Abstract
Aorta organ culture has been widely used as an ex vivo model for studying vessel pathophysiology. Recent studies show that the vascular smooth muscle cells (VSMCs) in organ culture undergo drastic dedifferentiation within the first few hours (termed early phenotypic modulation). Loss of tensile stress to which aorta is subject in vivo is the cause of this early phenotypic modulation. However, no underlying molecular mechanism has been discovered thus far. The purpose of the present study is to identify intracellular signals involved in the early phenotypic modulation of VSMC in organ culture. We find that the drastic VSMC dedifferentiation is accompanied by accelerated actin cytoskeleton dynamics and downregulation of SRF and myocardin. Among the variety of signal pathways examined, increasing actin polymerization by jasplakinolide is the only one hindering VSMC dedifferentiation in organ culture. Moreover, jasplakinolide reverses actin dynamics during organ culture. Latrunculin B (disrupting actin cytoskeleton) and jasplakinolide respectively suppressed and enhanced the expression of VSMC markers, SRF, myocardin, and CArG-box-mediated SMC promoters in a VSMC line. These results identify actin cytoskeleton degradation as a major intracellular signal for loss of tensile stress-induced early phenotypic modulation of VSMC in organ culture. This study suggests that disrupting actin cytoskeleton integrity may contribute to the pathogenesis of vascular diseases.
Keywords: Actin cytoskeleton, phenotype modulation, organ culture, tensile stress, stretch, Jasplakinolide
Introduction
Vascular smooth muscle cells (VSMC) play important roles in vessel contraction, the regulation of blood vessel tone, blood pressure and blood flow distribution (Albinsson and Hellstrand, 2007; Hayashi et al., 2001; Owens et al., 2004) . Unlike terminally differentiated skeletal and cardiac muscle cells, VSMCs retain the ability to modulate phenotypes ranging from the contractile state to the proliferative synthetic state in response to physiological and pathological stimuli. Under normal conditions, adult differentiated VSMC express a distinct repertoire of SMC specific marker genes including SM22α (SM22), SM α-actin (SMA), and SM myosin heavy chain (SM-MHC). In response to vascular injury, VSMC alter their phenotype towards a dedifferentiated state characterized by decreased expression of SMC marker genes and increased proliferation and migration. Such phenotypic modulation of VSMC is essential for vascular development and the progression of vascular diseases such as atherosclerosis and restenosis (Owens et al., 2004; Pipes et al., 2006).
Extensive studies have been carried out to characterize the molecular mechanisms that regulate VSMC phenotypic modulation (Pipes et al., 2006; Wang and Olson, 2004; Yoshida and Owens, 2005). The SRF-mediated transcriptional regulatory network plays the central role in regulating VSMC gene expression (Miano et al., 2007). SRF recruits myocardin, a potent VSMC transcriptional co-activator, and binds to the CArG box to activate the transcription of an array of VSMC contractile genes (Wang et al., 2001). The transcriptional activities of SRF and myocardin can be modulated by a variety of signal pathways through direct interaction with other transcription factors and cofactors, posttranslational modification and nuclear translocation (Wang and Olson, 2004).
Organ culture of aorta segments has been used as an ex vivo model for studying vascular pathophysiology, such as endothelium dysfunction (Alm et al., 2002; Nilsson et al., 2008), increased vasoconstriction (Cao et al., 2005a; Cao et al., 2006; Eskesen and Edvinsson, 2006), migration and proliferation of VSMC (Mekontso-Dessap et al., 2006; Slomp et al., 1996). It has been generally assumed that vascular smooth muscle cells (VSMCs) in organ culture and primary culture undergo gradual dedifferentiation. A recent study has distinguished two stages for VSMC dedifferentiation in organ culture: (i) early stage phenotypic modulation occurs within the first few hours following removal from the in vivo physiological cues, and involves the drastic downregulation of VSMC specific marker gene expression; (ii) late stage phenotypic modulation refers to VSMC dedifferentiation processes that take place in organ culture after many hours or even days and weeks (Guo et al., 2008). The molecular mechanisms underlying VSMC dedifferentiation at these two stages are distinct. While the activation of ERK and p38 MAPK pathways is involved in the late phenotypic modulation of VSMCs, the early phenotypic modulation is due to the loss of the mechanical forces to which VSMC are subject in vivo (Guo et al., 2008). However, little is known about the molecular mechanisms underlying the loss of mechanical force-induced early phenotypic modulation of VSMC.
It is well established that the actin cytoskeleton plays an integral role in VSMC function, including allowing vessel contraction and providing mechanical strength (Gunst and Zhang, 2008; Tang and Anfinogenova, 2008). VSMCs in the aorta are subject to both tensile stress due to blood pressure and shear stress resulting from blood flow. These stresses keep the VSMCs in a stretched state which in turn is required for maintaining VSMC differentiation (Birukov et al., 1998; Hellstrand and Albinsson, 2005). It has been suggested that tensile stress is the most important mechanical factor in VSMC differentiation (Guo et al., 2008). Thus, loss of tensile stress may regulate VSMC gene transcription via extracellular matrix (ECM)/Integerin/focal adhesion pathway (Gunst and Zhang, 2008; Tang and Anfinogenova, 2008). As a stretch sensing module, the actin cytoskeleton also contributes to stretch-induced contractile differentiation of VSMC via increased actin polymerization (Zeidan et al., 2003). The goal of present study is to identify the signal pathways involved in the early phenotypic modulation of VSMC in aorta organ culture. Among the variety of signal pathways examined, we find that jasplakinolide, an actin cytoskeleton polymerization inducer, is the only chemical blocking VSMC dedifferentiation. These results identify a novel role of actin cytoskeleton remodeling in mediating drastic VSMC dedifferentiation induced by the loss of tensile stresses in aorta organ culture.
Materials and Methods
Tissue preparation and aorta organ culture
C57BL/6J mice (Jackson Laboratory) were anaesthetized with CO2 and exsanguinated. The whole aorta was gently removed and freed of adhering tissue under a dissection microscope. The aortas were then cut into 2 mm cylindrical segments and incubated for 1, 2, 4, or 24 hours at 37°C in humidified 5% CO2. The resulting organ culture was performed in a 24-well plate, with two segments per well, containing 1 mL serum free Dulbecco's modified Eagle's medium (DMEM) containing L-glutamine (584 mg/L), penicillin (100 U/mL) and streptomycin (100 µg/mL) (Cao et al., 2005b; Xu et al., 2008).
Real-time reverse transcription (RT)-PCR
Total RNA from aorta or PAC1 cells was extracted and purified using RNeasy Fibrous Tissue Kit (Qiagen) and RNeasy Kit (Qiagen) respectively. cDNAs were synthesized using the Superscript II reverse transcriptase (Invitrogen). Real-time PCR was performed using the StepOnePlus system (Applied Biosystems) in the presence of SYBR Green. snRNA U6 was used as the internal control, and all PCR primers were designed to cover at least 2 exons (Table 1).
Table 1.
Sequences of primers used for real-time PCR
| Gene | Sequence | Amplicon size (bp) | GenBank accession # |
|---|---|---|---|
| U6 | Forward 5’-CTCGCTTCGGCAGCACATATACTA-3’ | 92 | NR_002752 |
| Reverse 5’-CGCTTCACGAATTTGCGTGTCATC-3’ | |||
| SMA | Forward 5’-ATCTTTTCCATGTCGTCCCAGTTG-3’ | 310 | NM_007392 |
| Reverse 5’-GAGAAGCCCAGCCAGTCG-3’ | |||
| SM22 | Forward 5’-TCCTTCCAGTCCACAAACGACCAA-3’ | 92 | NM_011526 |
| Reverse 5’-TTTGGACTGCACTTCTCGGCTCAT-3’ | |||
| SM22 | Forward 5’-TCCTTCCAGCCCACAAACGACCAA-3’ | 92 | NM_031549 |
| Reverse 5’-TTTGGACTGCACTTCTCGGCTCAT-3’ | |||
| SM-MHC | Forward 5’-AACGCCCTCAAGAGCAAACTCAGA-3’ | 161 | NM_013607 |
| Reverse 5’-TCCCGAGCGTCCATTTCTTCTTCA-3’ | |||
| SRF | Forward 5’-AAAGAAACCGTGAAAAGGCAACA-3’ | 229 | NM_020493 |
| Reverse 5’-CTGACAGCACCGTCTGGGACCGT-3’ | |||
| Myocd | Forward 5’-CAGTGAAGCAGCAAATGACTCGG-3’ | 230 | NM_145136 |
| Reverse 5’-GTCGTTGGCGTAGTGATCGAAGG-3’ | |||
Analysis of actin cytoskeleton dynamics
The G/F-actin ratio in vivo assay kit (Cytoskeleton, Denver, CO) was used to measure the actin cytoskeleton dynamics in the aorta cultured for 24 hours with and without 0.3 µM Jasp based on the manufacturer’s protocol. Briefly, pooled aortas were homogenized in 500 µl F-actin stabilization buffer. Lysates were centrifuged at 2,000 rpm for 5 min at 37°C to pellet unbroken cells, and then the supernatants were collected. To separate the F-actin from the G-actin, the supernatants were centrifuged at 100,000 g for 1 h at 37°C. The supernatants were then immediately collected, while the pellets were resuspended in ice-cold H2O plus 1 µM cytochalasin D and incubated on ice for 1 h to dissociate F-actin. The resuspended pellets were gently mixed every 15 min. To measure F/G-actin ratio, equal amounts of proteins from the supernatant (G-actin) and the resuspended pellet (F-actin) underwent immunoblot analysis using the actin antibody in the kit.
Cell culture and Luciferase assay
PAC1 (a pulmonary artery-derived SMC line) (Rothman et al., 1992) cells were maintained in DMEM (Invitrogen) with 10 % FBS at 37°C with 5 % CO2. The SMC promoter reporters (50ng) including SM22α, SM α-actin, SM-MHC, 4xCArGnear, and aortic carboxypeptidase-like protein (ACLP) (Layne et al., 2002; Li et al., 1996; Wang et al., 2001; Yoshida et al., 2004) were transiently transfected with 1 ng CMV-Renilla Luciferase Reporter (Promega) into PAC1 using the Lipofectamine and Plus transfection reagents (Invitogen) for 48 hours. 0.5 µM latrunculin B (LatB) or 0.3 µM jasplakinolide (Jasp) was added to the cultures for 6 hours before harvesting for luciferase assays. The promoter activities were determined by the firefly luciferase activity relative to the internal control renilla luciferase activity using the dual luciferase assay system described by the manufacturer (Promega, Madison, WI).
Data Analysis
At least three independent experiments were performed for all assays. The values presented are means ± standard errors of the means (SEMs). Statistical analysis was performed using one-way ANOVA. The difference was considered significant at p-value < 0.05.
Results and Discussion
Organ culture-induced drastic dedifferentiation of VSMC
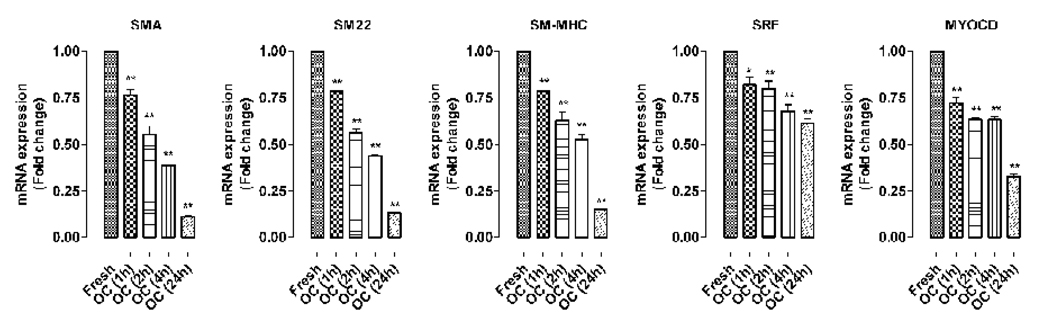
Aorta organ culture has been widely used as an ex vivo model for studying vessel pathophysiology including endothelium dysfunction, vasoconstriction, and migration and proliferation of VSMC. To evaluate the practicality of using aorta organ culture to study the molecular mechanisms of VSMC phenotypic modulation, we performed a time-course expression of the VSMC differentiation markers SMA, SM22, and SM-MHC upon removal of aorta from the mice. Consistent with a previous report (Guo et al., 2008), we found that mRNA expression of all the selected VSMC marker genes decreases by 25% in the first hour and steadily falls by 90% after 24 hours (Fig.1). Such drastic VSMC dedifferentiation can be observed in all aorta organ cultures irrespective of the genetic background of the mice.
Fig. 1.
The expression of VSMC-specific genes and key transcriptional regulators SRF and myocardin in organ culture (OC). The real time RT-PCR assay shows that mRNA levels of VSMC-specific gene (SMA, SM22, SM-MHC) and transcriptional regulator (SRF and myocardin) steadily decreases at 1, 2, 4 and 24 hours in aorta organ culture. “*” and “**” indicate p<0.05 and p<0.01 relative to the fresh control group.
Transcription factor SRF and its cofactor myocardin (Myocd), a master regulator for VSMC differentiation, play a central role in regulating VSMC gene transcription (Pipes et al., 2006; Wang et al., 2001; Wang et al., 2004). We observed that the expressions of SRF and myocardin also decrease significantly in organ culture within 24 hours. These results suggest that downregulation of SRF and myocardin contributes to VSMC dedifferentiation.
Role of actin cytoskeleton in VSMC dedifferentiation during organ culture
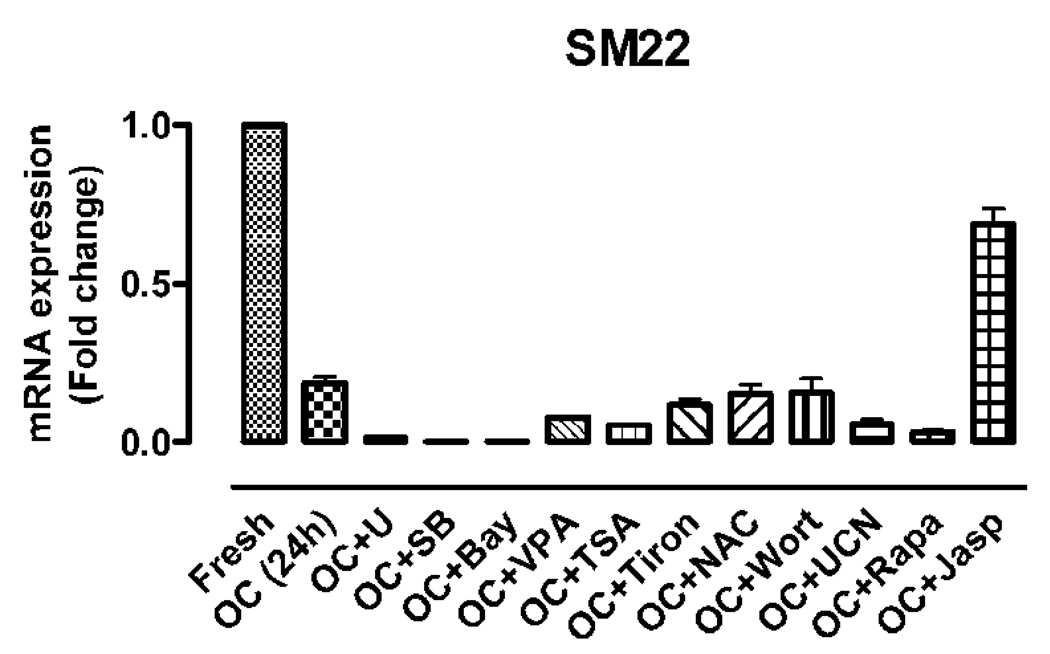
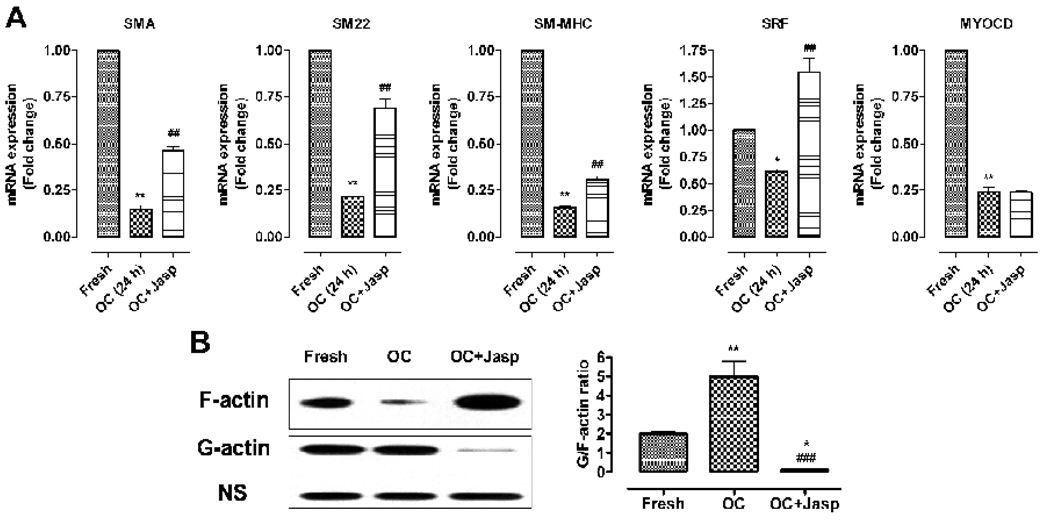
To explore the intracellular signals that elicit VSMC dedifferentiation during organ culture, we incubated the aorta segments in organ culture with a variety of chemicals such as UCN-01, U0126, SB203580, Bay 11–7082, Wortmannin, Rapamycin, Trichostatin A, valproic acid, Tiron, NAC, and Jasp (Table 2). These chemicals are known to modulate VSMC differentiation by inhibiting the signal pathways of protein kinase C (PKC), MAPK, NF-kappa B, PI3 kinase/Akt, mammalian target of rapamycin (mTOR), histone deacetylase (HDACs), reactive oxygen species (ROS) and cytoskeleton polymerization respectively (Hellstrand and Albinsson, 2005). We find that Jasp is the only one showing significant ability of hindering the downregulation of SMC-specific genes including SM22 at 24 hours in organ culture; none of the other chemicals appears to block VSMC dedifferentiation (Fig.2, 3A). Indeed, Jasp significantly blocks the drastic decreased transcription of SMA, SM22, and SM-MHC, but not of myocardin (Fig. 3A). Interestingly, Jasp treatment has the most significant effect on the expression of SM22 and SRF (Fig. 3A): Jasp blocks the downregulation of SM22 to 65% of the control and increases the transcription of SRF by 60% (Fig. 3A), consistent with the role of SM22 and SRF as sensors for cell shape change (Shapland et al., 1993) and actin cytoskeleton dynamics respectively (Sotiropoulos et al., 1999). This result suggests that disruption of actin cytoskeleton may account for the early phenotypic modulation of VSMC in the aorta organ culture.
Table 2.
Chemicals used in organ culture
| Inhibitors | Effect on pathway | Concentration used |
|---|---|---|
| UCN-01 | PKC inhibitor | 10−7 M |
| U0126 | ERK1/2 inhibitor | 10−5 M |
| SB203580 | p38 inhibitor | 10−5 M |
| Bay 11–7082 | NF-κB inhibitor | 10−5 M |
| Wortmannin | PI3K inhibitor | 10−6 M |
| Rapamycin | mTOR inhibitor | 10−7 M |
| Trichostatin A | HDAC inhibitor | 2×10−7 M |
| valproic acid | HDAC inhibitor | 2×10−3 M |
| Tiron | ROS scavenger | 10−2 M |
| NAC | ROS scavenger | 2×10−3 M |
Fig. 2.
The effect of chemicals on OC-induced downregulation of SMC marker genes. qRT-PCR was used to measure the expression of SM22 from aorta segments freshly isolated and in OC for 24 hours with the indicated chemicals. The values are expressed as comparison with the freshly isolated aorta.
Fig. 3.
Role of actin cytoskeleton dynamics in organ culture (OC)-induced dedifferentiation of VSMC. (A) the downregulation of VSMC-specific genes can be partially blocked by Jasplakinolide (Jasp), an actin polymerization inducer. (B) The actin cytoskeleton dynamics in organ culture was measured by the G-/F-actin ratio using western blot analysis and the intensity of each band was quantified by the Photoshop software. * and ** indicate p<0.05 and p<0.01 vs fresh group; ### indicates p<0.001 vs OC (24 h) group.
VSMCs are subject to constant intraluminal blood pressure in vivo; this results in tensile stress being applied to the VSMC. Loss of tensile stress has been recognized as the main cause triggering early phenotypic modulation of VSMC in organ culture (Birukov et al., 1998; Guo et al., 2008). It is known that the actin cytoskeleton contributes to stretch-induced contractile differentiation of VSMC via increased actin polymerization (Zeidan et al., 2003). The actin cytoskeleton, as a mechanotransduction sensing element, thus plays an important role in transcriptional regulation in response to the mechanically-induced signal transduction from the cell surface to the nucleus (Wang et al., 2009).
To determine whether actin cytoskeleton remodeling occurs in organ culture, we measured the amounts of F-actin and G-actin in the aorta 24 hours after organ culture. The monomer G-actin assembles into contractile F-actin (Gunst and Zhang, 2008). The amount of free G-actin and F-actin were analyzed by western blot analysis (Fig.3B). The G-/F-actin ratio increases significantly in organ culture, reflecting the increased actin depolymerization and accelerated actin cytoskeleton dynamics (Fig. 3B). This result suggests that loss of tensile stress induces actin cytoskeleton degradation. Indeed, Jasp treatment significantly decreases the G-/F-actin ratio (Fig. 3B). Taken together, these results suggest that change of actin cytoskeleton dynamics is one of the major intracellular signals for loss-of-stretch induced VSMC dedifferentiation during early phenotypic modulation in organ culture.
Manipulation of actin cytoskeleton affects VSMC differentiation
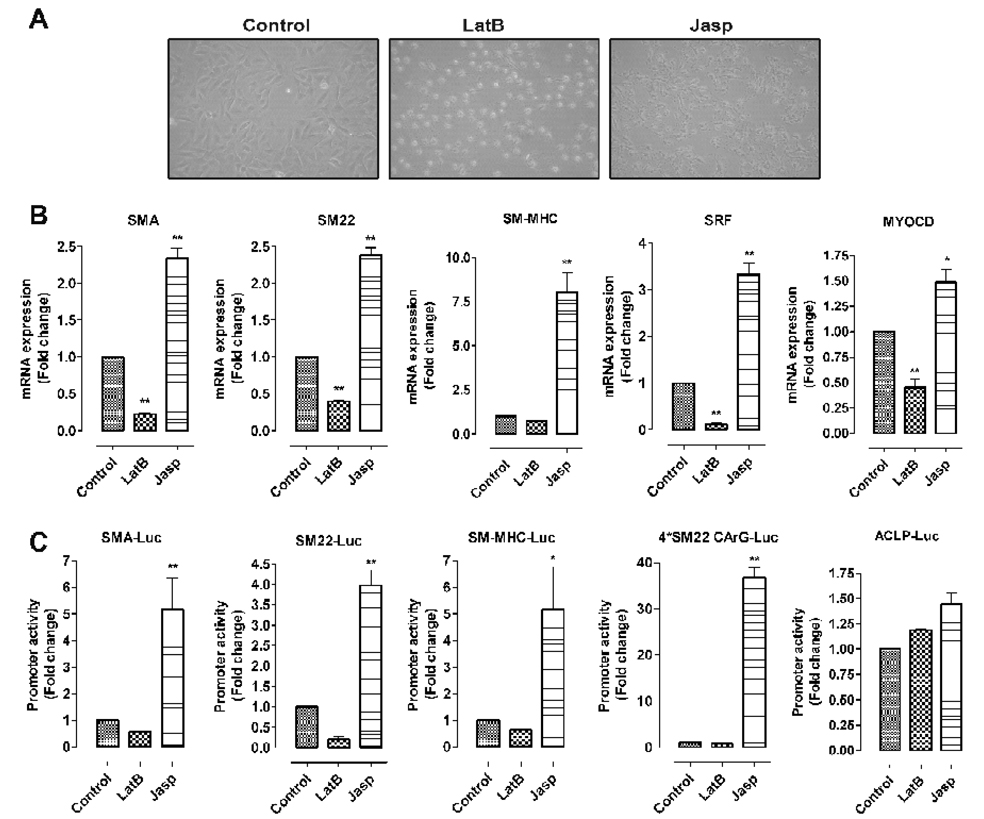
To explore the role of actin cytoskeleton in regulating VSMC gene transcription, we examined the effect of Jasp and LatB, two commonly used reagents known to respectively enhance and disrupt actin cytoskeleton (Kim et al., 2008), on VSMC gene transcription in vitro using the PAC1 line. After treatment with LatB, cultured VSMC (PAC1) show significant morphologic change: their shape changes from elongated to circular. By contrast, Jasp treatment changes the morphology from elongated to nearly linear shape (Fig. 4A). LatB and Jasp can respectively inhibit and increase the transcription of VSMC marker genes, SRF and myocardin (Fig. 4B). Studies of the transcriptional control of VSMC-specific gene expression have highlighted the importance of the CArG box in SMC gene regulation (Miano et al., 2007). Our transient transfection assays show that Jasp significantly increases the activities of CArG box-dependent SMC promoters, not those of the CArG box-independent ACLP promoter (Fig. 4C). These results indicate that reorganization of the actin cytoskeleton can modulate the expression of the two SMC key transcriptional factors SRF and myocardin as well as the transcriptional activities of CArG box-dependent promoters.
Fig. 4.
Manipulation of actin cytoskeleton affects VSMC differentiation in vitro. (A) latrunculin B (LatB, 0.5 µM for 6 hours) and Jasplakinolide (Jasp, 0.3 µM for 6 hours) induce significant morphology changes of PAC1 cells. (B–C) LatB and Jasp respectively reduce and enhance the transcription of VSMC-specific markers, SRF and myocardin by the real-time RT-PCR assay (B) and VSMC promoter activities (C) in PAC1 cells treated with LatB or Jasp for 6 hours. * and ** indicate p<0.05 and p<0.01 vs control.
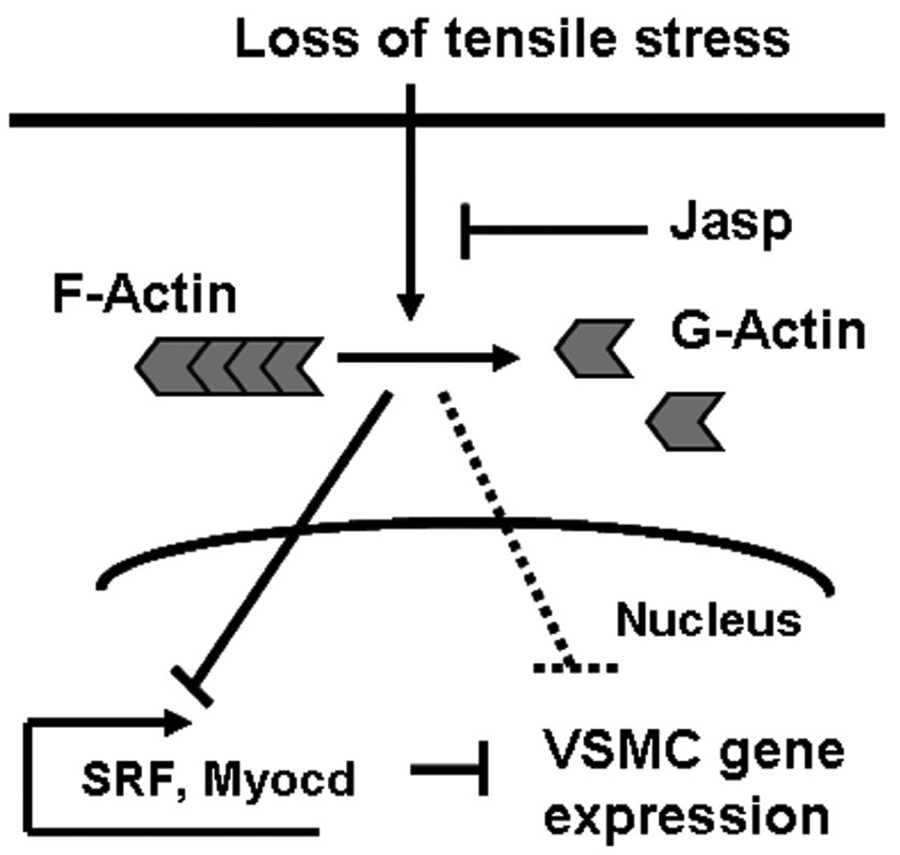
In summary, our study revealed actin cytoskeleton dynamics as a major intracellular signal in the early phenotypic modulation of VSMC in organ culture. We thus propose a potential mechanism whereby loss of tensile stress blocks VSMC transcription via disruption of the actin cytoskeleton (Fig. 5).
Fig. 5.
A potential mechanism for organ culture-induced early phenotypic modulation of VSMC. During organ culture, loss of tensile stress induces actin cytoskeleton dissociation that then leads to decreased expression of SRF and Myocd and the downregulation of VSMC gene transcription.
It should be noted that organ culture is still commonly used as an ex vivo model for studying VSMC pathophysiology. The present study highlights the drastic and rapid dedifferentiation of VSMC in aorta organ culture. Because loss of tensile stress induced stretch is inevitable for both primary culture and organ culture, it is conceivable that results obtained in vitro or ex vivo may be inconsistent with the results in vivo. This implies potential limitations for the use of organ culture in studying VSMC phenotypic changes. It is quite possible that disruption of actin cytoskeleton also contributes to other organ culture-induced pathophysiological processes such as endothelial dysfunction, abnormal vasoconstriction, and VSMC proliferation. Our analyses suggest that maintaining actin cytoskeleton integrity may provide a potential therapeutic target for future treatment of vascular diseases.
Conclusion
This study identified actin cytoskeleton reorganization as a driving mechanism of the drastic dedifferentiation of VSMC in organ culture. Our results emphasize the significance of disrupting actin cytoskeleton integrity in the pathogenesis of vascular diseases.
Acknowledgments
This work was supported by the grant from the National Heart, Lung, and Blood Institute (HL087014 to L.L). We would like to thank Giuseppe Rossi, Hong Jiang and Zhonghui Xu for editorial assistance, technical help and valuable discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albinsson S, Hellstrand P. Integration of signal pathways for stretch-dependent growth and differentiation in vascular smooth muscle. Am J Physiol Cell Physiol. 2007;293:C772–C782. doi: 10.1152/ajpcell.00622.2006. [DOI] [PubMed] [Google Scholar]
- Alm R, et al. Organ culture: a new model for vascular endothelium dysfunction. BMC Cardiovasc Disord. 2002;2:8. doi: 10.1186/1471-2261-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukov KG, et al. Intraluminal pressure is essential for the maintenance of smooth muscle caldesmon and filamin content in aortic organ culture. Arterioscler Thromb Vasc Biol. 1998;18:922–927. doi: 10.1161/01.atv.18.6.922. [DOI] [PubMed] [Google Scholar]
- Cao YX, et al. Enhanced transcription of contractile 5-hydroxytryptamine 2A receptors via extracellular signal-regulated kinase 1/2 after organ culture of rat mesenteric artery. Basic Clin Pharmacol Toxicol. 2005a;96:282–288. doi: 10.1111/j.1742-7843.2005.pto960402.x. [DOI] [PubMed] [Google Scholar]
- Cao YX, et al. Up-regulation of alpha1A-adrenoceptors in rat mesenteric artery involves intracellular signal pathways. Basic Clin Pharmacol Toxicol. 2006;98:61–67. doi: 10.1111/j.1742-7843.2006.pto_240.x. [DOI] [PubMed] [Google Scholar]
- Cao YX, et al. Induces vasodilatation of rat mesenteric artery in vitro mainly by inhibiting receptor-mediated Ca(2+)-influx and Ca(2+)-release. Arch Pharm Res. 2005b;28:709–715. doi: 10.1007/BF02969362. [DOI] [PubMed] [Google Scholar]
- Eskesen K, Edvinsson L. Upregulation of endothelin ETB receptor-mediated vasoconstriction in rat coronary artery after organ culture. Eur J Pharmacol. 2006;539:192–194. doi: 10.1016/j.ejphar.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Gunst SJ, Zhang W. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol. 2008;295:C576–C587. doi: 10.1152/ajpcell.00253.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, et al. The early- and late stages in phenotypic modulation of vascular smooth muscle cells: differential roles for lysophosphatidic acid. Biochim Biophys Acta. 2008;1781:571–581. doi: 10.1016/j.bbalip.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, et al. Phenotypic modulation of vascular smooth muscle cells induced by unsaturated lysophosphatidic acids. Circ Res. 2001;89:251–258. doi: 10.1161/hh1501.094265. [DOI] [PubMed] [Google Scholar]
- Hellstrand P, Albinsson S. Stretch-dependent growth and differentiation in vascular smooth muscle: role of the actin cytoskeleton. Can J Physiol Pharmacol. 2005;83:869–875. doi: 10.1139/y05-061. [DOI] [PubMed] [Google Scholar]
- Kim HR, et al. Cytoskeletal remodeling in differentiated vascular smooth muscle is actin isoform dependent and stimulus dependent. Am J Physiol Cell Physiol. 2008;295:C768–C778. doi: 10.1152/ajpcell.00174.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layne MD, et al. Characterization of the mouse aortic carboxypeptidase-like protein promoter reveals activity in differentiated and dedifferentiated vascular smooth muscle cells. Circ Res. 2002;90:728–736. doi: 10.1161/01.res.0000013289.97650.c8. [DOI] [PubMed] [Google Scholar]
- Li L, et al. Expression of the SM22alpha promoter in transgenic mice provides evidence for distinct transcriptional regulatory programs in vascular and visceral smooth muscle cells. J Cell Biol. 1996;132:849–859. doi: 10.1083/jcb.132.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekontso-Dessap A, et al. Vascular-wall remodeling of 3 human bypass vessels: organ culture and smooth muscle cell properties. J Thorac Cardiovasc Surg. 2006;131:651–658. doi: 10.1016/j.jtcvs.2005.08.048. [DOI] [PubMed] [Google Scholar]
- Miano JM, et al. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am J Physiol Cell Physiol. 2007;292:C70–C81. doi: 10.1152/ajpcell.00386.2006. [DOI] [PubMed] [Google Scholar]
- Nilsson D, et al. Endothelin receptor-mediated vasodilatation: effects of organ culture. Eur J Pharmacol. 2008;579:233–240. doi: 10.1016/j.ejphar.2007.09.031. [DOI] [PubMed] [Google Scholar]
- Owens GK, et al. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Pipes GC, et al. The myocardin family of transcriptional coactivators: versatile regulators of cell growth, migration, and myogenesis. Genes Dev. 2006;20:1545–1556. doi: 10.1101/gad.1428006. [DOI] [PubMed] [Google Scholar]
- Rothman A, et al. Development and characterization of a cloned rat pulmonary arterial smooth muscle cell line that maintains differentiated properties through multiple subcultures. Circulation. 1992;86:1977–1986. doi: 10.1161/01.cir.86.6.1977. [DOI] [PubMed] [Google Scholar]
- Shapland C, et al. Purification and properties of transgelin: a transformation and shape change sensitive actin-gelling protein. J Cell Biol. 1993;121:1065–1073. doi: 10.1083/jcb.121.5.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomp J, et al. Nature and origin of the neointima in whole vessel wall organ culture of the human saphenous vein. Virchows Arch. 1996;428:59–67. doi: 10.1007/BF00192928. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos A, et al. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell. 1999;98:159–169. doi: 10.1016/s0092-8674(00)81011-9. [DOI] [PubMed] [Google Scholar]
- Tang DD, Anfinogenova Y. Physiologic properties and regulation of the actin cytoskeleton in vascular smooth muscle. J Cardiovasc Pharmacol Ther. 2008;13:130–140. doi: 10.1177/1074248407313737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, et al. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell. 2001;105:851–862. doi: 10.1016/s0092-8674(01)00404-4. [DOI] [PubMed] [Google Scholar]
- Wang DZ, Olson EN. Control of smooth muscle development by the myocardin family of transcriptional coactivators. Curr Opin Genet Dev. 2004;14:558–566. doi: 10.1016/j.gde.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Wang N, et al. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- Wang Z, et al. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature. 2004;428:185–189. doi: 10.1038/nature02382. [DOI] [PubMed] [Google Scholar]
- Xu CB, et al. Lipid-soluble smoke particles upregulate vascular smooth muscle ETB receptors via activation of mitogen-activating protein kinases and NF-kappaB pathways. Toxicol Sci. 2008;106:546–555. doi: 10.1093/toxsci/kfn173. [DOI] [PubMed] [Google Scholar]
- Yoshida T, et al. Forced expression of myocardin is not sufficient for induction of smooth muscle differentiation in multipotential embryonic cells. Arterioscler Thromb Vasc Biol. 2004;24:1596–1601. doi: 10.1161/01.ATV.0000137190.63214.c5. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Owens GK. Molecular determinants of vascular smooth muscle cell diversity. Circ Res. 2005;96:280–291. doi: 10.1161/01.RES.0000155951.62152.2e. [DOI] [PubMed] [Google Scholar]
- Zeidan A, et al. Stretch-induced contractile differentiation of vascular smooth muscle: sensitivity to actin polymerization inhibitors. Am J Physiol Cell Physiol. 2003;284:C1387–C1396. doi: 10.1152/ajpcell.00508.2002. [DOI] [PubMed] [Google Scholar]