Abstract
Decades of research on the cellular mechanisms of memory have led to the widely-held view that memories are stored as modifications of synaptic strength. These changes involve presynaptic processes, such as direct modulation of the release machinery, or postsynaptic processes, such as modulation of receptor properties. Parallel studies have revealed that memories may also be stored by nonsynaptic processes, such as modulation of voltage-dependent membrane conductances, which are expressed as changes in neuronal excitability. Although in some cases nonsynaptic changes may function as part of the engram itself, they may also serve as mechanisms through which a neural circuit is set to a permissive state to facilitate synaptic modifications that are necessary for memory storage.
Introduction
Cellular and molecular studies implicate modulation of synaptic strength as the basis of learning and memory [1–5]. Changes in synaptic strength occur via a wide range of mechanisms that act at the level of the presynaptic neuron (e.g., direct modulation of release process and subsequent changes in amount of neurotransmitter released), or at the level of the postsynaptic neuron (e.g., modifications in function and/or number of neurotransmitter receptors) (Figure 1a). However, it is now clear that different forms of learning and patterns of neuronal activity also produce diverse and widespread nonsynaptic changes by modulating membrane components, including resting and voltage-dependent channels and ion pumps, which are often expressed as changes in excitability (Box 1; Figure 1b). Despite the growing number of studies reporting nonsynaptic changes, several aspects of this form of plasticity remain elusive. What is its relationship to synaptic plasticity and what is its functional relevance? Are nonsynaptic changes part of the engram (see Glossary) itself or do they act as a permissive mechanism to facilitate synaptic mechanisms? How can nonsynaptic changes achieve high degrees of specificity similar to those expressed by synaptic modifications? Here we review selected examples of early and more recent evidence of learning- and activity-induced nonsynaptic changes, and we discuss their potential relevance.
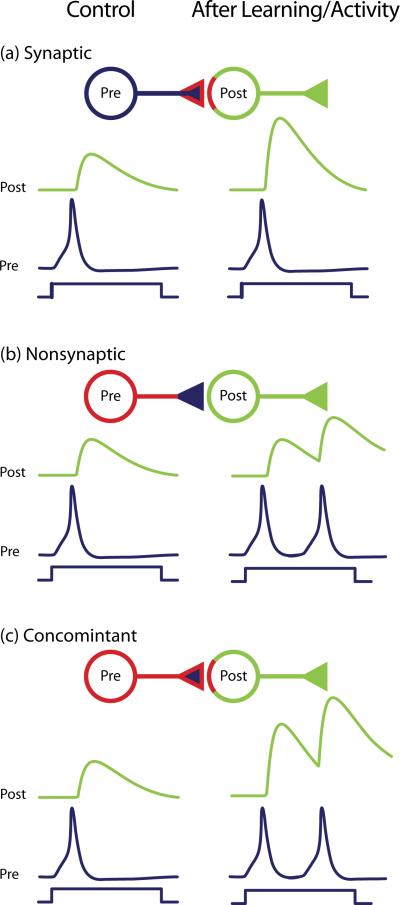
Figure 1.
Nonsynaptic and synaptic changes. The interaction between a presynaptic (Pre; blue) and a postsynaptic (Post; green) neuron can be modified by changes in synaptic strength (a), changes in excitability (b), or both (c). (a) When learning or neuronal activity activates a synaptic mechanism (red highlight on the presynaptic terminal and on the postsynaptic neuron), the strength of the synaptic connection is altered (enhanced in this case). (b) When learning or neuronal activity activates a nonsynaptic mechanism (red highlight on soma and neurites of the presynaptic neuron), the excitability is altered (increased, in this case). Consequently, the presynaptic neuron generates a greater number of APs during a fixed time interval. Although the synaptic response to individual APs is not changed, the greater number of evoked spikes produces summating synaptic potential and a resulting greater synaptic response in the postsynaptic neuron. (c) When both synaptic and nonsynaptic mechanisms are triggered by learning or neuronal activity (red highlight on soma, neurites and terminal of the pre- and post-synaptic neurons), each component may work synergistically to change (strengthen, in this case) the synaptic response in the postsynaptic neuron. Note that in this illustration the nonsynaptic change has been embedded arbitrarily in the presynaptic neuron, but it could equally have been embedded in the postsynaptic neuron. Indeed, the “presynaptic” neuron is postsynaptic to some other neuron.
Changes in excitability produced by learning
Although the role of synaptic changes in learning dates back to Ramón y Cajal [6] and Tanzi [7], data supporting both the role of synaptic and nonsynaptic changes began to emerge in the 1970s and 1980s. The breakthroughs occurred with the development of several model systems in which it was possible to monitor changes in neuronal properties produced by learning (see ref. [1] for early review).
Woody and his colleagues [8,9] provided early evidence for learning-dependent nonsynaptic plasticity in vertebrates by showing that classical conditioning of the cat eyeblink reflex was associated with increased excitability and input resistance in neurons in sensory-motor cortical areas and in facial nucleus. Compelling early evidence for nonsynaptic changes associated with learning was provided by Crow and Alkon [10] who found that classical conditioning in the mollusk Hermissenda produced changes in several membrane properties of the photoreceptors, including increases in spontaneous firing (Figure 2a) and input resistance [10]. Voltage-clamp analysis revealed that these effects were due to a reduction of two K+ currents (IA, and IK,Ca) [11–13]. These changes in excitability persisted when the photoreceptors were isolated from the nervous system, excluding the possibility that they were due to upstream modifications of tonic synaptic input to the photoreceptors (Box 1).
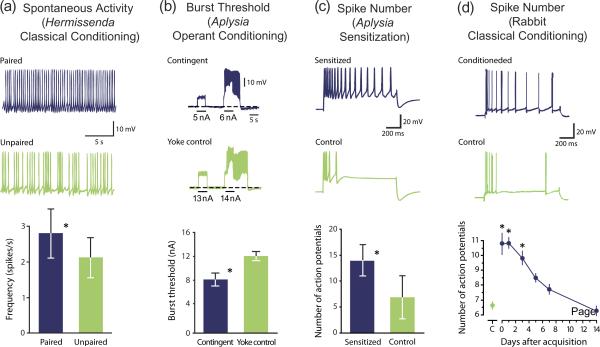
Figure 2.
Changes in excitability produced by different forms of learning. (a) Following classical conditioning, consisting of paired presentations of light and rotation, photoreceptors in Hermissenda exhibited a greater frequency of spontaneous firing (blue) than those produced by an unpaired procedure consisting of random presentation of light and rotation (green). (b) Operant conditioning of feeding behavior in Aplysia increased the excitability of neuron B51. B51 burst threshold was lower in trained (contingent) animals (blue) as compared to control (yoke control) animals (green). (c) Sensitization of the tail-siphon withdrawal reflex in Aplysia induced a long-term (24 h) increase in the excitability of the sensory neurons. The number of APs evoked during a 1-s, 2-nA depolarization was greater in sensitized animals (blue) as compared to untrained control animals (green). (d) Trace eyelid conditioning produced changes in the intrinsic excitability of pyramidal neurons in the CA1 area of the rabbit hippocampus. Selected examples of recordings from CA1 hippocampal neurons (top traces) illustrate that firing discharge was greater 24 h after acquisition in neurons from conditioned animals (blue), as compared to that of neurons from animals trained with a pseudoconditioning control procedure (green). The number of APs in CA1 neurons (bottom graph) from conditioned animals (blue circles) became indistinguishable from control (C) animals (green circles) 14 days after acquisition. Figure adapted from Refs. [8] for panel (a); [25], for panel (b); [22] for panel (c) and [47] for panel (d).
More recent studies revealed that all major forms of nonassociative and associative learning (see Glossary) produced modifications that were not limited to synaptic function. In the leech, both habituation and sensitization of the shortening reflex modified the excitability of S interneurons that mediate the reflex [14]. Habituation decreased the input resistance and the excitability, whereas sensitization produced the opposite effect [15]. In the rabbit, delay eyelid conditioning produced up to a 30 day reduction in spike threshold and afterhyperpolarization (AHP) of Purkinje cells [17]. In monkeys, changes in the membrane properties of motor neurons were produced by operant conditioning of the spinal stretch reflex [18,19].
Contribution of changes in excitability to the memory trace
Although learning-induced changes in excitability are ubiquitous, surprisingly little is known about their relevance to the memory. Are they part of the engram or do they serve some other function(s)? A definitive answer to this question is elusive because key tests of necessity and sufficiency have not been performed, and in only a few cases have quantitative correlation analyses been done. In Hermissenda, the firing frequency of the photoreceptors was correlated with the conditioned response [10], suggesting a causal relationship between excitability changes and behavior.
Recently, it was possible to artificially induce an excitability change at the single-cell level and to examine its role in the modifications produced by operant conditioning of feeding in the mollusk Aplysia. Operant conditioning of feeding decreased the burst threshold (Figure 2b) and increased the input resistance of neuron B51, a key “decision-making” neuron in the feeding circuit [20–23]. These changes, which were consistent with an increase in excitability, were intrinsic because they were expressed by isolated B51 in culture [22–24]. Injection of cAMP into a naïve B51 induced changes in excitability similar to those produced by operant conditioning [23,24] and increased the recruitment of B51 and the motor activity associated with feeding [23], thus recapitulating the memory phenotype. Although the above examples support the hypothesis that changes in excitability serve as a memory trace, the results need to be interpreted with caution because of the possibility of the concurrent presence of both synaptic and nonsynaptic mechanisms (see below).
Relationship between nonsynaptic and synaptic plasticity
Changes in nonsynaptic expression mechanisms frequently occur concomitantly with synaptic changes, which suggest that concurrent changes may be a common motif. However, the presence of both changes raises the question of whether the two can be mechanistically decoupled and the extent to which each contributes to the expression of memory. Plasticity at the Aplysia sensorimotor connection is an example of tight coupling between synaptic and nonsynaptic modifications (Figure 2c). One-day training, which produces persistent (24 h) long-term sensitization (LTS) of the tail-siphon withdrawal reflex, was accompanied by both facilitation of the sensorimotor synapse and increased sensory neuron (SN) excitability [25]. Similar effects were produced by sensitizing stimuli or stimuli that mimic sensitization training in reduced preparations [26–28]. Furthermore, multiple brief applications of serotonin (5-HT) to isolated SNs produced a long-term increase in excitability similar to that induced by training, suggesting that this modification was intrinsic [29]. In Aplysia, classical conditioning also produced similar nonsynaptic and synaptic changes of the SNs [30]. The changes in excitability produced by 5-HT are likely due to the modulation of multiple K+ conductances including IA, IK,Ca and IKs [31]. Similar biophysical changes were associated with LTS [32]. Although such changes in membrane currents would increase the excitability of the sensory neurons, they could also broaden the action potential (AP) and enhance neurotransmitter release [33]. Therefore, the modulation of presynaptic K+ currents might serve as a common mechanism for concomitantly regulating excitability and synaptic strength. A similar conclusion can be drawn from work in Hermissenda in which currents that are modulated to express changes in excitability also change transmitter release via spike broadening [34].
Changes in excitability are not always “lock stepped” mechanistically or temporally with modifications of synaptic strength. For example, excitability changes were dissociated with different training procedures. Four-day training, which led to LTS that persists for weeks [35–37], produced long-term facilitation of the sensorimotor synapse similar to that produced by the one-day training protocol, but without a change in excitability [37,38]. Indeed, there was an unexpected narrowing of the AP in the SNs [35,37,38]. These findings suggest that nonsynaptic and synaptic changes co-exist (Figure 1c) and may play distinct roles in the expression of the different temporal domains of the memory.
Concomitant synaptic and nonsynaptic changes, similar to those in Aplysia and Hermissenda, were identified in rats trained with a classical conditioning protocol consisting of odors (CS) paired with foot shocks (US). This paradigm led to an enhancement of both CS-evoked synaptic input and neuronal excitability in amygdala neurons [39]. These changes appear to share a common biochemical pathway. Both were prevented when dopamine signaling was pharmacologically blocked [39].
In addition to nonsynaptic and synaptic changes at the same site within a circuit, synaptic and nonsynaptic plasticity can be localized at different sites. Fear conditioning (see Glossary) led to synaptic and nonsynaptic changes in different anatomical regions with distinct contributions to behavioral modifications. Enhancement of the synaptic input to amygdala neurons appeared to serve as a mechanism for memory expression and maintenance [40]. Conversely, changes in excitability in the infralimbic (IL) prefrontal cortex appeared to contribute to extinction. The excitability of IL neurons was decreased following training, but increased during extinction [41]. These bidirectional changes in excitability were produced by modulation of IK,Ca underlying the slow AHP [41]. Neurons in the amygdala contribute to the storage of the learned fear with synaptic mechanisms, whereas neurons in the IL cortex contribute to the memory storage with a decrease in excitability and to extinction with an increase in excitability.
Recently, an unexpected relationship between nonsynaptic and synaptic mechanisms of plasticity was found in Aplysia following classical conditioning of feeding [42]. Classical conditioning strengthened the CS-evoked excitatory synaptic input to neuron B51, which was consistent with the observed augmented feeding response [42,43]. However, classical conditioning decreased the excitability of B51 [42], which, by itself, would tend to reduce feeding behavior. Nevertheless, classical conditioning increased the CS-evoked recruitment of B51 possibly by “overpowering” the diminished excitability. The physiological significance of this relationship between nonsynaptic and synaptic changes needs to be further investigated.
The above examples illustrate the diversity of relationships between nonsynaptic and synaptic plasticity, with examples in which the two mechanisms act synergistically to produce the expected behavioral response, and others in which each mechanism contributes to distinct aspects of memory, such as recall and extinction.
Insights from dissociations between nonsynaptic changes in excitability and the memory trace
Some examples of nonsynaptic changes can be directly linked to mechanisms of memory [10,23], but other dissociations are present and the relationship is less clear. These dissociations provided two insights about changes in excitability: first, they participate differently in different temporal domains of memory, and second, they may have a permissive role in regulating synaptic plasticity.
Examples of temporal dissociations between nonsynaptic changes and the memory trace come from invertebrates and vertebrates. In the mollusk Lymnaea, long-term memory for classical conditioning of feeding [44] was associated with a Na+-dependent depolarization in the modulatory neuron CGC [45,46], which was delayed until 24 h after training, but was then observed for the following 14 days [45]. Although the depolarization in CGC was not per se necessary for memory formation and maintenance at early times (<24 h), it appears to regulate the ability of the CS to elicit feeding at later times [45].
Brons and Woody [9] found that increased excitability produced by classical conditioning persisted after the response was extinguished. Because retraining after extinction resulted in a much faster rate of acquisition, (i.e., savings), the increase in excitability could function as a mechanism for savings [9]. Trace eyelid conditioning in the rabbit induced an increased excitability of hippocampal CA1 and CA3 pyramidal neurons [47,48] (Figure 2d) and a decrease of their AHP [47–49], which were different from those in the cerebellum following delay conditioning. For trace conditioning, the time course of the excitability change did not parallel that of the response, and it was not detected seven days after training at a time when the memory remained [47,48] (Figure 2d). In addition, the nonsynaptic changes were not restricted to a specific area, but were distributed throughout the dorsal hippocampus. These findings indicate that whereas changes in excitability are insufficient to account for the memory in trace conditioning, they may represent a storage mechanism for a temporally limited phase of the memory.
The above studies support the view that changes in excitability may promote the occurrence of other types of neuronal plasticity involved in memory. Indeed, as discussed previously [1], because of the voltage-dependent properties of the N-methyl-D-aspartate (NMDA)-type glutamate receptors, a change in membrane potential could modulate the probability of inducing LTP. Consequently, a nonsynaptic change, such as a tonic depolarization of the resting potential produced by one trial of a conditioning procedure, could facilitate the induction of synaptic plasticity on a second trial.
Olfactory discrimination learning provides another example of how changes in excitability play an indirect, but relevant, role in memory formation. Several consecutive days of training were necessary for a rat to discriminate between a first pair of odors, but once it reached good performance, its ability to discriminate between a new pair of odors improved dramatically (i.e., rule learning) [50]. Both spike frequency adaptation and AHP amplitude were reduced in pyramidal neurons of the olfactory cortex following discrimination training [51,52]. These changes, detectable for 1–3 days after training, decayed to baseline values by 5–7 days after training [51,52], suggesting that they are involved in rule learning rather than memory storage per se [50].
Computational work has explored possible relationships between nonsynaptic changes and memory. In an artificial neural network containing excitability changes and Hebbian learning rules, the excitability of a neuron may serve as a “label” to identify it as recently active [53]. This mechanism could bridge the gaps between temporally separated stimuli and help explain aspects of trace conditioning (see above) [54]. A similar computational model suggested that changes in excitability could regulate the threshold for the induction of synaptic plasticity [55], thus proposing intrinsic plasticity as a mechanism to prime circuits to a more permissive state for modification during learning [50].
In summary, results from both vertebrates and invertebrates reveal learning-induced changes in excitability that can function as part of the memory trace, either as primers to promote the occurrence of further neuronal changes or as a transient storage mechanism active for a temporally limited phase of the memory.
Activity-dependent modulation of nonsynaptic properties
Nonsynaptic changes associated with LTP and LTD
Specific patterns of neuronal stimulation induce either enhancement (LTP) or reduction (LTD) of synaptic strength [56,57], cellular phenomena that are believed to underlie aspects of learning and memory [3,56,58,59]. Although research on LTP and LTD largely focuses on the mechanisms underlying modulation of synaptic strength, several lines of evidence indicate that both LTP and LTD are accompanied by modifications of intrinsic excitability.
Bliss and Lømo [60] found an increase in the population spike that could not be entirely explained by the increase in the EPSP. This nonsynaptic component of LTP was termed EPSP-to-spike potentiation (E-S potentiation) [60]. Since then, E-S potentiation has been found in several brain regions using a variety of LTP-inducing protocols (Figure 3a). Early studies supported the hypothesis that E-S potentiation represents an increase in the intrinsic excitability of the postsynaptic neurons through modulation of voltage-gated channels [63–65]. Detailed biophysical and biochemical analyses of E-S potentiation indicate that in CA1 pyramidal neurons, LTP-induced increased excitability was associated with a shift in the activation curve of voltage-gated Na+ channels (gNa) [66]. The changes in excitability shared a similar signaling pathway with LTP, including activation of NMDA receptors, Ca2+ influx and activity of CaMKII [66]. A similar LTP-induced increase in excitability was associated with a shift in the activation curve of gNa in cultured CA1 neurons [67]. However, other studies found that the increase in excitability produced by LTP was associated with a decrease of the hyperpolarization-activated current Ih [68,69]. A decrease in Ih would increase the membrane input resistance and, because of less shunting, enhance synaptic depolarization and the ability to elicit APs [68]. Similarly, a shift in the activation curve of gNa would reduce the AP threshold and increase excitability [66]. Therefore, LTP-induced increased excitability could be achieved by synergistic modulation of multiple conductances, including gNa and gh. In addition to the hippocampus, synergistic changes in synaptic strength and excitability were found in the cerebellar cortex. High-frequency stimulation of mossy fibers led to both LTP and an enhancement of intrinsic excitability of granule cells [70].
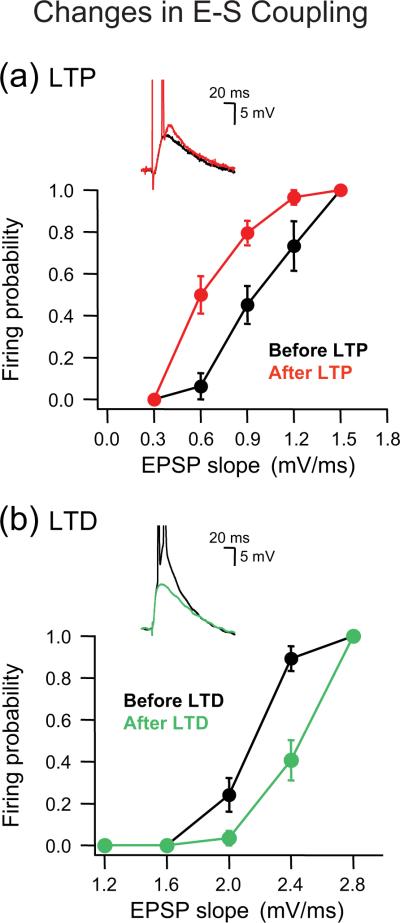
Figure 3.
Bidirectional changes in E-S coupling of CA1 hippocampal neurons associated with LTP and LTD. (a) Facilitation of dendritic integration followed LTP induction. Firing probability plotted as function of EPSP slope indicates that the E-S coupling curve (black circles, before LTP) shifted to the left after LTP induction (red circles, after LTP). The inset illustrates that an EPSP, subthreshold before LTP induction (black trace), became potentiated and generated spikes after LTP induction (red trace). (b) Depression of dendritic integration followed LTD induction. The E-S coupling curve (black circles, before LTD) generated as in (a) shifted to the right after LTD induction (green circles, after LTD). The inset illustrates that an EPSP, already suprathreshold to generate spikes before LTD induction (black trace), became depressed and insufficient to elicit spikes after LTD induction (green trace). Figure adapted from Ref. [73].
Apparent dissociations between changes in synaptic function and changes in excitability have also been found. In CA1 pyramidal neurons, subthreshold synaptic stimulation paired with back-propagating APs at theta frequency (i.e., theta-burst pairing TBP) produced both LTP and a decrease in somatic excitability due to an enhancement of Ih [71]. The increase in Ih decreased the membrane input resistance and reduced the ability to elicit APs [71]. The activity-dependent decrease in excitability was blocked by the Ih inhibitor ZD7288 without any effect on LTP [71]. Analogous decreased excitability was produced by a pattern of intracellular stimulation delivered at theta frequency (i.e., theta-burst firing) without pairing of the postsynaptic APs with synaptic stimulation [71].
Using a TBP similar to that used by Fan et al. [71], Campanac et al. also found an Ih-dependent decrease in excitability [68]. They hypothesized that this decrease in excitability opposite to LTP may represent a negative-feedback (homeostatic) mechanism to promote network stability, protecting the circuit from becoming saturated [68,71]. Whether LTP procedures increased or decreased excitability may depend on the degree of LTP. In CA1 neurons, near maximal TBP-induced LTP increased Ih and decreased excitability [68,71], whereas moderate LTP decreased Ih and produced E-S potentiation [68,69]. These findings suggest that differential regulation of Ih based on the degree of LTP could serve as a feedback mechanism to control neuronal activity. According to this model, strong LTP would limit neuronal activity via an increase in Ih, whereas moderate LTP would facilitate activity via a decrease in Ih.
LTD is also associated with changes in excitability. In CA1 neurons, LTD-induced reduction of the E-S coupling (Figure 3b) [69,72,73] was largely due to a decrease in excitability [72,74]. In contrast with the results by Daoudal et al. [72], Brager and Johnston found that LTD in CA1 neurons was associated with an increase in excitability due to an attenuation of Ih [75]. The discrepancies among LTD results have not been resolved, but one possible explanation may reside in the different induction protocols and in the varying degrees of LTD. The above examples emphasize the diversity of activity-dependent changes in excitability, both in terms of types of induction stimuli and molecular mechanisms, and revealed a complex relationship between changes in excitability and synaptic plasticity.
Other synaptically-driven nonsynaptic changes
Although the changes in excitability described above were expressed concurrently with modifications of synaptic strength (LTP or LTD), some examples exhibited alterations in excitability without apparent changes in synaptic strength. In CA1 neurons, E-S potentiation could be induced by short trains of synaptic stimulation of Schaffer collateral fibers at theta frequency [76]. Because the increase in E-S potentiation was not associated with a change in synaptic strength or with a generalized change in somatic excitability, these results suggest that a local increase in dendritic excitability can be produced by specific patterns of activity in the absence of synaptic plasticity. Neurons of the cerebellar deep nuclei exhibited a rapid, synaptically-driven increase in their excitability, which required Ca2+ influx through activation of NMDA receptors, but was not associated with synaptic facilitation [77]. The interneurons of the rat dentate gyrus exhibited a synaptically-driven long-term depolarization of their resting potential associated with an increase in the E-S coupling but not with synaptic potentiation of the perforant path [78]. This activity-dependent depolarization was produced by a decrease in the activity of the electrogenic Na+ pump [78] and required the rise of intracellular Ca2+ and activation of AMPA receptors. These results indicate that the modulation of excitability can contribute to information storage independently from synaptic plasticity. Similarly, in the leech, low-frequency repetitive stimulation of touch (T) neurons induced a Na+ pump-dependent increase in the amplitude of the AHP, which was associated with a persistent depression of the synaptic connections of T cells [79] and may be a cellular mechanism contributing to habituation. In contrast to repetitive stimulation, 5-HT, which mediates sensitization [14,80], reduced the AHP amplitude by inhibiting the Na+ pump [81].
The synaptic drive does not need to be excitatory to induce a change in intrinsic excitability. In the medial vestibular nucleus, brief periods of inhibitory synaptic input, or direct membrane hyperpolarization, triggered a long-lasting increase in excitability, termed firing rate potentiation [82]. Firing rate potentiation, which was due to a decrease in cytosolic Ca2+ that reduced CaMKII activity and attenuated BK-type IK,Ca [83], might contribute to motor learning in the vestibulo-ocular reflex.
Nonsynaptic changes independent from synaptic stimulation
As described above, changes in excitability induced by firing of CA1 pyramidal neurons were observed in the absence of synaptic stimulation [71]. Similarly to the Ih-dependent decrease in excitability induced by theta-burst firing, CA1 pyramidal neurons exhibit an activity-dependent decrease in firing when stimulated intracellularly at frequencies higher than theta [84]. High-frequency stimulation enhanced Ca2+ influx through CaV1/L-type Ca2+ channels, which potentiated the KV7/KCNQ K+ (M-type) channels function and reduced the firing frequency, thus serving as a homeostatic mechanism to regulate intrinsic excitability following prolonged high-frequency activity. Collectively, the results by Fan et al. [71] and Wu et al. [84] indicate that reduced excitability may be induced in the same neurons by different levels of neuronal activity through the modulation of distinct ion channels.
The effects of activity on excitability appear to differ in different brain regions. In the pyramidal neurons of layer V of the primary visual cortex, high-frequency intracellular depolarizations produced an enduring increase in excitability [85]. The increased excitability required Ca2+ entry and was not associated with changes in the passive membrane properties, suggesting the involvement of voltage-gated channels [85]. In the rat entorhinal cortex, persistent graded increases in firing frequency associated with a reduced slow AHP were induced in pyramidal neurons by repetitive depolarizing steps during blockage of glutamatergic and GABAergic neurotransmission in the presence of the cholinergic agonist carbachol [86]. These sustained levels of firing could be either increased or decreased in an input-specific manner and relied on activity-dependent changes of Ca2+-dependent cationic current [86]. This intrinsic ability of the neurons in entorhinal cortex to generate graded persistent activity has been proposed as a cellular mechanism for working memory [86]. Activity-dependent switching among stable states of activity has been reported in the endogenously bursting neuron R15 in Aplysia [87].
Site-specific changes in excitability
In contrast to mechanisms of memory storage based on changes in synaptic strength that have a potentially massive storage capacity, global changes in excitability would theoretically alter the throughput of all the synaptic inputs impinging on a neuron and have limited storage capacity. Recent findings may help to reconcile differences between models of memory storage based on synaptic vs. intrinsic plasticity. Dendritic integration (i.e, spatial and temporal summation of synaptic potentials at the dendrite) is also modulated in an activity-dependent manner in parallel with both LTP and LTD [69]. The linearity of the dendritic integration increased following induction of LTP and decreased following LTD. Importantly, these effects were restricted to the potentiated/depressed pathway [69]. Using changes in the EPSP amplitude/slope relationship to examine possible nonsynaptic changes associated with LTP and LTD, Campanac and Debanne showed that this parameter was facilitated after LTP (Figure 3a) and depressed after LTD (Figure 3b) [73]. These bidirectional modifications were specific to the synaptic input that was potentiated or depressed and were mimicked by blocking specific voltage-dependent channels, thus confirming the involvement of voltage-dependent nonsynaptic mechanisms in the plasticity of dendritic integration [73].
Simultaneous voltage recording and Ca2+ imaging from individual dendrites in CA1 neurons revealed that nonsynaptic plasticity could be spatially confined at the level of individual dendrites [88]. LTP induction was accompanied by a local increase in dendritic excitability that favored back propagation of APs into that dendritic region with a subsequent boost in Ca2+ influx [89]. A leftward shift in the inactivation curve of IA, and the consequent reduction in IA, accounted for the enhanced excitability [89]. This LTP-triggered increase in dendritic excitability was branch specific, localized at or in vicinity of the potentiated synaptic site [89,90], and required NMDA receptor activation and Ca2+ influx [91]. Local increase in dendritic excitability could facilitate propagation of synaptic potentials toward the site of AP initiation [88,90,92] and contribute to enhancing the coupling between synaptic potentials and spike, as observed following LTP induction. These results indicate that activity-dependent nonsynaptic changes can be expressed locally, even at individual dendritic branches [69,89,90], achieving degrees of specificity that resemble those observed for synaptic plasticity [4],
Data on CA1 pyramidal cells are still controversial as both local and cell-wide changes in excitability have been reported following LTP induction (see above) [71]. To reconcile this discrepancy, Sjöström et al. [92] suggested that compartment-specific changes in excitability may reflect distinct requirements for information storage. Local changes in excitability would favor synapse-specific synaptic plasticity [69,73,89–91]. For global changes in excitability, the degree of synaptic modification appears to determine the polarity of the nonsynaptic change. Moderate levels of synaptic plasticity produce nonsynaptic changes that would act synergistically with synaptic mechanisms [66,68]. Conversely, more robust levels of synaptic plasticity produce nonsynaptic changes that would act as a negative-feedback mechanism to enhance network stability in the presence of long-term synaptic plasticity, thus protecting the overall circuit activity from becoming saturated (following LTP) [71] or suppressed (following LTD) [75]. One aspect of activity-dependent nonsynaptic plasticity that requires further investigation is the specific role of distinct voltage-dependent conductances. Although at least three conductances, gNa, gh and gA, are implicated in activity-dependent changes in excitability of CA1 pyramidal neurons (see ref. [92] for recent review), the precise relationship among these conductances in increasing/decreasing neuronal excitability globally or locally is not understood.
Overall, these results indicate that excitability can be altered differently in different compartments within the same neuron down to the level of single dendritic branches [90], thus revealing degrees of modulation that are more complex and richer than originally envisioned.
Conclusions
Theories of memory storage have been inspired by the unique features of the synapse and its plasticity. However, analyses in both vertebrate and invertebrate model systems indicate that learning and memory, as well as patterns of electrical stimulation of neurons and neural pathways, also produce changes in excitability. These changes can be neuron wide or restricted to specific cellular compartments such as individual dendrites, thus affecting neuronal function and signal integration either globally or locally. The functional significance of the changes is less clear. Additional research is needed to assess the quantitative contribution and functional relevance of changes in intrinsic excitability (and synaptic plasticity) to specific examples of learning and memory.
Box 1 Measuring neuronal excitability.
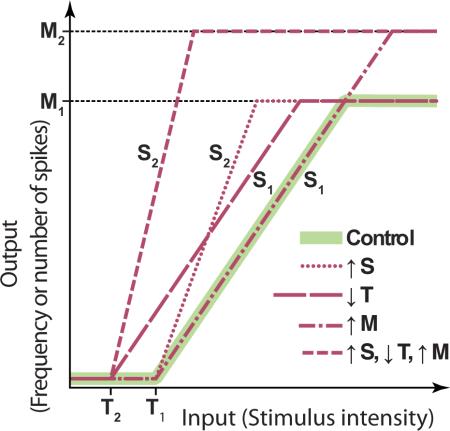
Excitability is a complex multidimensional phenomenon that describes the ways in which a neuron integrates and responds to stimuli. It depends on an ensemble of intrinsic properties including resting potential, leakage conductance (input resistance), membrane capacitance, membrane pumps, and time- and voltage- dependent membrane conductances. Demonstrating that changes in excitability are intrinsic to a neuron is a critical issue in the analysis of the mechanisms of learning and memory, although this stringent test has been performed only in a few of the many examples of learning-induced changes in excitability. Phenomenologically, excitability is frequently defined by using one or more of the parameters of the input-output (I-O) relationship of a neuron, which is determined by applying fixed-duration incremental depolarizing current pulses and counting the number of spikes elicited at each intensity. The I-O relationship can be roughly characterized by three parameters: a threshold (T) (i.e., the minimum amount of current or voltage required to elicit at least one spike), a slope term (S), and a maximum (M) (i.e., maximum number of spikes). The inset illustrates a hypothetical piecewise linear I-O relationship (green) and several ways in which a change in excitability could be expressed. The relationship shown is for a cell that is normally silent in the absence of stimulation (a neuron that has endogenous spontaneous spike activity would have an I-O relationship with T shifted to the left of the x axis). Learning or neuronal activity can shift the threshold (e.g., from T1 to T2), affect M (e.g., from M1 to M2) without altering T or S, or affect S (e.g., from S1 to S2) without altering M or T. Alternatively, a combination of these parameters can be modulated. In generating an I-O relationship, the choice of stimulus duration is generally made on an ad hoc basis. However, a I-O relationships can greatly dependent on the duration of the stimuli used to generate them because of the time-dependent activation and inactivation of the ionic conductances underlying neuronal excitability. Therefore, a change in excitability measured with one stimulus duration might not be detected with another. A corollary is that behavioral stimuli used to test memory may not “engage” a learning-induced excitability change unless the test stimuli lead to activation patterns similar to the artificial stimuli used to experimentally measure excitability. Therefore, it is difficult to interpret results when an experimentally measured excitability change does not correspond to a behavioral measure of memory. A limitation with many experimental analyses of excitability is the lack of a full exploration of the parameter space. In some cases only the threshold is examined and in other cases only the response to a single fixed-intensity stimulus. Consequently, excitability may be a more ubiquitous feature than currently appreciated. Finally, it should be noted that because of the complex geometry of neurons and the differential distribution of ion channels in different compartments and subcompartments of a neuron, any neuron could have a multiplicity of local I-O curves, each of which can be independently modulated.
Box 2 Outstanding questions.
What is the functional relevance of local vs. global changes in excitability on network activity and, also, on maintenance of the memory trace?
Can the occurrence of learning-dependent changes in excitability be specifically induced and blocked by pharmacological or genetic manipulations? If so, what are the consequences of these manipulations on the formation and maintenance of the memory trace?
To what extent are the molecular mechanisms underlying learning-dependent changes in excitability shared by those underlying learning-dependent synaptic plasticity?
Are changes in excitability, similar to those produced in vitro by patterns of neuronal activity, also produced by in vivo learning paradigms?
Acknowledgments
We thank D. Baxter, T. Crow, D. Johnston and M. Clarke for comments on an earlier draft of the manuscript. Supported by NIH grant MH58321.
Glossary
- Engram
is synonymous with memory trace and indicates the physical representation of a memory.
- Nonassociative learning
refers to a behavioral change that occurs in response to a single stimulus, or to two stimuli not temporally related. Habituation and sensitization are two examples of non associative learning.
- Habituation
is defined as the gradual decrease of a behavioral response to a weak or moderate stimulus that is presented repeatedly. Following habituation, the response may be restored to its initial state either passively with time (i.e., spontaneous recovery), or with the presentation of a novel stimulus (i.e., dishabituation).
- Sensitization
is defined as the enhancement of a behavioral response elicited by a weak stimulus following another, usually noxious stimulus. Sensitization can also develop in response to a moderate stimulus that is presented repeatedly at relatively short intervals.
- Associative learning
refers to the formation of an association either between two stimuli (i.e., classical conditioning), or between a behavior and a stimulus (i.e., operant conditioning).
- Classical conditioning
is the ability to associate a predictive stimulus with a subsequent salient event. In classical conditioning procedures, a novel or weak stimulus (conditioned stimulus; CS) is paired with a stimulus that generally elicits a reflexive response (unconditioned stimulus and response, respectively; US and UR). After sufficient training with contingent CS-US presentations (which may be a single trial), the CS becomes capable to elicit a response (conditioned response; CR), which often resembles the UR (or some aspect of it).
- Delay conditioning
is a classical conditioning procedure in which the US occurs during the presentation of the CS.
- Trace conditioning
is a classical conditioning procedure in which an interval (the trace) is imposed between the CS offset and the US onset. Following trace conditioning, an animal can learn to time its response to the CS according to the duration of the trace interval imposed between the CS and the US.
- Fear conditioning
is a form of classical conditioning in which an animal learns to express fear in response to a neutral stimulus (e.g. a tone; CS) after the stimulus is paired with an aversive event, such as an electrical shock (US).
- Operant conditioning
is a type of associative learning in which a behavior of an animal is followed by delivering either a desirable or an aversive stimulus, arranged by the experimenter. The desirable stimulus (e.g., food) will typically increase the future occurrence of the behavior (a process called positive reinforcement). An aversive stimulus (e.g., a noxious electric shock) will tend to decrease the future probability of the behavior (a process called punishment). A behavior can also be reinforced when it becomes contingent with the removal of an aversive stimulus from the animal's environment (i.e., negative reinforcement). Thus, through the processes of operant conditioning an animal learns the consequences of its behavior.
- Extinction
is an active process, by which a conditioned response becomes suppressed during repetitive presentations of the CS alone.
- Population spike
is the signal representing the summation of the evoked action potentials in postsynaptic neurons). LTP procedures can increase the amplitude of the population spike because more postsynaptic neurons are activated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Byrne JH. Cellular analysis of associative learning. Physiol. Rev. 1987;67:329–439. doi: 10.1152/physrev.1987.67.2.329. [DOI] [PubMed] [Google Scholar]
- 2.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 3.Martin SJ, Morris RG. New life in an old idea: the synaptic plasticity and memory hypothesis revisited. Hippocampus. 2002;12:609–636. doi: 10.1002/hipo.10107. [DOI] [PubMed] [Google Scholar]
- 4.Poirazi P, Mel BW. Impact of active dendrites and structural plasticity on the memory capacity of neural tissue. Neuron. 2001;29:779–796. doi: 10.1016/s0896-6273(01)00252-5. [DOI] [PubMed] [Google Scholar]
- 5.Fusi S, et al. Cascade models of synaptically stored memories. Neuron. 2005;45:599–611. doi: 10.1016/j.neuron.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Cajal S. R. y. Histologie du systeme nerveux. Vol. 2. Maline; Paris: 1911. [Google Scholar]
- 7.Tanzi E. I fatti e la induzioni nell'odierna istologia del sistema nervoso. Riv. Sper. Freniatr. Med. Leg. Alienazioni Met. Soc. Ital. Psichiatria. 1893;19:419–72. [Google Scholar]
- 8.Woody CD, Black-Cleworth P. Differences in excitability of cortical neurons as a function of motor projection in conditioned cats. J. Neurophysiol. 1973;36:1104–1116. doi: 10.1152/jn.1973.36.6.1104. [DOI] [PubMed] [Google Scholar]
- 9.Brons JF, Woody CD. Long-term changes in excitability of cortical neurons after Pavlovian conditioning and extinction. J. Neurophysiol. 1980;44:605–615. doi: 10.1152/jn.1980.44.3.605. [DOI] [PubMed] [Google Scholar]
- 10.Crow TJ, Alkon DL. Associative behavioral modification in Hermissenda: cellular correlates. Science. 1980;209:412–414. doi: 10.1126/science.209.4454.412. [DOI] [PubMed] [Google Scholar]
- 11.Alkon DL, et al. Primary changes of membrane currents during retention of associative learning. Science. 1982;215:693–695. doi: 10.1126/science.7058334. [DOI] [PubMed] [Google Scholar]
- 12.Alkon DL, et al. Reduction of two voltage-dependent K+ currents mediates retention of a learned association. Behav. Neural. Biol. 1985;44:278–300. doi: 10.1016/s0163-1047(85)90296-1. [DOI] [PubMed] [Google Scholar]
- 13.Farley J. Associative training results in persistent reductions in a calcium-activated potassium current in Hermissenda type B photoreceptors. Behav. Neurosci. 1988;102:784–802. [Google Scholar]
- 14.Sahley CL, et al. The S cell: an interneuron essential for sensitization and full dishabituation of leech shortening. J. Neurosci. 1994;14:6715–6721. doi: 10.1523/JNEUROSCI.14-11-06715.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burrell BD, et al. Non-associative learning and serotonin induce similar bidirectional changes in excitability of a neuron critical for learning in the medicinal leech. J. Neurosci. 2001;21:1401–1412. doi: 10.1523/JNEUROSCI.21-04-01401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JJ, Thompson RF. Cerebellar circuits and synaptic mechanisms involved in classical eyeblink conditioning. Trends Neurosci. 1997;20:177–181. doi: 10.1016/s0166-2236(96)10081-3. [DOI] [PubMed] [Google Scholar]
- 17.Schreurs BG, et al. Intracellular correlates of acquisition and long-term memory of classical conditioning in Purkinje cell dendrites in slices of rabbit cerebellar lobule HVI. J. Neurosci. 1998;18:5498–5507. doi: 10.1523/JNEUROSCI.18-14-05498.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolpaw JR. The complex structure of a simple memory. Trends Neurosci. 1997;20:588–894. doi: 10.1016/s0166-2236(97)01133-8. [DOI] [PubMed] [Google Scholar]
- 19.Carp JS, Wolpaw JR. Motoneuron plasticity underlying operantly conditioned decrease in primate H-reflex. J. Neurophysiol. 1994;72:431–442. doi: 10.1152/jn.1994.72.1.431. [DOI] [PubMed] [Google Scholar]
- 20.Nargeot R, et al. In vitro analog of operant conditioning in Aplysia. I. Contingent reinforcement modifies the functional dynamics of an identified neuron. J. Neurosci. 1999;19:2247–2260. doi: 10.1523/JNEUROSCI.19-06-02247.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nargeot R, et al. In vitro analog of operant conditioning in Aplysia. II. Modifications of the functional dynamics of an identified neuron contribute to motor pattern selection. J. Neurosci. 1999;19:2261–2272. doi: 10.1523/JNEUROSCI.19-06-02261.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brembs B, et al. Operant reward learning in Aplysia: Neuronal correlates and mechanisms. Science. 2002;296:1706–1709. doi: 10.1126/science.1069434. [DOI] [PubMed] [Google Scholar]
- 23.Mozzachiodi R, et al. Changes in neuronal excitability serve as a mechanism of long-term memory for operant conditioning. Nat. Neurosci. 2008;11:1146–1148. doi: 10.1038/nn.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorenzetti FD, et al. Molecular mechanisms underlying a cellular analog of operant reward learning. Neuron. 2008;59:815–828. doi: 10.1016/j.neuron.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cleary LJ, et al. Cellular correlates of long-term sensitization in Aplysia. J. Neurosci. 1998;18:5988–5998. doi: 10.1523/JNEUROSCI.18-15-05988.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walters ET, et al. Mechanoafferent neurons innervating tail of Aplysia. II. Modulation by sensitizing stimulation. J. Neurophysiol. 1983;50:1543–1559. doi: 10.1152/jn.1983.50.6.1543. [DOI] [PubMed] [Google Scholar]
- 27.Klein M, et al. Facilitatory transmitters and cAMP can modulate accommodation as well as transmitter release in Aplysia sensory neurons: evidence for parallel processing in a single cell. Proc. Natl. Acad. Sci. USA. 1986;83:7994–7998. doi: 10.1073/pnas.83.20.7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Critz SD, et al. Modulatory effects of serotonin, FMRFamide, and myomodulin on the duration of action potentials, excitability, and membrane currents in tail sensory neurons of Aplysia. J. Neurophysiol. 1991;66:1912–1926. doi: 10.1152/jn.1991.66.6.1912. [DOI] [PubMed] [Google Scholar]
- 29.Dale H, et al. Serotonin produces long-term changes in the excitability of Aplysia sensory neurons in culture that depend on new protein synthesis. J Neurosci. 1987;7:2232–2238. doi: 10.1523/JNEUROSCI.07-07-02232.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antonov I, et al. Activity-dependent presynaptic facilitation and Hebbian LTP are both required and interact during classical conditioning in Aplysia. Neuron. 2003;37:135–147. doi: 10.1016/s0896-6273(02)01129-7. [DOI] [PubMed] [Google Scholar]
- 31.Baxter DA, et al. Computational model of the serotonergic modulation of sensory neurons in Aplysia. J. Neurophysiol. 1999;82:2914–2935. doi: 10.1152/jn.1999.82.6.2914. [DOI] [PubMed] [Google Scholar]
- 32.Scholz KP, Byrne JH. Long-term sensitization in Aplysia: biophysical correlates in tail sensory neurons. Science. 1987;235:685–687. doi: 10.1126/science.2433766. [DOI] [PubMed] [Google Scholar]
- 33.Byrne JH, Kandel ER. Presynaptic facilitation revisited: state and time dependence. J. Neurosci. 1996;16:425–435. doi: 10.1523/JNEUROSCI.16-02-00425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gandhi CC, Matzel LD. Modulation of presynaptic action potential kinetics underlies synaptic facilitation of type B photoreceptors after associative conditioning in Hermissenda. J. Neurosci. 2000;20:2022–2035. doi: 10.1523/JNEUROSCI.20-05-02022.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frost WM, et al. Monosynaptic connections made by the sensory neurons of the gill and siphon-withdrawal reflex in Aplysia participate in the storage of long-term memory for sensitization. Proc. Natl. Acad. Sci. USA. 1985;82:8266–8269. doi: 10.1073/pnas.82.23.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wainwright ML, et al. Localized neuronal outgrowth induced by long-term sensitization training in Aplysia. J. Neurosci. 2002;22:4132–4141. doi: 10.1523/JNEUROSCI.22-10-04132.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wainwright ML, et al. Dissociation of morphological and physiological changes associated with long-term memory in Aplysia. J. Neurophysiol. 2004;92:2628–2632. doi: 10.1152/jn.00335.2004. [DOI] [PubMed] [Google Scholar]
- 38.Antzoulatos E, Byrne JH. Long-term sensitization training produces spike narrowing in Aplysia sensory neurons. J. Neurosci. 2007;27:676–683. doi: 10.1523/JNEUROSCI.4025-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenkranz JA, Grace AA. Dopamine-mediated modulation of odour-evoked amygdala potentials during Pavlovian conditioning. Nature. 2002;417:282–287. doi: 10.1038/417282a. [DOI] [PubMed] [Google Scholar]
- 40.Rogan MT, et al. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 2001;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- 41.Santini E, et al. Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. J. Neurosci. 2008;28:4028–4036. doi: 10.1523/JNEUROSCI.2623-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lorenzetti FD, et al. Classical and operant conditioning differentially modify the intrinsic properties of an identified neuron. Nat. Neurosci. 2006;9:17–19. doi: 10.1038/nn1593. [DOI] [PubMed] [Google Scholar]
- 43.Lechner HA, et al. Classical conditioning of feeding behavior in Aplysia: I. Behavioral analysis. J. Neurosci. 2000;20:3369–3376. doi: 10.1523/JNEUROSCI.20-09-03369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kemenes I, et al. Critical time-window for NO-cGMP-dependent long-term memory formation after one-trial appetitive conditioning. J. Neurosci. 2002;22:1414–1425. doi: 10.1523/JNEUROSCI.22-04-01414.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kemenes I, et al. Role of delayed nonsynaptic neuronal plasticity in long-term associative memory. Curr. Biol. 2006;16:1269–1279. doi: 10.1016/j.cub.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 46.Nikitin ES, et al. Persistent sodium current is a nonsynaptic substrate for long-term associative memory. Curr. Biol. 2008;18:1221–1226. doi: 10.1016/j.cub.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 47.Moyer JR, et al. Trace eyeblink conditioning increase CA1 excitability in a transient and learning specific manner. J. Neurosci. 1996;16:5536–5546. doi: 10.1523/JNEUROSCI.16-17-05536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson LT, et al. Transient changes in excitability of rabbit CA3 neurons with a time course appropriate to support memory consolidation. J. Neurophysiol. 1996;76:1836–1849. doi: 10.1152/jn.1996.76.3.1836. [DOI] [PubMed] [Google Scholar]
- 49.Coulter DA, et al. Classical conditioning reduces amplitude and duration of calcium-dependent afterhyperpolarization in rabbit hippocampal pyramidal cells. J. Neurophysiol. 1989;61:971–981. doi: 10.1152/jn.1989.61.5.971. [DOI] [PubMed] [Google Scholar]
- 50.Saar D, Barkai E. Long-term modifications in intrinsic neuronal properties and rule learning in rats. Eur. J. Neurosci. 2003;17:2727–2734. doi: 10.1046/j.1460-9568.2003.02699.x. [DOI] [PubMed] [Google Scholar]
- 51.Saar D, et al. Reduced after-hyperpolarization in rat piriform cortex pyramidal neurons is associated with increased learning capability during operant conditioning. Eur. J. Neurosci. 1998;10:1518–1523. doi: 10.1046/j.1460-9568.1998.00149.x. [DOI] [PubMed] [Google Scholar]
- 52.Saar D, et al. Long-lasting cholinergic modulation underlies rule learning in rats. J. Neurosci. 2001;21:1385–1392. doi: 10.1523/JNEUROSCI.21-04-01385.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Janowitz MK, van Rossum MC. Excitability changes that complement Hebbian learning. Network. 2006;17:31–41. doi: 10.1080/09548980500286797. [DOI] [PubMed] [Google Scholar]
- 54.Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn. Mem. 2003;10:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- 55.Triesch J. Synergies between intrinsic and synaptic plasticity mechanisms. Neural Comput. 2007;19:885–909. doi: 10.1162/neco.2007.19.4.885. [DOI] [PubMed] [Google Scholar]
- 56.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 57.Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol. Rev. 2001;81:1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- 58.Whitlock JR, et al. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 59.Pastalkova E, et al. Storage of spatial information by the maintenance mechanism of LTP. Science. 2007;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- 60.Bliss TV, Lømo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abraham WC, et al. Long-term potentiation involves enhanced synaptic excitation relative to synaptic inhibition in guinea-pig hippocampus. J. Physiol. 1987;394:367–380. doi: 10.1113/jphysiol.1987.sp016875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chavez-Noriega LE, et al. The EPSP-spike (E-S) component of long-term potentiation in the rat hippocampal slice is modulated by GABAergic but not cholinergic mechanisms. Neurosci. Lett. 1989;104:58–64. doi: 10.1016/0304-3940(89)90329-7. [DOI] [PubMed] [Google Scholar]
- 63.Taube JS, Schwartzkroin PA. Mechanisms of long-term potentiation: EPSP/spike dissociation, intradendritic recordings, and glutamate sensitivity. J. Neurosci. 1988;8:1632–1644. doi: 10.1523/JNEUROSCI.08-05-01632.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bernard C, Wheal HV. Simultaneous expression of excitatory postsynaptic potential/spike potentiation and excitatory postsynaptic potential/spike depression in the hippocampus. Neuroscience. 1995;67:73–82. doi: 10.1016/0306-4522(95)00008-7. [DOI] [PubMed] [Google Scholar]
- 65.Wathey JC, et al. Computer simulations of EPSP-spike (E-S) potentiation in hippocampal CA1 pyramidal cells. J. Neurosci. 1992;12:607–618. doi: 10.1523/JNEUROSCI.12-02-00607.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu J, et al. Activity-dependent long-term potentiation of intrinsic excitability in hippocampal CA1 pyramidal neurons. J. Neurosci. 2005;25:1750–1760. doi: 10.1523/JNEUROSCI.4217-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ganguly K, et al. Enhancement of presynaptic neuronal excitability by correlated presynaptic and postsynaptic spiking. Nat. Neurosci. 2000;3:1018–1026. doi: 10.1038/79838. [DOI] [PubMed] [Google Scholar]
- 68.Campanac E, et al. Downregulation of dendritic Ih in CA1 pyramidal neurons after LTP. J. Neurosci. 2008;28:8635–8643. doi: 10.1523/JNEUROSCI.1411-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Z, et al. Bidirectional changes in spatial dendritic integration accompanying long-term synaptic modifications. Neuron. 2003;37:463–972. doi: 10.1016/s0896-6273(02)01189-3. [DOI] [PubMed] [Google Scholar]
- 70.Armano S, et al. Long-term potentiation of intrinsic excitability at the mossy fiber-granule cell synapse of rat cerebellum. J. Neurosci. 2000;20:5208–5216. doi: 10.1523/JNEUROSCI.20-14-05208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fan Y, et al. Activity-dependent decrease of excitability in rat hippocampal neurons through increases in Ih. Nat. Neurosci. 2005;8:1542–1551. doi: 10.1038/nn1568. [DOI] [PubMed] [Google Scholar]
- 72.Daoudal G, et al. Bidirectional plasticity of excitatory postsynaptic potential (EPSP)-spike coupling in CA1 hippocampal pyramidal neurons. Proc. Natl. Acad. Sci. USA. 2002;99:14512–14517. doi: 10.1073/pnas.222546399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Campanac E, Debanne D. Spike timing-dependent plasticity: a learning rule for dendritic integration in rat CA1 pyramidal neurons. J. Physiol. 2008;586:779–793. doi: 10.1113/jphysiol.2007.147017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li CT, et al. Bidirectional modification of presynaptic neuronal excitability accompanying spike timing-dependent synaptic plasticity. Neuron. 2004;41:257–268. doi: 10.1016/s0896-6273(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 75.Brager DH, Johnston D. Plasticity of intrinsic excitability during long-term depression is mediated through mGluR-dependent changes in Ih in hippocampal CA1 pyramidal neurons. J. Neurosci. 2007;27:13926–13937. doi: 10.1523/JNEUROSCI.3520-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fink AI, O'Dell TJ. Short trains of theta frequency stimulation enhance CA1 pyramidal neuron excitability in the absence of synaptic potentiation. J. Neurosci. 2009;29:11203–11214. doi: 10.1523/JNEUROSCI.1450-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aizenman CD, Linden DJ. Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nat. Neurosci. 2000;3:109–111. doi: 10.1038/72049. [DOI] [PubMed] [Google Scholar]
- 78.Ross ST, Soltesz I. Long-term plasticity in interneurons of the dentate gyrus. Proc. Natl. Acad. Sci. USA. 2001;98:8874–8879. doi: 10.1073/pnas.141042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scuri R, et al. Activity-dependent increase of the AHP amplitude in T sensory neurons of the leech. J. Neurophysiol. 2002;88:2490–2500. doi: 10.1152/jn.01027.2001. [DOI] [PubMed] [Google Scholar]
- 80.Zaccardi ML, et al. Sensitization and dishabituation of swim induction in the Hirudo medicinalis: role of serotonin and cyclic AMP. Behav. Brain Res. 2004;153:317–326. doi: 10.1016/j.bbr.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 81.Catarsi S, Brunelli M. Serotonin depresses the after-hyperpolarization through the inhibition of the Na+/K+ electrogenic pump in T sensory neurones of the leech. J. Exp. Biol. 1991;155:261–273. doi: 10.1242/jeb.155.1.261. [DOI] [PubMed] [Google Scholar]
- 82.Nelson AB, et al. Long-lasting increases in intrinsic excitability triggered by inhibition. Neuron. 2003;40:609–620. doi: 10.1016/s0896-6273(03)00641-x. [DOI] [PubMed] [Google Scholar]
- 83.Nelson AB, et al. Decreases in CaMKII activity trigger persistent potentiation of intrinsic excitability in spontaneously firing vestibular nucleus neurons. Neuron. 2005;46:623–631. doi: 10.1016/j.neuron.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 84.Wu WW, et al. Coupling of L-type Ca2+ channels to KV7/KCNQ channels creates a novel, activity-dependent, homeostatic intrinsic plasticity. J. Neurophysiol. 2008;100:1897–1908. doi: 10.1152/jn.90346.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cudmore RH, Turrigiano GG. Long-term potentiation of intrinsic excitability in LV visual cortical neurons. J. Neurophysiol. 2004;92:341–348. doi: 10.1152/jn.01059.2003. [DOI] [PubMed] [Google Scholar]
- 86.Egorov AV, et al. Graded persistent activity in entorhinal cortex neurons. Nature. 2002;420:173–178. doi: 10.1038/nature01171. [DOI] [PubMed] [Google Scholar]
- 87.Lechner HA, et al. Bistability and its regulation by serotonin in the endogenously bursting neuron R15 in Aplysia. J. Neurophysiol. 1996;75:257–262. doi: 10.1152/jn.1996.75.2.957. [DOI] [PubMed] [Google Scholar]
- 88.Magee JC, Johnston D. Plasticity of dendritic function. Curr. Opin. Neurobiol. 2005;15:334–342. doi: 10.1016/j.conb.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 89.Frick A, et al. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nat. Neurosci. 2004;7:126–135. doi: 10.1038/nn1178. [DOI] [PubMed] [Google Scholar]
- 90.Losonczy A, et al. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature. 2008;452:436–441. doi: 10.1038/nature06725. [DOI] [PubMed] [Google Scholar]
- 91.Kim J, et al. Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons. Neuron. 2007;54:933–947. doi: 10.1016/j.neuron.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sjöström PJ, et al. Dendritic excitability and synaptic plasticity. Physiol. Rev. 2008;88:769–840. doi: 10.1152/physrev.00016.2007. [DOI] [PubMed] [Google Scholar]