Introduction
The function of lymphocytes is tightly regulated by signals that are initiated by antigen or antigen/MHC binding to cell surface antigen receptors. These signals are critical for initiation and reactivation of adaptive immune responses, but also play an essential role in establishing tolerance to self-antigens. Antigen receptors do not function alone in these decision-making processes. Rather the ultimate outcome of an encounter with antigen also depends upon coordinate reception of secondary and tertiary signals provided by cell associated ligands, e.g. CD40L, as well as cytokines, chemokines and TLR agonists.
Antigen receptors may function as simple binary switches that are “off” until antigen is bound, at which point they transduce an invariant “on” or “go” signal. Alternatively, signaling may change depending on parameters such as antigen structure or duration of interaction with the cell. For example, a time-honored hypothesis holds that tolerance results from antigen receptor stimulation for an extended period of time without reception of a second signal. A tenet of this hypothesis is that the antigen receptor's signaling function changes, e.g. is attenuated, in the absence of a secondary or tertiary queue. This presentation will focus on the mechanism of signal transduction by B lymphocyte antigen receptors, and how signaling function changes when cells are chronically stimulated by autoantigens, leading to an unresponsive state known as anergy. As time permits, we will discuss disruption of anergy by infectious agents that deliver signals to innate sensors such as TLR.
B cell antigen receptor structure and signaling
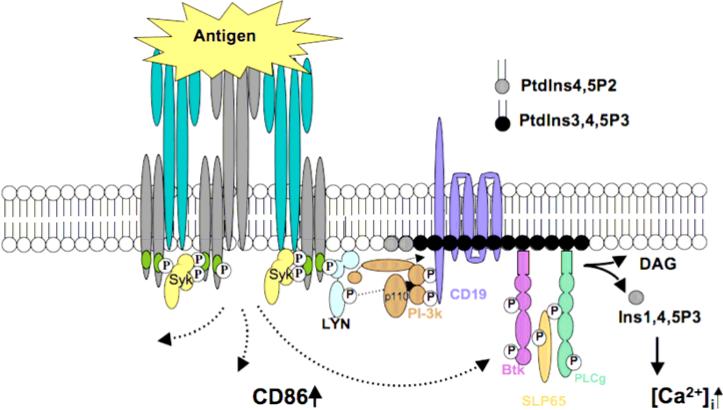
Cells of the immune system sense their specific antigen using cell surface receptors that are composed of multiple subunits. A division of labor exists within these receptors wherein antigen binding and signal transducing functions are vested in distinct, non-covalently substructures. B lymphocyte antigen receptors (BCR) are composed of a membrane bound form of immunoglobulin that serves as their antigen binding substructure, and a heterodimer of CD79a and b (Ig-α and β) that serves as their transducer substructure (figure 1). Unlike many growth factor receptors, antigen receptors do not contain “on-board” enzymatic activity, and thus must recruit effector enzymes that propagate and amplify signals. Key to this process are signaling motifs found in the cytoplasmic tails of the receptors transducer subunits. These motifs, designated “immunoreceptor tyrosine-based activation motifs” or ITAMs, contain two conserved tyrosine residues that when phosphorylated bind to downstream effectors via their type 2 SRC homology (SH2) domains. Most critical in signaling is the engagement of the tyrosine kinase SYK that binds via its paired SH2 domains to the precisely spaced phosphotyrosines in the ITAMs. Binding of SYK is the critical “tipping point” for cell activation through antigen receptors. ITAM phosphorylation leading to SYK activation is triggered by antigen aggregation of receptors, which by increasing local concentrations of ITAMs and receptor-associated SRC family kinases that phosphorylate them, promotes ITAM tyrosine phosphorylation. It is important to emphasize that SYK engagement absolutely requires dual phosphorylation of ITAMs.
Figure 1.
Silencing autoreactive B cells by anergy
Recent studies indicate that as many as 70% of newly generated B cell are autoreactive, and must be silenced to prevent development of autoimmune disease. While this silencing is known to occur by at least three mechanisms; receptor editing, clonal deletion and anergy, it appears that most autoreactive cells are silenced by anergy. Anergic B cells display a marker phenotype suggestive of developmental arrest at the transitional stage. However, this does not reflect developmental arrest because mature B cells also acquire this phenotype and become anergic upon encounter with antigen in the absence of second signal. Anergic B cells persist in the periphery for ~5 days and continue to express antigen receptors (BCR), many of which are unoccupied by antigen. However, these receptors do not transduce activating signals upon antigen binding. Interestingly however, maintenance of this unresponsive state requires chronic occupancy of small proportion of receptors by antigen. These finding are consistent with the two-signal model of tolerance induction, showing that chronic antigen receptor occupancy in the absence of secondary signals leads to a change, possibly attenuation, in antigen receptor signaling.
The BCR as a three position molecular switch
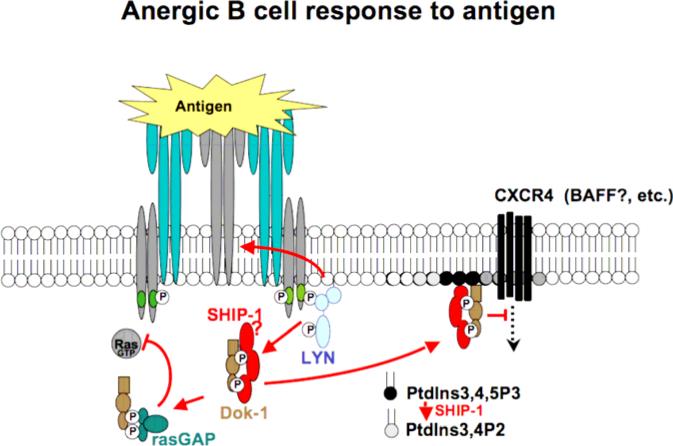
This presentation will focus on molecular mechanisms underlying transduction of BCR signals that enforce the antigen unresponsiveness of anergic B cells. Results suggest that the BCR functions minimally as a three position molecular switch. In unoccupied receptors, ITAM tyrosines are unphosphorylated and the receptor is therefore “off”. Acute occupancy of receptors leads to transduction of activating signals via biphosphorylation of ITAM tyrosines and consequent engagement of the SYK tyrosine kinase and downstream pathways. Chronic receptor occupancy leads to biased ITAM monophosphorylation and engagement of the Lyn tyrosine kinase and its stimulation of downstream inhibitory signaling circuitry (figure 2). By tyrosine phosphorylation of the inositol lipid phosphatase SHIP and its adaptor Dok-1, Lyn triggers a pathway that blocks signaling by receptors that require PtdIns3,4,5P3 for signaling. This regulation is imposed locally on, for example, signaling by co-aggregated BCR, and more broadly, inhibiting signaling by remotely stimulated chemokine, cytokine and perhaps BAFF receptors. It is likely that other Lyn targets, e.g. CD22 and coupled tyrosine phosphatases SHP1 and SHP2, also participate in maintenance of anergy.
Figure 2.
Infection and disruption of anergy
Acting through innate immune sensors, infectious agents such as gammaherpesvirus 68 are able to subvert anergy and trigger production of autoantibodies. This appears to involve virus-associated agonists for innate immune sensors, and occurs by engagement of B cell intrinsic sensing pathways that require MyD88, a TLR transducer. We will discuss preliminary evidence that activation of innate immune sensors disrupts anergy by complementing rather than correcting BCR signaling “defects” in anergic cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Recommended reading
B cell anergy: from transgenic models to naturally occurring anergic B cells Cambier JC, Gauld SB, Merrell KT, Vilen BJ. Nat Rev Immunol. 2007 Aug;7(8):633-43.