Abstract
The present study examined whether testosterone levels are influenced by being with a sexual and romantic partner after a period of sexual abstinence. Women in long distance relationships (n = 15) provided five saliva samples: at least one week before seeing their partner (and at least 2 weeks since their last visit), the day before seeing their partner, when they were with their partner but prior to engaging in sexual activity, the day after their first sexual activity, and three days after they were separated from their partners. Salivary testosterone was lowest when participants had been away from their partners for at least two weeks and highest the day before they were to see their partners and the day after sexual activity. Results from this study indicated that women’s testosterone increased both the day before they were with their partners and they day after they first engaged in sexual activity. However, something about initially reuniting with their partners returned their testosterone to baseline levels, which may be an effect of being in the same location as a partner, or just a state fluctuation due to nervousness or other psychological state.
Keywords: sexual behavior, testosterone, androgen, sexual anticipation, women, orgasm
Introduction
The relationship between human sexuality and androgens is complex and still not well understood. In 1970, an anonymous researcher published a case study in Nature in which he demonstrated that anticipation of sexual activity could increase testosterone levels in men, as measured indirectly by beard growth. Over the course of two years, the researcher spent periods of several weeks conducting research in isolation. He began weighing his beard clippings once daily as an androgen bioassay. On days when he was anticipating visiting his female sexual partner and on days immediately following sexual activity, his beard clippings were heavier, indicating higher levels of androgen production (Anonymous, 1970). This interesting case study led to a series of more controlled studies on the relation between sexual activity with a partner, partner presence, and androgens, particularly testosterone.
Several studies done in male rodents have shown that testosterone increases in anticipation of sexual activity (e.g. Graham & Desjardins, 1980), in the presence of a receptive female (e.g. Bonilla-Jaime et al., 2006) and in response to copulation (Kamel et al., 1975, Bonilla-Jaime et al., 2006). In studies of the effects of sexual activity on testosterone in human males, results have been varied. One early case study conducted with a male participant found that testosterone was higher in blood samples taken during (pre-orgasm) and after (post-orgasm) intercourse compared to control samples taken on non-sex days. There was no significant difference between pre- and post-orgasm samples, however (Fox et al., 1972). Two subsequent studies also found that testosterone was higher on nights when sex occurred (Dabbs & Mohammed, 1992, n = 4) and the morning after sex occurred (Kraemer et al., 1976, n = 20), compared to non-sex days. In contrast, two studies with eight and six male participants, respectively, found no increase in testosterone related to sexual intercourse (Lee et al., 1974, Stearns et al., 1973). All of the above studies on sexual intercourse used discrete sampling methods and, with the exception of Dabbs and Mohammed (1992) who sampled saliva, all of the studies examined plasma testosterone. For reviews on the effects of sexual activity and testosterone on men see Archer (2006) and van Anders and Watson (2006).
To our knowledge, there have been no studies of androgen responses to sexual activity in non-human females. Testosterone only seems to play a role in female sexual behavior when the behavior is decoupled from the estrous cycle (e.g., Rissman, 1995), although it has been argued that any effect of increased testosterone on female sexual motivation and behavior is a result of its aromatization to estradiol (e.g., Wallen, 2001). Studies of women’s testosterone responses to sexual activity have also shown variable results (for a review see van Anders & Watson, 2006). Using discrete sampling methods, three studies have looked at women’s testosterone levels in relation to sexual intercourse. One study found no change in plasma levels of testosterone after sexual intercourse (Lee et al., 1974), and two subsequent studies found testosterone increased around times of sexual activity. Of the two studies that noted increases, one found higher testosterone levels in a small sample of four women on nights when sex occurred compared to non-sex nights (Dabbs and Mohammed, 1992). In a larger sample of 16 women, van Anders et al. (2007) found testosterone was higher both before and after sexual intercourse compared to nights where the women engaged in control activities, providing evidence for both an anticipatory increase in testosterone and an overall elevation in testosterone with sexual activity. This study also compared women who did and did not have orgasms during sexual activity and found that women who experienced orgasms had higher overall testosterone the day of the sexual activity.
If there is an effect of sexual activity on testosterone in women, we expect that it will be most pronounced after a period of abstinence from sexual activity with a partner. Exton et al. (2001) found that when men masturbated after 3 weeks of abstinence from all sexual activity, their testosterone was higher than when masturbating during a non-abstinence period. Baseline testosterone was not different for the abstinence and non-abstinence conditions. Another study on abstinence found that when testosterone was measured daily, it was stable over the course of 8–16 days of abstinence, with the exception of a spike seven days following last ejaculation (Jiang et al., 2003).
Several animal studies have shown that being separated from a partner results in increased cortisol or corticosterone levels when compared to being separated from a sibling (prairie voles: Bosch et al., 2009) or even an infant (titi monkeys: Mendoza et al., 1986). The social isolation/partner separation paradigm used in animal research may be somewhat limited in its generalizability to humans, however, given that it is more analogous to being placed in solitary confinement. In studies of human partner separation, participants often have phone or email contact with their partners as well as other social contacts. A more relevant study of partner separation, conducted with human subjects, found that cortisol did not change in response to separation for people low in attachment anxiety, but people high in attachment anxiety showed increased cortisol in response to separation. Cortisol was lower for the high anxiety group both in preparation for separation and upon reunion and was highest while they were actually separated from their partners (Diamond et al., 2008). There is some evidence of an inverse relationship between cortisol and testosterone under conditions of stress (e.g. Cummings et al., 1983; for a review see Rivier & Rivest, 1991), so high cortisol could be linked with lower testosterone during separation.
Another study of partner separation looked at testosterone levels in couples who were in long distance or same-city relationships, as well as single people. Women in same city relationships had lower testosterone levels compared to single women, while those in long distance relationships were not significantly different from single women (van Anders & Watson, 2007). These findings suggest that women separated from their partners have higher basal testosterone than those in same-city relationships. We do not know how basal levels of testosterone may change in response to partner presence and sexual activity in women.
Several researchers have demonstrated that exposure to putative male pheromones can have an effect on women’s physiological (e.g., Jacob et al., 2001) and endocrine responses (e.g. Preti et al., 2003; Wyart et al., 2007). Other pheromonal research has demonstrated that being exposed to male sweat extract can alter menstrual cycle length in women (e.g., Cutler et al., 1986). These studies show that pheromonal or odor cues from being exposed to males can have an effect on a woman’s own physiology, which could be one possible mechanism that can explain any change in women’s testosterone levels. Of course, if there is a change before the woman is reunited with her partner, then the mechanism is likely to be rooted in changes resulting from changes in her psychological state.
The present study is an attempt to further explore the changes in testosterone that occur from being with a sexual and romantic partner. Specifically, we were interested in both the effects of partner presence and sexual activity after a period of separation on testosterone levels. We recruited women who were in long distance relationships, and whose partners visited for brief periods of time. Because past research suggests testosterone is highest on days when sexual activity occurs (van Anders et al., 2007), but does not necessarily change over the course of sexual activity (Lee et al, 1974), we sampled on specific days as opposed to immediately before and after intercourse. We hypothesized that, compared to when they were alone, salivary testosterone levels would be highest the day before seeing a partner as seen in Anonymous (1970) and throughout the visit, which would include sexual intercourse. We predicted testosterone would be lowest during the initial period of separation and after the visit.
Method
Participants
Participants were 17 women between the ages of 18 and 28 (M = 21.3, SD = 3.0) in monogamous, long distance relationships who saw their male sexual partners once per month or less. Two women did not complete the study. One participant broke up with her partner at the beginning of the visit. The other participant did not give a reason, and we were unable to contact her. Demographics and analyses are included only for the 15 women who completed all components of the study. Participants were recruited from an undergraduate research pool and from the community. Thirteen of the participants were Caucasian, one was Latina and one was Black. Eight participants had been in their current relationship for less than a year, three for 1–3 years, two for 3–5 years and two for more than 5 years. Thirteen of the participants reported heterosexual orientation and two reported bisexual orientation. Women who reported being heterosexual also all ranked themselves as a 0 on the Kinsey Scale. Of the two women who reported being bisexual, one ranked herself as a 2 and one as a 3 on the Kinsey scale. Prior to beginning the study, participants were asked to abstain from partner based sexual activity (e.g., phone sex) for two weeks, but were allowed to masturbate. To our knowledge, there has been no evidence that masturbation increases testosterone levels in women (e.g. Exton et al., 1999), and only one study showing changes in men (Purvis et al., 1976). Confirmatory analyses revealed no significant differences in testosterone levels at the initial sample (Alone) between those women who did (n = 6) and did not (n = 9) engage in masturbation, t(13) = 1.42, p = .97. Of the 15 participants, seven were on hormonal contraceptives and eight were freely cycling with regular menstrual cycles.
Procedures
All procedures were approved by the University of Texas at Austin Institutional Review Board. Participants were recruited through online advertisements, and flyers posted across campus. Callers were screened by trained female research assistants to determine if they qualified for the study. Inclusion criteria were as follows: between the ages of 18–45, currently in a sexually active long distance relationship, absence of any sexual problems, and no reported endocrine abnormalities. Initially we required that participants not use any form of hormonal contraception, but due to the difficulty of obtaining participants from this already exclusive population, we altered the criteria to allow for women on monophasic oral contraceptives. Although hormonal contraceptives alter the hormonal milieu in women by lowering testosterone levels (among other changes) (Panzer et al., 2006) the repeated measures design used in this study allowed us to examine the changes in testosterone that occurred for each woman. Also, a recent study has demonstrated that the degree of change in testosterone (in response to competition) is similar for women on and off hormonal contraceptives (Edwards & O’Neal, 2009). Since the present study also looks at changes in testosterone, it is possible that these findings would extend to other domains, such as response to sexual activity and partner presence.
Salivary testosterone levels were measured at the following five time points: (1) One week before partner visit (Alone), (2) One day before partner visit (Day Before), (3) With partner, but before sexual intercourse (Presence), (4) One day after the first sexual intercourse (Day After Intercourse), (5) Three days after their partners left (Post Visit). With the exception of the Presence sample, all samples were collected between the hours of 2:00 pm and 6:00 pm to control for diurnal variations in testosterone (Dabbs, 1990). The timing of the Presence sample was dependent on when the participant was reunited with her partner. For 12 women this fell between the hours of 4 pm and 12 am.
Upon qualification, women were scheduled to come into the lab at least one week prior to their next visit with their partner and at least two weeks since their last visit. All participants came into the lab for their initial visit 7–10 days before their partner arrived. During the initial visit, a researcher explained the study procedures and had participants read and sign a consent form. Women then provided the Alone saliva sample and filled out the demographics and saliva sample questionnaires. Participants were then scheduled for their next visit, which took place the day before each participant was reunited with her partner. Three participants were unable to find a convenient time to schedule the second visit, so they were given an envelope with instructions, questionnaires and 3 test tubes in order to complete the Day Before, Presence, and Day After Intercourse samples on their own.
During the second visit, participants provided the Day Before sample and filled out the associated saliva sample questionnaire. They were given an envelope with instructions, saliva sample questionnaires, and two test tubes for the Presence and Day After Intercourse saliva samples. The instructions provided information about the timing and storage of the saliva samples. The Presence sample was to be completed within 30 minutes of being with their partner, regardless of the time of day, and the Day After Intercourse sample was to be done the day after the first sexual intercourse. The Day After Intercourse was completed the day after the Presence sample for all participants. Participants were asked to freeze the sample within two hours of collection. Samples were kept in their household freezers and brought to the lab within one week.
Finally, women returned to the lab with the saliva samples and questionnaires three days after they were separated from their partners. During the same visit, they provided the final sample (Post Visit) and filled out the saliva sample questionnaire. Participants either received partial class credit or were paid $25 for their participation.
Materials
Saliva samples
Participants were asked not to smoke or to eat or drink anything but water, or to brush their teeth, or exercise one hour before providing each saliva sample. This is standard practice in salivary hormone research (e.g., Kirschbaum, et al., 1992). Participants salivated without stimulation into untreated polystyrene centrifuge tubes, providing 3 ml of saliva per sample. Women’s testosterone levels in saliva assays correlate with total testosterone concentration in serum (Granger et al., 2004). Once in the lab, samples were frozen at −20°Celsius until assay when they were thawed and centrifuged for 15 minutes at 3,000 rpm. Enzyme immunoassays (EIA) were performed in-house using kits purchased from Salimetrics (State College, PA). All assays were run in duplicate. Inter-assay C.V. was 7.5% at 200.52 pg/ml and 4.9% at 17.47 pg/ml and intra-assay C.V. was 2.86%.
Demographics & screening questionnaire
The demographics portion of the questionnaire asked participants their age, relationship information (length, status), sexual orientation (free-response & Kinsey scale), and ethnicity (free-response). The screening portion of the questionnaire asked participants about any medications they were currently taking (including herbal supplements), endocrine abnormalities, recent sexual activity, and menstrual cycle information (first day of last period, average cycle length). Participants were prescreened over the phone to determine eligibility, and this in person screening served as a verification of the original screen. No participants were excluded based on these questions.
Saliva sample questionnaire
This questionnaire was filled out each time the participant provided a saliva sample. Participants recorded the date and time of the sample and indicated whether they had eaten, drank, smoked, exercised, or brushed their teeth within the past hour. They were also asked to report any problems related to the sample. Depending on the sample there were further questions. The Presence sample questionnaire asked “How much time elapsed between this sample and you engaging in sexual activity with your partner. (Please answer after sexual activity has occurred).” The Day After Intercourse questionnaire included the following two questions, “How many times have you had sexual intercourse since the last saliva sample?” and “In your most recent sexual encounter with your partner, did you have an orgasm?” The Post Visit sample asked, “How many times in total did you engage in sexual activities with your partner?”
Data analysis
All data were analyzed using SPSS Version 12.0 (Chicago, IL). To control for the variability in individual participants’ testosterone levels, we standardized each person’s testosterone data by calculating the percent change over baseline for each sample, for each person using the following formula ((Sample−Alone)/Alone)*100. Analyses were conducted using percent change scores as in previous studies (e.g. van Anders et al. 2007; Carré & McCormick, 2008). Studies assessing the relationship between sexual function and androgens in women have shown that wide variability exists between women in hormonal measures of androgens (e.g., Guay, 2002; Labrie et al., 1997). Percent change scores, as opposed to raw scores, indicate the relative amount of change occurring for each person, taking into account variability in baseline levels.
Overall effects were tested using a repeated measures analysis of variance (ANOVA) with time as the within subjects variable, contraceptive status as the between subjects variable, and testosterone level as the dependent variable. Post hoc tests were done on the using paired samples t-tests.
Results
Because one participant had a testosterone level that was too low to measure at the Presence time point, we used the lower detection limit of the assay (6.1 pg/ml) as an estimated value for that sample. Results of analyses did not differ substantially with the elimination of this participant thus all results reported below include the full sample of 15 women. Participants’ average testosterone at the Alone sample was 34.2 pg/ml (SD = 13.2), and there was no difference between women on (M = 33.1, SD = 13.1) and off (M = 35.1, SD = 14.0) oral contraceptives, t (13) = .29, p = .78. The average salivary testosterone level for all data points across the study was 35.6 pg/ml (SD = 13.79).
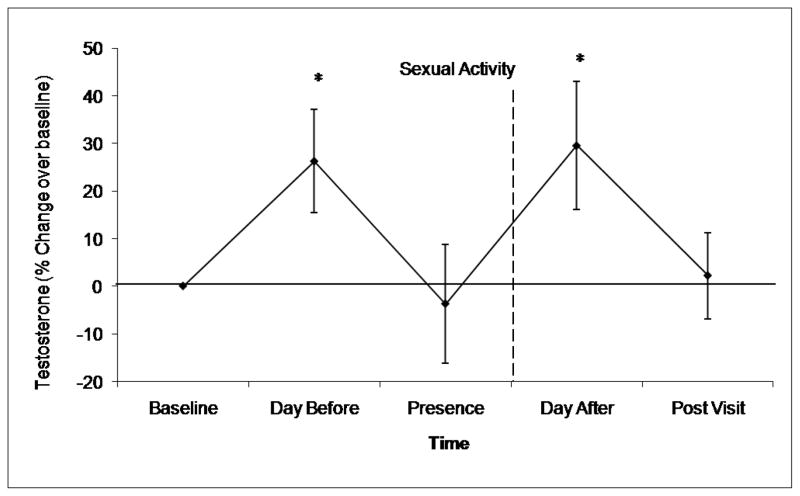
There was a significant change over time for the testosterone percent change data, F (4, 52) = 3.27, p = .018. There was no interaction between contraceptive status and time, F (4, 52) = 1.30, p = .28, nor was there a between groups effect of contraceptive status, F (1, 13) = .11, p = .75. We were interested in testing each time point against the participants’ testosterone levels at the Alone time point. Post hoc t-tests showed that there were significant differences between the Alone baseline and the Day Before time point, t (14) = −2.41, p = .03 and the Day After Intercourse time point, t (14) = −2.20, p = .04, but not between Alone and Presence, t (14) = .30, p = .77 or Alone and Post Visit, t (14) = −.24, p = .81 (Figure 1). The mean testosterone levels for each time point were as follows, Alone, M = 34.2 (SD = 13.2), Day Before = 39.4 (SD = 13.7), Presence = 31.2, (SD = 15.2), Day After Intercourse, 41.3 (SD = 14.8), Post Visit 31.6, (SD = 9.8).
Figure 1.
Women’s change in testosterone levels after (at least) two weeks of abstinence (Alone), the day before sexual activity (Day Before), when they are with their partner (Presence), the day after the initial sexual activity (Day After Intercourse) and three days after their last sexual activity (Post Visit). Testosterone levels were highest in anticipation of sexual activity and shortly after sexual activity. * Indicates values that were significantly greater than baseline at p < .05.
Post-hoc analyses were conducted to examine the effect of orgasm and number of sexual encounters on testosterone. All but four women reported having orgasms during the sexual encounters that occurred before they gave the Day After Intercourse sample. The mean testosterone level for the women who experienced orgasm was 41.8 (SD = 15.4) at the Day After Intercourse point, while the women who did not have an orgasm had a mean of 40.0 (SD = 15.5), which was not significantly different, t (13) = 0.19, p = .42. Participants engaged in a median of four sexual encounters during the course of the study. A correlation between the number of sexual encounters and the percent change in testosterone from the Day After Intercourse to the Post Visit was not significant, r(12) = .003, p = .99.
Discussion
The present study examined the effects partner presence and sexual activity on salivary testosterone levels in women compared to periods of partner absence. As hypothesized, testosterone was found to be low when they were separated from their partners and not close to being reunited. Levels were similar when participants had been alone without having partnered sex for at least two weeks and three days after they were separated from their partners, but also when they were first in the presence of their partners. We also hypothesized that testosterone would be highest during times immediately before and during partner togetherness, which included the Day Before, Presence and Day After Intercourse points. This hypothesis was only partially supported. We found that testosterone increased the Day Before and the Day After Intercourse relative to baseline, but returned to baseline levels at the Presence time point.
This study adds to the literature on the effects of partner presence and sexual activity on women’s testosterone. Especially notable is the evidence for an increase in testosterone the day before being reunited with a partner after three or more weeks of being alone and abstaining from partnered sexual activity. This is the second study to show that testosterone is higher before sexual activity when compared to a control condition (van Anders et al., 2007). Also interesting was the increase in testosterone seen the day after the first sexual activity, demonstrating that testosterone is elevated in periods of sexual activity compared to periods of abstinence. This replicates the case study of the male researcher (Anonymous 1970).
The finding that women had lower testosterone levels when first in the presence of their partner was unexpected. We hypothesized that participants would still be anticipating sexual activity, which we believed would increase testosterone as shown in previous studies. This result does coincide with the findings from the van Anders & Watson (2007) study where women in same city relationships had lower testosterone levels compared to both single women and women in long distance relationships who were not currently with their partners. They suggested that being in the same physical location as a partner can lower testosterone in women. Our results offer some support to this theory. It is possible that the increase seen after the first sexual activity is due to the sexual activity, while the lower level of testosterone seen during partner presence is the regular level for women in the presence of their partners.
Precisely why women would show lower levels of testosterone when in the presence of a partner is highly speculative. One possibility is that the decrease in testosterone is indicative of the woman’s mood or state of mind when they are first reunited with their partners. For example, there may be an acute effect of romantic emotions (i.e., love) that, at least temporarily, dampens testosterone. Alternatively, testosterone levels could be suppressed as a consequence of exposure to a man, potentially via pheromonal or odor cues. Several studies have shown that exposure to male odors or pheromones can alter endocrine secretion, although none of them have looked at testosterone specifically (e.g., Cutler et al., 1986; Preti et al., 2003). A third potential explanation is that participants were nervous when seeing their partners after a long absence. Anxiety or nervousness could have increased cortisol levels which, in turn, could have dampened testosterone levels. This sequence of events has been reported in men (Cummings et al., 1983).
van Anders et al. (2007) found that in their study, women who had orgasms had higher overall testosterone compared to women who did not on the day of sexual activity, but not the following day. We also found that testosterone was not higher the next day in women who experienced orgasm compared to those who did not report having an orgasm. However, only four of the women in our sample of 15 did not have an orgasm in the present study. Post-hoc analyses also indicated there was no significant relationship between the number of sexual encounters and the change in testosterone seen between the Day After Intercourse and three days following separation from their partner. This suggests that frequency of sexual intercourse does not serve as a “protective” factor in maintaining post-sex testosterone elevation.
A limitation of this study is that we did not have a control condition with which to reference the changes seen over time. However, because participants’ samples were taken at different time points over the span of two years, it is unlikely that historical time effects would be responsible for altering testosterone. Also, since almost all of the samples were taken at the same time of day, we would expect testosterone levels to be similar under control conditions as seen in other studies (e.g. Dabbs, 1990). We should also note that statistical analysis of the raw testosterone data showed that the increase from the Alone sample to the Day Before sample was not statistically significant, even though it was significant using the percent change data. This discrepancy indicates the importance of taking baseline levels into account for hormonal measures.
The present findings demonstrate significant increases in testosterone in anticipation of reuniting with a long distance partner, and also the day after first sexual activity. Testosterone increased from baseline the day before a partner’s visit, and showed the highest peak the day after sexual activity. Unexpectedly, initial partner presence decreased the anticipatory spike in testosterone back to baseline levels. Future research that examines the relationship between both psychological components of reuniting with a long distance partner (e.g., attachment anxiety, nervousness, romantic love) as well as other hormonal components (i.e., cortisol) would help elucidate the mechanisms underlying this decrease.
Acknowledgments
The authors would like to thank Sheilanova Molina, Eve Andrews, Ashley Garner, and Luke Thorstenson for their assistance on this project. This publication was made possible by a scholarship from the Natural Sciences and Engineering Research Council of Canada to the first author, and by Grant Number 5 RO1 HD051676-04 to the second author. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Natural Sciences and Engineering Research Council of Canada or the National Institute of Child Health and Human Development (NICHD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anonymous Effects of sexual activity on beard growth in man. Nature. 1970;226:869–870. doi: 10.1038/226869a0. [DOI] [PubMed] [Google Scholar]
- Archer J. Testosterone and human aggression: An evaluation of the challenge hypothesis. Neurosci Biobehav Rev. 2006;30:319–345. doi: 10.1016/j.neubiorev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Bonilla-Jaime J, Vazquez-Palacios G, Arteaga-Silva M, Retana-Marquez S. Hormonal responses to different sexually related conditions in male rats. Horm Behav. 2006;49:376–382. doi: 10.1016/j.yhbeh.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Nair HR, Ahern TH, Neumann ID, Young LJ. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology. 2009;34:1406–1415. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carré JM, McCormick CM. Aggressive behavior and change in salivary testosterone concentrations predict willingness to engage in a competitive task. Horm Behav. 2008;53:403–409. doi: 10.1016/j.yhbeh.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Cummings DC, Quigley ME, Yen SS. Acute suppression of circulating testosterone levels by cortisol in men. J Clin Endocrinol Metab. 1983;57:671–673. doi: 10.1210/jcem-57-3-671. [DOI] [PubMed] [Google Scholar]
- Cutler W, Preti G, Krieger A, Huggins G. Human axillary secretions influence women’s menstrual cycles: The role of donor extract from men. Horm Behav. 1986;20:463–473. doi: 10.1016/0018-506x(86)90008-5. [DOI] [PubMed] [Google Scholar]
- Dabbs JM., Jr Salivary testosterone measurements: Reliability across hours, days, and weeks. Physiol Behav. 1990;48:83–86. doi: 10.1016/0031-9384(90)90265-6. [DOI] [PubMed] [Google Scholar]
- Dabbs JM, Jr, Mohammed S. Male and female salivary testosterone concentrations before and after sexual activity. Physiol Behav. 1992;52:195–197. doi: 10.1016/0031-9384(92)90453-9. [DOI] [PubMed] [Google Scholar]
- Diamond LM, Hicks AM, Otter-Henderson KD. Every time you go away: Changes in affect, behavior, and physiology associated with travel-related separations from romantic partners. J Pers Soc Psychol. 2008;95:385–343. doi: 10.1037/0022-3514.95.2.385. [DOI] [PubMed] [Google Scholar]
- Edwards DA, O’Neal JL. Oral contraceptives decrease saliva testosterone but do not affect the rise in testosterone associated with athletic competition. Horm Behav. 2009;56:193–194. doi: 10.1016/j.yhbeh.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Exton MS, Bindert A, Kruger T, Scheller F, Hartmann U, Schedlowski M. Cardiovascular and endocrine alterations after masturbation-induced orgasm in women. Psychosom Med. 1999;61:280–289. doi: 10.1097/00006842-199905000-00005. [DOI] [PubMed] [Google Scholar]
- Exton MS, Krüger TH, Bursch N, Haake P, Knapp W, Schedlowski M, Hartmann U. Endocrine response to masturbation-induced orgasm in healthy men following a 3-week sexual abstinence. World J Urol. 2001;19:377–382. doi: 10.1007/s003450100222. [DOI] [PubMed] [Google Scholar]
- Fox CA, Ismail AAA, Love DN, Kirkham KE, Loraine JA. Studies on the relationship between plasma testosterone levels and human sexual activity. J Endocrinol. 1972;52:51–58. doi: 10.1677/joe.0.0520051. [DOI] [PubMed] [Google Scholar]
- Graham JM, Desjardins C. Classical conditioning: Induction of luteinizing hormone and testosterone secretion in anticipation of sexual activity. Science. 1980;210:1039–1041. doi: 10.1126/science.7434016. [DOI] [PubMed] [Google Scholar]
- Granger D, Shirtcliff E, Booth A, Kivlighan K, Schwartz E. The ‘trouble’ with salivary testosterone. Psychoneuroendocrinology. 2004;29:1229–1240. doi: 10.1016/j.psyneuen.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Guay AT. Screening for androgen deficiency in women: Methodological and interpretive issues. Fertil Steril. 2002;77:S83–S88. doi: 10.1016/s0015-0282(02)02965-5. [DOI] [PubMed] [Google Scholar]
- Jacob S, Hayreh DJS, McClintock MK. Context-dependent effects of steroid chemosignals on human physiology and mood. Phys Behav. 2001;74:15–27. doi: 10.1016/s0031-9384(01)00537-6. [DOI] [PubMed] [Google Scholar]
- Jiang M, Xin J, Zou Q, Shen JW. A research on the relationship between ejaculation and serum testosterone level in men. J Zhejiang Univ Sci. 2003;4:236–240. doi: 10.1631/jzus.2003.0236. [DOI] [PubMed] [Google Scholar]
- Kamel F, Mock E, Wright W, Frankel A. Alterations in plasma concentrations of testosterone, LH, and prolactin associated with mating in the male rat. Horm Behav. 1975;6:277–288. doi: 10.1016/0018-506x(75)90014-8. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wust S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosom Med. 1992;54:648–657. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Becker HB, Brodie HKH, Doering CH, Moos RH, Hamburg DA. Orgasmic frequency and plasma testosterone levels in normal human males. Arch Sex Behav. 1976;5:125–132. doi: 10.1007/BF01541869. [DOI] [PubMed] [Google Scholar]
- Labrie F, Bélanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrin Metab. 1997;82:2396–2402. doi: 10.1210/jcem.82.8.4160. [DOI] [PubMed] [Google Scholar]
- Lee PA, Jaffe RB, Midgley AR., Jr Lack of alteration of serum gonadotropins in men and women following sexual intercourse. Am J Obstet Gynecol. 1974;120:985–987. doi: 10.1016/0002-9378(74)90351-2. [DOI] [PubMed] [Google Scholar]
- Mendoza S, Mason W. Parental division of labour and differentiation of attachments in a monogamous primate (Callicebus moloch) Anim Behav. 1986;34:1336–1347. [Google Scholar]
- Panzer C, Wise S, Fantini G, Kang D, Munarriz R, Guay A, Goldstein I. Impact of oral contraceptives on sex hormone-binding globulin and androgen levels: A retrospective study in women with sexual dysfunction. J Sex Med. 2006;3:104–13. doi: 10.1111/j.1743-6109.2005.00198.x. [DOI] [PubMed] [Google Scholar]
- Preti G, Wysocki CJ, Barnhart KT, Sondheimer SJ, Leyden JJ. Male axillary extracts contain pheromones that affect pulsatile secretion of luteinizing hormone and mood in women recipients. Biol Reprod. 2003;68:2107–2113. doi: 10.1095/biolreprod.102.008268. [DOI] [PubMed] [Google Scholar]
- Purvis KB, Landgren M, Cekan Z, Diczfalusy E. Endocrine effects of masturbation in men. J Endocrinol. 1976;70:439–444. doi: 10.1677/joe.0.0700439. [DOI] [PubMed] [Google Scholar]
- Rissman EF. An alternative animal model for the study of female sexual behavior. Curr Dir Psychol Sci. 1995;4:6–10. [Google Scholar]
- Rivier C, Rivest S. Effects of stress on the activity of the hypothalamic-pituitary-gonadal axis: Peripheral and central mechanisms. Biol Reprod. 1991;45:523–532. doi: 10.1095/biolreprod45.4.523. [DOI] [PubMed] [Google Scholar]
- Stearns EL, Winter JSD, Faiman C. Effects of coitus on gonadotropin, prolactin and sex steroid levels in man. J Clin Endocrinol Metab. 1973;37:687–690. doi: 10.1210/jcem-37-5-687. [DOI] [PubMed] [Google Scholar]
- van Anders SM, Hamilton LD, Schmidt N, Watson NV. Associations between testosterone secretion and sexual activity in women. Horm Behav. 2007;51:477–482. doi: 10.1016/j.yhbeh.2007.01.003. [DOI] [PubMed] [Google Scholar]
- van Anders SM, Watson NV. Social neuroendocrinology: Effects of social contexts and behaviors on sex steroids in humans. Hum Nat. 2006;17:212–237. doi: 10.1007/s12110-006-1018-7. [DOI] [PubMed] [Google Scholar]
- van Anders SM, Watson NV. Testosterone levels in women and men who are single, in long-distance relationships, or same-city relationships. Horm Behav. 2007;51:286–291. doi: 10.1016/j.yhbeh.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Wallen K. Sex and context: Hormones and primate sexual motivation. Horm Behav. 2001;40:339–357. doi: 10.1006/hbeh.2001.1696. [DOI] [PubMed] [Google Scholar]
- Wyart C, Webster WW, Chen JH, Wilson SR, McClary A, Khan RM, Sobel N. Smelling a single component of male sweat alters levels of cortisol in women. J Neurosci. 2007;27:1261–1265. doi: 10.1523/JNEUROSCI.4430-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]