Abstract
This study was conducted to examine the relationship between whole blood serotonin level and behavioral symptoms in 78 subjects with autism. No significant associations were found between serotonin level and the primary behavioral outcome measures. However, a significant inverse relationship between serotonin level and self-injury was demonstrated.
Keywords: autism, serotonin, repetitive behavior, self-injury, aggression
2. INTRODUCTION
Autism is a severe neurodevelopmental disorder. Its etiology most probably resides primarily in complex genetics and genetic heterogeneity likely accounts for the historic difficulty in reliably identifying susceptibility genes. Autistic symptoms are also well recognized in many other genetic disorders with disparate etiologies, and it is possible that these symptoms represent a phenotype with a common neurochemical substrate (Chugani, 2002). Genetic analysis would greatly benefit from the identification of specific quantitative characteristics, such as biochemical measures. Quantitative measures such as whole blood serotonin may help identify a subgroup of patients with a homogeneous phenotype and provide a complementary strategy for genetic studies beyond relying on qualitative criteria such as those elicited from the widely used Autism Diagnostic Interview-Revised (ADI-R). Biochemical investigation in autism may also potentially identify subgroups that are pathophysiologically related and the use of such measures could hold promise in predicting autistic severity (Kuperman et al., 1985) and sibling recurrence (Piven et al., 1991).
Beginning with a study by Schain and Freedman in 1961, most investigators have found that serotonin levels measured in whole blood are significantly higher in autistic subjects as compared to normal controls, and approximately one-third of autistic individuals are considered to have hyperserotonemia. There is also a significant body of evidence to support the notion that serotonin plays an important role in brain development and affects a range of social behavior, affect regulation, aggression, and anxiety. Recent neuroimaging studies are noteworthy in their ability to demonstrate developmental changes in brain serotonin synthesis capacity and using positron emission tomography (PET), Chugani et al. (1999) found significant differences between autistic and nonautistic children in serotonin synthesis capacity. These results must be interpreted with caution because of the methodology employed, but may suggest that there is a disruption in the mechanisms that regulate serotonin synthesis during development in autism.
The published studies to date comparing levels of blood serotonin with behavioral symptoms of autism have failed to demonstrate any consistent pattern (Kuperman et al, 1987; Mulder et al, 2004; Hranilovic et al, 2007). One study (Kuperman et al., 1987) evaluated 25 boys with infantile autism and although there was no significant correlation between platelet-rich serotonin concentration and Autism Behavior Checklist (ABC) scale scores, four individual items on the ABC that reflect behavioral symptomatology were significantly associated with serotonin concentration. The relationship between serotonin levels and the behavioral symptoms in autism is of particular interest because this domain focuses on symptoms (e.g., repetitive and sensory circumscribed interests, compulsions, and motor stereotypies) which may respond to serotonergic medications. If behavioral symptoms are positively correlated with peripheral serotonin, for example, this may suggest increased uptake of serotonin and support the use of serotonin reuptake inhibitors. There have been relatively few controlled trials with serotonergic medications to date and demonstrating a relationship between the behavior domain and serotonin level may encourage additional trials in the future
3. METHODS AND MATERIALS
Subjects are part of an ongoing recruitment by the Mount Sinai Family Studies Research Center for multiplex families at the Seaver Autism Center for Research and Treatment/New York Autism Center for Excellence in collaboration with the Autism Genetic Research Exchange (AGRE). Affected families are required to have at least one case of autism and another case with autism or a sub threshold autism-related disorder. Individuals known to have medical conditions associated with autism were excluded (e.g., tuberous sclerosis, fragile X syndrome, phenylketonuria). Cases with suspected autism were assessed using the ADI-R. The ADI-R is an investigator-based, semi-structured instrument used to differentiate autistic disorder from non-autistic mental disability in individuals greater than 18 months old. An algorithm that incorporates ICD-10 and DSM-IV criteria and examines the three main symptom domains of autism (communication, reciprocal social interaction, and repetitive, restricted behaviors) is used for diagnosis (Lord et al., 1994).
Blood to assay serotonin was collected from a total of 154 individuals affected with autism or an autism-related disorder. Affected cases 15 years-old and younger were then screened to select only those with an ADI-R diagnosis of autism who were not taking serotonergic medication at the time of the assessment. A total of 78 subjects were included in the current analysis. Several measures were employed to assess behavior and included the ADI-R algorithm behavior domain, The Yale-Brown Obsessive-Compulsive Scale (YBOCS) compulsive subscales, and the Vineland Adaptive Behavior Scales (VABS) maladaptive behavior domain. Whole blood serotonin levels were measured using high-pressure liquid chromatography (HPLC) with fluorometric detection using methods described elsewhere (Cook et al., 1990). The relationship between whole blood serotonin and the behavioral symptoms of autism was analyzed using bivariate Spearman rank-order correlations to account for the non-normal distribution of serotonin level.
4. RESULTS
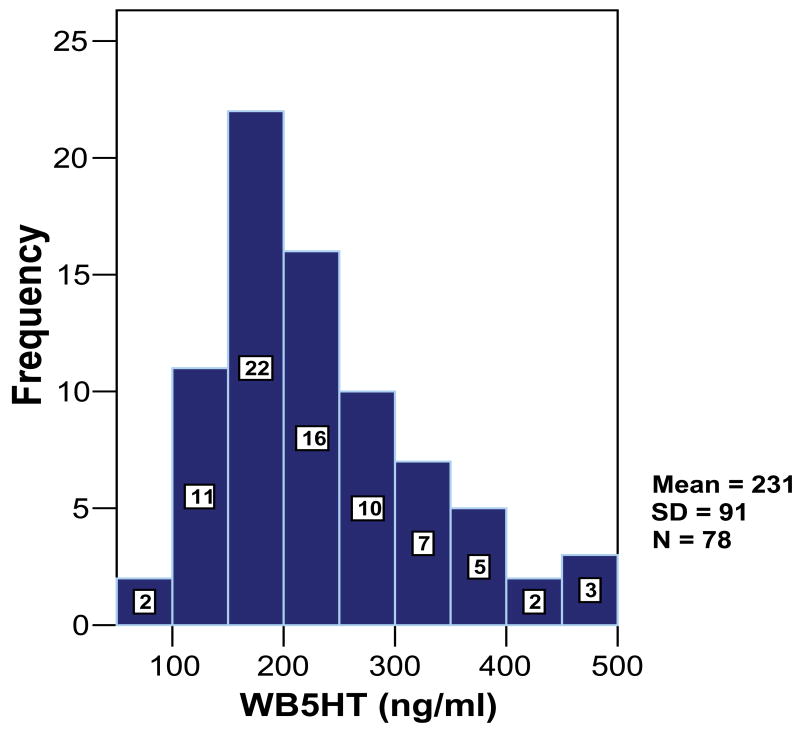
The 78 subjects with autism came from 51 families. The range of ages was from 2 to 15 years old (mean = 6.77, SD = 2.93). Sixty-five cases were male (83.3%) and 13 cases were female (16.7%). The ethnic composition of the population was 78.2% White, 7.7% Hispanic, and 2.6% Black. The remaining cases were classified as either “other” (2.6%), “unknown” (5.1%), or ethnicity data was missing (3.8%). Whole blood serotonin levels ranged from 78 to 471 ng/ml (see Figure 1) and approximately one-third of subjects had levels greater than 252 ng/ml (skewness=0.840, SE=0.272).
Figure 1.
The Distribution of Whole Blood Serotonin Level Among Subjects
Spearman correlations were computed to examine associations between whole blood serotonin and the behavioral symptoms of autism as measured by the ADI-R, YBOCS, and VABS (see Table 1). Using a Bonferroni correction, the significance level across all tests is 0.003. No significant effects were found for the potential confounders of age (r = 0.025, P = 0.825), gender (r = 0.036, P = 0.755), ethnicity (r = 0.024, P = 0.839), or family membership (r = −0.028, P = 0.808) on serotonin level. The absence of these confounders, which are typically significant in related studies, may be a result of relative demographic homogeneity of our sample.
Table 1.
Spearman Correlations Between Whole Blood Serotonin Level and Behavior Symptoms in Autism
| Variable | R | pa | df |
|---|---|---|---|
| ADI Behavior Total | −.073 | .524 | 76 |
| D1-circumscribed interests | .050 | .666 | 76 |
| D2-compulsions | −.065 | .569 | 76 |
| D3-motor stereotypes | −.179 | .116 | 76 |
| D4-repetitive/sensory interests | .056 | .629 | 76 |
| YBOCS Sum | −.118 | .413 | 50 |
| Time occupied by compulsions | .035 | .809 | 50 |
| interference due to compulsions | −.106 | .464 | 50 |
| distress due to compulsions | .036 | .802 | 50 |
| resistance against compulsions | .272 | .056 | 50 |
| degree of control over compulsions | .163 | .257 | 50 |
| VABS Maladaptive Behavior Domain | −.252 | .058 | 55 |
| ADI Aggression Composite | −.260 | .021 | 76 |
| Item 90: self-injury | −.342 | .002 | 76 |
| Item 91b: aggression to caregivers | −.227 | .045 | 76 |
| Item 91c: aggression to non-caregivers | −.073 | .523 | 76 |
Using a Bonferroni correction, the significance level across all tests is 0.003
No significant associations were found between serotonin level and the ADI-R algorithm behavior domain or any of the behavior subdomains (D1, circumscribed interests; D2, compulsions; D3, motor stereotypes; D4, repetitive/sensory interests). No significant associations were found between serotonin level and the YBOCS sum or any of the compulsion subscales of the YBOCS (time occupied by compulsions; interference due to compulsions; distress due to compulsions; resistance against compulsions; degree of control over compulsions). No significant associations were found between serotonin level and the VABS maladaptive behavior domain part 1 and 2.
A significant finding did emerge between serotonin level and one of the individual items on the ADI designed to elicit symptoms of aggression: whole blood serotonin level was inversely correlated with self injury. When looking at other ADI items that assess symptoms of aggression to others, findings were either nominally significant or not significant. Using a composite of aggressive items on the ADI encompassing aggression to self and others, the result was nominally significant, but only before applying the Bonferroni correction. Yet, this finding is likely driven by the relationship between serotonin level and self-injury.
5. DISCUSSION
In our sample, as it has currently been defined, no significant associations exist between whole blood serotonin levels and the ADI-R algorithm behavior domain, the YBOCS compulsive subscales, or the VABS maladaptive behavior domain. These results are consistent with previous studies to examine the relationship between platelet serotonin level and behavioral symptoms of autism where no significant correlations were detected on the primary behavioral outcome measures (Kuperman et al, 1987; Mulder et al, 2004; Hranilovic et al, 2007). However, the absence of significant findings in this study may also be a function of the relative homogeneity of the sample and a skewed distribution of serotonin levels. It remains possible that comparison to an aged-matched, normal control group would introduce sufficient power to detect significant associations between serotonin level and behavioral symptomatology. In addition, platelet counts were not obtained and calculation of serotonin per platelet was not possible to more fully characterize the distribution of the trait and examine possible behavioral correlates
Nevertheless, it is important to note that significant relationships are present between whole blood serotonin and some behavioral symptoms associated with autism. Our data indicate an inverse correlation between peripheral serotonin level and aggression to self. In addition, this finding appears to be relatively specific for self-injury because other items reflecting aggression were not significantly associated with serotonin level after Bonferroni correction. These results are also consistent with findings from Bodfish and colleagues (2000) that demonstrate self injury to be distinct from other repetitive behaviors. Similarly, although not statistically significant, many trends between serotonin level and behavior symptoms that are present in our data appear to follow an inverse relationship in contrast to what we hypothesized. Despite doing two-tailed analyses, our hypothesis may have been too directional and much literature to date supports the presence of an inverse relationship between aggression and serotonin or its metabolite (5-HIAA) in aggressive psychiatric populations, including personality disorders (Goveas et al., 2004) and suicide attempters (Tyano et al., 2005), as well as violent offenders (Linnoila et al., 1983). However, this is the first study that we are aware of to demonstrate a related finding in autism.
Behavioral symptoms have considerable impact on individuals with autism and their families and significantly affect quality of life and safety. Identifying symptom patterns, such as self-injury, that are associated with serotonin level may predict specific subgroups of patients with a more consistent response to medication, an important implication given previous reports of the heterogeneity of hyperserotonemia in autism (Cook et al., 1993). It will also be interesting in future studies to examine possible associations between self-injury in autistic populations and functional genetic polymorphisms that impact serotonergic regulation.
Table 2.
Descriptive Statistics
| Variable | N | Mean | SD | Skewness | |
|---|---|---|---|---|---|
| Statistic | Std. Error | ||||
| Serotonin Level | 78 | 230.784 | 90.501 | .840 | 0.272 |
| ADI Behavior Total | |||||
| D1-circumscribed interests | 78 | 1.44 | 1.325 | 0.486 | 0.272 |
| D2-compulsions | 78 | 1.01 | 1.075 | 0.682 | 0.272 |
| D3-motor stereotypes | 78 | 1.67 | 0.617 | −1.688 | 0.272 |
| D4-repetitive/sensory interests | 78 | 1.74 | 0.568 | −2.146 | 0.272 |
| YBOCS Sum | |||||
| time occupied by compulsions | 50 | 1.74 | 1.226 | 0.592 | 0.337 |
| interference due to compulsions | 50 | 1.54 | 1.313 | 0.191 | 0.337 |
| distress due to compulsions | 50 | 2.22 | 1.404 | −0.179 | 0.337 |
| resistance against compulsions | 50 | 2.80 | 1.457 | −0.916 | 0.337 |
| degree of control over compulsions | 50 | 2.46 | 1.358 | −0.345 | 0.337 |
| ADI Aggression Composite | 78 | 3.03 | 2.579 | 0.811 | 0.272 |
| Item 90: self-injury | 78 | 0.51 | 0.698 | 1.013 | 0.272 |
| Item 91b: aggression to caregivers | 78 | 1.50 | 1.985 | 2.780 | 0.272 |
| Item 91c: aggression to non-caregivers | 78 | 1.23 | 2.221 | 2.788 | 0.272 |
Acknowledgments
This work was supported in part by the AACAP/Eli Lily Young Investigator Award (Dr. Kolevzon), the Seaver Autism Center (Drs. Hollander and Silverman), and the National Institute of Mental Health through a STAART grant (U54 MH 066673). Dr. Hollander also receives support from the Orphan Products Division of the Food and Drug Administration grant #FD-R-001520-01-03, and an investigator initiated research grant from Lilly Research Labs. Dr. Cook receives support from the Jean Young and Walden W. Shaw Foundation and The Daniel X. & Mary Freedman Foundation for Academic Psychiatry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: comparisons to mental retardation. Journal of Autism and Developmental Disorders. 2000;30(3):237–43. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- 2.Chugani DC. Role of altered brain serotonin mechanisms in autism. Molecular Psychiatry. 2002;7(Suppl 2):S16–7. doi: 10.1038/sj.mp.4001167. [DOI] [PubMed] [Google Scholar]
- 3.Chugani DC, Muzik O, Behen M, Rothermel R, Janisse JJ, Lee J, Chugani HT. Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Annals of Neurology. 1999;45:287–295. doi: 10.1002/1531-8249(199903)45:3<287::aid-ana3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Cook EH, Arora RC, Anderson GM, Berry-Kravis EM, Yau S, Yeoh HC, Sklena PJ, Charak DA, Leventhal BL. Platelet serotonin studies in familial hyperserotonemia of autism. Life Sciences. 1993;52:2005–2015. doi: 10.1016/0024-3205(93)90685-v. [DOI] [PubMed] [Google Scholar]
- 5.Cook EH, Leventhal BL, Heller W, Metz J, Wainwright M, Freedman DX. Autistic children and their first-degree relatives: relationships between serotonin and norepinephrine levels and intelligence. Journal of Neuropsychiatry Clinical Neuroscience. 1990;2:268–274. doi: 10.1176/jnp.2.3.268. [DOI] [PubMed] [Google Scholar]
- 6.Goveas JS, Csernansky JG, Coccaro EF. Platelet serotonin content correlates inversely with life history of aggression in personality-disordered subjects. Psychiatry Research. 2004;126:23–32. doi: 10.1016/j.psychres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Hranilovic D, Bujas-Petkovic Z, Vragovic R, Vuk T, Hock K, Jernej B. Hyperserotonemia in adults with autistic disorder. Journal of Autism and Developmental Disorders. 2007;37(10):1934–40. doi: 10.1007/s10803-006-0324-6. [DOI] [PubMed] [Google Scholar]
- 8.Kuperman S, Beeghly JH, Burns TL, Tsai LY. Serotonin relationships of autistic probands and their first degree relatives. Journal of the American Academy of Child Psychiatry. 1985;24:186–190. doi: 10.1016/s0002-7138(09)60446-5. [DOI] [PubMed] [Google Scholar]
- 9.Kuperman S, Beeghly JH, Burns TL, Tsai LY. Association of serotonin concentration to behavior and IQ in autistic children. Journal of Autism and Developmental Disorders. 1987;17(1):133–140. doi: 10.1007/BF01487265. [DOI] [PubMed] [Google Scholar]
- 10.Linnoila M, Virkkunen M, Scheinin M, Nutila Am Rimon R, Goodwin FK. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from non-impulsive violent behavior. Life Sciences. 1983;33:2609–2614. doi: 10.1016/0024-3205(83)90344-2. [DOI] [PubMed] [Google Scholar]
- 11.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview – Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 12.Mulder EJ, Anderson GM, Kema IP, de Bildt A, van Lang ND, den Boer JA, Minderaa RB. Platelet serotonin levels in pervasive developmental disorders and mental retardation: diagnostic group differences, within-group distribution, and behavioral correlates. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(4):491–9. doi: 10.1097/00004583-200404000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Piven J, Tsai G, Nehme E, Coyle JT. Platelet serotonin, a possible marker for familial autism. Journal of Autism and Developmental Disorders. 1991;21(1):51–59. doi: 10.1007/BF02206997. [DOI] [PubMed] [Google Scholar]
- 14.Schain RJ, Freedman DX. Studies on 5-hydroxyindole metabolism in autistic and other mentally retarded children. Journal of Pediatrics. 1961;58:315–20. doi: 10.1016/s0022-3476(61)80261-8. [DOI] [PubMed] [Google Scholar]
- 15.Tyano S, Zalsman G, Ofek H, Blum I, Apter A, Wolovik L, Sher L, Sommerfeld E, Harell D, Weizman A. Plasma serotonin levels and suicidal behavior in adolescents. European Neuropsychopharmacology. 2006;16(1):49–57. doi: 10.1016/j.euroneuro.2005.05.005. [DOI] [PubMed] [Google Scholar]