Abstract
Adenosine 5′-triphosphate is frequently released by cells and acts as an agonist for G protein-coupled P2Y receptors and ligand-gated P2X cationic channels in numerous tissues. The breakdown of ATP by ectonucleotidases not only terminates its extracellular messenger functions, but also provides a pathway for the generation of two additional agonists: adenosine 5′-diphosphate, acting via some P2Y receptors, and adenosine, a native agonist for G protein-coupled adenosine receptors. In the pituitary gland, adenosine 5′-triphosphate is released from the endings of magnocellular hypothalamic neurons and by anterior pituitary cells through pathway(s) that are still not well characterized. This gland also expresses several members of each family of purinergic receptors. P2X and adenosine receptors are co-expressed in the somata and nerve terminals of vasopressin-releasing neurons as well as in some secretory pituitary cells. P2X receptors stimulate electrical activity and modulate InsP3-dependent calcium release from intracellular stores, whereas adenosine receptors terminate electrical activity. Calcium-mobilizing P2Y receptors are predominantly expressed in non-secretory cells of the anterior and posterior pituitary.
1. Introduction
Purines adenosine-5′-triphosphate (ATP), adenosine-5′-diphosphate (ADP) and adenosine, and pyrimidines uridine-5′-triphosphate (UTP) and uridine-5′-diphosphate (UDP) are not only energy sources and important intracellular molecules that control various cellular processes, but they are also extracellular messengers. The concept of purinergic signaling was first proposed by G. Burnstock [1]. Since then, the extracellular roles of purines and pyrimidines have been established in numerous tissues, including the pituitary gland. The effects of these messengers are mediated via two main groups of purinergic receptors: G protein-coupled adenosine receptors (ARs), also known as P1 receptors, and ATP, ADP, UTP and/or UDP-activated P2 receptors (P2Rs). ARs are a single family of receptors, whereas P2Rs belong to one of two families: G protein-coupled receptors (P2YRs) and ligand-gated ion channels (P2XRs) [2].
The release of endogenous nucleotides represents a critical component for the activation of these receptors. The roles of ATP as a neurotransmitter or co-transmitter and adenosine as an important neuromodulator are well established in the peripheral and central nervous systems. Cardiac tissue releases adenosine, whereas ATP is secreted by skeletal muscle, adrenal chromaffin cells, pancreatic beta cells, mast cells, blood cells, fibroblasts, and endothelial cells in response to mechanical stress, hypoxia, acidosis, osmotic shock or inflammation [3,4]. Several reports have also suggested that pyrimidines are secreted by nonexcitable cells, including endothelial, epithelial, and astrocytoma cells [5]. The duration and distance of ATP's actions are limited by several ectonucleotidases, which ensure that circulating levels of ATP are below that required for the global activation of purinergic receptors. The currently identified ectonucleotidases include members of the ectonucleotide triphosphate diphosphohydrolase family (eNTPDase) and several other subfamilies of enzymes. These enzymes not only hydrolyze extracellular ATP and/or ADP to adenosine 5′-monophosphate (AMP) and adenosine, but also metabolize other nucleotide tri- and diphosphates, including UTP and UDP [5,6].
Released ATP is a native agonist for P2XRs. Molecular, physiological, and pharmacological studies have revealed the existence of seven subunits, denoted as P2X1 through P2X7, as well as several spliced forms of these subunits. Each subunit is proposed to have two transmembrane helices connected by a large extracellular loop, with both N- and C-termini located in the cytoplasm. From the N-termini through the second transmembrane domain, the cloned subunits exhibit a relatively high level of amino acid sequence homology. In contrast, the C-termini vary in length and show no apparent sequence homology, except for the region nearest to transmembrane domain-2 [7]. The functional channels are composed of at least three subunits, which can form ion permeable pores through homo- and heteropolymerization [8,9]. P2XR subtypes differ with respect to their ligand-selectivity profiles, antagonist sensitivity, and cation selectivity. Their activation leads to an increase in the intracellular calcium concentration ([Ca2+]i), with Ca2+ influx occurring through the pores of these channels and voltage-gated Ca2+ channels, following the initial depolarization of cells by P2XR-generated Na+ currents [10,11]. These receptor channels are expressed in numerous tissues, including the central nervous system, sensory, motor and sympathetic neurons, chromaffin cells, smooth muscle, testis, colon, gut, bladder, thymus, pancreas, skin, and immune cells [12].
ATP, UTP, ADP, and UDP are native agonists for the P2YRs. To date, eight mammalian P2YRs have been identified and are denoted as P2Y1R, P2Y2R, P2Y4R, P2Y6R, P2Y11R, P2Y12R, P2Y13R, and P2Y14R [13]. The missing numbers represent either non-mammalian versions of these receptors or proteins that have some sequence homology to P2YRs but do not respond to nucleotide application. In contrast to P2XRs and ARs, the genes of all P2YRs, other than P2Y11R, do not contain introns in their coding sequence [12]. Four receptors, P2Y1R, P2Y2R, P2Y4R, and P2Y6R, signal through the Gq/11-dependent pathway, leading to the activation of phospholipase C and the generation of InsP3 and diacylglycerol. In excitable cells, InsP3-induced Ca2+ mobilization is frequently accompanied with an initial hyperpolarization of the cell membrane and an inhibition of voltage-gated Ca2+ influx mediated by Ca2+-controlled K+ channels. This is subsequently followed by a sustained depolarization of the plasma membrane and the facilitation of voltage-gated Ca2+ influx through poorly characterized pathways. Activation of MAP kinase and phospholipase D signaling pathways, both secondary to the activation of protein kinase C, has also been reported for P2YRs, as well as their coupling to Gi/o (P2Y13R) and Gs (P2Y11R) signaling pathways [14]. These receptors are expressed in the brain, lymph nodes, bone marrow, placenta, stomach, intestine, adipose tissue, epithelial, endothelial, and immune cells, osteoclasts and osteoblasts, plateles and other cell types [12].
Adenosine is a common agonist for four subtypes that comprise the AR family of G protein-coupled receptors, designated A1R, A2AR, A2BR, and A3R. Each of these receptor genes contains an intron within the coding region, and polymorphisms have been observed in the A1R and A2AR. Activation of these receptors leads to modulation of basal adenylyl cyclase activity. The A1R and A3R are negatively coupled to the adenylyl cyclase signaling pathway through pertussis toxin-sensitive Gi/o. The A2AR and A2BR, on the other hand, stimulate the activity of this enzyme through cholera toxin-sensitive Gs. The A2BR also signals through Gq/11-dependent phospholipase C. The intracellular pathways triggered by these receptors include modulation of the activity of voltage-gated Ca2+ channels and K+ channels, as well as activation of the MAP kinase family of signal transduction pathways. The physiological relevance of these receptors in modulation of cardiovascular, immune and central nervous system have been confirmed by transgenic knockout mice [2,12].
All elements required for purinergic signaling are present in the pituitary gland. ATP is released by magnocellular neurons in the posterior pituitary and by anterior pituitary cells. The pituitary gland also contains nucleotide and nucleoside-converting enzymes whose activities account for the formation of ADP and adenosine. The cells of this gland express numerous ARs, P2YRs, and P2XRs. In this paper, we will review the current state of knowledge regarding purinergic signaling in the magocellular neurons and the pituitary gland. Earlier work in this field has been summarized in several reviews [15-19]. For the role of purinergic signaling in parvocellular hypothalamic nuclei see [20,21].
2. Release and Extracellular Metabolism of ATP
The physiological sources of the extracellular nucleotides required for activation of purinergic receptors in pituitary cells remain largely uncharacterized. In general, neurons, neuroendocrine cells and platelets release ATP by Ca2+-controlled exocytosis of nucleotides stored within synaptic vesicles or dense core granules. The magnocellular neurons of the hypothalamus that control release of vasopressin and oxytocin also contain ATP and the specific pattern of action potentials originating from the cellular bodies of these neurons has been suggested to control the release of ATP [17]. However, inhibition of electrical activity in the magnocellular neurons only partially inhibits secretion, suggesting that afferent signals acting on nerve terminals in the neurohypophysis may also contribute to ATP release [22]. It has also been suggested that extracellular ATP levels could reach 4-40 μM, which are sufficient concentrations to activate the majority of P2Rs [23]. A more recent study confirmed that ATP released from the neurohypophysis during stimulation depolarizes nerve terminals and potentiates vasopressin secretion [24]. In addition to its action on nerve terminals in the posterior pituitary, released ATP probably acts on neurohypophysial astrocytes, also known as pituicytes, but does not affect oxytocin release [23,24].
We recently showed that ATP is also released by normal and immortalized anterior pituitary cells at resting conditions. Such basal ATP release was enhanced in cells treated with ARL67156, an inhibitor of ectonucleotidases [25]. GnRH-induced stimulation of gonadotropin release was accompanied by elevation in basal ATP release, raising the possibility that ATP is stored in the secretory vesicles of these cells [26]. This is consistent with an earlier study showing calcium-dependence of ATP release [27] and modulation of ATP release by prolactin secretagogues [28]. On the other hand, facilitation of prolactin release did not elevate ATP secretion in perifused pituitary cells, suggesting that it could also be released by another mechanism, possibly from pituicytes. Consistent with our findings, both secretory and non-secretory ATP release pathways were found to be operative in human astrocytes [29]. In other tissues, ABC-binding cassette transporters, hemichannels, and P2X7R, among others have been suggested to participate in non-vesicular ATP release [30]. Interestingly, pituitary cells express functional multidrug resistance proteins [31] and P2X7R [32], which could contribute to ATP rerlease. Finally, we showed that endogenous release of ATP by cultured cells is of sufficient amplitude to desensitize homomeric and heteromeric P2X3Rs, confirming that the measured ATP is biologically active [25]. Certainly, these in vitro conditions should not accurately reflect the in vivo situation, where the tissue ATP concentration represents a balance between rates of release, hydrolysis, and dilution into intercellular compartments.
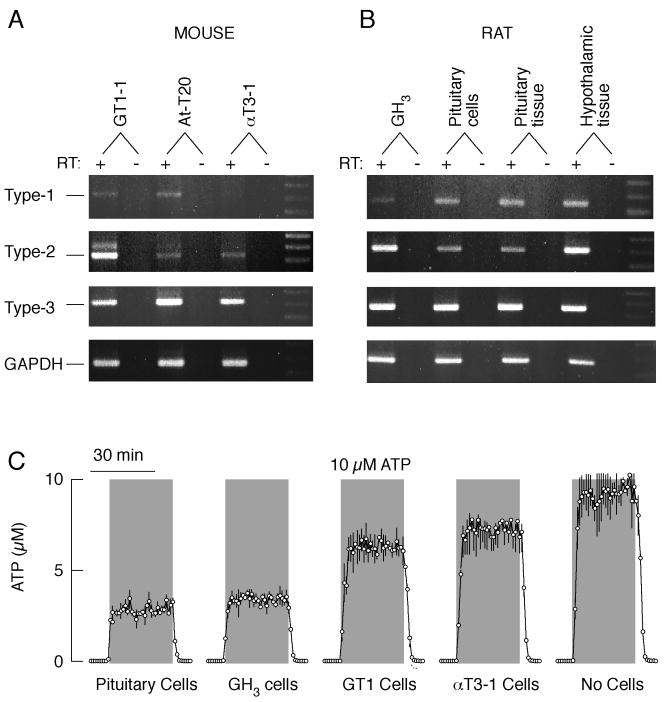
The action of ATP as an autocrine/paracrine factor is critically dependent on its rapid metabolism by ectonucleotidases [6]. These enzymes are also expressed on the plasma membrane of pituicytes and neurosecretory terminals [33]. Extracellularly added ATP is rapidly hydrolyzed by isolated neurohypophysis, indicating that the ectonucleotidases are functional. The group that discovered this also detected accumulation of adenosine when AMP was added to isolated neurohypophysis, suggesting that these enzymes provide a pathway for the activation of ARs in this tissue. This, in turn, is likely to account for the termination of ATP-induced vasopressin release [22]. mRNA transcripts for plasma membrane-located eNTPDases 1, 2 and 3 are expressed in hypothalamic and pituitary tissues, cultured pituitary cells and immortalized lacto-somatotrophs, corticotrophs, gonadotrophs, as well as in GnRH-secreting cells (Fig. 1A and B) [25]. Depending on the cell type and the rates of perifusion, pituitary cells were able to degrade between 30 and 70% of extracellularly added ATP (Fig. 1C). The ectonucleotide cascade not only terminates the extracellular messenger functions of ATP but also provides a pathway for the generation of ADP and adenosine, which in pituitary cells may activate some P2YRs and ARs, respectively (see below). Ecto-5′-nucleotidases (CD73), which generate adenosine from AMP, were found by immunocytochemistry in about 20% of anterior pituitary cells [34].
Fig. 1.
Expression and activity of ectonucleotidase in hypothalamic and pituitary tissues, pituitary cells, and immortalized hypiothalamic (GT1-1) and pituitary (At-T20, αT3-1, and GH3) cells. A and B, Expression of ectonucleotidase eNTPDase 1-3 transcripts in hypothalamic and pituitary tissues and cells. In both panels, DNA markers are shown in the last right lanes. In this and following figures, for negative controls, PCR was conducted performing first-stand cDNA synthesis without RT (-). C, Cell-type specificity of extracellular ATP metabolism. Chambers without and with cells (5×106 per column) were perifused with Krebs-Ringer buffer at flow rate of 0.8 ml/min. Samples were collected every minute and immediately tested for ATP concentrations. Gray areas indicate the duration of ATP application and its concentrations in the medium. Traces show measured ATP concentrations in effluents.
3. Expression and Signaling by P2XRs
Initial knowledge about the expression and role of P2Rs in the posterior pituitary was obtained in experiments using Ca2+ measurements and hormone secretion. These studies revealed that extracellularly added ATP lead to an increase in [Ca2+]i and vasopressin secretion, and that these actions were strongly and reversibly inhibited by the P2R blocker suramin (reviewed in [17]). P2XRs have been suggested to mediate the action of extracellular ATP on vasopressin release [35]. Initially, the role of P2X2Rs in ATP-induced increases in [Ca2+]i and peptide release from rat isolated neurohypophysial terminals had been suggested [23]. A more recent study indicated that a functional mixture of homomeric P2X2R and P2X3R mediates the majority of ATP's responses in vasopressinergic neurohypophysial terminals. The same study also provided the first electrophysiological evidence for the existence of P2XR currents in vasopressinergic neurohypophysial terminals and showed the lack of expression of P2XRs in terminals labeled for oxytocin [36]. ATP also plays a role in vasopressin release by activating postsynaptic receptors on perikarya or dendrites of supraoptic neurons [37]. At the present time, there is no evidence for the functional role of P2XRs in pituicytes.
Single-cell [Ca2+]i measurements, along with studies on hormone secretion in cells stimulated with various agonists in the presence and absence of P2XR inhibitors were also instrumental in the initial characterization of purinergic receptors in anterior pituitary cells [26,28,32,38,39]. These experiments revealed that functional P2XRs are operative in all secretory cell types and raised the possibility that several subtypes of these channels could be expressed in a cell type-specific manner. However, this method is of limited use for the identification of the receptor subtypes expressed, especially in characterizing the rapidly desensitizing homomeric and heteromeric P2XRs, and their roles in electrical activity [40].
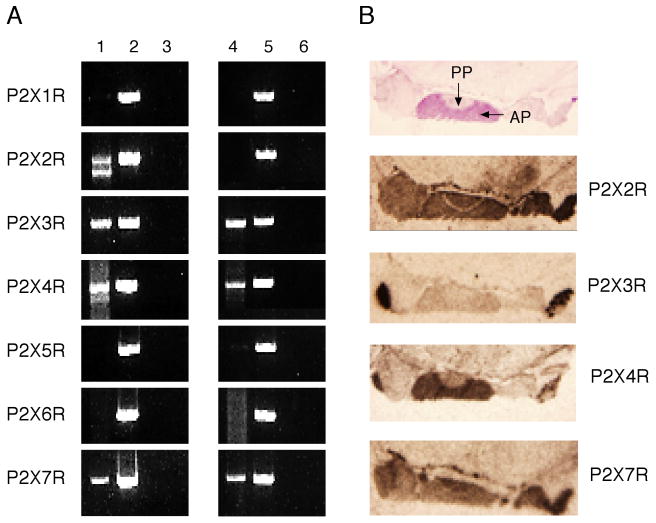
In more recent studies, we combined molecular biology techniques with electrophysiology to obtain additional data on expression and roles of these channels in pituitary cells. With the use of specific rat P2X primers and sequence analysis, P2X2, P2X3, P2X4, and P2X7 mRNA transcripts were detected in a mixed population of anterior pituitary cells (Fig. 2A, lane 1). Immortalized GH3 pituitary cells also expressed transcripts for P2X3, P2X4, and P2X7 subunits, but not the P2X2 subunit (Fig. 2A, lane 4). The mRNA levels of P2XRs in anterior pituitary cells were also examined by in situ hybridization. Figure 2A, top left panel, shows the position of the anterior and posterior pituitary. In parallel to RT-PCR analysis, the mRNA hybrids of the P2X2, P2X3, P2X4, and P2X7 subunits were also present in the anterior pituitary. The P2X4 mRNA subunit was more pronounced in the anterior than in the posterior pituitary, whereas the expression of the P2X3 subunit was relatively low in both areas. These mRNA expression patterns were consistent with immunohistochemical studies in the rat hypothalamo-neurohypophysial system at the electron microscope level. Immunoreactivity to P2X2Rs was localized in paraventricular and supraoptic nuclei, as well as in the posterior pituitary – in pituicytes and a subpopulation of neurosecretory axons [41]. Immunocytochemical labeling in vasopressinergic terminals also suggested the existence of P2X2, P2X3, P2X4, and P2X7 subunits [36].
Fig. 2.
Expression of P2XR mRNAs in the pituitary gland. A, Detection of mRNA transcripts for P2XRs in anterior pituitary cells (line 1) and a GH3 cell line (4). Lines 2 and 5 indicate positive controls and lines 3 and 6 negative controls. B, Detection of mRNA transcripts in the rat pituitary by in situ hybridization. PP, posterior pituitary, AP, anterior pituitary.
Abundant PCR products of two different sizes, approximately 1.6 and 1.4 kb long, were consistently amplified by the P2X2 primers in mixed rat pituitary cells (Fig. 1A). Earlier experiments revealed that the primary P2X2 gene transcript in rats undergoes extensive alternative splicing resulting in modified mRNA sequences. One of the spliced subunits, termed P2X2b, lacks a series of 69 C-terminal amino acids and creates a functional homomeric channel, which desensitizes more rapidly than the full-size receptor, termed P2X2a [42,43]. This form of the subunit was also detected in other tissues [44-46]. It has also been suggested that electrostatic charges of six amino acid side chains located close to the proximal splicing site play a critical role in controlling the rate of receptor desensitization [42].
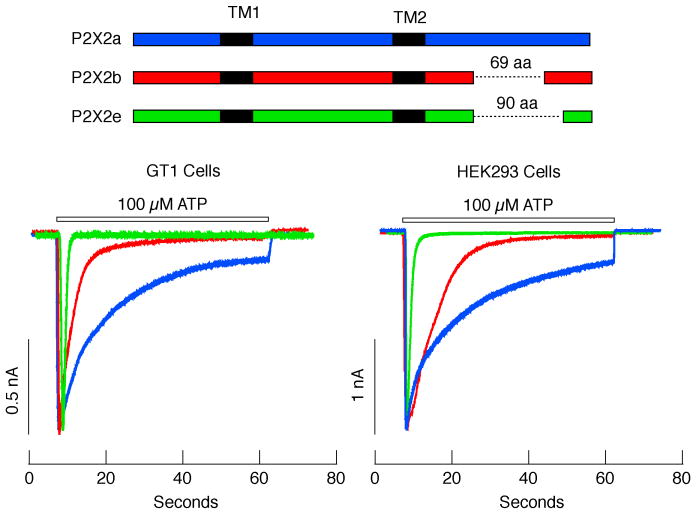
Recently, we also analyzed the expression of P2X2Rs in mouse pituitary cells and investigated the functional significance of the N- and C-termini specific structures of these receptors in terms of current, calcium signaling, and inter-subunit interactions in living cells. This study showed that the mouse pituitary expresses three forms of P2X2 subunits: the full size P2X2a and the spliced forms P2X2b and P2X2e, which are missing 69 and 90 residues in their C-termini, respectively. Furthermore, all three mouse forms have an additional 13 N-terminal residues not present in the rat pituitary gland. Electrophysiological experiments revealed that the rate of homomeric P2X2eR desensitization was comparable to the rates of rapidly desensitizing P2X1R and P2X3R. The rate of P2X2bR desensitization, however, was faster than P2X2aR but slower than P2X2eR (Fig. 3). These deletions in the C-terminal, in turn, effectively reduced the peak amplitude and duration of calcium signals [47]. The ion-permeating pores of P2XRs are formed by three subunits, and because the same primary P2X2 transcript can yield three different splicing variants, it is probable that a single pituitary cell expresses a series of homo- and heteromeric P2X2Rs. The structure of the N-terminal tail also influences the duration of P2X2R-mediated channel signaling in the continuous presence of an agonist. Specifically, deletion of the first 13 amino acids at the N-terminus of the mouse P2X2R resulted in accelerated desensitization rates of all C-terminal splicing isoforms [47].
Fig. 3.
Electrophysiological characterization of P2X2Rs cloned from mouse anterior pituitary cells. Top panel, Schematic representation of splice forms of P2X2R in pituitary cells. Horizontal dotted lines indicate missing sequences. Black areas indicate transmembrane domains (TM1 and TM2). Bottom panels, Patterns of ATP-induced current profiles in GT1 neurons (left) and HEK293 cells (right) expressing recombinant P2X2Rs. Green traces, P2X2e currents; red traces, P2X2bR currents; blue traces, P2X2aR current. Horizontal bars indicate duration of ATP application.
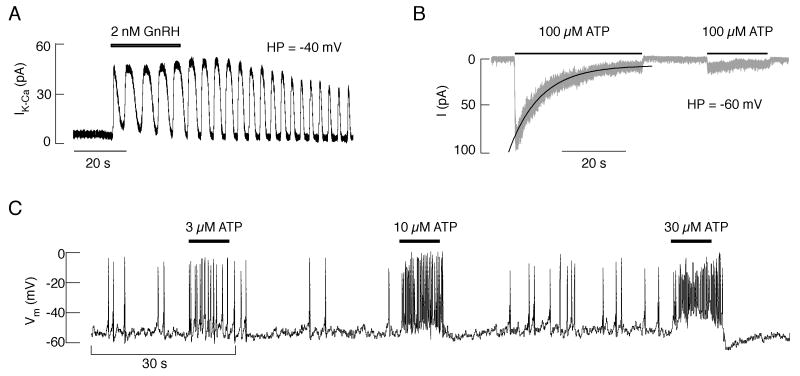
Single-cell patch-clamp analysis in gonadotrophs from embryonic, neonatal, and adult rats revealed that ATP application in all gonadotrophs generates a depolarizing and non-oscillatory current (Fig. 4B), in contrast to GnRH that triggered an oscillatory hyperpolarizing current (Fig. 4A). This clearly indicates that Ca2+-mobilizing P2YRs are not expressed in this particular cell type. The biophysical and pharmacological properties of ATP-induced depolarizing current (kinetics of activation, deactivation, desensitization, and resensitization) in gonadotrophs from adult and neonatal animals were comparable with those observed in cells expressing recombinant P2X2R. Sensitivity of ATP-evoked current to inhibition by pyridoxal 5-phosphate 6-azophenyl-2′,4′-disulfonic acid, reactive blue 2, and suramin, as well as the lack of effect of ivermectin, further confirmed this conclusion. ATP-induced rapid depolarization of gonadotrophs leads to the initiation of firing in quiescent cells, an increase in the frequency of action potentials in spontaneously active cells (Fig. 4C), and a transient stimulation of LH release. ATP also influenced GnRH-induced currents and membrane potential oscillations as well as LH release in an extracellular Ca2+-dependent manner. These InsP3-dependent oscillations were facilitated, slowed, or stopped, depending on the ATP concentration, the time of its application, and the level of Ca2+ content in intracellular stores. These results indicate that in gonadotrophs P2X2Rs could operate as pacemaking channels and modulators of GnRH-controlled electrical activity and secretion [48].
Fig. 4.
ATP-induced current and pacemaking activity in identified rat pituitary gonadotrophs. A, GnRH-induced outward oscillatory calcium-activated SK type potassium current. HP, holding potential. B, ATP-induced non-oscillatory inward current. Gray trace, experimental record; black solid line, the monoexponential fitting curve for the decay of current in the presence of ATP (desensitization). C, Concentration-dependent effects of ATP on the frequency of action potentials and the level and duration of afterhyperpolarization in a spontaneously active cell. Horizontal bars indicate duration of agonist application.
Approximately 90% of identified lactotrophs, from neonatal to adult, also responded to ATP application by generating inward depolarizing currents. Although the average peak amplitude of currents in response to supramaximal ATP concentrations was several-fold higher in gonadotrophs than in lactotrophs, the profiles of currents were highly comparable in the two cells types. Ivermectin, the specific P2X4R allosteric modulator, affected the ATP-induced current in lactotrophs but not in gonadotrophs. In the presence of ivermectin, there was large, roughly 6-fold increase in the maximum amplitude of current. Ivermectin also significantly increased the sensitivity of channels for ATP, delayed the deactivation of receptors, and enhanced ATP-induced PRL release. These results indicate that lactotrophs express homomeric and/or heteromeric P2X4Rs, which could operate as pacemaking channels [49].
4. Expression and Signaling by P2YRs
Since pituicytes are intimately associated with neurosecretory terminals, the released ATP from neuronal endings could affect their function in neurohypophysis. In the majority of pituicytes in primary culture, ATP triggers a rapid extracellular calcium-independent rise in [Ca2+]i, which is abolished in cells when phospholipase C and the endoplasmic reticulum calcium pump are blocked, indicating the functional operation of calcium-mobilizing P2YRs [50]. The stimulatory action of ATP is followed by potassium efflux [51]. The identification of the P2YR mediating the action of ATP in pituicytes has not been completed. The pharmacological profile of the calcium response in these cells suggests that ADP, UTP, and UDP are not endogenous agonists for these receptors [50].
Sheep and rat anterior pituitary cells also express P2YRs and their activation by ATP is associated with an elevation in [Ca2+]i due to InsP3-dependent release of Ca2+ from intracellular stores [52,53]. Molecular cloning and the functional characterization of rat P2YRs in the pituitary revealed the expression of P2Y2R with a pharmacological profile resembling that of the sheep pituitary [54]. For this type of receptor, ATP and UTP are equipotent agonists. The presence of UTP-sensitive P2YRs has been suggested in rat pituitary folliculo-stellate cells in primary culture [55]. The expression of these receptors has also been suggested in gonadotrophs [56] and lactotrophs [57], but our laboratory was unable to confirm these findings [26,58].
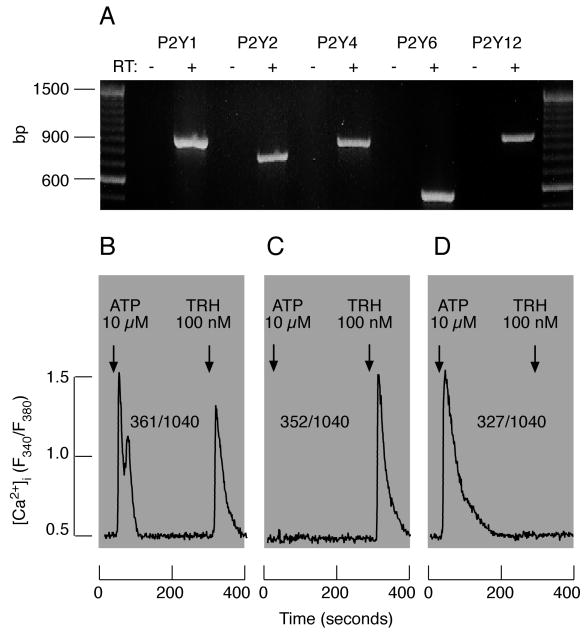
In addition to P2Y2R, RT-PCR analysis revealed the presence of transcripts for Gq-coupled calcium-mobilizing P2Y1R, P2Y4R, and P2Y6R, as well as Gi-coupled P2Y12R, in mixed anterior pituitary cells (Fig. 5A). Unlike UTP and UDP, in the absence of extracellular Ca2+, ATP and ADP triggered calcium signaling in about 40% of lactotrophs and prolactin release in mixed populations of secretory pituitary cells. A fraction of other unidentified cells also responded to ATP/ADP application with a rise in [Ca2+]i (Fig. 5B-D). The ligand-selectivity profile of calcium mobilization-dependent signaling and the blockade of these responses by pyridoxal 5-phosphate 6-azophenyl-2′,4′-disulphonic acid indicated that P2Y1Rs could be responsible for extracellular Ca2+-independent stimulation of prolactin release, but further pharmacological characterization of these receptors is required [58].
Fig. 5.
Characterization of P2YRs expressed in rat anterior pituitary cells. A, Detection of P2YR mRNA transcripts in a mixed population of anterior pituitary cells. B-D, ATP (10 μM)-induced calcium mobilization from endoplasmic reticulum in pituitary cells bathed in calcium-deficient medium. Numerators indicate number of cells responding to ATP and TRH (B, lactotrophs), TRH only (C, lactotrophs), and ATP only (D, unidentified pituitary cells). Arrows indicate moments of agonist applications, which were present in medium to the end of recordings.
5. Expression and Signaling by ARs
Extracellular ATP has a profound effect on the three-dimensional structure of pituicytes; it changes them from a flat shape to a stellate morphology. However, this action of ATP is not mediated by P2Rs, but rather requires both its hydrolysis by ectonucleotidases and the activation of ARs [59]. The action of adenosine in the posterior pituitary is not restricted to astroglial cells, but also activates ARs expressed in neurohypophysial terminals, leading to the inhibition of N-type calcium channels. In turn, this attenuates high K+-induced release of vasopressin and oxytocin by activating the A1R subtype [60]. Conversion of ATP to adenosine also terminates the stimulatory action of endogenously released and exogenously added ATP on vasopressin release from hypothalamo-neurohypophysial explants [61,62]. These results indicate the dual control of the stimulatory action of ATP on neuropeptide release by ectonucleotidases and the subsequent activation of A1Rs.
The expression and role of A1Rs in the anterior pituitary gland has been reviewed [18,19]. Briefly, functional receptors in immortalized pituitary cells were first described in 1985 [63]. Later, they were also identified in pituitary lactotrophs [64,65]. Pharmacological, electrophysiological, and secretory data indicated the negative coupling of these receptors to the adenylyl cyclase signaling pathway through pertussis toxin-sensitive G proteins, causing the abolition of spontaneous electrical activity, Ca2+ signaling, and hormone release. Cessation of spontaneous electrical activity by adenosine probably occurs indirectly, by the activation of voltage-gated K+ channels, and/or directly by the inhibition of voltage-gated Ca2+ channels. Pituitary cells also express A2AR, A2BR, and A3R [66-68]. The A2BRs are expressed in folliculo-stellate cells, stimulate IL-6 secretion [69], and trigger the formation of gap junctions [34]. A3Rs could contribute to the antiproliferative activity of adenosine in pituitary tumor cells [68].
6. Concluding Remarks
Once secreted by pituitary cells, the extracellular messenger actions of ATP are controlled by ecto-ATPases and ecto-5′-nucleotidase, which degrade ATP to ADP, AMP, and adenosine. While the capacity of these enzymes to hydrolyze ATP and generate adenosine from extracellular AMP is well established, further work is required to clarify the mechanism of ATP release by posterior and anterior pituitary cells. ATP, ADP, and adenosine are native agonists for pituitary cells and have dual actions on Ca2+ signaling: stimulatory and inhibitory, depending on the receptor subtype expressed. ATP activates P2XRs, which are expressed in all secretory anterior pituitary cells, as well as in nerve terminals in the posterior pituitary. There is molecular evidence for the expression of P2X2, P2X3, P2X4, and P2X7 subunits and immunocytochemical evidence for the expression of the P2X6 subunit. Electrophysiological studies have established the expression of P2X2Rs in vasopressinergic neurohypophysial terminals and gonadotrophs, as well as the expression of P2X4R in lactotrophs. These receptors facilitate the firing of action potentials, elevate [Ca2+]i, and stimulate hormone release. The identification of the P2XR subtype(s) expressed in somatotrophs, thyrotrophs, corticotrophs, and pituicytes necessitates further investigation. Molecular and physiological evidence for the expression of several Ca2+-mobilizing P2YRs has also been obtained. In contrast to P2XRs, the expression of these receptors is more limited in the anterior pituitary gland. A fraction of lactotrophs probably expresses P2Y1R, whereas folliculo-stellate cells express P2Y2Rs. Other secretory anterior pituitary cells also express P2YRs, but their identification will require additional studies. ADP is a potent agonist for the activation of P2Y1R in anterior pituitary cells. Posterior and anterior pituitary cells also express ARs. Among the members of this family of receptors, the best characterized are the A1Rs. These receptors are coupled to the Gi/o signaling pathway and effectively attenuate electrical activity through multiple mechanisms. Such organization of the purinergic pathway in the pituitary gland provides an effective but transient mechanism for the action of ATP as an agonist. The details of this mechanism include the prolonged activation of P2Y1R by ADP as well as termination of the stimulatory actions of P2XRs not only by hydrolysis of ATP, but also by the generation of adenosine and activation of A1Rs. Future work on the mechanism of purinergic release and the distribution of specific purinergic signaling elements should help us unravel the spatial and temporal aspects of ATP's actions in the pituitary gland.
Acknowledgments
Supported by the Intramural Research Program of the NICHD, NIH
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–81. [PubMed] [Google Scholar]
- 2.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–92. [PubMed] [Google Scholar]
- 3.Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol. 2001;38:900–8. doi: 10.1097/00005344-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res. 2001;26:959–69. doi: 10.1023/a:1012388618693. [DOI] [PubMed] [Google Scholar]
- 5.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–94. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- 7.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–67. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 8.Nicke A, Baumert HG, Rettinger J, Eichele A, Lambrecht G, Mutschler E, Schmalzing G. P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. Embo J. 1998;17:3016–28. doi: 10.1093/emboj/17.11.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicke A, Kerschensteiner D, Soto F. Biochemical and functional evidence for heteromeric assembly of P2X1 and P2X4 subunits. J Neurochem. 2005;92:925–33. doi: 10.1111/j.1471-4159.2004.02939.x. [DOI] [PubMed] [Google Scholar]
- 10.Koshimizu TA, Van Goor F, Tomic M, Wong AO, Tanoue A, Tsujimoto G, Stojilkovic SS. Characterization of calcium signaling by purinergic receptor-channels expressed in excitable cells. Mol Pharmacol. 2000;58:936–45. doi: 10.1124/mol.58.5.936. [DOI] [PubMed] [Google Scholar]
- 11.Samways DS, Egan TM. Acidic amino acids impart enhanced Ca2+ permeability and flux in two members of the ATP-gated P2X receptor family. J Gen Physiol. 2007;129:245–56. doi: 10.1085/jgp.200609677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–83. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer W, Krugel U. P2Y receptors: focus on structural, pharmacological and functional aspects in the brain. Curr Med Chem. 2007;14:2429–55. doi: 10.2174/092986707782023695. [DOI] [PubMed] [Google Scholar]
- 14.Erb L, Liao Z, Seye CI, Weisman GA. P2 receptors: intracellular signaling. Pflugers Arch. 2006;452:552–62. doi: 10.1007/s00424-006-0069-2. [DOI] [PubMed] [Google Scholar]
- 15.Stojilkovic SS, Tomic M, Van Goor F, Koshimizu T. Expression of purinergic P2X2 receptor-channels and their role in calcium signaling in pituitary cells. Biochem Cell Biol. 2000;78:393–404. [PubMed] [Google Scholar]
- 16.Stojilkovic SS, Koshimizu T. Signaling by extracellular nucleotides in anterior pituitary cells. Trends Endocrinol Metab. 2001;12:218–25. doi: 10.1016/s1043-2760(01)00387-3. [DOI] [PubMed] [Google Scholar]
- 17.Troadec JD, Thirion S. Multifaceted purinergic regulation of stimulus-secretion coupling in the neurohypophysis. Neuro Endocrinol Lett. 2002;23:273–80. [PubMed] [Google Scholar]
- 18.Rees DA, Scanlon MF, Ham J. Adenosine signalling pathways in the pituitary gland: one ligand, multiple receptors. J Endocrinol. 2003;177:357–64. doi: 10.1677/joe.0.1770357. [DOI] [PubMed] [Google Scholar]
- 19.Rees DA, Scanlon MF, Ham J. Novel insights into how purines regulate pituitary cell function. Clin Sci (Lond) 2003;104:467–81. doi: 10.1042/CS20030053. [DOI] [PubMed] [Google Scholar]
- 20.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 21.Stojilkovic SS. Purinergic regulation of hypothalamo-pituitary functions. Trends Endocrinol Metab. 2009 doi: 10.1016/j.tem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sperlagh B, Mergl Z, Juranyi Z, Vizi ES, Makara GB. Local regulation of vasopressin and oxytocin secretion by extracellular ATP in the isolated posterior lobe of the rat hypophysis. J Endocrinol. 1999;160:343–50. doi: 10.1677/joe.0.1600343. [DOI] [PubMed] [Google Scholar]
- 23.Troadec JD, Thirion S, Nicaise G, Lemos JR, Dayanithi G. ATP-evoked increases in [Ca2+]i and peptide release from rat isolated neurohypophysial terminals via a P2X2 purinoceptor. J Physiol. 1998;511(Pt 1):89–103. doi: 10.1111/j.1469-7793.1998.089bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knott TK, Marrero HG, Custer EE, Lemos JR. Endogenous ATP potentiates only vasopressin secretion from neurohypophysial terminals. J Cell Physiol. 2008;217:155–61. doi: 10.1002/jcp.21485. [DOI] [PubMed] [Google Scholar]
- 25.He ML, Gonzalez-Iglesias AE, Tomic M, Stojilkovic SS. Release and extracellular metabolism of ATP by ecto-nucleotidase eNTPDase 1-3 in hypothalamic and pituitary cells. Purinergic Signal. 2005;1:135–44. doi: 10.1007/s11302-005-6208-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomic M, Jobin RM, Vergara LA, Stojilkovic SS. Expression of purinergic receptor channels and their role in calcium signaling and hormone release in pituitary gonadotrophs. Integration of P2 channels in plasma membrane- and endoplasmic reticulum-derived calcium oscillations. J Biol Chem. 1996;271:21200–8. doi: 10.1074/jbc.271.35.21200. [DOI] [PubMed] [Google Scholar]
- 27.Chen ZP, Kratzmeier M, Levy A, McArdle CA, Poch A, Day A, Mukhopadhyay AK, Lightman SL. Evidence for a role of pituitary ATP receptors in the regulation of pituitary function. Proc Natl Acad Sci U S A. 1995;92:5219–23. doi: 10.1073/pnas.92.11.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nunez L, Villalobos C, Frawley LS. Extracellular ATP as an autocrine/paracrine regulator of prolactin release. Am J Physiol. 1997;272:E1117–23. doi: 10.1152/ajpendo.1997.272.6.E1117. [DOI] [PubMed] [Google Scholar]
- 29.Joseph SM, Buchakjian MR, Dubyak GR. Colocalization of ATP release sites and ecto-ATPase activity at the extracellular surface of human astrocytes. J Biol Chem. 2003;278:23331–42. doi: 10.1074/jbc.M302680200. [DOI] [PubMed] [Google Scholar]
- 30.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Andric SA, Kostic TS, Stojilkovic SS. Contribution of multidrug resistance protein MRP5 in control of cyclic guanosine 5′-monophosphate intracellular signaling in anterior pituitary cells. Endocrinology. 2006;147:3435–45. doi: 10.1210/en.2006-0091. [DOI] [PubMed] [Google Scholar]
- 32.Koshimizu TA, Tomic M, Wong AO, Zivadinovic D, Stojilkovic SS. Characterization of purinergic receptors and receptor-channels expressed in anterior pituitary cells. Endocrinology. 2000;141:4091–9. doi: 10.1210/endo.141.11.7737. [DOI] [PubMed] [Google Scholar]
- 33.Thirion S, Troadec JD, Nicaise G. Cytochemical localization of ecto-ATPases in rat neurohypophysis. J Histochem Cytochem. 1996;44:103–11. doi: 10.1177/44.2.8609366. [DOI] [PubMed] [Google Scholar]
- 34.Lewis BM, Pexa A, Francis K, Verma V, McNicol AM, Scanlon M, Deussen A, Evans WH, Rees DA, Ham J. Adenosine stimulates connexin 43 expression and gap junctional communication in pituitary folliculostellate cells. Faseb J. 2006;20:2585–7. doi: 10.1096/fj.06-6121fje. [DOI] [PubMed] [Google Scholar]
- 35.Kapoor JR, Sladek CD. Purinergic and adrenergic agonists synergize in stimulating vasopressin and oxytocin release. J Neurosci. 2000;20:8868–75. doi: 10.1523/JNEUROSCI.20-23-08868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knott TK, Velazquez-Marrero C, Lemos JR. ATP elicits inward currents in isolated vasopressinergic neurohypophysial terminals via P2X2 and P2X3 receptors. Pflugers Arch. 2005;450:381–9. doi: 10.1007/s00424-005-1471-x. [DOI] [PubMed] [Google Scholar]
- 37.Song Z, Sladek CD. Site of ATP and phenylephrine synergistic stimulation of vasopressin release from the hypothalamo-neurohypophyseal system. J Neuroendocrinol. 2006;18:266–72. doi: 10.1111/j.1365-2826.2006.01411.x. [DOI] [PubMed] [Google Scholar]
- 38.Villalobos C, Alonso-Torre SR, Nunez L, Garcia-Sancho J. Functional ATP receptors in rat anterior pituitary cells. Am J Physiol. 1997;273:C1963–71. doi: 10.1152/ajpcell.1997.273.6.C1963. [DOI] [PubMed] [Google Scholar]
- 39.Chung HS, Park KS, Cha SK, Kong ID, Lee JW. ATP-induced [Ca(2+)](i) changes and depolarization in GH3 cells. Br J Pharmacol. 2000;130:1843–52. doi: 10.1038/sj.bjp.0703253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He ML, Zemkova H, Koshimizu TA, Tomic M, Stojilkovic SS. Intracellular calcium measurements as a method in studies on activity of purinergic P2X receptor channels. Am J Physiol Cell Physiol. 2003;285:C467–79. doi: 10.1152/ajpcell.00042.2003. [DOI] [PubMed] [Google Scholar]
- 41.Loesch A, Miah S, Burnstock G. Ultrastructural localisation of ATP-gated P2X2 receptor immunoreactivity in the rat hypothalamo-neurohypophysial system. J Neurocytol. 1999;28:495–504. doi: 10.1023/a:1007009222518. [DOI] [PubMed] [Google Scholar]
- 42.Koshimizu T, Koshimizu M, Stojilkovic SS. Contributions of the C-terminal domain to the control of P2X receptor desensitization. J Biol Chem. 1999;274:37651–7. doi: 10.1074/jbc.274.53.37651. [DOI] [PubMed] [Google Scholar]
- 43.Koshimizu T, Tomic M, Koshimizu M, Stojilkovic SS. Identification of amino acid residues contributing to desensitization of the P2X2 receptor channel. J Biol Chem. 1998;273:12853–7. doi: 10.1074/jbc.273.21.12853. [DOI] [PubMed] [Google Scholar]
- 44.Brandle U, Spielmanns P, Osteroth R, Sim J, Surprenant A, Buell G, Ruppersberg JP, Plinkert PK, Zenner HP, Glowatzki E. Desensitization of the P2X(2) receptor controlled by alternative splicing. FEBS Lett. 1997;404:294–8. doi: 10.1016/s0014-5793(97)00128-2. [DOI] [PubMed] [Google Scholar]
- 45.Simon J, Kidd EJ, Smith FM, Chessell IP, Murrell-Lagnado R, Humphrey PP, Barnard EA. Localization and functional expression of splice variants of the P2X2 receptor. Mol Pharmacol. 1997;52:237–48. doi: 10.1124/mol.52.2.237. [DOI] [PubMed] [Google Scholar]
- 46.Parker MS, Larroque ML, Campbell JM, Bobbin RP, Deininger PL. Novel variant of the P2X2 ATP receptor from the guinea pig organ of Corti. Hear Res. 1998;121:62–70. doi: 10.1016/s0378-5955(98)00065-3. [DOI] [PubMed] [Google Scholar]
- 47.Koshimizu TA, Kretschmannova K, He ML, Ueno S, Tanoue A, Yanagihara N, Stojilkovic SS, Tsujimoto G. Carboxyl-terminal splicing enhances physical interactions between the cytoplasmic tails of purinergic P2X receptors. Mol Pharmacol. 2006;69:1588–98. doi: 10.1124/mol.105.019802. [DOI] [PubMed] [Google Scholar]
- 48.Zemkova H, Balik A, Jiang Y, Kretschmannova K, Stojilkovic SS. Roles of purinergic P2X receptors as pacemaking channels and modulators of calcium-mobilizing pathway in pituitary gonadotrophs. Mol Endocrinol. 2006;20:1423–36. doi: 10.1210/me.2005-0508. [DOI] [PubMed] [Google Scholar]
- 49.Varva V, Jindrichova M, Jelinkova I, Zemkova H. Inhibitory role of P2X6 subunit in rat heteromeric P2X4/P2X6 receptor channels. Society for Neuroscience Annual Meeting.2008. [Google Scholar]
- 50.Troadec JD, Thirion S, Petturiti D, Bohn MT, Poujeol P. ATP acting on P2Y receptors triggers calcium mobilization in primary cultures of rat neurohypophysial astrocytes(pituicytes) Pflugers Arch. 1999;437:745–53. doi: 10.1007/s004240050841. [DOI] [PubMed] [Google Scholar]
- 51.Troadec JD, Thirion S, Petturiti D, Poujeol P. Potassium efflux triggered by P2Y purinoceptor activation in cultured pituicytes. Pflugers Arch. 2000;440:770–7. doi: 10.1007/s004240000343. [DOI] [PubMed] [Google Scholar]
- 52.van der Merwe PA, Wakefield IK, Fine J, Millar RP, Davidson JS. Extracellular adenosine triphosphate activates phospholipase C and mobilizes intracellular calcium in primary cultures of sheep anterior pituitary cells. FEBS Lett. 1989;243:333–6. doi: 10.1016/0014-5793(89)80156-5. [DOI] [PubMed] [Google Scholar]
- 53.Davidson JS, Wakefield IK, Sohnius U, van der Merwe PA, Millar RP. A novel extracellular nucleotide receptor coupled to phosphoinositidase-C in pituitary cells. Endocrinology. 1990;126:80–7. doi: 10.1210/endo-126-1-80. [DOI] [PubMed] [Google Scholar]
- 54.Chen ZP, Krull N, Xu S, Levy A, Lightman SL. Molecular cloning and functional characterization of a rat pituitary G protein-coupled adenosine triphosphate (ATP) receptor. Endocrinology. 1996;137:1833–40. doi: 10.1210/endo.137.5.8612522. [DOI] [PubMed] [Google Scholar]
- 55.Uchiyama M, Nakajima Y, Sakuma Y, Kato M. Purinergic regulation of intracellular Ca2+ concentration of rat pituitary folliculo-stellate cells in primary culture. J Neuroendocrinol. 2001;13:378–85. doi: 10.1046/j.1365-2826.2001.00639.x. [DOI] [PubMed] [Google Scholar]
- 56.Chen ZP, Kratzmeier M, Poch A, Xu S, McArdle CA, Levy A, Mukhopadhyay AK, Lightman SL. Effects of extracellular nucleotides in the pituitary: adenosine triphosphate receptor-mediated intracellular responses in gonadotrope-derived alpha T3-1 cells. Endocrinology. 1996;137:248–56. doi: 10.1210/endo.137.1.8536620. [DOI] [PubMed] [Google Scholar]
- 57.Carew MA, Wu ML, Law GJ, Tseng YZ, Mason WT. Extracellular ATP activates calcium entry and mobilization via P2U-purinoceptors in rat lactotrophs. Cell Calcium. 1994;16:227–35. doi: 10.1016/0143-4160(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 58.He ML, Gonzalez-Iglesias AE, Stojilkovic SS. Role of nucleotide P2 receptors in calcium signaling and prolactin release in pituitary lactotrophs. J Biol Chem. 2003;278:46270–7. doi: 10.1074/jbc.M309005200. [DOI] [PubMed] [Google Scholar]
- 59.Rosso L, Peteri-Brunback B, Vouret-Craviari V, Deroanne C, Troadec JD, Thirion S, Van Obberghen-Schilling E, Mienville JM. RhoA inhibition is a key step in pituicyte stellation induced by A(1)-type adenosine receptor activation. Glia. 2002;38:351–62. doi: 10.1002/glia.10072. [DOI] [PubMed] [Google Scholar]
- 60.Wang G, Dayanithi G, Custer EE, Lemos JR. Adenosine inhibition via A(1) receptor of N-type Ca(2+) current and peptide release from isolated neurohypophysial terminals of the rat. J Physiol. 2002;540:791–802. doi: 10.1113/jphysiol.2002.016394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song Z, Sladek CD. Does conversion of ATP to adenosine terminate ATP-stimulated vasopressin release from hypothalamo-neurohypophyseal explants? Brain Res. 2005;1047:105–11. doi: 10.1016/j.brainres.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 62.Knott TK, Marrero HG, Fenton RA, Custer EE, Dobson JG, Jr, Lemos JR. Endogenous adenosine inhibits CNS terminal Ca(2+) currents and exocytosis. J Cell Physiol. 2007;210:309–14. doi: 10.1002/jcp.20827. [DOI] [PubMed] [Google Scholar]
- 63.Dorflinger LJ, Schonbrunn A. Adenosine inhibits prolactin and growth hormone secretion in a clonal pituitary cell line. Endocrinology. 1985;117:2330–8. doi: 10.1210/endo-117-6-2330. [DOI] [PubMed] [Google Scholar]
- 64.Yu WH, Kimura M, Walczewska A, Porter JC, McCann SM. Adenosine acts by A1 receptors to stimulate release of prolactin from anterior-pituitaries in vitro. Proc Natl Acad Sci U S A. 1998;95:7795–8. doi: 10.1073/pnas.95.13.7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scorziello A, Landolfi E, Grimaldi M, Meucci O, Ventra C, Avallone A, Postiglione A, Schettini G. Direct effect of adenosine on prolactin secretion at the level of the single rat lactotroph: involvement of pertussis toxin-sensitive and -insensitive transducing mechanisms. J Mol Endocrinol. 1993;11:325–34. doi: 10.1677/jme.0.0110325. [DOI] [PubMed] [Google Scholar]
- 66.Weaver DR. A2a adenosine receptor gene expression in developing rat brain. Brain Res Mol Brain Res. 1993;20:313–27. doi: 10.1016/0169-328x(93)90058-w. [DOI] [PubMed] [Google Scholar]
- 67.Dixon AK, Gubitz AK, Sirinathsinghji DJ, Richardson PJ, Freeman TC. Tissue distribution of adenosine receptor mRNAs in the rat. Br J Pharmacol. 1996;118:1461–8. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ohana G, Bar-Yehuda S, Barer F, Fishman P. Differential effect of adenosine on tumor and normal cell growth: focus on the A3 adenosine receptor. J Cell Physiol. 2001;186:19–23. doi: 10.1002/1097-4652(200101)186:1<19::AID-JCP1011>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 69.Rees DA, Lewis BM, Lewis MD, Francis K, Scanlon MF, Ham J. Adenosine-induced IL-6 expression in pituitary folliculostellate cells is mediated via A2b adenosine receptors coupled to PKC and p38 MAPK. Br J Pharmacol. 2003;140:764–72. doi: 10.1038/sj.bjp.0705488. [DOI] [PMC free article] [PubMed] [Google Scholar]