Abstract
Dilute capsaicin produces a differential effect on incision-related pain behaviors depending upon the test; it reduces heat hyperalgesia and guarding pain but not mechanical hyperalgesia. This suggests that common mechanisms for heat hyperalgesia and guarding pain occur, and distinct mechanisms exist for mechanical hyperalgesia. The purpose of the present study was to evaluate the effect of capsaicin treatment on the activity of cutaneous nociceptors sensitized by incision to understand the mechanisms for the selective action of dilute capsaicin on incisional pain. We compared the effect of 0.05% capsaicin versus vehicle treatment on pain behaviors after incision and on the activity of nociceptors from these same rats using the in vitro glabrous skin nerve preparation. Immunohistochemical expression of protein gene product 9.5 (PGP9.5), neurofilament 200, calcitonin gene related peptide (CGRP) and isolectin B4 (IB4) in skin was also evaluated 1 week after 0.05% capsaicin infiltration. Infiltration of 0.05% capsaicin decreased CGRP and IB4/PGP9.5-immunoreactivity of nociceptors in skin. The same dose of capsaicin that inhibited heat hyperalgesia and guarding behavior interfered with chemo- and heat sensitivity of C-fibers. Neither mechanical hyperalgesia nor mechanosensitivity of nociceptors was affected by capsaicin, suggesting that the concentration of capsaicin used in this study did not cause fiber degeneration. These results demonstrate that nociceptors desensitized by capsaicin contribute to heat hyperalgesia and guarding pain after plantar incision. These putative TRPV1-expressing C-fibers are sensitized to heat and acid after incision, and the transduction of heat and chemical stimuli after plantar incision is impaired by dilute capsaicin.
1. Introduction
Postoperative pain is a common problem and many patients experience moderate to severe pain after surgery despite intensive management [2]. This indicates that additional efforts are required to improve postoperative pain management. To understand the mechanisms of pain from a surgical incision and to study new therapies for postoperative pain treatment, we have developed and characterized a rat model of postoperative pain [8].
Our previous study [13] demonstrated that subcutaneous infiltration of dilute capsaicin (8-methy-N-vanillyl-6-nonenamide) solutions (0.025 – 0.10%) before incision produced a differential effect on incision-related pain behaviors depending upon the test; capsaicin reduced heat hyperalgesia and guarding pain but not mechanical hyperalgesia. These results suggest that common mechanisms for heat hyperalgesia and guarding pain occur, and that distinct mechanisms exist for mechanical hyperalgesia.
Since capsaicin sensitivity is considered to be a pharmacological trait of a subpopulation of nociceptive sensory neurons [9,30], we hypothesized that different populations of nociceptors make unique contributions to various incision-related pain behaviors. The purpose of the present study was to evaluate the effect of capsaicin treatment on the activity of cutaneous nociceptors to understand the mechanisms for the selective action of dilute capsaicin on incisional pain. We compared the effect of 0.05% capsaicin versus vehicle treatment on pain behaviors after incision and on the activity of nociceptors from these same rats using the in vitro glabrous skin-tibial nerve preparation. Heat, mechanical and chemical stimuli were tested. Immunohistochemical expression of protein gene product 9.5 (PGP9.5, a pan-axonal marker), neurofilament 200 (N52, a myelinated A-fiber marker), calcitonin gene related peptide (CGRP, for peptidergic fibers) and isolectin B4 (IB4, for non-peptidergic C-fibers) in skin was also evaluated 1 week after 0.05% capsaicin infiltration.
2. Materials and Methods
2.1. General
All experimental procedures were approved by The University of Iowa Animal Care and Use Committee, Iowa City Iowa. Rats were treated in accordance with the Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals issued by the International Association for the Study of Pain [41].
Adult male Sprague-Dawley rats (250-300 g; Harlan, Indianapolis, IN) were used. Rats were housed in groups of two to three in clear plastic cages, with a 12-h light-dark cycle. Food and water were available ad libitum.
2.2. Capsaicin infiltration
Capsaicin (Sigma, St. Louis, MO) was dissolved in vehicle consisting of 1.5% Tween 80, 1.5% alcohol and 97% saline. Under isoflurane anesthesia, 200 μl of 0.05% capsaicin (100 μg/200 μl) or an equal volume of the vehicle was infiltrated subcutaneously into the intended incision site of the right hind paw using a 0.5-ml insulin syringe with a 30-gauge needle. The persons performing behavioral and electrophysiological experiments were blinded to the treatment allocation.
2.3. Immunohistochemistry
One week after 0.05% capsaicin or vehicle injection, at the time an incision would have been made, sensory fiber immunohistochemistry was performed.
2.3.1. Immunohistochemistry staining protocol
Rats were anesthetized with sodium pentobarbital (150 mg/kg) and transcardiacally perfused with 0.1 M phosphate buffered saline, followed by 4% paraformaldehyde. The glabrous plantar skin samples were removed from capsaicin- and vehicle-injected hindpaws, and post-fixed in the same fixative for 4 hrs. After washing in gradually increasing concentrations of sucrose, samples were rapidly frozen in 2-methylbutane chilled at -80 °C. Consecutive sections (12 μm) were prepared and frozen at -80 °C until usage.
Briefly, endogenous peroxidases were inactivated by incubating the sections with 30% methanol containing 0.1% H2O2 (v/v) for 45 min. To block nonspecific reactions, sections were incubated in 0.1M phosphate buffered saline containing 1.5% bovine serum albumin and 0.1% Tween-20 for 1 hr. CGRP- and N52-positive fibers were visualized by confocal microscopy. Since many non-neuronal tissues were stained with IB4, PGP9.5 and IB4 double labeling was performed to localize IB4-positive nerve fibers in skin.
Three consecutive skin sections were used for each rat. Sections were processed for CGRP and N52 labeling by incubation in rabbit anti-CGRP polyclonal antibody (1:1000, Peninsula Laboratories Inc, San Carlos, CA) and mouse anti-N52 monoclonal antibody (1:1000, Sigma, Saint Louis, MO), respectively, at 4 °C overnight, followed by incubation in Cy3-conjugated goat anti-rabbit IgG (1:200, Jackson ImmunoRes, West Grove, PA) or Cy2-conjugated goat anti-mouse IgG (1:200, Jackson ImmunoRes, West Grove, PA) for 4 hrs. For IB4 and PGP9.5 double labeling, sections were incubated with biotinylated griffonia simplicifolia lectin I (1:8000, Vector Laboratories, Burlingame, CA) and rabbit anti-PGP9.5 antibody (1:1000, Chemicon, Temecula, CA), followed by Cy2-conjugated streptavidin (1:200, Jackson ImmunoRes, West Grove, PA) and Cy3-conjugated goat anti-rabbit IgG (1:200, Jackson ImmunoRes, West Grove, PA). For controls, sections were stained without primary or secondary antibodies; in addition. Sections from vehicle and capsaicin treatments were processed at the same time to minimize differences in conditions.
Skin sections were mounted in DPX and observed under Zeiss 510 confocal microscopy (Carl Zeiss MicroImaging, Inc., Thornwood, NY). Eight confocal slices were scanned every micrometer to 8 μm in depth. The 8 digital images were merged using LSM 510 software (Carl Zeiss MicroImaging, Inc., Thornwood, NY).
2.3.2. Immunoreactive area
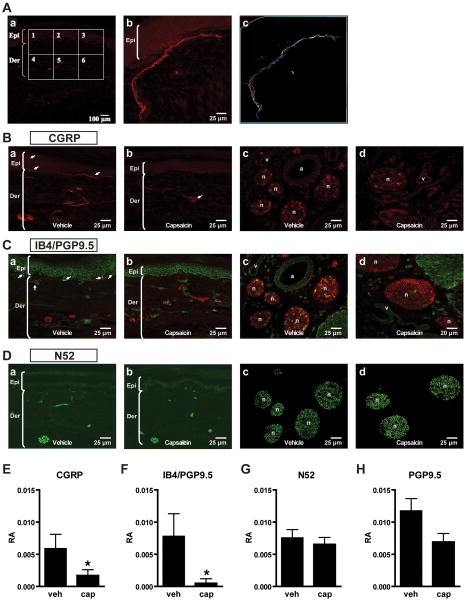
Under low power magnification, 6 adjacent squares (300 μm × 300 μm) were selected in the center of the each confocal image as shown in Fig. 1Aa. The top line of the first series of squares was placed at the stratum corneum-granulosum junction. The second row of squares was adjacent and deep to the first row, including deep dermis. These 6 areas were used to quantitate the area of immunoreactivity under higher magnification.
Fig. 1.
Effects of dilute capsaicin infiltration on afferent fiber immunoreactivity. A: Example for measuring immunoreactive area in skin sections. (Aa): Confocal image of a skin section for calcitonin gene related peptide (CGRP) fluorescent immunohistochemistry. Six adjacent squares (300 μm × 300 μm for each) were selected bordering the epidermis and dermis. (Ab,c): In each square, the total immunopositive area was determined by manually tracing CGRP-immunoreactive fibers. The relative area (RA) of immunoreactivity was calculated by dividing the total immunopositive area by the total skin area. B: CGRP fluorescent immunohistochemistry in plantar skin (Ba,b) and subcutaneous tissue containing large nerve bundles (Bc,d) 1 week after subcutaneous infiltration of vehicle versus 0.05% capsaicin. CGRP-immunolabeled fibers in plantar skin are indicated by arrows (Ba,b). C: Isolectin B4 (IB4) and protein gene product 9.5 (PGP9.5) fluorescent double labeling immunohistochemistry in plantar skin (Ca,b) and subcutaneous tissue (Cc,d) after vehicle versus capsaicin treatment. Double-labeled fibers (yellow) in the epidermal-dermal junction are indicated by arrows (Ca). D: Neurofilament 200 (N52) fluorescent immunohistochemistry in plantar skin (Da,b) and subcutaneous tissue (Dc,d) after vehicle vs. capsaicin treatment. E-H: The RA of immunoreactivity of CGRP- (E), IB4/PGP9.5- (F), N52- (G), and total PGP9.5-positive fibers (H) in vehicle-treated and 0.05% capsaicin-treated skin. Data are expressed as mean ± SE. *P < 0.05 vs. vehicle by unpaired t-test. Epi = epidermi; Der = dermis; n = nerve; a = artery; v = vein; veh = vehicle; cap = capsaicin.
CGRP-, IB4- and N52-immunoreactive areas were measured using Neurolucida software (Version 7, Microbrightfield Inc, Williston, VT). First, the area of each square was viewed under high power and the total skin area (dermis/epidermis) was measured. Next, immunoreactive nerve bundles were marked by manually tracing the immunopositive areas (see example, Fig. 1Ab,c), and the total immunopositive area was determined. The relative area (RA) was calculated by the formula: the total immunopositive area divided by the total skin area (RA = Total immunopositive area / Total skin area). Fig. 1Ab shows a large area of CGRP-immunoreactive nerve bundles in a square from a skin section. This square was chosen as an example of immunoreactive nerve bundles and is not representative. The skin area of the square was encircled (blue line, Fig. 1Ac) and total skin area was measured as 609194 μm2. The total immunopositive fiber area was calculated as 27352 μm2. In this example, RA = 27352/609194 = 0.04489. The average RA per rat was calculated by averaging the values of 18 squares from 3 consecutive skin sections (6 squares per section) that were obtained from each rat. Four rats were studied in the vehicle- and the 0.05% capsaicin-treated groups. The person quantifying the area of immunoreactivity was blinded to the treatment allocation, capsaicin versus vehicle.
Subcutaneous layers were also imaged. The nerve trunks that were visualized were usually cross sections. The area of these cross sections was not calculated and was only discussed qualitatively.
2.4. Plantar incision
One week after capsaicin or vehicle infiltration, a plantar incision was made as described previously [8]. Rats were anesthetized with 1.5-2% isoflurane delivered into a sealed box and then via a nose cone, and the surgical field was prepared in a sterile manner. A 1-cm longitudinal incision was made through skin and fascia of the plantar aspect of the right hindpaw beginning 1.0 cm from the end of heel. The flexor muscle was not incised. Previous studies showed persistent, robust mechanical and heat hyperalgesia with skin and fascia incision; guarding pain behavior is somewhat limited after skin and fascia incision only compared to skin, fascia and muscle incision [8,39]. After hemostasis with gentle pressure, the skin was apposed with 2 mattress sutures of 5-0 nylon on an FS-2 needle. The wound site was covered with topical antibiotic ointment.
2.5. Assessment of pain behaviors
Guarding pain behaviors and withdrawal responses to mechanical and heat stimuli were evaluated 1 day after incision.
To assess guarding pain behaviors, a cumulative pain score was determined as described previously [40]. Unrestrained rats were placed on a stainless steel mesh floor (8 × 8 mm grid). Using an angled magnifying mirror, the position of both paws of each animal were closely observed during a 1-min period repeated every 5 min for 30 min. Depending on the position in which each paw was found during the majority of the 1-min scoring period, a score of 0, 1, or 2 was given. Full weight bearing of the paw (score = 0) was present if the wound was blanched or distorted by the mesh. If the area of the wound touched the mesh without blanching or distorting, a 1 was given. If the paw was completely off the mesh, a score of 2 was recorded. The sum of the six scores (0–12) obtained during the 30-min session for each paw was obtained. The difference between the scores from the incised paw and non-incised paw was the cumulative pain score for that 30-min period. This score was doubled for the comparison with the cumulative pain scores for 1-hr session from previous studies [8,40]. Guarding pain was always performed first to avoid evoked responses influencing the pain score.
To assess mechanosensitivity, rats were placed on a plastic mesh floor covered with a clear plastic cage top (21 × 27 × 15 cm) and allowed to acclimate. Withdrawal response to punctate mechanical stimulation was determined using calibrated von Frey filaments applied from underneath the cage through openings (12 × 12 mm) in the plastic mesh floor to an area adjacent to the wound as described previously [39]. Each filament was applied once starting with 10 mN and continuing until a withdrawal response occurred or 250 mN was reached. If there was no withdrawal response to the 250 mN filament, 522 mN, the next filament was recorded. This was repeated a total of three times with a 10-min test free period between withdrawal responses. The lowest force from the three tests producing a response was considered the withdrawal threshold [39].
To assess heat withdrawal responses, rats were placed on a heat-tempered glass floor (3 mm thick) covered with clear plastic cage top (21 × 27 × 15 cm) and allowed to acclimate. Withdrawal latencies to heat were assessed by applying a focused radiant heat source underneath a glass floor on the middle of incision. The heat stimulus was a light from a 50-W projector lamp, with an aperture diameter of 6 mm. Paw withdrawal latencies were measured to the nearest 0.1 s. The latency time to evoke a withdrawal was determined with a cutoff value of 30 s. The intensity of the heat was adjusted to produce withdrawal latency in normal rat of 10-15 s. Three trials 10 min apart were used to obtain average paw withdrawal latency [39].
2.6. Electrophysiological studies
The same incised rats assessed for pain behaviors were studied in electrophysiological experiments using the glabrous in vitro skin-nerve preparation.
2.6.1. Preparation
The rat glabrous in vitro skin-nerve preparation, modeled as saphenous nerve-skin preparation [26], has been described elsewhere [4,12]. In brief, rats were euthanized in a carbon dioxide chamber; the medial and lateral plantar nerves and their innervated territory on the glabrous hindpaw skin were subcutaneously dissected until the nerve and skin could be removed. The skin was placed epidermal side down in the in vitro perfusion chamber, and superfused with modified Krebs–Hensleit solution (in mM: 110.9 NaCl, 4.8 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2SO4, 24.4 NaHCO3 and 20.0 glucose, pH 7.4), which was saturated with a gas mixture of 95% oxygen and 5% carbon dioxide. The temperature of the bath solution was maintained at 32 ± 0.5 °C. The plantar nerves were drawn through a small hole into the recording chamber containing a superficial layer of mineral oil and a bottom layer of modified Krebs-Hensleit solution. The nerve was desheathed on a mirror stage, and small filaments were repeatedly split with sharpened forceps to allow single fiber recording to be made using extracellular gold-wire recording electrodes. Neural activity was amplified (DAM50, Harvard Apparatus, Holliston, MA), filtered, and displayed using standard techniques. Amplified signals were led to a digital oscilloscope and an audiomonitor and fed into PC computer via a data acquisition system (spike2/CED1401 program, Cambridge Electronic Design Ltd., Cambridge, UK). Action potentials collected on a computer were analyzed off-line with a template-matching function of Spike 2 software (Cambridge Electronic Design Ltd.). If more than one fiber was present in a recording, data were analyzed only if the unit's action potential shape and amplitude could be easily discriminated from the other unit's action potential.
2.6.2. Identification of afferents
The mechanoreceptive fields of afferent units were identified by probing the dermis side of the skin with a blunt glass rod; thus, mechanosensitive afferents were recorded. Only units with a clearly distinguished signal to noise ratio (greater than 2:1) were further studied. Once the receptive field was identified, ongoing spontaneous activity was recorded over a 5-min period for each fiber before any modality testing. After recording of spontaneous activity a standard protocol of mechanical stimulation followed by heat stimulation was performed (n=89). In a separate group of fibers (n=51), a standard protocol for mechanical stimulation followed by chemical stimulation using lactic acid was performed after recording of spontaneous activity.
2.6.3. Feedback-controlled mechanical stimulation
To determine quantitative mechanosensitivity a servo force-controlled mechanical stimulator (Series 300B Dual Mode Servo System, Aurora Scientific, Aurora, Ontario, Canada) [20] was used. A flat-ended cylindrical metal probe (tip diameter 0.7 mm) attached to the tip of the stimulator arm was placed just close to the most sensitive spot of the receptive field so that no force was generated. A computer-controlled ascending series of sustained force stimuli (100 ms rise time, 1.9 s duration of sustained force plateau) was applied at 60-s intervals (10-120 mN range of force).
2.6.4. Feedback-controlled heat stimulation
After mechanical stimulation, a standard heat ramp was delivered by a feedback-controlled heat stimulator. To prevent measuring errors due to thermal convection, the receptive field of each unit was isolated with a small metal ring (5 mm internal diameter), which could seal by its own weight. The bath solution within the ring was manually removed with a syringe. A thermocouple was gently placed to measure subcutaneous temperature. A radiant lamp was placed in the translucent area underneath the organ bath and the light beam was focused onto the epidermal side of the skin. A computer-controlled standard heat ramp was applied starting from 33 to 48 °C in 15 s. We determined that the temperature of the epidermal side was about 2 °C higher than the dermal side. To avoid heat sensitization, fibers having receptive fields in the heat-stimulated area were avoided for subsequent recording.
2.6.5. Acid stimulation
In a separate group of fibers, after mechanical stimulation, chemosensitivity was assessed using pH 5.5 lactic acid. In our previous study, pH 5.5 lactic acid was shown to activate 9 of 14 C-fibers (64%) and 1 of 6 A-fibers (17%) from incised skin, and 3 of 10 C-fibers (30%) and none of 7 (0%) A-fibers from unincised skin, respectively [16].
To restrict the chemical stimuli to the isolated receptive field, a small metal ring (5 mm internal diameter), which could seal by its own weight, was used. In some cases, inert silicone grease was added to ensure a waterproof seal. After recording baseline for 5 min, the metal ring was placed and the modified Krebs-Hensleit solution inside the ring was removed with a syringe. Then pH 5.5 lactic acid (15 mM; 32 °C) was applied to the receptive filed for 5 min, followed by 5-min washout. To avoid sensitization/desensitization of nociceptors, fibers having receptive fields in the previously studied area were avoided for subsequent recording.
Lactic acid for chemical stimulation was made by replacing NaHCO3 (24.4 mM) normally contained in modified Krebs-Hensleit solution with L-lactic acid (Sigma, St. Louis, MO; 85% to a final concentration of 15mM). The pH of lactic acid was measured and adjusted to pH 5.5 with a few drops of 1N NaOH before application. The final osmolarity of the lactic acid solution was 312 mOsm; the sodium concentration was 125 mM.
2.6.6. Conduction velocity and fiber categorization
The conduction velocity of each unit was determined by monopolar electrical stimulation (5-20 V, 0.5-2.0 ms duration, 0.2-1.0 Hz) into the most mechanosensitive site in the receptive filed. Then the distance between the receptive field and the recording electrode (conduction distance) was divided by the latency of the action potential. Afferent fibers conducting slower than 2.5 m/s were classified as C-fibers, those conducting between 2.5 and 24 m/s as Aδ-fibers, and those conducting faster than 24 m/s as Aβ-fibers [22]. Units were classified as mechanosensitive nociceptors on the basis of their graded response throughout the innocuous and noxious range of mechanical force stimuli. Rapidly adapting fibers were not studied.
After completion of the recording protocol for each fiber, the distance between the incision and the residual indentation on skin created by the monopolar electrode was measured using a ruler with 0.5 mm graduation, and then the location of receptive filed in relation to the incision was marked on a schematic of the plantar hindpaw accordingly. Because this experiment was intended to evaluate the effect of capsaicin on mechanonociceptors, we limited the study to units whose receptive fields were located in the area affected by capsaicin (up to 10 mm from the incision).
2.6.7. Data analyses
Action potentials collected on a computer were analyzed off-line with a template-matching function of Spike 2 software. If a unit discharged at a rate of 0.1 imp/s or more without any intentional stimuli over a 5-min period, it was categorized as spontaneously active. For mechanical and heat responses, the quantitative analysis of unit discharges was carried out by averaging responses in 1-s bins and by counting total action potentials in a response. Background activity was subtracted from any evoked responses; thus, assuming background activity was sustained during the stimulus period. Threshold was determined as the lowest force stimulation or temperature which elicited the first action potential in a response. If background activity was present, threshold was determined by the lowest force or heat that increased background activity by at least two standard deviations greater than background average for 10 s (1s bins). For chemical responses, unit discharges were counted in 10-s bins and total responses were averaged during baseline, during acid application and after washout. A unit was considered activated (responsive) when it discharged greater than 0.1 imp/s during chemical stimulation. If background activity was present, the unit was regarded as responsive if the activity was increased at least two standard deviations greater than the background activity during the chemical stimulation period. To count impulses generated by acid in a unit with spontaneous activity, background activity was subtracted from the evoked responses during stimulation.
2.7. Statistical analyses
RAs of immunoreactivity after capsaicin or vehicle injection were compared by unpaired t-test. Among behavioral data, heat withdrawal latencies and guarding pain scores were compared using unpaired t-test, and von Frey thresholds were compared using Mann-Whitney test. Among electrophysiological data, conduction velocity and average spontaneous activity of afferents were compared by unpaired t-test. Heat threshold of C-fibers with or with spontaneous activity was also compared by unpaired t-test. A χ2 test was used to compare the percentage of total C- and Aδ-fibers, the percentage of spontaneously discharging units, the percentage of heat-sensitive units, the percentage of acid-responsive units and the percentage of fibers responding to each force level between the treatment groups. Mann-Whitney test was used to compare the mechanical and heat thresholds, and heat- and acid-evoked action potentials between the two treatment groups. The stimulus-response relationship for the mechanical responses was compared using two-way ANOVA with repeated measures on one factor. Data are presented as mean ± SE or median [range]. Statistical analysis was performed using SPSS 13.0 for Windows (SPSS Inc., Chicago, IL) and Prism 4.0 (GraphPad Software, Inc., San Diego, CA). P < 0.05 was considered statistically significant.
3. Results
3.1. Fluorescent immunohistochemical analyses for CGRP-, IB4- and N52-positive fibers
CGRP-immunoreactivity could be found in the epidermis and dermis in vehicle-treated skin (Fig. 1Ba). From CGRP-immunoreactive nerve bundles adjacent to the epidermal-dermal junction, some single fibers (arrows) extended vertically into the epidermis. One week after capsaicin treatment (Fig. 1Bb), no CGRP-immunoreactivity was detected in the epidermis or adjacent to the epidermal-dermal junction. Few CGRP-immunoreactive nerve bundles could be found in the dermis (arrow). Images from the subcutaneous tissue also showed differences between capsaicin and vehicle treatment (Fig. 1Bc,d). CGRP-immunoreactivity was evident in large nerve bundles 1 week after vehicle injection (Fig. 1Bc). In contrast, CGRP-positive staining was rarely observed in large nerve bundles 1 week after capsaicin infiltration (Fig. 1Bd).
IB4 (green) and PGP9.5 (red) double labeling detected a few IB4-positive nerve bundles (yellow) adjacent to the epidermal-dermal junction in vehicle-treated skin (Fig. 1Ca, arrows). In capsaicin-treated skin, no IB4-immunoreactive bundles were observed at the epidermal-dermal junction (Fig. 1Cb). Some PGP9.5-immunoreactive nerve bundles (red) remained after capsaicin treatment (Fig. 1Ca,b). In the subcutaneous tissue, both IB4-immunoreactivity (yellow) and PGP9.5-immunoreactivity (red) could be found within large nerve trunks after vehicle treatment (Fig 1Cc). However, only PGP9.5-immunoreactivity was present in these nerve trunks 1 week after 0.05% capsaicin treatment (Fig. 1Cd).
In vehicle-treated skin, N52-immunoreactive nerve bundles were present in the dermis (Fig. 1Da) and subcutaneous tissue (Fig. 1Dc). There were no N52-immunoreactive fibers visible in the epidermis. Capsaicin treatment did not change the N52-immunoreactivity in the dermis and subcutaneous tissue compared to vehicle treatment (Fig. 1Db, d).
As shown in Fig. 1E, the RA of CGRP-positive fibers was 0.0059 ± 0.0022 after vehicle injection. Capsaicin injection significantly reduced the RA to 0.0017 ± 0.0009 (P < 0.05 by unpaired t-test). The RAs of IB4-positive fibers were reduced from 0.0078 ± 0.0035 to 0.0005 ± 0.0007 by capsaicin injection (Fig. 1F, P < 0.01 by unpaired t-test). There were no significant changes in N52-positive fibers (Fig. 1G) and PGP9.5-positive fibers (Fig. 1H) after capsaicin injection. Although the total labeling of PGP9.5-positive fibers was reduced by capsaicin, it was not significant.
3.2. Behavioral studies
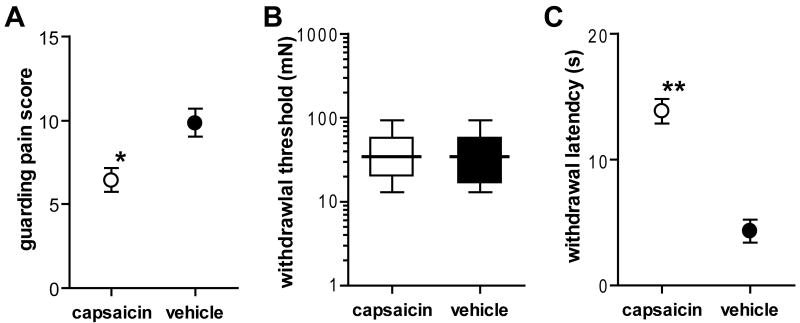
Our previous study demonstrated that pain behaviors both before and after plantar incision were modified by dilute capsaicin depending upon the test [13]. Capsaicin treatment increased the baseline heat withdrawal latency before incision, and attenuated the increase in guarding pain score and the decrease in the heat withdrawal latency after incision. Mechanical withdrawal threshold to von Frey filament application was not changed. In the present study, compared to the vehicle-treated rats, the mean guarding pain score on postoperative day 1 was significantly less in the capsaicin-treated rats (Fig. 2A; P < 0.01 by unpaired t-test). The median withdrawal thresholds to von Frey filament testing were not different between the two treatment groups (Fig. 2B). The mean withdrawal latency to radiant heat was significantly greater in the capsaicin-treated rats compared to the vehicle-treated rats (Fig. 2C; P < 0.001 by unpaired t-test).
Fig. 2.
Effects of capsaicin treatment on pain behaviors one day after plantar incision in rats undergoing in vitro primary afferent recording. A: Guarding pain score was significantly smaller in capsaicin group (n = 23, ○) compared to vehicle group (n = 26, ●) (*P < 0.01 vs. vehicle by unpaired t-test). B: Withdrawal threshold to von Frey filament application. C: Paw withdrawal latency to radiant heat was significantly greater in capsaicin group compared to vehicle group (**P < 0.001 vs. vehicle by unpaired t-test). Results in A and C are expressed as means (circles) ± SE (error bars). The data in B are expressed as median (horizontal line) with 1st and 3rd quartiles (boxes), and 10th and 90th percentiles (whiskers).
3.3. Electrophysiological studies
3.3.1. General properties of afferents
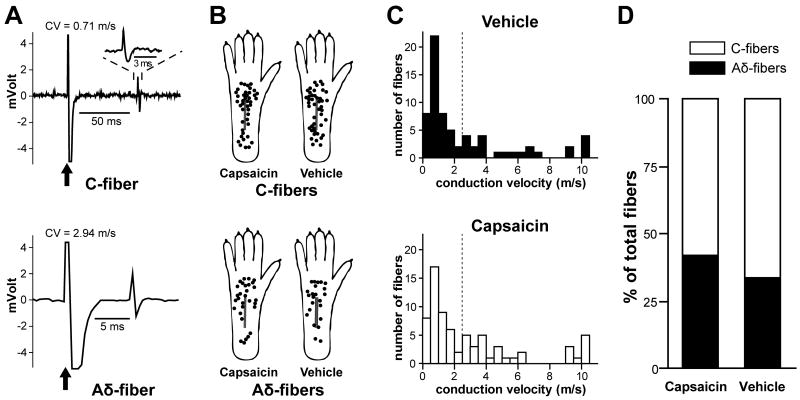
A total of 140 mechanosensitive nociceptors were identified and characterized from the right tibial nerve of 56 rats 1 day after incision; 87 were classified as C-fibers and 53 as Aδ-fibers based on conduction velocity (Fig. 3A). Seventy two fibers (42 C- and 30 Aδ-fibers) were studied from 27 capsaicin-treated rats; 68 fibers (45 C- and 23 Aδ-fibers) were studied from 29 vehicle-treated rats. There was no difference in the conduction velocity of afferents between the treatment groups (Table 1). No mechanosensitive nociceptors identified in this study conducted in the Aβ conduction velocity range. The receptive fields of all fibers were located in glabrous hindpaw skin, as shown in Fig. 3B. Conduction velocity distribution histograms are shown in Fig. 3C. Surprisingly the proportions of C and Aδ-fibers identified were not statistically different between the capsaicin- and vehicle-treated groups (Fig. 3D).
Fig. 3.
Receptive fields and percentage occurrence of mechanosensitive nociceptors. A: Sample recording traces showing the action potential evoked by electrical stimulation to identify the fiber type by conduction velocity. Arrows indicate stimulus artifacts. Inset displays an enlarged view of the action potential. The conduction velocity values of the C- and Aδ-fiber shown here were 0.71 and 2.94 m/s, respectively. CV = conduction velocity. B: Distribution of the receptive fields of C- and Aδ-fibers for capsaicin- and vehicle-treated rats. Each circle represents the center of a unit's mechanoreceptive filed. The receptive field area was not quantified. C: Conduction velocity distribution histograms. D: Percentage of occurrence of C- and Aδ-fibers identified.
Table 1.
Properties of mechanosensitive afferent fibers from incised skin of capsaicin- and vehicle-pretreated rats
| C-fibers Capsaicin |
Vehicle | Aδ-fibers Capsaicin |
Vehicle | |
|---|---|---|---|---|
| Total number of mechanonociceptors | 42 | 45 | 30 | 23 |
| Conduction velocity, m/s | 0.95 ± 0.08 (0.22-2.40) |
0.92 ± 0.08 (0.27-2.37) |
6.71 ± 0.90 (2.51-21.50) |
7.44 ± 1.29 (2.54-23.84) |
| Fibers with spontaneous activity | 16/42 (38.1%) |
22/45 (48.9%) |
4/30 (13.3%) |
6/23 (26.1%) |
| Median threshold (mN) | 40 | 40 | 40 | 40 |
| Heat-responsive fibers | 4/25 (16.0%)** | 26/29 (89.7%) | 2/21 (9.5%) | 2/14 (14.3%) |
| Acid-responsive fibers | 3/17 (17.6%)* | 9/16 (56.3%) | 0/9 (0.0%) | 1/9 (11.1%) |
Values for conduction velocity are means ± SE with ranges in parentheses; other values are number of fibers with percents in parentheses.
P < 0.05;
P < 0.001 vs. vehicle by χ2 test
3.3.2. Spontaneous discharge
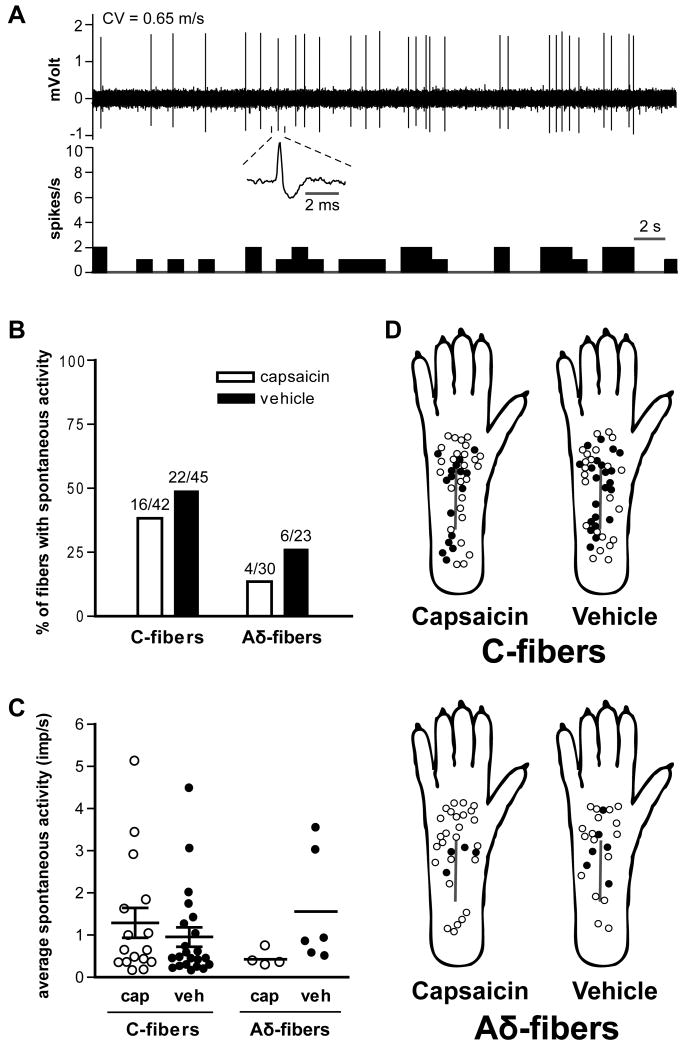
An example of a spontaneously discharging fiber is shown in Fig. 4A. This sample tracing was recorded from a C-fiber of a capsaicin-treated rat hindpaw. Twenty of 72 afferent fibers (27.8%) in the capsaicin group and 28 of 68 fibers (41.2%) in the vehicle group were spontaneously activity (Table 1). The prevalence of spontaneously discharging C- and Aδ-fibers and the average discharge rates were not significantly different between the two treatment groups (Fig. 4B). The discharge rates of spontaneously active C- and Aδ-fibers were not different between the treatment groups (Fig. 4C). The distribution of receptive fields of C- and Aδ-nociceptors with or without spontaneous activity is shown in Fig. 4D. Consistent with our previous study [4], in vehicle-treated skin, the average distance from the receptive fields to the incision was less in spontaneously active C-fibers (2.7 ± 0.5 mm, *P < 0.05 by unpaired t-test) compared to C-fibers without spontaneous activity (4.6 ± 0.6 mm). In the capsaicin group, the average distance from the incision was 3.0 ± 0.6 and 4.7 ± 0.6 mm in C-fibers with and without spontaneous activity, respectively.
Fig. 4.
Effect of capsaicin treatment on spontaneous activity of mechanosensitive afferent fibers one day after plantar incision. A: A sample recording of ongoing activity in a C-fiber from a capsaicin-treated rat. The average firing frequency in this example was 0.76 imp/s. The upper and lower panels show the digitized oscilloscope trace and spike density histogram (bin width = 1 s), respectively. Inset displays an enlarged view of the action potential. CV = conduction velocity. B: Prevalence of spontaneously discharge in C- and Aδ-fibers. C: Average activity of spontaneously discharging fibers. Bars represent mean and whiskers represent SE. Imp = impulse; cap = capsaicin; veh = vehicle. D: Distribution of the receptive fields of C- and Aδ-fibers with or without spontaneous activity, for capsaicin- and vehicle-treated rats. Solid circles represent receptive fields of afferent units with spontaneous activity and open circles represent those without spontaneous activity.
3.3.3. Response to heat stimulation
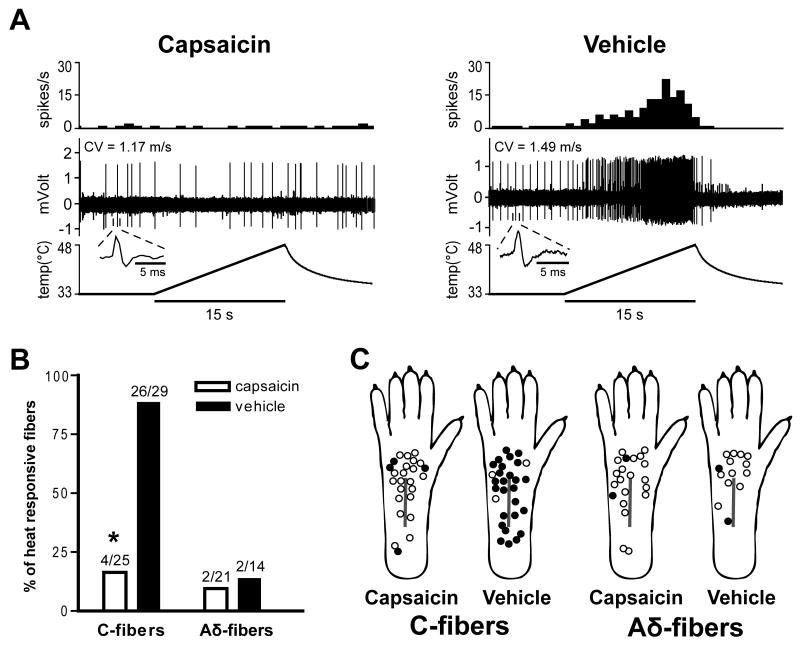
Mechanosensitive C-fibers in the capsaicin-treated group displayed a marked decrease in their heat-responsiveness 1 day after plantar incision. Heat responses of representative C-fibers in the capsaicin versus the vehicle group are shown in Fig. 5A. Few Aδ-fibers in both treatment groups were heat-responsive. Only 4 of 25 C-units (16.0%) in the capsaicin group responded to heat from 33 to 48 °C in 15 s compared to 26 of 29 (89.7%) in the vehicle group (Table 1 and Fig. 5B; P < 0.001 by χ2 test). There was no difference in the incidence of heat sensitivity of Aδ-fibers between the two treatment groups. The distribution of receptive fields of mechanosensitive afferent fibers with or without heat sensitivity is shown in Fig. 5C.
Fig. 5.
Effect of capsaicin treatment on heat sensitivity of afferent units one day after plantar incision. A: Representative recording traces showing heat response of C-fibers. Capsaicin treatment decreased heat-responsiveness of C-fibers compared to vehicle infiltration. The upper, middle and lower panels show the spike density histograms (bin width = 1 s), digitized oscilloscope tracings and the heat stimuli applied, respectively. Insets display single action potential. CV = conduction velocity. B: Prevalence of heat-responsive fibers in C- and Aδ-fibers. *P < 0.001 vs. vehicle by χ2 test. C: Distribution of the receptive fields of C- and Aδ-fibers with or without heat sensitivity, for capsaicin- and vehicle-treated rats. Solid circles represent receptive fields of heat-responsive afferent units, and open circles represent those of non-responsive units.
We previously reported that most C-fibers sensitized by heat were localized to ≤ 2 mm from the incision, in vitro, 1 day after plantar incision [4]. The heat-responsiveness of the C-fibers innervating > 2mm from the incision was not different than C-fibers from unincised skin. Based on these results, the incidence of heat-responsive units was evaluated after subdividing C-fibers into those having receptive fields ≤ 2mm and those having receptive fields > 2 mm from the incision in the present study. In C-fibers innervating ≤ 2mm from the incision, no units (0 of 9 units) in the capsaicin group and all units (10 units) in the vehicle group were heat-responsive (P < 0.001 by Fisher's exact test). Likewise, in C-fibers innervating > 2mm from the incision, the incidence of heat-responsive units was significantly lower in the capsaicin group (4 of 16 units, 25.0%; P < 0.001 by χ2 test) compared to the vehicle group (16 of 19 units, 84.2%).
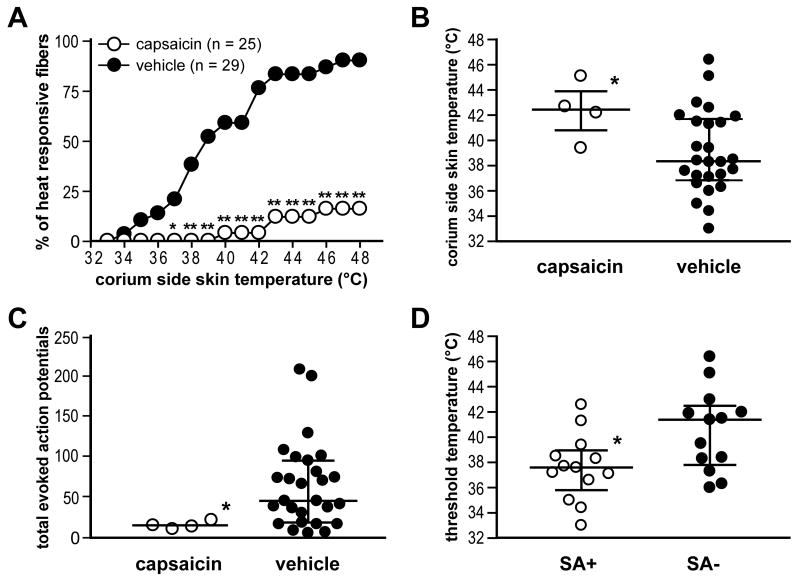
A summary of heat responses in mechanosensitive C-units is shown in Fig. 6. A smaller percentage of C-fibers in the capsaicin group responded to heat (37-48 °C) compared to C-fibers in the vehicle group (Fig. 6A; P < 0.05 at 37 °C; P < 0.01 from 38 to 48 °C by χ2 test). The median threshold temperature to excite single mechanosensitive C-fibers was significantly greater in the capsaicin group than in the vehicle group (Fig. 6B; P < 0.05 by Mann-Whitney test). Total heat evoked action potentials in the capsaicin group were significantly less than those in the vehicle group (Fig. 6C; P < 0.05 by Mann-Whitney test).
Fig. 6.
Capsaicin treatment decreases heat sensitivity of C-fibers one day after incision. A: The percentage of heat-responsive units at each temperature in C-fibers. A decreased percentage of fibers in capsaicin group (n = 25, ○) respond to 37-48 °C heat compared to those in vehicle group (n = 29, ●) (*P < 0.05; **P < 0.01 vs. vehicle by χ2 test). B: Comparison of threshold temperature for activation of C-fibers between treatment groups. *P < 0.05 vs. vehicle by Mann-Whitney test. C: Comparison of total evoked action potentials by heat-responsive C-fibers between treatment groups. *P < 0.05 vs. vehicle by Mann-Whitney test. In B and C, the horizontal line represents median and vertical line the interquartile range. D: Relation between spontaneous activity and heat sensitivity in C-fibers of vehicle group. The horizontal line represents mean and vertical line the SE. *P < 0.05 vs. SA- by unpaired t-test. SA+ = fibers with spontaneous activity; SA- = fibers without spontaneous activity.
In our previous study, we observed that 1 day after incision, spontaneously discharging C-fibers from incised rats had lower heat thresholds compared to the fibers without any spontaneous activity [4]. In this study, we again evaluated the relationship between spontaneous activity and heat sensitivity of C-fibers in the vehicle group. Since few C-fibers (4 of 25 fibers) in the capsaicin group were heat-responsive, we only included units in the vehicle group in this analysis. As shown in Fig. 6D, the heat response threshold of spontaneously discharging fibers was less than fibers without spontaneous activity (P < 0.05 by unpaired t-test).
3.3.4. Response to acid stimulation
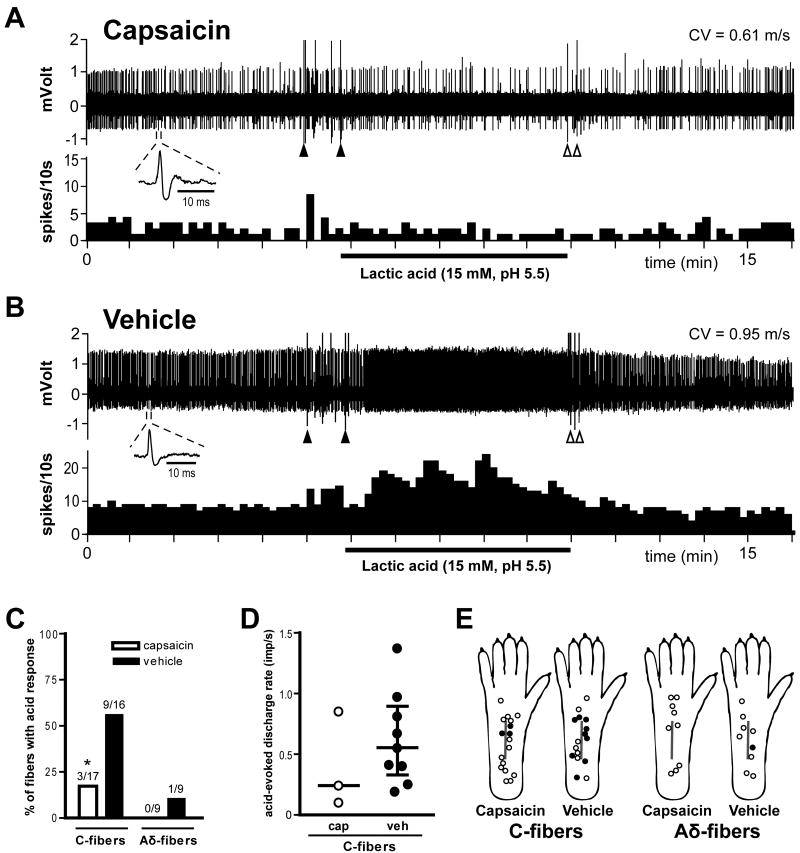
In our previous study, mechanosensitive C-fibers adjacent to the incision had increased responsiveness to lactic acid [16]. The example recordings in Fig. 7A and B show the responses of two single C-fibers to acid application from a capsaicin- and a vehicle-treated hindpaw. Lactic acid did not change the activity of nociceptors in skin treated with capsaicin, but the activity was increased during acid application in the vehicle-treated skin. Capsaicin decreased the proportion of C-fibers responding to lactic acid. Three of 17 C-units (17.6%) in the capsaicin group were responsive to pH 5.5 lactic acid compared to 9 of 16 (56.3%) in the vehicle group (Table 1 and Fig 7C; P < 0.05 by χ2 test). Few Aδ-fibers in either treatment group were acid-responsive (Fig. 7C). The evoked discharge rates of acid-responsive C-fibers during acid application were not different between the treatment groups (Fig. 7D). Fig. 7E shows the distribution of receptive fields of mechanosensitive nociceptors with or without acid-responsiveness. In C-fibers innervating ≤ 2mm from the incision, 3 of 10 units (30%) in the capsaicin group and 6 of 10 units (60%) in the vehicle group were acid-responsive. In C-fibers innervating > 2mm from the incision, no units (0 of 7 units) in the capsaicin group and 3 of 6 units (50%) in the vehicle group responded to lactic acid.
Fig. 7.
Effect of capsaicin treatment on the acid-responsiveness of afferent units one day after plantar incision. A and B: Sample recordings from two single C-fibers from a capsaicin- (A) and a vehicle-treated rat (B). The upper and lower panels show the digitized oscilloscope tracings and spike density histograms (bin width = 10 s), respectively. Insets display the action potentials of these units. Artifacts produced by placing and removal of the metal ring are marked by two black arrowheads and two white arrowheads, respectively. CV = conduction velocity. C: Percentage occurrence of acid-responsive units in C- and Aδ-fibers when tested with pH 5.5 lactic acid. * P < 0.05 vs. vehicle by χ2 test. D: acid-evoked discharge rate of each acid-responsive C-fiber during lactic acid application. Bars represent median and whiskers represent interquartile range. Imp = impulse; cap = capsaicin; veh = vehicle. E: Distribution of the receptive fields of C- and Aδ-fibers with or without responsiveness to pH 5.5 lactic acid, for capsaicin- and vehicle-treated rats. Each circle represents the center of a unit's mechanoreceptive field. Solid and open circles represent receptive fields of afferents with and without acid-responsiveness, respectively.
3.3.5. Response to mechanical stimulation
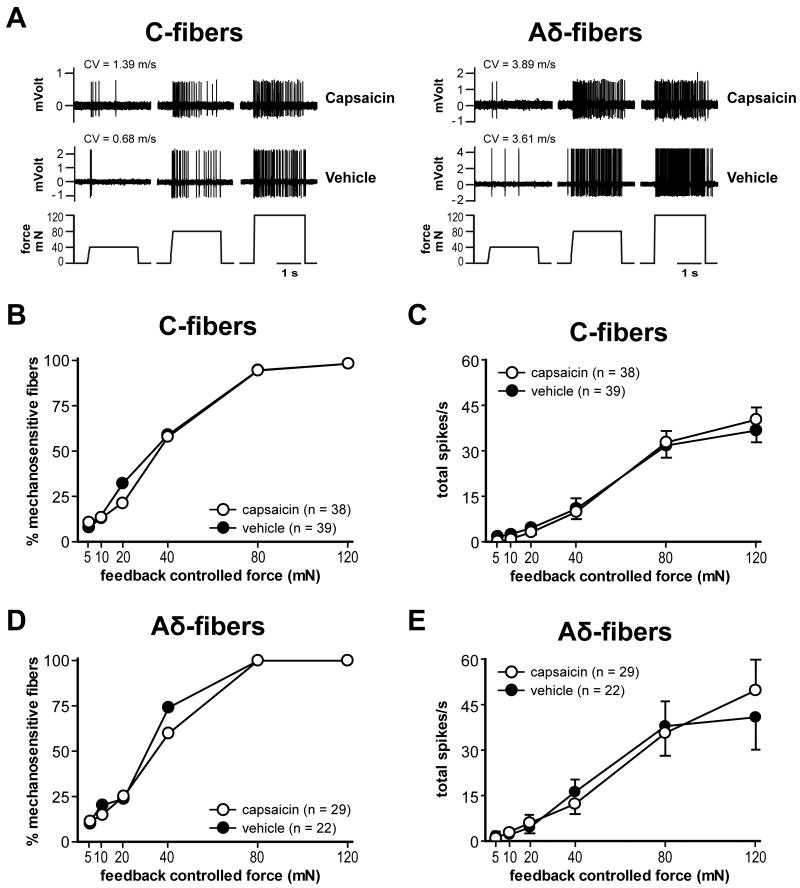
The example traces in Fig. 8A show the responses of nociceptors to force-controlled mechanical stimuli. A summary of mechanical responses of C- and Aδ-fibers is shown in Fig. 8B-E. The median mechanical response thresholds of C- and Aδ-fibers in the capsaicin group were 40 mN and 40 mN, respectively. In the vehicle group, the median response threshold was 40 mN and 40 mN, respectively. No significant difference was evident for both C- and Aδ-fibers when mechanical thresholds were compared between the two treatment groups (Table 1, PFig. 8B and D). The force-response curve of C- and Aδ-fibers showed increases in responses to greater mechanical forces (< 0.001 by two-way ANOVA with repeated measures on one factor; Fig. 8A, C and E). The stimulus-response functions of both C- and Aδ-fibers in the capsaicin group did not differ significantly from their corresponding vehicle groups (Fig. 8C and E).
Fig. 8.
Effect of capsaicin treatment on mechanosensitivity of afferents one day after plantar incision. A: Sample recording traces showing responses to mechanical stimuli. Both C- and Aδ-fibers from either capsaicin or vehicle treatment groups have force-related increase responses to the ascending series of mechanical stimuli (40, 80, and 120 mN). CV = conduction velocity. B and C: The percentage mechanosensitivity at each force (B) and stimulus-response function (C) of C-fibers in capsaicin (n = 38, ○) vs. vehicle group (n = 39, ●). D and E: The percentage of mechanosensitivity (D) and stimulus-response function (E) of Aδ-fibers in capsaicin (n = 29, ○) vs. vehicle group (n = 22, ●).
4. Discussion
In the present study, infiltration of 0.05% capsaicin decreased CGRP and IB4/PGP9.5-immunoreactivity of nociceptors in skin. However, electrophysiological data demonstrated that the same dose of dilute capsaicin did not decrease the prevalence of C-mechanonociceptors in skin, suggesting that C-fibers are not killed by capsaicin. This dose that inhibited heat hyperalgesia and guarding behaviors interfered with chemo- and heat sensitivity, but not mechanosensitivity of C-nociceptors. Responses of A-mechanonociceptors were not affected by capsaicin.
While we have previously demonstrated that dilute capsaicin modifies pain related behaviors both before and after the incision, the purpose of this study was to explore the possible neural mechanisms that explain the differential effect of capsaicin on the incision-induced pain behaviors. We used vehicle-treated, incised rats as a control group to study sensitized nociceptors, rather than capsaicin-treated, unincised rats, which would have studied normal nociceptors. That is, we compared the effect of capsaicin versus vehicle on the pain behaviors after incision, and evaluated the electrophysiological activity of nociceptors from these same rats in vitro.
Previous studies showed that C-mechanonociceptors are sensitized to heat and lactic acid application by incision [4,16]. The present study demonstrates that C-mechanonociceptors from incised rats are desensitized to heat and lactic acid by 0.05% capsaicin, which also reduces heat hyperalgesia and guarding pain. C-fibers desensitized by capsaicin are likely TRPV1-expressing nociceptors [29]. The TRPV1 receptor is a polymodal nocisensor responsive to various noxious stimuli, including noxious heat, low pH and capsaicin [10,33]. Several factors can sensitize TRPV1 receptors, including nerve growth factor, low pH and prostaglandin E2, and these are present in the incisional wound environment [17,34,35].
Most capsaicin-sensitive nociceptors are C-fibers, and especially when low concentrations are utilized, capsaicin is highly selective for activating or desensitizing C-fibers [14,18,31]. Previous studies have demonstrated, in vivo and in vitro, that over 90% of mechano-heat-sensitive C-fibers in rat skin are capsaicin-sensitive [18,28]. In our previous study using the rat in vitro glabrous skin-tibial nerve preparation, about 80% of C-mechanonociceptors from unincised skin were heat-sensitive [4]. If dilute capsaicin treatment destroyed the nerve terminal of TRPV1-containing fibers, thereby making them also unresponsive to mechanical stimuli, the incidence of C-mechanonociceptors would have decreased. However, dilute capsaicin did not affect the proportions of C- and Aδ-mechanonociceptors, implying that most if not all TRPV1-containing fibers maintain their mechanosensitivity after capsaicin infiltration.
The concentration of capsaicin infiltrated subcutaneously in this study may have selectively affected the transducer mechanism(s) for heat and acid while sparing mechanical transduction. Data from a previous study support these results; in cat cornea, topical application of 0.01 ∼ 1% capsaicin onto the receptive fields of polymodal nociceptors abolished responsiveness to heat and chemical but not mechanical stimuli in 85% of fibers [7]. Clearly, the capsaicin is not C-fiber destructive when infiltrated in a dose that inhibits heat hyperalgesia and guarding behaviors, and the effect is not simply loss of TRPV1 function on C-fibers because capsaicin treatment is more effective against guarding behavior after plantar incision than TRPV1 receptor blockade [36]. In agreement, in our recent study using TRPV1 knockout mice, while heat hyperalgesia induced by incision was completely abolished by loss of TRPV1, guarding pain behaviors, although small in mice, were not affected [6,24].
In this study, we quantified nerve fiber immunoreactivity in bundles by counting the volume of the immunoreactive fiber area in the epidermis, dermis and subcutaneous tissue. These fibers likely innervate skin and subcutaneous structures and should be vulnerable to subcutaneous injection of capsaicin because nerve fibers originating from dorsal root ganglia reach the subcutaneous tissue as nerve bundles largely running parallel to the skin surface [11]. Cutaneous nerves then form horizontal subepidermal nerve plexi before separating into single nerve fibers that pierce into the epidermis [19].
The electrophysiological data indicate that a decrease in nerve fiber immunoreactivity rather than nerve fiber degeneration occurs after dilute capsaicin treatment. Capsaicin can deplete of neuropeptides such as CGRP and block of axonal transport of macromolecules from dorsal root ganglia to peripheral axons [14,15]. This may explain the decreased area of CGRP- and IB4/PGP9.5-immunoreactivity of C-fibers [21]. In line with this hypothesis, 0.03% capsaicin applied intravesically increased the volume threshold for reflex micturition in rats and caused a profound reduction in CGRP- and substance P-immunoreactivity without causing significant ultrastructural changes of the bladder nerve fibers determined by electron microscopy, excluding terminal axon degeneration as the main mechanism of action [3]. Also, when a much higher concentration (1%) of capsaicin was topically applied to the eye of rats, electron microscopy showed no signs of axon degeneration in the cornea after 46 hrs, although swollen mitochondria with disorganized cristae could be found [32]. Taken together, the results of the preceding and present studies suggest that subcutaneous infiltration of dilute capsaicin before incision may decrease heat- and chemosenstivity of CGRP-expressing and IB4-binding C-fibers from incised tissue, without fiber loss or degeneration.
The role of C- versus A-fibers in mechanical hyperalgesia after incision is not clearly defined. It is possible both C- and A-nociceptors contribute to the mechanical sensitivity [5]. In the present study, dilute capsaicin did not affect in vitro mechanosensitivity of nociceptors; neither the proportions of mechanosensitive C- and Aδ-fibers, nor the stimulus-response functions were affected. These data may explain the lack of effect of capsaicin on the withdrawal response to punctate mechanical stimuli after incision. Also, moderate to poor efficacy of capsaicin in the treatment of neuropathic pain [23] could be at least partially explained by the lack of effect of capsaicin on mechanical responses of nociceptors, considering that mechanical hyperalgesia is one of the major symptoms of neuropathic pain.
Previously, in vivo and in vitro, we have shown that primary afferent nociceptors develop spontaneous activity after incision [4,25]. It has been hypothesized that the underlying mechanism responsible for the spontaneous activity might be lowering of the heat threshold of afferents to the point at which body temperature becomes an adequate stimulus for excitation [27]. Our previous report demonstrated that spontaneously discharging C-fibers from incised skin had lower heat response threshold compared to the C-fibers without spontaneous activity [4], supporting this hypothesis. The present study showed similar results; in the vehicle-treated group, the heat response threshold of spontaneously discharging C-fibers was less than C-fibers without spontaneous activity. However, dilute capsaicin greatly reduced heat responses but did not influence spontaneous activity of nociceptors. This indicates that the mechanisms for the in vitro spontaneous activity in cutaneous nociceptors after incision could not be solely explained by the lowering of the threshold temperature for activation of afferents. Multiple mechanisms appear to be involved in the development of spontaneous activity in nociceptors after incision, and they remain yet to be proven.
Spontaneous activity of nociceptors after incision has been suggested as one of the mechanisms for guarding pain behavior. However, in the present study, dilute capsaicin decreased guarding pain behavior but did not affect spontaneous activity of nociceptors. This finding suggests that mechanisms other than spontaneous activity of cutaneous nociceptors might be involved in the pathogenesis of nonevoked, guarding pain behavior after plantar incision. Recent studies support this concept [37,38].
In recent clinical trials, the efficacy of a single intraoperative wound instillation of capsaicin (15 ml of 0.006% solution) on postoperative pain was evaluated in adult male patients undergoing open mesh groin hernia repair [1]. The cumulative pain score was significantly lower during the first 3 days postoperatively in the capsaicin-treated group. Also, in a recent report of phase 3 trial evaluating the effect of capsaicin instilled into the surgical site (60ml of 0.025% solution) immediately before wound closure, capsaicin significantly reduced postoperative pain following total knee arthroplasty from 4 to 48 hrs after surgery [42]. Together these clinical studies and our data suggest that guarding behavior may translate to clinical postoperative pain.
5. Conclusion
The results of the present study demonstrate that nociceptors desensitized by capsaicin contribute to heat hyperalgesia and guarding pain after plantar incision. These fibers are sensitized to heat and acid after incision [4,16], and the transduction of heat and chemical stimuli after plantar incision is impaired by dilute capsaicin. Neither mechanical hyperalgesia nor mechanosensitivity of C-fibers is affected by capsaicin. Capsaicin treatment inhibits guarding and heat hyperalgesia, suggesting that the reduction of pain in patients after surgery may be related to loss of heat- and/or chemosensitivity but not mechanosensitivity of nociceptors. The same dose of dilute capsaicin that decreased CGRP- and IB4/PGP9.5-immunoreactivity did not affect the proportions of mechanosensitive C- and Aδ-fibers. The concentration of capsaicin infiltrated subcutaneously in this study might have selectively affected the transducer for heat and acid in TRPV1-containing nociceptors without producing degeneration. These results indicate that key nociceptors transmitting pain at rest in postoperative patients are likely TRPV1-expressing C-fibers; furthermore, we suggest that intact mechanonociceptor function after capsaicin treatment may provide clues to the limited anti-hyperalgesic effects of capsaicin in some clinical pain states.
Acknowledgments
Support: This work was performed and in supported by the Department of Anesthesia at the University of Iowa and by National Institutes of Health, Bethesda, Maryland grants GM-55831 to T.J.B. Dr. Kang and Dr. Wu contributed equally to the manuscript.
The authors are grateful to Dr. Herbert K. Proudfit, PhD for assistance with immunohistochemistry.
Footnotes
Dr. Brennan has served as a consultant to Anesevia, manufacturer of Adlea (capsaicin) during the completion of the studies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aasvang EK, Hansen JB, Malmstrom J, Asmussen T, Gennevois D, Struys MM, Kehlet H. The effect of wound instillation of a novel purified capsaicin formulation on postherniotomy pain: a double-blind, randomized, placebo-controlled study. Anesth Analg. 2008;107:282–91. doi: 10.1213/ane.0b013e31816b94c9. [DOI] [PubMed] [Google Scholar]
- 2.Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97:534–40. doi: 10.1213/01.ANE.0000068822.10113.9E. table of contents. [DOI] [PubMed] [Google Scholar]
- 3.Avelino A, Cruz F. Peptide immunoreactivity and ultrastructure of rat urinary bladder nerve fibers after topical desensitization by capsaicin or resiniferatoxin. Auton Neurosci. 2000;86:37–46. doi: 10.1016/S1566-0702(00)00204-6. [DOI] [PubMed] [Google Scholar]
- 4.Banik RK, Brennan TJ. Spontaneous discharge and increased heat sensitivity of rat C-fiber nociceptors are present in vitro after plantar incision. Pain. 2004;112:204–13. doi: 10.1016/j.pain.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Banik RK, Brennan TJ. Sensitization of primary afferents to mechanical and heat stimuli after incision in a novel in vitro mouse glabrous skin-nerve preparation. Pain. 2008;138:380–91. doi: 10.1016/j.pain.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banik RK, Brennan TJ. Trpv1 mediates spontaneous firing and heat sensitization of cutaneous primary afferents after plantar incision. Pain. 2009;141:41–51. doi: 10.1016/j.pain.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belmonte C, Gallar J, Pozo MA, Rebollo I. Excitation by irritant chemical substances of sensory afferent units in the cat's cornea. J Physiol. 1991;437:709–25. doi: 10.1113/jphysiol.1991.sp018621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 9.Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 10.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 11.Doucette R, Diamond J. Normal and precocious sprouting of heat nociceptors in the skin of adult rats. J Comp Neurol. 1987;261:592–603. doi: 10.1002/cne.902610410. [DOI] [PubMed] [Google Scholar]
- 12.Du J, Koltzenburg M, Carlton SM. Glutamate-induced excitation and sensitization of nociceptors in rat glabrous skin. Pain. 2001;89:187–98. doi: 10.1016/s0304-3959(00)00362-6. [DOI] [PubMed] [Google Scholar]
- 13.Hamalainen MM, Subieta A, Arpey C, Brennan TJ. Differential effect of capsaicin treatment on pain-related behaviors after plantar incision. J Pain. 2009;10:637–45. doi: 10.1016/j.jpain.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- 15.Jancso G. Pathobiological reactions of C-fibre primary sensory neurones to peripheral nerve injury. Exp Physiol. 1992;77:405–31. doi: 10.1113/expphysiol.1992.sp003603. [DOI] [PubMed] [Google Scholar]
- 16.Kang S, Brennan TJ. Chemosensitivity and mechanosensitivity of nociceptors from incised rat hindpaw skin. Anesthesiology. 2009;111:155–64. doi: 10.1097/ALN.0b013e3181a16443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapoor M, Kojima F, Yang L, Crofford LJ. Sequential induction of pro- and anti-inflammatory prostaglandins and peroxisome proliferators-activated receptor-gamma during normal wound healing: a time course study. Prostaglandins Leukot Essent Fatty Acids. 2007;76:103–12. doi: 10.1016/j.plefa.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kenins P. Responses of single nerve fibres to capsaicin applied to the skin. Neurosci Lett. 1982;29:83–8. doi: 10.1016/0304-3940(82)90369-x. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy WR. Opportunities afforded by the study of unmyelinated nerves in skin and other organs. Muscle Nerve. 2004;29:756–67. doi: 10.1002/mus.20062. [DOI] [PubMed] [Google Scholar]
- 20.Khalsa PS, LaMotte RH, Grigg P. Tensile and compressive responses of nociceptors in rat hairy skin. J Neurophysiol. 1997;78:492–505. doi: 10.1152/jn.1997.78.1.492. [DOI] [PubMed] [Google Scholar]
- 21.Kissin I. Vanilloid-induced conduction analgesia: selective, dose-dependent, long-lasting, with a low level of potential neurotoxicity. Anesth Analg. 2008;107:271–81. doi: 10.1213/ane.0b013e318162cfa3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leem JW, Willis WD, Chung JM. Cutaneous sensory receptors in the rat foot. J Neurophysiol. 1993;69:1684–99. doi: 10.1152/jn.1993.69.5.1684. [DOI] [PubMed] [Google Scholar]
- 23.Mason L, Moore RA, Derry S, Edwards JE, McQuay HJ. Systematic review of topical capsaicin for the treatment of chronic pain. Bmj. 2004;328:991. doi: 10.1136/bmj.38042.506748.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pogatzki-Zahn EM, Shimizu I, Caterina M, Raja SN. Heat hyperalgesia after incision requires TRPV1 and is distinct from pure inflammatory pain. Pain. 2005;115:296–307. doi: 10.1016/j.pain.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Pogatzki EM, Gebhart GF, Brennan TJ. Characterization of Adelta- and C-fibers innervating the plantar rat hindpaw one day after an incision. J Neurophysiol. 2002;87:721–31. doi: 10.1152/jn.00208.2001. [DOI] [PubMed] [Google Scholar]
- 26.Reeh PW. Sensory receptors in mammalian skin in an in vitro preparation. Neurosci Lett. 1986;66:141–6. doi: 10.1016/0304-3940(86)90180-1. [DOI] [PubMed] [Google Scholar]
- 27.Reeh PW, Petho G. Nociceptor excitation by thermal sensitization--a hypothesis. Prog Brain Res. 2000;129:39–50. doi: 10.1016/S0079-6123(00)29004-3. [DOI] [PubMed] [Google Scholar]
- 28.Seno N, Dray A. Capsaicin-induced activation of fine afferent fibres from rat skin in vitro. Neuroscience. 1993;55:563–9. doi: 10.1016/0306-4522(93)90524-j. [DOI] [PubMed] [Google Scholar]
- 29.Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- 30.Szolcsanyi J. A pharmacological approach to elucidation of the role of different nerve fibres and receptor endings in mediation of pain. J Physiol (Paris) 1977;73:251–9. [PubMed] [Google Scholar]
- 31.Szolcsanyi J. Selective responsiveness of polymodal nociceptors of the rabbit ear to capsaicin, bradykinin and ultra-violet irradiation. J Physiol. 1987;388:9–23. doi: 10.1113/jphysiol.1987.sp016598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szolcsanyi J, Jancso-Gabor A, Joo F. Functional and fine structural characteristics of the sensory neuron blocking effect of capsaicin. Naunyn Schmiedebergs Arch Pharmacol. 1975;287:157–69. doi: 10.1007/BF00510447. [DOI] [PubMed] [Google Scholar]
- 33.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–43. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 34.Woo YC, Park SS, Subieta AR, Brennan TJ. Changes in tissue pH and temperature after incision indicate acidosis may contribute to postoperative pain. Anesthesiology. 2004;101:468–75. doi: 10.1097/00000542-200408000-00029. [DOI] [PubMed] [Google Scholar]
- 35.Wu C, Boustany L, Liang H, Brennan TJ. Nerve growth factor expression after plantar incision in the rat. Anesthesiology. 2007;107:128–35. doi: 10.1097/01.anes.0000267512.08619.bd. [DOI] [PubMed] [Google Scholar]
- 36.Wu C, Gavva NR, Brennan TJ. Effect of AMG0347, a transient receptor potential type V1 receptor antagonist, and morphine on pain behavior after plantar incision. Anesthesiology. 2008;108:1100–8. doi: 10.1097/ALN.0b013e31817302b3. [DOI] [PubMed] [Google Scholar]
- 37.Xu J, Brennan TJ. Comparison of skin incision vs. skin plus deep tissue incision on ongoing pain and spontaneous activity in dorsal horn neurons. Pain. 2009;144:329–39. doi: 10.1016/j.pain.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu J, Brennan TJ. Guarding pain and spontaneous activity of nociceptors after skin versus skin plus deep tissue incision. Anesthesiology. doi: 10.1097/ALN.0b013e3181c2952e. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zahn PK, Brennan TJ. Primary and secondary hyperalgesia in a rat model for human postoperative pain. Anesthesiology. 1999;90:863–72. doi: 10.1097/00000542-199903000-00030. [DOI] [PubMed] [Google Scholar]
- 40.Zahn PK, Gysbers D, Brennan TJ. Effect of systemic and intrathecal morphine in a rat model of postoperative pain. Anesthesiology. 1997;86:1066–77. doi: 10.1097/00000542-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 42.Adlea Clinical Data. http://www.anesiva.com/wt/page/cl_dataadlea.