cut down or control substance use.” Master's levels psychometricians with formal training on the administration of the SCID conducted the interviews, and all results were reviewed by a supervisor for consistency in diagnosis.
2.2 Study Design
This study was approved by the Johns Hopkins Institutional Review Board and was conducted in accordance with the 1964 Declaration of Helsinki. Volunteers provided written informed consent and were paid for participating ($24/night). Volunteers participated as inpatients living on a residential research facility for approximately 6 weeks. During this time, they were not allowed to consume caffeine; cigarette smoking was allowed except for 1.5 hours before and during experimental sessions.
This study used a double-blind, placebo-controlled, between-group (CocAb vs. CocDep) design where each volunteer participated in one assessment session, one cocaine safety screening session, six cocaine sample sessions, three relapse sessions, three progressive ratio sessions and one lottery session. In addition to being blinded to doses, both the subjects and the staff interacting with them in conducting sessions and collecting data were blinded to diagnostic category. All sessions were conducted at least 24 hours apart with a maximum of four sessions per week. The assessment session took place a minimum of three days after admission. The cocaine safety session was conducted at least 24 hours after the assessment session. Volunteers then participated in a series of six pairs of sessions, each pair consisting of one sample session and one self-administration session the next day (using either a choice or PR procedure). The order of the self-administration sessions (Choice or PR) was counterbalanced across subjects. The order of cocaine dose within each self-administration procedure was randomized. A lottery session was the last session prior to discharge.
2.3 Experimental Sessions
Experimental sessions were conducted in an isolated room that provided a constant testing environment. The subject was seated in a comfortable chair in front of a personal computer (Apple IIGS, Apple Computer, Cupertino, CA, USA) that recorded subjective, observer-rated and physiological data. A research assistant was present throughout sessions and initiated the data collection, monitored the volunteer and answered observer-rated questions. A physician was present, administered the drug and monitored the ECG for up to 30 minutes after each infusion. A peripheral i.v. line remained in place throughout all sessions.
2.3.1 Baseline Assessment Session
During the first session, subjects completed a number of assessments and behavioral tasks in the absence of drug administration. These measures included the NEO personality assessment (Costa and McCrae, 1985), the Eysenck Impulsivity Scale, I-7 version (Eysenck and Eysenck, 1978; Eysenck et al., 1985), the Sensation Seeking Scale (Zuckerman, 1994), the Barratt Impulsivity Scale, BSI-11 version (Patton et al., 1995), and a Gambling Task. The first four instruments were used to assess trait characteristics. The Gambling Task (Bechara et al., 1994; Bechara et al., 1998) is a decision-making task requiring evaluation of long-term consequences. Subjects are instructed that the goal of the task is to earn as much “play” money as possible by selecting one card at a time from any of four decks until they are instructed to stop. The four decks differ in the 1) magnitude and frequency of rewards (in which the subject receives a certain amount of money), and 2) penalties (in which the subject must pay a certain amount of money). The primary outcome of performance is the number of cards selected from the decks that result in an overall gain (the advantageous decks) versus an overall loss (the disadvantageous decks).
2.3.2. Cocaine Dose Response Session
Volunteers received four injections of intravenous cocaine (0, 12.5, 25, and 50 mg/70 kg) in ascending order with 60-minutes separating each injection. Physiological and subjective data were evaluated 30-minutes prior to any drug administration and for 1-hour after each infusion.
2.3.3. Sample Sessions
Sample sessions were conducted the day before each relapse or progressive ratio session. Volunteers received a single injection (cocaine at 0, 12.5, or 25/70 kg) and were instructed that this same dose would be available during the session the following day. Physiological and subjective data were collected 30-minutes before and for 1-hour after the infusion. Forty-five minutes after the injection, subjects completed the Multiple-Choice Procedure (Griffiths et al., 1993). For this task, subjects were asked to make sequential choices on a questionnaire between cocaine and various amounts of money. The data are reported as “crossover” point, which is the dollar value at which the first choice for money rather than drug is made. They were instructed that at the end of the study, during the “Lottery Drawing,” one of the choices they have made will be drawn at random and that they will receive whatever they chose (i.e., cocaine or money).
2.3.4. Relapse Sessions
Volunteers participated in three relapse sessions, each following a sample session conducted the previous day. In each session, volunteers had seven opportunities to choose between the assigned cocaine dose (0, 12.5 or 25 mg/70kg) and varying amounts of money; the seven trials occurred at 15-minute intervals. The value of the money alternative decreased by $3 over consecutive trials as follows: $19, $16, $13, $10, $7, $4, and $1. The money and the drug vial were placed in front of the volunteer before they made their choice. If the subject chose cocaine, that dose was injected within two minutes. If money was chosen the cash was handed to the volunteer. At the end of session, the cash was collected and the volunteer was given a receipt of their earnings. These earnings were separate from study participation earnings and both were paid to the volunteer upon discharge. All seven choices were presented to the volunteer regardless of their choices. A maximum of $70 could be earned if they chose money for all seven trials. Subjective and physiological data were collected at baseline, following each choice trial and for 60 minutes following the last choice trial.
2.3.5. Progressive Ratio Sessions
Subjects participated in three progressive ratio sessions. After a 30-minute baseline assessment period, volunteers were allowed to perform a task (pressing the button on the computer mouse) in order to earn up to seven injections of the assigned cocaine dose. The number of button-presses required to receive an infusion increased with each infusion earned (50, 250, 500, 1000, 1500, 2000, and 2500 times), and the subject was informed of the requirements. Responses, which could be made only every 0.5 seconds, were displayed on the computer monitor until the response requirement was fulfilled at which point an icon (“Injection earned”) would appear. Within two minutes of earning the cocaine dose, the injection would be administered to the volunteer. A minimum interval of 15 minutes between infusions was enforced for safety reasons. The session concluded after the completion of all seven injections, after 30 minutes of no button pressing, after the volunteer reported that they would work no longer, or after a total session time of 5 hours (4 hours of access to cocaine + 30 minute baseline and 30 minute post-last injection).
2.4. Experimental Outcomes
2.4.1. Subject- and Observer-Rated Measures
Subject-rated measures included visual analog scales, a street value question and an adjective checklist. Six visual analog questions were asked to determine the subjective effects of each injection. These included “Do you feel any drug effect?, Do you feel a rush?, Does the drug have any good effects?, Does the drug have any bad effects?, How much do you like the drug?” and “How much do you desire cocaine right now?” The subjects responded by positioning an arrow along a 100-point line labeled with “not at all” at one end and “extremely” at the other. The visual analog scales were administered at baseline (i.e., 15 minutes before first injection), once each minute for 10 minutes after the start of the injection, and 15, 30, and 45 minutes post-injection during the safety and sample sessions. During the relapse and progressive ratio sessions, the visual analog scales were completed at baseline and once each minute for 10 minutes after the start of each choice injection. If the money was selected during relapse sessions, the volunteer was only asked “How much do you desire cocaine right now?” once each minute for 10 minutes immediately following their choice of money. Street value was measured by asking “What is the street value of the dose you just received?” 10 minutes after each infusion during all sessions. The subject-rated adjective checklist consisted of 22 items rated from 0 (indicating “not at all”) to 4 (indicating “extremely”). The items were: craving for cocaine, dizzy/lightheaded, drug effect, dry mouth, excited, fearful, feel a thrill, feeling of power, fidgety, irritable, jittery, nervous, numbness, seeing/hearing things, sleepy, stimulated, stomach upset/nausea, suspicious, sweating, thirsty, tingling, tremor. Observer adjectives were rated on the same 5-point scale, and included drug effect, difficulty concentrating, fidgety, talkative, positive vocalizations, tremor/shaky, sweating, edgy/irritable and moody. Subjective- and observer-rated adjectives were administered at baseline and 10, 30, and 45 minutes after each infusion during the safety and sample sessions. For the relapse and progressive ratio sessions, the adjective checklists were administered at baseline and 10 minutes after each injection.
2.4.2. Physiological Measures
Heart rate, blood pressure (BP), and skin temperature were collected every minute using an automatic physiological monitoring device (Noninvasive Patient Monitor model 506, Criticare Systems, Waukesha, Wis.) that was interfaced with a Macintosh computer. Respiratory rate was taken by an observer who visually observed and counted the number of breaths taken by the volunteer for a 30-second period. Pupil diameter was determined from photographs taken in constant room lighting with a Polaroid camera (Polaroid Corp., Cambridge, Mass.) using a twofold magnification. Respiratory rate and pupil diameter were recorded 15 minutes before (baseline) infusion, 10, 15, 30, and 45 minutes after each infusion during the dose-response and lottery sessions. These same measures were recorded at baseline and 10 minutes after each infusion during the sample, relapse, and progressive ratio sessions.
2.5 Drugs
Cocaine powder (Mallinckrodt, Inc., St. Louis, MO) was aseptically prepared under a horizontal laminar flow hood by dissolving it in sterile saline to deliver a dose of 12.5, 25 or 50 mg/70 kg in 1 ml and filtering the solution through a 0.22-μm Millex-GS Millipore filter (Millipore, Bedford, MA) into a sterile, pryrogen-free vial (Lyphomed, division of Fujisawa USA, Inc., Deerfield, IL). The same volume of sterile saline served as the placebo. Cocaine and placebo were administered intravenously at a constant rate by a physician into a venous catheter in the arm over 60 seconds. During injections, the flow rate of the saline drip was increased to prevent backflow and ensure rapid delivery into the circulatory system. All injections were followed by a 1 ml saline flush through the catheter port.
2.6 Safety Criteria
A physician monitored the ECG continuously for a minimum of 15 minutes following each infusion. Prior to drug administration, the volunteer's heart rate had to be below 130 beats/minute, systolic BP <165 mm Hg, and diastolic BP <100 mm Hg to receive the next dose. If these criteria were not met, the injection was delayed until the physician judged it safe to proceed. Any subject with an abnormal ECG, increased heart rate greater than [(220-subjects age) × 0.85], systolic BP >180 mm Hg, or diastolic BP >120 mm Hg for 4 or more consecutive minutes did not receive the next injection and was terminated from the study.
2.7 Statistical Analysis
The two groups were compared on demographics and trait characteristics using Chi Square tests for dichotomous variables or independent t-tests with testing for equality of variance (Levene, 1960) for continuous variables. Baseline measures collected during the dose response session were examined for group differences with independent t-tests. Data collected from the dose response session (excluding baseline) were analyzed as raw time course data with 3-factor ANOVA (group [2], dose [4] and time) for mixed designs. The physiological measures collected on a minute-by-minute basis were summarized before analysis to yield average values for baseline, and time points for 5, 10, 15, 20, 30 and 40 minutes after each infusion. Subsequent analyses of transformed data, such as peaks and peak change from baseline were analyzed with two-factor mixed ANOVA (group [2] and dose [4]). Data from the relapse choice and progressive ratio sessions were analyzed with 2-factor ANOVA (group and dose) for mixed designs. Significant main and interaction effects were further explored by use of Tukey post-hoc tests. Spearman's rho was employed to examine the correlation between cocaine self-administration in the two procedures. All multi-level analyses were run with SAS Proc Mixed software. All analyses were conducted using SAS 9.1 for Windows and were considered significant when p ≤ 0.05.
3. Results
3.1 Subjects
3.1.1. Recruitment
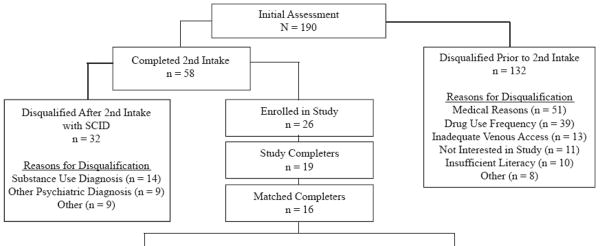
The results from the subject enrollment process are illustrated in the upper portion of Table 1. One hundred and ninety people completed an initial in-person assessment. Out of the 190 subjects screened 132 were disqualified for reasons unrelated to the SCID interview outcome [i.e., failure to meet the cocaine criteria, medical problems (e.g., abnormal EKG, hypertension), seeking treatment, lost interest in the study, or lost to follow-up]. Fifty-eight subjects met all preliminary study criteria and went on for the SCID diagnosis interview. Of those, 32 were disqualified because of failure to meet the cocaine abuse/dependence diagnosis or because they had an exclusionary psychiatric disorder. Twenty-six subjects (14%) were enrolled as inpatients and 19 completed the protocol. Of the seven discharged early, four were for non-medical reasons, one because he was informed of his positive HIV status, and two exceeded the BP safety criteria during testing.
Table 1.
Disposition of subjects during the multi-stage screening and evaluation process (upper half) and demographic and cocaine use characteristics of the subjects meeting criteria for the Cocaine Abuse group and Cocaine Dependence group who were matched and completed the study (lower half).
 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cocaine Abuse Group | Cocaine Dependence Group | ||||||||||||
| Subject | Sex | Racea | Age | Education (years) | Days Cocaine Use in Past 30* | Years Since First Use | Subject | Sex | Racea | Age | Education (years) | Days Cocaine Use in Past 30* | Years Since First Use |
| 1 | F | W | 44 | 12 | 3 | 8 | 9 | F | H | 27 | 9 | 20 | 7 |
| 2 | M | W | 23 | 12 | 1 | 2 | 10 | F | W | 40 | 15 | 30 | 5 |
| 3 | M | AA | 37 | 12 | 3 | 12 | 11 | M | AA | 29 | 14 | 30 | 12 |
| 4 | M | W | 40 | 12 | 4 | 15 | 12 | M | AA | 36 | 12 | 30 | 13 |
| 5 | M | AA | 35 | 10 | 9 | 15 | 13 | M | AA | 35 | 12 | 28 | 14 |
| 6 | M | W | 42 | 12 | 8 | 18 | 14 | M | W | 41 | 12 | 30 | 21 |
| 7 | M | W | 38 | 8 | 4 | 22 | 15 | M | W | 37 | 12 | 30 | 23 |
| 8 | F | AA | 42 | 10 | 10 | 23 | 16 | F | W | 36 | 12 | 30 | 18 |
| 6-M | 5-W | 37.6 | 11 | 5.25 | 14.38 | 5-M | 4-W | 35.1 | 12.25 | 28.5 | 14.13 | ||
| 2-F | 3-AA | (±6.61) | (±1.51) | (±3.28) | (±7.03) | 3-F | 3-AA | (±4.88) | (±1.75) | (±3.51) | (±6.33) | ||
| 1-H | |||||||||||||
Race: AA (African American), W (Caucasian), H (Hispanic)
No Group differences except where noted (*).
Independent-Group T-tests p<0.05
3.1.2. Safety
This study was originally designed to include a fourth cocaine dose (50 mg/70 kg) in the self-administration sessions. During enrollment of the first nine subjects, six doses were delayed and 17 doses were withheld due to elevated cardiovascular readings. These series of discharges and delays led to a protocol amendment that eliminated the highest cocaine dose (50mg/70kg) from any further self-administration sessions. All self-administration data from the 50mg/70kg dose were subsequently excluded from the analysis. Two elevated BP incidents occurred after repeated injections of cocaine (i.e., one subject was receiving 25mg/70kg, and the other 50mg/70kg) during progressive-ratio sessions. For the latter subject, on the last session, he chose to receive one 50mg/70kg dose before his BP elevated beyond the safety criteria; however, this was his last session so he was not excluded. After the elimination of the highest dose, there were an additional eight doses delayed and two withheld, but no additional volunteers were discharged due to cardiovascular reactions.
3.1.3. Matching
Nineteen subjects completed the study and were considered for matching. Subjects were first matched on years of cocaine use to ensure that they had comparable opportunities to develop cocaine dependence based upon duration of exposure. Subjects were then successfully matched on gender, age and race. Two groups of eight CocAb and CocDep users were matched and are reported here.
The lower portion of Table 1 shows subject demographics and drug use characteristics for the 16 matched subjects. The two groups did not differ on these characteristics (p > 0.05) except for their self-report of past 30-days of cocaine use (t[14] = 13.69; p < 0.0001; this was an a priori criterion by which the groups were defined). All subjects reported their preferred route of cocaine use to be smoking, except for one subject in the CocDep group who reported using intravenously. Self-reported use of other drugs in the last 30 days was low for both groups (6 or fewer occasions for alcohol, heroin, benzodiazepines and marijuana in both groups).
3.2. Cocaine Dose Response
3.2.1. Baseline Measures
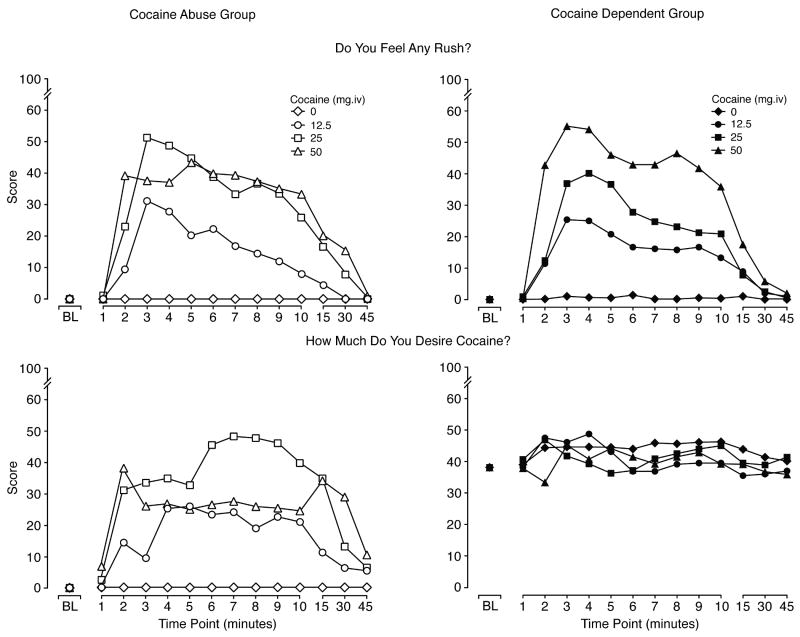
Few differences between the two groups were noted at pre-injection baseline (data collected at the start of the dose response session prior to the first injection). CocDep users reported a significantly greater “desire for cocaine” (visual analog scale; t[14] = 2.43; p = 0.045) compared to the CocAb group (see baseline data in Figure 1). Observer ratings of “edgy/irritable” revealed modestly lower, but statistically different, scores for the dependent users [t(14) = -2.65; p = 0.033].
Figure 1.
Data illustrate the mean scores for visual analog ratings of “Do you feel any rush?” (upper panels) and “How much do you desire cocaine right now?” (lower panels) for CocAb (n=8; right panels) and CocDep (n=8; left panels) users. Time course data are shown for up to 45 minutes after each of four consecutive injections of 0, 12.5, 25 and 50 mg/70 kg, i.v. given 1 hr apart. For ratings of “rush,” significant main effects were found for dose (F[3, 42]=13.9; p <0.0001) and time (F[12, 168]=18.3; p <0.0001). For ratings of desire for cocaine, significant main effects were found for group (F[1,14]=6.7; p = 0.022), time (F[12, 168]=4.1; p <0.0001) and a group by time interaction (F[12,168]=2.3; p = 0.009).
3.2.2. Visual Analog Scales
Cocaine produced significant dose- and time-dependent increases on ratings of “any drug effect,” “high,” “rush,” “good effects” and “liking for cocaine” (p <0.0001). There were no significant group differences (or interactions by group) on these measures. Data shown in Figure 1 (upper panel) for the visual analog time course for “rush” are representative of the other measures listed above, whereby scores were more clearly dose-related for the CocDep subjects (right panel), while the two higher doses (25 mg/70 kg and 50 mg/70 kg) were rated more similarly by the CocAb group (left panel; statistical outcomes shown in legend). In contrast to the other visual analog measures, there was a significant difference between the two groups on ratings of “How much do you desire cocaine?” (Figure 1; lower panels), whereby CocDep users reported sustained and relatively high scores for “desire” throughout the test session. Baseline data are included in Figure 1 (but were not included in the dose analysis) to illustrate that this group difference was present before any cocaine was administered (as described above). As can be seen, CocDep users reported elevated ratings of desire at baseline (see baseline analysis above), and ratings remained high following all injections with no evidence of a dose- or time-effect. In contrast, the CocAb group reported baseline levels of zero and cocaine produced dose-dependent and time-dependent increases in ratings of desire. Ratings of “desire” declined by 45 minutes for the CocAb group, while scores showed no evidence of diminishing for the CocDep group.
3.2.3. Adjective Checklists
Cocaine produced significant increases on a number of expected outcomes with the adjective rating scales (main effect of dose; df [3,42]; p <0.05 for each). For example, subject-rated scales revealed significant cocaine effects on ratings of “dizzy/lightheaded,” “tremor,” “jittery,” “fidgety,” “feel a thrill,” “stimulated” and “drug effect;” observers rated significant cocaine-related increases for “drug effect” only.
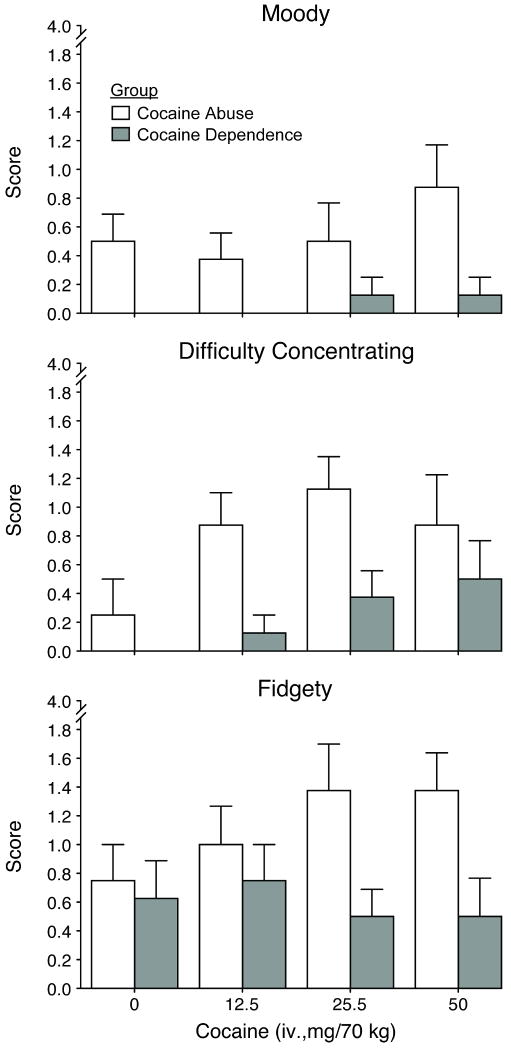
There were also a number of significant differences (p <0.05) between the groups (i.e., main effect of group) on the adjective rating scales with regard to response to cocaine. Subject-ratings were significantly higher for ratings of “suspicious” (F=7.9) in the CocAb group compared to CocDep group. Ratings for “crave cocaine” (F=6.0) were significantly higher in the CocDep group, similar to those reported for “desire” above, whereby the dependent users had elevated scores at baseline and their scores did not increase as a function of cocaine dose compared to the CocAb group. Both observers and subjects provided significantly higher scores for “sweating” (F=7.1) for the CocDep compared to the CocAb group. The observers also rated significantly higher scores for the CocAb group on measures of “moody,’ “difficulty concentrating,’ “fidgety” (see Figure 2 and legend for statistical outcomes), “drug effect” (F[1,14]= 4.7), and “edgy/irritable” (F=12.2).
Figure 2.
Mean peak data (±1 S.E.M.; n=8/group) are shown as a function of cocaine dose for three observer adjective rating scales collected during the dose response sessions. Significant differences between the groups were found on time course, peak analysis or both for ratings of “moody” (F[1,14]=4.98; p = 0.043), “difficulty concentrating” (F=5.9; p = 0.029) and fidgety (F=5.21; p = .039).
3.2.4. Street Value
Estimates of cocaine's street value increased as a function of dose. Moreover, there was a significant group × dose interaction (F[3,42]=5.1; p=0.005) characterized by CocDep group assigning higher average street values for active cocaine compared to the CocAb group, with the greatest difference (nearly 3-fold) occurring at the 50 mg/70 kg dose (means of $10.88 versus $30.38 for CocAb versus CocDep, respectively).
3.2.5. Physiological Measures
The time course analyses revealed significant dose-related increases (df=3, 42; p < 0.0001 main effects of dose) on physiological measures, including heart rate (F=22.6), systolic (F=26.4) and diastolic BP (F=12.8), and pupil diameter (F=6.5). However, no group differences were observed on these measures.
3.3. Cocaine Self-administration
Data on the pharmacodynamic effects of cocaine from the relapse and progressive ratio sessions are not presented because there were varied patterns of cocaine administration between volunteers and across conditions.
3.3.1. Relapse Procedure
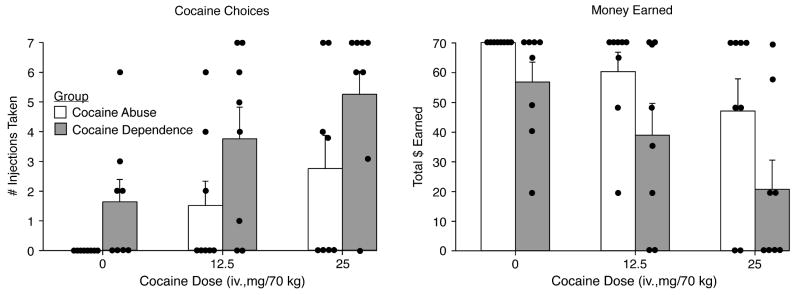
Figure 3 illustrates self-administration outcomes from the Relapse Choice Procedure for total injections taken (left panel) and money earned (right panel). The average number of injections taken (of seven available during each test session) increased significantly across both groups as a function of cocaine dose (see figure legends for statistical outcome). However, there was a significant difference (F[1,14] = 5.27; p = 0.038) between the two groups on the number of injections taken, whereby the CocDep group reliably chose to take more cocaine than the CocAb group, with nearly an average two-fold difference between the groups for both active doses. The individual subject data are also shown in the figure (as small circles) and reveal that the CocAb group selected all of the money alternatives when placebo was available, while half of the CocDep users chose placebo instead of money two or more times during the session. Moreover, more CocAb subjects chose to take no active cocaine doses, whereas there were very few instances when CocDep users chose money only. Also shown in Figure 3 is that the total amount of money earned decreased significantly in a dose-dependent fashion for both groups (each money vs. dollar choice is independent so the number of injections versus dollar earned do not covary precisely); however, the difference between the groups failed to reach significance (p = 0.055).
Figure 3.
Data are shown from the Relapse Choice procedure for mean number of cocaine choices (left panel) and average total money earned (right panel) for the CocAb and CocDep users. Average data are shown in the bars and the single solid dots represent the individual subjects. For the number of cocaine choices, there was a significant effect of group (F[1,14] 5.3; p = 0.038) and dose (F[2, 28] 3.4; p = 0.049). For the money earned, there was a significant effect of dose (F[2, 28]3.69; p = 0.038) and a trend for group (F[1,14]4.4; p = 0.055). Post-hoc analyses revealed that these differences reached significance on both outcomes for the 25 mg/70 kg condition (p <0.05).
3.3.2. Progressive Ratio Procedure
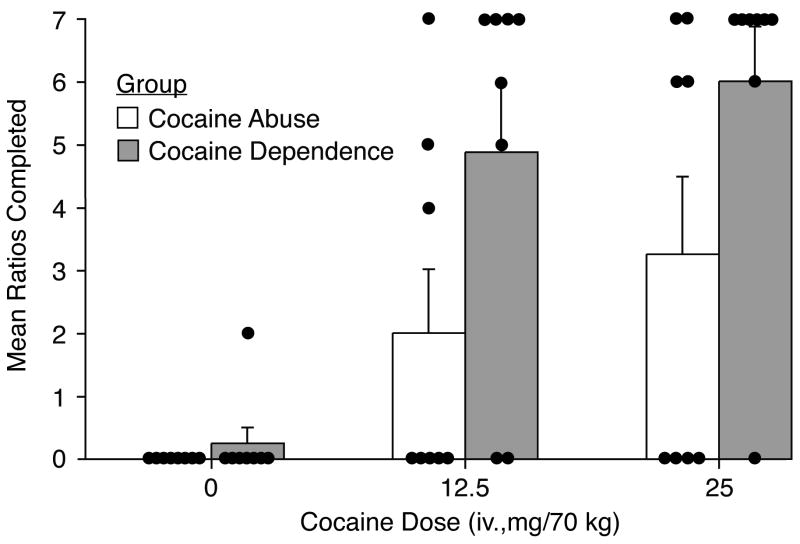
Figure 4 illustrates the individual data and mean number of ratios completed in progressive ratio sessions. Cocaine self-administration showed significant dose-related increases [F(2,28) = 8.43; p = 0.001]. While group differences appear similar to those observed in the Relapse Choice Procedure (i.e., with the CocDep group working to complete more ratios to receive cocaine compared to the CocAb group), this did not reach statistical significance (p = 0.056). As with the Relapse Procedure, there were more individuals in the CocAb group who earned no active injections (i.e., completed no ratios) compared to the CocDep group, in this case, in the absence of an alternative reinforcer.
Figure 4.
Data are shown from the Progressive Ratio procedure for the mean (±1 S.E.M.; n=8/group) number of ratios completed (i.e., injections earned). Average data are shown in the bars and the single solid dots represent the individual subjects. For the ratios completed, there was a significant effect of dose (F[2,28]=8.4; p = 0.001) but only trend towards a group effect (F[1,14]=3.92; p = 0.056).
Three individuals from the CocAb abstained from cocaine completely in both procedures; the other abstainers in the Relapse Choice and the Progressive Ratio sessions (1 CocAb and 2 CocDep) were separate individuals. When comparing the primary outcome of the Relapse Choice procedure (# of injections taken) to that of the Progressive Ratio procedure (# of ratios completed) collapsed across all doses, there was a strong positive correlation (Spearman's rho=.76; p<. 001) between these two measures of cocaine self-administration.
3.4 Multiple-Choice Procedure
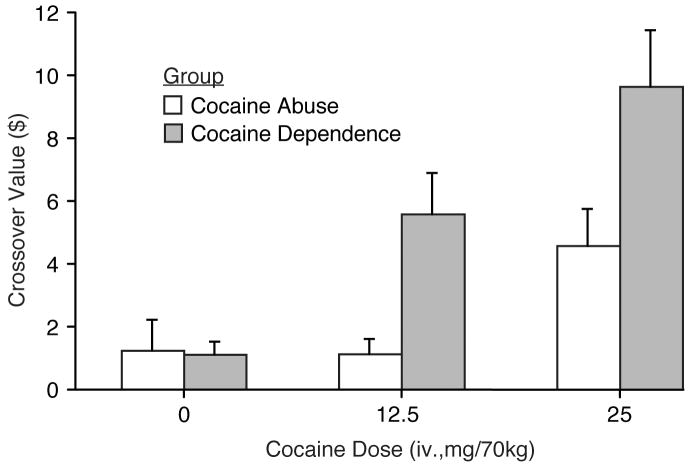
The Multiple-Choice questionnaire was collected during each sample session (i.e., six times- three preceding Progressive Ratio sessions and three preceding Relapse Choice Sessions). As shown in Figure 5, mean crossover points increased in a dose dependent fashion for both groups (see figure legend for statistical outcomes). There was also a significant difference between the two groups with the crossover points for the CocDep group greater than those for the CocAb group. Overall, the findings for the Multiple-Choice Procedure were similar to those for the cocaine self-administration procedures.
Figure 5.
Data shown are mean crossover values (±1 S.E.M.; n=8/group) from the Multiple-Choice Procedure collected at the end of the sample sessions preceding the Relapse Choice Procedure and the Progressive Ratio Procedure; as there was no significant difference on crossover values as a function of session type that followed (for the same doses); means shown here are collapsed across the two procedures. There was a significant effect of cocaine dose (F[2,28]=6.3; p = .006) and a significant difference between the CocAb and CocDep groups (F[1,14]=4.9; p = 044) on crossover values.
3.5 Trait and Impulsivity Measures
Table 2 presents the outcomes for the trait and impulsivity measures. There were no significant baseline differences between the groups on scales measuring impulsivity [Eysenck Scale and Barratt Impulsivity Scale] nor sensation-seeking behavior [Zuckerman Sensation Seeking Scale]. The NEO showed no group difference on any subscale.
Table 2.
Data are shown for the mean (±1 SEM) scores for the CocAb (n=8) versus CocDep (n=8) groups for the baseline characteristic measures on four questionnaires. There were no significant differences between the two groups.
| Non-Dependent Users | Dependent Users | |
|---|---|---|
| Barratt Impulsiveness | 64.6 (±2.1) | 64.8 (±3.4) |
| Eysenck Impulsivity Scale | ||
| Impulsiveness | 7.3 (±1.1) | 8.0 (±1.5) |
| Venturesomeness | 9.9 (±0.7) | 10.3 (±1.6) |
| Empathy | 11.5 (±1.1) | 12.9 (±0.7) |
| Zuckerman Sensation Seeking | ||
| Thrill & Adventure | 6.6 (±0.7) | 6.1 (±1.4) |
| Experience Seeking | 5.5 (±0.6) | 4.9 (±0.9) |
| Disinhibition | 4.5 (±0.8) | 5.8 (±0.8) |
| Boredom Susceptibility | 1.9 (±0.6) | 2.1 (±0.4) |
| Total Score | 18.5 (±1.7) | 18.9 (±2.3) |
| NEO | ||
| Neuroticism | 79.8 (±5.3) | 86.1(±7.9) |
| Extraversion | 113.1 (±2.5) | 114.4(±3.8) |
| Openness | 103.6 (±3.2) | 99.4(±1.8) |
| Agreeableness | 120.3 (±5.3) | 112.6(±5.7) |
| Conscientiousness | 118.6 (±5.1) | 112.5(±5.1) |
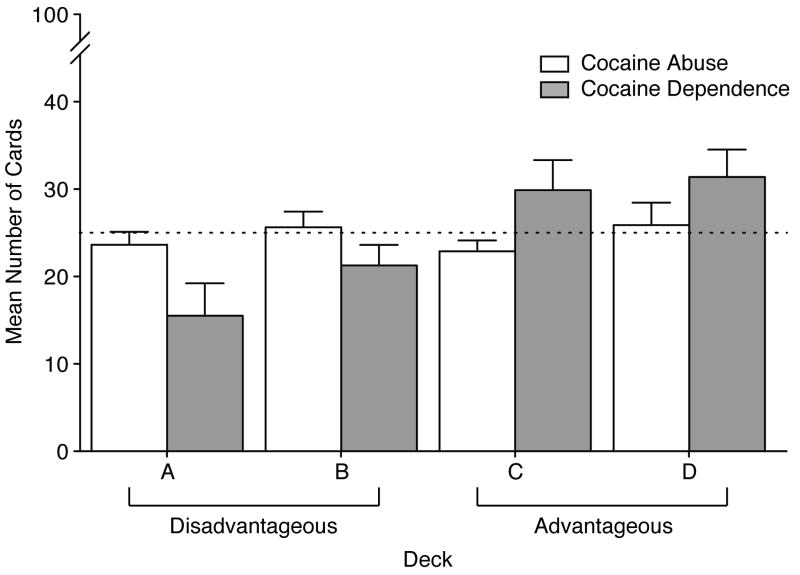
Results for the gambling task card selection are shown in Figure 6 for the two groups. Random selection of cards from the 100 trials would yield approximately 25 cards (chance, indicated by the dotted line) from each of the four decks. The CocAb group chose more cards from the disadvantageous decks (Decks A and B) and fewer cards from the advantageous decks (Decks C and D) compared to the CocDep group. These overall differences did not reach statistical significance. However, for both Decks A and B, no selection differences were observed between groups in the first bins of card choices (i.e., 1-20), but the CocDep group showed significant reductions in disadvantageous card choice in some subsequent bin trials (21-40, 81-100; p <. 05). Overall, these choices led the CocDep group to earn more winnings ($415.63) compared to CocAb group which lost money overall (-387.50).
Figure 6.
Mean data mean (±1 S.E.M.; n=8/group) are shown for the Gambling task (Bechara et al., 1994; Bechara et al., 1998) for the dependent (white bars) and non-dependent (gray bars) groups. The dotted line indicates the card choice that would occur by chance for the disadvantageous card decks (A and B) and the advantageous card decks (C and D). The overall choice behavior did not differ significantly between the groups.
4. Discussion
This study examined differences between individuals with lengthy histories of cocaine use on their pharmacodynamic response to cocaine, their cocaine self-administration behavior under multiple conditions, and baseline characteristics related to impulsivity and sensation seeking. Two groups of current users, those meeting DSM-IV criteria for cocaine dependence and those meeting only criteria for cocaine abuse were compared. The groups did not differ significantly with regard to their reported duration of cocaine use (both averaging slightly more than 14 years of use) or their average age of initial cocaine use (21.0 vs. 23.5 for the CocDep and CocAb groups, respectively), and thus individuals in both groups had equivalent opportunities to develop cocaine dependence. While the groups did not differ significantly on baseline trait measures, they did exhibit differences in response to experimenter-administered cocaine and differed significantly in their cocaine self-administration behavior with these latter differences corresponding to their self-reported cocaine use frequency.
While experimenter-administered cocaine produced its expected dose- and time dependent effects (i.e., cardiopressor effects, subjective reports of euphoria), the CocAb and CocDep groups exhibited differences on some, but not most, pharmacodynamic responses. The absence of differences on any physiological measure (e.g., heart rate, blood pressure, mydriasis) suggests there was no differential biological or metabolic response. However, the CocAb subjects' ratings indicated that cocaine produced significantly greater suspiciousness, while the observers provided modestly, but significantly, higher ratings for that same group on difficulty concentrating, irritability, moodiness and strength of drug effect compared to the dependent group. Combined, these findings suggest that the CocAb subjects, while not differentially sensitive to the direct euphorigenic effects of cocaine, may actually exhibit increased sensitivity to the anxiogenic or unpleasant effects of cocaine compared to the dependent users or perhaps tolerance to these specific effects in the dependent subjects. In perhaps the only other study published to date examining the response to cocaine in sporadic versus heavy users, Mendelson and colleagues reported that a single challenge with a moderate dose (28 mg/70 kg) of intravenous cocaine in a group of male occasional cocaine users resulted in significantly greater cardiovascular responses, higher ratings on subjective measures of euphoria and “high,” and a greater increase in ACTH release compared to the cocaine-dependent subjects (Mendelson et al., 1998). The authors interpreted these findings as evidence of tolerance to cocaine in the heavier users. While those data, showing differential sensitivity to the positive subjective effects of cocaine, are seemingly at odds with the present findings where no differences on positive subjective outcomes were found, they are consistent with more experienced users exhibiting a decreased response to cocaine. However, the history of exposure, including frequency and duration of cocaine use, differed substantively between the two studies. In the earlier study, the occasional users group reported only 5-10 occasions of cocaine use in the preceding year. The CocAb subjects in the present study were more frequent users (1-2 times/weekly) and had a history of regular cocaine use that was longer even than the heavy user group in the study by Mendelson and colleagues.
With respect to desire or craving for cocaine, the CocDep users reliably reported significantly greater levels of desire compared to the CocAb group. These elevated ratings in desire, however, were evident at baseline and were not increased after challenge with active cocaine. Examination of all of the sessions (dose response and all sample sessions [latter data not shown]) indicated that these differences persisted over the course of the inpatient stay. In contrast, the CocAb subjects reported baseline ratings of zero for desire for cocaine prior to injection, and active cocaine doses triggered generally dose-related increases in ratings of desire and craving, consistent with earlier reports of cocaine-induced cocaine craving (Jaffe et al., 1989). Moreover, CocDep users valued cocaine to a greater degree on estimates of the street value of the dose received suggesting that they both wanted cocaine more and valued it more highly when received, and these data were concordant with the findings from the Multiple-Choice Procedure which revealed higher crossover values (the point at which money is chosen over drug) in the CocDep group.
Both the Relapse Choice Procedure and the Progressive Ratio Procedure yielded reliable and dose-dependent cocaine self-administration in these volunteers. Importantly, findings for the two procedures were generally concordant suggesting that both have good face validity and utility as laboratory models as there was a strong positive relationship between self-administration in the two procedures, and both were predicted by the subjects' self-report of cocaine prior to study entry. Two differences between the outcomes for the procedures are notable. First, the average cocaine intake was reliably (but not significantly) higher when each active dose was made available in the Progressive Ratio Procedure compared to the Relapse Choice Procedure (and this was true for both groups) suggesting that the availability of an alternative reinforcer (i.e., money) in the Relapse Choice Procedure may have reduced cocaine taking as has been previously reported (Higgins et al., 1994). Second, of the dependent users, only one individual was willing to work for placebo in the Progressive Ratio Procedure, while one-half of the group chose placebo at least once in the Relapse Choice Procedure. In no instance did the CocAb users choose or work for placebo. Responding for placebo could indicate greater drug-related conditioned responses in the CocDep user group and could even reflect the “loss of control” feature of drug dependence. Most importantly, the self-administration data revealed that the CocDep users rarely abstained from choosing active cocaine when available (in contrast to the CocAb subjects) and reliably self-administered more cocaine (on average twice as much) than the CocAb users. Thus, the self-reported history of greater cocaine use in the natural environment by the dependent users was also captured in these laboratory analogs of cocaine use, which proved sensitive at detecting group differences.
This study found no significant differences between the two groups of subjects on measures of sensation seeking or impulsivity. These attributes have been the subject of much research and are often reported as risk factors for the development of substance abuse or dependence (see for recent review Verdego-Garcia et al., 2008). However, with regard to cocaine users, there are a limited number of studies with which to compare the present findings and perhaps none that have examined differences between cocaine dependent and non-dependent active users. Studies have reported that cocaine dependent individuals have higher scores on the Eysenck, the Barratt and the Zuckerman scales when compared to non-drug using controls (Coffey et al., 2003; Patkar et al., 2004). Also, studies of cocaine-dependent individuals enrolled in outpatient clinical trials have reported that greater impulsivity, as assessed by these questionnaires, was significantly correlated with greater cocaine use and poorer retention in treatment (Moeller et al., 2001; Patkar et al., 2004). Importantly, average scores for these trait questionnaires in the present study were comparable to those reported previously for cocaine dependent individuals (Moeller et al., 2001; Petry, 2001; Patkar et al., 2004). With regard to these measures, the present study focused on only a small but carefully selected subgroup of individuals and may be hampered by limited power; however, group differences were evident on a number of other outcomes. Thus, while these trait measures may be predictive of risk for experimentation and response to treatment, they did not differentiate the two distinct diagnostic categories of cocaine abuse and dependence.
The Gambling task (Bechara et al., 1994; Bechara et al., 1998), widely used to assess decision making and sensitivity to future consequences, did not reveal statistically significant differences between the two groups in their overall card choices. Inspection of the data reveals that the CocAb group did not vary their card selection as a function of advantage or disadvantage of the decks (i.e., their card selection was nearly always close to chance), whereas the CocDep individuals showed a pattern of selection more consistent with choosing to increase earnings (i.e., fewer cards from the disadvantageous decks than the advantageous decks). There were, however, some significant differences between the groups for selection of disadvantageous cards across trials within the session, whereby the CocDep users chose fewer disadvantageous cards on successive trials suggesting better acquisition or sensitivity to the contingencies, compared to the relatively unchanged selection by the CocAb group. As with the trait measures, studies have reported generally poorer decision making on the gambling task in cocaine-dependent individuals when compared to non-drug using controls (Verdejo-Garcia et al., 2007; van der Plas et al., 2008). A recent study reported this same finding but went further to compare decision-making when the reward was hypothetical or when monetary contingencies were in place (Vadhan et al., 2009). That report indicated that the performance of cocaine dependent individuals with lengthy cocaine use histories (average of 20.5 years), while deficient in the hypothetical rewards task, did not differ from the controls when there was an opportunity to earn real money. This plasticity in responding with cocaine-dependent individuals is consistent with the observation in the present study that cocaine dependent individuals were able to alter their response across trials to improve outcomes.
In conclusion, this study did not provide support for the hypothesis that characteristics related to impulsivity or sensation seeking will distinguish between individuals with longstanding histories of cocaine use who meet DSM-IV criteria for abuse versus those who escalate to develop cocaine dependence. While few differences in pharmacodynamic response to cocaine were found between the groups, the composite profile suggests that the CocAb subjects may be more sensitive to the unpleasant effects of cocaine; it is possible that this may represent a protective mechanism against escalation of use. Importantly, this study demonstrates that cocaine dependent individuals report greater desire for cocaine, value cocaine more highly based upon street value estimates and the Multiple-Choice Procedure, and self-administer greater amounts of cocaine compared to those meeting criteria only for cocaine abuse. A primary distinguishing feature of dependence is the loss of control over drug use. The present study found that cocaine dependent individuals were more likely to take all of the available cocaine (and some even chose placebo) compared to their non-dependent counterparts in both the presence and absence of alternative reinforcers and may be reflective of the loss of control over drug use. While few laboratory studies have employed these procedures to examine individual differences in drug taking in humans, this study highlights the power and sensitivity of these methods and their potential utility for exploring factors that mediate the complex behaviors of drug abuse and dependence. Finally, cocaine self-administration under controlled circumstances in the laboratory reflected the differential rates of self-reported cocaine use in the natural environment between groups contributing further evidence of the validity of these laboratory models.
Acknowledgments
The authors would like to thank the following staff members who contributed to this project: John Yingling for technical assistance, Abby Yuscavage, Lisa Notes, Abigail Robarts, Victoria Casselton, Anthony Fluty and the Residential Nursing Staff for research support, and Jeff McCagh, Pharm.D. for pharmacy support.
Role of Funding Source Funding Source: This work was supported by grants from the National Institute on Drug Abuse (NIDA), R0114653 (SLW) and T320007209 (ECD); NIDA had no further role in study design; in the collection, analysis and interpretation of the data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Conflicts of Interest: All of the authors declare that they have no conflicts of interest to report that could inappropriately influence, or be perceived to influence, this work.
Contributors: Dr. Walsh secured funding for the project. Drs. Walsh, Donny and Bigelow participated in the design the study. Dr. Walsh and Mr. Nuzzo undertook the statistical analysis. Dr. Umbricht provided medical support and supervised dosing sessions. Dr. Walsh wrote the manuscript. All authors have contributed to and approve the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
List of References
- APA. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-R. American Psychiatric Association; Washington, D.C.: 2000. [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Tranel D, Anderson SW. Dissociation of working memory from decision making within the human prefrontal cortex. J Neurosci. 1998;18:428–437. doi: 10.1523/JNEUROSCI.18-01-00428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady JV, Griffiths RR. Behavioral procedures for evaluating the relative abuse potential of CNS drugs in primates. Fed Proc. 1976;35:2245–2253. [PubMed] [Google Scholar]
- Brook JS, Whiteman M, Finch S, Cohen P. Longitudinally foretelling drug use in the late twenties: adolescent personality and social-environmental antecedents. J Gen Psychol. 2000;161:37–51. doi: 10.1080/00221320009596693. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exper Clin Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. The NEO Personality Inventory Manual. Psychological Assessment Resources; Odessa, Florida: 1985. [Google Scholar]
- Dusenbury L, Khuri E, Millman RB. Adolescent substance abuse: A sociodevelopmental perspective. In: Lowinson JH, Ruiz P, Millman RB, editors. Substance Abuse A Comprehensive Textbook. Williams & Wilkins; Baltimore, MD: 1992. pp. 832–842. [Google Scholar]
- Eysenck SBG, Eysenck HJ. Impulsiveness and venturesomeness: Their position in a dimensional system of personality description. Psychol Rep. 1978;43:1247–1255. doi: 10.2466/pr0.1978.43.3f.1247. [DOI] [PubMed] [Google Scholar]
- Eysenck SBG, Pearson PR, Easting G, Allsopp JF. Age norms for impulsiveness, venturesomeness and empathy in adults. Personality Indiv Differences. 1985;5:613–619. [Google Scholar]
- Fischman MW, Foltin RW, Nestadt G, Pearlson GD. Effects of desipramine maintenance on cocaine self-administration. J Pharmacol Exper Ther. 1990;253:760–770. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Effects of buprenorphine on the self-administration of cocaine by humans. Behav Pharmacol. 1994;5:79–89. doi: 10.1097/00008877-199402000-00009. [DOI] [PubMed] [Google Scholar]
- Friedman AS, Glassman K. Family risk factors versus peer risk factors for drug abuse. A longitudinal study of an African American urban community sample. J Subst Abuse Treat. 2000;18:267–275. doi: 10.1016/s0740-5472(99)00072-0. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Liebson IA. Suppression of ethanol self-administration in alcoholics by contingent time-out from social interactions. Behav Res Therapy. 1974;12:327–334. doi: 10.1016/0005-7967(74)90007-2. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Troisi JR, Silverman K, Mumford GK. Multiple-choice procedure: an efficient approach for investigating drug reinforcement in humans. Behav Pharmacol. 1993;4:3–13. [PubMed] [Google Scholar]
- Higgins ST, Bickel WK, Hughes JR. Influence of an alternative reinforcer on human cocaine self-administration. Life Sci. 1994;55:179–187. doi: 10.1016/0024-3205(94)00878-7. [DOI] [PubMed] [Google Scholar]
- Jaffe JH. Trivializing dependence. Brit J Addict. 1990;85:1425–1427. doi: 10.1111/j.1360-0443.1990.tb01624.x. [DOI] [PubMed] [Google Scholar]
- Jaffe JH, Cascella NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacol. 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- Jellinek EM. The disease concept of alcoholism. Hillhouse Press; New Brunswick, NJ: 1960. [Google Scholar]
- Johanson CE, Balster RL, Bonese K. Self-administration of psychomotor stimulant drugs: The effects of unlimited access. Pharmacol Biochem Behav. 1976;4:45–51. doi: 10.1016/0091-3057(76)90174-x. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Epstein EE, McCrady BS. Loss of control and inability to abstain: the measurement of and the relationship between two constructs in male alcoholics. Addiction. 1995;90:1025–1036. doi: 10.1046/j.1360-0443.1995.90810252.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Corey LA, Prescott CA, Neale MC. Genetic and environmental risk factors in the aetiology of illicit drug initiation and subsequent misuse in women. Brit J Psychiatry. 1999;175:351–356. doi: 10.1192/bjp.175.4.351. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse, and dependence in a U.S. population-based sample of male twins. Arch Gen Psychiatry. 2000;57:261–269. doi: 10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostatis. Neuropsychopharmacol. 2000;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278:45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- Levene H. Contributions to Probability and Statistics: Essays in honor of Harold Hotelling. Stanford University Press; 1960. [Google Scholar]
- Lyvers M. “Loss of control” in alcoholism and drug addiction: A neuroscientific interpretation”. Exper Clin Psychopharmacol. 2000;8:225–249. doi: 10.1037//1064-1297.8.2.225. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Sholar M, Mello NK, Teoh SK, Sholar JW. Cocaine tolerance: Behavioral, cardiovascular, and neuroendocrine function in men. Neuropsychopharmacol. 1998;18:263–271. doi: 10.1016/S0893-133X(97)00146-2. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski JG. The impact of impulsivity on cocaine use and retention in treatment. J Sub Abuse Treatment. 2001;21:193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Genes and addiction. Nature Genet. 2000;26:277–281. doi: 10.1038/81570. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Murray HW, Mannelli P, Gottheil E, Weinstein SP, Vergare MJ. Pre-treatment measures of impulsivity, aggression and sensation seeking are associated with treatment outcome for African-American cocaine-dependent patients. J Addict Dis. 2004;23:109–122. doi: 10.1300/J069v23n02_08. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol. 1995;51:768–775. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Petry NM. Substance abuse, pathological gambling, and impulsiveness. Drug Alcohol Depend. 2001;63:29–38. doi: 10.1016/s0376-8716(00)00188-5. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Silverman K, Kirby KN, Griffiths RR. Modulation of drug reinforcement by behavioral requirements following drug ingestion. Psychopharmacol. 1994;114:243–247. doi: 10.1007/BF02244844. [DOI] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacol. 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Harley RM, Xian H, Eisen S, Goldberg J, True WR, Faraone SV. Genetic and environmental influences on transitions in drug use. Behav Genetics. 1999;29:473–479. doi: 10.1023/a:1021635223370. [DOI] [PubMed] [Google Scholar]
- Vadhan NP, Hart CL, Haney M, van Gorp WG, Foltin RW. Decision-making in long-term cocaine users: Effects of a cash monetary contingency on Gambling task performance. Drug Alcohol Depend. 2009;102:95–101. doi: 10.1016/j.drugalcdep.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Plas EAA, Crone EA, Wery PM, van den Wildenberg WPM, Tranel D, Bechara A. Executive control deficits in substance-dependent individuals: A comparison of alcohol, cocaine, and methamphetamine and of men and women. J Clin Exper Neuropsychol. 2008;26:1–14. doi: 10.1080/13803390802484797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdego-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: Review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Benbrook A, Funderburk F, David P, Cadet JL, Bolla KI. The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing with the Iowa Gambling Task. Drug Alcohol Depend. 2007;90:2–11. doi: 10.1016/j.drugalcdep.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacol. 2002;26:479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Behavioral expressions and biosocial bases of sensation seeking. Cambridge University Press; New York, New York: 1994. [Google Scholar]