Abstract
Activation of neurons in the bed nucleus of the stria terminalis (BNST) plays a critical role in stress and anxiety-related behaviors. Previously, we have shown that serotonin (5-HT) can directly modulate BNST neuronal excitability by an action at postsynaptic receptors. In this study we built upon that work to examine the effects of 5-HT on excitatory neurotransmission in an in vitro BNST slice preparation. Bath application of 5-HT reversibly reduced the amplitude of evoked excitatory postsynaptic currents (eEPSCs). These effects were mimicked by the 5-HT1B/D receptor agonist, sumatriptan, and by the 5-HT1B receptor selective agonist, CP93129. Conversely, the effects of 5-HT and sumatriptan could be blocked by the 5-HT1B receptor selective antagonist, GR55562. In contrast, the 5-HT1A receptor agonist 8-OH DPAT or antagonist WAY 100635 could not mimic or block the effect of 5-HT on eEPSCs. Together, these data suggest that the 5-HT-induced attenuation of eEPSCs was mediated by 5-HT1B receptor activation. Moreover, sumatriptan had no effect on the amplitude of the postsynaptic current elicited by pressure applied AMPA, suggesting a possible presynaptic locus for the 5-HT1B receptor. Furthermore, 5-HT, sumatriptan and CP93129 all increased the paired pulse ratio of eEPSCs while they concomitantly decreased the amplitude of eEPSCs, suggesting that these agonists act to reduce glutamate release probability at presynaptic locus. Consistent with this observation, sumatriptan decreased the frequency of miniature EPSCs, but had no effect on their amplitude. Taken together, these results suggest that 5-HT suppresses glutamatergic neurotransmission in the BNST by activating presynaptic 5-HT1B receptors to decrease glutamate release from presynaptic terminals. This study illustrates a new pathway by which the activity of BNST neurons can be indirectly modulated by 5-HT, and suggests a potential new target for the development of novel treatments for depression and anxiety disorders.
Keywords: serotonin, excitatory postsynaptic currents, presynaptic receptor, patch clamp recording, anxiety disorders
Introduction
Serotonin (5-HT) neurotransmission is critically involved in stress and anxiety-like behaviors (Ressler and Nemeroff, 2000, Lowry et al., 2005), and selective serotonin reuptake inhibitors (SSRIs) are the most widely used medications for the treatment of anxiety disorders. However, the mechanisms of SSRIs are not well understood and sometimes controversial. This is partially due to the diversity of 5-HT receptors expressed in the central nervous system: there being seven 5-HT receptor families and 14 subtypes (Hoyer et al., 2002). Moreover, the 5-HT effects in the central nervous system are further complicated because 5-HT acts not only as a neurotransmitter but also a neuromodulator (see (Fink and Gothert, 2007) for a review). Hence, 5-HT not only affects neuronal excitability through activating postsynaptic receptors, including 5-HT1A, 2A, 2C, 3, 4, 7 (Rainnie, 1999b, Craven et al., 2001, Chapin et al., 2002, Levita et al., 2004, Xiang et al., 2005, Mlinar et al., 2006, Hashimoto and Kita, 2008, Guo et al., 2009, Hammack et al., 2009), but growing evidence indicates that 5-HT can also affect presynaptic excitatory or inhibitory neurotransmission in the CNS (Koyama et al., 2002, Bouryi and Lewis, 2003, Hashimoto and Kita, 2008).
The bed nucleus of the stria terminalis (BNST) is an important forebrain region involved in the modulation of anxiety-like behaviors (Lee and Davis, 1997, Treit et al., 1998, Sullivan et al., 2004b, Davis, 2006, Meloni et al., 2006) and the mediation of the stress response (Casada and Dafny, 1991, Hammack et al., 2004, Hammack et al., 2009). It is a pivotal relay of cortical information to the paraventricular nucleus of hypothalamus (PVN) (Herman et al., 1994, Choi et al., 2007), and lesions of the BNST decreased the stress response of rats (Fendt et al., 2003, Hammack et al., 2004). Conversley, stimulation of the BNST mimics the cardiovascular responses to stress (Dunn and Williams, 1995), and stress is a major precipitating factor in the development of anxiety-like behavior (Shekhar et al., 2005). The BNST receives substantial serotonergic innervation from the dorsal raphé nucleus (DRN), and expresses multiple 5-HT receptors (Vertes, 1991, Halberstadt and Balaban, 2008). In addition, 5-HT fibers target multiple BNST neurons including those neurons expressing the stress hormone, corticotrophin releasing factor (CRF) (Phelix et al., 1992b, 1992a). Hence, the BNST might be a critical region for 5-HT modulation of stress-induced anxiety-like behaviors.
Previously we have shown that 5-HT has a direct postsynaptic effect on BNST neurons (Rainnie, 1999a, Levita et al., 2004, Guo et al., 2009), and can either hyperpolarize or depolarize BNST neurons depending on the 5-HT receptor subtype(s) activated. However, the majority of BNST neurons are inhibited by activation of postsynaptic 5-HT1A receptors (Levita et al., 2004). Significantly, we have shown that local infusion of a 5-HT1A agonist, 5-carboxamidotryptamine (5-CT), into the BNST attenuates the acoustic startle response in rats, suggesting that 5-HT1A receptor-mediated inhibition of BNST neurons has an anxiolytic action.
In addition to a direct modulation through activation of postsynaptic 5-HT receptors, the excitability of BNST neurons could also be modulated indirectly by affecting synaptic transmission in this nucleus. The BNST receives substantial glutamatergic input from the prefrontal cortex (PFC), basolateral amygdale (BLA), and hippocampus (Weller and Smith, 1982, Dong et al., 2001a, Walker et al., 2003, Massi et al., 2008), and recent studies have shown that this input can be modulated by several neurotransmitters and neuromodulators including glutamate itself, norepinephrine, dopamine, corticotrophin releasing factor (CRF), and cannabinoids (Egli et al., 2005, Grueter and Winder, 2005, Muly et al., 2007, Kash et al., 2008, Massi et al., 2008, McElligott and Winder, 2008, 2009). However, no study to date has examined the effects of 5-HT on synaptic transmission in the BNST. In the present study, we directly addressed this issue by using patch clamp recordings from an in vitro BNST slice preparation to examine the effect of 5-HT on glutamatergic neurotransmission and identify the underlying 5-HT receptor subtype(s) mediating the effect.
Experimental procedures
Animals
Male Sprague-Dawley rats (5–7 weeks old, Charles River, Raleigh, NC) were used in this experiment. Animals were housed 4–5 per cage and had access to food and water ad libitum. All experimental protocols strictly conform to National Institutes of Health guidelines for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee of Emory University. Cares were taken to minimize the stress and suffering of rats before sacrificing.
Slice preparation
Slices containing the anterolateral BNST were obtained as previously described (Rainnie, 1999a, Muly et al., 2007). Briefly, under isoflurane anesthesia (Fisher Scientific, Hanoverpark, IL, USA), animals were decapitated and the brains rapidly removed and immersed in a cold (4°C) 95%–5% oxygen/carbon dioxide oxygenated “cutting solution” with the following composition (in mM): NaCl (130), NaHCO3 (30), KCl (3.50), KH2PO4 (1.10), MgCl2 (6.0), CaCl2 (1.0), glucose (10), supplemented with kynurenic acid (2.0). Slices containing the BNST were cut at a thickness of 350 μM using a Leica VTS-1000 vibratome (Leica Microsystems Inc., Bannockburn, IL, USA). Slices were kept in oxygenated “cutting solution” at room temperature for 1 h before transferring to regular artificial cerebrospinal fluid (ACSF) containing (in mM): NaCl (130), NaHCO3 (30), KCl (3.50), KH2PO4 (1.10), MgCl2 (1.30), CaCl2 (2.50), and glucose (10). Slices were kept in the regular ACSF for at least 30 min before recording.
Patch clamp recording
Individual slices were transferred to a recording chamber mounted on the fixed stage of a Leica DMLFS microscope (Leica Microsystems Inc., Bannockburn, IL, USA), where they were maintained fully submerged and continuously perfused with oxygenated 32 °C ACSF at a flow rate of 1–2 ml/min. Individual BNST neurons were identified by using differential interference contrast (DIC) optics and infrared (IR) illumination with an IR sensitive CCD camera (Orca ER, Hamamatsu, Tokyo Japan). All cells recorded were confined to the anterolateral BNST (BNSTAL) as previously reported (Levita et al., 2004, Hammack et al., 2007). Patch pipettes were pulled from borosilicate glass and had a resistance of 4–6 MΩ. The patch recording solution had the following composition (in mM): 130 K-gluconate, 2 KCl, 10 HEPES, 3 MgCl2, 2 K-ATP, 0.2 NaGTP, and 5 phosphocreatine, titred to pH 7.3 with KOH, and 290 mOsm. Data acquisition and analysis were performed using a MultiClamp 700B amplifier in conjunction with pClamp 10.0 software and a DigiData 1320A AD/DA interface (Molecular Devices, Burlingame, CA, USA). Whole cell patch clamp recordings were obtained and whole cell currents were filtered at 2 kHz and digitized at 10–20 kHz. The membrane potential was held at −60 mV for all neurons if not specified. Only those BNST neurons which had a stable membrane potential more negative than −55 mV and an action potential that overshot by > 10 mV were used. Access resistance was monitored throughout the experiments and neurons showing more than a 15% change of access resistance were discarded.
Recording of evoked EPSCs
Excitatory postsynaptic currents (EPSCs) onto BNST neurons were evoked as previously described (Muly et al., 2007). In brief, a concentric bipolar stimulation electrode (FHC, Bowdoinham, ME USA) was placed on the afferent fibers of the stria terminalis. Evoked EPSCs (eEPSCs) were recorded in the presence of the GABAA receptor antagonist SR 95531 (5 μM) to block GABAA receptor-mediated inhibitory postsynaptic currents (IPSCs). One train of five single square wave pulses (150 μs, 0.2 Hz) was delivered every 2 min throughout the experiment to induce EPSCs. For analysis the peak eEPSC amplitude was calculated as the mean response to each series of 5 stimulations. The mean of three stable eEPSCs obtained immediately before drug treatment was considered as baseline eEPSCs. All eEPSCs values were normalized to the baseline amplitude and expressed as the percentage of baseline.
Paired pulse paradigm
To examine the potential involvement of presynaptic 5-HT receptors, a paired pulse paradigm was employed in which two stimuli were delivered with an inter-stimulus-interval (ISI) of 50 ms. Five pairs of stimuli were delivered with an interval of 5 s between each pair and were averaged to measure the peak amplitude of both eEPSCs. The paired pulse ratio (PPR) was then calculated as the peak amplitude of the second EPSC (P2) divided by the first, EPSC1 (P1). Alterations in the PPR are thought to represent changes in release probability in the presynaptic terminal (Hess et al., 1987, Manabe et al., 1993).
Miniature EPSCs recording
Miniature EPSCs (mEPSCs) were examined in the presence of TTX (1 μM) and SR 95531 (5 μM). Two sessions of 1 min recording captured before 5-HT application were used as baseline. Another two sessions of 1 min recordings were captured during 5-HT application. All mEPSCs were detected offline using the MiniAnalysis program 6.0 (Synaptosoft Inc., Decatur, GA). The mEPSCs frequency and amplitude were represented as the mean values of two sessions.
Drug application
The following drugs were obtained from 1) Sigma-Aldrich (St. Louis, MO): serotonin, tetrodotoxin (TTX), DNQX (6,7-dinitroquinoxaline-2,3-dione), 8-OH-DPAT (8-hydroxy-2-(din-propylamino) tetraline), WAY 100635 (N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridyl)cyclohexanecarboxamide), AMPA (α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate); and 2) Tocris Bioscience (Ellisville, MO): CP 93129 (1,4-Dihydro-3-(1,2,3,6-tetrahydro-4-pyridinyl)-5H-pyrro l[3,2-b]pyridin-5-one dihydrochloride), GR55562 (3-[3-(Dimethylamino)propyl]-4-hydroxy-N-[4-(4-pyridinyl)phenyl]benzamide dihydrochloride), RS CPP ((RS)-3-(2-Carboxypiperazin-4-yl)-propyl-1-phosphonic acid), SR 95531(6-Imino-3-(4-methoxyphenyl)-1(6H)-pyridazinebutanoic acid hydrobromide). Sumatriptan was a generous gift from Glaxo Wellcome. Drugs were made as concentrated stock solutions in distilled H2O, except DNQX, which was made in 100% DMSO. The final test concentration of DMSO was no more than 0.1%. All drugs were applied in the ACSF using a continuous gravity fed bath application unless specifically stated.
Pressure-application of AMPA was used to directly elicit postsynaptic AMPA currents. Briefly, a modified patch electrode (resistance ~3 MΩ) was filled with ACSF containing AMPA (1 mM) and placed close to the recorded BNST neuron. AMPA receptor-mediated currents were evoked by pressure ejection (20–100 ms, 5–20 psi) using a picospritzer II (Parker Hannifin Instrumentation, Cleveland, OH). The transient pressure application was repeated every minute before and during drug application. After recording four stable baseline AMPA currents, sumatriptan was added to perfusion ACSF and the amplitude of the AMPA currents in the presence of sumatriptan were examined.
Statistics
All data are expressed as the mean ± S.E.M. For the evoked EPSCs, the amplitudes were normalized and expressed as the percentage of the baseline EPSC amplitude. All statistical tests were conducted using Excel 2003 or Graphpad Prism 4.0. A Student’s t-test or paired t-test was used to detect significant differences for un-paired or paired data. A p value <0.05 was considered statistically significant for all cases.
Results
eEPSCs in the BNST
Monosynaptic EPSCs were induced in 90% of BNST neurons by stimulating the stria terminalis (ST). The baseline eEPSCs amplitude was set to 60% of maximal eEPSCs amplitude, with amplitudes ranging from 160–500 pA. In 10% of BNST neurons stimulation of the ST evoked a polysynaptic EPSC and hence, these cells were not included in our analysis. Consistent with previous reports (Rainnie 1999, Egli and Winder, 2003, Egli et al., 2005), the EPSCs evoked at holding potential of −60 mV were nearly completely blocked by prior application of the AMPA/KA receptor antagonist DNQX (20 μM), whereas prior application of the NMDA receptor antagonist RS-CPP (10 μM) has no or little effect on the eEPSCs (Figure 1A, B), suggesting that the eEPSCs are mediated primarily by AMPA/KA receptor activation at rest.
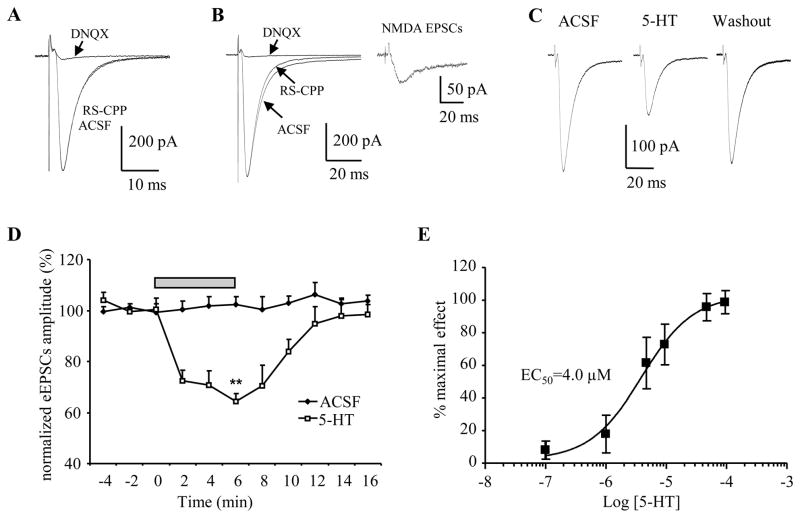
Figure 1. 5-HT reduced the amplitude of eEPSCs in the BNST.
Whole cell patch clamp recordings were made on BNST slices and EPSCs were evoked by stimulating the stria terminalis. Membrane potential was held at −60 mV. A) In one BNSTAL neuron, application of RS-CPP (10 μM) had no noticeable effect on the eEPSCs, whereas DNQX (20 μM) nearly completely blocked eEPSCs. Notice that the eEPSCs traces in the ACSF and RS-CPP were overlapped. B) In another BNSTAL neuron, RS-CPP application caused a leftward shift of the decay phase of eEPSCs, whereas did not affect the rising phase and peak amplitude of eEPSCs. Subsequently application of DNQX blocked the residual component of eEPSCs. Inset: the NMDA receptor-mediated EPSCs was revealed by subtracting the eEPSCs traces obtained before and during RS-CPP application. C) Representative traces showing the eEPSCs recordings before, during and after 5-HT application. 5-HT suppressed the amplitude of eEPSCs, an effect that reversed after 10 min of wash with ACSF. D) The time course of 5-HT (50 μM) action on eEPSCs amplitude indicated a fast activating and recovery pattern. **, p<0.01 vs baseline. E) Dose-response curve of the inhibiting effect of 5-HT on eEPSCs in BNST, the IC50 was 4.0 μM. The numbers of neurons tested in each 5-HT concentration were between 6 and 10.
Effect of 5-HT on eEPSCs
Bath application of 5-HT (50 μM) caused a significant reduction in the amplitude of the eEPSCs in BNST neurons (Figure 1C, D). In all neurons tested, application of 5-HT (50 μM) consistently reduced the amplitude of eEPSCs to 64.2±3.1% of baseline level (baseline 255±26 pA, 5-HT 164±18 pA; p<0.001, n=10, Figure 1D). As illustrated in Figure 1D, the inhibitory effect of 5-HT on the eEPSCs amplitude was reversible and showed complete recovery following 10 minutes of wash out with control ACSF (baseline 300±42 pA, washout 295±33 pA; 98.5±3.9% of baseline, p=0.5 vs. baseline, p<0.05 vs. 5-HT, n=4). Importantly, the amplitude of eEPSCs did not change during a similar period of continuous perfusion with ACSF, suggesting the 5-HT-induced attenuation of eEPSCs is not due to “run-down” of eEPSCs. Next, we examined the dose-response relationship of the 5-HT-induced modulation of eEPSCs amplitude. The inhibitory effect of 5-HT on eEPSCs was clearly dose-dependent (see Figure 1E). A low concentration of 5-HT (0.1 μM) evoked only a slight decrease (93.7±4.0% of baseline, n=7) in the amplitude of the eEPSCs, and the 5-HT effect reached a plateau level at 50–100 μM. Fitting a sigmoid curve to the dose-response relationship revealed an EC50 of 4.0 μM for the 5-HT induced suppression of eEPSCs amplitude (Figure 1E).
Receptor(s) which mediate the 5-HT effect
Multiple subtypes of seven distinct 5-HT receptor families have been identified and cloned (5-HT1-7; (Teitler and Herrick-Davis, 1994, Hoyer et al., 2002)). Previous studies had indicated that the Gi-coupled 5-HT1A,1B,1D receptors were most often associated with an inhibitory effect on glutamatergic neurotransmission (Fitzgerald and Sanes, 1999, Pickard et al., 1999), whereas the Gq-coupled 5-HT2A receptor subtype has been reported to enhance glutamate transmission (Fitzgerald and Sanes, 1999, Smith et al., 2001, Hasuo et al., 2002, Harsing, 2006). Consequently, we next tested the ability of several 5-HT1 receptor subtype selective agonists and antagonists to mimic and/or block the 5-HT induced attenuation of eEPSCs amplitude. We first tried to mimic the 5-HT response with the mixed 5-HT1B/D agonist, sumatriptan, which inhibits excitatory transmission in multiple brain regions (Jennings et al., 2004, Jeong et al., 2008). Bath application of sumatriptan caused a dose-dependent reduction in the amplitude of the eEPSCs. Low dose of sumatriptan (1 μM) caused a slight, but non-significant decrease in the amplitude of the eEPSCs (baseline 242±46 pA, sumatriptan 1 μM 233±54 pA; 95.7±5.3% of baseline, n=6, p=0.21). A higher dose of sumatriptan (10 μM) significantly reduced the amplitude of eEPSCs in all neurons tested (baseline 242± 27 pA, sumatriptan 10 μM 159±25 pA; 64.6±4.5% of baseline, n=11, p<0.001)(Figure 2A,C), which is close to the effect induced by 50 μM 5-HT (64.2±3.1% of baseline). Importantly, the effect of sumatriptan was reversible and recovered to 92.5±4.5% of baseline after 10 minutes of washout with control ACSF (baseline 244±37 pA, washout 229±41 pA; p=0.18 vs baseline, p<0.01 vs sumatriptan, n=8).
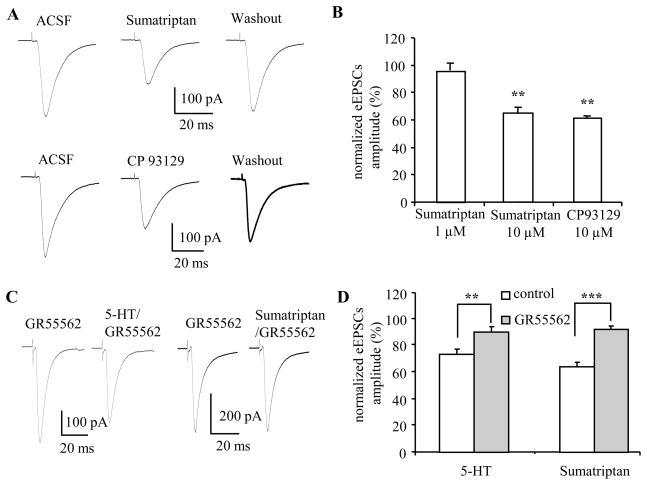
Figure 2. 5-HT1B receptor agonist mimicked and 5-HT1B antagonist blocked the effect of 5-HT on eEPSCs in BNST neurons.
A) Representative traces showing application of sumatriptan (10 μM) or CP93129 (10 μM) reversibly reduced the amplitude of eEPSCs; the effect could be reversed after 10 min washout with ACSF. B) Group data showing 10 μM sumatriptan (n=11) and 10 μM CP93129 (n=8) significantly reduced the amplitude of eEPSCs, while 1 μM sumatriptan (n=6) only slightly reduced eEPSCs amplitude (p>0.05). C, D) Pre-application of 5-HT1B antagonist GR55562 (10 μM) blocked the effect of 5-HT and sumatriptan on eEPSCs. **, p<0.01; ***, p<0.001
As sumatriptan is a mixed agonist with high affinity for both 5-HT1B and 5-HT1D receptors (Peroutka and McCarthy, 1989, Lesage et al., 1998), we next examined the effect on the eEPSCs amplitude of exogenous application of the selective 5-HT1B receptor agonist, CP93129 (Matsubara et al., 1991, Chadha et al., 2000). Application of CP93129 (10 μM) mimicked the inhibitory effect of both 5-HT and sumatriptan on the amplitude of eEPSCs (Figure 2B). Here, the eEPSCs amplitude was depressed to 61.4±2.0% of baseline levels by CP39129 (baseline 300±10 pA, CP 93129 183± 9 pA; n=10, p<0.001). In comparison to 5-HT and sumatriptan, the recovery rate from CP93129 application was much slower, showing only partial recovery of the eEPSCs amplitude after 10 min of washout (baseline 322 ± 8 pA, washout 236 ± 11 pA; 74±4% of baseline, p<0.01 vs baseline, p<0.01 vs CP93129, n=5). These results suggest that activation of 5-HT1B rather than 5-HT1D receptors mediated the inhibitory effect of 5-HT on eEPSCs. To test this hypothesis, we next examined the effect of prior application of the selective 5-HT1B receptor antagonist GR55562 on the modulatory actions of 5-HT and sumatriptan.
Application of GR55562 (10 μM) alone had no effect on the amplitude of eEPSCs (baseline 309±20 pA, GR55562 320±23 pA; 103.9±4.7% of baseline, n=14, p=0.5). However, prior application of GR55562 significantly attenuated the inhibitory effect of 5-HT on eEPSCs amplitude (Figure 2C). In the presence of GR55562 (10 μM), 5-HT (50 μM) reduced the mean eEPSCs amplitude to 80.0±3.5% of baseline (GR55562 358±31 pA, 5-HT in GR55562 287±29 pA, n=8, p<0.01)(Figure 2C), whereas 5-HT (10 μM) only reduced eEPSCs to 91±4% of baseline (GR55562 321±31 pA, 5-HT in GR55562 291±32 pA, p>0.05, n=7), which is significantly different from the 5-HT (10 μM) effect in control ACSF (74±4% of baseline, n=9, p<0.01) (Figure 2D). Similarly, the effect of sumatriptan (10 μM) was almost completely abolished by prior application of GR55562. In the presence of GR55562 application of sumatriptan reduced the eEPSCs amplitude to 93.3±2.4% of baseline (GR5562 270±20 pA, sumatriptan in GR55562 251±19 pA; p>0.05, n=6) (Figure 2C, D), which is significantly different from that of sumatriptan application in control ACSF (64.2±3.1%, p<0.001). Hence, the inhibitory effect of sumatriptan on the eEPSCs amplitude was most likely due to its activity at 5-HT1B rather than 5-HT1D receptors.
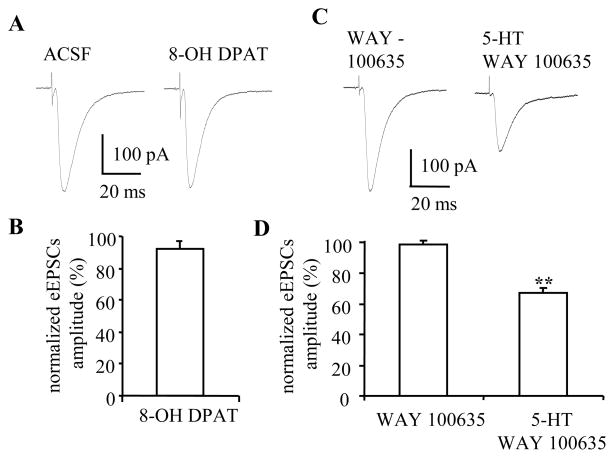
Evidence from other brain regions suggested that activation of 5-HT1A receptors can also modulate glutamate release (Koyama et al., 2002, Fink and Gothert, 2007). Hence, we next examined the effect of application of the selective 5-HT1A receptor agonist, 8-OH DPAT, on the eEPSCs amplitude. At 10 μM, 8-OH DPAT caused a small but non-significant reduction of the eEPSCs amplitude (baseline 283±33 pA, 8-OH DPAT 258±29 pA, 92.2±5.1% of baseline, p=0.2, n=6) (Figure 3A, C). We then tested whether prior application of the selective 5-HT1A receptor antagonist, WAY 100635, could block the inhibitory effect of 5-HT on the amplitude of eEPSCs. Application of WAY 100635 (200 nM) alone had no effect on the amplitude of eEPSCs (baseline 210±22 pA, WAY 100635 204±18 pA, p=0.42, n=6). Subsequent application of 5-HT (50 μM) in the presence of WAY 100635 reduced the amplitude of eEPSCs to 67.0±3.3% of baseline (5-HT 135±10 pA, n=6, p<0.01) (Figure 3B, D), which was not significantly different from the 5-HT response in control ACSF (Figure 1D). These result suggest that 5-HT1A receptor activation is unlikely to contribute to the inhibitory effect of 5-HT on excitatory transmission in the BNST. However, the small residual effect of 5-HT on eEPSCs in the presence of GR55562 might also indicate the contribution of other 5-HT receptor subtype(s).
Figure 3. Suppression effect of 5-HT on eEPSCs could not be mimicked or blocked by 5-HT1A receptor agonist or antagonist.
A, B) 5-HT1A receptor agonist, 8-OH DPAT (10 μM) has no apparent effect on eEPSCs. C, D) In the presence of 5-HT1A receptor antagonist WAY100635 (200 nM), 5-HT (50 μM) suppressed the amplitude of eEPSCs (n=6, p<0.01), an effect showed no difference from that of 5-HT in control ACSF.
Presynaptic action of 5-HT
Although 5-HT1B receptor modulation of glutamate transmission has been reported to have a presynaptic locus in many regions of the central nervous system (Maroteaux et al., 1992, Boschert et al., 1994, Sari et al., 1997, Muramatsu et al., 1998, Riad et al., 2000, Shen and Johnson, 2008), 5-HT1B mRNA is also expressed in BNST tissue (Guo et al., 2009, Hammack et al., 2009). Hence, the 5-HT1B receptor-induced modulation of glutamate transmission could have either a pre- or postsynaptic locus. To determine the site of 5-HT1B receptor action we performed the following experiments.
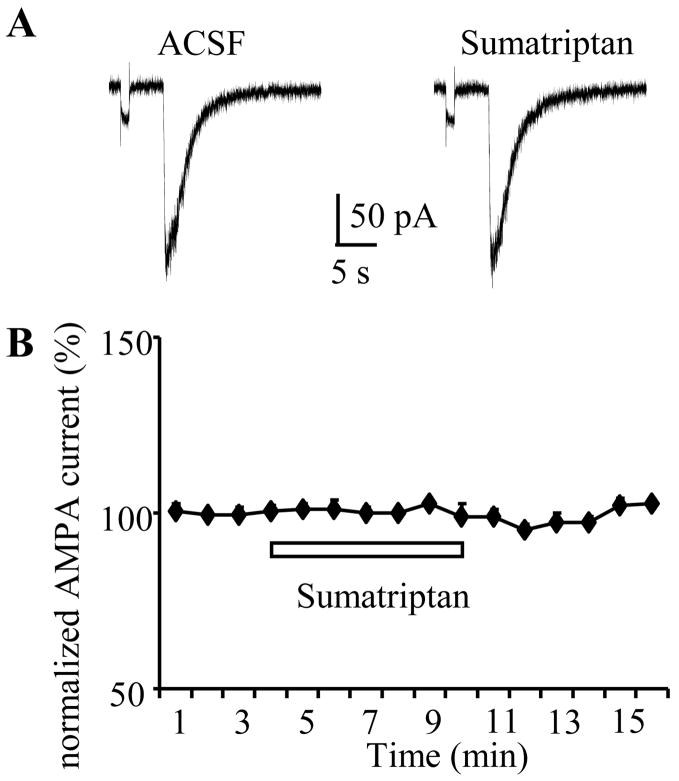
First, we examined the effect of sumatriptan on direct postsynaptic AMPA currents evoked by pressure-application of AMPA at a holding potential of −60 mV (Figure 4A). As summarized above, the eEPSCs were mediated mainly by AMPA/KA receptors but not NMDA receptors at rest, thus we used AMPA instead of glutamate to avoid the excitotoxicity associated with NMDA receptor activation. If 5-HT1B receptor activation reduced eEPSCs through a postsynaptic mechanism, sumatriptan application should also decrease the amplitude of pressure application-induced AMPA currents. Pressure ejected AMPA (1 mM) evoked an inward current with a mean amplitude of 219±22 pA (n=5). Bath application of sumatriptan (10 μM) had no effect on postsynaptic AMPA currents (220 ±20 pA, 100.4±0.4% of baseline, n=5, p=0.4; Figure 4B), suggesting that postsynaptic 5-HT1B receptors are unlikely to contribute to the 5-HT induced modulation of eEPSCs.
Figure 4. Sumatriptan has no effect on postsynaptic AMPA current induced by local pressure ejection of AMPA.
Postsynaptic AMPA currents were induced by puff-application of AMPA (1 mM) onto recorded neurons every 1 min. A) Upper traces showed the inward currents induced by puff application of AMPA in ACSF and during sumatriptan application. B) No changes of postsynaptic AMPA current was observed during sumatriptan application.
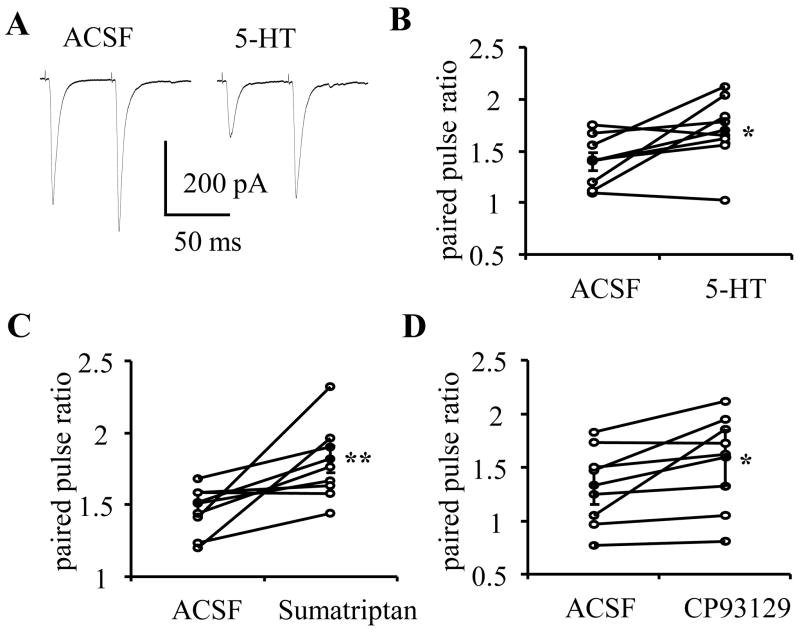
Second, we used a paired pulse paradigm in which two stimuli were delivered with an inter-stimulus-interval of 50 ms (see Methods). Here, an alteration of PPR is used as a measure of a change in release probability from presynaptic terminals. Application of 5-HT (50 μM) reduced amplitude of eEPSCs and concomitantly caused a significant increase in the PPR compared to baseline (baseline 1.35±0.11; 5-HT 1.71±0.16, p<0.05, n=8, see Figure 5A, B). Similarly, application of sumatriptan (10 μM; baseline 1.51±0.08, sumatriptan 1.82±0.09, p<0.01, n=9, Figure 5C), or CP93129 (baseline 1.33±0.17; CP93129 10 μM 1.59±0.26; p<0.05, n=8, Figure 5D) also caused a significant increase in PPR. Together the increased PPR and decreased eEPSCs amplitude suggest that activation of 5-HT1B receptors located on presynaptic terminals function to inhibit glutamate release.
Figure 5. 5-HT, sumatriptan and CP93129 increased the paired-pulse ratio.
A) Increased PPR was observed associated with the decrease of eEPSCs amplitude. Group data showed the increased PPR during 5-HT (B), sumatriptan (C) and CP 93129 (D) application. Open circle showed the PPR of individual neurons, filled circle showed the mean PPR. *, ** paired t-test, p<0.05, 0.01 respectively.
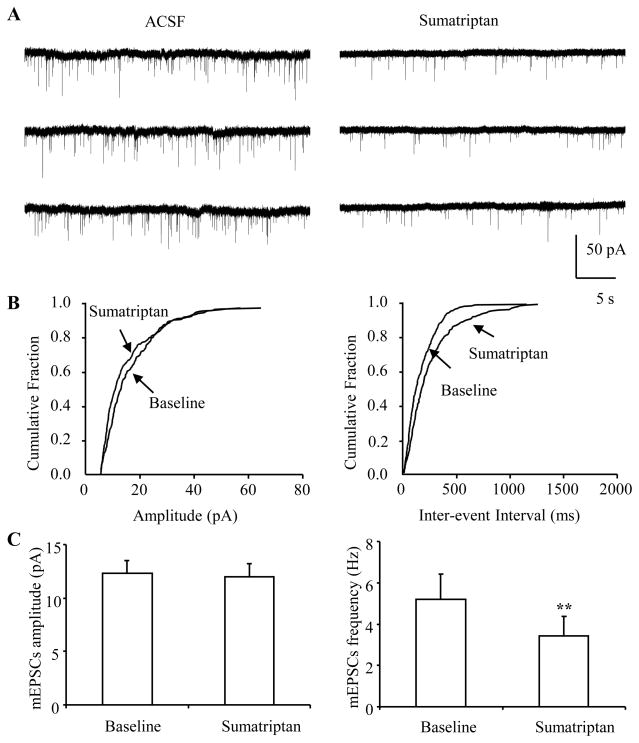
To confirm a presynaptic site of action, we examined the effects of sumatriptan application on the frequency and amplitude of spontaneous miniature EPSCs (mEPSCs) recorded in the presence of TTX (1 μM) and the GABAA receptor antagonist SR95531 (5 μM). As shown in Fig. 6A, sumatriptan (10 μM) decreased the frequency of mEPSCs observed in BNST neurons. A cumulative fraction plot showed an increase of inter-event-interval during sumatriptan application (Figure 6B right). In all neurons tested sumatriptan consistently reduced the frequency of mEPSCs to an average of 63.7±3.7% of baseline (baseline 5.2±1.2 Hz, sumatriptan 3.5±0.9 Hz, n=11, p<0.001), but had no effect on the amplitude of mEPSCs (baseline 12.3±1.2 pA, sumatriptan 12.0±1.2 pA; 97.5±2.3% of baseline; n=11, p=0.41) (Figure 6C). The data are consistent with the sumatriptan-induced increase in PPR, and add further support for a presynaptic site of action for 5-HT1B receptor activation.
Figure 6. Sumatriptan decreased the frequency of mEPCSs.
Spontaneous mEPSCs in BNST were recorded in the presence of TTX (1 μM) and GABAA antagonist SR 95531 (5 μM). A) Traces showing mEPSCs recorded from BNST neurons, and sumatriptan application reduced the frequency of mEPSCs (right). B) Cumulative plots showed the increase of inter-event interval of mEPSCs. C) Group data indicated sumatriptan reduced the frequency but have no effect on the amplitude of mEPSCs. n=11, **, paired t-test p<0.01
Discussion
In this study we have shown that 5-HT acts to attenuate excitatory neurotransmission in the BNST, an effect that was reversible, could be mimicked by 5-HT1B receptor agonists, and blocked by the selective 5-HT1B receptor antagonist, GR55562. Furthermore, we provide evidence that 5-HT1B receptor activation had no effect on postsynaptic AMPA currents, but increased the PPR of eEPSCs, and decreased the frequency of mEPSCs, suggesting that 5-HT acts on presynaptic 5-HT1B receptors to decrease glutamate release from presynaptic terminals.
Glutamatergic input onto BNST neurons
Tract tracing studies have shown that the stria terminalis represents the major afferent input to the BNSTAL from key components of the limbic circuit including the BLA, PFC, and hippocampus (Weller and Smith, 1982, Dong et al., 2001a, Walker et al., 2003, Massi et al., 2008). In this study, EPSCs were evoked by stimulating the stria terminalis and thus most likely represent the response to excitatory input originating from these upstream regions. Importantly, in almost all neurons examined stimulation of the stria terminalis evoked a monosynaptic EPSC as previously reported (Muly et al., 2007), suggesting that this represents a direct excitatory input onto BNST neurons. Previous studies showed AMPA/KA and NMDA receptors mediate the EPSPs/EPSCs in the BNST (Dumont et al., 2005, Egli et al., 2005, Dumont et al., 2008, Massi et al., 2008) and the AMPA/NMDA ratio shows a dynamic change in cocaine self-administration (Dumont et al., 2005) or morphine dependent rats (Dumont et al., 2008). However, the near total blockade of eEPSCS by DNQX and the little effect of RS-CPP on eEPSCs at the resting membrane potential of BNSTAL neurons suggest that at rest AMPA/KA receptor activation mediates the eEPSCs, which consistent with the AMPA receptor dependency showed by Winder and colleagues (Egli and Winder, 2003, Egli et al., 2005).
Presynaptic action of 5-HT1B in modulating glutamate release
Inhibition of excitatory synaptic transmission can result from either pre- and/or post-synaptic mechanisms. In this study, converging evidence suggest that the inhibitory effect of 5-HT on eEPSCs was mediated by a presynaptic mechanism, most likely by decreasing the probability of glutamate release from presynaptic terminals. Furthermore, we report that 5-HT1B receptor agonists and antagonist mimicked or blocked 5-HT effect on eEPSCs, an effect that was not mimicked by 5-HT1A receptor agonist or antagonist. This result is consistent with previous studies from other brain regions showing a 5-HT1B receptor-mediated decrease in excitatory neurotransmission (Singer et al., 1996, Muramatsu et al., 1998, Pickard et al., 1999). Noticeably, although GR55562 effectively blocked the 5-HT effect on eEPSCs, the small residual effect of 5-HT suggest that the contribution of another 5-HT receptor subtype could not be excluded. Nevertheless, this study provided the first evidence for a 5-HT1B receptor-mediated presynaptic inhibition of glutamate release in the BNST.
Consistent with our results, binding studies have shown a high level of 5-HT1B receptor binding sites in the BNST (Bonaventure et al., 1997, Cloez-Tayarani et al., 1997, Cloez-Tayarani et al., 1998). 5-HT1B receptors are widely distributed in the central nervous system, however they are mainly expressed on axon terminals (Maroteaux et al., 1992, Boschert et al., 1994, Sari et al., 1997, Manrique et al., 1999, Riad et al., 2000). In many regions 5-HT1B receptors are thought to act as terminal autoreceptors to regulate 5-HT release (Sari et al., 1997). However, the results outlined above further suggest that in the BNST some of these 5-HT1B binding sites are located on excitatory axon terminals. Indeed, in situ hybridization studies have shown that 5-HT1B receptor mRNA is highly expressed in each of the regions sending major excitatory inputs to the BNSTAL, including the BLA, prefrontal cortex, and hippocampus, (Cloez-Tayarani et al., 1997). It is possible that in projection neurons from these regions mature 5-HT1B receptor protein is transported to the axon terminals that innervate the BNSTAL. Moreover, our own RT-PCR study has shown 5-HT1B mRNA expression in the BNST (Guo et al., 2009, Hammack et al., 2009). As the majority of BNST neurons are GABAergic (McDonald, 1983, Cullinan et al., 1993, Sun and Cassell, 1993), 5-HT1B receptor activation may also function to regulate intrinsic or extrinsic release of GABA from axon terminals of BNST neurons.
Functional significance of 5-HT1B receptor-mediated modulation of glutamatergic transmission in the BNST
Accumulating evidence indicates that the BNST plays an important role in the adaptive response to stress (Vyas et al., 2003, Sullivan et al., 2004a, Pego et al., 2008), anxiety-like behaviors (Walker et al., 2003, Hammack et al., 2004) and drug-seeking behaviors (Aston-Jones and Harris, 2004, Smith and Aston-Jones, 2008). As mentioned above, the BNST receives excitatory inputs mainly from key structures of limbic circuit and then relays these inputs to multiple subcortical structures including the PVN, the ventral tegmental area (VTA), the parabrachial nuclei (PbN), the dorsal Raphé nucleus (DRN), as well as the nucleus of the solitary tract (NTS), (Holstege et al., 1985, Berk, 1987, Peyron et al., 1997, Dong et al., 2001b, Georges and Aston-Jones, 2002, Walker et al., 2003, Dong and Swanson, 2004, Choi et al., 2007). Each of these structures is intimately involved in regulating the autonomic and endocrine response of an organism to environmental pressure (see (Herman et al., 2003, Walker et al., 2003, Lowry et al., 2008) for reviews). Hence, the BNST may serve as a gate-control in these important affective circuits. Recently, Massi and colleagues reported that presynaptic CB1 receptors attenuate glutamate release from prefrontal cortical afferents making synapses onto BNST neurons that project to the VTA. These authors suggest that CB1 modulation of glutamate release in the BNST may play a critical role in modulating reward pathways. We propose that serotonergic modulation of presynaptic glutamate release may serve a similar function in regulating the behavioral response not only to appetitive stimuli but also aversive environmental stimuli.
Decreased brain 5-HT levels are thought to be one of the main causes of depression and anxiety disorders (Ressler and Nemeroff, 2000). Serotonergic afferents in the BNST originate from neurons located in the caudal DRN (Parent et al., 1981, Phelix et al., 1992a, Commons et al., 2003). We and others have shown that 5-HT fibers in the BNST make contact with BNST neurons including those that contain the stress hormone CRF (Phelix et al., 1992b, 1992a, Hammack et al., 2009). Hence, release of 5-HT within the BNST could have a significant impact on the overt behavioral response to sensory stimuli. Previously we have shown that local inhibition of the BNSTAL following infusion of a 5-HT1A receptor agonist has an anxiolytic action (Levita et al., 2004). Here we extended this study to show that 5-HT can also reduce the excitability of the BNSTAL by activating heterosynaptic 5-HT1B receptors located on glutamatergic afferents. We propose that the anxiolytic action of 5-HT in the anterolateral BNST would represent the combined action of reducing intrinsic neuronal excitability as well as reducing the excitatory drive onto these same neurons (see Figure 7 for a schematic diagram). By extrapolation, therefore, changes in the expression of 5-HT1B receptor expression may result in a significant alteration in the behavioral response to affective stimuli.
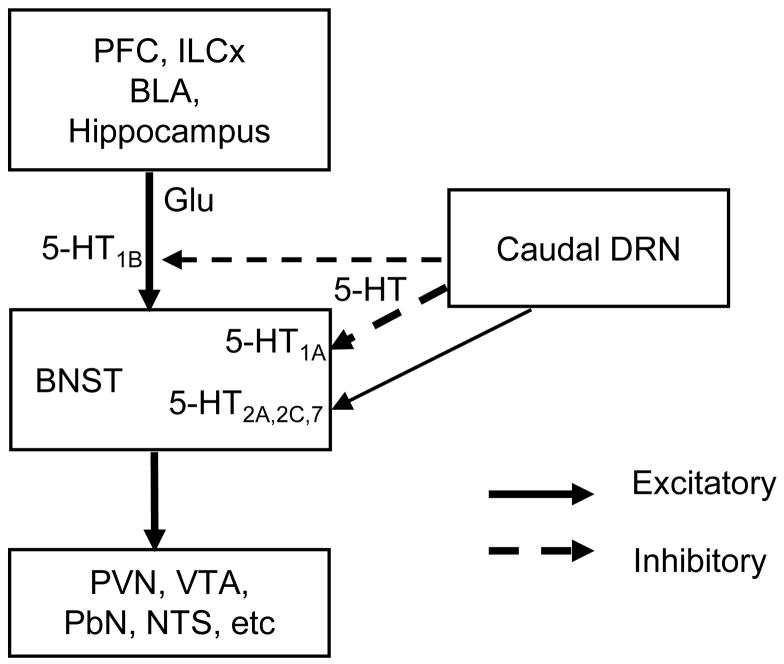
Figure 7. Schematic diagram of serotonergic modulation of neuronal excitability in the BNST.
BNST receives glutamatergic inputs from key structures of limbic system and projects to downstream targets involved in stress response, addiction and reward system. Information integration in BNST can dramatically affect the function of downstream nuclei. Serotonergic inputs from caudal DRN innervate the BNST and modulate BNST neuronal excitability through pre- or postsynaptic mechanisms. Presynaptically, 5-HT activates 5-HT1B receptor located on glutamatergic inputs to reduce the glutamate release, thus reduce the excitability of BNST neurons. Postsynaptically, activation of 5-HT1A receptor inhibits and 5-HT2A,2C,7 receptors excites BNST neurons.
Indeed, alterations in 5-HT1B receptor expression has been observed in patients with bipolar disorder and schizophrenia (Lopez-Figueoa et al., 2004), and substance abuse-disorder and major depression appear to be associated with a 5-HT1B receptor polymorphism (Huang et al., 2003). Moreover, emerging preclinical behavioral studies indicate that the 5-HT1B receptor plays a role in the modulation of aggression, anxiety, stress sensitivity and drug reinforcement (Clark and Neumaier, 2001, Przegalinski et al., 2004, Tatarczynska et al., 2004, Olivier and van Oorschot, 2005, Svenningsson et al., 2006, Muraki et al., 2008). The results of the current study suggest that modulation of 5-HT1B receptor activation in the BNSTAL may represent a novel target for the development of treatment strategies for stress and anxiety-like behaviors. The effects of BNST 5-HT1B receptor activation in anxiety models need to be examined in future studies.
We have proposed that the BNSTAL and the DRN are reciprocally linked in a closed-loop feedback system (Hammack et al., 2009), whereby stress activation of the BNST CRF neurons would ultimately result in downstream excitation of DRN 5-HT neurons, followed by feedback release of 5-HT in BNST. Release of 5-HT into the BNST would attenuate the glutamatergic drive onto BNST neurons via activation of presynaptic 5-HT1B receptors, and also move these same neurons away from action potential generation through postsynaptic 5-HT1A receptor activation. Concurrent activation of 5-HT1A and 5-HT1B receptors would then act to attenuate anxiety-like behavior.
Selective serotonin reuptake inhibitors (SSRIs) are one of the most widely prescribed antidepressant and anxiolytic treatments, which act to increase extracellular levels of 5-HT by inhibiting its reuptake into presynaptic terminals. As a region with substantial 5-HT projections and a high expression of the 5-HT transporter protein, extracellular 5-HT levels would be expected to increase in the BNST during SSRIs treatment. Hence, some of the therapeutic actions of the SSRIs may result, in part, from increasing levels of 5-HT in the BNST effectively reducing the activity of BNST neurons through a synergistic activation of pre- and postsynaptic receptors. However, following prolonged periods of stress 5-HT receptor expression in the BNST is altered in favor of an excitatory response to 5-HT, due to a down-regulation of 5-HT1A receptor mRNA and an up-regulation of 5-HT7 receptor mRNA (Hammack et al, 2009). Hence, the effects of traditional SSRIs may be compromised and the development of novel drugs that selectively target defined 5-HT receptor populations in the BNSTAL may represent a new approach for anxiolytic pharmacotherapy.
Acknowledgments
The authors want to sincerely thank Mr. Steve Ryan for critical review of the manuscript. This work was supported by National Institute of Mental Health (MH072908) to DGR, and Center for Behavioral Neuroscience STC Center: NSF Agmt #IBN-9876754, National Primate Research Center base grant #RR-00165, Animal Resource Program at NIH.
Abbreviations
- 5-CT
5-carboxamidotryptamine
- 5-HT
5-hydroxytryptamine, serotonin
- ACSF
artificial cerebrospinal fluid
- BNST
the bed nucleus of the stria terminalis
- CRF
corticotrophin-releasing factor
- DRN
dorsal Raphé nucleus
- eEPSCs
evoked excitatory postsynaptic currents
- EPSCs
excitatory postsynaptic currents
- ILCx
infralimbic cortex
- IPSCs
inhibitory postsynaptic currents
- mEPSCs
miniature excitatory postsynaptic currents
- PbN
parabrachial nuclei
- PFC
prefrontal cortex
- PPR
paired-pulse ratio
- PVN
paraventricular nucleus of the hypothalamus
- SSRIs
selective serotonin reuptake inhibitors
- TTX
tetrodotoxin
- VTA
ventral tagmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aston-Jones G, Harris GC. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology. 2004;47 (Suppl 1):167–179. doi: 10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Berk ML. Projections of the lateral hypothalamus and bed nucleus of the stria terminalis to the dorsal vagal complex in the pigeon. J Comp Neurol. 1987;260:140–156. doi: 10.1002/cne.902600111. [DOI] [PubMed] [Google Scholar]
- Bonaventure P, Schotte A, Cras P, Leysen JE. Autoradiographic mapping of 5-HT1B- and 5-HT1D receptors in human brain using [3H]alniditan, a new radioligand. Receptors Channels. 1997;5:225–230. [PubMed] [Google Scholar]
- Boschert U, Amara DA, Segu L, Hen R. The mouse 5-hydroxytryptamine1B receptor is localized predominantly on axon terminals. Neuroscience. 1994;58:167–182. doi: 10.1016/0306-4522(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Bouryi VA, Lewis DI. The modulation by 5-HT of glutamatergic inputs from the raphe pallidus to rat hypoglossal motoneurones, in vitro. J Physiol. 2003;553:1019–1031. doi: 10.1113/jphysiol.2003.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casada JH, Dafny N. Restraint and Stimulation of Bed Nucleus of the Stria Terminalis Produce Similar Stress-Like Behaviors. Brain Research Bulletin. 1991;27:207–212. doi: 10.1016/0361-9230(91)90069-v. [DOI] [PubMed] [Google Scholar]
- Chadha A, Sur C, Atack J, Duty S. The 5HT(1B) receptor agonist, CP-93129, inhibits [(3)H]-GABA release from rat globus pallidus slices and reverses akinesia following intrapallidal injection in the reserpine-treated rat. Br J Pharmacol. 2000;130:1927–1932. doi: 10.1038/sj.bjp.0703526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin EM, Haj-Dahmane S, Torres G, Andrade R. The 5-HT(4) receptor-induced depolarization in rat hippocampal neurons is mediated by cAMP but is independent of I(h) Neurosci Lett. 2002;324:1–4. doi: 10.1016/s0304-3940(02)00113-1. [DOI] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci. 2007;27:2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MS, Neumaier JF. The 5-HT1B receptor: behavioral implications. Psychopharmacol Bull. 2001;35:170–185. [PubMed] [Google Scholar]
- Cloez-Tayarani I, Cardona A, Rousselle JC, Massot O, Edelman L, Fillion G. Autoradiographic characterization of [3H]-5-HT-moduline binding sites in rodent brain and their relationship to 5-HT1B receptors. Proc Natl Acad Sci U S A. 1997;94:9899–9904. doi: 10.1073/pnas.94.18.9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloez-Tayarani I, Cardona A, Sarhan H, Rousselle JC, Massot O, Edelman L, Fillion G. Mapping of 5-HT-moduline binding sites in guinea-pig brain by film and digital autoradiography. Brain Research. 1998;798:311–315. doi: 10.1016/s0006-8993(98)00393-x. [DOI] [PubMed] [Google Scholar]
- Commons KG, Connolley KR, Valentino RJ. A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology. 2003;28:206–215. doi: 10.1038/sj.npp.1300045. [DOI] [PubMed] [Google Scholar]
- Craven RM, Grahame-Smith DG, Newberry NR. 5-HT1A and 5-HT2 receptors differentially regulate the excitability of 5-HT-containing neurones of the guinea pig dorsal raphe nucleus in vitro. Brain Res. 2001;899:159–168. doi: 10.1016/s0006-8993(01)02221-1. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Watson SJ. Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol. 1993;332:1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. American Psychologist. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001a;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001b;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J Comp Neurol. 2004;468:277–298. doi: 10.1002/cne.10949. [DOI] [PubMed] [Google Scholar]
- Dumont EC, Mark GP, Mader S, Williams JT. Self-administration enhances excitatory synaptic transmission in the bed nucleus of the stria terminalis. Nat Neurosci. 2005;8:413–414. doi: 10.1038/nn1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Rycroft BK, Maiz J, Williams JT. Morphine produces circuit-specific neuroplasticity in the bed nucleus of the stria terminalis. Neuroscience. 2008;153:232–239. doi: 10.1016/j.neuroscience.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn JD, Williams TJ. Cardiovascular-Responses to Electrical-Stimulation of the Bed Nucleus of the Stria Terminalis. Journal of Comparative Neurology. 1995;352:227–234. doi: 10.1002/cne.903520206. [DOI] [PubMed] [Google Scholar]
- Egli RE, Kash TL, Choo K, Savchenko V, Matthews RT, Blakely RD, Winder DG. Norepinephrine modulates glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2005;30:657–668. doi: 10.1038/sj.npp.1300639. [DOI] [PubMed] [Google Scholar]
- Egli RE, Winder DG. Dorsal and ventral distribution of excitable and synaptic properties of neurons of the bed nucleus of the stria terminalis. J Neurophysiol. 2003;90:405–414. doi: 10.1152/jn.00228.2003. [DOI] [PubMed] [Google Scholar]
- Fendt M, Endres T, Apfelbach R. Temporary inactivation of the bed nucleus of the stria terminalis but not of the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. Journal of Neuroscience. 2003;23:23–28. doi: 10.1523/JNEUROSCI.23-01-00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink KB, Gothert M. 5-HT receptor regulation of neurotransmitter release. Pharmacol Rev. 2007;59:360–417. doi: 10.1124/pr.107.07103. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KK, Sanes DH. Serotonergic modulation of synapses in the developing gerbil lateral superior olive. J Neurophysiol. 1999;81:2743–2752. doi: 10.1152/jn.1999.81.6.2743. [DOI] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, Winder DG. Group II and III metabotropic glutamate receptors suppress excitatory synaptic transmission in the dorsolateral bed nucleus of the stria terminalis. Neuropsychopharmacology. 2005;30:1302–1311. doi: 10.1038/sj.npp.1300672. [DOI] [PubMed] [Google Scholar]
- Guo JD, Hammack SE, Hazra R, Levita L, Rainnie DG. Bi-directional modulation of bed nucleus of stria terminalis neurons by 5-HT: molecular expression and functional properties of excitatory 5-HT receptor subtypes. Neuroscience. 2009;164:1776–1793. doi: 10.1016/j.neuroscience.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Balaban CD. Selective anterograde tracing of nonserotonergic projections from dorsal raphe nucleus to the basal forebrain and extended amygdala. Journal of Chemical Neuroanatomy. 2008;35:317–325. doi: 10.1016/j.jchemneu.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Guo JD, Hazra R, Dabrowska J, Myers KM, Rainnie DG. The response of neurons in the bed nucleus of the stria terminalis to serotonin: implications for anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1309–1320. doi: 10.1016/j.pnpbp.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Mania I, Rainnie DG. Differential expression of intrinsic membrane currents in defined cell types of the anterolateral bed nucleus of the stria terminalis. J Neurophysiol. 2007;98:638–656. doi: 10.1152/jn.00382.2007. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Watkins LR, Maier SF. Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behavioral Neuroscience. 2004;118:443–448. doi: 10.1037/0735-7044.118.2.443. [DOI] [PubMed] [Google Scholar]
- Harsing LG. The pharmacology of the neurochemical transmission in the midbrain raphe nuclei of the rat. Curr Neuropharmacol. 2006;4:313–339. doi: 10.2174/157015906778520764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Kita H. Serotonin activates presynaptic and postsynaptic receptors in rat globus pallidus. J Neurophysiol. 2008;99:1723–1732. doi: 10.1152/jn.01143.2007. [DOI] [PubMed] [Google Scholar]
- Hasuo H, Matsuoka T, Akasu T. Activation of presynaptic 5-hydroxytryptamine 2A receptors facilitates excitatory synaptic transmission via protein kinase C in the dorsolateral septal nucleus. J Neurosci. 2002;22:7509–7517. doi: 10.1523/JNEUROSCI.22-17-07509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Watson SJ. Involvement of the Bed Nucleus of the Stria Terminalis in Tonic Regulation of Paraventricular Hypothalamic Crh and Avp Messenger-Rna Expression. Journal of Neuroendocrinology. 1994;6:433–442. doi: 10.1111/j.1365-2826.1994.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Hess G, Kuhnt U, Voronin LL. Quantal analysis of paired-pulse facilitation in guinea pig hippocampal slices. Neurosci Lett. 1987;77:187–192. doi: 10.1016/0304-3940(87)90584-2. [DOI] [PubMed] [Google Scholar]
- Holstege G, Meiners L, Tan K. Projections of the bed nucleus of the stria terminalis to the mesencephalon, pons, and medulla oblongata in the cat. Exp Brain Res. 1985;58:379–391. doi: 10.1007/BF00235319. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Jennings EA, Ryan RM, Christie MJ. Effects of sumatriptan on rat medullary dorsal horn neurons. Pain. 2004;111:30–37. doi: 10.1016/j.pain.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Jeong HJ, Chenu D, Johnson EE, Connor M, Vaughan CW. Sumatriptan inhibits synaptic transmission in the rat midbrain periaqueductal grey. Mol Pain. 2008;4:54. doi: 10.1186/1744-8069-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Nobis WP, Matthews RT, Winder DG. Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1-dependent process. J Neurosci. 2008;28:13856–13865. doi: 10.1523/JNEUROSCI.4715-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Matsumoto N, Murakami N, Kubo C, Nabekura J, Akaike N. Role of presynaptic 5-HT1A and 5-HT3 receptors in modulation of synaptic GABA transmission in dissociated rat basolateral amygdala neurons. Life Sci. 2002;72:375–387. doi: 10.1016/s0024-3205(02)02280-4. [DOI] [PubMed] [Google Scholar]
- Lee YL, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. Journal of Neuroscience. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage AS, Wouters R, Van Gompel P, Heylen L, Vanhoenacker P, Haegeman G, Luyten WH, Leysen JE. Agonistic properties of alniditan, sumatriptan and dihydroergotamine on human 5-HT1B and 5-HT1D receptors expressed in various mammalian cell lines. Br J Pharmacol. 1998;123:1655–1665. doi: 10.1038/sj.bjp.0701766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levita L, Hammack SE, Mania I, Li XY, Davis M, Rainnie DG. 5-hydroxytryptamine1A-like receptor activation in the bed nucleus of the stria terminalis: electrophysiological and behavioral studies. Neuroscience. 2004;128:583–596. doi: 10.1016/j.neuroscience.2004.06.037. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ, Shekhar A. Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Ann N Y Acad Sci. 2008;1148:86–94. doi: 10.1196/annals.1410.004. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- Manabe T, Wyllie DJ, Perkel DJ, Nicoll RA. Modulation of synaptic transmission and long-term potentiation: effects on paired pulse facilitation and EPSC variance in the CA1 region of the hippocampus. J Neurophysiol. 1993;70:1451–1459. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- Manrique C, Hery F, Faudon M, Francois-Bellan AM. Indirect evidence for an association of 5-HT(1B) binding sites with retinal and geniculate axon terminals in the rat suprachiasmatic nucleus. Synapse. 1999;33:314–323. doi: 10.1002/(SICI)1098-2396(19990915)33:4<314::AID-SYN8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Maroteaux L, Saudou F, Amlaiky N, Boschert U, Plassat JL, Hen R. Mouse 5HT1B serotonin receptor: cloning, functional expression, and localization in motor control centers. Proc Natl Acad Sci U S A. 1992;89:3020–3024. doi: 10.1073/pnas.89.7.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massi L, Elezgarai I, Puente N, Reguero L, Grandes P, Manzoni OJ, Georges F. Cannabinoid receptors in the bed nucleus of the stria terminalis control cortical excitation of midbrain dopamine cells in vivo. J Neurosci. 2008;28:10496–10508. doi: 10.1523/JNEUROSCI.2291-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara T, Moskowitz MA, Byun B. CP-93,129, a potent and selective 5-HT1B receptor agonist blocks neurogenic plasma extravasation within rat but not guinea-pig dura mater. Br J Pharmacol. 1991;104:3–4. doi: 10.1111/j.1476-5381.1991.tb12374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Neurons of the bed nucleus of the stria terminalis: a golgi study in the rat. Brain Res Bull. 1983;10:111–120. doi: 10.1016/0361-9230(83)90082-5. [DOI] [PubMed] [Google Scholar]
- McElligott ZA, Winder DG. Alpha1-adrenergic receptor-induced heterosynaptic long-term depression in the bed nucleus of the stria terminalis is disrupted in mouse models of affective disorders. Neuropsychopharmacology. 2008;33:2313–2323. doi: 10.1038/sj.npp.1301635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott ZA, Winder DG. Modulation of glutamatergic synaptic transmission in the bed nucleus of the stria terminalis. Prog Neuropsychopharmacol Biol Psychiatry. 2009 doi: 10.1016/j.pnpbp.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni EG, Gerety LP, Cohen BM, Carlezon WA. Anxiety-like effects of corticotropin-releasing factor (CRF) are reduced by 6-hydroxydopamine lesions of the bed nucleus of the stria terminalis (BNST) Neuropsychopharmacology. 2006;31:S109–S109. [Google Scholar]
- Mlinar B, Mascalchi S, Mannaioni G, Morini R, Corradetti R. 5-HT4 receptor activation induces long-lasting EPSP-spike potentiation in CA1 pyramidal neurons. Eur J Neurosci. 2006;24:719–731. doi: 10.1111/j.1460-9568.2006.04949.x. [DOI] [PubMed] [Google Scholar]
- Muly EC, Mania I, Guo JD, Rainnie DG. Group II metabotropic glutamate receptors in anxiety circuitry: correspondence of physiological response and subcellular distribution. J Comp Neurol. 2007;505:682–700. doi: 10.1002/cne.21525. [DOI] [PubMed] [Google Scholar]
- Muraki I, Inoue T, Koyama T. Effect of co-administration of the selective 5-HT1A receptor antagonist WAY 100,635 and selective 5-HT1B/1D receptor antagonist GR 127,935 on anxiolytic effect of citalopram in conditioned fear stress in the rat. Eur J Pharmacol. 2008;586:171–178. doi: 10.1016/j.ejphar.2008.01.040. [DOI] [PubMed] [Google Scholar]
- Muramatsu M, Lapiz MD, Tanaka E, Grenhoff J. Serotonin inhibits synaptic glutamate currents in rat nucleus accumbens neurons via presynaptic 5-HT1B receptors. Eur J Neurosci. 1998;10:2371–2379. doi: 10.1046/j.1460-9568.1998.00248.x. [DOI] [PubMed] [Google Scholar]
- Olivier B, van Oorschot R. 5-HT1B receptors and aggression: a review. Eur J Pharmacol. 2005;526:207–217. doi: 10.1016/j.ejphar.2005.09.066. [DOI] [PubMed] [Google Scholar]
- Parent A, Descarries L, Beaudet A. Organization of ascending serotonin systems in the adult rat brain. A radioautographic study after intraventricular administration of [3H]5-hydroxytryptamine. Neuroscience. 1981;6:115–138. doi: 10.1016/0306-4522(81)90050-6. [DOI] [PubMed] [Google Scholar]
- Pego JM, Morgado P, Pinto LG, Cerqueira JJ, Almeida OF, Sousa N. Dissociation of the morphological correlates of stress-induced anxiety and fear. Eur J Neurosci. 2008;27:1503–1516. doi: 10.1111/j.1460-9568.2008.06112.x. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ, McCarthy BG. Sumatriptan (GR 43175) interacts selectively with 5-HT1B and 5-HT1D binding sites. Eur J Pharmacol. 1989;163:133–136. doi: 10.1016/0014-2999(89)90406-8. [DOI] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1997;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Phelix CF, Liposits Z, Paull WK. Monoamine innervation of bed nucleus of stria terminalis: an electron microscopic investigation. Brain Res Bull. 1992a;28:949–965. doi: 10.1016/0361-9230(92)90218-m. [DOI] [PubMed] [Google Scholar]
- Phelix CF, Liposits Z, Paull WK. Serotonin-CRF interaction in the bed nucleus of the stria terminalis: a light microscopic double-label immunocytochemical analysis. Brain Res Bull. 1992b;28:943–948. doi: 10.1016/0361-9230(92)90217-l. [DOI] [PubMed] [Google Scholar]
- Pickard GE, Smith BN, Belenky M, Rea MA, Dudek FE, Sollars PJ. 5-HT1B receptor-mediated presynaptic inhibition of retinal input to the suprachiasmatic nucleus. J Neurosci. 1999;19:4034–4045. doi: 10.1523/JNEUROSCI.19-10-04034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przegalinski E, Papla I, Siwanowicz J, Filip M. Effects of 5-HT1B receptor ligands microinjected into the ventral tegmental area on the locomotor and sensitizating effects of cocaine in rats. Eur Neuropsychopharmacol. 2004;14:217–225. doi: 10.1016/S0924-977X(03)00106-8. [DOI] [PubMed] [Google Scholar]
- Rainnie DG. Neurons of the bed nucleus of the stria terminalis (BNST). Electrophysiological properties and their response to serotonin. Ann N Y Acad Sci. 1999a;877:695–699. doi: 10.1111/j.1749-6632.1999.tb09304.x. [DOI] [PubMed] [Google Scholar]
- Rainnie DG. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J Neurophysiol. 1999b;82:69–85. doi: 10.1152/jn.1999.82.1.69. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12 (Suppl 1):2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, el Mestikawy S, Hamon M, Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol. 2000;417:181–194. [PubMed] [Google Scholar]
- Sari Y, Lefevre K, Bancila M, Quignon M, Miquel MC, Langlois X, Hamon M, Verge D. Light and electron microscopic immunocytochemical visualization of 5-HT1B receptors in the rat brain. Brain Res. 1997;760:281–286. doi: 10.1016/s0006-8993(97)00400-9. [DOI] [PubMed] [Google Scholar]
- Shen KZ, Johnson SW. 5-HT inhibits synaptic transmission in rat subthalamic nucleus neurons in vitro. Neuroscience. 2008;151:1029–1033. doi: 10.1016/j.neuroscience.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JH, Bellingham MC, Berger AJ. Presynaptic inhibition of glutamatergic synaptic transmission to rat motoneurons by serotonin. J Neurophysiol. 1996;76:799–807. doi: 10.1152/jn.1996.76.2.799. [DOI] [PubMed] [Google Scholar]
- Smith BN, Sollars PJ, Dudek FE, Pickard GE. Serotonergic modulation of retinal input to the mouse suprachiasmatic nucleus mediated by 5-HT1B and 5-HT7 receptors. J Biol Rhythms. 2001;16:25–38. doi: 10.1177/074873040101600104. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DE, Johnson LR, Hou M, Ledoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004a;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DEA, Johnson LR, Hou M, Ledoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004b;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Sun N, Cassell MD. Intrinsic GABAergic neurons in the rat central extended amygdala. J Comp Neurol. 1993;330:381–404. doi: 10.1002/cne.903300308. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M, Vaugeois JM, Nomikos GG, Greengard P. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science. 2006;311:77–80. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- Tatarczynska E, Klodzinska A, Stachowicz K, Chojnacka-Wojcik E. Effect of combined administration of 5-HT1A or 5-HT1B/1D receptor antagonists and antidepressants in the forced swimming test. Eur J Pharmacol. 2004;487:133–142. doi: 10.1016/j.ejphar.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Teitler M, Herrick-Davis K. Multiple serotonin receptor subtypes: molecular cloning and functional expression. Crit Rev Neurobiol. 1994;8:175–188. [PubMed] [Google Scholar]
- Treit D, Aujla H, Menard J. Does the bed nucleus of the stria terminalis mediate fear behaviors? Behavioral Neuroscience. 1998;112:379–386. doi: 10.1037//0735-7044.112.2.379. [DOI] [PubMed] [Google Scholar]
- Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res. 2003;965:290–294. doi: 10.1016/s0006-8993(02)04162-8. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Weller KL, Smith DA. Afferent connections to the bed nucleus of the stria terminalis. Brain Res. 1982;232:255–270. doi: 10.1016/0006-8993(82)90272-4. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Wang L, Kitai ST. Modulation of spontaneous firing in rat subthalamic neurons by 5-HT receptor subtypes. J Neurophysiol. 2005;93:1145–1157. doi: 10.1152/jn.00561.2004. [DOI] [PubMed] [Google Scholar]