Abstract
Retinal neovascularization is a leading cause of visual disability. Retinal diseases involving neovascularization all follow the same progression, beginning with vascular inflammatory reactions and injury of the vascular endothelium and ending with neovascularization, fibrosis and retinal detachment. Understanding the mechanisms underlying this process is critical to its prevention and treatment. Research using retinopathy models has revealed that the NOX2 NADPH oxidase has a key role in inducing production of reactive oxygen species and angiogenic cytokines and causing vascular inflammatory reactions and neovascularization. This prospective review addresses the potential role of the urea/ornithine pathway enzyme arginase in this process. Studies of peripheral vessels isolated from diabetic animals have shown that increased arginase activity causes vascular endothelial cell dysfunction by decreasing availability of L-arginine to endothelial cell nitric oxide synthase which decreases nitric oxide bioavailability and increases oxidative stress. Increasing arginase activity also increases formation of polyamines and proline, which can induce cell growth and fibrosis. Studies in models of retinopathy show that increases in oxidative stress and signs of vascular inflammation are correlated with increases in arginase activity and arginase 1 expression and that decreasing arginase expression or inhibiting its activity blocks these effects. Furthermore, the induction of arginase during retinopathy is blocked by knocking out NOX2 or inhibiting NADPH oxidase activity. These observations suggest that NADPH oxidase-induced activation of the arginase pathway has a key role in causing retinal vascular dysfunction during retinopathy. Limiting the actions of arginase could provide a new strategy for treating this potentially blinding condition.
Vascular inflammatory reactions and ischemic retinopathy
Cellular inflammation at the blood-microvascular endothelial cell interface is a common feature of retinal diseases characterized by hyperpermeablity and neovascularization. Recent studies in models of ischemic retinopathy suggest that inflammatory reactions play a key role in initiating vascular injury and dysfunction.[2, 28, 53, 98] Various chemokines and cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, vascular endothelial cell growth factor (VEGF) and monocyte chemotactic protein (MCP)-1 are upregulated in ischemic retinopathy and have been reported to cause pathologic inflammatory reactions [47, 49, 50, 54, 98]. The increase in inflammatory mediators results in increased endothelial cell expression of adhesion molecules, such as intercellular adhesion molecule (ICAM)-1 and platelet endothelial cell adhesion molecule (PECAM) and accumulation of adherent leukocytes in the retinal blood vessels (leukostasis) [48]. Stasis of leukocytes and their activation are thought to contribute to increases in vascular permeability and subsequent neovascularization.
Reactive oxygen species and ischemic retinopathy
Increased production of reactive oxygen species (ROS) has been strongly implicated in the pathogenesis of vascular inflammatory reactions, injury and dysfunction, both within and outside the retina [27, 33, 36, 40]. In models of diabetes oxidative stress can occur due to decreased activity of antioxidant enzymes [9, 67], formation of advanced glycation end products, glucose auto-oxidation [92] and activation of protein kinase C [20, 41]. All of these events have been suggested to be initiated by superoxide overproduction by the mitochondrial electron-transport chain [3]. However, data showing increased activity of NADPH oxidase in diabetic patients and animals and high glucose-treated endothelial cells [27, 32, 42, 81] suggest that NADPH oxidase is also an important source of ROS. Moreover, studies in models of endothelial cell dysfunction indicate that the mitochondria are a target of NADPH oxidase and that NADPH oxidase-dependent formation of peroxynitrite contributes to endothelial dysfunction by causing mitochondrial injury and activation of mitochondrial oxidase [23, 34].
The NADPH oxidase → ROS signaling pathway has been strongly implicated in vascular disease. Current literature suggests that the most relevant NOX isoforms in endothelial cells are NOX1, NOX2 and NOX4. NOX1 and 4 are expressed at higher levels than NOX2 in normal endothelial cells [82, 90]. However, studies have shown that NOX2 is critically involved in vascular inflammatory reactions in models of OIR, diabetic retinopathy as well as in endotoxin induced retinal inflammation [4–6]. Increased expression of the NADPH oxidase catalytic subunit NOX2 has been shown to be correlated with pathological angiogenesis and vascular inflammatory reactions in models of retinopathy [5, 6]. Inhibiting NADPH oxidase activity by deleting its catalytic subunit NOX2 or by specific inhibitors prevents vitreoretinal neovascularization in mice with oxygen-induced retinopathy (OIR) [5] as well as reducing cytokine expression, retinal inflammatory reactions and vascular injury in models of diabetic retinopathy and endotoxin-induced uveitis [6].
Activity of the inducible isoform of NO synthase is another prominent source of oxidative stress during inflammatory conditions, including retinopathy. The initial phase of the inflammatory reaction is characterized by expression of inducible NOS. This enzyme produces large quantities of NO (in the millimolar range), which protects against infections due to its cytostatic and cytotoxic activities towards pathogens [76]. Whereas NO produced at low levels by the activity of constitutive NOS isoforms (endothelial NOS and neuronal NOS) can mediate its effects directly by interactions with metal-containing proteins, high levels of NO produced by inducible NOS can act indirectly via the generation of reactive nitrogen oxide species (RNOS), which are formed through the interaction of NO with molecular oxygen or superoxide. High levels of RNOS can also cause tissue damage due to lipid peroxidation, DNA damage, oxidation of thiols and nitration of tyrosine residues [69, 96]
L-arginine, NOS and vascular dysfunction
Vascular endothelial cell dysfunction is defined as impaired endothelial cell-dependent relaxation due to decreased production and/or bioavailability of NO. NO production by endothelial NOS is critically involved in maintaining the integrity and stability of the vascular endothelium, preventing platelet aggregation and leukocyte adhesion and maintaining blood flow. Endothelial cell dysfunction is a prominent feature of diseases characterized by oxidative stress and vascular inflammatory reactions and has been noted in various disease models, including diabetes, ischemia/reperfusion injury and in a variety of organ beds including the retina [30, 62, 89, 99, 100].
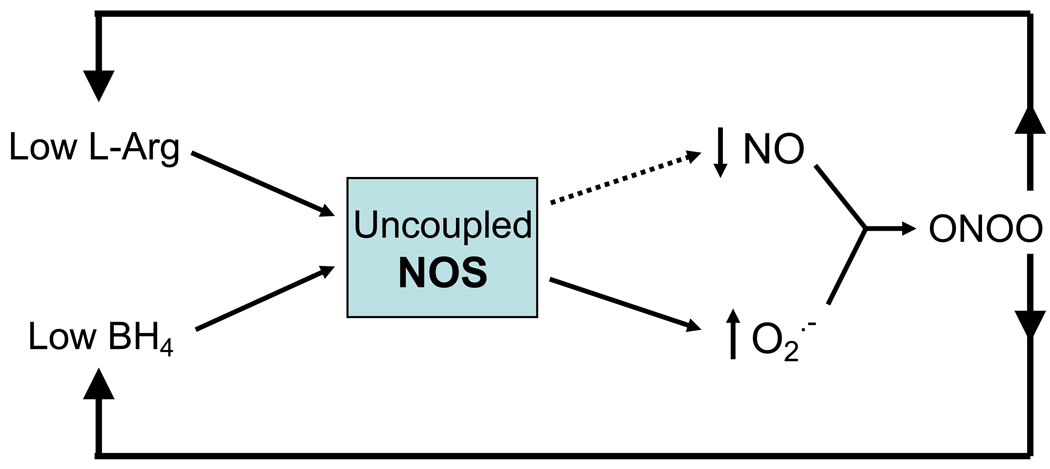
L-arginine is the substrate required by endothelial NOS along with oxygen to produce NO. Thus, maintaining an adequate cellular supply of L-arginine is critical for normal vascular function. Deficiencies in L-arginine supply have been strongly implicated in vascular diseases, including diabetes, hypertension, atherosclerosis and reperfusion injury [16, 19, 94]. If the supply of L-arginine does not meet the needs of active NOS, NOS becomes “uncoupled”, producing less NO and using more molecular oxygen as an alternative substrate to form superoxide instead of NO (Figure 1). This superoxide reacts very rapidly with NO to form the potent RNOS peroxynitrite. This state of imbalance between L-arginine supply and NOS activity can occur when cellular transport of L-arginine is inhibited, with prolonged and elevated NOS activity [1, 51], reduced recycling of L-citrulline back to L-arginine and/or elevated catabolism of L-arginine by arginase [8, 17, 95].
Figure 1.
Scheme showing immediate causes and effects of NOS uncoupling. Decreases in the NOS substrate L-Arg or cofactor tetrahydrobiopterin (BH4) results in decreases in NO and increases in superoxide (O2˙− ). The O2˙− reacts rapidly with NO to form the highly reactive nitrogen species peroxynitrite (ONOO ). This further decreases bioavailability of NO for downstream signaling events.
Supplemental L-arginine treatment has been reported to prevent endothelial cell dysfunction and restore endothelial cell-dependent vasodilation in diabetes [7, 64, 72], hypertension [77, 87], reperfusion injury [94] and heart failure [74] when given acutely. However, studies in animals and a recent clinical trial have found no benefit with chronic oral administration of supplemental L-arginine [22, 45, 65, 78, 85]. These contradictory results can be explained by enhanced hepatic catabolism of oral L-arginine by arginase to urea and ornithine when exposed to L-arginine loads [43, 68, 79, 91].
While the above data strongly implicate L-arginine deficiency in endothelial cell dysfunction, measurement of L-arginine levels in endothelial cells has shown that intracellular L-arginine concentration (0.1 – 1 mM) greatly exceeds the Km of endothelial NOS for L-arginine [~ 5 µM] [73]. Accordingly, endothelial NOS should be saturated with substrate under all but the most extreme conditions of L-arginine deficiency. This contradiction between the low Km of endothelial NOS for L-arginine and the positive effects of supplemental L-arginine in acutely reversing vascular dysfunction and augmenting the actions of endothelial NOS agonists has been termed the “L-arginine paradox.” This seeming conflict may be explained by data showing that L-arginine metabolism varies greatly within the endothelial cell due to intracellular compartmentalization and sequestration [21]. Studies showing that a complex exists between endothelial NOS and the major L-arginine system y+ transporter protein (CAT1) and that both molecules are located within plasma membrane caveolae [66] provide further evidence of L-arginine compartmentalization. Co-localization of arginase with endothelial NOS and neuronal NOS has also been reported [18, 84].
Arginine metabolism/arginase
L-arginine is metabolized by NOS to produce NO and L-citrulline. L-citrulline can be recycled back to L-arginine by argininosuccinate synthase and argininosuccinate lyase, which are co-localized in caveolae with endothelial NOS and the L-arginine transporter CAT1 [66, 80]. L-arginine is also substrate for arginase, which catalyzes conversion of L-arginine to ornithine and urea [44]. There are two arginase isoforms which are encoded by separate genes. Arginase 1 (Arg1) is a cytosolic enzyme that constitutes a majority of total body arginase activity. It is strongly expressed in the liver and is central to the urea cycle, but is also localized in other tissues. Arginase 2 (Arg2) is mainly a mitochondrial enzyme which is strongly expressed in the kidney, but is also present in other cells. Arg1 activation provides substrate for the ornithine decarboxylase (ODC) pathway, producing polyamines. Arg2 plays a role in the production of proline, a critical component of collagen, through the ornithine aminotransferase (OAT)/pyrroline-5-carboxylate reductase pathway [25, 59]. Both ODC and OAT pathways can promote cell growth. Therefore, elevated arginase activity is associated with excess cell growth and collagen accumulation--fibrosis.
Arginase and NOS activity
As a competitive consumer of L-arginine, arginase has been shown to inhibit the normal function of NOS in many diseases [24]. Competition between NOS and arginase for L-arginine within the cell to produce either NO or ornithine/urea is quite feasible given their individual enzymatic properties. Although the affinity of L-arginine is much higher for NOS (Km ~ 6 µM) than for arginase (Km ~ 5 mM), the maximum activity (Vmax) for arginase is greater than 1000 times that for NOS, indicating similar rates of substrate utilization at physiological L-arginine levels [11].
Decreased plasma levels of L-arginine have been reported in diabetic animals and patients [35, 71] and in vascular tissue of STZ-diabetic rats [71]. Increased arginase activity seems to be involved. Diabetic rats have elevated liver arginase activity, as well as elevated manganese content which may stimulate the enzyme [12, 83]. In diabetic patients, increased arginase activity has been reported in red blood cells [46] and in penile vessels and the latter alteration has been shown to be associated with erectile dysfunction [10]. Inhibition of arginase has also been shown to block endotoxin or TNF-α induced leukocyte binding to cultured endothelial cells in vitro [38]. Studies also have shown elevated arginase activity and expression in retinas of streptozotocin-induced diabetic mice and endotoxin-treated mice which are correlated with signs of retinal inflammation and vascular dysfunction and apparent decreases in NO formation and/or bioavailability [14, 101].
Studies indicate that NO has a dual role in physiological and pathological responses depending on the specific source and amount produced. The constitutively expressed NOSs, endothelial NOS and neuronal NOS, are calcium-dependent and regulated to produce low levels of NO. NO from endothelial NOS is responsible for maintaining blood flow and preventing platelet aggregation and leukostasis [57]. NO from neuronal NOS is involved in neural signaling, but is also expressed in smooth muscle cells where it has a key role in regulating vascular responses to tissue hypoxia [93]. On the other hand, inducible NOS is calcium-independent, constitutively active and produces large amounts of NO. It is not expressed in normal retinas, but is induced in retinal glia and microglial cells during disease and inflammatory conditions.
In the normal wound healing process associated with inflammatory conditions, the activity of inducible NOS and arginase are temporally regulated. The early phase of the inflammatory response is characterized by activity of inducible NOS in generating large amounts of NO needed to eliminate pathogens. The later repair phase is characterized by activation of arginase which converts arginine to ornithine and urea. As explained above, ornithine is converted to polyamines which promote cell growth and to proline which is required for collagen synthesis. Thus, activity of arginase has a key role in cell growth and extracellular matrix formation during the wound healing process. Under normal conditions the balance between the activity of inducible NOS and arginase is reciprocally regulated. For example, NOS metabolism of L-arginine can control arginase activity through the production of NG-hydroxyl-L-arginine (NOHA), intermediate product in the generation of NO and potent inhibitor of arginase. NOS-derived NO has been found to inhibit the function of the arginase pathway in generating polyamines due to nitrosylation of ODC which inhibits its activity reversibly [76]. NO can also dampen inflammatory reactions by causing nitrosylation of the NF-κB subunits p65 and p50. This reduces their DNA binding which in turn decreases transcription of inducible NOS and other inflammatory mediators [18, 52, 63]. On the other hand excessive arginase activity can reduce L-arginine availability to NOS, leading to uncoupling which would limit the production of NOHA and NO, reducing nitrosylation and thereby releasing arginase and other inflammatory mediators from normal feed-back control regulation.
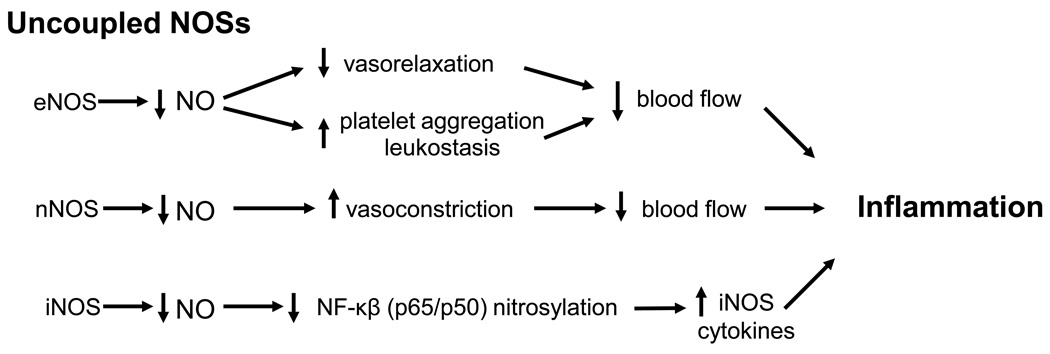
In summary, NOS uncoupling can contribute to disease progression by reducing bioavailable NO and by increasing reactive oxygen and nitrogen species which can have multiple adverse effects. Uncoupling of endothelial NOS can contribute to ischemia/hypoxia and tissue damage by reducing endothelial cell-dependent vasorelaxation and inducing platelet aggregation and leukostasis, whereas uncoupling of neuronal NOS could exacerbate the damage by altering smooth muscle cell responses to hypoxia, leading to vasoconstriction and further hypoxia/ischemia (Figure 2, Table 1). Uncoupling of inducible NOS could increase NF-κB transcriptional activity due decreased nitrosylation of p65 and p50 which would further increase the formation of inducible NOS and other inflammatory mediators. In addition, NOS uncoupling could further enhance activity of the arginase/polyamine pathway by reducing NO-mediated nitrosylation and NOHA formation. Finally, NO from all three NOSs will react rapidly with superoxide to form peroxynitrate, a highly reactive inflammatory and toxic molecule [86].
Figure 2.
Suggested mechanisms by which uncoupling of endothelial NOS (eNOS), neuronal NOS (nNOS) and inducible NOS (iNOS) contributes to inflammation and tissue injury.
Table 1.
Effects of excessive arginase activity
| 1. Reduces the NOS substrate L-arginine, leading to decreased NO production and increased production of O2˙− by uncoupled NOS. |
|
| 2. Increased levels of arginase products – ornithine, polyamines and proline. |
|
| Results – pathological vascular growth and fibrosis. |
Arginase and retinopathy
Ischemic retinopathies are characterized by a progression of vascular damage, beginning with inflammatory reactions, endothelial cell dysfunction and reduced blood flow, which can lead to pathological neovascularization and fibrovascular scarring, culminating in retinal detachment and blindness (for review, please see [29]). Based on the known function of arginase in other tissues, excessive arginase activity could play role at each of these stages. For example, during the inflammatory stage, excessive arginase activity could enhance cytokine formation by limiting NO production by iNOS which would reduce nitrosation of the NF-κB subunits, thereby blocking the normal feedback inhibition of NF-κB activity. In addition, excessive arginase activity at the level of the retinal endothelial and smooth muscle cells could exacerbate retinal inflammation by reducing NO production by endothelial and neuronal NOS which would decrease blood flow and promote platelet aggregation and leukocyte adhesion to the vessel wall. The ensuing retinal ischemia would further increase the retinal injury and could contribute to pathological neovascular growth due to the induction of VEGF expression. Excessive arginase activity could also contribute to pathological vascular growth and fibrovascular scarring by increasing the formation of polyamines and proline, which promote cell growth and collagen formation, respectively.
The concept that over-active arginase causes retinal disease by reducing NO formation may appear paradoxical given that numerous groups have reported increased levels of NO products (nitrate/nitrite and the peroxynitrite biomarker nitrotyrosine) in models of diabetic retinopathy, EIU and OIR [13, 53, 56, 97]. Studies showing NOS gene deletion or treatment with NOS inhibitors decreases peroxynitrite formation and reduces signs of EIU [31] diabetic retinopathy [26, 53, 58, 102] and OIR [13] strongly support the role of nitrative stress in retinopathy. However, it is important to note that most studies showing increased “NO levels” during retinopathy have used photometric assays to measure total tissue nitrite levels after reduction of nitrate to nitrite. Given that nitrate formation is favored in the presence of excess superoxide[15], the values obtained in such analyses represent relative levels of total NO formed rather than bio-available NO. Recent studies using animal and tissue culture models of diabetic retinopathy indicate that the amount of bioavailable NO is substantially diminished by high glucose or diabetes whereas NOS-dependent formation of superoxide is increased, implying that NOS is uncoupled [14]. Furthermore, the decline in NO is blocked by treatment with a specific arginase inhibitor, consistent with the proposed action of arginase in causing NOS uncoupling.
Surprisingly, in spite of the well established role of arginase in inflammation, vascular dysfunction, pathological cell growth and fibrosis, almost nothing is known about its role in retinopathy. A PubMed search on arginase and retina revealed only 9 papers published between 1974 and 2009. Most of these are descriptive reports on the distribution of the enzymes of arginine metabolism in ocular structures. However, one paper describes the co-induction of inducible NOS and other arginine metabolic enzymes in rats with EIU [55]. Another reports on the role for polyamines in excitotoxic cell death [70]. In both reports, Arg1 was increased and localized mainly to Muller cells. Polyamines have been shown to be toxic for microglial cells, but not for astrocytes or Muller cells [70, 88]. While the specific role of arginase activity in retinal fibrosis has not been investigated, arginase provides the substrate for the collagen precursor proline and appears to have a critical role in excess collagen deposition and coronary fibrosis in a mouse model of diabetic heart disease [39]. As explained above, the actions of polyamines in promoting vascular growth are well known. In agreement, studies have shown that polyamine antagonists block both retinal and choroidal angiogenesis [60, 61]. Further work is necessary to determine the specific role of arginase activity in causing increased collagen formation and retinal fibrosis in models of retinopathy.
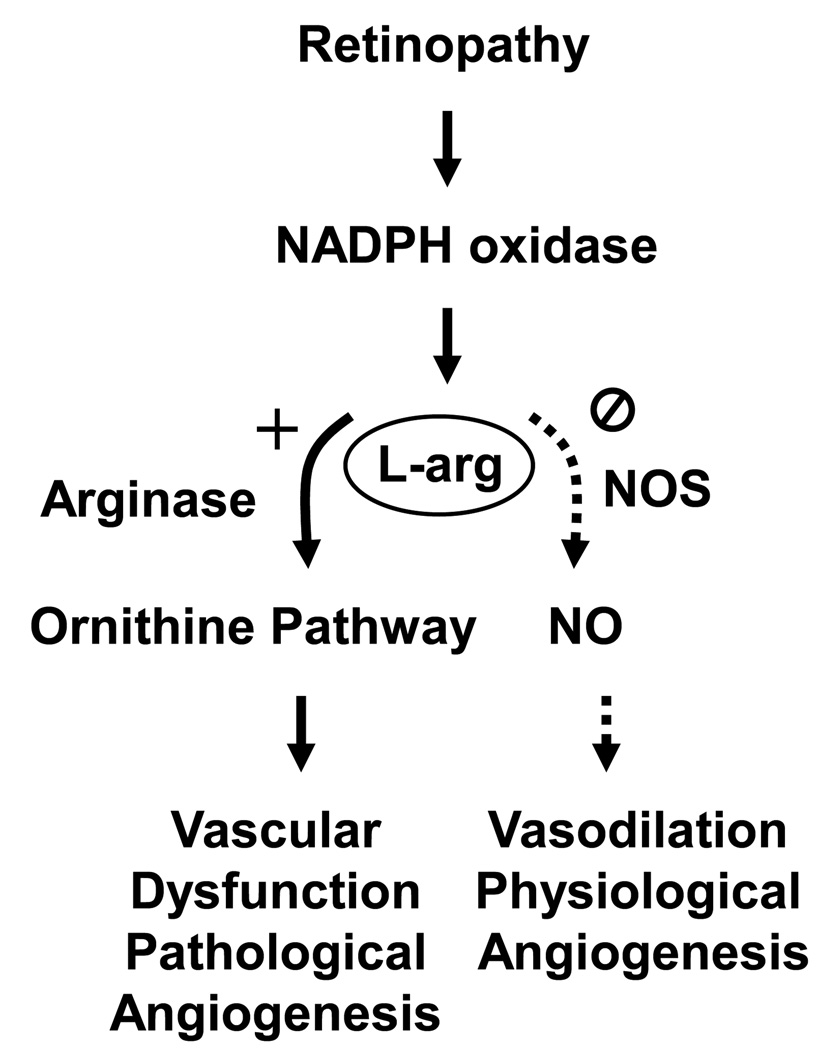
The potential role of altered eNOS function in retinopathy has been investigated in several models. Impairment of endothelial cell-dependent vasorelaxation to acetylcholine is clearly evident in retinal arteries of streptozotocin-induced diabetic rats as compared with non-diabetic controls [37, 100]. Based on the discovery that diabetes causes endothelial NOS uncoupling and vascular endothelial cell dysfunction by activating arginase and thereby decreasing availability of L-arginine to endothelial NOS, decreasing NO formation and increasing ROS [75], studies of diabetes-induced endothelial cell dysfunction were undertaken in aortas from double transgenic mice completely deficient in Arg2 (Arg2−/−) and partially deficient in Arg1 (Arg1+/−). [Complete deletion of Arg1 is lethal at 2 – 3 wks]. The results of this analysis showed that diabetes-induced aortic endothelial cell dysfunction is abrogated in the diabetic Arg1+/−Arg2−/− mice [39]. This result suggests that activity of arginase has a fundamental role in diabetes-induced vascular injury. Moreover, studies in a mouse model for oxygen-induced retinopathy indicate that inhibiting arginase activity reduced vitreoretinal neovascularization (Zhang and Caldwell, in progress). Given that each of the ischemic retinopathies follows the same pathological progression, beginning with reduced blood flow and signs of vascular endothelial cell injury and inflammation and progressing to neovascularization and fibrosis, it is likely that activation of the arginase pathway is critically involved. This hypothesis has been supported by studies showing that arginase activity and Arg1 expression are increased in models of EIU and diabetic retinopathy and in cultured retinal endothelial cells, Muller cells and microglial cells exposed to high glucose or cytokines and that Arg2 is not affected [14, 101]. Studies using retinal models also indicate that increases in arginase activity and Arg1 expression are correlated with increases in cytokine expression and with increased oxidative stress, reduced NO, vascular dysfunction and pathological angiogenesis. Furthermore, deletion of one copy of the Arg1 gene and both copies of Arg2 decreases cytokine production in both diabetic retinopathy and EIU [14, 101]. Further studies demonstrate that the increase in Arg1 is abrogated in mice that lack the catalytic subunit of NADPH oxidase NOX2. Cytokine-induced expression of Arg1 was also blocked in cells treated with an inhibitor of NADPH oxidase, providing further support for the potential role of NADPH oxidase derived ROS in increasing arginase activity. Taken together, these observations suggest that NADPH oxidase-induced activation of the arginase pathway has a key role in the progression from retinal vascular dysfunction, to pathological angiogenesis and retinal fibrosis during ischemic retinopathy (Figure 3).
Figure 3.
Working model showing proposed mechanisms underlying pathological angiogenesis during ischemic retinopathy. Abbreviations: NOS: nitric oxide synthase, NO: nitric oxide, L-Arg: L-arginine
Conclusion
Recent studies showing that arginase activity is required for the development of retinal inflammatory reactions in models of diabetes, endotoxin-induced inflammation and OIR imply a role for the arginase pathway in retinopathy. The observation that NOX2-dependent increases in oxidative stress are involved in increasing arginase activity implies a molecular link between NADPH oxidase-dependent oxidative stress, overactive arginase and ischemic retinopathy. The hypothesis that activation of arginase is involved in retinal vascular injury is a new concept in the field of retinopathy research. Further study is required to confirm these observations and define the specific mechanisms that regulate arginase expression and activity in the retina. This work could help to explain the progression from sub-clinical vascular inflammation to vascular injury and retinopathy. Given that the early signs of retinopathy are shared by blinding retinal diseases that affect children, working aged adults and the elderly [29], further investigation of the arginase pathway is highly significant to the development of new treatments and strategies for clinical intervention.
Acknowledgments
This work was supported by National Eye Institute Grants R01 EY04618 and R01 EY11766, and Veterans Administration Merit Review Award (R.B.C.); National Heart, Lung, and Blood Institute Grant R01 HL70215 (R.W.C); the Greater Southeast Affiliate's Postdoctoral Fellowship AHA0725604B (W. Z.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
References
- 1.Abou-Mohamed G, Johnson JA, Jin L, El-Remessy AB, Do K, Kaesemeyer WH, Caldwell RB, Caldwell RW. Roles of superoxide, peroxynitrite, and protein kinase C in the development of tolerance to nitroglycerin. J Pharmacol Exp Ther. 2004;308:289–299. doi: 10.1124/jpet.103.056119. [DOI] [PubMed] [Google Scholar]
- 2.Adamis AP. Is diabetic retinopathy an inflammatory disease? The British journal of ophthalmology. 2002;86:363–365. doi: 10.1136/bjo.86.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aiello LP, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, 3rd, Klein R. Diabetic retinopathy. Diabetes Care. 1998;21:143–156. doi: 10.2337/diacare.21.1.143. [DOI] [PubMed] [Google Scholar]
- 4.Al-Shabrawey M, Bartoli M, El-Remessy AB, Ma G, Matragoon S, Lemtalsi T, Caldwell RW, Caldwell RB. Role of NADPH Oxidase and STAT3 in Statin-mediated Protection Against Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 2008;49:3231–3238. doi: 10.1167/iovs.08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Shabrawey M, Bartoli M, El-Remessy AB, Platt DH, Matragoon S, Behzadian MA, Caldwell RW, Caldwell RB. Inhibition of NAD(P)H oxidase activity blocks vascular endothelial growth factor overexpression and neovascularization during ischemic retinopathy. The American journal of pathology. 2005;167:599–607. doi: 10.1016/S0002-9440(10)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Shabrawey M, Rojas M, Sanders T, Behzadian MA, El-Remessy AB, Bartoli M, Parpia AK, Liou G, Caldwell RB. Role of NADPH Oxidase in Retinal Vascular Inflammation. Invest. Ophthalmol. Vis. Sci. 2008;49:3239–3244. doi: 10.1167/iovs.08-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angulo J, Rodriguez-Manas L, Peiro C, Neira M, Marin J, Sanchez-Ferrer CF. Impairment of nitric oxide-mediated relaxations in anaesthetized autoperfused streptozotocin-induced diabetic rats. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:529–537. doi: 10.1007/pl00005289. [DOI] [PubMed] [Google Scholar]
- 8.Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Champion HC, Hare JM. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108:2000–2006. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia S, Shukla R, Venkata Madhu S, Kaur Gambhir J, Madhava Prabhu K. Antioxidant status, lipid peroxidation and nitric oxide end products in patients of type 2 diabetes mellitus with nephropathy. Clin Biochem. 2003;36:557–562. doi: 10.1016/s0009-9120(03)00094-8. [DOI] [PubMed] [Google Scholar]
- 10.Bivalacqua TJ, Hellstrom WJ, Kadowitz PJ, Champion HC. Increased expression of arginase II in human diabetic corpus cavernosum: in diabetic-associated erectile dysfunction. Biochem Biophys Res Commun. 2001;283:923–927. doi: 10.1006/bbrc.2001.4874. [DOI] [PubMed] [Google Scholar]
- 11.Boger RH. Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the "L-arginine paradox" and acts as a novel cardiovascular risk factor. J Nutr. 2004;134:2842S–2847S. doi: 10.1093/jn/134.10.2842S. discussion 2853S. [DOI] [PubMed] [Google Scholar]
- 12.Bond JS, Failla ML, Unger DF. Elevated manganese concentration and arginase activity in livers of streptozotocin-induced diabetic rats. J Biol Chem. 1983;258:8004–8009. [PubMed] [Google Scholar]
- 13.Brooks SE, Gu X, Samuel S, Marcus DM, Bartoli M, Huang PL, Caldwell RB. Reduced severity of oxygen-induced retinopathy in eNOS-deficient mice. Investigative ophthalmology & visual science. 2001;42:222–228. [PubMed] [Google Scholar]
- 14.Caldwell RB, Zhang W, Rojas M, Bartoli M, El-Remessy AB, Tsai N, Lemtalsi T, Iddings J, Romero MJ, Caldwell RW. Impaired nitric oxide bioavailability in diabetic retinopathy: Involvement of arginase activity. Investigative ophthalmology & visual science. 2009;49 E Abstract. [Google Scholar]
- 15.Chen B, Keshive M, Deen WM. Diffusion and reaction of nitric oxide in suspension cell cultures. Biophysical journal. 1998;75:745–754. doi: 10.1016/S0006-3495(98)77564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen PY, Sanders PW. L-arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J Clin Invest. 1991;88:1559–1567. doi: 10.1172/JCI115467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chicoine LG, Paffett ML, Young TL, Nelin LD. Arginase inhibition increases nitric oxide production in bovine pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L60–L68. doi: 10.1152/ajplung.00194.2003. [DOI] [PubMed] [Google Scholar]
- 18.Ckless K, van der Vliet A, Janssen-Heininger Y. Oxidative-nitrosative stress and post-translational protein modifications: implications to lung structure-function relations. Arginase modulates NF-kappaB activity via a nitric oxide-dependent mechanism. American journal of respiratory cell and molecular biology. 2007;36:645–653. doi: 10.1165/rcmb.2006-0329SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Creager MA, Cooke JP, Mendelsohn ME, Gallagher SJ, Coleman SM, Loscalzo J, Dzau VJ. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest. 1990;86:228–234. doi: 10.1172/JCI114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curtis TM, Scholfield CN. The role of lipids and protein kinase Cs in the pathogenesis of diabetic retinopathy. Diabetes Metab Res Rev. 2004;20:28–43. doi: 10.1002/dmrr.431. [DOI] [PubMed] [Google Scholar]
- 21.Cynober L, Le Boucher J, Vasson M. Arginine metabolism in mammals. J. Nutr. Biochem. 1995;6:402–413. [Google Scholar]
- 22.De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 24.Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clinical and experimental pharmacology & physiology. 2007;34:906–911. doi: 10.1111/j.1440-1681.2007.04638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durante W, Liao L, Reyna SV, Peyton KJ, Schafer AI. Transforming growth factor-beta(1) stimulates L-arginine transport and metabolism in vascular smooth muscle cells: role in polyamine and collagen synthesis. Circulation. 2001;103:1121–1127. doi: 10.1161/01.cir.103.8.1121. [DOI] [PubMed] [Google Scholar]
- 26.El-Remessy AB, Behzadian MA, Abou-Mohamed G, Franklin T, Caldwell RW, Caldwell RB. Experimental diabetes causes breakdown of the blood-retina barrier by a mechanism involving tyrosine nitration and increases in expression of vascular endothelial growth factor and urokinase plasminogen activator receptor. The American journal of pathology. 2003;162:1995–2004. doi: 10.1016/S0002-9440(10)64332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellis EA, Guberski DL, Somogyi-Mann M, Grant MB. Increased H2O2, vascular endothelial growth factor and receptors in the retina of the BBZ/Wor diabetic rat. Free Radic Biol Med. 2000;28:91–101. doi: 10.1016/s0891-5849(99)00216-6. [DOI] [PubMed] [Google Scholar]
- 28.Ferguson TA, Apte RS. Angiogenesis in eye disease: immunity gained or immunity lost? Seminars in immunopathology. 2008;30:111–119. doi: 10.1007/s00281-008-0113-8. [DOI] [PubMed] [Google Scholar]
- 29.Friedlander M. Fibrosis and diseases of the eye. J Clin Invest. 2007;117:576–586. doi: 10.1172/JCI31030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garhofer G, Zawinka C, Resch H, Kothy P, Schmetterer L, Dorner GT. Reduced response of retinal vessel diameters to flicker stimulation in patients with diabetes. The British journal of ophthalmology. 2004;88:887–891. doi: 10.1136/bjo.2003.033548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goureau O, Bellot J, Thillaye B, Courtois Y, de Kozak Y. Increased nitric oxide production in endotoxin-induced uveitis. Reduction of uveitis by an inhibitor of nitric oxide synthase. J Immunol. 1995;154:6518–6523. [PubMed] [Google Scholar]
- 32.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 33.U.P.D.S.U. Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 34.Guzik TJ, Harrison DG. Vascular NADPH oxidases as drug targets for novel antioxidant strategies. Drug discovery today. 2006;11:524–533. doi: 10.1016/j.drudis.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Hagenfeldt L, Dahlquist G, Persson B. Plasma amino acids in relation to metabolic control in insulin-dependent diabetic children. Acta Paediatr Scand. 1989;78:278–282. doi: 10.1111/j.1651-2227.1989.tb11070.x. [DOI] [PubMed] [Google Scholar]
- 36.Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:E14–E22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- 37.Horio N, Clermont AC, Abiko A, Abiko T, Shoelson BD, Bursell SE, Feener EP. Angiotensin AT(1) receptor antagonism normalizes retinal blood flow and acetylcholine-induced vasodilatation in normotensive diabetic rats. Diabetologia. 2004;47:113–123. doi: 10.1007/s00125-003-1262-x. [DOI] [PubMed] [Google Scholar]
- 38.Horowitz S, Binion DG, Nelson VM, Kanaa Y, Javadi P, Lazarova Z, Andrekopoulos C, Kalyanaraman B, Otterson MF, Rafiee P. Increased arginase activity and endothelial dysfunction in human inflammatory bowel disease. American journal of physiology. 2007;292:G1323–G1336. doi: 10.1152/ajpgi.00499.2006. [DOI] [PubMed] [Google Scholar]
- 39.Iddings JA, Romero MJ, Caldwell RB, Caldwell RW. Arginase I is Involved in Diabetes-Induced Vascular Endothelial Dysfunction. Circulation. 2008;118:S-552. [Google Scholar]
- 40.Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 41.Inoguchi T, Sonta T, Tsubouchi H, Etoh T, Kakimoto M, Sonoda N, Sato N, Sekiguchi N, Kobayashi K, Sumimoto H, Utsumi H, Nawata H. Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: role of vascular NAD(P)H oxidase. J Am Soc Nephrol. 2003;14:S227–S232. doi: 10.1097/01.asn.0000077407.90309.65. [DOI] [PubMed] [Google Scholar]
- 42.Inoguchi T, Tsubouchi H, Etoh T, Kakimoto M, Sonta T, Utsumi H, Sumimoto H, Yu HY, Sonoda N, Inuo M, Sato N, Sekiguchi N, Kobayashi K, Nawata H. A possible target of antioxidative therapy for diabetic vascular complications-vascular NAD(P)H oxidase. Curr Med Chem. 2003;10:1759–1764. doi: 10.2174/0929867033457133. [DOI] [PubMed] [Google Scholar]
- 43.Jean C, Rome S, Mathe V, Huneau JF, Aattouri N, Fromentin G, Achagiotis CL, Tome D. Metabolic evidence for adaptation to a high protein diet in rats. J Nutr. 2001;131:91–98. doi: 10.1093/jn/131.1.91. [DOI] [PubMed] [Google Scholar]
- 44.Jenkinson CP, Grody WW, Cederbaum SD. Comparative properties of arginases. Comparative biochemistry and physiology. 1996;114:107–132. doi: 10.1016/0305-0491(95)02138-8. [DOI] [PubMed] [Google Scholar]
- 45.Jeremy RW, McCarron H, Sullivan D. Effects of dietary L-arginine on atherosclerosis and endothelium-dependent vasodilatation in the hypercholesterolemic rabbit. Response according to treatment duration, anatomic site, and sex. Circulation. 1996;94:498–506. doi: 10.1161/01.cir.94.3.498. [DOI] [PubMed] [Google Scholar]
- 46.Jiang M, Jia L, Jiang W, Hu X, Zhou H, Gao X, Lu Z, Zhang Z. Protein disregulation in red blood cell membranes of type 2 diabetic patients. Biochem Biophys Res Commun. 2003;309:196–200. doi: 10.1016/s0006-291x(03)01559-6. [DOI] [PubMed] [Google Scholar]
- 47.Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. The American journal of pathology. 2001;158:147–152. doi: 10.1016/S0002-9440(10)63952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B, Kern TS, Adamis AP. A central role for inflammation in the pathogenesis of diabetic retinopathy. Faseb J. 2004;18:1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 49.Joussen AM, Poulaki V, Mitsiades N, Kirchhof B, Koizumi K, Dohmen S, Adamis AP. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. Faseb J. 2002;16:438–440. doi: 10.1096/fj.01-0707fje. [DOI] [PubMed] [Google Scholar]
- 50.Joussen AM, Poulaki V, Qin W, Kirchhof B, Mitsiades N, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP. Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. The American journal of pathology. 2002;160:501–509. doi: 10.1016/S0002-9440(10)64869-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaesemeyer WH, Ogonowski AA, Jin L, Caldwell RB, Caldwell RW. Endothelial nitric oxide synthase is a site of superoxide synthesis in endothelial cells treated with glyceryl trinitrate. Br J Pharmacol. 2000;131:1019–1023. doi: 10.1038/sj.bjp.0703665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kelleher ZT, Matsumoto A, Stamler JS, Marshall HE. NOS2 regulation of NF-kappaB by S-nitrosylation of p65. J Biol Chem. 2007;282:30667–30672. doi: 10.1074/jbc.M705929200. [DOI] [PubMed] [Google Scholar]
- 53.Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Experimental diabetes research. 2007;2007:95–103. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kociok N, Radetzky S, Krohne TU, Gavranic C, Joussen AM. Pathological but not physiological retinal neovascularization is altered in TNFRp55-receptor-deficient mice. Investigative ophthalmology & visual science. 2006;47:5057–5065. doi: 10.1167/iovs.06-0407. [DOI] [PubMed] [Google Scholar]
- 55.Koga T, Koshiyama Y, Gotoh T, Yonemura N, Hirata A, Tanihara H, Negi A, Mori M. Coinduction of nitric oxide synthase and arginine metabolic enzymes in endotoxin-induced uveitis rats. Experimental eye research. 2002;75:659–667. doi: 10.1006/exer.2002.2062. [DOI] [PubMed] [Google Scholar]
- 56.Kowluru RA, Tang J, Kern TS. Abnormalities of retinal metabolism in diabetes and experimental galactosemia. VII. Effect of long-term administration of antioxidants on the development of retinopathy. Diabetes. 2001;50:1938–1942. doi: 10.2337/diabetes.50.8.1938. [DOI] [PubMed] [Google Scholar]
- 57.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leal EC, Manivannan A, Hosoya K, Terasaki T, Cunha-Vaz J, Ambrosio AF, Forrester JV. Inducible nitric oxide synthase isoform is a key mediator of leukostasis and blood-retinal barrier breakdown in diabetic retinopathy. Investigative ophthalmology & visual science. 2007;48:5257–5265. doi: 10.1167/iovs.07-0112. [DOI] [PubMed] [Google Scholar]
- 59.Li H, Meininger CJ, Hawker JR, Jr, Haynes TE, Kepka-Lenhart D, Mistry SK, Morris SM, Jr, Wu G. Regulatory role of arginase I and II in nitric oxide, polyamine, and proline syntheses in endothelial cells. Am J Physiol Endocrinol Metab. 2001;280:E75–E82. doi: 10.1152/ajpendo.2001.280.1.E75. [DOI] [PubMed] [Google Scholar]
- 60.Lima e Silva R, Kachi S, Akiyama H, Shen J, Hatara MC, Aslam S, Gong YY, Khu NH, Lauer TW, Hackett SF, Marton LJ, Campochiaro PA. Trans-scleral delivery of polyamine analogs for ocular neovascularization. Experimental eye research. 2006;83:1260–1267. doi: 10.1016/j.exer.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 61.Lima e Silva R, Saishin Y, Saishin Y, Akiyama H, Kachi S, Aslam S, Rogers B, Deering T, Gong YY, Hackett SF, Lai H, Frydman BJ, Valasinas A, Marton LJ, Campochiaro PA. Suppression and regression of choroidal neovascularization by polyamine analogues. Investigative ophthalmology & visual science. 2005;46:3323–3330. doi: 10.1167/iovs.04-1210. [DOI] [PubMed] [Google Scholar]
- 62.Mandecka A, Dawczynski J, Blum M, Muller N, Kloos C, Wolf G, Vilser W, Hoyer H, Muller UA. Influence of flickering light on the retinal vessels in diabetic patients. Diabetes Care. 2007;30:3048–3052. doi: 10.2337/dc07-0927. [DOI] [PubMed] [Google Scholar]
- 63.Marshall HE, Stamler JS. Inhibition of NF-kappa B by S-nitrosylation. Biochemistry. 2001;40:1688–1693. doi: 10.1021/bi002239y. [DOI] [PubMed] [Google Scholar]
- 64.Matsunaga T, Okumura K, Ishizaka H, Tsunoda R, Tayama S, Tabuchi T, Yasue H. Impairment of coronary blood flow regulation by endothelium-derived nitric oxide in dogs with alloxan-induced diabetes. J Cardiovasc Pharmacol. 1996;28:60–67. doi: 10.1097/00005344-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 65.Mayhan WG, Patel KP, Sharpe GM. Effect of L-arginine on reactivity of hamster cheek pouch arterioles during diabetes mellitus. Int J Microcirc Clin Exp. 1997;17:107–112. doi: 10.1159/000179217. [DOI] [PubMed] [Google Scholar]
- 66.McDonald KK, Zharikov S, Block ER, Kilberg MS. A caveolar complex between the cationic amino acid transporter 1 and endothelial nitric-oxide synthase may explain the "arginine paradox". J Biol Chem. 1997;272:31213–31216. doi: 10.1074/jbc.272.50.31213. [DOI] [PubMed] [Google Scholar]
- 67.Merzouk S, Hichami A, Madani S, Merzouk H, Berrouiguet AY, Prost J, Moutairou K, Chabane-Sari N, Khan NA. Antioxidant status and levels of different vitamins determined by high performance liquid chromatography in diabetic subjects with multiple complications. Gen Physiol Biophys. 2003;22:15–27. [PubMed] [Google Scholar]
- 68.Morris SM., Jr. Regulation of enzymes of urea and arginine synthesis. Annu Rev Nutr. 1992;12:81–101. doi: 10.1146/annurev.nu.12.070192.000501. [DOI] [PubMed] [Google Scholar]
- 69.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiological reviews. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pernet V, Bourgeois P, Di Polo A. A role for polyamines in retinal ganglion cell excitotoxic death. Journal of neurochemistry. 2007;103:1481–1490. doi: 10.1111/j.1471-4159.2007.04843.x. [DOI] [PubMed] [Google Scholar]
- 71.Pieper GM, Dondlinger LA. Plasma and vascular tissue arginine are decreased in diabetes: acute arginine supplementation restores endothelium-dependent relaxation by augmenting cGMP production. J Pharmacol Exp Ther. 1997;283:684–691. [PubMed] [Google Scholar]
- 72.Pieper GM, Peltier BA. Amelioration by L-arginine of a dysfunctional arginine/nitric oxide pathway in diabetic endothelium. J Cardiovasc Pharmacol. 1995;25:397–403. doi: 10.1097/00005344-199503000-00008. [DOI] [PubMed] [Google Scholar]
- 73.Pollock JS, Forstermann U, Mitchell JA, Warner TD, Schmidt HH, Nakane M, Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991;88:10480–10484. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rector TS, Bank AJ, Mullen KA, Tschumperlin LK, Sih R, Pillai K, Kubo SH. Randomized, double-blind, placebo-controlled study of supplemental oral L-arginine in patients with heart failure. Circulation. 1996;93:2135–2141. doi: 10.1161/01.cir.93.12.2135. [DOI] [PubMed] [Google Scholar]
- 75.Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, Caldwell RB, Caldwell RW. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res. 2008;102:95–102. doi: 10.1161/CIRCRESAHA.107.155028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Satriano J. Arginine pathways and the inflammatory response: interregulation of nitric oxide and polyamines: review article. Amino acids. 2004;26:321–329. doi: 10.1007/s00726-004-0078-4. [DOI] [PubMed] [Google Scholar]
- 77.Schlaich MP, Parnell MM, Ahlers BA, Finch S, Marshall T, Zhang WZ, Kaye DM. Impaired L-arginine transport and endothelial function in hypertensive and genetically predisposed normotensive subjects. Circulation. 2004;110:3680–3686. doi: 10.1161/01.CIR.0000149748.79945.52. [DOI] [PubMed] [Google Scholar]
- 78.Schulman SP, Becker LC, Kass DA, Champion HC, Terrin ML, Forman S, Ernst KV, Kelemen MD, Townsend SN, Capriotti A, Hare JM, Gerstenblith G. L-arginine therapy in acute myocardial infarction: the Vascular Interaction With Age in Myocardial Infarction (VINTAGE MI) randomized clinical trial. Jama. 2006;295:58–64. doi: 10.1001/jama.295.1.58. [DOI] [PubMed] [Google Scholar]
- 79.Socha HM, Romero MJ, Caldwell RW. Oral citrulline administration enhances NO-dependent vasodilation. FASEB (Exptl. Biol.) 2006;2006 [Google Scholar]
- 80.Solomonson LP, Flam BR, Pendleton LC, Goodwin BL, Eichler DC. The caveolar nitric oxide synthase/arginine regeneration system for NO production in endothelial cells. J Exp Biol. 2003;206:2083–2087. doi: 10.1242/jeb.00361. [DOI] [PubMed] [Google Scholar]
- 81.Sonta T, Inoguchi T, Tsubouchi H, Sekiguchi N, Kobayashi K, Matsumoto S, Utsumi H, Nawata H. Evidence for contribution of vascular NAD(P)H oxidase to increased oxidative stress in animal models of diabetes and obesity. Free Radic Biol Med. 2004;37:115–123. doi: 10.1016/j.freeradbiomed.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 82.Sorescu D, Somers MJ, Lassegue B, Grant S, Harrison DG, Griendling KK. Electron spin resonance characterization of the NAD(P)H oxidase in vascular smooth muscle cells. Free Radic Biol Med. 2001;30:603–612. doi: 10.1016/s0891-5849(00)00507-4. [DOI] [PubMed] [Google Scholar]
- 83.Spolarics Z, Bond JS. Comparison of biochemical properties of liver arginase from streptozocin-induced diabetic and control mice. Arch Biochem Biophys. 1989;274:426–433. doi: 10.1016/0003-9861(89)90455-4. [DOI] [PubMed] [Google Scholar]
- 84.Steppan J, Ryoo S, Schuleri KH, Gregg C, Hasan RK, White AR, Bugaj LJ, Khan M, Santhanam L, Nyhan D, Shoukas A, Hare JM, Berkowitz DE. Arginase modulates myocardial contractility by a nitric oxide synthase 1-dependent mechanism. Proc Natl Acad Sci U S A. 2006;103:4759–4764. doi: 10.1073/pnas.0506589103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Susic D, Francischetti A, Frohlich ED. Prolonged L-arginine on cardiovascular mass and myocardial hemodynamics and collagen in aged spontaneously hypertensive rats and normal rats. Hypertension. 1999;33:451–455. doi: 10.1161/01.hyp.33.1.451. [DOI] [PubMed] [Google Scholar]
- 86.Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 87.Taddei S, Virdis A, Mattei P, Ghiadoni L, Sudano I, Salvetti A. Defective L-arginine-nitric oxide pathway in offspring of essential hypertensive patients. Circulation. 1996;94:1298–1303. doi: 10.1161/01.cir.94.6.1298. [DOI] [PubMed] [Google Scholar]
- 88.Takano K, Ogura M, Yoneda Y, Nakamura Y. Oxidative metabolites are involved in polyamine-induced microglial cell death. Neuroscience. 2005;134:1123–1131. doi: 10.1016/j.neuroscience.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 89.Tilton RG, Chang K, Corbett JA, Misko TP, Currie MG, Bora NS, Kaplan HJ, Williamson JR. Endotoxin-induced uveitis in the rat is attenuated by inhibition of nitric oxide production. Investigative ophthalmology & visual science. 1994;35:3278–3288. [PubMed] [Google Scholar]
- 90.Van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal. 2005;7:308–317. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 91.van de Poll MC, Soeters PB, Deutz NE, Fearon KC, Dejong CH. Renal metabolism of amino acids: its role in interorgan amino acid exchange. Am J Clin Nutr. 2004;79:185–197. doi: 10.1093/ajcn/79.2.185. [DOI] [PubMed] [Google Scholar]
- 92.Vlassara H, Palace MR. Diabetes and advanced glycation endproducts. J Intern Med. 2002;251:87–101. doi: 10.1046/j.1365-2796.2002.00932.x. [DOI] [PubMed] [Google Scholar]
- 93.Ward ME, Toporsian M, Scott JA, Teoh H, Govindaraju V, Quan A, Wener AD, Wang G, Bevan SC, Newton DC, Marsden PA. Hypoxia induces a functionally significant and translationally efficient neuronal NO synthase mRNA variant. J Clin Invest. 2005;115:3128–3139. doi: 10.1172/JCI20806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weyrich AS, Ma XL, Lefer AM. The role of L-arginine in ameliorating reperfusion injury after myocardial ischemia in the cat. Circulation. 1992;86:279–288. doi: 10.1161/01.cir.86.1.279. [DOI] [PubMed] [Google Scholar]
- 95.White AR, Ryoo S, Li D, Champion HC, Steppan J, Wang D, Nyhan D, Shoukas AA, Hare JM, Berkowitz DE. Knockdown of arginase I restores NO signaling in the vasculature of old rats. Hypertension. 2006;47:245–251. doi: 10.1161/01.HYP.0000198543.34502.d7. [DOI] [PubMed] [Google Scholar]
- 96.Wu G, Morris SM., Jr. Arginine metabolism: nitric oxide and beyond. The Biochemical journal. 1998;336(Pt 1):1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yomura Y, Shoji Y, Asai D, Murakami E, Ueno S, Nakashima H. Direct, real-time, simultaneous monitoring of intravitreal nitric oxide and oxygen in endotoxin-induced uveitis in rabbits. Life sciences. 2007;80:1449–1457. doi: 10.1016/j.lfs.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 98.Yoshida S, Yoshida A, Ishibashi T, Elner SG, Elner VM. Role of MCP-1 and MIP-1alpha in retinal neovascularization during postischemic inflammation in a mouse model of retinal neovascularization. J Leukoc Biol. 2003;73:137–144. doi: 10.1189/jlb.0302117. [DOI] [PubMed] [Google Scholar]
- 99.Yu DY, Su EN, Cringle SJ, Yu PK. Isolated preparations of ocular vasculature and their applications in ophthalmic research. Progress in retinal and eye research. 2003;22:135–169. doi: 10.1016/s1350-9462(02)00044-7. [DOI] [PubMed] [Google Scholar]
- 100.Yu PK, Yu DY, Cringle SJ, Su EN. Tetrahydrobiopterin reverses the impairment of acetylcholine-induced vasodilatation in diabetic ocular microvasculature. J Ocul Pharmacol Ther. 2001;17:123–129. doi: 10.1089/10807680151125438. [DOI] [PubMed] [Google Scholar]
- 101.Zhang W, Baban B, Rojas M, Tofigh S, Virmani SK, Patel C, Behzadian MA, Caldwell RW, Caldwell RB. Arginase activity mediates retinal inflammation in endotoxin-induced uveitis. Am. J. Pathol. 2009;175(No 2) doi: 10.2353/ajpath.2009.081115. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zheng L, Du Y, Miller C, Gubitosi-Klug RA, Ball S, Berkowitz BA, Kern TS. Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin-induced diabetes. Diabetologia. 2007;50:1987–1996. doi: 10.1007/s00125-007-0734-9. [DOI] [PubMed] [Google Scholar]