Abstract
In many eukaryotes, phospholipids (PLs) and sphingolipids (SLs) are abundant membrane components and reservoirs for important signaling molecules. In Leishmania, the composition, metabolism, and function of PLs and SLs differ significantly from those in mammalian cells. Although only a handful of enzymes have been experimentally characterized, available data suggest many steps of PL/SL metabolism are critical for Leishmania viability and/or virulence, and could be a source for new drug targets. Further studies of genes involved in the synthesis (de novo and salvage) and degradation of PLs and SLs will reveal their diverse effects on Leishmania pathogenesis.
Keywords: Leishmania, phospholipid, sphingolipid, plasmalogen, Trypanosoma, virulence
1. Introduction
Phospholipids (PLs) are major components of biological membranes with importance extending above and beyond basic structural roles. First, degradation of glycerophospholipids generates a plethora of signaling molecules including inositol 1,4,5-triphosphate, 1,2-diacylglycerol [1, 2], arachidonic acid, lyso-phospholipids, and phosphatidic acid [3, 4]. In addition, PLs such as phosphatidylinositol (PI) can be modified by a group of PI kinases (PI3K, PI4K, and PIPKs) into bioactive phosphoinositides (PIP, PIP2, and PIP3); PI kinases are activated by small G-proteins and regulate a number of cellular processes including membrane trafficking and cytoskeleton remodeling [5–7]. Furthermore, the exposure of phosphatidylserine (PS) on the outer surface of cells is believed to trigger the removal of apoptotic cells by phagocytes [8–10]. Parasitic protozoans including Leishmania have been reported to mimic such PS exposure to avoid destruction and gain entry to host phagocytes [11–13].
Sphingolipids (SLs) constitute another major group of membrane components in eukaryotes. In mammals, virtually every metabolite in SL biosynthesis or degradation has potent activities. These compounds (ceramide, ceramide-1-phosphate, sphingosine, and sphingosine-1-phosphate) mediate many signaling pathways including apoptosis, cell-to-cell recognition, growth, and differentiation [14, 15]. In particular, ceramide (generated from de novo synthesis or degradation of high order SLs) is a potent secondary messenger capable of activating protein phosphatases PP1 and PP2A [16], protein kinase Czeta [17], and lysosomal protease Cathepsin D [18].
Trypanosomatid protozoans including Trypanosoma brucei, Trypanosoma cruzi, and Leishmania species are important human pathogens. While synthesis of the lipid anchor for parasite surface molecules such as lipophosphoglycan (LPG) and glycosylphosphatidylinositol (GPI)-anchored proteins has received considerable attention [19], metabolism of PLs and SLs in Leishmania has been relatively understudied. Recent developments in genome sequencing (complete for L. major, L. braziliensis, and L. infantum, ongoing for several other species) and lipidomic tools have forged a powerful platform for the study of lipid metabolism in Leishmania. Gaining new knowledge on PL and SL metabolism will not only provide fundamental insight into the molecular bases of Leishmania pathogenesis, but also may reveal new targets for selective drugs.
2. Composition of PLs and SLs in Leishmania
The analysis of lipid composition and structures requires a combination of chromatography and mass spectrometry/NMR. The use of electrospray ionization mass spectrometry coupled with tandem mass spectrometry (ESI/MS/MS) and collision–induced dissociation, has allowed rapid identification and comprehensive description of individual PLs and SLs [20, 21]. While promising, some challenges remain as some lipid species yield useful diagnostic daughter ions less efficiently than others. For example, in some ESI/MS/MS analyses, the cellular level of PS is below the limit of detection in L. major promastigotes [22, 23]. In these cases, data from alternative analytic methods are needed to give an accurate picture of the overall lipid distribution and abundance. The discussion below reflects a consensus view of PL/SL distribution in Leishmania.
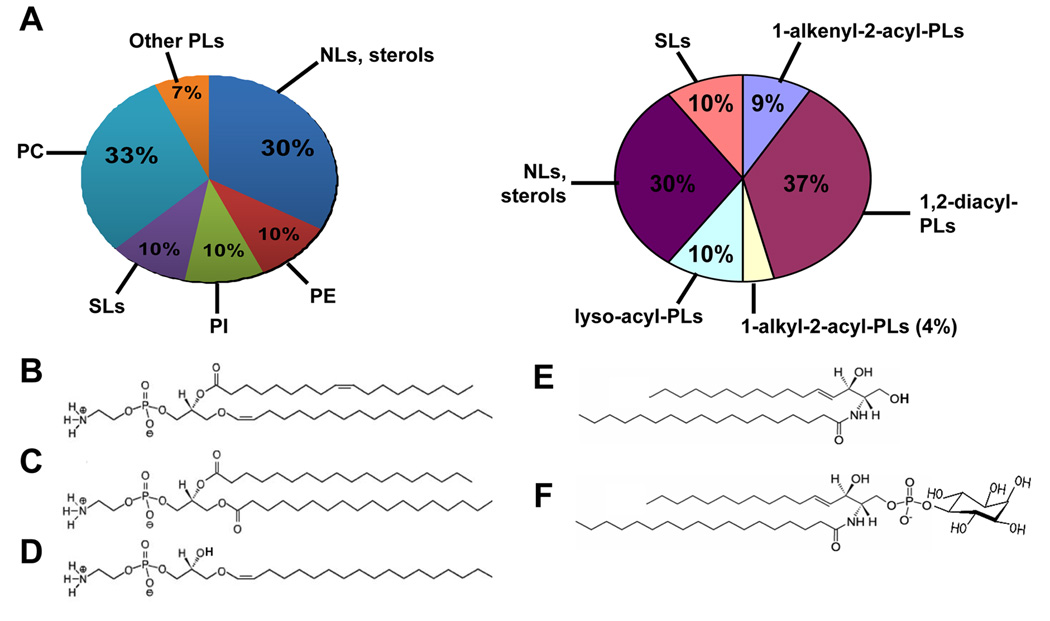
Overall, PLs account for ~70% of total cellular lipids in Leishmania [24, 25] (Fig. 1A). Major PL species classified according to the ‘headgroup’ include phosphatidylcholine (PC, 30–40%), phosphatidylethanolamine (PE, ~10%), and PI (~10%) (Fig. 1A, left) [22, 25, 26]. PLs may also be classified based on their lipid anchors, consisting of 1,2-diacyl, 1-alkyl-2-acyl, 1-alkenyl-2-acyl (plasmalogens), or in some cases lyso-alkyl or lyso-acyl groups (Fig. 1A, right). Interestingly, the majority (80–90%) of PE in L. major belongs to plasmenylethanolamine (PLE) or ethanolamine plasmalogen (1-O-1'-alkenyl-2-acyl-phosphotidylethanolamine) [22, 27] (Fig. 1B). PLE is also more abundant than diacyl-PE in the procyclic and blood stream forms of T. brucei [28–30]. In contrast, plasmalogen lipids (PLE and choline plasmalogen) only constitute ~20% of total PLs in mammals, although their levels can reach over 50% in heart and nervous tissues [31].
Figure 1.
PLs and SLs in L. major. (A) Lipid composition in L. major promastigotes based on the headgroups (left) or anchors (right) of PLs. Structures of each class of lipids were determined by ESI/MS/MS and abundance was estimated as previously described [22, 23, 26]. Other PLs include phosphatidylglycerol (PG), cardiolipin (CL), and phosphatidic acid. (B)–(F) Molecular species of PE and SLs in L. major. (B) A PLE: 1-O-octadec-1’-enyl 2-octadecenoyl sn-glycero-3-phosphoethanolamine (p18:0/18:1-PE). (C) A diacyl PE: 1,2-distearoyl sn-glycero-3-phosphoethanolamine (18:0/18:0-PE). (D) A lyso-PE: 1-O-octadec-1’-enyl 2-lyso sn-glycero-3-phosphoethanolamine (p18:0/lyso-PE). (E) A ceramide: N-stearoylhexadecesphing-4-enine (d16:1/18:0-Cer). (F) An IPC: phosphoryl inositol N-stearoylhexadecesphing-4-enine (d16:1/18:0-IPC).
Notably, side chains of Leishmania PE can be modified to form cis-9,10-methyleneoctadecanoic acid (4–11%), a cyclopropane fatty acid commonly found in bacteria and other Trypanosomatids [24, 32]. These cyclopropane-containing PEs confer acid resistance in E. coli [33], although their role is not known in Leishmania. In addition to PLE, 1,2-diacyl PE and 1-O-1'-alkenyl-2-lyso PE are also present in Leishmania at much lower levels [22](Fig. 1C–D).
The most abundant glycerophospholipid in Leishmania is PC, consisting of 1,2-diacyl and 1-lyso-2-acyl species with unusually long and unsaturated fatty acid species [22]. The functions of these heterogeneous PC species are not well understood, although their polyunsaturated fatty acid chains could modulate membrane physiology by reducing the melting point and confer resistance to host-derived oxidants. PI is another major group of glycerophospholipids which contains a combination of 1,2-diacyl, 1-alkyl-2acyl and 1-lyso-2-acyl species [26] (Fig. 1A). In Leishmania, diacyl PI mostly exists as the un-conjugated or free form, whereas alkyl-acyl- and lyso-PI are widely found in the anchors of surface glycoconjugates including glycosylinositolphospholipids (GIPLs), GPI-containing membrane proteins, and LPG [34, 35]. Leishmania parasites also synthesize low levels of phosphatidylglycerol (PG), cardiolipin (CL), and phosphatidic acid. Functions of these minor classes of phospholipids are not well understood, although PG and CL are known to be important components of mitochondrial membrane in many eukaryotes.
In mammalian cells, PS is asymmetrically distributed across the membrane bilayer and the exposure of PS on the outside surface of cells is an early sign of apoptosis [10]. PS has been detected in Leishmania by chromatography-based methods [25, 36], or flow cytometry based on reactivity with either annexin V (which binds to PS and other anionic PLs in the presence of Ca2+) or a PS-specific mAb [12, 37]. No obvious orthologues for the bacterial CDP-diacylglycerol: serine O-phosphatidyltransferase (PS synthase 1) are evident in the TriTryp genomes (L. major, T. brucei, and T. cruzi). Nonetheless, these parasites contain a putative base exchange enzyme (also called PS synthase 2) and a putative PS decarboxylase, suggesting they possess an active PS metabolism [23](Fig. 2). At present, no data address the question of whether PS is synthesized by Leishmania or acquired from the host. In T. brucei, recent reports indicate PS can be synthesized by head group exchange with PE [28] and is more abundant in the bloodstream form then in procyclics [30].
Figure 2.
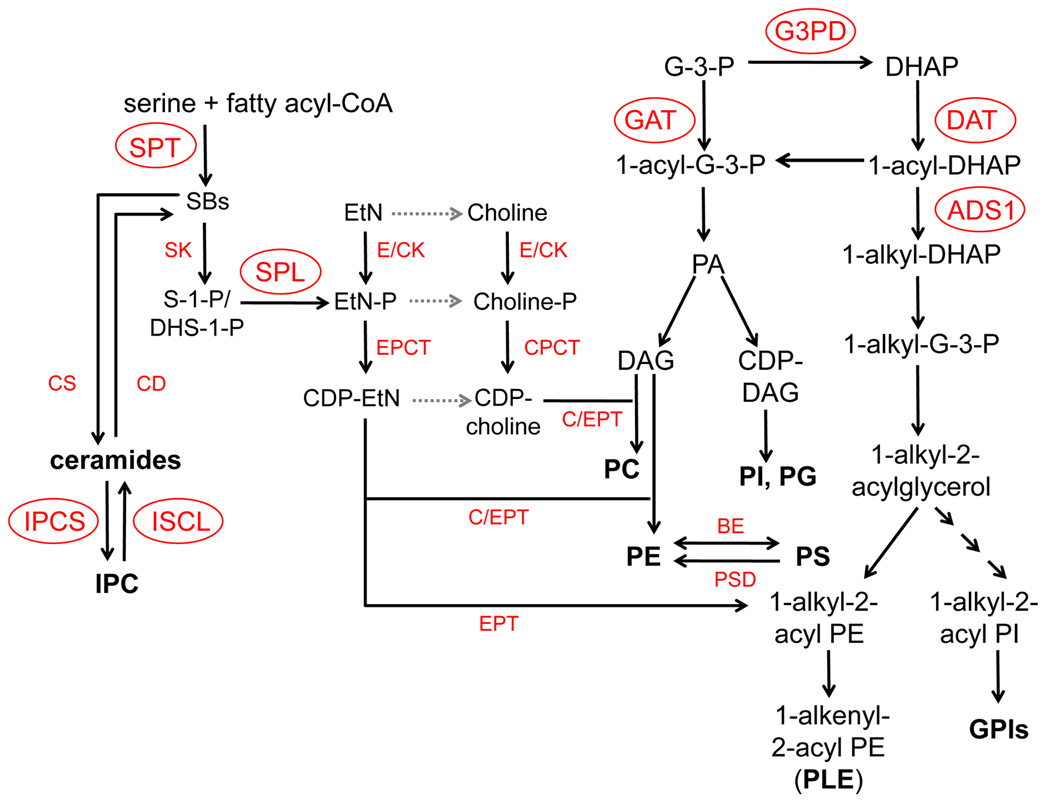
Predicted metabolism of SLs and PLs in Leishmania. DAG: diacylglycerol; G-3-P: glycerol-3-phosphate; DHAP: dihydroxyacetonephosphate. Enzymes of which candidate genes have been identified in the L. major geneDB are indicated. Gray dotted arrows represent pathways where the putative enzymes have yet to be identified. Experimentally characterized enzymes/genes (circled) include DAT (dihydroxyacetone phosphate acyltransferase, system ID: LmjF34.1090), GAT (glycerol-3-phosphate acyltransferase, LmjF03.0080), ADS1 (1-alkyl dihydroxyacetone phosphate synthase 1, LmjF30.0120), G3PD (glycerol-3-phosphate dehydrogenase, LmjF10.0510), SPT (serine palmitoyltransferase, LmjF34.3740 and LmjF35.0320), SPL (sphingosine-1-phosphate lyase, LmjF30.2350), IPCS (IPC synthase, LmjF35.4990), and ISCL (inositol sphingolipidphospholipase C-like, LmjF08.0200). Other enzymes (that have yet to be characterized although their putative genes have been identified from L. major genome) include CS (ceramide synthase); CD (ceramidase); SK (sphingosine kinase); E/CK (ethanolamine/choline kinase); EPCT (ethanolamine-phosphate cytidylyltransferase); CPCT (cholinephosphate cytidylyltransferase); EPT (ethanolamine-specific phosphotransferase); C/EPT (choline/ethanolamine phosphotransferase); BE: (base exchange enzyme or PS synthase); and PSD (PS decarboxylase).
PS exposure in Leishmania has attracted great interest recently due to its ability to serve as a ‘deactivating’ signal for host cells, following the paradigm of PS exposure on apoptotic mammalian cells limiting macrophage activation [38, 39]. This may occur indirectly through exposure of PS on Leishmania infected host cells, which are then engulfed by phagocytic cells. This ‘Trojan Horse’ model suggests that Leishmania co-opts host cells to gain safe entry into macrophages without activation. Other studies raise the possibility that PS exposure on the parasite surface directly interacts with host cells, leading to the release of anti-inflammatory cytokines such as TGF-β and the down-regulation of pro-inflammatory cytokine TNF-α in host phagocytes [12]. Similar apoptotic mimicry has been observed during the internalization of Leishmania amazonensis amastigotes [40]. Nonetheless, further studies are needed to determine whether PS exposure on the parasite is actively controlled. Two recent studies suggest that dying parasites with PS exposure may contribute to increased lesion pathology in Leishmania infections [12, 13]. However since dead antigen co-inoculated with parasites also can result in increased lesion pathology [41, 42], further work is needed to establish the role of parasite PS exposure in pathogenicity.
Unlike mammals or plants, Leishmania parasites do not synthesize sphingomyelin or complex glyco-SLs. Instead, the majority of SLs in Leishmania belong to unglycosylated inositol phosphorylceramide (IPC) and ceramide (Fig. 1E–F)[27, 43, 44]. IPC is absent in mammals but common in fungi, where it exists as free or glycosylated forms [45, 46]. In L. major, IPC species include phosphoryl inositol N-stearoylhexadecesphing-4-enine (d16:1/18:0-IPC) and phosphoryl inositol N-stearoylsphingosine (d18:1/18:0-IPC), with the former being the dominant species (Fig. 1F). Since the long chain base (sphingoid base) of SLs is synthesized through the condensation of serine and fatty acyl-CoA, it is concluded that Leishmania enzyme preferentially incorporates myristoyl-CoA (C14) over palmitoyl-CoA (C16) into their long chain base and ceramide (Fig. 1E) [47]. This differs from mammalian cells which mostly use palmitoyl-CoA to synthesize their SLs [48]; or yeasts (S. cerevisiae) in which palmitoyl Co-A is the preferred substrate [49] and in vivo sphingoid bases arise primarily from C16 and C18 acyl-CoA [50].
In addition to IPC, neutral glycosphingolipids have been isolated from the amastigote forms of Leishmania amazonensis in hamster foot lesions [51]. Structure of these surface neutral glycosphingolipids was partially elucidated by negative ion fast atom bombardment mass spectrometry. The glycan moieties presented linear sequences of hexoses and N-acetylhexosamines ranging from four to six sugar residues, and the ceramide moieties consisted of a d18:1 sphingosine and fatty acids of C24:1 or C16:0 [52]. These glycosphingolipids may play a role in parasite invasion, as monoclonal antibodies specific for their carbohydrate epitopes blocked macrophage invasion by amastigotes [51]. Origin of these neutral glycosphingolipids is not known, as they may be synthesized by the parasites or salvaged from the mammalian host.
Overall, the composition of PLs and SLs in Leishmania is quite different from that in mammals, which may reflect the difference in membrane physiology between parasites and hosts. As major membrane components, these lipids may determine the membrane permeability and fluidity and have profound impact in vesicular trafficking, nutrient acquisition through endocytosis, or cell differentiation which involves extensive membrane remodeling/reorganization and macroautophagy. In addition, the polyunsaturated fatty acid chains found in PC could confer resistance to host-derived oxidants. The difference between Leishmania and mammals in lipid composition could be exploited towards selective chemotherapy. For example, lysophospholipid analogs including miltefosine, edelfosine, and ilmofosine have been used successfully as oral drugs to treat cutaneous and visceral leishmaniasis [53, 54]. These compounds possess potent leishmaniacidal activity, although the molecular mechanism of such activity is still controversial. Amongst their various effects in Leishmania parasites, inhibition of membrane lipid synthesis and induction of apoptosis or necrosis have been observed [55–57]. Therefore, understanding the roles of lipid metabolism in Leishmania parasites could have important implications in guiding the development of novel PL analogues that possess anti-protozoan activity.
3. Metabolism
3.1 Acquisition and/or synthesis of PL and SL headgroups (EtN, choline, and inositol)
EtN, choline, and inositol are essential nutrients which form the headgroups of most PLs and GPIs. As discussed in 3.3, Leishmania can convert serine into EtN via SL metabolism, which is the major pathway for EtN production in promastigotes [23]. It is not clear whether Leishmania parasites can synthesize choline de novo, although EtN can be incorporated to PC (Fig. 2). In L. mexicana and T. brucei, inositol is synthesized from glucose 6-phosphate using two sequentially acting enzymes: inositol-3-phosphate synthase which converts glucose 6-phosphate to inositol 3-phosphate, and then inositol monophosphatase which dephosphorylates inositol 3-phosphate to generate inositol [58, 59]. In addition to biosynthesis, these head group are also incorporated in Leishmania parasites via membrane transporters. The uptake of inositol is mediated by a high-affinity myo-inositol/H+ transporter driven by proton gradient [60]. The uptake of EtN and choline has been biochemically characterized in Leishmania [61, 62], although their respective transporters have yet to be identified.
3.2 Enzymes involved in the synthesis of PE
In eukaryotes, PE may be synthesized via three routes: 1) through the Kennedy pathway: EtN ⇒ ethanolamine phosphate (EtN-P) ⇒ CDP-EtN ⇒ PE (Fig. 2) [63]; 2) through the decarboxylation of PS (by PS decarboxylase) which occurs in mitochondria or Golgi in many eukaryotes [64] (localization is unknown in Trypanosomatids); and 3) via reversible head group exchange (base exchange) between PE, PC, and PS [65]. In T. brucei, recent evidence suggests that Kennedy pathway is the dominant route for PE synthesis and essential for survival and mitochondrial function [28, 30, 66]. In L. major, SL and PE metabolism are linked through EtN-P, which can be generated from the degradation of phosphorylated sphingoid bases (Fig. 2) [23]. Most candidate genes for PE synthesis (Fig. 2) have been identified from Leishmania genomes, and a handful of them have been experimentally characterized in Leishmania or T. brucei, as described below.
3.2.1 Glycerol-3-phosphate acyltransferase (GAT) and glycerol-3-phosphate dehydrogenase (G3PD)
Synthesis of diacyl- and ether PLs starts with glycerol-3-phosphate (G3P) and dihydroxyacetone phosphate (DHAP) (Fig. 2), which can be generated from the metabolism of sugars. GAT catalyzes the acylation of G3P at the sn-1 position to produce 1-acyl-G3P (Fig. 2). 1-acyl-G3P can also be generated from 1-acyl-DHAP by a NADPH-dependent 1-alkyl/1-acyl-DHAP reductase. In mammals and yeast, 1-acyl-G3P is used for the biosynthesis of phosphatidic acid, diacylglycerol, triacylglycerol, and ester PLs (diacyl-PC, PE, PI, and PG) (Fig. 2) [67, 68]. L. major GAT exhibits a low-affinity for G3P and prefers unsaturated fatty acyl-CoA (C16:1, C18:1, C18:2) as substrates [69]. DHAP, however, is not a preferred substrate for this enzyme. Deletion of LmGAT resulted in a major reduction (~50%) in triacylglycerol synthesis but had little effect on diacyl- (PC and PI) or ether PLs [69]. In addition, null mutants of LmGAT contained normal levels of LPG and GPI-anchored proteins (which contain ether lipids in their GPI-anchors) and their virulence was not affected in mice. These results suggest that DHAP, not G-3-P, is the primary precursor for PL biosynthesis in Leishmania. Alternatively, L. major may contain an as yet unidentified GAT that is responsible for the initial step of ester phospholipid synthesis.
L. major contains a mitochondrial FAD-dependent glycerol-3-phosphate dehydrogenase (G3PD) [70] (Fig. 2); in addition, two more putative G3PD genes have been identified from the L. major genome. One or more of these enzymes may be involved in the G3P/DHAP shuttle between glycosome and mitochondrion [70].
3.2.2 Dihydroxyacetone phosphate acyltransferase (DAT) and 1-alkyl-DHAP synthase (ADS1)
In many organisms, the de novo synthesis of ether PLs starts with the acylation of DHAP by the enzyme dihydroxyacetone phosphate acyltransferase (DAT) [71](Fig. 2). The resulting product, 1-acyl-DHAP, is converted to 1-alkyl-DHAP by a FAD-dependent alkyl DHAP synthase (ADS1) [22, 72], and further reduced to 1-alkyl-glycerol-3-phosphate (1-alkyl-G3P) by an NADPH-dependent 1-alkyl/acyl-DHAP reductase (Fig. 2). 1-Alkyl-G3P serves as the obligate precursor for all ether PLs, including PLE, alkyl-acyl-PI, and alkyl-lyso-PI species which are incorporated into the GPI-anchors in Leishmania (Fig. 2) [19]. L. major DAT is unusually large (1436 amino acids) with an N-terminal domain of ~650 amino acids which does not have similarity to any known proteins. This glycosome-localized DAT has a high specificity for DHAP (but not G-3-P) and palmitoyl-CoA. Deletion of the DAT gene (LmjF34.1090) in L. major results in a complete loss of DAT activity [73], suggesting it is the sole functional enzyme. Significantly, DAT mutants could not synthesize ether PLs, yet the production of diacyl PLs was not affected [74]. Genetic analysis suggests LmDAT is synthetic lethal with LmGAT [74]. Together, these data imply both DAT and GAT are involved in the initiation of diacyl PL synthesis and one enzyme can compensate the loss of the other. Finally, null mutants of DAT grew slowly as promastigotes with increased cell death during stationary phase and failed to induce pathology in BALB/c mice [73], suggesting ether PLs are important for promastigote virulence.
ADS1 catalyzes the reduction of 1-acyl-DHAP to 1-alkyl-DHAP, the first committed step of ether lipid synthesis. Similar to DAT, L. major ADS1 lacks obvious transmembrane domains but contains a tripeptide SKI at the C terminus, which could serve as a targeting signal for the glycosome. Biochemical analysis revealed that L. major ADS1utilizes 1-palmitoyl-DHAP (the main product of DAT) as a preferred substrate. Lipid analysis of ADS1 mutants demonstrates that this enzyme is required for the synthesis of ether PLs and their derivatives including PLE, LPG, and GIPLs [22] but not diacyl-PC or lyso-PCs. Compared to DAT, defects of ADS1 null mutants were less severe as these cells grew reasonably well as promastigotes, suggesting ether lipids are not essential for survival or in vitro growth [22]. Notably, ADS1 mutants (ads1−) were sensitive to complement and unable to survive the initial phase of macrophage infection; however, amastigotes of ads1− were infective in both macrophage and mouse infections [22]. These virulence defects were similar to a LPG-deficient mutant lpg1− [75], suggesting PLE or GIPLs are not required for Leishmania (amastigotes) growth in macrophages or in mouse infections.
3.2.3 Other enzymes involved in PE metabolism
Both L. major and T. brucei genomes contain two EtN/choline kinases (E/CKs), which catalyze the initial steps of Kennedy pathway to produce EtN/choline phosphate (Fig. 2). TbEK1 (T. brucei EtN kinase 1) was shown to be an EtN-specific kinase, whereas TbEK2 was able to phosphorylate both EtN and choline, with choline being the preferred substrate [76]. In addition, recent evidence suggests that in T. brucei procyclic forms, the last step in the Kennedy pathway, i.e. the conversion from CDP-EtN to PE, is mediated by two separate activities, one leading to PLE and the other leading to diacyl-PE [28]. Furthermore, RNAi or conditional knockout of ethanolamine-phosphate cytidylyltransferase (EPCT) led to disrupted mitochondrial morphology and function, and eventually cell death in T. brucei [28, 66][30]. Future studies will determine whether the separation of PLE and PE synthesis is due to compartmentalization or substrate specificity. Orthologs of both TbEPT and TbC/EPT are present in Leishmania (Fig. 2) and functional characterizations of these genes will unravel their specificities.
Leishmania genomes also contain two genes predicted to encode enzymes that may be involved in the synthesis of PS and/or PE: a based exchange enzyme which swaps headgroups between PS and PE, and a PS decarboxylase which converts PS to PE. Given the importance of PS in parasite-host interaction [38, 39], functional studies are needed to reveal the contribution of these enzymes in PL metabolism and Leishmania infection.
3.3 Enzymes involved in SL metabolism and EtN production: serine palmitoyltransferase (SPT) and sphingosine-1-phosphate lyase (SPL)
De novo SL biosynthesis starts with the condensation of L-serine and palmitoyl-CoA into 3-ketosphinganine. This is catalyzed by a pyridoxal 5′-phosphate-dependent enzyme serine palmitoyltransferase (SPT, EC 2.3.1.50) consisting of two subunits, SPT1 and SPT2 [77, 78] (Fig. 2). Two ORFs with homology to SPT1 and SPT2 have been identified from the L. major genome with SPT2 being the putative catalytic subunit. It is noteworthy that the main IPC species in L. major contains C16 as the long chain base, suggesting that L. major SPT prefers myristoyl-CoA over palmitoyl-CoA as a substrate [27, 47]. Deletion of SPT2 in L. major results in a complete loss of IPC and other SLs. Although viable as promastigotes, these spt2− mutants failed to differentiate to infective metacyclic parasites and died rapidly. Importantly, spt2− parasites maintained `lipid rafts' as defined by Triton X-100 detergent resistant membrane formation (see more in the next section).
To further examine the potential role of SLs in differentiation and virulence, another mutant which is defective in the degradation of SLs (spl−) was generated by deleting the gene encoding for sphingosine-1-phosphate lyase (SPL) [23]. SPL is responsible for the breakdown of phosphorylated sphingoid bases, such as sphingosine-1-phosphate (S-1-P) or dihydrosphingosine-1-phosphate (DHS-1-P), to produce EtN-P and hexadecenal/hexadecanal (Fig. 2). Surprisingly, despite their distinct cellular SL levels (spt2− promastigotes are SL-null whereas spl− mutants show slight increased IPC levels), both mutants contain less PLE compared WT parasites in stationary phase and fail to differentiate to infective metacyclics [23]. More importantly, defects in PLE synthesis and metacyclogenesis can be fully restored in both mutants through the supplementation of ethanolamine (EtN) or EtN-P, the degradative product of SPL (Fig. 2). These data indicate that unlike most other eukaryotes, the primary role of SL metabolism in Leishmania major promastigotes is to effectively convert serine into EtN, which is the main source of EtN for promastigotes (Fig. 2).
3.4 Enzymes involved in the metabolism of IPC: IPC synthase and inositol sphingolipidphospholipase C-like protein (ISCL)
IPC, the end product of SL biosynthesis in Leishmania, is generated by the transfer of a phosphorylinositol headgroup from PI to ceramide, a process catalyzed by the IPC synthase. In fungi, this enzyme has been established as a target for the development of anti-fungal compounds including aureobasidin A1 [79]. Based on sequence analysis, L. major IPC synthase contains six predicted transmembrane regions and two putative lumenal motifs (each contains histidine and aspartate residues which mediates nucleophilic attack on lipid phosphate ester bonds) which constitute the catalytic domain [29]. This predicted topology is shared by the sphingolipid synthases in T. brucei (a family of four) and is more conserved with respect to mammalian sphingomyelin synthases than to fungal IPC synthases [29, 80, 81]. Expression of L. major IPC synthase in a mammalian cell line (HEK293) or in blood stream trypanosomes leads to the synthesis of an IPC-like species [80][29]. In addition, L. major IPC synthase partially complements the pleomorphic defects in cell morphology and division observed in AUR1 mutants of S. cerevisiae [80, 82]. Further biochemical and genetic analyses are needed to determine whether IPC synthesis is essential for Leishmania, especially in the amastigote stage.
The abundance of non-mammalian IPC in Leishmania has suggested to many that IPC synthesis may be a good target for chemotherapeutic intervention [80, 83], analogous to proposals arising from the toxicity and specificity of the yeast IPC synthase inhibitor aureobasidin A [79, 84]. Although several studies point to significant toxicity of auerobasidin A against various Leishmania species, that toxicity can occur at concentrations not affecting IPC synthesis [26, 80, 85]. For instance, while auerobasidin A inhibits L. major promastigote growth with an EC50 of 0.6 µM, mass spectrometry analysis revealed no effect on IPC synthesis at this concentration, and IPC synthesis inhibition was not seen until >5.0 µM auerobasidin A [26]. Consistent with these findings, studies of heterologous expressed IPC synthase suggests an enzymatic IC50 on the order of 100 µM [80]. Similarly, the T. cruzi IPC synthase is insensitive to auerobasidin A [86, 87]. Lastly, auerobasidin A was similarly toxic to the spt2− parasites, which lack IPC [26, 44]. These findings suggest that the major inhibitory activity of auerobasidin A against L. major in vivo may involve a target other than IPC synthase, while not yet excluding this enzyme as a potential amastigote target for more effective inhibitors in the future.
Despite the lack of sphingomyelin synthesis, L. major promastigotes possess a potent sphingomyelinase (SMase) activity which is dependent on the ISCL (Inositol phosphoSphingolipid phospholipase C-Like) protein. ISCL is also required for the degradation of IPC, although its activity with sphingomyelin is 10–20 fold greater than that seen with IPC [88]. Such dual activity is also observed from the yeast homolog, ScISC1p, which is both a neutral SMase and an IPC hydrolase [89]. Null mutants of ISCL (iscl−) showed modest accumulation of IPC, but grew and differentiated normally in vitro. Interestingly, iscl− mutants did not induce lesion pathology in the susceptible BALB/c mice, yet persisted indefinitely at low levels at the site of infection [88]. Notably, the acute virulence of iscl− was completely restored by the expression of ISCL or heterologous mammalian or fungal SMases, but not by fungal proteins exhibiting only IPCase activity [88]. Since Leishmania do not synthesize sphingomyelin, together, these findings strongly suggest the degradation of host-derived sphingomyelin plays a pivotal role in the proliferation of Leishmania in mammalian hosts and the manifestation of acute disease pathology.
3.5 Salvage of PLs and SLs
In addition to de novo synthesis, Leishmania parasites also acquire PLs and SLs through salvage. Promastigotes of spt2− and spl− (neither can convert SL metabolites to EtN) grow at near wild type (WT) rates during log phase and contain normal amount of PLE and other Leishmania-specific ester PLs [23, 27], indicating these parasites can salvage lipid metabolites from the culture medium which contains fetal bovine serum. Amastigotes of spt2− and spl− are morphologically normal and fully virulent in both macrophage and mouse infections [26]. Importantly, despite the lack of de novo synthesis, amastigotes of spt2− contain significant amounts of IPC (~108 molecules per cell) [26], a SL not synthesized by mammalian cells; both mutants also make WT-level of PLE (1~2 ×108 molecules per cell) in amastigote stages [26]. Together, these data suggest amastigotes actively acquire and remodel host lipids and convert them into parasite-specific SLs/PLs. So far, amastigotes from all species of Leishmania contain host-derived SLs and PLs [90, 91], which could be degraded to generate intermediates for lipid synthesis, or serve as precursors for headgroup remodeling. Uptake of PLs may involve membrane-associated ATP-binding cassette (ABC) transporters. Genomes of L. major and L. infantum contain a family (42 in total) of these transporters. Several of these ABC transporters have been characterized and they possess activity to inwardly translocate PC, PE, PS, alkyl-glycerophosphocholine, and miltefosine [92–95]. Other members of the family, such as the ABCG-like transporters, confer resistance to alkyl-phospholipids when overexpressed [96, 97]. Therefore, these ABC transporters are not only involved in transbilayer lipid movement, but also play a role in the uptake and efflux of drugs.
4. Functions
4.1 Anchors for surface glycoconjugates
In Leishmania, surface virulence factors including LPG, GIPLs, and GPI-proteins all contain ether PLs in their anchors. Specifically, the lipid moieties of GIPLs and GPI-anchored proteins are sn-1-alkyl-2-acyl-PIs [19, 98], whereas that of LPG is sn-1-alkyl-2-lyso-PI and the alkyl group is C24–26 [99, 100]. The long alkyl chain in LPG has been implicated, biochemically, in the down-regulation of host cell responses, including the inhibition of protein kinase C and nitric oxide (NO) production [101, 102]. However, genetic studies with the ADS1 mutants (null for most GPI-anchored molecules) in L. major indicate lyso-alkyl- or alkylacyl-GPI anchors are not solely responsible for the inhibition of macrophage activation or amastigote survival in mammalian hosts [22].
In Trypanosomatids, EtN is a component of the core structure in the GPI-anchored proteins consisting of EtN-phosphate-6-mannose-α1,2-mannose-α1,6-mannose-α1,4-glucosamine-α1,6-myoinositol-1-phospholipid [19]. The EtN moiety was transferred from PE onto a GPI precursor lipid, followed by the attachment of the precursor to a polypeptide in the endoplasmic reticulum [62, 103]. In T. brucei, inhibition of PE and PI metabolism has negative impact on the synthesis of GPI-anchored virulence factors [30, 104, 105].
Finally, IPC is the end product of SL biosynthesis in Leishmania and to date, there is no evidence showing that IPC or ceramide can serve as anchors for glycoconjugates. Trypanosoma cruzi and Trichomonas vaginalis, however, do use IPC as a carrier for LPG-like surface molecules [106, 107]. Synthesis of these IPC-anchored glycoconjugates is not well characterized, although current data suggest the ceramide moiety in T. cruzi GPI is likely added through a remodeling process [108], whereas T. vaginalis ceramide-GPIs is probably made de novo since key enzymes in the standard diacyl GPI pathway are missing in the genome.
4.2 Metacyclogenesis
In L. major, the exit of replicating promastigotes from the cell cycle into stationary growth phase is accompanied by differentiation to the infective metacyclic form (a process known as metacyclogenesis). Notably, the cellular level of PLE rises significantly (2–3 fold) as promastigotes progress into stationary phase, suggesting this lipid may play critical role(s) as parasites undergo differentiation. PLE possesses different physical properties compared with diacyl PE [109], including an ability to promote rapid membrane fusions [110], and a propensity to form and/or interact with organized membrane microdomains. These unique membrane properties could play an important role during metacyclogenesis, a time marked by greatly increased membrane trafficking and remodeling. The onset of stationary phase also coincides with an increase in macroautophagy activity, where a key step involves the conjugation of PE to ATG8 proteins [111]; macroautophagy mutants in L. major show interesting defects in metacyclogenesis and infectivity, suggesting PE may be involved in promastigote differentiation[112].
4.3 Organization of lipid rafts
As in other organisms, Leishmania SLs (mostly IPC) and sterols are enriched in organized membrane microdomains termed lipid rafts, whereas ester PLs such as diacyl PC are usually found in a more fluid and disordered state due to their unsaturated fatty acid chains and low melting temperatures [113, 114]. While less extensively studied, ether PLs including PLE are preferentially associated with lipid rafts and may promote the formation of raft-like domains [115–117]. Previous studies have shown that the organization of Leishmania membrane differs greatly between procyclics and metacyclics. For example, LPG is not associated with lipid rafts in procyclics but is enriched in detergent resistant membrane (DRM) faction in metacyclics [113]. Interestingly, it has been suggested that the macroscopically visible alterations in the metacyclic surface arise from the formation of lipid rafts [118], although this must be reconciled with studies in other organisms suggesting that lipid rafts may be considerably smaller [119].
The generation of viable and largely healthy ads1− and spt2− parasites provided an excellent oppotunity to address an important question: can lipid rafts be formed in the absence of ether PLs or SLs in the context of a living organism? Previous studies employing a limited number of markers suggested that Leishmania could maintain lipid rafts despite the complete loss of either of these lipid classes [22, 27]. Most studies relied upon the relatively non-stringent method of DRM extraction, as raft proteins are usually found in insoluble fractions after mild treatment with a non-ionic detergent (e. g. Triton X100). Similar results were obtained with the spt2− mutant with detergent free approaches [27].
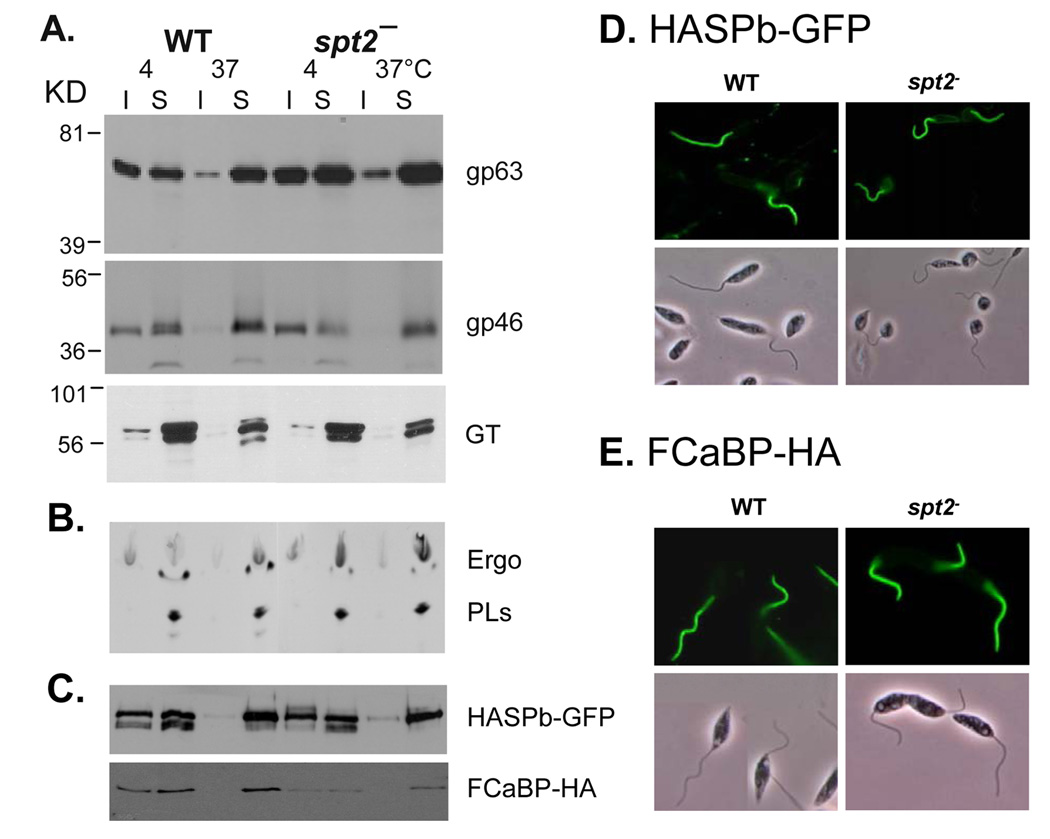
Recently we extended these findings to include other ‘raft markers’. As shown in Fig. 3A, two GPI-anchored proteins, GP63 and GP46, were strongly associated with DRM at 4 °C but not 37 °C in both WT and SL-null parasites (spt2−), whereas a non-raft membrane protein, glucose transporter [120], was clearly not associated with DRM. In addition, both WT and spt2− DRMs were rich in ergosterol (the dominant sterol species in Leishmania) but not ester PLs (Fig. 3B), suggesting the loss of SLs does not affect the composition of raft lipids in Leishmania. To examine whether engineered reporter proteins are targeted to rafts in the absence of SLs, transgenic parasites expressing either a GFP-tagged L. major HASPB fusion protein [121] or a HA-tagged T. cruzi Calcium-binding protein FCaBP [122] were subjected to detergent extraction. Results showed both were associated with DRM as expected, and targeted to the flagellum, which are known to be enriched in lipid rafts (Fig. 3D–E)[123]. Significantly, these behaviors were maintained in the SL-null spt2− cells (Figs. 3C–E), again pointing to a remarkable ability to maintain lipid raft-like targeting in the absence of SLs.
Figure 3.
SL-null L. major generates normal DRM rafts. (A) TX100 insoluble (I) and soluble (S) fractions from log phase parasites (WT and spt2−) were analyzed by western-blot to assess the distribution of gp63, gp46, and glucose transporter (GT). (B) Log phase WT and spt2− parasites were labeled with [1,2]14C-acetate and extracted with 1% TX100 at 4 °C or 37 °C. Detergent insoluble materials were separated from soluble materials by centrifugation. Lipids from both insoluble and soluble fractions were extracted and analyzed by thin layer chromatography. Ergo: ergosterol; PLs: phospholipids. (C) WT and spt2− parasites with episomally expressed HASPB-GFP or FCaBP-HA were subjected to detergent extraction and western-blot analysis as described in (A), using anti-GFP and anti-HA antibodies. In addition, these parasites were subjected to fluorescence microscopy, as shown in D (GFP epifluorescence) and E (primary antibody: anti-HA mAb; secondary antibody: FITC-labeled goat-antimouse IgG). Leishmania culture, DRM isolation, and western-blot were performed as previously described [27]. To isolate metacyclics, spt2− mutants were grown to stationary phase in the presence of 500 µM of EtN as previously described [23].
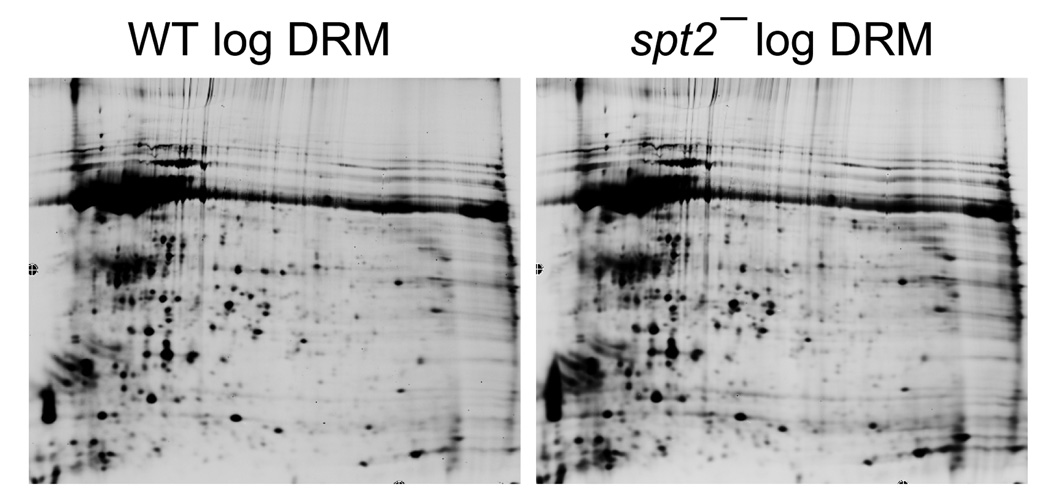
Furthermore, preliminary results from unbiased proteomic studies of DRMs from WT vs. spt2− parasites showed a remarkable similarity (>98% of protein spots showed similar abundance between WT and spt2−, Fig. 4). Together, these data suggest that SLs play a relatively minor role in the formation of lipid rafts and the targeting of raft proteins.
Figure 4.
2D gel analysis of DRM fraction in L. major. DRM fractions were prepared from log phase WT and spt2− parasites as described [27] and subjected to 2D gel electrophoresis. Among all detectable spots, only ~1.2% showed greater than 2.5-fold variation between WT and spt2−.
Similar to spt2−, the absence of ether PLs in ads1− does not affect the formation of lipid rafts as defined by buoyant DRM criterion [22, 74]. Together, these data suggest the rather unique redundancy in lipid composition (the combination of ether PLs, SLs, and ergosterol) may render Leishmania parasites more resistant to lipid perturbations that are generally fatal to other organisms.
5. Further characterization of PL and SL metabolism
Leishmania PCs contain relatively long and unsaturated fatty acid species, including C22:5 and C22:6 [22]. Long chain fatty acids are also found in the lyso-PI anchor of LPG [100, 124]. The L. major genome contains 13 candidate genes for fatty acid elongases and 12 for fatty acid desaturases. Some elongases are potentially involved in the synthesis of bulk fatty acids using butyl-CoA as a precursor, a process first described in T. brucei [125], while others may only elongate palmitoyl-CoA or stearoyl-CoA. Further genetic, biochemical, and lipidomic studies are needed to address the substrate specificity of these enzymes and the roles of long, unsaturated fatty acids in Leishmania.
In Trypanosoma brucei, PE is a direct precursor of the EtN-phosphoglycerol moiety found on the side chain of Glu362 of eukaryotic elongation factor 1A (eEF1A), as RNAi knock down of enzymes in the Kennedy pathways led to inhibition of eEF1A modification [126]. The function of EtN-phosphoglycerol modification is not clear, although similar eEF1A modifications have been identified in mammalian cells [127, 128] and plants [129].
While several studies have focused on SL degradation [23, 88], very little is known about the enzymes involved in PL degradation. The activity of phospholipase D has been detected in L. donovani and this activity increases in response to acute osmotic stress [130]. T. brucei contains a membrane bound glycosyl-phosphatidylinositol phospholipase C (GPI-PLC) which can cleave dimyristoylglycerol from the variant surface glycoprotein and protein-GPI precursors [131, 132]. Interestingly, L. major cells expressing GPI-PLC have a GPI-deficient phenotype [133]. PL degradation may possess additional functions. For example, living inside the host phagolysosomes, which is rich in lipids and amino acids but poor in sugars [134, 135], Leishmania amastigotes may depend on salvaged host lipids for carbon sources. Twenty-one putative lipase genes have been identified in the L. major gene DB and some could be responsible for the production fatty acids and other nutrients from scavenged host lipids.
6. Comparison of PL and SL metabolism between L. major and T. brucei
6.1 De novo SL biosynthesis is essential for T. brucei but not L. major
Unlike L. major, which remained fully viable as replicating procyclics in the absence of de novo SL synthesis [27, 44], inhibition of SPT by myriocin led to aberrant cytokinesis, characterized by delayed kinetoplast segregation and emergence of cells with abnormal DNA content, followed by cell death [136, 137]. Similarly, RNAi silencing of SL synthases (which are responsible for the synthesis of high order SLs in T. brucei including IPC and sphingomyelin) in bloodstream T. brucei led to rapid growth arrest and eventual cell death [29]. Thus unlike Leishmania where IPC synthesis is not essential in culture and possibly neither for mammalian infectivity, inhibition of SL synthesis may provide valid drug targets in T. brucei.
6.2 Developmental stage-dependent PL and SL composition
As described earlier for Leishmania (section 4.2), developmental stage-dependent modulation of lipid metabolism has also been observed in T. brucei. For example, procyclic trypanosomes contain IPC and sphingomyelin, while surprisingly bloodstream-stage parasites contain sphingomyelin and ethanolamine phosphorylceramide, but no detectable IPC [29]. As an extracellular parasite, the lack of IPC may help bloodstream trypanosomes avoid triggering host immune response.
Concluding remarks
Lipids are essential components of life and the recent advance of genome sequencing, molecular biology, and lipidomic tools have greatly facilitated the study on lipid metabolism in Leishmania. In addition to being membrane components, PLs and SLs play multiple roles in Leishmania cell biology: PI serves as anchors for important surface virulence factors; EtN synthesis through SL degradation is essential for the survival and differentiation of stationary phase promastigotes; uptake and turnover of SLs/PLs may be an essential route of nutrient uptake. Compared to other organisms, Leishmania membrane is rather unique in that the loss of ether PLs or SLs does not have significant effect on the formation and functions of lipid rafts. Additional roles will be elucidated in the near future and we expect the new knowledge to have important implications in the development of novel drugs.
Acknowledgements
We thank Drs. Reid Townsend for the 2D gel analysis of Leishmania DRMs; D. McMahon-Pratt and S. Landfear for antisera to GP46 and glucose transporter; D. Engman for the molecular construct containing T. brucei FCaBP gene; and F. Matthew Kuhlmann for comments on this manuscript. This work was supported by NIH grant AI31078 to SMB and a TTU Research Development grant to KZ.
Abbreviations
- PL
phospholipid
- SL
sphingolipid
- PE
phosphatidylethanolamine
- PC
phosphatidylcholine
- PI
phosphatidylinositol
- PS
phosphatidylserine
- IPC
inositol phosphorylceramide
- PLE
plasmenylethanolamine
References
- 1.Majerus PW, et al. The metabolism of phosphoinositide-derived messenger molecules. Science. 1986;234:1519–1526. doi: 10.1126/science.3024320. [DOI] [PubMed] [Google Scholar]
- 2.Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakraborti S. Phospholipase A(2) isoforms: a perspective. Cell Signal. 2003;15:637–665. doi: 10.1016/s0898-6568(02)00144-4. [DOI] [PubMed] [Google Scholar]
- 4.Divecha N, Irvine RF. Phospholipid signaling. Cell. 1995;80:269–278. doi: 10.1016/0092-8674(95)90409-3. [DOI] [PubMed] [Google Scholar]
- 5.Coppolino MG, et al. Inhibition of phosphatidylinositol-4-phosphate 5-kinase Ialpha impairs localized actin remodeling and suppresses phagocytosis. J Biol Chem. 2002;277:43849–43857. doi: 10.1074/jbc.M209046200. [DOI] [PubMed] [Google Scholar]
- 6.Santarius M, Lee CH, Anderson RA. Supervised membrane swimming: small G-protein lifeguards regulate PIPK signalling and monitor intracellular PtdIns(4,5)P2 pools. Biochem J. 2006;398:1–13. doi: 10.1042/BJ20060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krauss M, et al. Stimulation of phosphatidylinositol kinase type I-mediated phosphatidylinositol (4,5)-bisphosphate synthesis by AP-2mu-cargo complexes. Proc Natl Acad Sci U S A. 2006;103:11934–11939. doi: 10.1073/pnas.0510306103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fadok VA, et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 9.Balasubramanian K, Mirnikjoo B, Schroit AJ. Regulated externalization of phosphatidylserine at the cell surface: implications for apoptosis. J Biol Chem. 2007;282:18357–18364. doi: 10.1074/jbc.M700202200. [DOI] [PubMed] [Google Scholar]
- 10.Vance JE, Steenbergen R. Metabolism and functions of phosphatidylserine. Prog Lipid Res. 2005;44:207–234. doi: 10.1016/j.plipres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Nguewa PA, et al. Programmed cell death in trypanosomatids: a way to maximize their biological fitness? Trends Parasitol. 2004;20:375–380. doi: 10.1016/j.pt.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 12.van Zandbergen G, et al. Leishmania disease development depends on the presence of apoptotic promastigotes in the virulent inoculum. Proc Natl Acad Sci U S A. 2006;103:13837–13842. doi: 10.1073/pnas.0600843103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wanderley JL, et al. Cooperation between apoptotic and viable metacyclics enhances the pathogenesis of Leishmaniasis. PLoS One. 2009;4:e5733. doi: 10.1371/journal.pone.0005733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hannun YA, Obeid LM. The Ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem. 2002;277:25847–25850. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- 15.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 16.Chalfant CE, et al. Long chain ceramides activate protein phosphatase-1 and protein phosphatase-2A. Activation is stereospecific and regulated by phosphatidic acid. J Biol Chem. 1999;274:20313–20317. doi: 10.1074/jbc.274.29.20313. [DOI] [PubMed] [Google Scholar]
- 17.Wang G, et al. Direct binding to ceramide activates protein kinase Czeta before the formation of a pro-apoptotic complex with PAR-4 in differentiating stem cells. J Biol Chem. 2005;280:26415–26424. doi: 10.1074/jbc.M501492200. [DOI] [PubMed] [Google Scholar]
- 18.Heinrich M, et al. Ceramide as an activator lipid of cathepsin D. Adv Exp Med Biol. 2000;477:305–315. doi: 10.1007/0-306-46826-3_33. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson MA. The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions of trypanosome research. J Cell Sci. 1999;112(Pt 17):2799–2809. doi: 10.1242/jcs.112.17.2799. [DOI] [PubMed] [Google Scholar]
- 20.Pulfer M, Murphy RC. Electrospray mass spectrometry of phospholipids. Mass Spectrom Rev. 2003;22:332–364. doi: 10.1002/mas.10061. [DOI] [PubMed] [Google Scholar]
- 21.Hsu FFT, J . Applications in Biochemistry, Biology and Medicine. Vol. 3. New York: Elsevier Science; 2005. Tandem mass spectrometry with electrospray ionization of sphinomyelins. The Encyclopedia of Mass Spectrometry; pp. 430–447. [Google Scholar]
- 22.Zufferey R, et al. Ether phospholipids and glycosylinositolphospholipids are not required for amastigote virulence or for inhibition of macrophage activation by Leishmania major. J Biol Chem. 2003;278:44708–44718. doi: 10.1074/jbc.M308063200. [DOI] [PubMed] [Google Scholar]
- 23.Zhang K, et al. Redirection of sphingolipid metabolism toward de novo synthesis of ethanolamine in Leishmania. Embo J. 2007;26:1094–1104. doi: 10.1038/sj.emboj.7601565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beach DH, Holz GG, Jr, Anekwe GE. Lipids of Leishmania promastigotes. J Parasitol. 1979;65:201–216. [PubMed] [Google Scholar]
- 25.Wassef MK, Fioretti TB, Dwyer DM. Lipid analyses of isolated surface membranes of Leishmania donovani promastigotes. Lipids. 1985;20:108–115. doi: 10.1007/BF02534216. [DOI] [PubMed] [Google Scholar]
- 26.Zhang K, et al. Leishmania salvage and remodelling of host sphingolipids in amastigote survival and acidocalcisome biogenesis. Mol Microbiol. 2005;55:1566–1578. doi: 10.1111/j.1365-2958.2005.04493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang K, et al. Sphingolipids are essential for differentiation but not growth in Leishmania. Embo J. 2003;22:6016–6026. doi: 10.1093/emboj/cdg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Signorell A, et al. Phosphatidylethanolamine in Trypanosoma brucei is organized in two separate pools and is synthesized exclusively by the Kennedy pathway. J Biol Chem. 2008;283:23636–23644. doi: 10.1074/jbc.M803600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutterwala SS, et al. Developmentally regulated sphingolipid synthesis in African trypanosomes. Mol Microbiol. 2008;70:281–296. doi: 10.1111/j.1365-2958.2008.06393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gibellini F, Hunter WN, Smith TK. The ethanolamine branch of the Kennedy pathway is essential in the bloodstream form of Trypanosoma brucei. Mol Microbiol. 2009;73:826–843. doi: 10.1111/j.1365-2958.2009.06764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagan N, Zoeller RA. Plasmalogens: biosynthesis and functions. Prog Lipid Res. 2001;40:199–229. doi: 10.1016/s0163-7827(01)00003-0. [DOI] [PubMed] [Google Scholar]
- 32.Fish WR, et al. The cyclopropane fatty acid of trypanosomatids. Mol Biochem Parasitol. 1981;3:103–115. doi: 10.1016/0166-6851(81)90010-4. [DOI] [PubMed] [Google Scholar]
- 33.Chang YY, Cronan JE., Jr Membrane cyclopropane fatty acid content is a major factor in acid resistance of Escherichia coli. Mol Microbiol. 1999;33:249–259. doi: 10.1046/j.1365-2958.1999.01456.x. [DOI] [PubMed] [Google Scholar]
- 34.Schneider P, et al. Structure of the glycosyl-phosphatidylinositol membrane anchor of the Leishmania major promastigote surface protease. J Biol Chem. 1990;265:16955–16964. [PubMed] [Google Scholar]
- 35.McConville MJ, et al. The glycoinositol phospholipids of Leishmania mexicana promastigotes. Evidence for the presence of three distinct pathways of glycolipid biosynthesis. J Biol Chem. 1993;268:15595–15604. [PubMed] [Google Scholar]
- 36.Ramos RG, et al. Comparison between charged aerosol detection and light scattering detection for the analysis of Leishmania membrane phospholipids. J Chromatogr A. 2008;1209:88–94. doi: 10.1016/j.chroma.2008.07.080. [DOI] [PubMed] [Google Scholar]
- 37.de Freitas Balanco JM, et al. Apoptotic mimicry by an obligate intracellular parasite downregulates macrophage microbicidal activity. Curr Biol. 2001;11:1870–1873. doi: 10.1016/s0960-9822(01)00563-2. [DOI] [PubMed] [Google Scholar]
- 38.van Zandbergen G, Solbach W, Laskay T. Apoptosis driven infection. Autoimmunity. 2007;40:349–352. doi: 10.1080/08916930701356960. [DOI] [PubMed] [Google Scholar]
- 39.Wanderley JL, et al. Apoptotic mimicry: an altruistic behavior in host/Leishmania interplay. Braz J Med Biol Res. 2005;38:807–812. doi: 10.1590/s0100-879x2005000600001. [DOI] [PubMed] [Google Scholar]
- 40.Wanderley JL, et al. Mimicry of apoptotic cells by exposing phosphatidylserine participates in the establishment of amastigotes of Leishmania (L) amazonensis in mammalian hosts. J Immunol. 2006;176:1834–1839. doi: 10.4049/jimmunol.176.3.1834. [DOI] [PubMed] [Google Scholar]
- 41.Rogers M, et al. Proteophosophoglycans regurgitated by Leishmania-infected sand flies target the L-arginine metabolism of host macrophages to promote parasite survival. PLoS Pathog. 2009;5:e1000555. doi: 10.1371/journal.ppat.1000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogers ME, et al. Transmission of cutaneous leishmaniasis by sand flies is enhanced by regurgitation of fPPG. Nature. 2004;430:463–467. doi: 10.1038/nature02675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaneshiro ES, Jayasimhulu K, Lester RL. Characterization of inositol lipids from Leishmania donovani promastigotes: identification of an inositol sphingophospholipid. J Lipid Res. 1986;27:1294–1303. [PubMed] [Google Scholar]
- 44.Denny PW, et al. Sphingolipid-free Leishmania are defective in membrane trafficking, differentiation and infectivity. Mol Microbiol. 2004;52:313–327. doi: 10.1111/j.1365-2958.2003.03975.x. [DOI] [PubMed] [Google Scholar]
- 45.Steiner S, et al. Isolation and partial characterization of a major inositol-containing lipid in baker's yeast, mannosyl-diinositol, diphosphoryl-ceramide. Proc Natl Acad Sci U S A. 1969;64:1042–1048. doi: 10.1073/pnas.64.3.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith SW, Lester RL. Inositol phosphorylceramide, a novel substance and the chief member of a major group of yeast sphingolipids containing a single inositol phosphate. J Biol Chem. 1974;249:3395–3405. [PubMed] [Google Scholar]
- 47.Hsu FF, et al. Characterization of Inositol Phosphorylceramides from Leishmania major by Tandem Mass Spectrometry with Electrospray Ionization. J Am Soc Mass Spectrom. 2007 doi: 10.1016/j.jasms.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams RD, Wang E, Merrill AH., Jr Enzymology of long-chain base synthesis by liver: characterization of serine palmitoyltransferase in rat liver microsomes. Arch Biochem Biophys. 1984;228:282–291. doi: 10.1016/0003-9861(84)90069-9. [DOI] [PubMed] [Google Scholar]
- 49.Pinto WJ, Wells GW, Lester RL. Characterization of enzymatic synthesis of sphingolipid long-chain bases in Saccharomyces cerevisiae: mutant strains exhibiting long-chain-base auxotrophy are deficient in serine palmitoyltransferase activity. J Bacteriol. 1992;174:2575–2581. doi: 10.1128/jb.174.8.2575-2581.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dickson RC, et al. Synthesis of mannose-(inositol-P)2-ceramide, the major sphingolipid in Saccharomyces cerevisiae, requires the IPT1 (YDR072c) gene. J Biol Chem. 1997;272:29620–29625. doi: 10.1074/jbc.272.47.29620. [DOI] [PubMed] [Google Scholar]
- 51.Straus AH, et al. Stage-specific glycosphingolipids from amastigote forms of Leishmania (L.) amazonensis. Immunogenicity and role in parasite binding and invasion of macrophages. J Biol Chem. 1993;268:13723–13730. [PubMed] [Google Scholar]
- 52.Straus AH, et al. Glycosphingolipid antigens from Leishmania (L.) amazonensis amastigotes. Binding of anti-glycosphingolipid monoclonal antibodies in vitro and in vivo. Braz J Med Biol Res. 1997;30:395–399. doi: 10.1590/s0100-879x1997000300014. [DOI] [PubMed] [Google Scholar]
- 53.Sindermann H, et al. Miltefosine (Impavido): the first oral treatment against leishmaniasis. Med Microbiol Immunol. 2004;193:173–180. doi: 10.1007/s00430-003-0201-2. [DOI] [PubMed] [Google Scholar]
- 54.Soto J, Berman J. Treatment of New World cutaneous leishmaniasis with miltefosine. Trans R Soc Trop Med Hyg. 2006;100 Suppl 1:S34–S40. doi: 10.1016/j.trstmh.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 55.Azzouz S, et al. Aspects of the cytological activity of edelfosine, miltefosine, and ilmofosine in Leishmania donovani. J Parasitol. 2006;92:877–883. doi: 10.1645/GE-632R1.1. [DOI] [PubMed] [Google Scholar]
- 56.Verma NK, Dey CS. Possible mechanism of miltefosine-mediated death of Leishmania donovani. Antimicrob Agents Chemother. 2004;48:3010–3015. doi: 10.1128/AAC.48.8.3010-3015.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rakotomanga M, Saint-Pierre-Chazalet M, Loiseau PM. Alteration of fatty acid and sterol metabolism in miltefosine-resistant Leishmania donovani promastigotes and consequences for drug-membrane interactions. Antimicrob Agents Chemother. 2005;49:2677–2686. doi: 10.1128/AAC.49.7.2677-2686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin KL, Smith TK. The myo-inositol-1-phosphate synthase gene is essential in Trypanosoma brucei. Biochem Soc Trans. 2005;33:983–985. doi: 10.1042/BST0330983. [DOI] [PubMed] [Google Scholar]
- 59.Ilg T. Generation of myo-inositol-auxotrophic Leishmania mexicana mutants by targeted replacement of the myo-inositol-1-phosphate synthase gene. Mol Biochem Parasitol. 2002;120:151–156. doi: 10.1016/s0166-6851(01)00435-2. [DOI] [PubMed] [Google Scholar]
- 60.Mongan TP, et al. Substrate specificity of the Leishmania donovani myo-inositol transporter: critical role of inositol C-2, C-3 and C-5 hydroxyl groups. Mol Biochem Parasitol. 2004;135:133–141. doi: 10.1016/j.molbiopara.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 61.Zufferey R, Mamoun CB. Choline transport in Leishmania major promastigotes and its inhibition by choline and phosphocholine analogs. Mol Biochem Parasitol. 2002;125:127–134. doi: 10.1016/s0166-6851(02)00220-7. [DOI] [PubMed] [Google Scholar]
- 62.Rifkin MR, Fairlamb AH. Transport of ethanolamine and its incorporation into the variant surface glycoprotein of bloodstream forms of Trypanosoma brucei. Mol Biochem Parasitol. 1985;15:245–256. doi: 10.1016/0166-6851(85)90088-x. [DOI] [PubMed] [Google Scholar]
- 63.Kennedy EP, Weiss SB. The function of cytidine coenzymes in the biosynthesis of phospholipides. J Biol Chem. 1956;222:193–214. [PubMed] [Google Scholar]
- 64.Schuiki I, Daum G. Phosphatidylserine decarboxylases, key enzymes of lipid metabolism. IUBMB Life. 2009;61:151–162. doi: 10.1002/iub.159. [DOI] [PubMed] [Google Scholar]
- 65.Vance JE. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: two metabolically related aminophospholipids. J Lipid Res. 2008;49:1377–1387. doi: 10.1194/jlr.R700020-JLR200. [DOI] [PubMed] [Google Scholar]
- 66.Signorell A, et al. Perturbation of phosphatidylethanolamine synthesis affects mitochondrial morphology and cell-cycle progression in procyclic-form Trypanosoma brucei. Mol Microbiol. 2009;72:1068–1079. doi: 10.1111/j.1365-2958.2009.06713.x. [DOI] [PubMed] [Google Scholar]
- 67.Dircks L, Sul HS. Acyltransferases of de novo glycerophospholipid biosynthesis. Prog Lipid Res. 1999;38:461–479. doi: 10.1016/s0163-7827(99)00012-0. [DOI] [PubMed] [Google Scholar]
- 68.Kent C. Eukaryotic phospholipid biosynthesis. Annu Rev Biochem. 1995;64:315–343. doi: 10.1146/annurev.bi.64.070195.001531. [DOI] [PubMed] [Google Scholar]
- 69.Zufferey R, Mamoun CB. The initial step of glycerolipid metabolism in Leishmania major promastigotes involves a single glycerol-3-phosphate acyltransferase enzyme important for the synthesis of triacylglycerol but not essential for virulence. Mol Microbiol. 2005;56:800–810. doi: 10.1111/j.1365-2958.2005.04579.x. [DOI] [PubMed] [Google Scholar]
- 70.Guerra DG, et al. The mitochondrial FAD-dependent glycerol-3-phosphate dehydrogenase of Trypanosomatidae and the glycosomal redox balance of insect stages of Trypanosoma brucei and Leishmania spp. Mol Biochem Parasitol. 2006;149:155–169. doi: 10.1016/j.molbiopara.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 71.Hajra AK, Bishop JE. Glycerolipid biosynthesis in peroxisomes via the acyl dihydroxyacetone phosphate pathway. Ann N Y Acad Sci. 1982;386:170–182. doi: 10.1111/j.1749-6632.1982.tb21415.x. [DOI] [PubMed] [Google Scholar]
- 72.Lux H, et al. Ether--lipid (alkyl-phospholipid) metabolism and the mechanism of action of ether--lipid analogues in Leishmania. Mol Biochem Parasitol. 2000;111:1–14. doi: 10.1016/s0166-6851(00)00278-4. [DOI] [PubMed] [Google Scholar]
- 73.Zufferey R, Ben Mamoun C. Leishmania major expresses a single dihydroxyacetone phosphate acyltransferase localized in the glycosome, important for rapid growth and survival at high cell density and essential for virulence. J Biol Chem. 2006;281:7952–7959. doi: 10.1074/jbc.M512911200. [DOI] [PubMed] [Google Scholar]
- 74.Zufferey R, Al-Ani GK, Dunlap K. Leishmania dihydroxyacetonephosphate acyltransferase LmDAT is important for ether lipid biosynthesis but not for the integrity of detergent resistant membranes. Mol Biochem Parasitol. 2009;168:177–185. doi: 10.1016/j.molbiopara.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spath GF, et al. Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite Leishmania major. Proc Natl Acad Sci U S A. 2000;97:9258–9263. doi: 10.1073/pnas.160257897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gibellini F, Hunter WN, Smith TK. Biochemical characterization of the initial steps of the Kennedy pathway in Trypanosoma brucei: the ethanolamine and choline kinases. Biochem J. 2008;415:135–144. doi: 10.1042/BJ20080435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hanada K. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim Biophys Acta. 2003;1632:16–30. doi: 10.1016/s1388-1981(03)00059-3. [DOI] [PubMed] [Google Scholar]
- 78.Obeid LM, Okamoto Y, Mao C. Yeast sphingolipids: metabolism and biology. Biochim Biophys Acta. 2002;1585:163–171. doi: 10.1016/s1388-1981(02)00337-2. [DOI] [PubMed] [Google Scholar]
- 79.Nagiec MM, et al. Sphingolipid synthesis as a target for antifungal drugs. Complementation of the inositol phosphorylceramide synthase defect in a mutant strain of Saccharomyces cerevisiae by the AUR1 gene. J Biol Chem. 1997;272:9809–9817. doi: 10.1074/jbc.272.15.9809. [DOI] [PubMed] [Google Scholar]
- 80.Denny PW, et al. The protozoan inositol phosphorylceramide synthase: a novel drug target that defines a new class of sphingolipid synthase. J Biol Chem. 2006;281:28200–28209. doi: 10.1074/jbc.M600796200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huitema K, et al. Identification of a family of animal sphingomyelin synthases. Embo J. 2004;23:33–44. doi: 10.1038/sj.emboj.7600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hashida-Okado T, et al. AUR1, a novel gene conferring aureobasidin resistance on Saccharomyces cerevisiae: a study of defective morphologies in Aur1p-depleted cells. Mol Gen Genet. 1996;251:236–244. doi: 10.1007/BF02172923. [DOI] [PubMed] [Google Scholar]
- 83.Mina JG, et al. The Trypanosoma brucei sphingolipid synthase, an essential enzyme and drug target. Mol Biochem Parasitol. 2009;168:16–23. doi: 10.1016/j.molbiopara.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 84.Takesako K, et al. Aureobasidins, new antifungal antibiotics. Taxonomy, fermentation, isolation, and properties. J Antibiot (Tokyo) 1991;44:919–924. doi: 10.7164/antibiotics.44.919. [DOI] [PubMed] [Google Scholar]
- 85.Tanaka AK, et al. Inhibition of Leishmania (Leishmania) amazonensis growth and infectivity by aureobasidin A. J Antimicrob Chemother. 2007;59:487–492. doi: 10.1093/jac/dkl518. [DOI] [PubMed] [Google Scholar]
- 86.Salto ML, et al. Formation and remodeling of inositolphosphoceramide during differentiation of Trypanosoma cruzi from trypomastigote to amastigote. Eukaryot Cell. 2003;2:756–768. doi: 10.1128/EC.2.4.756-768.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Figueiredo JM, et al. Characterization of the inositol phosphorylceramide synthase activity from Trypanosoma cruzi. Biochem J. 2005;387:519–529. doi: 10.1042/BJ20041842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang O, et al. Degradation of Host Sphingomyelin is Essential for Leishmania Virulence. PLoS Pathogens. 2009 doi: 10.1371/journal.ppat.1000692. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sawai H, et al. Identification of ISC1 (YER019w) as inositol phosphosphingolipid phospholipase C in Saccharomyces cerevisiae. J Biol Chem. 2000;275:39793–39798. doi: 10.1074/jbc.M007721200. [DOI] [PubMed] [Google Scholar]
- 90.Henriques C, et al. Biochemical analysis of proteins and lipids found in parasitophorous vacuoles containing Leishmania amazonensis. Parasitol Res. 2003;89:123–133. doi: 10.1007/s00436-002-0728-y. [DOI] [PubMed] [Google Scholar]
- 91.Winter G, et al. Surface antigens of Leishmania mexicana amastigotes: characterization of glycoinositol phospholipids and a macrophage-derived glycosphingolipid. J Cell Sci. 1994;107(Pt 9):2471–2482. doi: 10.1242/jcs.107.9.2471. [DOI] [PubMed] [Google Scholar]
- 92.Parodi-Talice A, et al. The overexpression of a new ABC transporter in Leishmania is related to phospholipid trafficking and reduced infectivity. Biochim Biophys Acta. 2003;1612:195–207. doi: 10.1016/s0005-2736(03)00131-7. [DOI] [PubMed] [Google Scholar]
- 93.Ouellette M, Drummelsmith J, Papadopoulou B. Leishmaniasis: drugs in the clinic, resistance and new developments. Drug Resist Updat. 2004;7:257–266. doi: 10.1016/j.drup.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 94.Araujo-Santos JM, et al. The overexpression of an intracellular ABCA-like transporter alters phospholipid trafficking in Leishmania. Biochem Biophys Res Commun. 2005;330:349–355. doi: 10.1016/j.bbrc.2005.02.176. [DOI] [PubMed] [Google Scholar]
- 95.Perez-Victoria FJ, et al. Phospholipid translocation and miltefosine potency require both L. donovani miltefosine transporter and the new protein LdRos3 in Leishmania parasites. J Biol Chem. 2006;281:23766–23775. doi: 10.1074/jbc.M605214200. [DOI] [PubMed] [Google Scholar]
- 96.Castanys-Munoz E, et al. A novel ATP-binding cassette transporter from Leishmania is involved in transport of phosphatidylcholine analogues and resistance to alkyl-phospholipids. Mol Microbiol. 2007;64:1141–1153. doi: 10.1111/j.1365-2958.2007.05653.x. [DOI] [PubMed] [Google Scholar]
- 97.Castanys-Munoz E, et al. Characterization of an ABCG-like transporter from the protozoan parasite Leishmania with a role in drug resistance and transbilayer lipid movement. Antimicrob Agents Chemother. 2008;52:3573–3579. doi: 10.1128/AAC.00587-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ilgoutz SC, McConville MJ. Function and assembly of the Leishmania surface coat. Int J Parasitol. 2001;31:899–908. doi: 10.1016/s0020-7519(01)00197-7. [DOI] [PubMed] [Google Scholar]
- 99.McConville MJ, et al. Structures of the glycoinositolphospholipids from Leishmania major. A family of novel galactofuranose-containing glycolipids. J Biol Chem. 1990;265:7385–7394. [PubMed] [Google Scholar]
- 100.Orlandi PA, Jr, Turco SJ. Structure of the lipid moiety of the Leishmania donovani lipophosphoglycan. J Biol Chem. 1987;262:10384–10391. [PubMed] [Google Scholar]
- 101.Proudfoot L, et al. Regulation of the expression of nitric oxide synthase and leishmanicidal activity by glycoconjugates of Leishmania lipophosphoglycan in murine macrophages. Proc Natl Acad Sci U S A. 1996;93:10984–10989. doi: 10.1073/pnas.93.20.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Giorgione JR, Turco SJ, Epand RM. Transbilayer inhibition of protein kinase C by the lipophosphoglycan from Leishmania donovani. Proc Natl Acad Sci U S A. 1996;93:11634–11639. doi: 10.1073/pnas.93.21.11634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Menon AK, et al. Phosphatidylethanolamine is the donor of the terminal phosphoethanolamine group in trypanosome glycosylphosphatidylinositols. Embo J. 1993;12:1907–1914. doi: 10.1002/j.1460-2075.1993.tb05839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Martin KL, Smith TK. Phosphatidylinositol synthesis is essential in bloodstream form Trypanosoma brucei. Biochem J. 2006;396:287–295. doi: 10.1042/BJ20051825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martin KL, Smith TK. The glycosylphosphatidylinositol (GPI) biosynthetic pathway of bloodstream-form Trypanosoma brucei is dependent on the de novo synthesis of inositol. Mol Microbiol. 2006;61:89–105. doi: 10.1111/j.1365-2958.2006.05216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Costello CE, et al. Structural characterization of novel inositol phosphosphingolipids of Tritrichomonas foetus and Trichomonas vaginalis. Glycobiology. 1993;3:261–269. doi: 10.1093/glycob/3.3.261. [DOI] [PubMed] [Google Scholar]
- 107.Previato JO, et al. Glycoinositolphospholipid from Trypanosoma cruzi: structure, biosynthesis and immunobiology. Adv Parasitol. 2004;56:1–41. doi: 10.1016/s0065-308x(03)56001-8. [DOI] [PubMed] [Google Scholar]
- 108.Bertello LE, et al. Inositolphosphoceramide is not a substrate for the first steps in the biosynthesis of glycoinositolphospholipids in Trypanosoma cruzi. Mol Biochem Parasitol. 2004;133:71–80. doi: 10.1016/j.molbiopara.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 109.Lohner K. Is the high propensity of ethanolamine plasmalogens to form non-lamellar lipid structures manifested in the properties of biomembranes? Chem Phys Lipids. 1996;81:167–184. doi: 10.1016/0009-3084(96)02580-7. [DOI] [PubMed] [Google Scholar]
- 110.Glaser PE, Gross RW. Plasmenylethanolamine facilitates rapid membrane fusion: a stopped-flow kinetic investigation correlating the propensity of a major plasma membrane constituent to adopt an HII phase with its ability to promote membrane fusion. Biochemistry. 1994;33:5805–5812. doi: 10.1021/bi00185a019. [DOI] [PubMed] [Google Scholar]
- 111.Williams RA, et al. Characterization of unusual families of ATG8-like proteins and ATG12 in the protozoan parasite Leishmania major. Autophagy. 2009;5:159–172. doi: 10.4161/auto.5.2.7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Besteiro S, et al. Endosome sorting and autophagy are essential for differentiation and virulence of Leishmania major. J Biol Chem. 2006;281:11384–11396. doi: 10.1074/jbc.M512307200. [DOI] [PubMed] [Google Scholar]
- 113.Denny PW, Field MC, Smith DF. GPI-anchored proteins and glycoconjugates segregate into lipid rafts in Kinetoplastida. FEBS Lett. 2001;491:148–153. doi: 10.1016/s0014-5793(01)02172-x. [DOI] [PubMed] [Google Scholar]
- 114.Yoneyama KA, et al. Characterization of Leishmania (Viannia) braziliensis membrane microdomains, and their role in macrophage infectivity. J Lipid Res. 2006;47:2171–2178. doi: 10.1194/jlr.M600285-JLR200. [DOI] [PubMed] [Google Scholar]
- 115.Mattjus P, Slotte JP. Does cholesterol discriminate between sphingomyelin and phosphatidylcholine in mixed monolayers containing both phospholipids? Chem Phys Lipids. 1996;81:69–80. doi: 10.1016/0009-3084(96)02535-2. [DOI] [PubMed] [Google Scholar]
- 116.Ohvo-Rekila H, et al. Cholesterol interactions with phospholipids in membranes. Prog Lipid Res. 2002;41:66–97. doi: 10.1016/s0163-7827(01)00020-0. [DOI] [PubMed] [Google Scholar]
- 117.Pike LJ, et al. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry. 2002;41:2075–2088. doi: 10.1021/bi0156557. [DOI] [PubMed] [Google Scholar]
- 118.Denny PW, Smith DF. Rafts and sphingolipid biosynthesis in the kinetoplastid parasitic protozoa. Mol Microbiol. 2004;53:725–733. doi: 10.1111/j.1365-2958.2004.04208.x. [DOI] [PubMed] [Google Scholar]
- 119.Yuan C, et al. The size of lipid rafts: an atomic force microscopy study of ganglioside GM1 domains in sphingomyelin/DOPC/cholesterol membranes. Biophys J. 2002;82:2526–2535. doi: 10.1016/S0006-3495(02)75596-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Langford CK, et al. Functional expression and subcellular localization of a high-Km hexose transporter from Leishmania donovani. Biochemistry. 1995;34:11814–11821. doi: 10.1021/bi00037a020. [DOI] [PubMed] [Google Scholar]
- 121.Denny PW, et al. Acylation-dependent protein export in Leishmania. J Biol Chem. 2000;275:11017–11025. doi: 10.1074/jbc.275.15.11017. [DOI] [PubMed] [Google Scholar]
- 122.Godsel LM, Engman DM. Flagellar protein localization mediated by a calcium-myristoyl/palmitoyl switch mechanism. Embo J. 1999;18:2057–2065. doi: 10.1093/emboj/18.8.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tyler KM, et al. Flagellar membrane localization via association with lipid rafts. J Cell Sci. 2009;122:859–866. doi: 10.1242/jcs.037721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McConville MJ, et al. Structure of the lipophosphoglycan from Leishmania major. J Biol Chem. 1990;265:19611–19623. [PubMed] [Google Scholar]
- 125.Lee SH, et al. Fatty acid synthesis by elongases in trypanosomes. Cell. 2006;126:691–699. doi: 10.1016/j.cell.2006.06.045. [DOI] [PubMed] [Google Scholar]
- 126.Signorell A, et al. Phosphatidylethanolamine is the precursor of the ethanolamine phosphoglycerol moiety bound to eukaryotic elongation factor 1A. J Biol Chem. 2008;283:20320–20329. doi: 10.1074/jbc.M802430200. [DOI] [PubMed] [Google Scholar]
- 127.Rosenberry TL, et al. Biosynthetic incorporation of [3H]ethanolamine into protein synthesis elongation factor 1 alpha reveals a new post-translational protein modification. J Biol Chem. 1989;264:7096–7099. [PubMed] [Google Scholar]
- 128.Whiteheart SW, et al. Murine elongation factor 1 alpha (EF-1 alpha) is posttranslationally modified by novel amide-linked ethanolamine-phosphoglycerol moieties. Addition of ethanolamine-phosphoglycerol to specific glutamic acid residues on EF-1 alpha. J Biol Chem. 1989;264:14334–14341. [PubMed] [Google Scholar]
- 129.Ransom WD, et al. Phosphoglycerylethanolamine posttranslational modification of plant eukaryotic elongation factor 1alpha. Plant Physiol. 1998;117:949–960. doi: 10.1104/pp.117.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Blum JJ, et al. Phospholipase D (PLD) is present in Leishmania donovani and its activity increases in response to acute osmotic stress. J Eukaryot Microbiol. 2001;48:102–110. doi: 10.1111/j.1550-7408.2001.tb00421.x. [DOI] [PubMed] [Google Scholar]
- 131.Bulow R, Overath P. Purification and characterization of the membrane-form variant surface glycoprotein hydrolase of Trypanosoma brucei. J Biol Chem. 1986;261:11918–11923. [PubMed] [Google Scholar]
- 132.Hereld D, et al. A phospholipase C from Trypanosoma brucei which selectively cleaves the glycolipid on the variant surface glycoprotein. J Biol Chem. 1986;261:13813–13819. [PubMed] [Google Scholar]
- 133.Mensa-Wilmot K, et al. A glycosylphosphatidylinositol (GPI)-negative phenotype produced in Leishmania major by GPI phospholipase C from Trypanosoma brucei: topography of two GPI pathways. J Cell Biol. 1994;124:935–947. doi: 10.1083/jcb.124.6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McConville MJ, et al. Living in a phagolysosome; metabolism of Leishmania amastigotes. Trends Parasitol. 2007;23:368–375. doi: 10.1016/j.pt.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 135.Opperdoes FR, Coombs GH. Metabolism of Leishmania: proven and predicted. Trends Parasitol. 2007;23:149–158. doi: 10.1016/j.pt.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 136.Fridberg A, et al. Sphingolipid synthesis is necessary for kinetoplast segregation and cytokinesis in Trypanosoma brucei. J Cell Sci. 2008;121:522–535. doi: 10.1242/jcs.016741. [DOI] [PubMed] [Google Scholar]
- 137.Sutterwala SS, et al. De novo sphingolipid synthesis is essential for viability, but not for transport of glycosylphosphatidylinositol-anchored proteins, in African trypanosomes. Eukaryot Cell. 2007;6:454–464. doi: 10.1128/EC.00283-06. [DOI] [PMC free article] [PubMed] [Google Scholar]