Abstract
The aim of this study was to determine how distinctive patterns of unhelpful beliefs about sleep endorsed by insomnia patients relate to their presenting symptoms and treatment responses. A sample of 281 primary insomnia sufferers completed items comprising the Dysfunctional Beliefs About Sleep scale (DBAS-16). Their resultant scores on the four DBAS-16 subscales were then subjected to a cluster analysis, which resulted in the identification of four distinctive age-matched subgroups. Two subgroups were characterized by pathologically elevated scores on at least two of the DBAS-16 subscales, whereas the other two subgroups had subscale scores that closely resembled those of a normative sample. Subsequent comparisons showed the insomnia subgroups differed in regard to their insomnia severity, use of prescribed medication for sleep, depression and anxiety symptoms, and daytime sleepiness. Furthermore, comparisons of treatment outcomes (i.e. analysis of change scores and normative comparisons) across clusters showed that the subgroups did not benefit equally from a standardized form of Cognitive-Behavioral Therapy (CBT) for insomnia. Findings demonstrate the contribution of specific sleep-related beliefs on presenting insomnia symptoms and suggest the potential usefulness of tailoring CBT protocols to match the needs of distinctive insomnia subtypes.
Keywords: Primary insomnia, Dysfunctional beliefs, Cluster analysis, Cognitive-behavioral treatment
INTRODUCTION
Sleep-related beliefs, excessive arousal, and sleep-disruptive behaviors all have been mentioned as putative factors contributing to the etiology and maintenance of persistent insomnia (Espie, 2002; Harvey, 2002; Morin, 1993). Although the specific interrelation of these factors has not been yet fully explored, studies have suggested meaningful associations between some them. For instance, research addressing the role of aberrant beliefs about sleep has suggested their contribution to sleep-related anxiety and sleep-disruptive practices that perpetuate insomnia (Morin, Stone, Trinkle, Mercer, & Remsberg, 1993).
To date much of the research designed to explore the role of cognitive factors in insomnia has highlighted the range of distinctive beliefs that may contribute to sleep difficulties (Carney & Edinger, 2006; Jansson & Linton, 2007; Morin, Vallieres, & Ivers, 2007). Furthermore, it has generally been presumed that these distinctive types of beliefs or themes may have particular correlates at a behavioral or affective level (Carney & Edinger, 2006). For example, the belief that getting eight hours of sleep is absolutely necessary to feel refreshed the following day may spawn sleep-related “performance anxiety” when the individual is lying in bed incapable of falling asleep and thinking about the scheduled ensuing rising time. Similarly, the belief that a lack of energy is the irremediable consequence of poor sleep may lead to sleep-disruptive practices, such as napping or taking high doses of caffeine during the day as a way to counteract this feared effect.
Of course, not all insomnia sufferers hold the same types of unhelpful beliefs or endorse those to a similar degree (Edinger, Carney, & Wohlgemuth, 2008). Unfortunately, no studies to date have examined whether insomnia patients can be differentiated from each other on the basis of the types of unhelpful beliefs they endorse or whether distinctive patterns of unhelpful sleep-related beliefs may connote noteworthy differences among insomnia sufferers regarding their presenting clinical features. Admittedly, such a pattern-oriented approach would provide a more comprehensive perspective on the psychological functioning of the insomnia sufferer, as opposed to a variable-oriented approach, which is focused mainly on the variance explained by different types of factors, i.e. dysfunctional beliefs, regardless their particular combination.
Recognizing the role that unhelpful beliefs play in perpetuating insomnia, it is also important to consider how belief-targeted insomnia therapies, such as Cognitive Behavioral Treatment (CBT), affects these beliefs and whether changes in these belief after treatment are associated with other positive outcomes. Although limited in number, studies of this nature have confirmed both assertions: that sleep-related beliefs of primary insomnia sufferers change over the course of CBT and that reductions in the level of endorsement of such beliefs are associated with both objective and subjective sleep improvements (Edinger, Carney et al., 2008; Edinger, Wohlgemuth, Radtke, Marsh, & Quillian, 2001b; Morin, Blais, & Savard, 2002). As such, these findings can be interpreted in, at least, two directions: altering the individuals’ dysfunctional beliefs about sleep may give way to the elimination, for example, of the sleep-disruptive habits these beliefs may sustain or, alternately, sleep improvements may alter the dysfunctional beliefs.
Whatever the mechanism of change is, it is a matter of fact that not all patients respond optimally to this form of treatment (Harvey & Tang, 2003; Morin, Culbert, & Schwartz, 1994). Traditionally, the cognitive component included in most CBT protocols aims to alter faulty beliefs and misconceptions about sleep by either a fairly standardized cognitive restructuring protocol (Morin, Colecchi, Stone, Sood, & Brink, 1999) or by providing psychoeducation that targets specific generic myths and misconceptions endorsed frequently by insomnia patients (Edinger, Wohlgemuth, Radtke, Marsh, & Quillian, 2001a). Admittedly, these relative rigid and standardized verbal methods may effectively address certain problematic beliefs while overlooking others that may contribute to the overall insomnia problem (Bennett-Levy et al., 2004). In keeping with this assumption, we could hypothesize that individuals endorsing distinctive arrays of unhelpful beliefs may not only differ in their presenting insomnia symptoms, but they may also respond differently to a fixed form of CBT for insomnia. Therefore, by isolating the contribution of specific sets of beliefs to the phenomenon of insomnia and exploring their relationship with treatment outcome, we may be taking a step towards the identification of the subsets of patients who benefit most and least from a standardized CBT approach.
In this study we used a statistical cluster analysis to determine whether distinct patterns of beliefs and attitudes towards sleep could be identified in a sample of primary insomnia sufferers. We then examined the relationship of the identified profiles with other insomnia-related variables and with treatment outcome after CBT. Hypotheses tested included: 1) Insomnia sufferers can be grouped and distinguished on the basis of the type of sleep-related beliefs they endorse; 2) the level of endorsement as well as the main themes of dysfunctional sleep-related beliefs endorsed by the identified subgroups will be meaningfully related to other insomnia-related variables, such as overall insomnia severity, daytime symptoms and the use of substances that aid or interfere with sleep; and 3) it is expected that the response of the subgroups to a standardized form of CBT will differ since they will endorse different patterns of dysfunctional sleep-related beliefs.
METHOD
Design
This study consisted of secondary analyses of data obtained from four insomnia-focused research studies. One of the studies was a basic research study designed to compare the sleep patterns and traits of insomnia sufferers and normal sleepers (Edinger, Means, Carney, & Krystal, 2008). The remaining three studies (Edinger & Sampson, 2003; Edinger, Wohlgemuth, Radtke, Coffman, & Carney, 2007; Edinger et al., 2001a) were treatment trials designed to assess the efficacy of various formats or “doses” of CBT. The current study used a combination of cross-sectional and longitudinal statistical analyses to address the above-mentioned research objectives. All study procedures were reviewed and approved by the Institutional Review Board of Duke University Medical Center in Durham, NC. Participants were reimbursed for study-related parking expenses and were not charged for research-related evaluation or therapy procedures they received. In addition, all participants enrolled in the basic research study as well as those placed on a wait-list in one of the three treatment studies received some financial compensation for their time and the inconvenience they incurred as a result of study participation.
Participants
Participants (Total N = 385) were drawn from studies conducted at the Veteran’s Affairs Medical Center and Duke University Medical Center in Durham, NC. Included in this study were individuals who met DSM-IV-TR criteria (Association, 1994) for primary insomnia as well as a group of non-complaining normal sleepers. The normal sleepers (N = 104; 52 women) as well as 101 (52 women) insomnia sufferers were taken from the above-referenced basic research study, whereas the remaining 180 (96 women) participants were drawn from the CBT treatment trials mentioned. All participants underwent thorough screening procedures for their respective studies including structured psychiatric (Spitzer, Williams, Gibbons, & First, 1996) and sleep disorder (Schramm et al., 1993) interviews to rule out current psychiatric conditions, substance abuse problems, or medically-based sleep disorders (e.g. sleep apnea, restless legs, etc). Furthermore, all but the 19 participants drawn from the smallest treatment trial (Edinger & Sampson, 2003) additionally underwent one or more nights of polysomnographic screening and a medical examination with a physician to confirm the absence of medically-based sleep disorders and other sleep-disruptive medical conditions. Further details concerning the screening procedures and inclusion/exclusion criteria used for selecting these samples can be found elsewhere (Edinger, Means et al., 2008; Edinger & Sampson, 2003; Edinger et al., 2007; Edinger et al., 2001a).
The group of normal sleepers had a mean age = 47.2 years. (SD = 16.8 years) and completed an average of 15.9 (SD = 2.8 years) years of formal education. Most individuals (n = 85) in this group were Caucasians, fourteen were African Americans and the remaining were a mixture of other ethnic groups. The insomnia sufferers had a mean age = 53.0 years (SD = 13.6 years) and averaged 16.0 years (SD = 3.0 years) of education. Two hundred and forty of them were Caucasians, 28 were African Americans and the remainder had other ethnic backgrounds. Statistical comparisons showed the insomnia sample was significantly older (F(1, 383) = 11.94, P = 0.0006) and had a greater proportion of women (χ2 (1) = 5.77, P = 0.02) than did the normal sample but they did not differ significantly in regard to the years of education they completed (P = 0.65)
Measures
Dysfunctional Attitudes and Beliefs about Sleep questionnaire (DBAS)
All participants completed the original standard form (Morin, 1993) of the DBAS during the initial screening period of their respective study participation. However, inasmuch as a revised 16-item version (Morin et al., 2007) of this instrument has recently become available, the 16 individual items of the DBAS-16 were extracted for use in this study. Participants indicated the level of agreement or disagreement with each DBAS item on a 100 mm visual analogue scale accompanying each question. A ruler is referenced to the 100 mm line and a score at the “Strongly Disagree” pole was scored as a 0, whereas a score at the “Strongly Agree” pole was scored as 100, and all other intermediate scores refer to the place on the ruler corresponding to the participants response mark. Theme, or subscale, scores were then calculated by taking the mean scores on the items comprising the following DBAS-16 subscales: (1) Expectations for sleep (2 items); (2) Worry/helplessness about sleep (6 items); (3) Consequences of insomnia (5 items); and (4) Medication (3 items). Individuals participating in the CBT trials mentioned above also completed the DBAS at post-treatment and the six month follow-up time points. The follow-up DBAS-16 scores of this subset of individuals were also selected for use herein.
Insomnia Symptom Questionnaire (ISQ)
The ISQ was completed by all participants on one or more occasions to provide a measure of global subjective insomnia symptoms. Individuals participating in the CBT treatment trials mentioned above not only completed the ISQ as part of their initial screening, but also they did it at post-treatment and the six month follow-up time points. This instrument contains 13 Likert-style items designed to assess respondents’ perceptions about their daytime functioning and nighttime sleep (Spielman, Saskin, & Thorpy, 1987). However, we altered the format of the ISQ so that each item was accompanied by a 100 mm visual analogue scale (i.e. horizontal line), which was labeled “not at all” at its left extreme and “frequently” at its right extreme. Respondents were instructed to draw a vertical line through the point on each item’s analogue scale (i.e. 100 mm line) to indicate their responses. The distance from the left end of the line to the response line served as an analogue measure of the degree to which the respondent had the symptom noted by the item. The average score across the 13 items represented the respondent’s overall ISQ for each administration. Initial screening ISQ and also the six month follow-up ISQ for the group of individuals receiving CBT were selected for use herein.
Stanford Sleepiness Scale (SSS)
All participants completed the SSS during the initial screening period of their respective study participation. The SSS is a seven-point self-rating scale that quantifies progressive steps in sleepiness (Hoddes, Zarcone, Smythe, Phillips, & Dement, 1973). The scale consists of seven statements ranging from 1 to 7, with “1” indicating high alertness and “7” indicating imminent sleep. The respondent had to choose the number associated with the one of seven statements that best describes their typical level of alertness at four time points: 9 AM, 12 PM, 6 PM and 9 PM. The final score was the average of scores assigned to each of the four time points.
Beck Depression Inventory (BDI)
As part of their original study participation, all participants completed the original version of this instrument , i.e., the BDI-I, on one or more occasions. This is a well-validated instrument (Beck, Steer, & Garbin, 1988) that contains 21 items assessing symptoms of Major Depressive Disorder, including depressed mood, cognitive symptoms such as hopelessness, suicidal ideation, sleep disturbance, appetite changes, and reduced libido. Those in the basic research study completed the BDI-I on one occasion for research purposes, whereas those in the treatment studies completed this instrument both before and after receiving insomnia treatment to assess treatment effects. In the latter case, only the pre-treatment BDI-I was selected for use herein.
State-Trait Anxiety Inventory (STAI)
The 40-item State-Trait Anxiety Inventory (Spielberger, Gorsuch, & Lushene, 1970) was completed by all participants on one or more occasions to provide a measure of their current and usual levels of anxiety. As was the case for the BDI, those in the basic research study completed the STAI on one occasion for research purposes, whereas those in the treatment studies completed this instrument both before and after receiving insomnia treatment. In the latter case, only the pre-treatment STAI was selected for use. Standard administration/scoring procedures were employed to derive measures of state and trait anxiety from this instrument.
Sleep History Questionnaire
As part of the requirements of their respective parent studies, all participants completed a 10-page questionnaire including questions about sleep/wake symptoms, sleep habits, consumption of items that aid or interfere with sleep, and sleep expectations. For the purposes of this investigation, only those responses to a subset of questionnaire items with direct pertinence to the study objectives were considered for group comparisons. These included relevant sleep-related data, such as the number of nights per week with sleep onset latency (SOL) or awakenings during the night (WASO) lasting longer than 30 minutes, the number of nights per week with an early morning awakening, the number of nights per month using prescribed medication for sleep, and the duration of the sleep problem. Participants were also queried about daily caffeine consumption and about the number of days per month they consumed alcoholic beverages. Although we have not formally investigated the psychometric properties of these items, they all consist of reasonably simple and face-valid questions (items available from the author upon request).
Treatment
A subset of individuals included in this study received from 1 and 8 sessions of Cognitive Behavioral Therapy (CBT) for their insomnia (n=92). Twenty of these were enrolled in our first CBT study (Edinger et al., 2001a) and received 6 weekly sessions of this intervention. Another 9 were enrolled in our second study (Edinger & Sampson, 2003) designed to test an abbreviated, 2-session CBT and a third cohort of 63 patients were randomized to 1, 2, 4 or eight CBT sessions presented over an eight-week time period (Edinger et al., 2007). Regardless of the particular “treatment dosing” strategy employed, all of these individuals were provided an insomnia therapy designed to correct dysfunctional sleep-related cognitions and sleep-disruptive habits. The “cognitive therapy” component of this treatment consisted of a sleep education module, prepared in lay terms, which was delivered via an audio tape recording during the first treatment session and reinforced by each participant’s assigned therapist during the initial session and any follow-up sessions that the patient received. The taped education module was designed to correct generic unhelpful beliefs about sleep commonly reported by insomnia patients. Specifically, this tape included information about inter-individual variation in nightly sleep requirements, the effects of normal aging on sleep, the effects of mild sleep deprivation on subsequent sleep and daytime functioning, the influence of endogenous circadian rhythms, and the benefits of maintaining a regular sleep-wake cycle. During the remainder of the initial session as well as during any subsequent sessions, the therapists referred to information presented on this tape to address relevant dysfunctional sleep-related cognitions subsequently presented by their assigned study patients during treatment. In addition to this cognitive intervention, participants assigned to CBT also received standard stimulus control and sleep-restriction instructions beginning with the first treatment session and reinforced in any subsequent sessions they were provided. Since our methods for implementing these latter interventions across sessions have been well-described elsewhere (Edinger & Sampson, 2003; Edinger et al., 2007; Edinger et al., 2001a), they will not be reiterated here.
Data analyses
As noted previously, our primary purpose for this study was that of identifying subgroups of patients that have distinctive patterns of unhelpful beliefs. To address this objective we computed the four DBAS-16 subscale scores for each of the 281 insomnia sufferers comprising our insomnia sample and then subjected these data to a hierarchical cluster analysis using Ward’s method. The Euclidean distance was employed as the measure of similarity. The procedure adopted for identifying the optimal number of clusters which best fits the latent structure of the data set was two-fold: 1) to look at the dendrogram for large changes in the fusion level, and 2) examining the plot of the pseudo-t squared statistic. Differences between cluster groups on each of the four DBAS-16 subscales were examined by one-way analyses of variance (ANOVA) and subsequent Student-Newman-Keuls tests. Additionally, we performed a series of one-way ANOVAs and chi-square analyses to ascertain whether clusters differed on demographic factors that could account for their distinctive characteristics. These were gender, age and years of education completed.
Since there is not an established cutoff point implying “dysfunctionality” or “normality” for any of the 4 subscales of the DBAS-16, to characterize the resulting clusters regarding their scores on each of the subscales we used a social comparison approach (Seggar, Lambert, & Hansen, 2002). Specifically, we first computed the mean and standard deviation of each DBAS-16 subscale in our sample of normal sleepers. Given the noted age and gender differences between our insomnia and normal sleeper samples, we first tested the effects of age and gender on the DBAS-16 subscales scores within our normative group. Specifically, we divided our normal sample into two age groups (those < 40 years old vs. those ≥ 40) and conducted a series of 2 (age group) × 2 (gender) ANOVAs using the DBAS-16 subscale scores as dependent measures. Results of these analyses showed no significant main or interaction effects (all Ps ≥0.12) due to age or gender implying that these variables did not affect DBAS-16 subscale scores within the normal group. Given these results, we computed the mean and standard deviation of each DBAS-16 subscale within our entire sample of normal sleepers and used these values as social standards by which to compare the scores of the insomnia sample. We then designated one standard deviation above the mean of the normal sleeper sample as the “normative cutoff” for each DBAS-16 subscale. Subsequently, we compared the mean values of each cluster against these cutoffs. Those values falling at or below the cutoff were regarded as “within the normative range,” whereas those falling above the cutoff were considered “above the normal range.” In addition, we regarded scores as “below the normal endorsement” status if the cluster’s mean was below the normal range, defined as Mean – 1 standard deviation of the normal sleepers sample. We plotted each cluster’s mean score along with the normal range of scores (Mean ± 1 standard deviation) for each subscale. In addition to these plots, we conducted a series of analyses to compare the cluster subgroups on a range of variables that were not included in the clustering process. These analyses were carried out by means of one-way ANOVA and Student-Newman-Keuls post-hoc tests.
In our final set of analyses we examined the relationship between cluster group membership and outcome in the subset of our participants who received CBT (n=92) for their insomnia complaints. To compare the treatment outcomes of the different cluster groups, we performed two sets of statistical comparisons in which our primary endpoint was insomnia severity, as measured by the ISQ. In our first set of statistical comparisons, we conducted a factorial mixed-design repeated measures ANOVA to determine if the 4 clusters differed in their ISQ changes from baseline to the follow-up time point. A post-hoc ANOVA of baseline-to-follow-up change scores and subsequent Student-Newman-Keuls comparisons was employed to ascertain any differential CBT response across the cluster subgroups. For all statistical hypothesis tests described above, a two-tailed P value of ≤ 0.05 was regarded as statistically significant.
With our second set of these analyses we aimed to ascertain the clinical relevance of the treatment outcome across clusters by using the method known as Normative comparisons (Kendall, Marrs-Garcia, Nath, & Sheldrick, 1999). This approach aims to determine whether treated individuals display levels of symptomatology that are equivalent to or deviant from the levels shown by a normal population. Kendall’s method involves comparing treated patients with a normative sample. In order to conclude that a treated group is clinically equivalent to a normal group, a two-fold criterion must be met: 1) the statistical equivalency t test (set) must be significant, suggesting that the difference between the treated group mean and the normal group mean lies within a specified range of closeness, and 2) a traditional t test (tradt) must indicate that the mean values of these two groups are not significantly different. We used this procedure to evaluate which of the clusters reached endpoint ISQ scores that could be considered clinically equivalent to the normal sleepers scores. When specifying the range of closeness required to perform the statistical equivalency t test, Kendall et al. (1999) suggest a range of one standard deviation from the normative sample’s mean. Nevertheless, they also note that, ultimately, the defined range must be tailored to the particular comparison. Inasmuch as our “normative” group consisted of a sample of very well-screened normal sleepers free of any insomnia symptoms and other comorbidities, we considered that comparing the treated sample’s mean within the range of one standard deviation of the mean of this “supernormal” sample would be an overly stringent criterion. Keeping this in mind and considering other approaches to clinical significance of treatment outcomes (e.g., Jacobson & Truax, 1991), we chose a more lenient criterion of two standard deviations from the mean of the normal sleepers sample as the range of closeness acceptable for being regarded as “normative”. We, thus, defined the range of closeness for our analyses to be up to two standard deviations above the ISQ score mean (M = 22.64) for the normal sleepers group. Since higher scores on the ISQ represent more severe insomnia, we conducted upper tailed statistical equivalence t tests (set), followed by traditional t tests (tradt). In the presence of unequal variances, we computed Satterthwaite’s approximate t test (Hayes, 2004) instead of computing a pooled t test. To correct for type I error, we applied a Bonferroni correction and accepted P<0.0125 as significant (corrected P-value for 4 comparisons corresponding to P < 0.05 was calculated as follows: 1 − (1 − 0.05)1/4).
Lastly, for purely descriptive purposes, we computed the mean scores of the treated subgroups on the 4 scales of the DBAS-16 at follow-up and, again, plotted these values along with the “normal range” obtained in the sample of normal sleepers. The SAS version 9.1 computer package was utilized (SAS Institute, 2006) for all the statistical analyses.
RESULTS
Cluster analysis results
An inspection of the dendrogram and the pseudo t2 statistic included in the SAS software indicated that our insomnia sample could optimally be divided into five clusters or subgroups. To ascertain the manner in which the DBAS-16 subscale scores of these five subgroups differed, we conducted a statistical procedure known as multivariate profile analysis (Johnson & Wichern, 1992) to compare the DBAS-16 profiles of the five cluster groups. Results suggested significant effects for omnibus tests of “flatness”,(F(3, 274) = 172.63, P = 0.0001), “parallelism” (F(12, 725)= 38.37, P = 0.0001), and overall “elevation” (F(4, 276) = 132.59, P = 0.0001). The initial effect implied that one or more of the group profiles departed significantly from a flat profile; the second effect suggested that scale-to-scale patterns or profile “shapes” of two or more of the clusters differed significantly; and the final effect suggested that the groups differed significantly on one or more of the DBAS-16 subscale score elevations. Means and standard deviations for each cluster on the four DBAS-16 subscales are presented in Table 1. One-way ANOVAs indicated that clusters differed significantly in terms of their mean scores for each subscale: Expectations, F(4, 276) =74.27, P<0.0001, Worry/Helplessness, F(4, 276) =50.80, P<0.0001, Consequences, F(4, 276) =90.29, P<0.0001, and Medication, F(4, 276) =66.18, P<0.0001. Cluster-by-cluster differences on the four subscales are also provided in Table 1. As shown, the majority of the pairwise comparisons were statistically significant, meaning that most of the clusters’ mean scores on each DBAS-16 subscale differed from each other. However, the pairwise comparisons showed no differences between cluster 2 and cluster 3 means on the Worry/helplessness and Medication scales; cluster 2 didn’t differ either from cluster 4 on the Consequences scale. Furthermore, cluster 3 and cluster 4 did not differ significantly from each other in regard to their mean scores on the Expectations scale.
Table 1.
Cluster comparison on DBAS-16 subscales and demographic variables. Values are raw means and standard deviations unless specified otherwise
| Cluster 1 (n=70) |
Cluster 2 (n=51) |
Cluster 3 (n=37) |
Cluster 4 (n=93) |
Cluster 5 (n=30) |
|
|---|---|---|---|---|---|
| Expectations | 50.10 c (21.31) |
81.05 a (11.83) |
30.12 d (17.44) |
33.81 d (19.08) |
67.40 b (16.08) |
| Worry/Helplessness | 54.20 a (10.90) |
32.94 d (9.81) |
28.92 d (10.15) |
37.22 c (9.30) |
45.20 b (14.50) |
| Consequences | 54.92 b (18.04) |
44.46c (10.12) |
16.77 d (7.44) |
41.96 c (10.93) |
71.35 a (11.35) |
| Medication | 40.53 a (15.20) |
27.07 b (16.45) |
12.18 d (7.96) |
23.09 b,c (12.31) |
19.46 c (9.01) |
| Gender: male, % | 62.86 | 41.18 | 37.84 | 44.09 | 43.33 |
| Age: in years | 55.40 a (12.33) |
47.31b (14.91) |
56.65 a (14.28) |
52.85 a,b (12.63) |
52.73 a,b (13.94) |
| Number education years |
15.05 b (3.02) |
17.43 a (2.53) |
16.00 a,b (2.19) |
16.06 a,b (2.73) |
15.70 b (4.07) |
Means with different letters are significantly different according to Student-Newman-Keuls tests
Means with different letters are significantly different according to Student-Newman-Keuls tests
Means with different letters are significantly different according to Student-Newman-Keuls tests
Means with different letters are significantly different according to Student-Newman-Keuls tests
Demographic comparisons
We examined whether there were significant differences among the 5 identified clusters with respect to basic socio-demographic characteristics, including age, gender and years of education. Although there were no significant differences regarding the gender compositions of the clusters, individuals in cluster 2 were significantly younger (F(4, 276) =3.57, P=0.007) than individuals in cluster 1 and 3, and they had significantly more years of education (F(4, 250) =4.66, P=0.001) than did those comprising clusters 1 and 5. Because these age and educational differences could confound subsequent pre-planned comparisons among the clusters, we dropped cluster 2 from subsequent analyses. Since the remaining 4 clusters did not differ significantly in regard to these socio-demographic characteristics, they were retained to address the study questions
Characterization of clusters
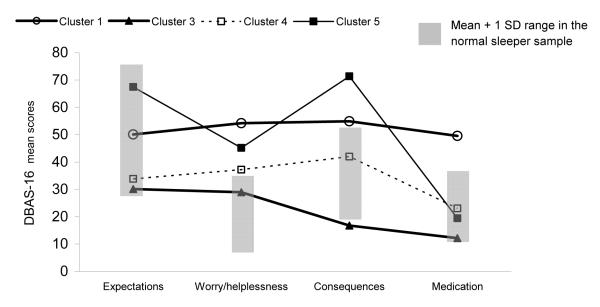
Figure 1 shows the profiles of the four clusters defined by their DBAS-16 subscale mean scores. Also shown in this figure is the presumed normative range (gray shaded areas) for each subscale based on the mean ± 1 standard deviation of scores obtained by our normal sleeper sample for each of the four subscales.
Figure 1.
Graphical display of the mean scores of the 4 clusters on each DBAS-16 subscale along with a normal range
Cluster 1 comprised 70 individuals (24.9% of the total sample) whose mean scores fell above the normal range for three of the four DBAS-16 subscales: Worry/helplessness, Consequences, and Medication. Since the features that distinguished this cluster from the other three (Clusters 3, 4 and 5) were mainly higher scores on the subscale addressing beliefs about the biological nature of insomnia and the need of medication to treat it and on the subscale Worry/helplessness, this cluster was named “Worried and medication biased”.
Cluster 3 Included 13.2% of the sample (n=37). In most respects, this group closely resembled the normal sleepers profile. However, the group comprising this cluster showed lower endorsement of beliefs related to the negative consequences of insomnia than did the normal group. This group was thus labeled “Low endorsement”.
Cluster 4 included 93 individuals (33.1% of the total sample) who just scored within the abnormal range on the DBAS-16 Worry/Helplessness scale. Hence, this group was named “Mild sleep worries”.
Cluster 5 was the smallest of the four clusters retained and accounted for 10.7% of the total sample (n=30). When compared to normal sleepers, these individuals were mainly characterized by strong beliefs about negative insomnia consequences as well as worry/helplessness about insomnia. We then named this cluster “Worried and symptom focused”.
Clusters validity
In a test of cluster validity, we conducted a series of ANOVAs using a range of questionnaire data not used in the analysis to identify the cluster subgroups. Initially we conducted a series of 4 (cluster group) × 2 (gender) ANOVAs so as to allow us to test both subgroup and gender effects. However, as main and interaction effects for gender tended to be non-significant (Ps > 0.05) we dropped gender as a factor from our final set of analyses and used simple one-way ANOVAS for these comparisons.
Our results showed significant differences between the clusters on several of the external variables considered in this study including: number of nights per week with insomnia symptoms (SOL or WASO > 30 minutes), F(3, 203)=3.70, P=0.01; insomnia severity, as measured by the Insomnia Symptom Questionnaire, F(3, 226)=20.25, P<0.0001; subjective daytime sleepiness, as measured by the Stanford Sleepiness Scale, F(3, 204)=6.24, P=0.0004; depression, F(3, 210)=3.59, P=0.01; trait, F(3, 200)=6.07, P=0.0006, and state, F(3, 210)=4.39, P=0.005 anxiety scores; and the frequency at which they used prescribed medications for sleep, F(3, 205)=5.49, P=0.001. Table 2 summarizes pairwise comparisons of the four cluster groups on these external variables. Compared with each of the other three groups, the “Low endorsement” group reported a significantly lower number of nights per week with insomnia symptoms. By contrast, all four clusters differed from each other regarding insomnia severity, as measured by the ISQ: the highest mean score was obtained by the “Worried and symptom-focused” group whereas the “Low endorsement” group showed the lowest scores on this measure.
Table 2.
Cluster comparison on external validation variables. Values are raw means and standard deviations
| Variable | Cluster 1 (n=70) Worried and medication biased |
Cluster 3 (n=37) Low endorsement |
Cluster 4 (n=93) Mild sleep worries |
Cluster 5 (n=30) Worried and symptom focused |
|---|---|---|---|---|
| Insomnia severity | ||||
| SOL or WASO* > 30 min: number of nights/week |
5.60a (1.95) | 4.27b (2.44) | 5.21a (1.89) | 5.68a (1.79) |
| Early morning awakenings : number of nights/week |
3.95 (2.15) | 3.34 (2.31) | 3.75 (2.32) | 3.95 (2.36) |
| Global insomnia symptoms: ISQ† |
58.20b (13.30) | 42.37d (10.62) | 52.53c (11.86) | 63.36a (12.43) |
| Duration of sleep problems : in years |
12.82 (11.78) | 10.36 (10.26) | 11.33 (10.05) | 11.95 (9.43) |
| Daytime measures | ||||
| Daytime sleepiness : SSS# |
3.12a (1.15) | 2.50b (0.53) | 2.69b (0.78) | 3.21a (0.85) |
| Depression symptoms: BDI |
6.59a (4.27) | 4.20b (2.68) | 5.69b (4.06) | 7.03a (4.47) |
| Anxiety symptoms: | ||||
| STAI State | 35.78a (7.49) | 29.37b (7.40) | 33.68a (8.65) | 34.17a (9.44) |
| STAI Trait | 37.56a (8.92) | 30.71b (6.79) | 34.70a (6.65) | 35.43a (7.07) |
| Substance use | ||||
| Prescribed medication for sleep: number of nights/month |
5.31a (9.02) | 0.40b (1.10) | 1.90b (5.04) | 2.35b (5.85) |
| Caffeine consumption: number of drinks/day |
2.60 (5.30) | 2.63 (2.01) | 1.98 (1.95) | 1.88 (1.91) |
| Alcohol consumption: number of days/month |
1.21 (5.60) | 0.09 (0.51) | 1.17 (4.33) | 0.85 (2.49) |
Means with different letters are significantly different according to Student-Newman-Keuls tests
Means with different letters are significantly different according to Student-Newman-Keuls tests
Means with different letters are significantly different according to Student-Newman-Keuls tests
Means with different letters are significantly different according to Student-Newman-Keuls tests
SOL: Sleep onset latency; WASO: Wake time after sleep onset
ISQ: Insomnia Symptom Questionnaire. Scores can range from 0 to 100, with higher scores indicating greater insomnia severity
SSS: Stanford Sleepiness Scale. Scores can range from 1 to 7, with higher scores indicating greater sleepiness
In regard to daytime measures, the two elevated profiles, the “Worried and medication-biased” group and the “Worried and symptom-focused” group, scored significantly higher than the “Mild sleep worries” and the “Low endorsement” groups on the BDI and the SSS. On the trait and state anxiety inventories, the “Low endorsement” cluster obtained significantly lower scores than did each of the other clusters. Finally, individuals in the “Worried and medication-biased” group reported significantly more nights per month of sleep medication use than did those in other groups.
Cluster membership and treatment outcome
As noted previously, we used a mixed-design repeated measures ANOVA to test for differential CBT treatment responses of the four cluster subgroups. In an initial set of these analyses we included gender, cluster group, and time (baseline vs. follow-up) as independent factors and used the number of CBT treatment sessions received as a covariate so as to control for the varying treatment doses the patients had received in their respective studies. However, no significant effects were noted for gender or the covariate, treatment dose, so they were dropped from our final analysis to simplify the presentation of our results. The final 2 (Time assessment) × 4 (clusters) ANOVA conducted showed significant main effects for Cluster (F(3, 88)= 6.17, P=0.0007) , and Time (F(1, 88)= 94.55, P=0.0001), as well as a significant interaction effect (F(3, 88)= 4.82 P=0.0038). Nevertheless, this interaction effect, indicating that changes on ISQ scores from baseline to follow-up varied significantly across clusters, obviated interpreting the two main effects. Student-Newman-Keuls tests of baseline-to-follow-up change scores indicated that the “Worried and symptom-focused” group mean change score, −30.72 points, was significantly higher than the mean change scores of the other three groups.
Regarding the normative comparison analysis, statistical equivalency t test was statistically significant for the “Low endorsement” group, set(116)=3.85, P<0.0125, “Mild sleep worries” group, set(140)=3.25, P<0.0125, and “Worried and symptom-focused” group, set(115)=2.25, P<0.0125, suggesting that the difference between the follow-up ISQ mean of these clusters and the mean of the normal sleepers group mean lied within the specified range of closeness. However, the second criteria, that is, the traditional t test comparison indicating that the difference between the cluster’s mean and the normal sleepers mean doesn’t differ from zero, was just met by the “Worried and symptom-focused group”, tradt(115)= −2.73, P=0.017. Thus, according to this procedure, we concluded that this group was the only one among the 4 clusters whose mean score on the ISQ could be considered clinically equivalent to the mean score of the group of normal sleepers.
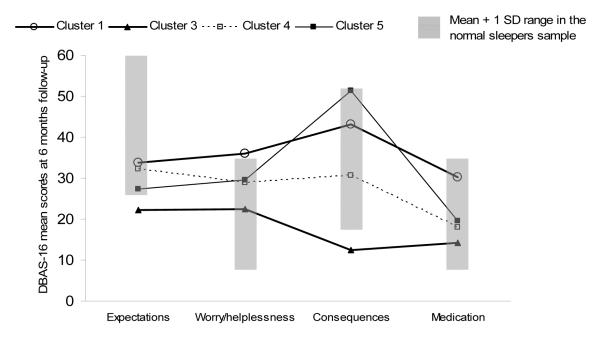
Finally, a visual inspection of the mean scores of the treated subgroups on the 4 scales of the DBAS-16 at follow-up showed that, with the exception of the “Worried and medication-biased” group, none of the groups obtained mean scores above the defined normal range.
DISCUSSION
Just as diverse as the manifestations of insomnia can be, the main themes of sleep-related cognitions can also be strikingly heterogeneous among insomnia sufferers. Indeed, our results suggest that individuals suffering from persistent insomnia can be classified into subgroups based on their endorsement of the specific types of dysfunctional beliefs measured by the DBAS-16 scale. Moreover, our data indicate that such classification may have use in understanding the phenomenology of insomnia and predicting treatment response.
Using the scores our study participants obtained on each DBAS-16 subscale, our analytic approach yielded four empirically-derived subgroups within our larger insomnia sample. In the absence of interpretative guidelines for the DBAS-16 scores (Morin et al., 2007), we compared the DBAS-16 profiles of each or our insomnia subgroups with a “normative profile” obtained in a sample of normal sleepers. Our subsequent inspection of the pattern of means for the four subgroups showed two of our subgroups had elevated profiles characterized by above-the-norm scores on at least two of the four DBAS-16 subscales. Based on the themes of elevated DBAS-16 scores shown by these subgroups, we assigned them the names “Worried and medication biased” and “Worried and symptom focused.” “Mild sleep worries” and “Low endorsement” were the labels assigned to the other two subgroups that were mainly characterized by DBAS-16 scores closely resembling those of the normal sleepers sample.
As shown in Table 2, the patterns of beliefs exhibited by these four subgroups were meaningfully related to measures of insomnia severity, daytime functioning, and use of prescribed medication for sleep, thus supporting their validity (Clatworthy, Buick, Hankins, Weinman, & Horne, 2005). Consistent with previous findings (Jansson & Linton, 2007), there was a positive relationship between higher endorsement of dysfunctional beliefs and increased functional impairment. When compared to the other three groups, the “Low endorsement” group reported less severe nocturnal sleep disturbances and milder daytime symptoms on the range of questionnaires used to assess such factors. These results emphasize the relative “normality” of this group concerning beliefs about sleep and suggest its association with a less complicated clinical picture of insomnia.
The “Mild sleep worries” group showed less severe daytime symptoms (i.e., sleepiness and depression) than did the two subgroups with elevated profiles, the “Worried and medication biased” and the “Worried and symptom focused”. Interestingly, these three groups didn’t differ in terms of reported nighttime symptoms. When looking at their mean DBAS-16 scores, we found that the “Mild sleep worries” group endorsed statements about the putative negative consequences and effects of insomnia less strongly than did these two other groups.
In view of these results, we could speculate that the increased self-reported daytime impairment found in the two groups with the more elevated DBAS-16 profiles may reflect a cognitive bias. Perhaps, endorsing beliefs about the consequences of insomnia, such as “I cannot function without a good night of sleep”, may indeed trigger self-fulfilling prophecies. That is, during the day, individuals endorsing this belief may selectively monitor body sensations for signs of fatigue or sleepiness in order to confirm their hypothesis (Harvey, Sharpley, Ree, Stinson, & Clark, 2007; Harvey, Tang, & Browning, 2005). This cognitive bias could also explain why some with insomnia fail to demonstrate strong evidence of objective daytime deficits (Bonnet, 2005). If this hypothesis is correct, then addressing and modifying these sorts of dysfunctional beliefs could play a pivotal role at improving some daytime deficits shown by some insomnia patients.
Of course, an alternative view of these results could also be tenable. Instead of conceptualizing sleep-related beliefs as a factor biasing the perception of daytime functioning, one could argue that these beliefs about the negative consequences of insomnia are just a reflection of the reality for this group of insomnia sufferers. Nonetheless, the cross-sectional nature of these results falls short of demonstrating a cause-effect relationship between specific beliefs and self-reported deficit.
The two groups characterized by abnormally high scores on two or more of the DBAS-16 subscales also differed from each other in terms of insomnia severity. The “Worried and symptom-focused” group reported more severe insomnia, according to their ISQ scores, than the “Worried and medication-biased” group. By contrast, it was the “Worried and medication-biased” group who reported taking sleep medications on a more regular basis. Once again, the specific content of the sleep-related beliefs endorsed dovetailed with an external behavioral or affective measure. Nevertheless little is known about the directionality of this relationship. Thus, prospective studies on this topic are clearly warranted.
The ability to derive different subtypes of insomnia sufferers using cluster analysis does not guarantee their clinical significance. The practical utility of these typologies should ultimately depend on their ability to assign different groups to different treatment approaches, in order to facilitate a more rational allocation of resources and maximize treatment efficacy. Given this consideration it is noteworthy that our subgroups responded differently to the specific form of CBT delivered. The group with more severe insomnia, the “Worried and symptom-focused” group, exhibited the greatest reduction on the ISQ scores at the 6-month follow-up. This finding is generally consistent with previous research indicating that greater sleep disturbance is positively associated with large symptom reduction after CBT (Espie, Inglis, & Harvey, 2001). Moreover, although research has pointed out that lower endpoint scores are less likely in patients with more severe insomnia (Espie et al., 2001), this group was the only one achieving scores on the ISQ at follow-up that could be considered clinically equivalent to the scores of a sample of normal sleepers, according to the Kendall et al. criteria (1999) we used for making this judgment.
By and large, it appears that insomnia sufferers fitting the “Worried and symptom-focused” profile are good responders to the form of CBT delivered in this study. Although the present study has not directly addressed this issue, a consideration of changes in DBAS-16 scores in this group may help in understanding their better treatment response. This group had the greatest mean score on the Expectations scale before treatment (see Figure 1). A visual inspection of Figure 2 indicates that their mean score on this scale decreased sharply after treatment. It has been hypothesized that when insomnia sufferers hold strongly unrealistic expectations about sleep (e.g. I need eight hours of sleep), they tend to worry excessively when such requirements are not met (Morin et al., 2007). In keeping with this observation, it seems reasonable to speculate that, after such unrealistic expectations have been challenged by psychoeducation, the level of worry/helplessness about insomnia may be reduced, and this belief change may lead to a reduced global appraisal of insomnia severity.
Figure 2.
Graphical display of the mean scores at 6 month-follow up of the 4 clusters on each DBAS-16 subscale along with a normal range
It is also important to mention that the “cognitive therapy” component of this treatment consisted of a standardized sleep education module. This module was structured to target a range of generic unhelpful beliefs common among insomnia sufferers in general, such as the inter-individual variation in nightly sleep requirements, the effects of normal aging on sleep, the effects of mild sleep deprivation on subsequent sleep and daytime functioning, the influence of endogenous circadian rhythms, and the benefits of maintaining a regular sleep-wake cycle. This cognitive approach may be effective for addressing a range of “cognitive treatment targets”, but may ignore additional problematic beliefs present in some insomnia subtypes. In fact, the “Worried and medication biased” group had scores on the Worry/helplessness scale that still remained above the norm after treatment. This group has also been characterized as most extreme in endorsing statements about medication, which may be related to beliefs about low self-efficacy and helplessness regarding the management of their insomnia (Belanger, Morin, Bastien, & Ladouceur, 2005). The verbal method used in this study seems not to have been sufficient to reduce the level of worry/helplessness in this group to a normative level. The belief “I’d better take sleeping pills” may need other types of intervention, rather than the psychoeducation component included in the current form of CBT. Other cognitive strategies commonly used in CBT for insomnia include cognitive restructuring and behavioral experiments (Bennett-Levy et al., 2004; Morin et al., 2009). The identification of the cognitive strategy that seems to be best for shifting specific sets of beliefs may be another interesting research approach.
Admittedly, this study has some limitations that merit consideration. One initial difficulty is the use of a self-report measure as the treatment outcome variable. This type of measure might lead to a reporting bias, i.e. improvements in beliefs about sleep may be associated to improvements in the self-report of insomnia. Such a bias could partially explain why individuals within an elevated DBAS-16 profile obtained higher change scores on the ISQ after CBT. Previous research has shown that changes in sleep cognitions are more strongly associated with subjective than with objective sleep improvements (Morin et al., 2002; Pat-Horenczyk, 1998). Thus, the use of self-report measures may also impose some caution about the generalizability of the results to objective measures, i.e., polysomnography-derived measures.
Another limitation concerns the use of an abbreviated version of the DBAS. In fact, the 16-item version used in this study includes a small number of items for assessing dysfunctional beliefs and, thus, may have not covered some unhelpful beliefs that can be relevant in understanding insomnia presentation and treatment outcome. However, the reduced version was preferred for use in this study since it has been well-validated recently (Morin et al., 2007). Moreover, being shorter, it certainly reduces respondent’s burden and, as such, it is bound to be used more frequently in future research about insomnia. Thus, the use of this abbreviated version may facilitate comparison of results across upcoming studies.
Additionally, although the response format for the most current version of the DBAS-16 is a Likert-type scale ranging from 0 to 10 (Morin et al., 2007) , we used the response format provided by the 30-item version, i.e., 100 mm visual analogue scale. We elected to keep this format for our analyses so as to not alter the original responses provided by participants. Whereas some scoring differences may emerge between the 2 response formats, this effect does not systematically favor one version over the other (Morin et al., 2007). Nonetheless, future work should investigate if similar profiles emerge when using the actual DBAS-16 Likert response format. We also acknowledge that our sample was only moderate in size and not ethnically diverse. Further, it included well-screened and more highly selected patients than typically found in the general medical setting. Hence, whether the patterns of dysfunctional beliefs shown by unsolicited insomnia sufferers are similar to those found in this study is yet to be determined.
This research presents a person-oriented approach in which patterns of beliefs are examined, rather than single measures of beliefs across insomnia sufferers. Despite the limitations mentioned above, our results point out that primary insomnia sufferers have heterogeneous patterns of unhelpful beliefs and that distinctive belief patterns are meaningfully related to other insomnia characteristics. The potential implications of these findings are that, when using the DBAS-16 as a research or clinical instrument, it may be informative to look at scores at individual subscales, in addition to the total DBAS-16 score, to identify the profile patterns noted herein. Moreover, our data provide some guidance regarding patterns of unhelpful beliefs that may predict different response to CBT for persistent insomnia. Future studies to aid in adapting these findings to clinical venues seem warranted.
ACKNOWLEDGEMENTS
This research was supported by the Department of Veterans Affairs (Grant # 0009) and the National Institute of Mental Health (Grant # 48187) awarded to Jack D. Edinger: PI. M. Montserrat Sánchez-Ortuño was supported by a research fellowship award from Fundación Séneca, Murcia, Spain. The views reported herein are exclusively those of the authors and do not reflect the views of the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- American Psychiatric Association, A. P., editor. Diagnostic and statistical manual of mental disorders. 4th ed American Psychiatric Association; Washington, D.C.: 1994. [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8(1):77–100. [Google Scholar]
- Belanger L, Morin CM, Bastien C, Ladouceur R. Self-Efficacy and Compliance With Benzodiazepine Taper in Older Adults With Chronic Insomnia. Health Psychology. 2005;24(3):281–287. doi: 10.1037/0278-6133.24.3.281. [DOI] [PubMed] [Google Scholar]
- Bennett-Levy J, Butler G, Fennell M, Hackman A, Mueller M, Westbrook D. Oxford guide to behavioural experiments in cognitive therapy. Oxford University Press; New York, NY: 2004. [Google Scholar]
- Bonnet M. Burden of chronic insomnia on the individual. National Institutes of Health; Bethesda, MD: 2005. [Google Scholar]
- Carney CE, Edinger JD. Identifying critical beliefs about sleep in primary insomnia. Sleep. 2006;29(4):444–453. [PubMed] [Google Scholar]
- Clatworthy J, Buick D, Hankins M, Weinman J, Horne R. The use and reporting of cluster analysis in health psychology: a review. British Journal of Health Psychology. 2005;10(Pt 3):329–358. doi: 10.1348/135910705X25697. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Carney CE, Wohlgemuth WK. Pretherapy cognitive dispositions and treatment outcome in cognitive behavior therapy for insomnia. Behavior Therapy. 2008;39(4):406–416. doi: 10.1016/j.beth.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Means MK, Carney CE, Krystal AD. Psychomotor performance deficits and their relation to prior nights’ sleep among individuals with primary insomnia. Sleep. 2008;31(5):599–607. doi: 10.1093/sleep/31.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger JD, Sampson WS. A primary care “friendly” cognitive behavioral insomnia therapy. Sleep. 2003;26(2):177–182. doi: 10.1093/sleep/26.2.177. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Wohlgemuth WK, Radtke RA, Coffman CJ, Carney CE. Dose-response effects of cognitive-behavioral insomnia therapy: a randomized clinical trial. Sleep. 2007;30(2):203–212. doi: 10.1093/sleep/30.2.203. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Cognitive behavioral therapy for treatment of chronic primary insomnia: a randomized controlled trial. Journal of the American Medical Association. 2001a;285(14):1856–1864. doi: 10.1001/jama.285.14.1856. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Does cognitive-behavioral insomnia therapy alter dysfunctional beliefs about sleep? Sleep. 2001b;24(5):591–599. doi: 10.1093/sleep/24.5.591. [DOI] [PubMed] [Google Scholar]
- Espie CA. Insomnia: conceptual issues in the development, persistence, and treatment of sleep disorder in adults. Annual Review of Psychology. 2002;53:215–243. doi: 10.1146/annurev.psych.53.100901.135243. [DOI] [PubMed] [Google Scholar]
- Espie CA, Inglis SJ, Harvey L. Predicting clinically significant response to cognitive behavior therapy for chronic insomnia in general medical practice: analysis of outcome data at 12 months posttreatment. Journal of Consulting and Clinical Psychology. 2001;69(1):58–66. doi: 10.1037//0022-006x.69.1.58. [DOI] [PubMed] [Google Scholar]
- Harvey AG. A cognitive model of insomnia. Behaviour Research and Therapy. 2002;40(8):869–893. doi: 10.1016/s0005-7967(01)00061-4. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Sharpley AL, Ree MJ, Stinson K, Clark DM. An open trial of cognitive therapy for chronic insomnia. Behaviour Research and Therapy. 2007;45(10):2491–2501. doi: 10.1016/j.brat.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Tang NK. Cognitive behaviour therapy for primary insomnia: can we rest yet? Sleep Medicine Reviews. 2003;7(3):237–262. doi: 10.1053/smrv.2002.0266. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Tang NK, Browning L. Cognitive approaches to insomnia. Clinical Psychology Review. 2005;25(5):593–611. doi: 10.1016/j.cpr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Statistical Methods for Communication Science. Lawrence Erlbaum Associates, Inc.; Mahwah, NJ: 2004. [Google Scholar]
- Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10(4):431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Jacobson NS, Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology. 1991;59(1):12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- Jansson M, Linton SJ. Psychological mechanisms in the maintenance of insomnia: arousal, distress, and sleep-related beliefs. Behaviour Research and Therapy. 2007;45(3):511–521. doi: 10.1016/j.brat.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Wichern DW. Applied multivariate statistical analysis. 3rd ed Prentice Hall; Englewood Cliffs, New Jersey: 1992. [Google Scholar]
- Kendall PC, Marrs-Garcia A, Nath SR, Sheldrick RC. Normative comparisons for the evaluation of clinical significance. Journal of Consulting and Clinical Psychology. 1999;67(3):285–299. doi: 10.1037//0022-006x.67.3.285. [DOI] [PubMed] [Google Scholar]
- Morin CM. Insomnia: Psychological assessment and management. Guildford Publications; New York: 1993. [Google Scholar]
- Morin CM, Blais F, Savard J. Are changes in beliefs and attitudes about sleep related to sleep improvements in the treatment of insomnia? Behavioural Research and Therapy. 2002;40(7):741–752. doi: 10.1016/s0005-7967(01)00055-9. [DOI] [PubMed] [Google Scholar]
- Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. Journal of the American Medical Association. 1999;281(11):991–999. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. American Journal of Psychiatry. 1994;151(8):1172–1180. doi: 10.1176/ajp.151.8.1172. [DOI] [PubMed] [Google Scholar]
- Morin CM, Stone J, Trinkle D, Mercer J, Remsberg S. Dysfunctional beliefs and attitudes about sleep among older adults with and without insomnia complaints. Psychology and Aging. 1993;8(3):463–467. doi: 10.1037//0882-7974.8.3.463. [DOI] [PubMed] [Google Scholar]
- Morin CM, Vallieres A, Guay B, Ivers H, Savard J, Merette C, Bastien C, Baillargeon L. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. Journal of the American Medical Association. 2009;301(19):2005–2015. doi: 10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CM, Vallieres A, Ivers H. Dysfunctional beliefs and attitudes about sleep (DBAS): validation of a brief version (DBAS-16) Sleep. 2007;30(11):1547–1554. doi: 10.1093/sleep/30.11.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pat-Horenczyk R. Changes in attitudes toward insomnia following cognitive intervention as part of a withdrawal treatment from hypnotics. Behavioural and Cognitive Psychotherapy. 1998;26(4):345–357. [Google Scholar]
- SAS Institute . Statistical Software System. Version 9.1 SAS Institute, Inc.; Cary, N.C.: 2006. [Google Scholar]
- Schramm E, Hohagen F, Grasshoff U, Riemann D, Hajak G, Weess HG, Berger M. Test-retest reliability and validity of the Structured Interview for Sleep Disorders According to DSM-III-R. American Journal of Psychiatry. 1993;150(6):867–872. doi: 10.1176/ajp.150.6.867. [DOI] [PubMed] [Google Scholar]
- Seggar LB, Lambert MJ, Hansen NB. Assessing clinical significance: Application to the Beck Depression Inventory. Behavior Therapy. 2002;33(2):253–269. [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait-Anxiety-Inventory. Consulting Psychologist Press; Palo Alto, California: 1970. [Google Scholar]
- Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987;10(1):45–56. [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbons M, First MB. Instruction Manual for the Structured Clinical Interview for DSM-IV (SCID-IV). (SCID 1996 Revision) Biometrics Research Department, New York Psychiatric Institute; New York: 1996. [Google Scholar]