Abstract
Objective
The goals of this study were to determine if endothelial nitric oxide synthase (eNOS) affects both early and late collateral arterial adaptation and blood flow recovery after severe limb ischemia, and to determine if eNOS-derived NO was necessary for recruitment of CXCR4+ VEGFR1+ hemangiocytes to the site of ischemia.
Methods and Results
Two studies were carried out. In the first study, hindlimb ischemia was induced by unilateral femoral artery excision in 3 groups: C57Bl6 (wild type), eNOS-/-, and C57Bl/6 mice treated with L-NAME from 1 day prior to excision through day 3 after excision (early L-NAME group). These 3 groups were studied on the 3rd day after induction of ischemia. In the second study, hindlimb ischemia was induced in 2 groups: C57Bl/6 mice (wild type) and C57Bl/6 mice treated with L-NAME from the 3rd through the 28th day after induction of ischemia. These 2 groups were studied on the 28th day after induction of ischemia. Dependent variables included hindlimb perfusion, collateral artery diameter, and the number and location of hemangiocytes within the ischemic hindlimb.
Results
In the first study, the eNOS-/- and early L-NAME treatment groups developed toe gangrene by the 2nd day of ischemia. These groups demonstrated less blood flow recovery and smaller collateral artery diameter than the wild type group. Hemangiocytes were present within the adventitia of collateral arteries in the wild type group, but were only sparsely present, in a random pattern, in the eNOS-/- and early L-NAME treatment groups. In the second study, the late L-NAME group showed less blood flow recovery and smaller collateral artery diameter on the 28th day of ischemia than the wild type group. Hemangiocytes were present in a peri-capillary distribution in the wild type group, but present only sparsely in the late L-NAME treatment group.
Conclusion
eNOS-derived NO is necessary for both the early (day 3) and late (day 28) adaptive responses to hindlimb ischemia. NO is necessary for normal hemangiocyte recruitment to the ischemic tissue.
Keywords: endothelium, nitric oxide, collateral artery, hindlimb ischemia, gangrene
Introduction
Critical limb ischemia secondary to peripheral arterial disease is a debilitating and potentially lethal disease. Critical limb ischemia is responsible for more than 150,000 amputations per year in the United States.1 In many cases surgical or catheter based revascularization procedures are not possible, necessitating amputation. For these reasons the development of molecular or cell-based therapies to enhance collateral artery enlargement and angiogenesis continues to be an area of substantial scientific and clinical interest.
Enlargement of the diameter of existing collateral arteries is the means by which blood flow downstream from the site of conduit artery occlusion is restored.2,3 At least three mechanisms participate in this process: shear stress,4,5 inflammation,6 and recruitment of bone marrow-derived vascular progenitor cells to areas of ischemia.7-10 Nitric oxide (NO) is a component in each of these mechanisms.11-14 During the early adaptive response to ischemia increased shear stress within collateral arteries leads to NO-induced vasodilation that results in a transient, temporary increase in flow through the collaterals.11 During the late adaptive response to ischemia NO participates in the recruitment of vascular progenitor cells that generate collateral artery remodeling, a process termed arteriogenesis, wherein net conductance across the collateral vessels is permanently increased.12-14
It is not universally accepted that NO derived from the endothelial isoform of nitric oxide synthase (eNOS) is requisite for arteriogenesis.11 Additionally, the role of NO in recruitment of hemangiocytes, a critically important subset of vascular progenitor cells,10,15 has not been defined. This study was designed to determine if eNOS-derived NO is essential for both the early and late adaptive response to ischemia, i.e., to arteriogenesis, and to determine the role of NO in hemangiocyte recruitment. We hypothesized that eNOS-derived NO production is essential for collateral artery remodeling, or arteriogenesis, during the late adaptive response to ischemia. We further hypothesized that recruitment of bone marrow-derived hemangiocytes to the ischemic hindlimb vasculature requires the presence of eNOS-derived NO.
Materials and Methods
Experimental animals and procedures
The care of mice complied with the National Research Council's Guide for the Care and the Use of Laboratory Animals. All protocols were approved by the Institutional Animal Care and Use Committee at the University of Massachusetts, Worcester, MA. C57Bl/6 mice and eNOS-/- mice, purchased from Jackson Laboratories (Bar Harbor, ME) were fed standard chow and maintained on a 12 hour light/dark cycle. Hindlimb ischemia was generated by ligation of the femoral artery at the inguinal ligament and popliteal fossa, followed by excision of the artery and all branches.16 The procedure was carried out under 3% isoflurane anesthesia, while burprenorphine was administered during the immediate postoperative period.
Experimental protocols
Separate studies were carried out to evaluate the early and late adaptive responses to acute hindlimb ischemia. The early adaptive response to ischemia (vasodilation) was studied 3 days after unilateral femoral artery excision in three groups: C57Bl/6 mice (wild type group); mice with targeted disruption of the eNOS loci (eNOS-/- group); and C57Bl/6 mice treated with L-NAME, 1 g/L added to the drinking water 24 hours prior to femoral artery excision (early L-NAME group). The L-NAME treatment was continued until sacrifice on day 3 after induction of ischemia, a time selected because preliminary work indicated that mice in the eNOS-/- and early L-NAME groups uniformly developed limb gangrene by this time. The late adaptive response to ischemia (arteriogenesis) was studied 28 days after induction of ischemia in two groups: C57Bl/6 mice (wild type group) and C57Bl/6 mice treated with L-NAME, 1 g/L in drinking water beginning on day 3 after induction of hindlimb ischemia (late L-NAME group). The L-NAME treatment was continued until sacrifice. Day 28 after induction of hindlimb ischemia was chosen as the time for study in the late adaptive response experiment based on published reports which indicate that post-ischemia arteriogenesis is evident at that time.3 The late adaptive response to ischemia was not studied in eNOS-/- mice because these animals consistently developed significant autoamputation by the 3rd day after induction of ischemia and we did not believe it was ethically appropriate to keep these animals beyond this 3 day period.
Laser Doppler perfusion imaging (LDPI)
A laser Doppler perfusion imager (Moor Instruments, Ltd., Devon, UK) was used to estimate blood flow within calf muscles. LDPI-derived data strongly correlate with calf muscle blood flow measured directly using microspheres and the method is widely used to quantify perfusion in the rodent hindlimb.16 Hair from limbs was removed by depilatory cream and mice were placed on a heating plate at 37°C to minimize temperature variation, and studies were conducted under 1.5% isoflurane anesthesia. Data are presented as the ratio of perfusion to the ischemic hindlimb and the contra-lateral non-ischemic hindlimb.
Immunostaining of thigh collateral arteries
The thigh muscles were harvested on day 3 or 28 after induction of ischemia in the early or late adaptive response groups, respectively. Collateral arteries were identified by double staining with antibodies for CD31 (PECAM-1, a marker of endothelial cells) and smooth muscle actin (both antibodies from BD Biosciences, San Jose, CA). Collateral artery diameter was measured in 10 randomly selected low power fields using precalibated microscope calipers (Carl Zeiss, Göttingen, GR) and the average diameter for the ischemic and contra-lateral non-ischemic hindlimb determined for each mouse. Collateral arterial diameter was expressed as a ratio of the diameters in ischemic and non-ischemic hindlimbs. All measurements were made in a blinded manner.
Immunostaining for hemangiocytes
The presence of hemangiocytes was measured in thigh muscles on day 3 or 28 after the induction of ischemia in the early and late adaptive response groups, respectively. Hemangiocytes were identified by double staining for CXCR4 and VEGFR1 (both antibodies from BD Bioscience, San Jose, CA).10 The number of hemangiocytes was determined in 10 randomly selected high power fields in both ischemic and contra-lateral, non-ischemic thigh muscle, and the average calculated. Observations were made in a blinded manner.
Confocal microscopy
A Leica DM IRBE confocal microscope was used (Leica Microsystems, Wetzler, Germany). Cy3-conjugated CXCR4 and FITC-conjugated VEGFR1 antibodies (BD Bioscience, San Jose, CA) were used to identify hemangiocytes. Nuclei were identified by Draq5 staining (Biostatus Limited, Leicestershire, UK).
Nitrate/nitrite (NOx) assay
Serum samples were processed using a Nitrate/Nitrite Fluorometric Assay Kit (Cayman Chemical, Ann Arbor, MI), which determines the concentration of NO2- and NO3-, the final products generated during NO metabolism in vivo. Fluorescence was detected using a Biotek Synergy 2 detector (BioTek Instruments Inc., Winooski, VT).
Statistical Analysis
Differences among groups at each time point were analyzed by means of ANOVA for comparisons between 3 groups of mice, and Student's t-test for comparisons between 2 groups where appropriate. If the ANOVA f-statistic was significant (p<.05), then post-hoc Student-Neuman-Keuls tests were carried out to determine the sites of significance at the p<.05 level threshold.
Results
Early adaptive response to hindlimb ischemia
The serum NOx concentration in the eNOS-/- group was 67% less than that noted in the wild type group. Treatment of C57Bl/6 mice with L-NAME reduced serum NOx by 71% on day 1 and 66% on day 3. These levels were similar to those noted in the eNOS-/- group (Fig. 1). Both the eNOS-/- and early L-NAME treatment groups demonstrated significant toe necrosis by the second day after induction of ischemia, whereas wild type mice did not (Fig. 2A). All three groups demonstrated a significant reduction of flow to the ischemic hindlimb immediately after femoral artery excision, whereas flow to the contra-lateral hindlimb did not change; consequently, the ischemic/non-ischemic limb flow ratio decreased in all three groups. This circumstance remained unchanged in all three groups through day 2. On day 3, however, the wild type group demonstrated an increase in flow to the ischemic hindlimb, resulting in an ischemic/non-ischemic flow ratio that was more than twofold greater than that noted in the eNOS-/- or L-NAME groups (Fig. 2B). The ischemic/non-ischemic collateral artery diameter ratio was 45% lower in the eNOS-/- group and 55% lower in the early L-NAME group than in the wild type group on day 3 (Fig. 2C). The number of CXCR4+ VEGFR1+ hemangiocytes within the ischemic thigh muscle was 86% less in the eNOS-/- and early L-NAME treatment groups than in the wild type group (Fig. 3A). These cells were clearly aligned along collateral arteries in the ischemic thigh musculature in the wild type group (Fig. 3B). Confocal microscopy revealed that hemangiocytes were primarily present within the adventitia of collateral arteries within the ischemic thigh in the wild type group (Fig. 3C).
Figure 1. Serum NOx concentration in wild type, eNOS-/-, and early L-NAME treatment groups.
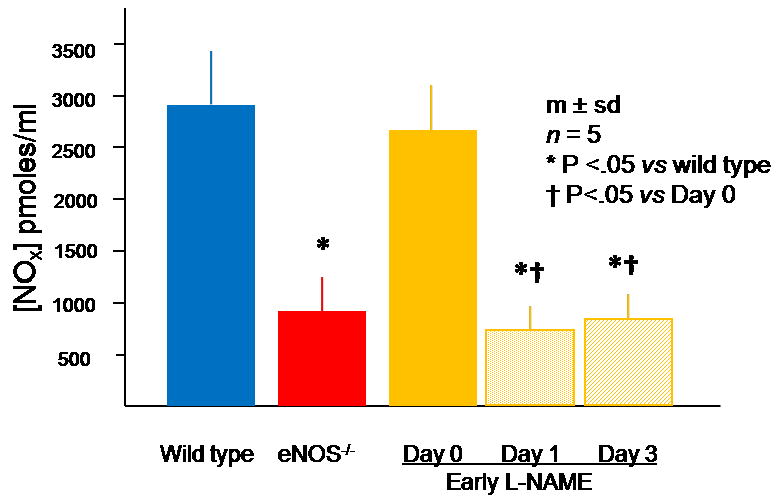
The eNOS-/- group had a serum NOx level lower than wild type mice. L-NAME significantly reduced serum NOx levels within 1 day after beginning L-NAME treatment. This reduction was sustained throughout the duration of L-NAME treatment.
Figure 2. Responses of wild type, eNOS-/- and early L-NAME treatment groups to hindlimb ischemia.
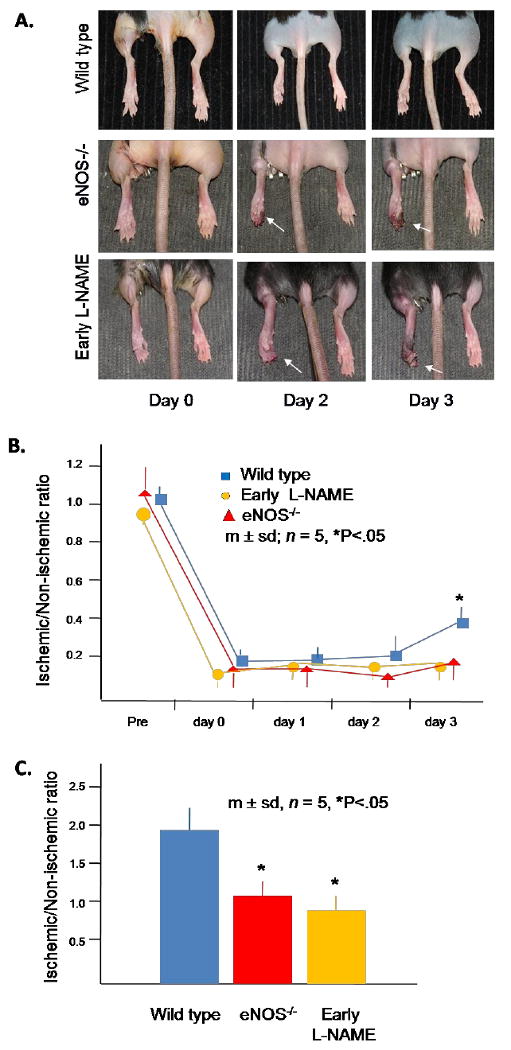
(A) Effects of femoral artery excision on hindlimb tissue integrity. The ischemic hindlimb is shown on the left and white arrows denote evidence of tissue necrosis. These photographs are representative of all animals in each group.
(B) LDPI data. Each mouse underwent 3 scans at each time point and the ratio of the LDPI signal from the ischemic and non-ischemic hindlimb calculated; the average ratio was used as a single data point for each mouse. (C) Collateral artery diameter on day 3 after induction of hindlimb ischemia. Data for each mouse was determined as the average of the largest arteries identified within 10 randomly selected low power fields by a blinded observer. The ratio of the average diameter in the ischemic thigh to the average diameter in the non-ischemic thigh was determined for each mouse and used as a single data point.
Figure 3. Presence of hemangiocytes in the thigh musculature in wild type, eNOS-/-, and early L-NAME treatment groups on day 3 after induction of hindlimb ischemia.
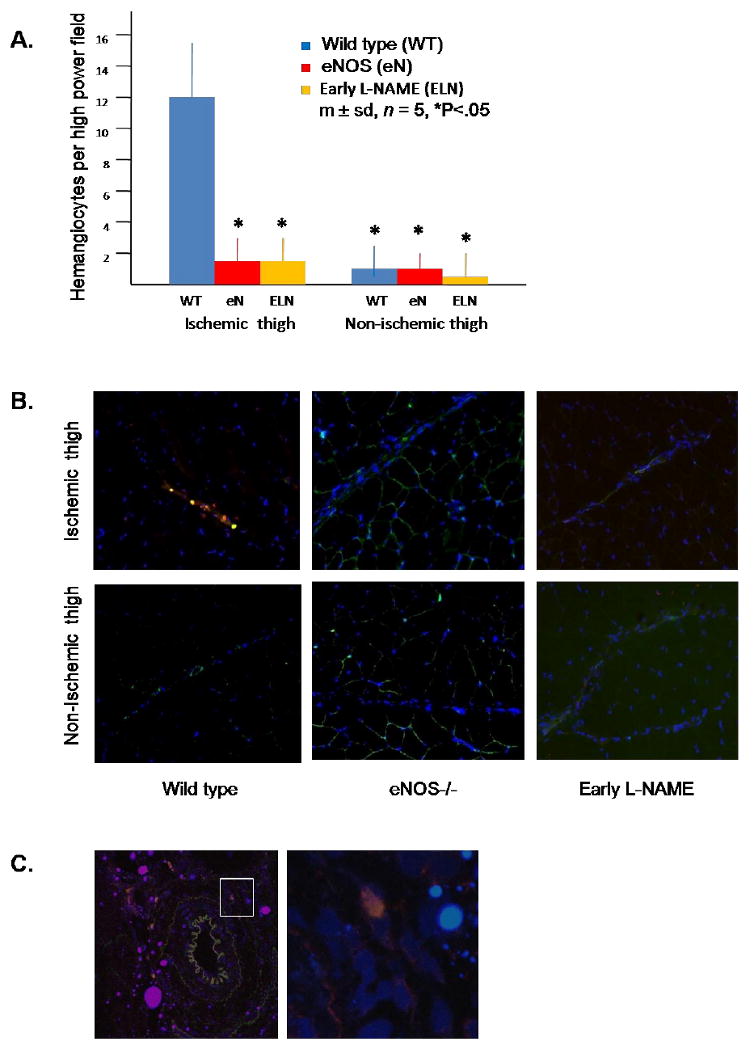
(A) Quantitation of hemangiocytes. Hemangiocytes were defined as cells that co-localized CXCR4 and VEGFR1. Data for each mouse was determined as the average number of hemangiocytes present in 10 randomly selected high power fields, determined by a blinded observer. (B) Representative photomicrographs of hemangiocyte immunostaining. VEGFR1 is stained green, CXCR4 is stained red, and nuclei are stained blue. A collateral vessel is present in each photomicrograph, evidenced by the linear cord of blue stained nuclei. Note the present of double stained hemangiocytes clustered along the collateral artery in the ischemic thigh of the control mouse (200×). (C) Confocal microscopy of a collateral artery within the ischemic thigh of the wild type group. The left hand photo is shown at 200× magnification. The area delineated by the white square in the left hand photo is shown at 400× in the right hand photo. Note the presence of a hemangiocyte in the adventitial area of a collateral artery. This adventitial distribution of hemangiocytes was commonly observed in the ischemic thigh muscle of wild type mice, but was never observed in eNOS-/- or early L-NAME treatment groups.
Late adaptive response to hindlimb ischemia
L-NAME treatment in the late adaptive response experiment differed insofar as L-NAME treatment was initiated on the third day after induction of hindlimb ischemia after the LDPI measurement was obtained. Blood flows to the ischemic and non-ischemic hindlimbs were similar in the wild type and late L-NAME treatment groups through day 7 after induction of hindlimb ischemia. However, beginning on day 14, blood flow to the ischemic hindlimb became significantly greater in the wild type group than the late L-NAME group, whereas flows to the contra-lateral non-ischemic hindlimb were unchanged; accordingly, the ischemic/non-ischemic flow ratios in the L-NAME group were 33% and 44% less than of those in the wild type group on days 14 and 28, respectively (Fig. 4A). The ischemic/non-ischemic thigh collateral diameter ratio was 60% less in the late L-NAME group than the wild type group on day 28 after induction of hindlimb ischemia (Fig. 4B). The number of CXCR4+ VEGFR1+ hemangiocytes present within the ischemic thigh muscles was 56% less in late L-NAME treatment group than in the wild type group on day 28 after induction of hindlimb ischemia, whereas the number of hemangiocytes present within the contra-lateral non-ischemic thigh muscles was similar in both groups (Fig. 5A). Interestingly, the distribution of these cells was different than that observed in the early adaptive response study (on day 3 after induction of hindlimb ischemia). Thus, hemangiocytes were present between myofibers, at the sites occupied by capillaries (Fig. 5B). Confocal microscopy confirmed the peri-capillary location of these hemangiocytes (Fig. 5C).
Figure 4. Response of wild type and late L-NAME treatment groups to hindlimb ischemia.
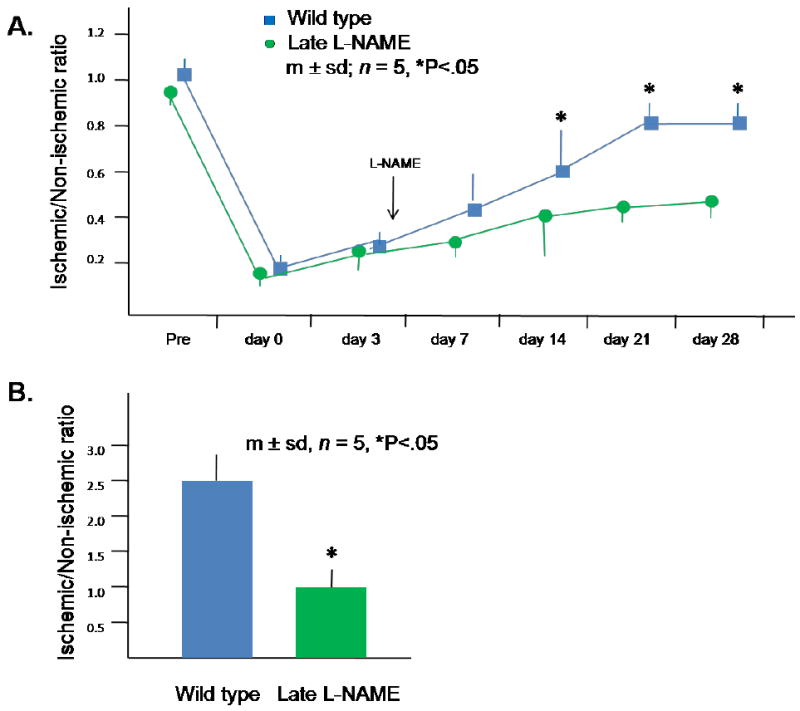
(A) LDPI data. Each mouse underwent 3 scans at each time point and the ratio of the LDPI signal from the ischemic and non-ischemic hindlimb calculated; the average ratio was used as a single data point for each mouse. (B) Collateral artery diameter on day 28 after induction of hindlimb ischemia. Data for each mouse was determined as the average of the largest arteries identified within 10 randomly selected low power fields by a blinded observer. The ratio of the average diameter in the ischemic thigh to the average diameter in the non-ischemic thigh was determined for each mouse and used as a single data point.
Figure 5. Presence of hemangiocytes in the thigh musculature in control and late L-NAME treatment groups 28 days after induction of hindlimb ischemia.
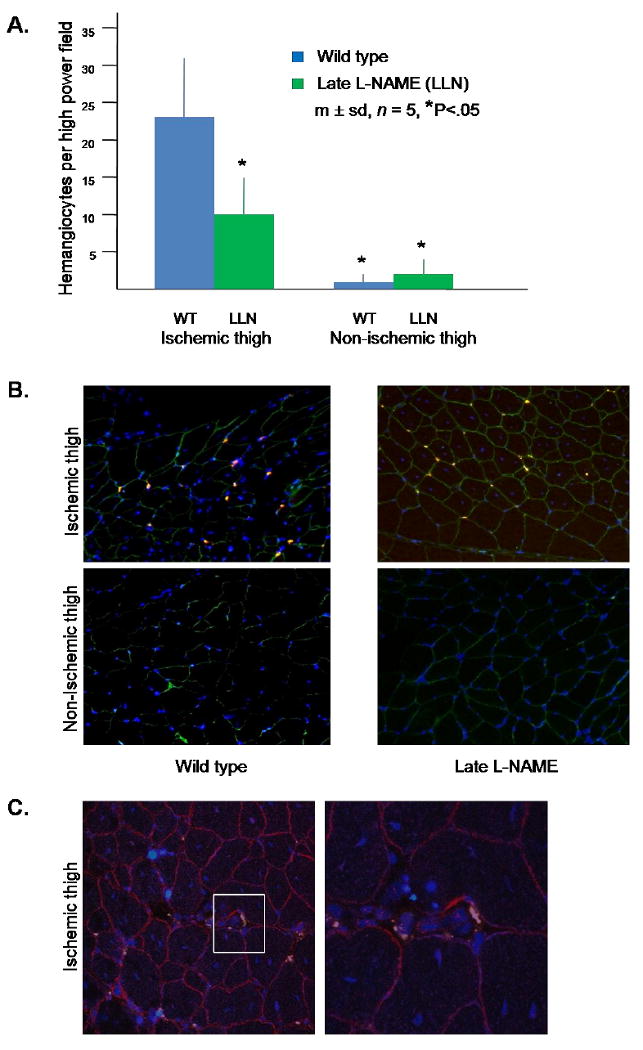
(A) Quantitation of hemangiocytes. Hemangiocytes were defined as cells that co-localized CXCR4 and VEGFR1. Data for each mouse was determined as the average number of hemangiocytes present in 10 randomly selected high power fields, determined by a blinded observer. (B) Representative photomicrographs of hemangiocyte immunostaining. VEGFR2 is stained green, CXCR4 is stained red, and nuclei are stained blue. In contrast to the data obtained at 3 days after the induction of hindlimb ischemia (Fig. 2.B.), note that hemangiocytes are present in the areas between myocytes, i.e., at the sites of capillaries (200×). (C) Confocal microscopy of the ischemic thigh of the wild type group. The left hand photo is shown at 200× magnification. The area delineated by the white square in the left hand photo is shown at 400× in the right hand photo. Note the presence of hemangiocytes in the peri-capillary region.
Discussion
Adaptations within existing collateral arteries occur following femoral artery occlusion to restore vascular conductance and hence limb blood flow. The early adaptation is vasodilation, while the late adaption is arteriogenesis, the later being a process of collateral artery remodeling.3 It has been established that eNOS-derived NO mediates collateral artery vasodilation during the early adaptive response to ischemia; however, the role of eNOS-derived NO in arteriogenesis remains unclear.11,17-21 The present studies support a role for eNOS-derived NO in both early and late collateral artery adaptations. In addition, this study provides the first evidence that eNOS is requisite for recruitment of hemangiocytes, a unique subset of bone marrow-derived vascular progenitor cells,10,15 to the ischemic vasculature.
Occlusion of the femoral artery increases the rate of blood flow through pre-existing, small diameter collateral arteries. This circumstance increases wall shear stress in these vessels, a mechanostimulus that increases eNOS expression and activity, the latter via eNOS phosphorylation.5,17 These events significantly enhance NO production and induce vasodilation of existing collateral arteries; new collateral arteries are not created during this process of arteriogenesis.3,5,17 The present findings are fully consistent with this process, as collateral artery diameter and perfusion within the ischemic hindlimb were less on day 3 after induction of ischemia in the eNOS-/- and early L-NAME treatment groups than in the wild type group.
It is known that the early post-ischemic, NO-induced vasodilation of collateral arteries is short-lived.3,11,18 The primary stimulus for this event, shear stress, dissipates during vasodilation because shear stress is inversely proportional to the 4th power of the vessel radius.18 This temporary vasodilation gives way to collateral artery remodeling, or arteiogenesis, a process that permanently increases net collateral artery conductance.2,3 Heretofore it was not clear if eNOS-derived NO participates in the process of arteriogenesis. Yu et al.19 and Lloyd et al.20 demonstrated reduced post-ischemic arteriogenesis when eNOS activity was blocked or eliminated; in contrast, however, Mees et al.11 failed to demonstrate an effect of eNOS-derived NO beyond 2 weeks after induction of ischemia. The present findings support a role for NO during arteriogenesis because collateral artery diameter and blood flow within the ischemic hindlimb were significantly less in the late L-NAME treatment group than the wild type group 28 days after induction of ischemia. Collectively, our findings indicate that NO is an active participant in post-ischemic vascular adaptation for at least the first month after induction of ischemia.
An alternative means to evaluate the role of eNOS in post-ischemic arteriogenesis is by the enhancement of eNOS activity, a circumstance that might be predicted to increase collateral artery growth and blood flow recovery. This predication has been confirmed: administration of Ad-eNOS to the ischemic hindlimb,21 overexpression of constitutively active eNOS in the ischemic hindlimb,11 or dietary supplementation with L-arginine 17 significantly improve post-ischemic arteriogenesis. Restitution of eNOS activity or administration of exogenous NO in the late L-NAME treatment group could provide additional support for the experimental hypothesis, if it restored arteriogenesis. However, co-administration of L-arginine and an arginine analogue such as L-NAME under in vivo conditions, or systemic administration of NO donor agents such as sodium nitroprusside, S-nitrosoglutathione, or 3-morpholinosydnonimine could also generate confounding results because it would not be clear if these agents reached the ischemic hindlimb.
Femoral artery excision establishes an inflammatory state within the ischemic tissue; indeed, monocytic 6 and lymphocytic 23 infiltration are requisite for post-ischemic arteriogenesis and angiogenesis. Activity of the Ca2+-independent isoform of nitric oxide synthase (iNOS) is increased in homogenates of ischemic hindlimb muscle prepared 14 days after induction of ischemia; protein and mRNA expression of iNOS were not determined, nor was the specific localization of the iNOS enzyme within the tissue (personal communication, Jinglian Yan, Worcester, MA, 2009). The arginine analogue L-NAME interacts with the arginine binding sites of both eNOS and iNOS and, while then IC50 for L-NAME for each enzyme (3.1 μM for iNOS, 0.35 μM for eNOS) indicate a 10-fold selectivity of eNOS > iNOS,24 it is likely that some L-NAME-induced inhibition of iNOS occurred in this study. In contemplating a possible role for iNOS-derived NO in arteriogenesis, consideration must be given to the site of NO production as the exceedingly brief T1/2 of NO precludes a direct effect at a distance far removed from its point of origin. Additional studies of iNOS are warranted, with specific attention to the location and cellular source iNOS expression within the ischemic hindlimb; moreover, iNOS inhibition with 1400W, which has a ∼105 fold specificity for iNOS > eNOS,24 might clarify the putative role of iNOS-derived NO in arteriogenesis.
An important and novel finding from the present experiments is that eNOS-derived NO is requisite for recruitment of hemangiocytes to the site of ischemic injury. Hemangiocytes, first described by Jin et al.10 represent a unique subset of bone marrow derived progenitor cells that participate in post-ischemic vascular repair.10,15 Hemangiocytes differ from endothelial progenitor cells (EPC) based on surface antigens; thus, hemangiocytes express CXCR4 and VEGFR1, whereas EPC express CD31, CD133, and VEGFR2.7,8 It is well established that eNOS-derived NO participates in the recruitment of EPC to the site of ischemic vascular injury.12-14 The present findings indicate a similar interaction for hemangiocytes. Specifically, hemangiocytes were evident in the adventitia of collateral arteries in the wild type group by day 3 after induction of hindlimb ischemia, whereas these cells were present only sporadically within the ischemic thigh when eNOS-derived NO was not present, i.e., in the eNOS-/- or early L-NAME treatment groups. Mobilization and homing of CXCR4 hemangiocytes is also contingent upon the chemokine SDF-1α 10 and an interaction between eNOS-derived NO and SDF-1α has been described;13 in this context, measurement of SDF-1α in the ischemic hindlimb of eNOS-/- mice and the use of CXCR4 blocking antibodies in this group represent two potentially useful lines of further investigation.
Another novel finding from these experiments is the change in location of hemangiocytes during the course of post-ischemic vascular recovery: on day 3 these cells were present along collateral arteries, the site anticipated were these cells participating in arteriogenesis, whereas on day 28 these cells were present in a peri-capillary location. Angiogenesis, defined as the de novo generation of new capillaries is an essential part of post-ischemic vascular adaptation.3 The capillary/myofiber ratio, a marker for angiogenesis, was not measured in this study; however, the presence of hemangiocytes in the peri-capillary regions between muscle cells might indicate that angiogenesis had occurred. Moreover, we propose that NO is requisite for recruitment of hemangiocytes to the peri-capillary location, as evidenced by the significant reduction of peri-capillary hemangiocytes in the late L-NAME treatment group. Most of the hemangiocytes seen within capillaries exhibited fragmentation of their nuclei, an observation that suggests apoptosis. It has been proposed that progenitor cells serve a paracrine effect; thus, these cells affect existing vascular cells, inducing repair and replication, rather than becoming permanently incorporated into existing vascular tissue.26
Interestingly, gangrene of the toes consistently occurred in eNOS-/- and early L-NAME treated mice by day 3 after induction of hindlimb ischemia, but was never observed in late L-NAME treated animals. One explanation for this finding is that delay in initiating L-NAME treatment until the third post-ischemic day allowed sufficient time for NO-induced recruitment of hemangiocytes to pre-existing collateral arteries in a manner similar to that observed in the wild type group studied on day 3. These cells have the potential to restructure the ischemic vasculature by direct12-14 and paracrine 26 actions, improving vascular conductance and hence perfusion. An alternative explanation is that the eNOS-/- and early L-NAME treatment groups were unable to generate the immediate eNOS-derived vasodilation of collateral arteries, a circumstance that would lead to a profound compromise in downstream perfusion.
We recognize two limitations to the interpretation of our results. First, the technique of femoral artery excision produces a sudden and very severe level of ischemia, whereas critical limb ischemia in humans is a disease process that develops over a period of years.1 A more gradual, clinically duplicative model of femoral arterial occlusion has been described in rats that causes less inflammation and less tissue necrosis, but lower levels of blood flow recovery than after acute occlusion.16 This model uses an ameroid constrictor, however, which is too large for use in the mouse hindlimb. Second, we did not employ perfusion fixation at an in situ pressure during preparation of the thigh muscles prior to collateral artery diameter measurement but instead this tissue was prepared at an effective pressure of 0 mmHg. This approach certainly limited the diameter of collateral arteries at the time of measurement. However, the difference in collateral artery diameter among study groups was quite substantial; moreover, collateral wall thickness did not vary among groups. It is thus unlikely that the use of perfusion fixation would have altered the outcome of collateral artery diameter.
The present findings are relevant to human peripheral arterial disease (PAD). PAD patients who develop intermittent claudication or critical limb ischemia have been shown to have endothelial dysfunction characterized by decreased NO bioavailability.27 This reduced NO bioavailability likely results in inadequate collateral artery enlargement in PAD. In addition, reduced NO bioavailability may be an impediment to the efficacy of molecular or chemical therapeutics in PAD. Clinical trials administering either recombinant vascular endothelial growth factor (rhVEGF)28 or recombinant fibroblast growth factor (rFGF2)29 to PAD patients have demonstrated therapeutic efficacy only after 60 – 90 days of treatment. Both VEGF and FGF2 exert their effects via upregulation and activation of eNOS. 30,31 The present study indicates that eNOS-derived NO is requisite for acute adaptation to severe ischemia to prevent tissue necrosis and for sustained arteriogenesis. We propose that treatment with agents known to enhance eNOS activity may prove a useful adjunctive therapy in PAD. Only through a complete understanding of the specific and temporally distinct eNOS-related recovery responses can we hope to achieve effective molecular and cell-based therapies for human patients.
Acknowledgments
We thank Jianming Li and Phong Dargon for assistance in obtaining confocal microscopy images.
Sources of Funding This work was funded by HL75353 (LM).
Footnotes
Clinical Relevance Paragraph This study demonstrates that eNOS-derived NO is requisite for both the early and late vascular recovery phases in response to hindlimb ischemia. Moreover, it demonstrates that recruitment of hemangiocytes, a specific subset of vascular progenitor cells that display VEGFR1 and CXCR4 surface antigens, is dependent on the presence of NO. These findings enhance understanding of the basic biological mechanisms in the recovery from ischemia. The need for eNOS-derived NO suggests that clinical strategies to enhance post-occlusive flow recovery in peripheral artery disease might be more successful if eNOS and/or NO are applied as a part of the treatment paradigm. Hemangiocytes are an important part of the cellular response to ischemia. The present findings underscore their importance and suggest that preservation or enhancement of eNOS within the ischemic limb might improve the recruitment of these critical reparative cells to the site of ischemic injury.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schainfeld RM, Isner JM. Critical limb ischemia: nothing to give at the office? Ann Intern Med. 1999;130:442–444. doi: 10.7326/0003-4819-130-5-199903020-00017. [DOI] [PubMed] [Google Scholar]
- 2.Helisch A, Schaper W. Arteriogenesis: the development and growth of collateral arteries. Microcirculation. 2003;10:83–97. doi: 10.1038/sj.mn.7800173. [DOI] [PubMed] [Google Scholar]
- 3.Scholz D, Ziegelhoeffer T, Helisch A, Wagner S, Friedrich C, Podzuweit T, Schaper W. Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol. 2002;34:775–787. doi: 10.1006/jmcc.2002.2013. [DOI] [PubMed] [Google Scholar]
- 4.Cooke JP. Flow, NO, and atherogenesis. Proc Natl Acad Sci. 2003;100:768–770. doi: 10.1073/pnas.0430082100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pipp F, Boehm S, Cai WJ, Adili F, Ziegler B, Karanovic G, Ritter R, Balzer J, Scheler C, Schaper W, Schmitz-Rixen T. Elevated fluid shear stress enhances postocclusive collateral artery growth and gene expression in the pig hindlimb. Arterioscler Thromb Vasc Biol. 2004;24:1664–1668. doi: 10.1161/01.ATV.0000138028.14390.e4. [DOI] [PubMed] [Google Scholar]
- 6.Voskuil M, van Royen N, Hoefer IE, Seidler R, Guth BD, Bode C, Schaper W, Piek JJ, Buschmann IR. Modulation of collateral artery growth in a porcine hindlimb ligation model using MCP-1. Am J Physiol. 2003;284:H1422–1428. doi: 10.1152/ajpheart.00506.2002. [DOI] [PubMed] [Google Scholar]
- 7.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatterman G, Isner J. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 8.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 9.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 10.Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, Hattori K, Zhang F, Hicklin DJ, Wu Y, Zhu Z, Dunn A, Salari H, Werb Z, Hackett NR, Crytal RG, Lyden D, Rafii S. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nature Medicine. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mees B, Wagner S, Ninci E, Tribulova S, Martin S, van Haperen R, Kostin S, Heil M, de Crom R, Schaper W. Endothelial nitric oxide synthase activity is essential for vasodilation during blood flow recovery but not for arteriogenesis. Arterioscler Thromb Vasc Biol. 2007;27:1–8. doi: 10.1161/ATVBAHA.107.145375. [DOI] [PubMed] [Google Scholar]
- 12.Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher A, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nature Medicine. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 13.Kaminski A, Ma N, Donndorf P, Lindenblatt N, Feldmeier F, Ong L, Furlani D, Skrabal C, Liebold A, Vollmar B, Steinhoff G. Endothelial NOS is required for SDF-1α/CXCR4-mediated peripheral endothelial adhesion of c-Kit+ bone marrow stem cells. Lab Invest. 2008;88:58–69. doi: 10.1038/labinvest.3700693. [DOI] [PubMed] [Google Scholar]
- 14.Rafii D, Psaila B, Butler J, Jin DK, Lyden D. Regulation of vasculogenesis by platelet-mediated recruitment of bone marrow-derived cells. Arterioscler Thromb Vasc Biol. 2008;28:217–222. doi: 10.1161/ATVBAHA.107.151159. [DOI] [PubMed] [Google Scholar]
- 15.Wragg A, Mellad J, Beltran L, Konoplyannikov M, San H, Boozer S, Deans R, Mathur A, Lkederman R, Kovacic J, Boehm M. VEGFR1/CXCR4-positive progenitor cells modulate local inflammation and augment tissue perfusion by a SDF-1-dependent mechanism. J Mol Med. 2008;86:1221–1232. doi: 10.1007/s00109-008-0390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang GL, Chang DS, Sarkar R, Wang R, Messina LM. The effect of gradual or acute arterial occlusion on skeletal muscle blood flow, arteriogenesis, and inflammation in rat hindlimb ischemia. J Vasc Surg. 2005;41:312–320. doi: 10.1016/j.jvs.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearny M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eitenmuller I, Volger O, Kluge A, Troidl K, Barancik M, Cai WJ, Heil M, Pipp F, Fischer S, Horrevoets AJ, Schmitz-Rixen T, Schaper W. The range of adaptation by collateral vessels after femoral artery occlusion. Circ Res. 2006;99:656–662. doi: 10.1161/01.RES.0000242560.77512.dd. [DOI] [PubMed] [Google Scholar]
- 19.Yu J, Demuink E, Zhuang Z, Drinane M, Kauser K, Rubanyi G, Qian H, Murata T, Escalante B, Sessa WC. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci. 2005;102:10999–11004. doi: 10.1073/pnas.0501444102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith R, Lin KF, Agata J, Chao L, Chao J. Human endothelial nitric oxide synthase gene delivery promotes angiogenesis in a rat model of hindlimb ischemia. Arterioscler Thromb Vasc Biol. 2002;22:1279–1285. doi: 10.1161/01.atv.0000026613.18742.67. [DOI] [PubMed] [Google Scholar]
- 21.Brevetti LS, Chang DS, Tang GL, Sarkar R, Messina LM. Overexpression of endothelial nitric oxide synthase increases skeletal muscled blood flow and oxygenation in severe rat hind limb ischema. J Vasc Surg. 2003;38:820–826. doi: 10.1016/s0741-5214(03)00555-x. [DOI] [PubMed] [Google Scholar]
- 22.Topper JN, Gimbrone MA., Jr Blood flow and vascular gene expression: fluid shear stress as a modulator of endothelial phenotype. Mol Med Today. 1999;5:40–46. doi: 10.1016/s1357-4310(98)01372-0. [DOI] [PubMed] [Google Scholar]
- 23.Van Weel V, Toes RE, Seghers L, Deckers MR, de Vries PH, Eilers J, et al. Natural killer cells and CD4+ T-cells modulate collateral artery development. Arterioscler Thromb Vasc Biol. 2007;27:2310–2318. doi: 10.1161/ATVBAHA.107.151407. [DOI] [PubMed] [Google Scholar]
- 24.Alderton WK, Cooper CE, Kowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ceradini D, Gurtner G. Homing to hypoxia: HIF-1 as a mediator of progenitor cell recruitment to injured tissue. Trends Cardiovasc Med. 2005;15:57–63. doi: 10.1016/j.tcm.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Heil M, Ziegelhoeffer T, Mees B, Schaper W. A different outlook on the role of bone marrow stem cells in vascular growth: Bone marrow delivers software not hardware. Circ Res. 2004;94:573–574. doi: 10.1161/01.RES.0000124603.46777.EB. [DOI] [PubMed] [Google Scholar]
- 27.Böger R, Bode-Böger S, Thiele W, Junker W, Alexander K, Frölich J. Biochemical evidence of impaired nitric oxide synthesis in patients with peripheral arterial disease. Circulation. 1997;95:2068–2073. doi: 10.1161/01.cir.95.8.2068. [DOI] [PubMed] [Google Scholar]
- 28.Henry T, Annex B, McKendall G, Azarin M, Lopex J, Giordano F, Shah P, Willerson J, Benza R, Berman D, Gibson M, Bajamonde A, Rundle A, Fine J, McCluskey E. The VIVA trial: Vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation. 2003;107:1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 29.Simons M, Annex B, Laham R, Kleiman N, Henry T, Dauerman H, Udelson J, Gervino E, Pike M, Whitehorse M, Moon T, Chronos N. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: Double-blind, randomized, controlled clinical trial. Circulation. 2002;105:788–793. doi: 10.1161/hc0802.104407. [DOI] [PubMed] [Google Scholar]
- 30.Hiasa KI, Ishibashi M, Ohtani K, Inuoe S, Zhao Q, Kitamoto S, Sata M, Ichiki T, Takeshita A, Egashira K. Gene transfer of stromal cell-derived factor-1a enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation. 2004;109:2454–2461. doi: 10.1161/01.CIR.0000128213.96779.61. [DOI] [PubMed] [Google Scholar]
- 31.Ahmad S, Hewett PW, Wang P, Al-Ani B, Cudmore M, Fujisawa T, Haigh JJ, le Noble F, Wang L, Mukhopadhyay D, Ahmed A. Direct evidence for endothelial vascular endothelial growth factor receptor -1 function in nitric oxide-mediated angiogenesis. Circ Res. 2006;99:715–722. doi: 10.1161/01.RES.0000243989.46006.b9. [DOI] [PubMed] [Google Scholar]


