Abstract
A recently reported selective agonist of the human A3 adenosine receptor (hA3AR), MRS5127, (1′R,2′R,3′S,4′R,5′S)-4′-[2-chloro-6-(3-iodobenzylamino)-purine]-2′,3′-O-dihydroxy-bicyclo-[3.1.0]hexane, was radioiodinated and characterized pharmacologically. It contains a rigid bicyclic ring system in place of a 5′-truncated ribose moiety, and was selected for radiolabeling due to its nanomolar binding affinity at both human and rat A3ARs. The radioiodination of the N6-3-iodobenzyl substituent by iododestannylation of a 3- (trimethylstannyl)benzyl precursor was achieved in 73% yield, measured after purification by HPLC. [125I]MRS5127 bound to the human A3AR expressed in membranes of stably transfected HEK 293 cells. Specific binding was saturable, competitive, and followed a one-site binding model, with a Kd value of 5.74 ± 0.97 nM. At a concentration equivalent to its Kd, non-specific binding comprised 27±2% of total binding. In kinetic studies, [125I]MRS5127 rapidly associated with the hA3AR (t1/2 = 0.514 ± 0.014 min), and the affinity calculated from association and dissociation rate constants was 3.50 ± 1.46 nM. The pharmacological profile of ligands in competition experiments with [125I]MRS5127 was consistent with the known structure-activity-relationship profile of the hA3AR. [125I]MRS5127 bound with similar high affinity (Kd, nM) to recombinant A3ARs from mouse (4.90 ± 0.77), rabbit (2.53 ± 0.11), and dog (3.35 ± 0.54). For all of the species tested, MRS5127 exhibited A3AR agonist activity based on negative coupling to cAMP production. Thus, [125I]MRS5127 represents a new species-independent agonist radioligand for the A3AR. The major advantage of [125I]MRS5127 compared with previously used A3AR radioligands is its high affinity, low degree of non-specific binding, and improved A3AR selectivity.
Keywords: nucleoside, G protein-coupled receptor, adenosine receptor, radioligand binding
1. Introduction
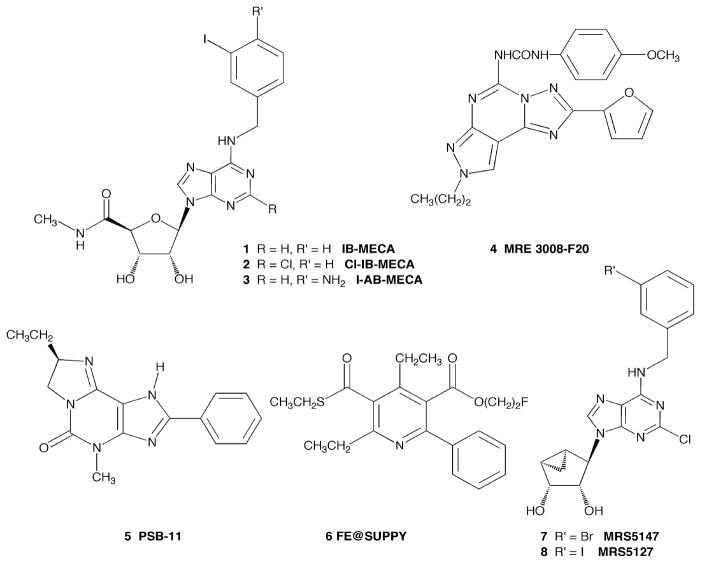
Modulation of the A3 adenosine receptor (A3AR) is being explored in preclinical and clinical studies for the treatment of a variety of diseases [1, 2]. Selective agonists 1 and 2 (Figure 1) are undergoing clinical trials for hepatocarcinoma, rheumatoid arthritis (phase IIB completed), psoriasis, and dry eye disease [3, 4]. Other target diseases for selective A3AR agonists and antagonists that might be the subject of future clinical trials are neurodegeneration [5, 6], inflammatory bowel disease [7], other autoimmune inflammatory diseases [8], and cancer [9]. The level of expression of the A3AR was found to be elevated in tumors, neutrophils, and synoviocytes in the disease state [9–12]. The A3AR expression level correlated to the responsiveness in arthritis patients to therapy with the A3AR agonist IB-MECA 1 [4].
Figure 1.
Structures of nucleoside and non-nucleoside, high affinity ligands for the A3AR. Compounds 3–6 have previously been prepared in radioactive form for use in receptor labeling studies.
The most widely used radioligand for the study of the A3AR is the high affinity agonist [125I]I-AB-MECA 3 (Kd ~ 1 nM at human (h), mouse (m), and rat (r) A3ARs) [13, 14]. The disadvantage of this compound is its low selectivity for the A3AR. Thus, it is useful for characterization of the A3AR in cell lines overexpressing the receptor and in various cells expressing the A3AR at high levels, such as eosinophils and neutrophils [15], but not in most native tissues. [3H]HEMADO (2-hexyn-1-yl-N6-methyladenosine), a tritiated radioligand of high affinity and selectivity was reported to be a useful radioligand for the hA3AR and demonstrated to have low nonspecific binding [16]. However, the greatly decreased affinity of adenosine agonists at the rat A3AR in comparison to the human A3AR has been noted consistently for adenosine analogs substituted at the 6 position with small alkyl moieties and at the 5′ and 2 positions with a range of structures [17–20]. Several antagonist radioligands have been used previously in in vitro studies, such as the pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine derivative [3H]MRE 3008F20 4 and the 4,5,7,8-tetrahydro-1H-imidazo[2,1-i]purin-5-one derivative [3H]PSB-11 5 [21, 22]. The disadvantage of these structurally diverse heterocyclic antagonists is their low affinity for the A3AR in nonhuman tissue. For example, the affinity of MRE 3008F20 at the rat A3AR is >10 μM [23]. Recently, a 18F-labeled radioligand, the 6-phenylpyridine derivative 6, suitable for PET (positron emission tomography) studies in both human and murine species was reported [24].
A new approach to designing ligands for the A3AR that bind selectively to several species homologues of this receptor is based on 5′-truncated nucleoside derivatives. Recently, we have extended this truncation approach to selective A3AR ligands containing the rigid (N)- methanocarba(bicyclo[3.1.0]hexane) ring system as a ribose substitute [25, 26]. This bicyclic ring system maintains a conformation that is preferred at the A3AR increasing selectivity, even in the absence of a 5′-N-methyluronamide group. Some members of this series were found to have reduced intrinsic activity for the A3AR or to function as full antagonists [25, 26]. One member of this series, the partial agonist MRS5147 7, was labeled with 76Br for use as a PET ligand of high affinity [27]. [76Br]MRS5147 bound to human and rat A3ARs with Ki values of 0.62 and 5.2 nM, respectively. The corresponding 3-iodo derivative MRS5127 8 also displays high affinity at both the h and r A3ARs [25, 26]. MRS5127 8 was highly A3AR-selective; its affinity at three human AR subtypes was determined to be: hA1= 3040 ± 610 nM; hA2A= 1080 ± 310 nM; hA3= 1.44 ± 0.60 nM. By Schild analysis of [35S]GTPγS binding to membranes from CHO cells expressing the hA3AR, MRS5127 appeared to be an antagonist [25]. However, further analysis determined that it is a partial agonist stimulating cAMP production in transfected cells with 45% efficacy compared to the full agonist NECA [26]. In this study, we have synthesized a radioiodinated form of this truncated rigid carbocyclic nucleoside derivative for in vitro studies and have characterized its binding properties at the A3AR in several species.
2. Materials and methods
2.1. Chemical synthesis
2.1.1. Materials and instrumentation
Hexamethyltin and other reagents, including pharmacological agents, were purchased from Sigma-Aldrich Chemical Company (St. Louis, Missouri), except where noted. MRS5127 8 was prepared as reported [25]. Sodium [125I]iodide (17.4 Ci/mg) in NaOH (1.0×10−5 M) was supplied by Perkin Elmer Life and Analytical Science (Boston, Massachusetts). 1H NMR spectra were obtained with a Varian Gemini 300 spectrometer using CDCl3 and CD3OD as solvents. Chemical shifts are expressed in δ values (ppm) with tetramethylsilane (δ 0.00) for CDCl3 and water (δ 3.30) for CD3OD. TLC analysis was carried out on aluminum sheets precoated with silica gel F254 (0.2 mm) from Aldrich. HPLC mobile phases for unlabeled material consisted of CH3CN/tetrabutyl ammonium phosphate (5 mM) from 20/80 to 60/40 in 20 min, flow rate 1.0 ml/min. High-resolution mass measurements were performed on Micromass/Waters LCT Premier Electrospray Time of Flight (TOF) mass spectrometer coupled with a Waters HPLC system. cLogP was calculated using CS ChemBioDraw Ultra V 12.0 (CambridgeSoft).
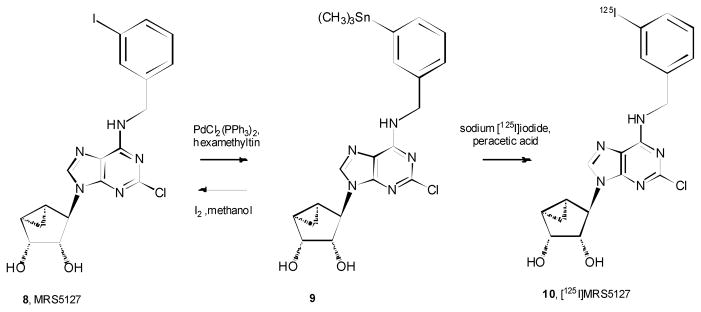
2.1.2. Preparation of 9: (1′R, 2′R, 3′S, 4′R, 5′S)-4′-[2-Chloro-6-(3-trimethylstannylbenzylamino)purine]-2′,3′-O-dihydroxybicyclo-[3.1.0]hexane (1)
MRS5127 8 (8.95 mg, 0.018 mmol), PdCl2(PPh3)2 (2.7 mg), and hexamethyltin (11 μL, 0.054 mmol) were mixed together in anhydrous dioxane (2 ml), and the resulting reaction mixture was stirred at 70° C for 2 h. The mixture was concentrated under reduced pressure. The product was purified by flash chromatography by using CHCl3: MeOH (10:1) as the eluant to afford the stannyl derivative 1 (9.3 mg, 90%) as an oil. 1H NMR (300 MHz, CDCl3), 7.81 (s, 1H), 7.53 (s, 1H), 7.34 (m, 2H), 7.33 (m, 1H), 6.49 (br s, 1H), 4.88 (br s, 2H), 4.00 (m, 2H), 3.71 (s, 1H), 3.65 (m, 1H), 3.47 (m, 1H), 2.02 (m, 1H), 1.96 (s, 1H), 1.64 (m, 1H), 1.28 (m, 2H), 0.81 (m, 1H), 0.29 (s, 9H). HRMS (M + 1)+: calculated for C21H27ClIN5O2Sn+ (M+H)+ 535.6338, found 536.0823 HPLC: Rt = 22.1 min. HPLC system: 5 mM TBAP/CH3CN from 80/20 to 60/40 in 25 min, then isocratic for 2 min; flow rate of 1 ml/min.
2.1.3. Regeneration of MRS5127 (8)
The trimethylstannyl intermediate 9 (0.1 mg) was reconverted to MRS5127 upon dissolving in MeOH (0.1 ml) followed by treatment with I2 (0.1 M in MeOH, 0.1 ml) for 10 min at room temperature (Figure 2). The structure was confirmed by HPLC and high resolution mass spectroscopy (HRMS). HRMS (M + 1)+: calculated for C18H18ClIN5O2+ (M+H)+: 498.0194; found, 498.0194. HPLC: Rt = 16.6 min (same system as above).
Figure 2.
Synthesis and subsequent radioiodination of the stannyl precursor 9.
2.1.4. Radiochemical synthesis of [125I]MRS5127 (10)
A solution of the stannyl derivative 9 in acetonitrile (4 μl, corresponding to 20 μg, 37 nmol) was added to ~75μl Na125I solution (20 mCi, 9 nmol, PerkinElmer) in a glass container. Then, a mixture of peracetic acid/acetonitrile/glacial acetic acid (using 32 wt.% peracetic acid from Sigma-Aldrich) was prepared in the ratio of 5/85/10 (v/v). An aliquot (5μl) of this peracetic acid solution (80μg, 1.05 μmol) was added to the reaction mixture with stirring. After 10 min the reaction mixture was diluted with 0.2 ml water, and the entire quantity was injected onto an HPLC to yield 14.6 mCi (73% yield) of 10. The HPLC system was different from the one specified for the nonradiochemical syntheses. The HPLC mobile phase consisted of water/acetonitrile, both containing 0.1% trifluoroacetic acid, in a gradient from 60/40 to 30/70 over 40 min at flow rate of 1.0 ml/min. For confirmation of the identity of the radioactive product, a mixture of 8 and 10 eluted as a single peak (retention time 13 min). The HPLC column was 4.6 × 250 mm, 300Å, 5μ, C-18 from MAC-MOD Analytical, Inc. (Chadd’s Ford, PA). The radioactive fraction was prepared as a solution in 1×10−5 M NaOH.
2.2. Pharmacological assays
2.2.1. Radioligand binding assays
HEK 293 cells stably expressing recombinant human, mouse, rabbit, or dog ARs were cultured in DMEM supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 400 μg/ml G418 [25, 28–30]. After harvest and homogenization in 10 mM Na+-HEPES buffer (pH 7.4) containing 10 mM EDTA and 0.1 mM benzamidine, the cell lysates were centrifuged at 20,000 g for 25 min at 4°C, and the pellet was re-suspended in 10 mM Na+-HEPES buffer (pH 7.4) containing 1 mM EDTA and 0.1 mM benzamidine. The suspension was re-homogenized and then centrifuged at 20,000 g for 25 min at 4°C. The resultant pellets were resuspended in buffer containing 10% sucrose and stored at −80°C. Membranes were isolated from mouse brain tissue using a similar protocol.
Cell membranes (100 μL) were incubated in 10 mM Na+-HEPES buffer (pH 7.4) containing 5 mM MgCl2, 5 units/ml adenosine deaminase, and [125I]MRS5127. In saturation binding assays, the concentration of [125I]MRS5127 ranged from ~0.5 – 40 nM following dilution (10–20-fold) with the non-radiolabeled compound. Specific [125I]MRS5127 binding fit optimally to a single-site binding model in all assays: y = (Bmax*[L])/(Kd+[L]), from which Bmax and Kd values were obtained. In competition binding assays, competitors were included in the incubations at concentrations spanning at least 6 orders of magnitude adjusted appropriately around the IC50 of each compound. The IC50 values were calculated using non-linear regression analysis by fitting the data to: binding = nonspecific binding + (total binding − specific binding)/(1 + 10x−logIC50). Ki values were calculated using the Cheng-Prusoff equation [31].
2.2.2. cAMP accumulation assays
HEK 293 cells were detached from cell culture plates, resuspended in serum-free DMEM containing 25 mM HEPES (pH 7.4), 1 unit/ml adenosine deaminase, and 20 μM Ro 20,1724 to inhibit phosphodiesterases, and then transferred to polypropylene tubes (200,000 cells/tube). The cells were co-stimulated with forskolin (10 μM) and AR agonists for 15 min at 37° C with shaking, after which the assays were terminated by adding 500 μL 0.1 N HCl. The lysates were centrifuged at 4,000 × g for 10 min after which cAMP was determined in the supernatants using a competitive binding assay, as described previously [25]. The data were expressed as the amount of cAMP that accumulated during the 15 min incubation time, i.e., pmol/15 min/200,000 cells. EC50 values were calculated by fitting the data to: V = Vmin + (Vmax−Vmin)/(1 + 10x−logEC50).
3. Results
3.1. Chemistry
Since MRS5127 already contains an iodine atom that is associated with high A3AR affinity and selectivity, that position was selected for convenient radiolabeling. A versatile method for rapidly introducing radioactive iodine on an aromatic ring is to use a stannyl precursor. The feasibility of this route was demonstrated through a ‘cold’ iodination reaction (Figure 2). The trimethylstannyl precursor 9 was generated in one step and in a good yield of 90% from MRS5127 using a palladium reagent and hexamethyltin. Protection of the hydroxyl groups or the exocyclic amine of this adenosine analogue was not necessary. Compound 9 was stable upon storage at −80°C for several months. This intermediate 9 rapidly and efficiently reverted to MRS5127 upon treatment with iodine. The radioiodination of MRS5127 was accomplished through standard methods of iododestannylation of 9 using sodium iodide as a source of 125I [32, 33]. The radiochemical yield of HPLC-purified product 10 was 73%.
3.1. Pharmacology
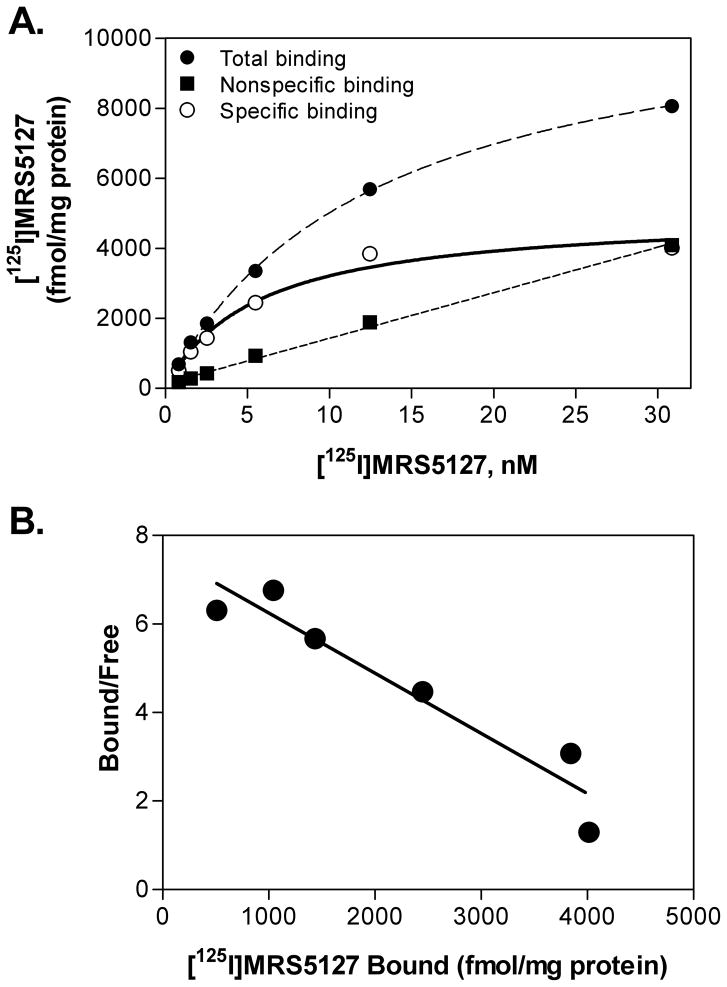
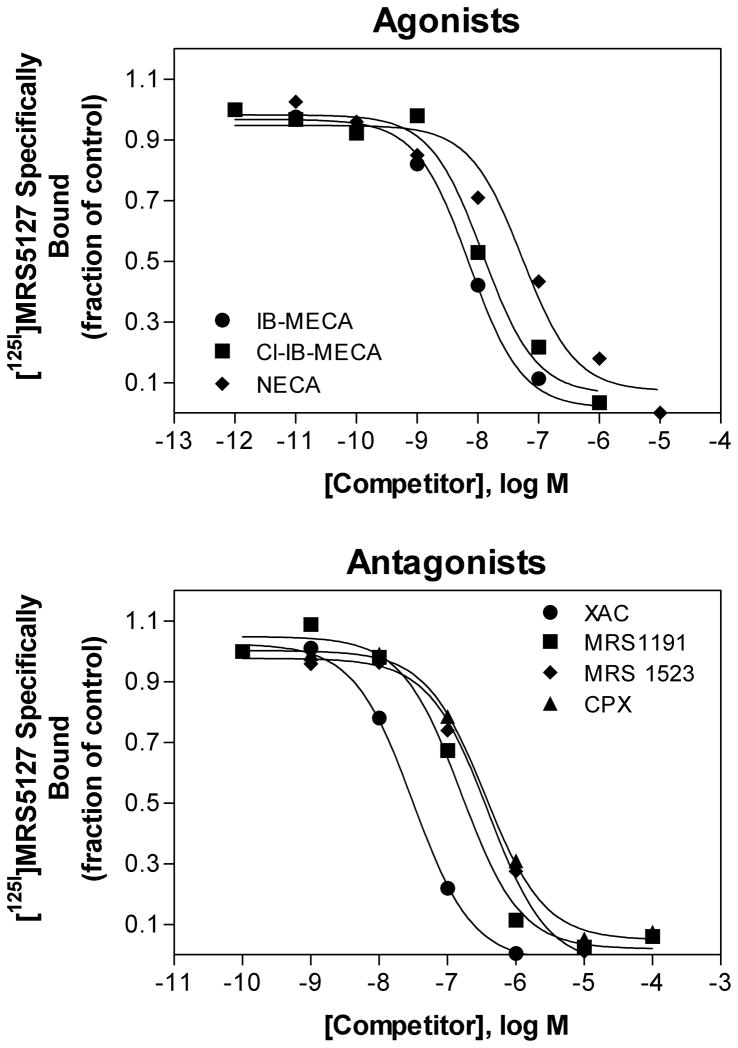
Equilibrium saturation studies of the binding of [125I]MRS5127 to the hA3AR were performed (Fig. 3A). Specific binding was observed that fit optimally to a single-site binding model demonstrated most clearly following Scatchard transformation of the data (Fig. 3B). Nonspecific binding amounted to 27 ± 2% of total binding at a concentration of 5 nM when the glass fiber filters were pre-incubated with polyethyleneimine (0.05%). The Kd value was determined to be 5.74 ± 0.97 nM. No specific binding was observed using non-transfected HEK 293 cells (data not shown). The ability of various known AR agonists and antagonists to compete for [125I]MRS 5127 binding to the hA3AR was tested. Fig. 4 shows that the rank order of potencies for agonists was (Ki, nM): IB-MECA (7.67±0.98 > Cl-IB-MECA (11.1±0.80 > NECA (51.7± 19.7), and for antagonists was XAC (25.4±3.7 > MRS1191 (56.7±13.0 > MRS1523 (246±66 > CPX (358±52, which correlates well with the known structure-activity-relationship profile of the hA3AR established in previous reports [1].
Figure 3.
(A) Saturation binding of [125I]MRS5127 at the human A3AR, and (B) Scatchard transformation of the same data. [125I]MRS5127 (0.8 – 32 nM) was incubated with membranes (50 μg) prepared from HEK 293 cells stably transfected with the human A3AR cDNA for 2 h at room temperature. Total binding, specific binding, and nonspecific binding defined by including 100 μM NECA, are presented in A. The data shown are representative of three experiments conducted independently.
Figure 4.
Competition for binding of [125I]MRS5127 at the human A3AR by the agonists IB-MECA, Cl-IB-MECA, and NECA, and by the antagonists XAC, MRS 1191, MRS 1523, and CPX. Membranes (50 μg) were incubated with [125I]MRS5127 (0.2 nM) for 2 h at room temperature. Nonspecific binding was determined with 100 μM NECA. The data shown are representative of 3 independent experiments.
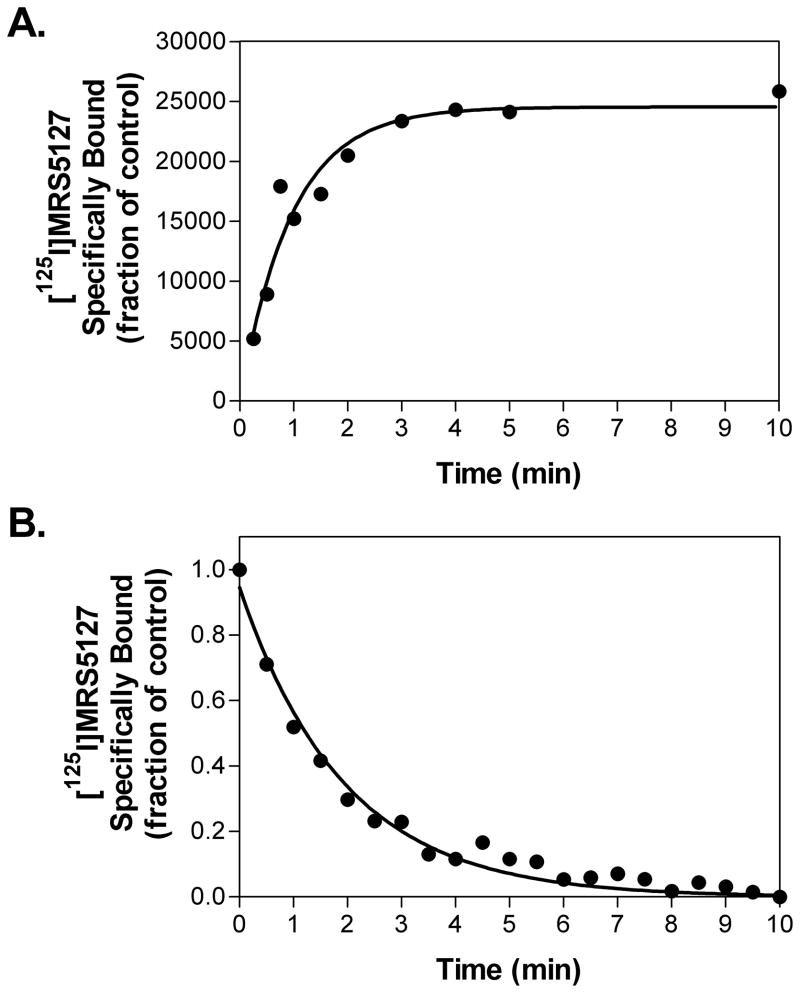
In association kinetic experiments (Fig. 5A), [125I]MRS5127 rapidly reached maximal binding in 3 min at room temperature when included in assays at 2–4 nM, with a t1/2 of 0.56 ± 0.08 min. After 120 min of incubation, the dissociation was initiated with the addition of 100 μM NECA at various time points as indicated in Fig. 5B. The association and dissociation rate constants were 0.244 ± 0.095 min−1 nM−1 and 0.514 ± 0.014 min−1, respectively. The Kd value calculated from the kinetic data was 3.50 ± 1.46 nM, which is in close agreement with the value obtained from equilibrium binding analysis.
Figure 5.
Association (A) and dissociation (B) kinetics of [125I]MRS5127 binding to membranes prepared from HEK 293 cells stably transfected with the human A3AR. [125I]MRS5127 (0.2 nM) was incubated with membranes (50 μg) at room temperature. Dissociation was initiated by the addition of 100μM NECA. The results shown are representative of 3–4 experiments conducted independently.
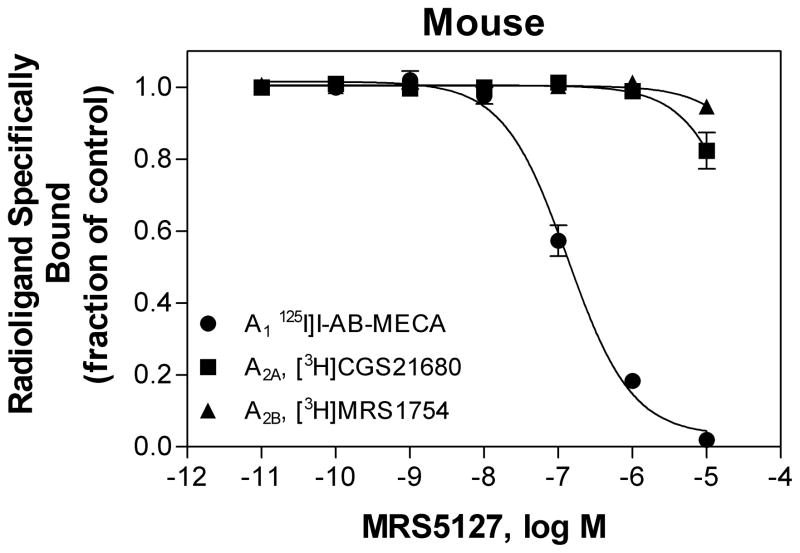
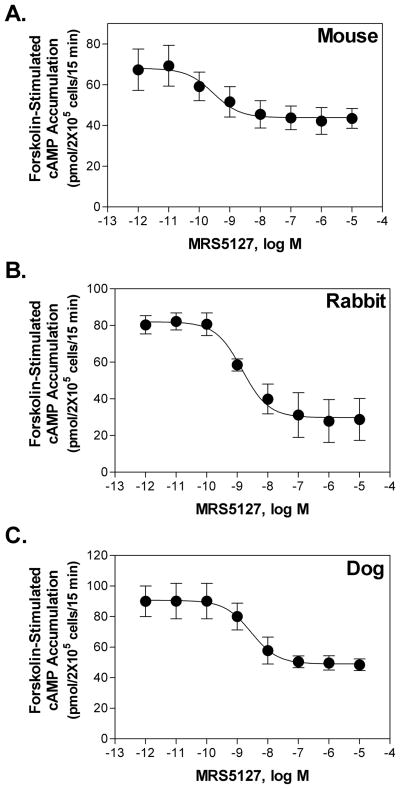
The binding of [125I]MRS5127 was further tested in saturation binding assays using membranes prepared from HEK 293 cells expressing recombinant mouse, rabbit, or dog A3ARs. Similar to the human A3AR, [125I]MRS5127 bound to the A3AR from all species with equal high affinity (Table 1). The selectivity of MRS5127 for recombinant mA3ARs was investigated by assessing its affinity for mA1, A2A, and A2BARs in competition binding assays using membranes from transfected HEK 293 cells and [125I]I-AB-MECA to label mA1ARs, [3H]CGS21680 to label mA2AARs, and [3H]MRS1754 to label A2BARs (Fig. 6). MRS5127 did not bind to mouse A2AAR or A2BARs. For the mA1AR, the affinity (Ki value) was calculated to be 140 ± 26 nM, being ~30-fold lower compared to the mA3AR. Thus, MRS5127 displays remarkable selectivity for the murine A3AR, although it is less than that reported previously for human receptors [25]. cAMP assays were conducted with transfected HEK 293 cells to confirm that MRS5127 functions as an agonist for mouse (EC50 = 1.34 ± 0.07 nM), rabbit (1.53 ± 0.59 nM), and dog A3ARs (2.79 ± 0.41 nM; Fig. 7).
Table 1.
Equilibrium binding data with [125I]MRS5127 for mouse, rabbit, and dog A3ARs
| Kd (nM) | Bmax (fmol/mg protein) | |
|---|---|---|
| Mouse | 4.90 ± 0.77 | 3,213 ± 326 |
| Rabbit | 2.53 ± 0.11 | 4,622 ± 199 |
| Dog | 3.35 ± 0.54 | 2,401 ± 618 |
[125I]MRS5127 was incubated with membranes (50 μg) prepared from HEK 293 cells transfected with mouse, rabbit, or dog A3AR cDNAs for 2 h at room temperature. Nonspecific binding was determined using 100 μM NECA. N = 3.
Figure 6.
Competition of MRS5127 for binding to mouse A1, A2A, and A2BARs. [125I]I-AB-MECA (A1; 0.3 nM), [3H]CGS21680 (A2A; 10 nM), or [3H]MRS1754 (2 nM) were incubated with membranes (50 μg) prepared from HEK 293 cells stably transfected with the respective mouse AR cDNAs for 2 h at room temperature. Non-specific binding was determined with 100 μM NECA. The data shown are representative of 3 experiments conducted independently.
Figure 7.
Functional agonism of MRS5127 for the mouse (A), rabbit (B), and dog (C) A3AR assessed in cAMP assays. HEK 293 cells stably expressing A3ARs were incubated with forskolin (10 μM) and increasing concentrations of MRS5127 for 15 min in the presence of Ro 20-1724 (20 μM). The amount of cAMP that accumulated over 15 min was measured using a protein binding assay, as described in METHODS. The data shown are the mean ± SEM of 3–4 independent experiments.
4. Discussion
MRS5127 is a member of a new series of adenine nucleoside ligands for the A3AR that contains a rigid (N)-methanocarbo(bicyclo[3.1.0]hexane) ring system substituted for the ribose moiety. It contains a 4′-truncated ribose-like moiety, which in various nucleoside analogue series has been shown to reduce relative efficacy at the A3AR. In previous studies, this compound was shown to display high affinity for human and rat A3ARs, with exceptional selectivity versus the other AR subtypes including the A1AR [25, 26]. The presence of an N6-halobenzyl group precludes the large species dependence of A3AR affinity observed for nucleoside derivatives that are N6-substituted with methyl and other small alkyl groups [17–20].
This study reports the synthesis and characterization of a radioiodinated form of MRS5127 - [125I]MRS5127 – for use as a probe for the A3AR in in vitro binding studies. The radioiodination of MRS5127 was accomplished through an iodostannylation approach of a 3-(trimethylstannyl)benzyl precursor 9. In binding studies using recombinant ARs, [125I]MRS5127 was found to bind with high affinity (Kd = 5.74 ± 0.97 nM) to the human A3AR as well as the A3AR from three other species including the mouse. Non-specific binding was low, especially when the opportunity for nonspecific binding was reduced by presoaking filters with polyethyleneimine. MRS5127 functioned species-independently as an agonist for the A3AR. The cLogP of MRS5127 was found to be 2.26, which is in the optimal range of 2–3 for bioavailability of small molecules. In contrast, the cLogP values of I-AB-MECA, PSB-11, and MRE 3008-F20 are −0.47, 1.40, and 3.70, respectively.
Similar to previous findings with human ARs, [125I]MRS5127 was found to be selective for the murine A3AR, with low affinity for the mA1AR and essentially no binding activity for the other AR subtypes. Thus, due to its high affinity, its uniformity across species, and its low degree of non-specific binding, [125I]MRS5127 represents a new chemical tool for characterizing A3ARs that has advantages over other radioligands that have been used previously [14, 16, 21, 22, 25, 32]. [125I]MRS5127 should be particularly useful for characterizing A3ARs in native tissues that express multiple different AR subtypes.
[125I]I-AB-MECA is the most commonly used radiolabel for the A3AR. This radioligand binds with high affinity to the A3AR from all species and also displays low non-specific binding [14]. The major disadvantage of [125I]I-AB-MECA is that its selectivity versus the A1AR is low. In fact, [125I]I-AB-MECA exhibits sufficiently high affinity (~5 nM) that it is also commonly used as a radioligand for the A1AR. When brain autoradiography was attempted using [125I]I-AB-MECA, it was found to mostly bind to the A1AR [34].
A similar stannylation method has been used previously to efficiently radioiodinate MRS1898, a derivative of MRS5127 that contains a 5′-methylaminocarbonyl substitution on the bicyclo ring constituent [32]. Similar to the present investigation, the labeling process was found to be simple, of high yield, and very efficient with no need for chemical protection. While [125I]MRS1898 proved to exhibit high affinity for the A3AR from both the human and rat, unlike [125I]MRS5127 its usefulness as a radioligand is limited by high non-specific binding [32].
This study established that a representative member of the series of truncated (N)- methanocarba nucleosides displays considerable agonist efficacy for the A3AR from three different species (mouse, rabbit, and dog) based on negative coupling to cAMP production. The efficacy of MRS5127 was similar to that of the 3′-amino- substituted A3AR agonist CP532-903 ([27]; data not shown). A similar functional assay (i.e., cAMP accumulation) with human receptors indicated that two members of the series, MRS5127 and its corresponding N6-3-bromobenzyl analogue MRS5147, were agonist ligands with roughly half efficacy in comparison to NECA [26]. However, an antagonist KB of 8.9 nM was determined with MRS5127 in [35S]GTPγS exchange assays with membranes from CHO cells expressing the hA3AR [25]. In these studies, the degree of activation of [35S]GTPγS binding by MRS5127 and similar 4′– truncated (N)-methanocarba nucleoside analogues was not substantial [25]. The finding of greater efficacy in the cAMP assay may be explained by amplification between receptor-mediated G protein activation and second messenger generation or by limited sensitivity of the [35S]GTPγS exchange assay.
In conclusion, this study reports the characterization of [125I]MRS5127 as a new agonist radioligand for the A3AR. The major advantage of [125I]MRS5127 is its low degree of non-specific binding and its improved selectivity versus the other AR subtypes. Furthermore, the generality of agonism across species in this series of truncated nucleosides is clearly demonstrated. Thus, this series of small molecules promises to be useful in both radioactive and unlabeled form as pharmacological probes for the A3AR.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Institute of Diabetes and Digestive and Kidney Diseases (KAJ) and by NIH R01 HL077707 (JAA). We thank Dr. Artem Melman (Clarkson University) for preparation of synthetic intermediates, and Dr. Zhan-Guo Gao and Dr. Athena Keene-Klutz for helpful discussion.
ABBREVIATIONS
- AR
adenosine receptor
- CHO
Chinese hamster ovary
- DMEM
Dulbecco’s modified Eagle’s medium
- IB-MECA
N6-(3-iodobenzyl)-5′-N-methylcarboxamidoadenosine
- I-AB-MECA
N6-(4-amino-3-iodobenzyl)-5′-N-methylcarboxamidoadenosine
- MRE 3008F20
5-N-(4-methoxyphenylcarbamoyl)amino-8-propyl-2-(2-furyl)pyrazolo [4,3-e]-1,2,4- triazolo[1,5-c]pyrimidine
- MRS1191
1,4-dihydro-2-methyl-6-phenyl-4-(phenylethynyl)-3,5-pyridinedicarboxylic acid, 3-ethyl- 5-(phenylmethyl) ester
- MRS1220
N-[9-chloro-2-(2-furanyl)[1,2,4]triazolo[1,5-c]quinazolin-5-yl]benzeneacetamide
- MRS1523
5-propyl-2-ethyl-4-propyl-3-(ethylsulfanylcarbonyl)-6-phenylpyridine-5-carboxylate
- MRS5127
(1′R,2′R,3′S,4′R,5′S)-4′-[2-chloro-6-(3-iodobenzylamino)-purine]-2′,3′-O-dihydroxybicyclo-[3.1.0]hexane
- MRS1754
8-[4-[[(4-cyano)phenylcarbamoylmethyl]oxy]phenyl]-1,3-di-(n-propyl)xanthine
- NECA
5′-N-ethylcarboxamidoadenosine
- PSB-11
8-ethyl-4-methyl-2-phenyl-(8R)-4,5,7,8-tetrahydro-1H-imidazo[2,1-i]-purin-5-one
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–64. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muller CE. Medicinal chemistry of adenosine A3 receptor ligands. Curr Top Med Chem. 2003;3:445–62. doi: 10.2174/1568026033392174. [DOI] [PubMed] [Google Scholar]
- 3.Bar-Yehuda S, Stemmer SM, Madi L, Castel D, Ochaion A, Cohen S, et al. The A3 adenosine receptor agonist CF102 induces apoptosis of hepatocellular carcinoma via de-regulation of the Wnt and NF-κB signal transduction pathways. Int J Oncol. 2008;33:287–95. [PubMed] [Google Scholar]
- 4.Silverman MH, Strand V, Markovits D, Nahir M, Reitblat T, Molad Y, et al. Clinical evidence for utilization of the A3 adenosine receptor as a target to treat rheumatoid arthritis: data from a phase II clinical trial. J Rheumatol. 2008;35:41–8. [PubMed] [Google Scholar]
- 5.Chen GJ, Harvey BK, Shen H, Chou J, Victor A, Wang Y. Activation of adenosine A3 receptors reduces ischemic brain injury in rodents. J Neurosci Res. 2006;84:1848–55. doi: 10.1002/jnr.21071. [DOI] [PubMed] [Google Scholar]
- 6.Von Lubitz DK, Lin RC, Popik P, Carter MF, Jacobson KA. Adenosine A3 receptor stimulation and cerebral ischemia. Eur J Pharmacol. 1994;263:59–67. doi: 10.1016/0014-2999(94)90523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzman J, Yu JG, Suntres Z, Bozarov A, Cooke H, Javed N, et al. ADOA3R as a therapeutic target in experimental colitis: proof by validated high-density oligonucleotide microarray analysis. Inflamm Bowel Dis. 2006;12:766–89. doi: 10.1097/00054725-200608000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Young HW, Sun CX, Evans CM, Dickey BF, Blackburn MR. A3 adenosine receptor signaling contributes to airway mucin secretion after allergen challenge. Am J Respir Cell Mol Biol. 2006;35:549–58. doi: 10.1165/rcmb.2006-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gessi S, Merighi S, Varani K, Cattabriga E, Benini A, Mirandola P, et al. Adenosine receptors in colon carcinoma tissues and colon tumoral cell lines: focus on the A3 adenosine subtype. J Cell Physiol. 2007;211:826–36. doi: 10.1002/jcp.20994. [DOI] [PubMed] [Google Scholar]
- 10.Madi L, Cohen S, Ochayin A, Bar-Yehuda S, Barer F, Fishman P. Overexpression of A3 adenosine receptor in peripheral blood mononuclear cells in rheumatoid arthritis: involvement of nuclear factor-κB in mediating receptor level. J Rheumatol. 2007;34:20–6. [PubMed] [Google Scholar]
- 11.Madi L, Ochaion A, Rath-Wolfson L, Bar-Yehuda S, Erlanger A, Ohana G, et al. The A3 adenosine receptor is highly expressed in tumor versus normal cells: potential target for tumor growth inhibition. Clin Cancer Res. 2004;10:4472–9. doi: 10.1158/1078-0432.CCR-03-0651. [DOI] [PubMed] [Google Scholar]
- 12.Ochaion A, Bar-Yehuda S, Cohen S, Amital H, Jacobson KA, Joshi BV, et al. The A3 adenosine receptor agonist CF502 inhibits the PI3K, PKB/Akt and NF-κB signaling pathway in synoviocytes from rheumatoid arthritis patients and in adjuvant-induced arthritis rats. Biochemical pharmacology. 2008;76:482–94. doi: 10.1016/j.bcp.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melman A, Gao ZG, Kumar D, Wan TC, Gizewski E, Auchampach JA, et al. Design of (N)-methanocarba adenosine 5′-uronamides as species-independent A3 receptor-selective agonists. Bioorganic & medicinal chemistry letters. 2008;18:2813–9. doi: 10.1016/j.bmcl.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olah ME, Gallo-Rodriguez C, Jacobson KA, Stiles GL. 125I-4-aminobenzyl-5′-N-methylcarboxamidoadenosine, a high affinity radioligand for the rat A3 adenosine receptor. Mol Pharmacol. 1994;45:978–82. [PMC free article] [PubMed] [Google Scholar]
- 15.Kohno Y, Ji X, Mawhorter SD, Koshiba M, Jacobson KA. Activation of A3 adenosine receptors on human eosinophils elevates intracellular calcium. Blood. 1996;88:3569–74. [PMC free article] [PubMed] [Google Scholar]
- 16.Klotz KN, Falgner N, Kachler S, Lambertucci C, Vittori S, Volpini R, et al. [3H]HEMADO--a novel tritiated agonist selective for the human adenosine A3 receptor. Eur J Pharmacol. 2007;556:14–8. doi: 10.1016/j.ejphar.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 17.Gao ZG, Blaustein JB, Gross AS, Melman N, Jacobson KA. N6-Substituted adenosine derivatives: selectivity, efficacy, and species differences at A3 adenosine receptors. Biochemical pharmacology. 2003;65:1675–84. doi: 10.1016/s0006-2952(03)00153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohno M, Gao ZG, Van Rompaey P, Tchilibon S, Kim SK, Harris BA, et al. Modulation of adenosine receptor affinity and intrinsic efficacy in adenine nucleosides substituted at the 2-position. Bioorganic & medicinal chemistry. 2004;12:2995–3007. doi: 10.1016/j.bmc.2004.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tchilibon S, Kim SK, Gao ZG, Harris BA, Blaustein JB, Gross AS, et al. Exploring distal regions of the A3 adenosine receptor binding site: sterically constrained N6-(2-phenylethyl)adenosine derivatives as potent ligands. Bioorganic & medicinal chemistry. 2004;12:2021–34. doi: 10.1016/j.bmc.2004.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu R, Frazier CR, Linden J, Macdonald TL. N6-ethyl-2-alkynyl NECAs, selective human A3 adenosine receptor agonists. Bioorganic & medicinal chemistry letters. 2006;16:2416–8. doi: 10.1016/j.bmcl.2006.01.110. [DOI] [PubMed] [Google Scholar]
- 21.Muller CE, Diekmann M, Thorand M, Ozola V. [3H]8-Ethyl-4-methyl-2-phenyl-(8R)-4,5,7,8-tetrahydro-1H-imidazo[2,1-i]-purin-5-one ([3H]PSB-11), a novel high-affinity antagonist radioligand for human A3 adenosine receptors. Bioorganic & medicinal chemistry letters. 2002;12:501–3. doi: 10.1016/s0960-894x(01)00785-5. [DOI] [PubMed] [Google Scholar]
- 22.Varani K, Merighi S, Gessi S, Klotz KN, Leung E, Baraldi PG, et al. [3H]MRE 3008F20: a novel antagonist radioligand for the pharmacological and biochemical characterization of human A3 adenosine receptors. Mol Pharmacol. 2000;57:968–75. [PubMed] [Google Scholar]
- 23.Yang H, Avila MY, Peterson-Yantorno K, Coca-Prados M, Stone RA, Jacobson KA, et al. The cross-species A3 adenosine-receptor antagonist MRS 1292 inhibits adenosine-triggered human nonpigmented ciliary epithelial cell fluid release and reduces mouse intraocular pressure. Curr Eye Res. 2005;30:747–54. doi: 10.1080/02713680590953147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wadsak W, Mien LK, Shanab K, Ettlinger DE, Haeusler D, Sindelar K, et al. Preparation and first evaluation of [18F]FE@SUPPY: a new PET tracer for the adenosine A3 receptor. Nucl Med Biol. 2008;35:61–6. doi: 10.1016/j.nucmedbio.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Melman A, Wang B, Joshi BV, Gao ZG, Castro S, Heller CL, et al. Selective A3 adenosine receptor antagonists derived from nucleosides containing a bicyclo[3.1.0]hexane ring system. Bioorganic & medicinal chemistry. 2008;16:8546–56. doi: 10.1016/j.bmc.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tosh DK, Chinn M, Ivanov AA, Klutz AM, Gao ZG, Jacobson KA. Functionalized congeners of A3 adenosine receptor-selective nucleosides containing a bicyclo[3.1.0]hexane ring system. J Med Chem. doi: 10.1021/jm900426g. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiesewetter DO, Lang L, Ma Y, Bhattacharjee AK, Gao ZG, Joshi BV, et al. Synthesis and characterization of [76Br]-labeled high-affinity A3 adenosine receptor ligands for positron emission tomography. Nucl Med Biol. 2009;36:3–10. doi: 10.1016/j.nucmedbio.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Auchampach JA, Jin X, Wan TC, Caughey GH, Linden J. Canine mast cell adenosine receptors: cloning and expression of the A3 receptor and evidence that degranulation is mediated by the A2B receptor. Mol Pharmacol. 1997;52:846–60. doi: 10.1124/mol.52.5.846. [DOI] [PubMed] [Google Scholar]
- 29.Takano H, Bolli R, Black RG, Jr, Kodani E, Tang XL, Yang Z, et al. A1 or A3 adenosine receptors induce late preconditioning against infarction in conscious rabbits by different mechanisms. Circ Res. 2001;88:520–8. doi: 10.1161/01.res.88.5.520. [DOI] [PubMed] [Google Scholar]
- 30.Wan TC, Ge ZD, Tampo A, Mio Y, Bienengraeber MW, Tracey WR, et al. The A3 adenosine receptor agonist CP-532,903 [N6-(2,5-dichlorobenzyl)-3′-aminoadenosine-5′-N-methylcarboxamide] protects against myocardial ischemia/reperfusion injury via the sarcolemmal ATP-sensitive potassium channel. J Pharmacol Exp Ther. 2008;324:234–43. doi: 10.1124/jpet.107.127480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochemical pharmacology. 1973;22:3099–108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 32.Gao ZG, Teng B, Wu H, Joshi BV, Griffiths GL, Jacobson KA. Synthesis and pharmacological characterization of [125I]MRS1898, a high-affinity, selective radioligand for the rat A3 adenosine receptor. Purinergic Signal. 2009;5:31–7. doi: 10.1007/s11302-008-9107-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaidyanathan G, Zalutsky MR. Preparation of N-succinimidyl 3-[*I]iodobenzoate: an agent for the indirect radioiodination of proteins. Nat Protoc. 2006;1:707–13. doi: 10.1038/nprot.2006.99. [DOI] [PubMed] [Google Scholar]
- 34.Rivkees SA, Thevananther S, Hao H. Are A3 adenosine receptors expressed in the brain? Neuroreport. 2000;11:1025–30. doi: 10.1097/00001756-200004070-00026. [DOI] [PubMed] [Google Scholar]