Abstract
Human embryonic kidney (HEK293) cells are widely used for the heterologous expression of voltage- and ligand-gated ion channels. Patch clamp analysis of HEK293 cells in the whole-cell configuration identified voltage-gated, rapidly inactivating inward currents. Peak current amplitudes ranged from less than 100 pA to more than 800 pA, with the majority (84 of 130 cells) in the 100–400 pA range. Transient inward currents were separated into three components on the basis of sensitivity to cadmium and tetrodotoxin (TTX). Application of cadmium (300 μM) reduced current amplitude to 65% of control, consistent with the existence of current carried by a cadmium -sensitive nonspecific cation channel previously identified in HEK293 cells. Application of TTX (500 nM) reduced current amplitude by 47%, consistent with the existence of current carried by a TTX-sensitive voltage-gated sodium channel. Joint application of cadmium and TTX was additive, reducing current amplitude to 28% of control. The residual cadmium- and TTX-resistant current represents a third pharmacologically distinct component of the rapidly inactivating inward current that was not characterized further. The pyrethroid insecticide tefluthrin (10 μM) prolonged the inactivation of transient currents and induced slowly decaying tail currents, effects that are characteristic of sodium channel modification by pyrethroids. The use of sodium channel isoform-specific primers in polymerase chain reaction amplifications on HEK293 cell first-strand cDNA detected the consistent expression of the human Nav1.7 sodium channel isoform in cells that expressed the TTX-sensitive component of current. These results provide evidence for an endogenous TTX-sensitive sodium current in HEK293 cells that is associated primarily with the expression of the Nav1.7 sodium channel isoform.
Keywords: HEK293 cells, sodium channels, Nav1.7, tetrodotoxin, tefluthrin
HEK293 cells, derived from human embryonic kidney cells by transformation with sheared adenovirus type 5 DNA [5], are employed extensively as a platform for the transient or stable heterologous expression of neuroreceptor and ion channel proteins [11]. Despite their origin HEK293 cells exhibit some characteristics of neurons, expressing more than 60 neuronal genes including neurofilament proteins and neuroreceptor and ion channel subunits [9, 11]. Moreover, electrophysiological studies confirm the functional expression of endogenous voltage-gated calcium and potassium channels in HEK293 cells [2, 6].
There has been no characterization of endogenous voltage-gated sodium channels in HEK293 cells, although there are anecdotal reports of small, endogenous tetrodotoxin (TTX)-sensitive currents [12] and these cells are known to express the sodium channel β1A auxiliary subunit [7]. A recent study [8] identified a rapidly inactivating endogenous cation current in HEK293 cells that superficially resembles currents carried by voltage-gated sodium channels. However, this current is distinguished from sodium currents by its sensitivity to block by cadmium, insensitivity to block by TTX, and lack of selectivity for sodium ions. This nonspecific cation current is associated with the expression of channels formed by polycystin-2.
Here we provide evidence for an endogenous, rapidly inactivating inward current in HEK293 cells that includes two components distinguished by their sensitivity to cadmium and TTX as well as a third, minor component that is insensitive to block by either cadmium or TTX. The presence of the TTX-sensitive component of the current was correlated primarily with the expression of the human Nav1.7 sodium channel isoform. These data provide evidence for the expression of endogenous voltage-gated sodium channels in HEK293 cells.
HEK293 cells (CRL-1573, lot number 7681666) were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and cultured at 37°C in DMEM (ATCC) supplemented with 10% FBS (ATCC) in a humidified atmosphere of 5% CO2/95% air. Upon receipt cells were passaged twice and then frozen under liquid N2 for future use; these stocks were considered to be at “laboratory passage one.” Cells for experimental use were derived from frozen stocks and maintained in continuous culture for up to 30 passages. Cells were trypsinized and plated in 35mm polystyrene dishes one to two days before electrophysiological experiments.
Whole cell patch clamp recordings were conducted at room temperature (21–25°C) using an Axopatch 200B amplifier (Molecular Devices, Foster City, CA). Cells were perfused at ~350 μl/min with extracellular solution that contained (mM) NaCl (140), KCl (5), CaCl2 (2), MgCl2 (1), and HEPES (10) and was adjusted to pH 7.40 using NaOH. The intracellular solution contained (in mM) NaCl (35), CsF (105), MgCl2 (2), EGTA (10), and HEPES (10) and was adjusted to pH 7.20 using CsOH. The final osmolarity of both solutions was 295 – 305 mOsm. Fire-polished patch electrodes were fabricated from borosilicate glass capillaries using a P-87 puller (Sutter Instruments, Novato, CA) to give a resistance of 1–2 MΩ when filled with intracellular solution. Output signals were filtered at 2 kHz and sampled at 50 kHz (DigiData 1322A; Molecular Devices). Voltage errors were minimized using 70–80% series resistance compensation. Leak currents were corrected using the P/4 method [3]. Membrane potentials were not corrected for junction potential (~2.3mV at 23.5°C). Stock solutions of TTX, CdCl2, and tefluthrin (all from Sigma Chemical Co., St. Louis, MO) were diluted to final concentrations in extracellular solution and applied through the perfusion system. Data were acquired and analyzed using pClamp 10.2 (Molecular Devices) and Origin 7.0 (OriginLab Corp., Northampton, MA) software. The Boltzmann equation [y=(A1−A2)/(1+e(x−x0)/dx)+A2] was used to fit conductance-voltage and sodium current inactivation data.
First-strand cDNA from HEK293 cells, synthesized using the SuperScripTM III CellsDirect cDNA Synthesis System (Invitrogen, Carlsbad, CA), was employed as the template in polymerase chain reaction amplifications using pairs of oligonucleotide primers developed previously for the specific amplification of each of the nine human sodium channel α subunit isoforms (Nav1.1 – Nav1.9) [4]. Reactions were initiated by denaturing for 2 min at 94 °C followed by 40 cycles of amplification (30 sec at 94 °C, 30 sec at 60 °C, 1 min at 72 °C) and final extension for 7 min at 72 °C. Amplification products (207–347 bp) were analyzed by electrophoresis in 2% agarose gels.
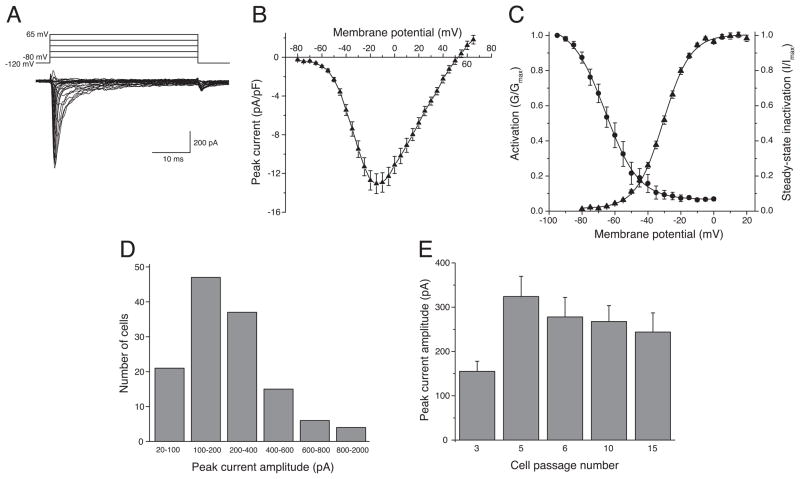
We detected transient cation currents with peak amplitudes of at least 20 pA in all 130 cells examined in this study. Currents evoked by step depolarizations from a holding potential of −120 mV to a range of test potentials activated and inactivated within 20 ms (Fig. 1A). Peak inward transient currents were typically observed upon depolarization to potentials near −10 mV with a mean maximal current density of 13.1 ± 1.0 pA/pF (n = 67) and a reversal potential of ~51 mV (Fig. 1B). Fig. 1C summarizes the voltage-dependent activation and steady-state inactivation of peak transient currents in HEK293 cells. Boltzmann fits of conductance–test potential relationships from activation experiments (as in Figs. 1A and 1B) gave a half-maximal potential for activation of −30.7 ± 0.2 mV with a slope factor of 8.22 ± 0.22 (n = 67). Similarly, Boltzmann fits of current–prepulse potentials relationships from inactivation experiments gave a half-maximal potential for steady-state inactivation of −65.9 ± 0.4 mV with a slope factor of 10.12 ± 0.33 (n = 16). The steady-state inactivation of transient currents was incomplete, giving a residual inactivation-resistant current (approximately 7% of maximal peak current) following a conditioning prepulse to 0 mV.
Fig. 1.
Properties of transient inward currents in HEK293 cells. (A) Representative current traces recorded during a 40-ms step depolarization from −120 mV to test potentials from −85 mV to 65 mV in 5-mV increments. (B) Current-voltage plot of multiple data sets such as those in panel A. Values are means ± SE of 67 separate determinations with different cells and are normalized to the capacitance (in pF) measured for each cell. (C) Voltage dependence of activation and steady-state inactivation. For activation, normalized conductance (G/Gmax) was derived from the current-voltage relationship (as in Fig. 1B) by dividing peak transient current (INa) by the driving force (V − Vrev) and normalizing to the maximum conductance observed in each cell. For inactivation, peak transient currents were measured during a test depolarization to −10 mV following a 200-ms conditioning prepulse to potentials from −95 mV to 0 mV in 5-mV increments and normalized to the maximal peak transient current in each experiment (I/Imax). Values for G/Gmax and I/Imax were plotted as a function of test (activation) or prepulse (inactivation) potential and curves were drawn by fitting mean values to the Boltzmann equation. Each data point is the mean ± SE of 67 (activation) or 16 (steady-state inactivation) determinations with different cells. (D) Amplitude distribution of transient inward currents measured upon depolarization from −120 mV to −10mV in 130 cells. (E) Plot of the mean amplitudes of transient inward currents as a function passage number for cells in continuous culture. Each value is the mean ± SE of 13–45 determinations with different cells.
Whereas all of the cells examined in this study expressed detectable transient currents, the amplitude of these currents varied widely from cell to cell. Fig. 1D summarizes the frequency distribution of current amplitudes. The majority of cells (84 of 130, 64.6%) expressed currents with amplitudes in the 100–400 pA range, but a few cells expressed much larger currents. Fig. 1E illustrates the relationship between mean peak current amplitude and cell passage number in continuous culture. Current amplitudes increased approximately two-fold between three and five laboratory passages in culture and declined slightly thereafter.
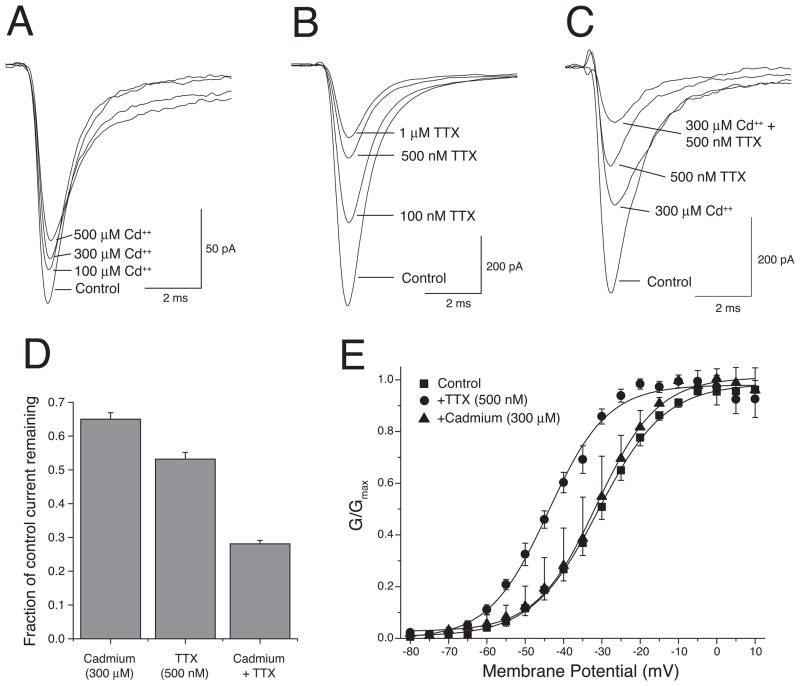
The previous report of an endogenous, cadmium-sensitive nonspecific cation current in HEK293 cells [8] led us assess the cadmium sensitivity of the transient currents expressed in our cell populations. In contrast to the results of Pelucchi et al. [8], peak transient currents were only partially blocked by cadmium. Fig. 2A shows the effect of 100 μM, 300 μM and 500 μM cadmium on peak transient currents in a representative cell; a small number of experiments with cadmium concentrations as high as 2 mM failed to increase block beyond that observed with 300–500 μM cadmium (data not shown). Results with a large number of cells show that 300 μM cadmium blocked only ~35% of the transient current (Fig. 2D). The failure of cadmium to completely block these currents led us to examine the effects of TTX, a potent and effective blocker of voltage-gated sodium channels that was not effective in blocking the previously-described cadmium-sensitive currents [8]. Fig. 2B shows the effect of 100 nM, 500 nM and 1 μM TTX on peak transient currents in a representative cell; results with a large number of cells show that 500 nM TTX blocked ~47% of the transient current (Fig. 2D).
Fig. 2.
Effects of cadmium and TTX on transient inward currents. (A) Representative current traces elicited by depolarization from −120 mV to −10 mV before cadmium exposure and after exposure to three cadmium concentrations. (B) Representative current traces elicited by depolarization from −120 mV to −10 mV before TTX exposure and after exposure to three TTX concentrations. (C) Representative current traces elicited by depolarization from −120 mV to −10 mV before cadmium or TTX exposure and after exposure to cadmium alone, TTX alone, and cadmium plus TTX. (D) The fraction of transient inward current remaining following exposure to cadmium (300 μM), TTX (500 nM), or cadmium (300 μM) plus TTX (500 nM). Values are means ± SE of 22 (cadmium), 30 (TTX), or 19 (cadmium + TTX) determinations with different cells. (E) Plots of normalized peak conductance (G/Gmax) obtained from experiments performed in the absence (as in Fig. 1A) or presence of TTX (500 nM), or cadmium (300 μM) plotted as a function of test potential. Values are means ± SE of 12 (control), 7 (TTX), or 5 (cadmium) determinations; control values represent the pooled controls from separate experiments with TTX or cadmium. Curves were drawn by fitting mean values to the Boltzmann equation.
The combined effects of cadmium and TTX on transient currents were independent and approximately additive. Fig. 2C illustrates the effects of 300 μM cadmium, 500 nM TTX, and the combination of these two treatments on peak transient currents recorded from a representative cell. Experiments with a large number of cells showed that cadmium and TTX together at these concentrations blocked ~72% of the transient current (Fig. 2D). Washout experiments (data not shown) demonstrated that the effects of individual and combined treatments with cadmium and TTX were completely reversible and that the extent of block in the presence of both compounds was not affected by prior application of either compound. The residual current that was resistant to both cadmium and TTX was not large enough to permit its further characterization.
Exposure of cells to TTX (500 nM) also altered the voltage dependence of activation of the transient current (Fig. 2E). Paired experiments in individual cells before and after TTX exposure (n = 7) showed that TTX caused a ~14.5 mV hyperpolarizing shift in the voltage dependence of activation, a shift that was statistically significant (paired t-test, P < 0.001; 6 d.f.). In contrast, similar paired experiments with 300 μM cadmium had no effect on the voltage dependence of activation (Fig. 2E). These results show that the TTX- and cadmium-resistant currents are carried by separate populations of channels with different gating properties.
The sensitivity of transient currents to TTX led us to assess whether these currents exhibited other pharmacological properties of voltage-gated sodium channels. Tefluthrin, like other pyrethroid insecticides, binds to voltage-gated sodium channel α subunits and produces characteristic changes in channel gating by slowing the rate of inactivation, inducing sodium tail currents, and shifting the voltage dependence of activation [10, 13]. Exposure to tefluthrin (10 μM) slowed the time course of transient current inactivation during a depolarizing pulse and also induced a persistent sodium tail current following repolarization (Fig. 3A). In some cells (as in Fig. 3A), tefluthrin also appeared to increase the amplitude of the peak current. Currents measured in the presence of tefluthrin evoked by step depolarizations from a holding potential of −120 mV to a range of test potentials (Fig. 3B) activated and inactivated very slowly compared to control currents (see Fig. 1A). Paired experiments in individual cells before and after tefluthrin exposure (n = 8) showed that tefluthrin produced a statistically significant (paired t-test, P < 0.001; 7 d.f.) ~19 mV hyperpolarizing shift in the voltage dependence of activation (Fig. 3C). We also performed a more limited set of experiments to assess the effects of tefluthrin in the presence of cadmium. Tefluthrin affected the cadmium-resistant component of current in a manner identical to that shown in Fig. 3 for the composite transient current (data not shown). All of these effects of tefluthrin are completely consistent with the actions of this compound on sodium channels in GT1-7 neurons [13] and on rat and human Nav1.3 sodium channels transiently expressed in Xenopus laevis oocytes [10].
Fig. 3.
Effects of tefluthrin on transient inward currents. (A) Representative currents recorded before and after exposure of a cell to 10 μM tefluthrin. (B) Representative current traces recorded in the presence of 10 μM tefluthrin during a 40-ms step depolarization from −120 mV to test potentials from −85 mV to 65 mV in 5-mV increments. (C) Plots of normalized peak conductance (G/Gmax) obtained from experiments performed in the absence (as in Fig. 1A) or presence (as in Fig. 3B) of 10 μM tefluthrin plotted as a function of test potential. Values are means ± SE of 8 paired determinations. Curves were drawn by fitting mean values to the Boltzmann equation.
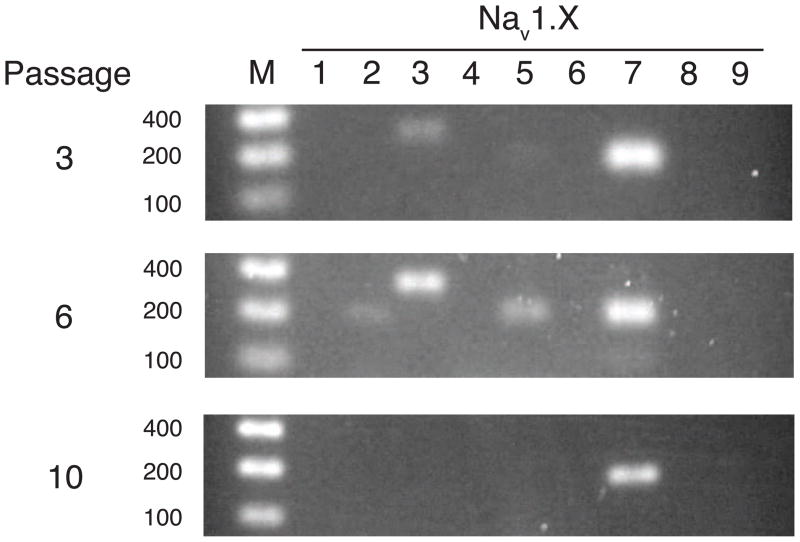
We used pairs of oligonucleotide primers specific for each of the nine human voltage-gated sodium channel α subunit isoforms in the RT-PCR analysis of sodium channel isoform expression in HEK293 cells after 3–15 laboratory passages in continuous culture. The Nav1.7 isoform was present in all first-strand cDNA pools examined (Fig. 4). The Nav1.2, Nav1.3, and Nav1.5 isoforms were also detected in cells after 3–6 passages (Fig. 4) and were also occasionally detected in cells grown continuously for 10–15 passages (data not shown). Although not rigorously quantitative, the relative abundance of the PCR products obtained from different isoforms suggests that the Nav1.7 isoform was consistently the most abundant at the level of translational expression. Despite the lack of confirming data on the relative abundance of different sodium channel isoform proteins, these results nevertheless support the conclusion that the TTX-sensitive voltage-gated sodium currents in these cells are associated primarily, but not exclusively, with the expression of the Nav1.7 sodium channel α subunit isoform.
Fig. 4.
Detection of the expression of individual sodium channel α subunit isoforms in HEK293 cells after 3, 6, or 10 laboratory passages in continuous culture by RT-PCR and electrophoresis in 2% agarose gels. Lanes: M, markers (100, 200, and 400 bp); 1–9, isoform-specific PCR products for Nav1.1 – Nav1.9, respectively.
The data presented here provide the first detection and characterization of an endogenous TTX-sensitive voltage-gated sodium current in HEK293 cells as one component of a heterogeneous transient inward current. The voltage-gated sodium current component was identified by its insensitivity to cadmium, sensitivity to TTX, and characteristic modification by the pyrethroid insecticide tefluthrin. Moreover, RT-PCR analyses detected the robust translational expression of the Nav1.7 sodium channel α subunit isoform in all first-strand cDNA preparations from these cells and also detected low levels of translational expression of the Nav1.2, Nav1.3 and Nav1.5 isoforms in template preparations from some batches of cells.
The TTX-sensitive voltage-gated sodium current was the major component (~50%) of a pharmacologically heterogeneous current that contained at least two other components. A portion of the total current (~35%) was blocked by cadmium. This component may represent the nonspecific, cadmium-sensitive transient cation current described recently in HEK293 cells [8]. It is possible that Nav1.5 sodium channels, which are sensitive to cadmium and resistant to TTX [1] and were detected at low relative abundance by RT-PCR in some batches of cells (see Fig. 4), may also contribute to the cadmium-sensitive current in some cells. A third, minor component of the transient current was insensitive to both cadmium and TTX. However, the amplitudes of the residual currents were too small to permit further characterization. Overall, the average maximal density of the composite transient inward current in the present study (13.1 pA/pF; see Fig. 1B) was more than five-fold greater than that reported previously for the cadmium-sensitive nonspecific cation current (2.35 pA/pF) [8].
Both the reproducible detection and large amplitude of endogenous voltage-gated sodium currents reported here are surprising in light of previous studies, which describe either the inconsistent detection of small-amplitude TTX-sensitive currents [12] or the expression of transient inward currents that are completely insensitive to TTX [8]. These discrepancies suggest that different lineages of HEK293 cells, obtained from different sources and cultured under different conditions, may vary greatly in the extent to which they express endogenous sodium or nonspecific cation currents. A similar lack of agreement exists between different studies regarding the expression of endogenous γ-aminobutyric acid-gated chloride currents in HEK293 cells [11]. Nevertheless, the expression of voltage-gated sodium channels and currents in the HEK293 cell line employed in this study is generally consistent with numerous other studies documenting the endogenous expression of a variety of neuronal proteins and mRNAs in these cells [11].
The existence of endogenous voltage-gated sodium currents in at least some lineages of HEK293 cells has important implications for the use of these cells as a platform for the heterologous expression of cloned voltage-gated sodium channels. Preliminary studies in this laboratory showed that the translational expression of endogenous sodium channel mRNAs persists in cells following stable transformation with individual rat sodium channel α subunit isoforms (B. He and D. M. Soderlund, unpublished). For the study of whole-cell sodium currents, the impact of endogenous currents can be minimized by selecting transformed cells that express currents with amplitudes significantly greater than those measured for endogenous currents in control cells. However, cells with endogenous currents should be avoided for studies of heterologously-expressed sodium channels at the single-channel level.
Acknowledgments
This work was supported in part by grants (R01-ES013686 and R01-ES014591) from the National Institute of Environmental Health Sciences, National Institutes of Health. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Environmental Health Sciences. We thank P. Adams and S. Kopatz for technical assistance and S. McCavera and R. von Stein for helpful discussions and critical reviews of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Backx PH, Yue DT, Lawrence JH, Marban E, Tomaselli GF. Molecular localization of a ion-binding site within the pore of mammalian sodium channels. Science. 1992;257:248–251. doi: 10.1126/science.1321496. [DOI] [PubMed] [Google Scholar]
- 2.Berjulow S, Doring F, Froschmayr M, Grabner M, Glossmann H, Hering S. Endogenous calcium channels in human embryonic kidney (HEK293) cells. Br J Pharmacol. 1996;118:748–754. doi: 10.1111/j.1476-5381.1996.tb15463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bezanilla F, Armstrong CM. Inactivation of the sodium channel. J Gen Physiol. 1977;70:549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardenas L, Seda M, Noheda P, Buschmann H, Cintado CG, Martin JD, Pinto FM. Molecular diversity of voltage-gated α and β subunit mRNAs in human tissues. Eur J Pharmacol. 2006;541:9–16. doi: 10.1016/j.ejphar.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 5.Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–77. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 6.Jiang B, Sun X, Cao K, Wang R. Endogenous Kv channels in human embryonic kidney (HEK-293) cells. Mol Cell Biochem. 2002;238:69–79. doi: 10.1023/a:1019907104763. [DOI] [PubMed] [Google Scholar]
- 7.Moran O, Nizzari M, Conti F. Endogenous expression of the β1A sodium channel subunit in HEK-293 cells. FEBS Lett. 2000;473:132–134. doi: 10.1016/s0014-5793(00)01518-0. [DOI] [PubMed] [Google Scholar]
- 8.Pelucchi B, Aguiari G, Pignatelli A, Manzati E, Witzgall R, del Senno L, Belluzzi O. Nonspecific cation current associated with native polycystin-2 in HEK-293 cells. J Am Soc Nephrol. 2006;17:388–397. doi: 10.1681/ASN.2004121146. [DOI] [PubMed] [Google Scholar]
- 9.Shaw G, Morse S, Ararat M, Graham FL. Preferential transformation of human neuronal cells by human adeonviruses and the origin of HEK 293 cells. FASEB J. 2002;16:869–871. doi: 10.1096/fj.01-0995fje. [DOI] [PubMed] [Google Scholar]
- 10.Tan J, Soderlund DM. Human and rat Nav1.3 voltage-gated sodium channels differ in inactivation properties and sensitivity to the pyrethroid insecticide tefluthrin. Neurotoxicology. 2009;30:81–89. doi: 10.1016/j.neuro.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas P, Smart TG. HEK293 cell line: a vehicle for the expression of recombinant proteins. J Pharm Toxicol Methods. 2005;51:187–200. doi: 10.1016/j.vascn.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Ukomandu C, Zhou J, Sigworth FJ, Agnew WS. μI Na+ channels expressed transiently in human embryonic kidney cells: biochemical and biophysical properties. Neuron. 1992;8:663–676. doi: 10.1016/0896-6273(92)90088-u. [DOI] [PubMed] [Google Scholar]
- 13.Wu SN, Wu YH, Chen BS, Liu YC. Underlying mechanism of action of tefluthrin, a pyrethroid insecticide, on voltage-gated ion currents and on action currents in pituitary tumor (GH3) cells and GnRH-secreting (GT1-7) neurons. Toxicology. 2009;2009:70–77. doi: 10.1016/j.tox.2009.01.009. [DOI] [PubMed] [Google Scholar]