Abstract
Context
Hypothermia improves neurological outcome for comatose survivors of out-of-hospital cardiac arrest. Use of computer controlled high surface area devices for cooling may lead to faster cooling rates and potentially improve patient outcome.
Objective
To compare the effectiveness of surface cooling with the standard blankets and ice packs to the Arctic Sun, a mechanical device used for temperature management.
Design, Setting, and Patients
Multi-center randomized trial of hemodynamically stable comatose survivors of out-of-hospital cardiac arrest.
Intervention
Standard post-resuscitative care inducing hypothermia using cooling blankets and ice (n=30) or the Arctic Sun (n=34).
Main Outcome Measures
The primary end point was the proportion of subjects who reached a target temperature within 4 hours of beginning cooling. The secondary end points were time interval to achieve target temperature (34°) and survival to 3 months.
Results
The proportion of subjects cooled below the 34°C target at 4 hours was 71% for the Arctic Sun group and 50% for the standard cooling group (p=0.12). The median time to target was 54 minutes faster for cooled patients in the Arctic Sun group than the standard cooling group (p<0.01). Survival rates with good neurological outcome were similar; 46% of Arctic Sun patients and 38% of standard patients had a cerebral performance category of 1 or 2 at 30 days (P=0.6).
Conclusions
While the proportion of subjects reaching target temperature within 4 hours was not significantly different, the Arctic Sun cooled patients to a temperature of 34° C more rapidly than standard cooling blankets.
Trial Registration
ClinicalTrials.gov NCT00282373, registered January 24, 2006.
Introduction
Induction of mild hypothermia (a core temperature of 32-34 °C) improves neurological outcome in comatose survivors of out-of hospital cardiac arrest.1 This therapy is being adopted around the world, with most centers utilizing simple external cooling devices such as cooling blankets and ice bags.2 However, these simple methods of cooling, while easy to implement, were not specifically designed for rapid cooling of critically ill patients and do not provide precision body temperature control during maintenance and reversal of hypothermia.
The Arctic Sun® (Medivance Corp, Louisville, Co.) is a United States Food and Drug Administration cleared temperature management system. It differs from standard cooling blankets by: 1) producing higher cold fluid flow rates, 2) utilizing conductive, adherent gel pads and 3), and implements a precise temperature feedback-control mechanism. These factors may allow a more rapid induction of cooling and improved control of temperature during hypothermia maintenance and re-warming than standard cooling blankets.
The objective of this study was to compare the Arctic Sun to standard cooling blankets and ice bags for induction and maintenance of therapeutic hypothermia after cardiac arrest. We hypothesized that a greater proportion of patients would reach target temperature within 4 hours when cooled with the Arctic Sun device as compared to patients cooled by standard cooling blankets. We further hypothesized that the Arctic Sun would cool patients faster and would improve the maintenance of patients within the therapeutic target range (32-34°C), with less out of the target range temperature fluctuations when compared to standard cooling.
Methods
This was a multi-center randomized controlled trial. Patients were assigned to hypothermic treatment with either the Arctic Sun or standard cooling blankets by a centralized telephone system which utilized a fixed block randomization scheme with an allocation ratio of 1:1 for each center. Six university-affiliated hospitals participated. All hospitals had protocols for cooling post-cardiac arrest patients, but their experience ranged from 0 to 15 patients prior to the study. Most centers had been cooling patients less than a year. The institutional review board approved the protocol at each site, and informed consent was obtained from the next-of-kin prior to subject randomization. We included comatose patients aged 18 years or older who had return of spontaneous circulation after an out-of-hospital cardiac arrest from a presumed cardiac cause. Patients with all initial rhythms were included. Inclusion and exclusion criteria are shown in Table 1.
Table 1.
Inclusion and exclusion criteria.
| Inclusion |
| 1.First attempt at resuscitation (ACLS or CPR) by emergency medical personnel initiated within 15 minutes of collapse. 2.Restoration of spontaneous circulation (ROSC) within 60 minutes of collapse. 3.Time from restoration of spontaneous circulation to initiation of cooling ≤ 6 hours. |
| Exclusion |
| 1.Temperature of less than 35°C on admission. 2.Comatose or vegetative state prior to cardiac arrest. 3.Positive pregnancy test. 4.Purposeful response to verbal commands after ROSC and prior to initiation of hypothermia. 5.Evidence of hypotension (MAP<60) for more than 30 minutes after the return of ROSC and prior to initiation of hypothermia. 6.Evidence of hypoxia (oxygen saturation < 85% despite supplemental oxygen) for more than 15 minutes after ROSC and prior to initiation of hypothermia. 7.Terminal illness that preceded the arrest (life expectancy < 1 yr). 8.Patients experiencing cardiogenic shock. 9.Patients continuing to experience refractory ventricular arrhythmias at the time of enrollment. 10.Patients receiving 2 or more high-dose vasopressors. 11.Active bleeding or known pre-existing coagulapathy. 12.Patient history of cold agglutinin disease. 13.Patient history of Raynaud’s disease 14.Patient history of Sickle Cell disease. 15.Evidence of compromised skin integrity or irregularities (such as uticaria, rash, lacerations, burns, abrasions). 16.Patient weight ≥ 114 kg (250 lb) or < 50 kg (110 lb). |
Interventions
All patients had a bladder temperature probe inserted to continuously measure core temperature and provide feedback to the cooling devices. No patients received an intravenous iced saline infusion. For the cooling blanket group, two standard cooling blankets were utilized, one wrapped around the upper torso and the other on the lower torso/thighs and set for maximum cooling. The cooling protocol included the application of bags containing ice to the axillae and groin and changed every 30-60 minutes as needed. Once the core temperature was below 34°, ice packs were removed and the cooling blanket was set to the automatic mode with a target bladder temperature of 33.5°. For patients randomized to the Arctic Sun, cooling pads were applied per the manufacturer’s instructions to the back, chest and thighs. The automatic mode was set to a target temperature of 33.5° degrees, and the maximum cooling rate was used. No ice bags were used in the Arctic Sun patients.
All patients were paralyzed and sedated during the induction and maintenance period. The sedative and paralytic agents were not specified by the study protocol to allow each institution to use their local guidelines. Patients were maintained in the target range between 32 and 34 ° C. Twenty-four hours after the initiation of cooling, the maintenance period ended and rewarming was initiated. For the standard cooling group, the temperature on the water blanket was increased by 1 ° C every 2-4 hours. For the Arctic Sun group, rewarming was begun by setting the target temperature at 36 degrees and the rewarming rate at 0.25 to 0.5 ° C per hour. Once the patient’s temperature reached 36 ° C, the device was turned off.
Measurements
Demographic variables and the circumstances of cardiac arrest were collected utilizing the Utstein criteria for all patients. Temperature was electronically recorded using a bladder probe. Nurses recorded temperature and vital signs every 15 minutes during cooling, every 30 minutes during the first 2 hours once target was reached and hourly until the rewarming phase was completed. The patients had standardized laboratory monitoring including: CBC, comprehensive metabolic panels, electrolytes, arterial blood gases, lipase, lactate and PT/PTT. Neurological outcomes were determined by unblinded investigators using the Glasgow Coma Score at baseline, 36 and 72 hrs, and 7 days (or at discharge if prior to 7 days). The Glasgow-Pittsburgh Cerebral Performance Categories (CPC) and Overall Performance Categories (OPC) were performed by an unblinded investigator at baseline, discharge, 30 days and 90 days by review of medical records or phone contact if the patient was no longer hospitalized. Complications were identified by the site investigator using a closed end checklist, including sepsis, pneumonia, bleeding, pancreatitis, renal failure, arterial hypoxemia, seizures, significant arrhythmia and pressure sores.
Outcomes
The primary outcome was a comparison of the proportion of patients achieving a target temperature of less than 34 ° C within 4 hours of initiation of cooling. The secondary outcomes were comparisons of the median time to target temperature, the incidence of overshoot during induction (any recorded temperature <32° C during induction) and the proportions of subjects who had an out of range (>34 ° or < 32 °) temperature recorded during the maintenance period.
Blinding
Neither investigators or the subjects’ families were blinded to study intervention because management of the different approaches to cooling was the primary purpose of the trial. The outcomes were assessed by study personnel who were aware of the group assignment for the subject.
Analysis
For our primary outcome, we performed an intent-to-treat analysis comparing the proportions of patients reaching the target temperature in each group using chi-square and calculated the absolute risk difference with 95% confidence intervals. Patients who were withdrawn prior initiation of cooling were analyzed as treatment failures in the primary outcome analysis. The time-to-target temperature (defined as the number of minutes from the start of cooling to bladder temperature below 33.9 °C) for patients who were cooled was compared using a Log-Rank test. Other categorical outcomes were compared between groups by determining the absolute risk difference with 95% confidence intervals. A 95% confidence interval for the risk difference that excluded 0 was considered statistically significant. Descriptive statistics for potential confounding variables include means or proportions with 95% confidence intervals.
Power
the selected sample size of 64 subjects provided 80% power with an alpha of 0.05 to detect a 30% increase in the proportion of patients reaching target temperature within 4 hours assuming a rate of 60% in the control group.
Results
Sixty four patients were enrolled between November 2004 and March 2007. Thirty patients were randomized to standard cooling blankets, and thirty four patients were randomized to the Arctic Sun. Three patients were removed from the protocol after randomization but before initiation of cooling: One in the Arctic Sun arm was removed because a CT scan obtained showed a subarachnoid hemorrhage; one patient in the Arctic Sun group was removed after family withdrew care after initially consenting to participate; another patient in the cooling blanket group recovered neurological function prior to cooling.
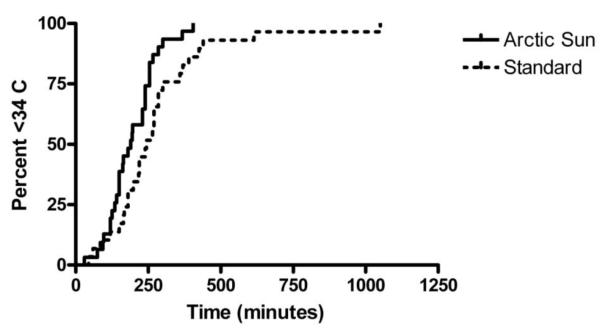
Patients had similar baseline characteristics (Table 2). A total of 24/34 (71%) patients treated with the Arctic Sun reached the target temperature within 4 hours compared to 15/30 (50%) in the standard water blanket group. The proportion reaching target within 4 hours was 21% higher for the Arctic Sun group, but this was not statistically different (95% CI of the difference −3.4% to 44.6%). The median time to target was 54 minutes faster for cooled patients in the Arctic Sun group than the standard cooling group (190 min [IQR 135,155]) vs 244 min [IQR 180, 300], p<0.01 by log-rank test). Time to achieve target temperature curves are shown in Figure 1.
Table 2.
Baseline characteristics of patients randomized to each treatment group.
| Arctic Sun | Standard | |
|---|---|---|
| Count of subjects | 34 | 30 |
| Age (mean ± SD) | 57±14 years | 64±14 |
| Male | 21 (62%) | 22 (73%) |
| Caucasian | 23 (68%) | 24 (80%) |
| Weight (mean ± SD) | 84±18 Kg | 91±18 Kg |
|
Temperature at initiation of cooling (mean ± SD) |
36±0.9 C | 36±0.8 C |
|
Temperature at start of cooling |
||
| Medical History | ||
| Diabetes | 11 (32%) | 13 (43%) |
| Coronary artery disease |
13 (38%) | 18(60%) |
| Cerebral vascular disease |
4 (12%) | 6 (20%) |
| Location of arrest | ||
| Home | 19 (55%) | 12 (40%) |
| Public place | 10 (29%) | 16 (53%) |
| Work | 5 (15%) | 2 (7%) |
| Witnessed arrest | 34 (100%) | 30 (100%) |
| Initial rhythm | ||
| VF/VT | 23 (68%) | 19 (63%) |
| Asystole | 7 (21%) | 5 (17%) |
| PEA | 4 (12%) | 6 (20%) |
|
Mean ± SD time from collapse to ROSC |
28±15 min | 26±14 min |
|
Mean ± SD time from collapse to start of cooling |
251±78 min | 245±89 min |
| Fibrinolysis | 3 (9%) | 2 (7%) |
| PCI | 9 (26%) | 12 (40%) |
Abbreviations: PCI, percutaneous coronary intervention; PEA, pulseless electrical activity; ROSC, return of spontaneous circulation; SD, standard deviation; VF/VT, ventricular fibrillation or ventricular tachycardia
Figure 1.
Time to achieve a core temperature <34 °C for patients cooled with the Arctic Sun and standard cooling methods.
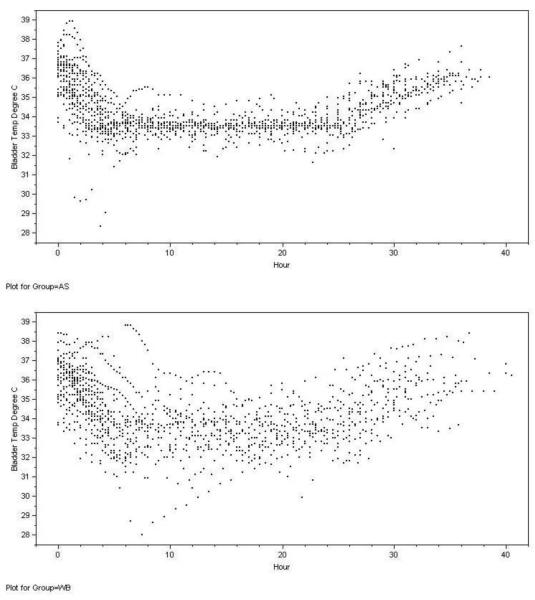
Hypothermia maintenance was terminated early in two subjects (one in each group) due to arrhythmias and hypotension in one case and because family requested therapy be stopped in the other. The performance of the two approaches for maintaining temperature in the target range is shown in Table 4. The temperatures at each time point for each subject in the two groups are shown in Figure 2. The rate of overshoot (below 32 °C) during induction was 8/29 (28%) in the standard cooling group and 1/32 (3%) in the Arctic Sun group (Risk difference 24%, 95% CI 7 to 42%, p=0.01). During maintenance of hypothermia 17/29 (58%) standard cooling patients and 5/32 (15%) Arctic Sun patients had a temperature below 32°C recorded (Risk difference 43%, 95% CI 21 to 65%), while 23/29 (79%) in the standard cooling group and 10/32 (31%) in Arctic Sun group had temperatures above 34 C recorded (Risk difference 48%, 95% CI 26 to 70%). Once rewarming began, the median (IQR) time to 36 degrees for the Arctic Sun was 600 (420-680) minutes while the median (IQR) time was 300 (180-600) minutes for the standard cooling blanket group. This difference was not statistically significant (P=0.07 Log Rank).
Table 4.
Serious adverse events (SAE) recorded during induction and maintenance of cooling in each treatment group.
| SAE | Arctic Sun Group n=32 |
Control Group n=29 |
Total n=61 |
|---|---|---|---|
| Death | 16 (50%) | 16 (55%) | 32 (53%) |
| Seizures / Status epilepticus | 6 (19%) | 5 (17%) | 11 (18%) |
| Pneumonia | 7 (22%) | 3 (10%) | 10 (16%) |
| Significant Arrhythmias | 2 (6%) | 5 (17%) | 7 (12%) |
| Renal failure | 2 (6%) | 3 (10%) | 5 (8%) |
| Stroke, ischemic | 4 (13%) | 1 (3%) | 5 (8%) |
| Sepsis / Septic Shock | 3 (9%) | 1 (3%) | 4 (7%) |
| Cardiac Arrest | 0 | 2 (7%) | 2 (3%) |
| Bleed, GI | 0 | 2 (7%) | 2 (3%) |
| Fever, unknown origin | 1 (3%) | 1 (3%) | 2 (3%) |
| Infection, UTI | 2 (6%) | 0 | 2 (3%) |
| Cardiogenic Shock | 0 | 2 (7%) | 2 (3%) |
| Anemia | 2 (6%) | 0 | 2 (3%) |
| Bacteremia | 1 (3%) | 0 | 1 (2%) |
| Bleed, Hematuria | 0 | 1 (3%) | 1 (2%) |
| MI, acute | 1 (3%) | 0 | 1 (2%) |
| Pneumothorax | 0 | 1 (3%) | 1 (2%) |
| Respiratory failure | 0 | 1 (3%) | 1 (2%) |
| EKG changes / ST elevation | 0 | 1 (3%) | 1 (2%) |
| Thrombosis, Deep Vein | 1 (3%) | 0 | 1 (2%) |
| Pleural effusion | 1 (3%) | 0 | 1 (2%) |
| Infection, sinusitus | 1 (3%) | 0 | 1 (2%) |
| Heparin Induced Thrombocytopenia | 1 (3%) | 0 | 1 (2%) |
| ANY SAE | 24 (75%) | 25 (86%) | 49 (80%) |
Figure 2.
Patient temperatures during induction, cooling and rewarming for Arctic Sun (AS) and water blanket (WB).
Overall survival and survival with a good three month neurological outcome (CPC 1 or 2) was similar for cooled patients between the two treatment arms (p=0.6 Fischer’s Exact test). Survival was higher for patients who presented with ventricular fibrillation or ventricular tachycardia than for patients who presented with asystole or pulseless electrical activity (Table 3). Only 1 patient had a CPC of 3 or 4 at 90 day follow-up.
Table 3.
Proportion of cooled patients with good neurological outcomes (CPC 1 or 2) at 90 days for each treatment by presenting cardiac rhythm.
| Arctic Sun n/total (%) |
Control n/total (%) |
|
|---|---|---|
| VF/VT | 14/23 (61%) | 10/19 (53%) |
| Asystole/PEA | 1/9 (9%) | 1/10 (10%) |
| Overall | 15/32 (47%) | 11/29 (38%) |
Abbreviations: VF/VT, ventricular fibrillation or ventricular tachycardia; PEA, pulseless electrical activity
Serious adverse events (SAEs) were common and typical for post-cardiac arrest patients. Three quarters of patients in the Arctic Sun group had an SAE (95% CI 57 to 89%) while 86% of the standard treatment group had a SAE. The proportion of patients with SAEs was similar between the two cooling methods. Only a minority of adverse events were rated by the investigator as related to hypothermia treatment. The most common adverse events are shown in Table 4. There were no skin related SAEs in either group.
Protocol violations occurred during the study. Interruptions in cooling were reported for 9 subjects (median duration 39 minutes, interquartile range of 54 minutes) in the Arctic Sun group and 3 subjects (median duration 30 minutes, interquartile range of 14 minutes) in the standard cooling group. Most of these interruptions were for radiological imaging such as head or chest CT scans. Another reported protocol violation was delay in paralytic administration, which occurred in 3 Arctic Sun patients and 2 cooling blanket patients. Finally, 2 patients (one in each group) who were enrolled were ultimately determined to be over the maximum weight of 250 lbs and four patients (all in the Arctic Sun group) who completed the study were ultimately determined to have non-cardiac etiology of their cardiac arrest (two drug overdose and two asphyxia). These subjects were included in the intent-to-treat analysis.
Discussion
The Arctic Sun and standard cooling blankets were both effective at cooling comatose patients resuscitated from cardiac arrest. We were able to reach 33.9°C in all cooled patients by 18 hours and in 90% of subjects by 6.5 hours following initiation of the therapy. This was substantially better than the HACA study where almost 20% of patients never reached their target temperature.3 The Arctic Sun cooled patients more rapidly than standard cooling blankets, with a median time to target temperature almost an hour shorter than the standard cooling group.
While the median time to target temperature was shorter in the Arctic Sun group, we were not able to demonstrate a statistically significant increase in the proportion of patients who reached target temperature within 4 hours of starting cooling. Our study was powered to detect a thirty percent increase in the proportion reaching target assuming a control rate of 60%. In retrospect, this was an over-ambitious estimation for our control group. In the HACA study only 25% of reached a core temperature <34° C within 4 hours.3 Our performance was better than this (50% of control patients reached < 34 ° C within 4 hours), but a post-hoc power calculation suggests that with 50% of control subjects reaching target by 4 hours, we only had a 50% chance to find a significant difference between the treatment arms.
The surprisingly slow rate of cooling in both groups may have been due to several factors. First, the majority of the institutions involved were relatively inexperienced with hypothermia. This may have decreased the overall performance of all centers. A report in 2001 at a center that implemented a new protocol using cooling blankets found a median time to target of 300 minutes, a number significantly longer than the control group in this study. In the HACA clinical trial the median time from initiation of cooling to reach 34 C was approximately 375 minutes. However, a subsequent report from one of the sites using the Arctic Sun reported a median time to target temperature of 137 minutes4. While some of the improvement may be due to the use of a different device, we believe that much of the improved performance is due to increased experience. Unfortunately, we were not able to assess the learning curve in our cohort. We only had one center that enrolled more than 11 subjects so it was not uncommon for centers to have several months pass between enrollments. Thus any given care team would likely care for only one or two cooling subjects over the course of the study, and there was little opportunity to see improvement over time. We believe that sites where cooling is used infrequently may find cooling times longer than those reported by centers where cooling is used more frequently.
A second issue that may have delayed cooling in both groups was the unexpected delay in the initiation of paralytics for some subjects. Our study did not have a standardized protocol for paralytics (each facility had institutional protocols and it would have been difficult to modify these protocols for a single trial.) While this is a limitation of our study, our protocol required that all patients be paralyzed throughout the cooling period. As we did not anticipate that there would be a delay in paralytic administration, our research protocol did not systematically record the time of paralytic administration. However, in 8% of subjects the investigator specifically noted that paralytics were delayed more than 2 hours into the cooling period. This delay was often related to obtaining paralytic infusions from the pharmacy. As non-paralyzed patients may shiver during cooling, these patients might be less likely to reach target temperature within four hours. Similarly, the inclusion of two patients above the maximum weight may have also resulted in longer cooling times. It is also possible is that our unblinded design encouraged investigators to be more aggressive in the non-Arctic Sun group. For example, while we recommended that ice be only applied to the groin and axilla, in some cases where cooling was not progressing quickly enough the investigators may have applied more ice to increase the cooling rate. As ice was not used in the Arctic Sun group, this may have biased our results in favor of the water blanket group. Finally, there were several interruptions in cooling for both groups, and this would lower the number of patients reaching target temperature by the 4 hour cutoff.
Another limitation to the generalizability of our findings is that we did not supplement the cooling devices with iced saline infusions during the induction period. The rapid infusion of 30 ml/kg of iced saline lowered core temperature by approximately 1.5 ° C.5 The use of these protocols would likely shorten the time to target temperature and increase the number of subjects reaching 33.9 ° in both groups.
The Arctic Sun provides much more precise control of temperature than standard cooling blankets. While the majority of patients in the control group had one or more hourly readings outside of the target range, only a minority of Arctic Sun patients had hourly temperatures above 34°C or below 32°C.
Overall survival and neurological outcomes were similar between the Arctic Sun and standard cooling. This was expected, as our study was not powered to show a difference in neurological outcomes. The high proportion of patients with good neurological outcomes (CPC 1 or 2) is similar to those reported in other controlled studies of hypothermia 1;3 and higher than those reported in one multi-center registry.6 As in other studies, the majority of the good outcomes were observed in patients with ventricular fibrillation or ventricular tachycardia.
Serious adverse events were common and related to the critical condition of study participants. Only a minority of adverse events were rated by the investigator as related to treatment. The rates were similar between the two cooling methods, and the types of adverse events were similar to those reported in other hypothermia studies.1 Institutions implementing a hypothermia protocol should be aware that many patients will have complications such as seizures, dysrhythmias and pneumonia that are commonly seen in post arrests patients independent of treatment with hypothermia3;6
The clinical relevance of more rapid cooling, minimizing temperature fluctuations and controlled rewarming remain uncertain in humans. Several preclinical studies have suggested benefit with rapid institution of hypothermia. In canine models, immediate post-resuscitation hypothermia improves neurological outcomes and survival compared to delayed hypothermia.7;8 Rodent models have also shown improved outcomes with early hypothermia after cardiac arrest. Functional outcome, quantitative EEG and histology were much better in animals treated with hypothermia immediately after ROSC compared to those treated one hour after ROSC.9 In a forebrain ischemia model, rats treated with hypothermia 1 hour after injury had better neurological function than those treated 4 hour after injury.10 Recent preclinical studies using focal ischemia and intra-cerebral hemorrhage models suggest that prolonged hypothermia may be beneficial models even with treatment delays. If these effects generalize to global ischemia, it is possible that longer treatment may compensate for treatment delays.11;12 Overall, we believe preclinical studies suggest a benefit of early versus late hypothermia and the most recent clinical guidelines recommend initiation as early as possible,13 the most optimal time of initiation of hypothermia in humans is still to be established.
The relative importance of rewarming rate is also unclear. Animal models suggest that gradual rewarming maintains the normal cerebrovascular regulation.14 There are several clinical conditions similar to cardiac arrest where controlled rewarming has been studied. In human trials of patients undergoing cardiopulmonary bypass gradual rewarming was associated with improved neurocognitive function in adult9;15 and children16, while increased intracranial pressure (ICP) was noted during rewarming in patients treated with hypothermia after large cerebral infarctions. In that study, the rewarming related ICP elevation was associated with deaths.17 While it is not clear that these findings can be generalized to post-cardiac arrest patients treated with hypothermia, the most recent guidelines recommend controlled rewarming at a rate of 0.3-0.5 °C/hour.13 In the current study nearly 85% were rewarmed at a rate of 0.2 to 0.8 °C/hour.
In conclusion, both cooling blankets and the Arctic Sun were able to effectively cool patients after cardiac arrest. While the proportion of patients reaching target temperature by 4 hours was similar, the Arctic Sun had a median time to target that was almost an hour faster than standard cooling blankets. The Arctic Sun provided more precise temperature control, and fewer patents were overcooled. Finally, centers considering implementation of a cooling protocol should be aware that the cooling times reported by centers that have experience with hypothermia may not be immediately reproduced by centers at the initiation of a hypothermia protocol.
Acknowledgement
This study was supported by a research grant from Medivance Incorporated to each institution. Medivance assisted with the study design and training of the investigators. The data were collected at the sites by the investigators and centrally tabulated by Medical Device Consultants, Inc. Medivance reviewed the final manuscript but did not make the final determination of publication. The final manuscript was approved by all authors. The project described was supported in part by Award Number K08DA020573 to Dr. Heard from the National Institute On Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Institutes or the National Institutes of Health. The authors would like to thank Mary Holden RN for her contribution to the design and performance of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest Statement: Dr. Paradis has acted as a paid consultant to Medivance Inc. All authors received research support for the performance of the study from Medivance Inc.
Reference List
- 1.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 2.Abella BS, Rhee JW, Huang KN, Vanden Hoek TL, Becker LB. Induced hypothermia is underused after resuscitation from cardiac arrest: a current practice survey. Resuscitation. 2005;64:181–6. doi: 10.1016/j.resuscitation.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–56. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 4.Haugk M, Sterz F, Grassberger M, Uray T, Kliegel A, Janata A, Richling N, Herkner H, Laggner AN. Feasibility and efficacy of a new noninvasive surface cooling device in post-resuscitation intensive care medicine. Resuscitation. 2007;75:76–81. doi: 10.1016/j.resuscitation.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Bernard S, Buist M, Monteiro O, Smith K. Induced hypothermia using large volume, ice-cold intravenous fluid in comatose survivors of out-of-hospital cardiac arrest: a preliminary report. Resuscitation. 2003;56:9–13. doi: 10.1016/s0300-9572(02)00276-9. [DOI] [PubMed] [Google Scholar]
- 6.Arrich J. Clinical application of mild therapeutic hypothermia after cardiac arrest. Crit Care Med. 2007;35:1041–7. doi: 10.1097/01.CCM.0000259383.48324.35. [DOI] [PubMed] [Google Scholar]
- 7.Kuboyama K, Safar P, Radovsky A, Tisherman SA, Stezoski SW, Alexander H. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med. 1993;21:1348–58. doi: 10.1097/00003246-199309000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Nozari A, Safar P, Stezoski SW, Wu X, Kostelnik S, Radovsky A, Tisherman S, Kochanek PM. Critical time window for intra-arrest cooling with cold saline flush in a dog model of cardiopulmonary resuscitation. Circulation. 2006;113:2690–6. doi: 10.1161/CIRCULATIONAHA.106.613349. [DOI] [PubMed] [Google Scholar]
- 9.Jia X, Koenig MA, Shin HC, Zhen G, Pardo CA, Hanley DF, Thakor NV, Geocadin RG. Improving neurological outcomes post-cardiac arrest in a rat model: immediate hypothermia and quantitative EEG monitoring. Resuscitation. 2008;76:431–42. doi: 10.1016/j.resuscitation.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colbourne F, Corbett D. Delayed postischemic hypothermia: a six month survival study using behavioral and histological assessments of neuroprotection. J Neurosci. 1995;15:7250–60. doi: 10.1523/JNEUROSCI.15-11-07250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacLellan CL, Girgis J, Colbourne F. Delayed onset of prolonged hypothermia improves outcome after intracerebral hemorrhage in rats. J Cereb Blood Flow Metab. 2004;24:432–40. doi: 10.1097/00004647-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 12.MacLellan CL, Clark DL, Silasi G, Colbourne F. Use of prolonged hypothermia to treat ischemic and hemorrhagic stroke. J Neurotrauma. 2009;26:313–23. doi: 10.1089/neu.2008.0580. [DOI] [PubMed] [Google Scholar]
- 13.Castren M, Silfvast T, Rubertsson S, Niskanen M, Valsson F, Wanscher M, Sunde K. Scandinavian clinical practice guidelines for therapeutic hypothermia and post-resuscitation care after cardiac arrest. Acta Anaesthesiol Scand. 2009;53:280–8. doi: 10.1111/j.1399-6576.2008.01881.x. [DOI] [PubMed] [Google Scholar]
- 14.Ueda Y, Suehiro E, Wei EP, Kontos HA, Povlishock JT. Uncomplicated rapid posthypothermic rewarming alters cerebrovascular responsiveness. Stroke. 2004;35:601–6. doi: 10.1161/01.STR.0000113693.56783.73. [DOI] [PubMed] [Google Scholar]
- 15.Sahu B, Chauhan S, Kiran U, Bisoi A, Lakshmy R, Selvaraj T, Nehra A. Neurocognitive function in patients undergoing coronary artery bypass graft surgery with cardiopulmonary bypass: the effect of two different rewarming strategies. J Cardiothorac Vasc Anesth. 2009;23:14–21. doi: 10.1053/j.jvca.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Sahu B, Chauhan S, Kiran U, Bisoi A, Ramakrishnan L, Nehra A. Neuropsychological function in children with cyanotic heart disease undergoing corrective cardiac surgery: effect of two different rewarming strategies. Eur J Cardiothorac Surg. 2009;35:505–10. doi: 10.1016/j.ejcts.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 17.Schwab S, Schwarz S, Spranger M, Keller E, Bertram M, Hacke W. Moderate hypothermia in the treatment of patients with severe middle cerebral artery infarction. Stroke. 1998;29:2461–6. doi: 10.1161/01.str.29.12.2461. [DOI] [PubMed] [Google Scholar]