Abstract
A bulky platinum triamine complex, [Pt(Me5dien)(NO3)]NO3 (Me5dien = N,N,N′,N′,N″ - pentamethyldiethylenetriamine) has been prepared and reacted in D2O with N-acetylmethionine (N-AcMet) and guanosine 5′-monophosphate (5′-GMP); the reactions have been studied using 1H NMR spectroscopy. Reaction with 5′-GMP leads to two rotamers of [Pt(Me5dien)(5′-GMP-N7)]+. Reaction with N-AcMet leads to formation of [Pt(Me5dien)(N-AcMet-S)]+. When a sample with equimolar mixtures of [Pt(Me5dien)(D2O)]2+, 5′-GMP, and N-AcMet was prepared, [Pt(Me5dien)(5′-GMP-N7)]+ was the dominant product observed throughout the reaction. This selectivity is the opposite of that observed for a similar reaction of [Pt(dien)(D2O)]2+ with 5′-GMP and N-AcMet. To our knowledge, this is the first report of a platinum(II) triamine complex that reacts substantially faster with 5′-GMP than with N-AcMet; the effect is most likely due to steric clashes between the methyl groups of the Me5dien ligand and the N-AcMet.
Keywords: nuclear magnetic resonance, ligand binding, anticancer drug, platinum
Cisplatin and its analogs are known to form 1,2-intrastrand cross-links with guanine that lead to DNA distortion and are considered responsible for cytotoxicity. However, reaction with proteins, especially at methionine residues, occurs readily [1, 2]. Protein adducts could be responsible for toxicity or resistance [3]. Previous small molecule studies have shown that [Pt(dien)Cl]Cl (dien = diethylenetriamine) reacts faster both with methionine (Met) [4] and with S-methylglutathione [5] than with guanosine 5′-monophosphate (5′-GMP).
We previously found that bulk on a platinum diamine compound can affect the N-AcMet adducts that can form. [Pt(N,N,N′,N′ - tetramethylethylenediamine)(D2O)2]2+ reacts with N-AcMet in only a 1:1 ratio even when excess N-AcMet is present, forming a chelate via the sulfur and carboxylate oxygen atoms [6]. Molecular mechanics calculations suggested severe steric clashes in a 2:1 N-AcMet:Pt complex. Later work with [Pt(N,N-diethylethylenediamine)(D2O)2]2+ indicated only one geometric isomer of the observed sulfur-oxygen chelate, namely the one with the sulfur atom trans to the diethyl nitrogen [7]. Thus, coordination of a methionine sulfur atom cis to a bulky amine nitrogen is apparently slowed considerably.
Because the bulk did not prevent the formation of a 2:1 guanine:Pt product for [Pt(N,N,N′,N′-tetramethylethylenediamine)(D2O)2]2+ [6, 8], we hypothesized that bulk affects the rate of reaction with methionine more than with guanine. We have therefore prepared [Pt(Me5dien)I]2[PtI6] and converted it into [Pt(Me5dien)(NO3)]NO3 according to previous methods, except that silver nitrate was substituted for silver triflate [9]. In D2O, the 1H NMR spectrum of [Pt(Me5dien)(NO3)]NO3 is similar to that of the previously reported [Pt(Me5dien)(D2O)]2+ species formed from the triflate complex. Formation of [Pt(Me5dien)(D2O)]2+ was expected since the reported equilibrium constant for nitrate association with platinum suggests less than 1% coordination of the nitrate should occur with the platinum concentrations utilized here [10].
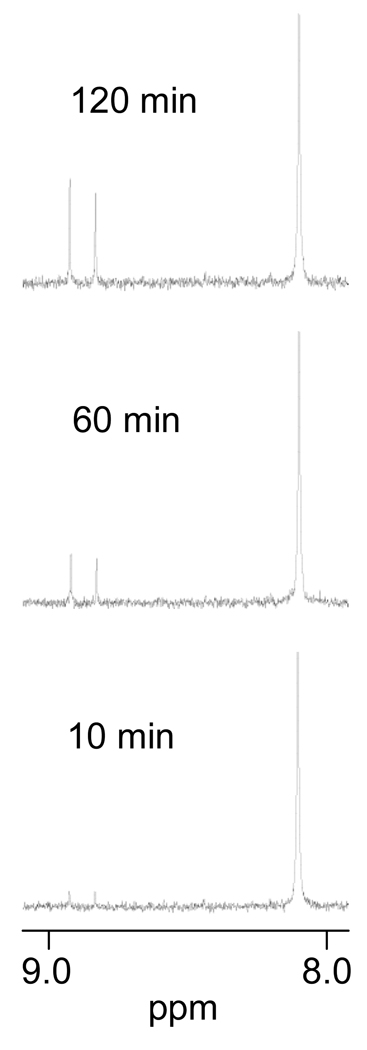
The [Pt(Me5dien)(D2O)]2+ complex (Me5dien = N,N,N′,N′,N″-pentamethyldiethylenetriamine) was reacted with 5′-GMP and N-AcMet at pH of 4 in separate reactions. The reaction of [Pt(Me5dien)(D2O)]2+ with 5′-GMP at concentrations of 3.33 mM each led to the appearance of two small new sets of NMR resonances within 10 minutes, with 5′-GMP H8 signals shifted downfield to 8.84 and 8.93 ppm (Figure 1). Previously it was shown that the Me5dien ligand does not have C2-symmetry [9], and thus these resonances are assigned to rotamers of the [Pt(Me5dien)(5′-GMP-N7)]+ complex. Increasing the temperature of the sample to 60 °C did not result in significant broadening or coalescence of the signals, indicating that exchange between the rotamers is very slow. Such slow rotation has been observed previously for [Pt(2,2′-bipiperidine)(5′-GMP)2] [11].
Figure 1.
Partial 1H NMR spectra of the reaction of [Pt(Me5dien)(D2O)]2+ with 5′-GMP. Initial concentrations of each reactant are 3.33 mM.
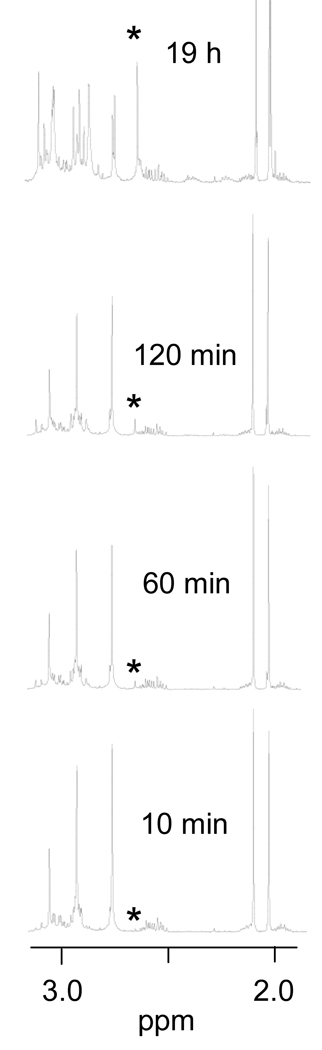
Reaction of [Pt(Me5dien)(D2O)]2+ with N-AcMet at 3.33 mM concentrations of each produced one new set of resonances that were barely visible within 10 minutes (Figure 2); these signals remained very small even after 3 hours. The downfield shift of the S-CH3 resonance to 2.66 ppm indicates that the complex is [Pt(Me5dien)(N-AcMet-S)]+. Formation of this complex appeared to be significantly slower than formation of the 5′-GMP complex, as evidenced by the extent of reaction of each at 120 min (Figure 1 and Figure 2). We estimated rate constants of 2 × 10−2 and 4 × 10−3 M−1 s−1 for 5′-GMP and N-AcMet, respectively. When 5’-GMP was added to [Pt(Me5dien)(N-AcMet-S)]+, slow displacement of the N-AcMet was observed; after 5 days, ~20% of the N-AcMet had been displaced. Previously, it was observed that 5’-GMP displaced L-methionine from [Pt(dien)(Met-S)]+.[4]
Figure 2.
Partial 1H NMR spectra of the reaction of [Pt(Me5dien)(D2O)]2+ with N-AcMet. Initial concentrations of each reactant are 3.33 mM. The S-CH3 NMR signal of the product is indicated by an asterisk.
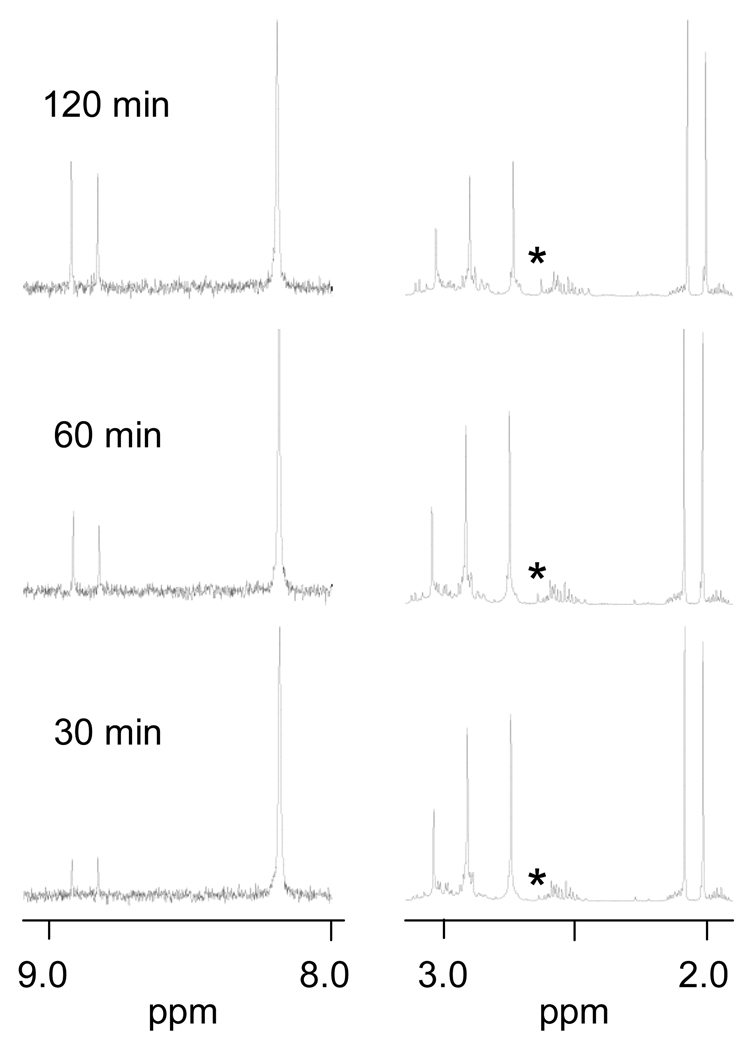
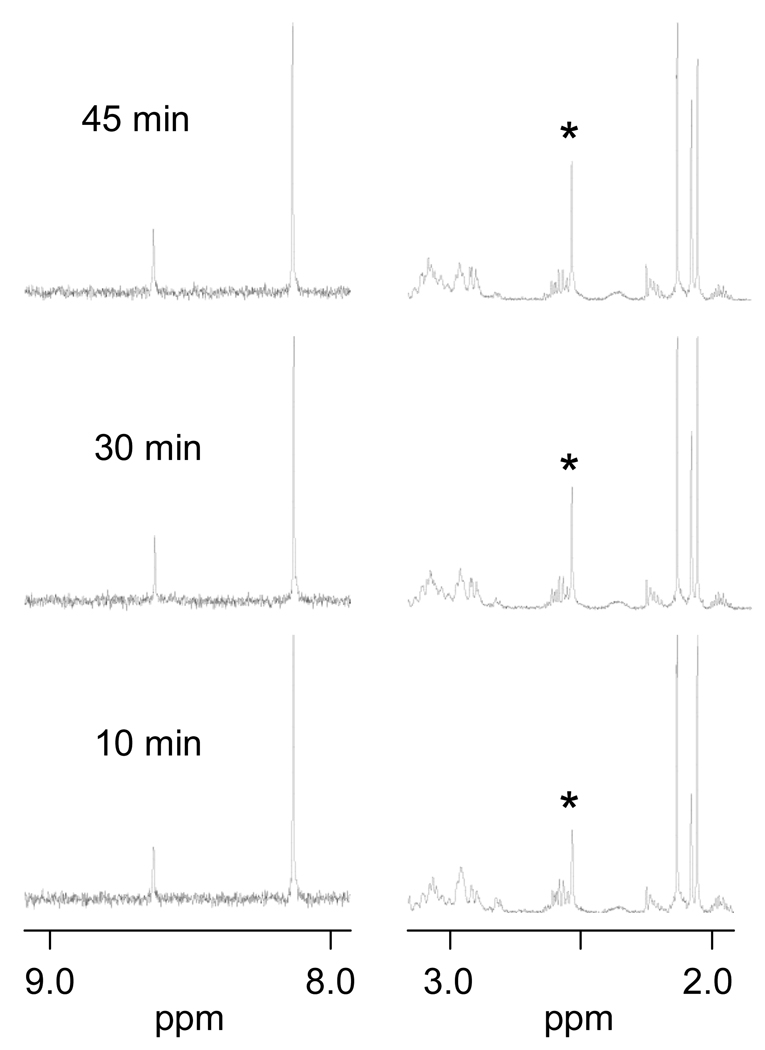
Next, [Pt(Me5dien)(D2O)]2+ was reacted with a mixture of 5′-GMP and N-AcMet to give an initial concentration of 3.33 mM of each reactant. As can be seen in Figure 3, this complex clearly reacts much faster with 5′-GMP than with N-AcMet throughout the reaction, which is the opposite trend observed previously for [Pt(dien)Cl]Cl [4, 5]. To determine whether the observed change was due to the carrier ligand (dien vs. Me5dien) or the leaving ligand (Cl− vs. D2O), we also reacted [Pt(dien)Cl]Cl with AgNO3 to produce [Pt(dien)(NO3)]NO3, which in solution should produce [Pt(dien)(D2O)]2+. The [Pt(dien)(D2O)]2+ was then reacted with a mixture of 5′-GMP and N-AcMet. As seen in Figure 4, [Pt(dien)(D2O)]2+ reacted faster with N-AcMet than with 5′-GMP, similar to [Pt(dien)Cl]Cl. Thus, the difference in leaving ligand cannot explain the strong preference for 5′-GMP by the [Pt(Me5dien)(D2O)]2+ complex, and the effect is attributed to the carrier ligand.
Figure 3.
Partial 1H NMR spectra of the reaction of [Pt(Me5dien)(D2O)]2+ with 5′-GMP and N-AcMet. Initial concentrations of each reactant are 3.33 mM. The S-CH3 NMR signal of the product is indicated by an asterisk.
Figure 4.
Partial 1H NMR spectra of the reaction of [Pt(dien)(H2O)]2+ with 5′-GMP and N-AcMet. Initial concentrations of each reactant are 2 mM. The S-CH3 NMR signal of the product is indicated by an asterisk.
Replacement of the amine hydrogens of dien with methyl groups would have several possible effects. First, a difference in observed trans effect would be possible, as Me5dien has an a methyl group on the N trans to the leaving ligand; however, we would not expect a substantial change in selectivity for GMP over N-AcMet to be caused by this modest change in the trans ligand. Second, the methyl groups are electron donating and could reduce the charge density on the platinum atom. A previous study [12] showed that a methyl group added to the trans nitrogen of dien led to a decreased rate of reaction with several small nucleophiles; however, the selectivity for nucleophiles was not substantially altered. We expect that the electron donating effect would not be sufficient to alter selectivity for GMP over N-AcMet in the present case, especially since both ligands should have a -1 charge at pH 4.
Third, the loss of hydrogen bond donors (i.e. amine hydrogens) from the carrier ligand could influence the reaction with both 5′-GMP and N-AcMet, which could accept hydrogen bonds via the phosphate and carboxylate groups, respectively. Hydrogen bonding has already been indicated in platinum and palladium complexes with 5′-GMP [13, 14], and thus it is unlikely that eliminating the hydrogen bonding ability of the dien ligand would dramatically increase selectivity for GMP. Finally, steric clashes between the carrier ligands and the entering ligand would be more likely for Me5dien than dien. Our previous studies have indicated that steric clashes are relevant to the types of N-AcMet adducts that form in platinum(II) diamine complexes [6, 7]. Studies from Canovese et al. utilized a dien derivative with ethyl groups cis to the entering ligand position, and there was a noticeable selectivity for smaller nucleophiles [12]; thus, severe steric clashes between the methyl groups of Me5dien and the methionine ligand seems to be a reasonable explanation for our findings.
To our knowledge, this is the first report of a platinum(II) triamine complex that reacts substantially faster with 5′-GMP than with N-AcMet at pH 4. Previous studies with [Pt(ethylenediamine)(H2O)2]2+ and [Pt(1,2-diaminocyclohexane)(H2O)2]2+ at pH 2 found rate constants with L-methionine at 25 °C were similar (0.70 and 0.85 M−1s−1, respectively) , as were rate constants for 5′-GMP at 40 °C (3.9 and 5.8 M−1 s−1, respectively) [15]. The additional bulk in [Pt(1,2-diaminocyclohexane)(H2O)2]2+ is on the carbon atoms instead of the diamine nitrogen atoms, suggesting bulk at the nitrogen atoms is needed for the dramatic increase in 5′-GMP selectivity observed here.
Reaction with 5′-GMP represents the major site of reaction of cisplatin with DNA, whereas methionine is a primary target of reaction with protein. It is possible that platinum complexes with bulky amine ligands would tend to react with proteins at residues other than methionine; a previous study found that the presence of a 4-picoline or piperidine ligand on cis-platinum(II) diamine complexes resulted in a decreased reaction with Met1 of ubiquitin[16]. Furthermore, it is conceivable that sufficiently bulky platinum complexes would show increased selectivity for reaction with DNA relative to proteins.
Acknowledgement
Financial support for this research was provided by awards from Research Corporation (Cottrell College Grant 6242) and the NIH (R15 AG16192-01).
Table of Abbreviations
- Me5dien
N,N,N′,N′,N″ - pentamethyldiethylenetriamine
- dien
Diethylenetriamine
- N-AcMet
N-acetylmethionine
- Met
Methionine
- 5’-GMP
Guanosine 5’-monophosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Appleton TG. In: Cisplatin: Chemistry and Biochemistry of a Leading Anticancer Drug. Lippert B, editor. Weinheim: Wiley-VCH; 1999. pp. 363–376. [Google Scholar]
- 2.Reedijk J, Teuben JM. In: Cisplatin: Chemistry and Biochemistry of a Leading Anticancer Drug. Lippert B, editor. Weinheim: Wiley-VCH; 1999. pp. 339–362. [Google Scholar]
- 3.Kartalou M, Essigmann JM. Mutat. Res. 2001;478:23–43. doi: 10.1016/s0027-5107(01)00141-5. [DOI] [PubMed] [Google Scholar]
- 4.Barnham KJ, Djuran MI, Murdoch PD, Sadler PJ. J. Chem. Soc. Chem. Comm. 1994:721–722. [Google Scholar]
- 5.Djuran MI, Lempers ELM, Reedijk J. Inorg. Chem. 1991;30:2648–2652. [Google Scholar]
- 6.Williams KM, Rowan C, Mitchell J. Inorg. Chem. 2004;43:1190–1196. doi: 10.1021/ic035212m. [DOI] [PubMed] [Google Scholar]
- 7.Williams KM, Chapman DJ, Massey SR, Haare C. J. Inorg. Biochem. 2005;99:2119–2126. doi: 10.1016/j.jinorgbio.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Cramer RE, Dahlstrom PL. J. Am. Chem. Soc. 1979;101:3679–3681. [Google Scholar]
- 9.Cini R, Intini FP, Maresca L, Pacifico C, Natile G. Eur. J. Inorg. Chem. 1998:1305–1312. [Google Scholar]
- 10.Appleton TG, Berry RD, Davis CA, Hall JR, Kimlin HA. Inorg. Chem. 1984;23:3514–3521. [Google Scholar]
- 11.Ano SO, Intini FP, Natile G, Marzilli LG. J. Am. Chem. Soc. 1998;120:12017–12022. [Google Scholar]
- 12.Canovese L, Cusumano M, Giannetto A. J. Chem. Soc., Dalton Trans. 1983;1983:195. [Google Scholar]
- 13.Kim SH, Martin RB. Inorg. Chim. Acta. 1984;91:11–18. [Google Scholar]
- 14.Marcelis ATM, Erkelens C, Reedijk J. Inorg. Chim. Acta. 1984;91:129–135. [Google Scholar]
- 15.Summa N, Schiessl W, Puchta R, Hommes NvE, Eldik Rv. Inorg. Chem. 2006;45:2948–2959. doi: 10.1021/ic051955r. [DOI] [PubMed] [Google Scholar]
- 16.Najajreh Y, Peleg-Shulman T, Moshel O, Farrell N, Gibson D. J. Biol. Inorg. Chem. 2003;8:167–175. doi: 10.1007/s00775-002-0402-y. [DOI] [PubMed] [Google Scholar]