Abstract
Objective(s)
Glucocorticoid administration to women in preterm labor improves neonatal mortality and morbidity. Fetal exposure to glucocorticoid levels higher than those appropriate to the current gestational stage has multiple organ systems effects. Some, e.g., fetal hypertension, are maximal at lower than the clinical dose. We hypothesized that the clinical dose has supramaximal lung maturational effects.
Study Design
We evaluated the full, half and a quarter the clinical betamethasone dose (12mg/70kg or 170μg/kg i.m. twice 24h apart) on fetal sheep lung pressure volume curves (PVC) after 48h exposure at 0.75 gestation. We measured key mRNAs and protein products that affect lung function and total lung dipalmitoyl phosphatidyl choline (DPPC).
Results
Full and half doses had similar PVC and total lung DPPC effects. mRNA for SPA, B and D and elastin rose in a dose dependent fashion.
Conclusion
Half the clinical betamethasone dose produces maximal PVC improvement in fetal sheep at 0.75 gestation.
BACKGROUND AND OBJECTIVE
Preterm birth occurs in 10% of live births and is a major cause of neonatal mortality and morbidity that result primarily from pulmonary immaturity and intraventricular hemorrhage1. NIH recommends routine glucocorticoid administration to women at risk of premature delivery between 24-34 weeks gestation to accelerate fetal lung maturation and decrease the incidence of complications of pulmonary immaturity2. Antenatal glucocorticoid therapy reduces infant mortality approximately 30%, neonatal respiratory distress syndrome approximately 50%, and both intracranial hemorrhage and periventricular leukomalacia approximately 70%3.
Glucocorticoids improve maximal neonatal lung volume by increasing lung compliance as a result of increased surfactant protein production and accelerated cytoarchitectual maturation4. Lung compliance is primarily determined by the surface tension of the surfactant film and mechanical properties of the continuous fiber network forming the structural skeleton of the pulmonary interstitium. Maturation of the fetal lung involves the mesenchyme as well as the surfactant proteins5.
Since synthetic glucocorticoids rapidly cross the placenta6 their administration to the pregnant mother inevitably exposes the fetus to glucocorticoid concentrations that are inappropriate for the current stage of fetal maturation. Indeed this is the purpose of the treatment. Extensive studies have indicated that this exposure results in an altered trajectory of development of several fetal systems7. Maternal administration of betamethasone to the pregnant baboon, in weight adjusted doses equivalent to those given to women in premature labor, elevates fetal blood pressure8 and decreases key neuronal proteins in the fetal brain9.
The present study determined in vivo changes following 48 h exposure to betamethasone at the full, half and a quarter the dose administered to women in premature labor on fetal lung pressure volume curves in sheep, the species in which the original observation was made by Liggins that glucocorticoids accelerate maturation of the fetal lung10. We also evaluated changes in mRNA for surfactant proteins A, B, C, D, elastin and the glucocorticoid receptor and protein changes by western analysis for surfactant proteins A, C and D. We hypothesized that the routine therapy of two 12 mg doses of betamethasone phosphate alone given intramuscularly to the mother 24 hours apart is supramaximal for fetal lung maturation.
MATERIALS AND METHODS
Care and use of animals and tissue collection
We studied a total of 30 pregnant Rambouillet-Columbia ewes of known gestational age. Betamethasone phosphate (170μg/kg – n = 8; 85 μg/kg - n = 8; 42.5 μg/kg - n = 7 or vehicle - n = 7 injected i.m) at 8.00 AM on two consecutive days beginning at 110 days gestation. The 170 μg/kg starting point was chosen as it represents the dose given to an “average” 70 kg woman (12mg / 70 kg). All procedures were approved by the University of Wyoming Institutional Animal Care and Use Committee.
Cesarean Section
48 h after the initial betamethasone dose, the fetus was removed at caesarean section under general anesthesia. Pregnant sheep were tranquilized with ketamine hydrochloride (10 mg/kg). Following intubation, isofluorane (2%, 2 l/min) was administered to the ewe to maintain a surgical plane of anesthesia throughout surgery. The maternal abdomen and uterus were opened and the fetus was euthanized by exsanguination while still under isofluorane general anesthesia. In the cases of twin pregnancy the second fetus remained in utero until processing of the first was complete, at which time the second was euthanized in the same manner. The mother was euthanized by intravenous administration of Beuthanasia solution (390 mg Pentobarbitone, 50 mg/ml Phenytonin) at 10 ml/50 Kg. In the 15 of the 30 ewes with twin fetuses, one fetus was chosen at random to be the first examined.
Pressure volume curves
The fetal chest was opened by sectioning the ribs on both sides of the sternum. The thoracic cavity was then flushed with cold saline at 4°C to slow mRNA and protein degradation. The fetal trachea was cannulated with polyvinyl tubing which was advanced until the tip was just above the carina. The fetal lungs were inflated with air to produce 5 cm increments in pressure until 40 cm H2O were reached. The air volume required to produce the rise in pressure on the inflation curve was recorded in 5 cm pressure differences. The process was reversed for deflation. Lung samples were then taken from the middle right lobe for molecular biology and protein analysis. The left lobe was fixed in 4 % phosphate buffered paraformaldehyde solution.
Quantitative RT-PCR
Lung total RNA was isolated from each sample using Tri Reagent (Sigma, St. Louis, MO). RNA concentration was determined spectrophotometrically at 260 and 280 nm. Primers for amplification of the 6 genes studied were designed using Beacon Designer software (BIO-RAD, Hercules, CA) from Genbank human cDNA sequences. Primer uniqueness was established using BLAST (NCBI). Table 1 shows each gene name, gene ID, GenBank accession number, forward and reverse primer sequences (5’ to 3’) with the primer first base number, PCR product length, primer melting temperature, and primer annealing temperature.
Table 1.
QRT-PCR Primers for surfactant protein (SP) A SP-A, SP-B, SP-C, SP-D, Elastin and glucocorticoid receptor (GR): Tm – melting temperature; Ta - annealing temperature.
| GENE | GENE ID | Accession # | Primer Sequence | Length | Tm | Ta |
|---|---|---|---|---|---|---|
| Elastin |
ELN |
M26188 |
27-CTCGGAGTTGGAGGACTG | 203 |
91 |
61 |
| 229-AAGGGCTTGGGAGGTTTG | ||||||
| SP-A |
SFTPA1 |
AF076633 |
563-CCATTACCAGCATCGTGAAG | 200 |
87 |
61 |
| 762-CAGGCAGTTCTTGTCATTCC | ||||||
| SP-B |
SFTPB |
AF107544 |
293-AGGAGTGCGATGTTCTTC | 200 |
89 |
61 |
| 492-CATCTTGTCCAGCAGAGG | ||||||
| SP-C |
SFTPC |
AF076634 |
124-ATCGTGGTTGTGGTTGTG | 260 |
89.5 |
61 |
| 383-ATAATGTAGCAGCAGGTTCC | ||||||
| SP-D |
SFTPD |
AJ133002 |
13-CGGAGTGTCGGGAAGAAG | 184 |
89 |
54 |
| 196-TGGTATCGGTCATGCTCAG | ||||||
| GR | NR3C1 | S44554 | 268-TTGGCAGGAGAGGATGATTC | 396 | 85 | 61 |
| 663-AACAGGAATTGGTGGAATGAC |
First strand synthesis was performed using 250ng total RNA, 100 ng of random hexamers, and 50 units SuperScript II Reverse transcriptase (Invitrogen, Carlsbad, CA). A control reaction lacking reverse transcriptase was included. After cDNA synthesis, RNA was removed by incubation with 2 units RNase H at 37° C for 20 min. Quantitative PCR was performed as described previously 11 using an iCycler iQ real-time PCR detection system (Bio-Rad, Hercules, CA). All samples were run in triplicate. 18S rRNA was used as an internal control (Ambion, Austin, TX).
PCR products were size fractionated in 1.3% agarose gels to confirm amplification of a single product of the expected size. PCR product sequences were validated by excision from the gel, purification using QIAquick gel extraction kit (QIAGEN, Valencia, CA) and sequencing by the NYU DNA sequencing facility. Sequences were subjected to a BLAST search (NCBI) to confirm identity with the corresponding GenBank sheep sequences (Table 1 accession numbers).
Western Blot Analysis of Surfactant proteins A, C and D
Immunoblots for detection of surfactant proteins (SP) A, C, and D were performed as detailed previously using 50 μg of total lung protein 12. Images were optically scanned (Hewlitt-Packard 5200C with HP PrecisionScan software v2.02) and digitized. Antigen signal was quantified by pixel density (Un Scan It gel v5.1; Silk Scientific, Orem, UT). Quantification was performed only after linearity was established between amount of protein and film exposure time. Pixel density data from all images are presented as the ratio of surfactant protein to β-actin.
Measurement of dipalmitoyl phosphatidyl choline (DPPC)
Dipalmitoyl phosphatidyl choline (DPPC) analysis was analyzed as in previous studies13.
Statistical Analysis
Measurements performed in twins were averaged to represent the pregnancy prior to analysis. Differences in treatment groups were compared using ANOVA and Bonferroni multiple comparison tests comparing BM dose to saline control. Preliminary comparisons showed no differences according to fetal sex so data were pooled. The distribution of singletons and twins in each treatment group were: control: n = 7 (3,4); 42.5 ug/kg: n = 7 (2,5); 85 ug/kg: n = 8 (2,6); 170 ug/kg: n = 8 (3,5). Pearson correlations were used to assess the relationship between BM dose and QRT-PCR data from surfactant proteins. Data are presented as mean ± SEM. Significance was set at p < 0.05.
RESULTS
Effects of fetal exposure to betamethasone on fetal body and organ weights
Table 2 provides the fetal body weight and organ weights for the four groups of animals. There were no significant differences between the groups.
Table 2.
Fetal organ weights at the different betamethasone treatments adjusted to maternal body weight. No differences were noted between fetal sexes therefore data were subsequently combined. S: singleton pregnancy, T: twin pregnancy.
| |
Saline N=7 (3S 4T) |
42.5 μg/kg N=7 (2S 5T) |
85 μg/kg N=8 (2S 6T) |
170 μg/kg N=8 (3S 5T) |
|---|---|---|---|---|
| Fetus (g) | 1704.8 ± 54.0 | 1672.7 ± 37.9 | 1674.8 ± 46.5 | 1709.8 ± 75.9 |
| Brain (g) | 35.2 ± 0.9 | 36.4 ± 0.9 | 37.0 ± 1.1 | 36.1 ± 0.6 |
| Heart RV (g) | 3.3 ± 0.1 | 3.2 ± 0.2 | 3.4 ± 0.2 | 3.4 ± 0.21 |
| Heart LV (g) | 4.4 ± 0.2 | 4.3 ± 0.2 | 4.7 ± 0.1 | 4.9 ± 0.21 |
| Heart total (g) | 13.5 ± 0.7 | 13.9 ± 0.6 | 14.7 ± 0.4 | 13.5 ± 0.7 |
| Lungs (g) | 72.9 ± 3.2 | 74.5 ± 3.0 | 67.9 ± 2.7 | 69.5 ± 3.5 |
| Liver (g) | 77.1 ± 3.3 | 78.9 ± 2.6 | 79.3 ± 2.8 | 75.6 ± 2.7 |
| Kidney R (g) | 7.9 ± 0.3 | 7.1 ± 0.2 | 7.4 ± 0.4 | 7.4 ± 0.5 |
| Kidney L (g) | 7.9 ± 0.3 | 7.1 ± 0.2 | 7.5 ± 0.4 | 7.6 ± 0.6 |
| Kidney total (g) | 15.8 ± 0.6 | 14.1 ± 0.3 | 14.9 ± 0.8 | 14.9 ± 1.1 |
| Adrenal R (g) | 0.08 ± 0.01 | 0.08 ± 0.00 | 0.09 ± 0.00 | 0.08 ± 0.00 |
| Adrenal L (g) | 0.10 ± 0.01 | 0.09 ± 0.00 | 0.09 ± 0.01 | 0.09 ± 0.01 |
| Adrenal total (g) | 0.18 ± 0.01 | 0.16 ± 0.01 | 0.18 ± 0.01 | 0.17 ± 0.01 |
| LW:BW ratio | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.04± 0.00 |
Fetal lung pressure volume relationship
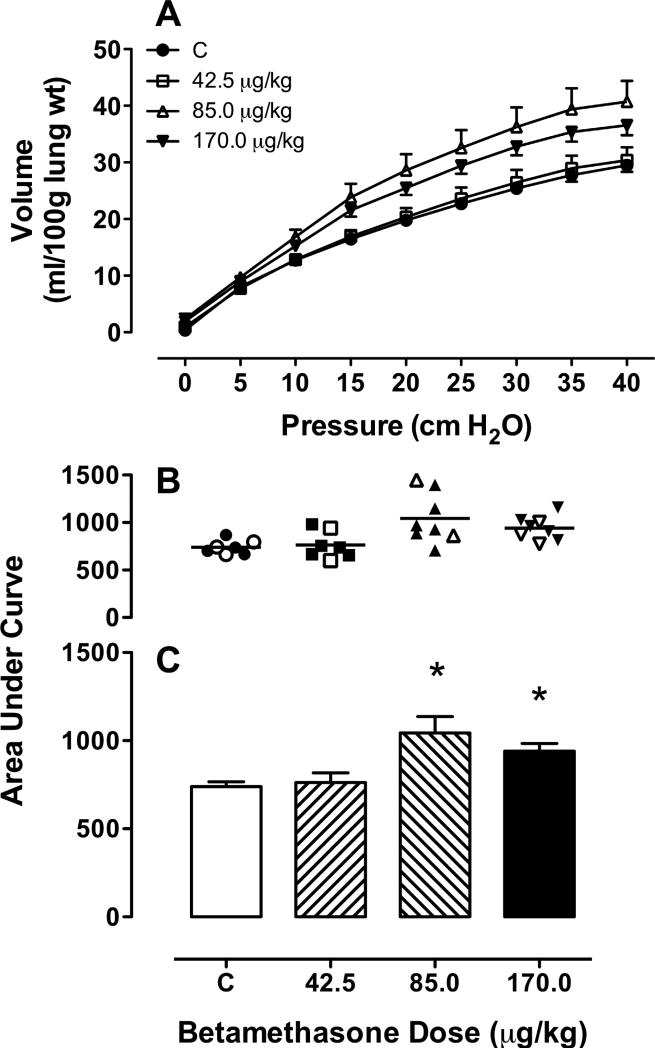
Fig 1 shows that the pressure – volume relationship for control and each dose of BM in the fetal lungs. In panel A the deflation limb of the pressure–volume curves for each treatment is shown. In panel B the area under the curve for each pregnancy in each treatment groups is depicted with the open symbols representing singletons and the closed symbols representing the values from twin pregnancies. Greater compliance is demonstrated in lungs of both the 85 and 170 μg/kg betamethasone doses when compared to saline control (Fig 1, panel C). The 42.5 μg/kg dose was not different from control (*; p < 0.05).
Figure 1. Pressure-volume relationships in fetal sheep lungs removed from fetuses of ewes treated with a single injection of betamethasone adjusted to maternal weight.
(0 μg/kg [C: control], N=7 (3,4); 42.5 μg/kg, N=7 (2,5); 85 μg/kg, N=8 (2,6); 170 μg/kg, N=8 (3,5)) at 8.00 AM on day 110 and again at 8.00 AM on day 111. The fetus was delivered by Cesarean Section and the lungs evaluated 48 h after the first dose. A deflation arm of the pressure-volume relationship (symbols for doses as indicated); B area under the curve for each BM dose; • average of twins; ○ singletons; C mean area under the curve for each BM dose. M ± SEM. * p<0.05 comparison to control.
Changes in surfactant protein mRNA and protein
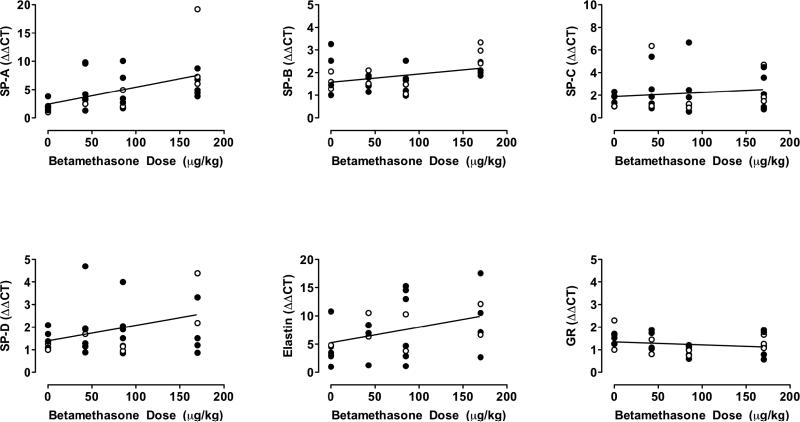
Fig 2 demonstrates that mRNA for surfactant proteins A, B, D and elastin showed a significant linear increase with betamethasone dose (p < 0.05).
Figure 2. Correlations between maternal weight adjusted betamethasone dose and mRNA of surfactant proteins, elastin and glucocorticoid receptor.
• average of twins; ○ singletons. SP-A r = 0.50 p = 0.005; SP-B r = 0.37 p = 0.046; SP-C r = 0.14 p = 0.469; SP-D r = 0.38 p=0.038; Elastin r = 0.39 p = 0.032; GR r = −.20 p= 0.301.
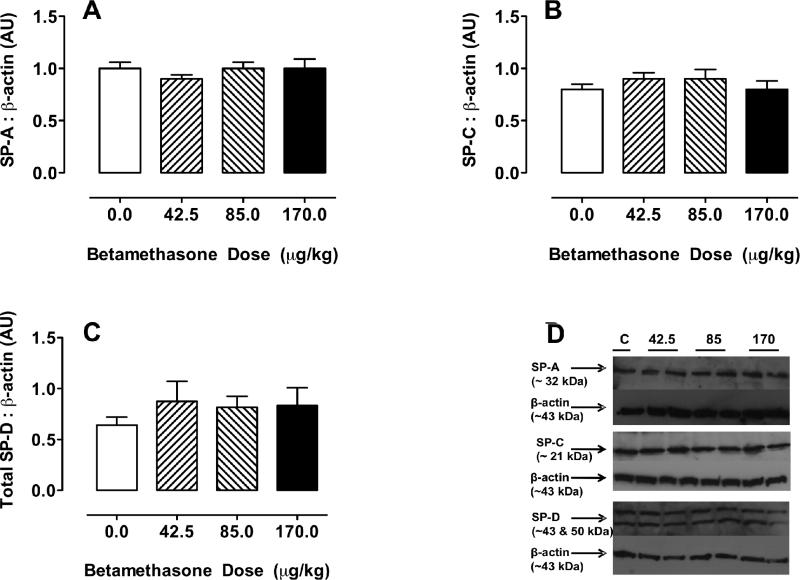
Fig 3 Panels A, B and C illustrate the abundance of surfactant proteins A, C and D from whole lung homogenate in response to increasing doses of betamethasone. The western analyses demonstrated protein bands of the expected sizes for each surfactant protein: SPA (~32 kDa), SPC (~21 kDa), and SPD (~43 and 50 kDa). β-actin was observed as a single band at ~43 kDa. SPA and SPC were quantified as the ~32 and ~21 kDa bands relative to β-actin while SPD is expressed as the sum of the ~43 and ~50 kDa bands relative to β-actin. When corrected for b-actin there were no differences between groups or compared with the control group for SP-A, SP-C, SP-D.
Figure 3. Surfactant protein (SP) in fetal sheep lungs removed from fetuses of ewes treated with a single injection of either vehicle or betamethasone (BM).
A SP-A; B SP-B; and C SP-C relative to E-actin in fetal sheep lungs removed from fetuses of ewes treated with a single injection of either vehicle C or BM adjusted to maternal weight (0 μg/kg [C: control], N=7 (3,4); 42.5 μg/kg, N=7 (2,5); 85 μg/kg, N=8 (2,6); 170 μg/kg, N=8 (3,5)) at 8.00 AM on day 110 and again at 8.00 AM on day 111. The fetus was delivered by Cesarean Section and the lungs evaluated for protein content by Western analysis 48 h after the first dose. There were no differences between groups.
Effect of differing doses of BM on total lung DPPC
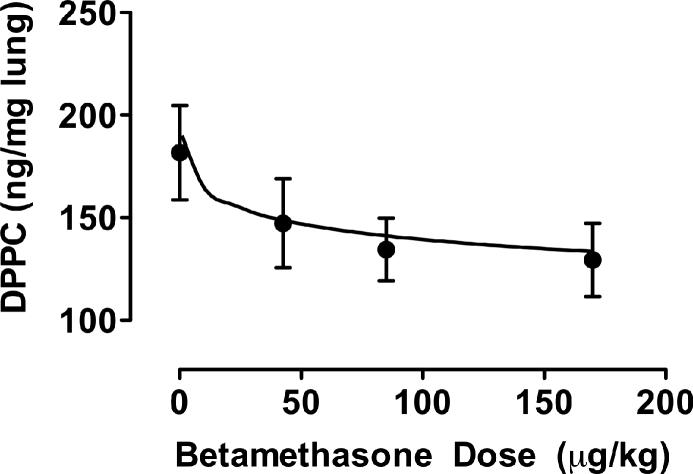
Fig 4 shows that total lung DPPC was significantly higher in the lungs of the control fetuses than at all three other doses of betamethasone which did not differ from each other (p < 0.05).
Figure 4. Fetal whole lung disaturated phophatidyl choline (DPPC).
DPPC in fetuses of pregnant ewes treated with different doses of betamethasone (BM) adjusted to maternal body weight (0 μg/kg [C: control], N=7 (3,4); 42.5 μg/kg, N=7 (2,5); 85 μg/kg, N=7 (1,6); 170 μg/kg, N=7 (2,5)). Mean ± SEM. Regression function: DPPC = −11.9 ln(BM) + 190. r = 0.993.
COMMENT
The efficacy of administration of synthetic glucocorticoids to women threatening premature labor is well documented, but several features of therapy have not been rigorously addressed2. Firstly, there are few systematic dose response studies in the clinical setting14. Therefore the optimum steroid dose in terms of health cost and benefit to the developing fetus at any gestational age has not been established. Secondly, given the different treatment regimens and accompanying variation in responses, the human situation does not allow study of acute or long-term fetal and post-natal non-pulmonary effects. Also, it is difficult to answer these questions since the dose of synthetic glucocorticoid administered is not normalized for maternal weight. Further, there are no data that provide guidance as to whether multiple pregnancy affects the optimal dose. Finally, different drug formulations – phosphate alone or phosphate/acetate combination – are used. In the USA combined formulations are more common while in Europe the phosphate alone is generally administered.
We have focused on dose effectiveness and hypothesized that the routine therapy of two 12 mg doses of betamethasone phosphate alone given intramuscularly to the mother 24 h apart is supramaximal for fetal lung maturation. Our observation that there were no effects of any betamethasone dose on overall fetal or organ weight likely reflects the short 48h interval between exposure and tissue collection/testing since weight changes have previously been described 15-17. Our data clearly show that half the normal, weight adjusted, clinical dose, given as the phosphate, produces maximal fetal lung PVC maturation and has effects on mRNA for key genes, which while not maximal may be adequate functionally. The magnitude of the change in both the PVC and mRNA expression likely reflects the gestational age used in the present study. It is of interest that there was no significant increase in abundance of protein for key genes studied in the 48h of glucocorticoid exposure. Improved PVC function may result from changes in membrane fluidity, post-translational processing or intracellular protein trafficking.
The average saturation of fetal lung phosphatidyl choline (PC) increases with gestational age, as does the amount of palmitate, the fatty acyl component of DPPC in lung surfactant PC18. On this basis, any acceleration of fetal lung maturation in response to glucocorticoid exposure would be predicted to be accompanied by increased DPPC, however our data show a decrease, also maximal at the half dose. A similar, dose dependent drop in saturated PC was observed following betamethasone administration to pregnant rabbits19, suggesting that glucocorticoid effects on key lung lipids may diverge from those of normal lung maturation. Neither ourselves nor the authors of this rabbit study can explain this seemingly paradoxical response. Perhaps with maturation, DPPC incorporation into lamellar bodies is more efficient after glucocorticoid exposure and a feedback mechanism decreases DPPC production. In addition it may be that betamethasone releases DPPC from type 2 cells to be carried away in lung fluid as the fetus breathes leaving less in lung tissue itself. In retrospect it would have been interesting to measure lung liquid DPPC levels. At this early stage of development there would have been little on-going surfactant synthesis, so cellular stores could easily have been depleted. Changes in lung compliance are influenced by (a) alterations in lung structure (thinning of saccular walls), and (b) intra-alveolar surfactant (i.e., external to the type 2 cell). There is evidence that the betamethasone effect on lung compliance is as much, if not more, due to acceleration of structural lung maturity than changes in surfactant availability. There is little evidence in our study of increased surfactant synthesis (DPPC or SP) by betamethasone. The explanation that betamethasone, at this fetal age, inhibits DPPC synthesis in type 2 cells, is unlikely given the effects on lung compliance. We can speculate that glucocorticoid -induced accelerated surfactant lipid synthesis outpaces palmitate supply, which is replaced by other available acyl moieties such as stearate or oleate, also normal lung surfactant lipid components. To answer this question it would be useful to evaluate lipids other than DPPC. Whatever the explanation, these DPPC data show that the half dose is as effective as the full dose.
Glucocorticoids acutely affect brain structure and function, and perturb cerebral maturation16;17;20. We have evaluated the relationship of the fetal hypertension produced when betamethasone is administered to the pregnant ewe to the resulting fetal plasma betamethasone concentration6. We administered equal total amounts of betamethasone, either as the fast releasing form, betamethasone phosphate or as the slow-release betamethasone depot preparation, consisting of 50% betamethasone phosphate and 50% betamethasone acetate. Plasma betamethasone concentrations achieved with the depot preparation were roughly half those measured after the same dose of soluble preparation but the fetal blood pressure rise was the same after both preparations, clearly indicating that the 12 mg per day betamethasone phosphate dose currently administered is greater than that needed for a maximal fetal response – in blood pressure at least.
Fetal exposure to glucocorticoids at levels higher than appropriate for the current stage of development delay brain growth in human fetuses as reflected by reduced head circumference at birth15;21 and reduce brain weight in monkey fetuses22;23. Delay of human brain maturation has also been shown in magnetic resonance images by the demonstration of reduced brain surface area and cortical folding complexity 24. Another study has demonstrated that consequences of antenatal glucocorticoid exposure on hypothalamo-pituitary-adrenal function persist for at least six months after birth “indicating that studies on long-term effects are warranted” 25. In this context it is important to note that neonatal dexamethasone treatment in premature infants results in long-lasting programming effects on hypothalamo-pituitary-adrenal axis and immune function at school age26. The offspring of the original clinical trial of antenatal glucocorticoid therapy have only been followed up for a little over thirty years and thus unwanted side-effects still have the majority of the life-span in which to emerge.
Although not an aim of the present study, it will be important to compare the various different steroid formulations in the context of health benefit:cost. We have studied maternal and fetal kinetics of betamethasone acetate and betamethasone phosphate-acetate combination in pregnant sheep6. With both combinations the highest maternal concentrations were obtained after 15 min followed by an exponential decline (t1/2 approximately 3 h) and betamethasone was no longer detectable after 8 to 12 h. Independent of the dose administered, betamethasone was first detectable in fetal plasma at 1 h and peaked at 3 h and was no longer detectable at 8 h. The combined phosphate acetate formulation produced maternal and fetal betamethasone concentrations one half of those obtained with betamethasone phosphate indicating slow release from the acetate over the first 8 h.
In another study, ewes carrying singleton pregnancies at 122 days gestation were randomized to single doses of either 0.25 or 0.5 mg/kg of betamethasone/acetate formulation, 0.5 mg/kg betamethasone phosphate or 0.25 mg/kg betamethasone acetate given 48h before delivery by cesarian section27. The effects of these treatments on fetal lung maturation were compared with saline placebo and two doses of 0.5 mg/kg betamethasone phosphate/acetate given 48 and 24h before delivery. The best lung maturation was observed with the two doses of betamethasone phosphate/acetate. Single doses of the betamethasone phosphate/acetate mixture and betamethasone acetate alone also induced lung maturation. There were no consistent responses to either dose of betamethasone phosphate. The authors conclude that betamethasone phosphate alone is ineffective with the dosing schedule used while betamethasone acetate induces lung maturation. However, the best responses were observed from the mixture of betamethasone phosphate/acetate. While these observations are of great value it is clear from our study that betamethasone phosphate given in adequate doses can be effective in improving pressure volume curves.
Acknowledgments
Supported by HL 068649
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
PAPER PRESENTATION INFORMATION
This research was presented at the 56th Annual Scientific Meeting of the Society for Gynecologic Investigation, Glasgow, United Kingdom, March 17-21, 2009.
Reprints for this article will not be available
Condensation
Half the clinical dose of betamethasone on a weight adjusted basis is as efficacious as the full dose in improving fetal sheep pressure volume curves.
Reference List
- 1.Lorenz JM, Wooliever DE, Jetton JR, Paneth N. A Quantitative Review of Mortality and Developmental Disability in Extremely Premature Newborns. Arch Pediatr Adolesc Med. 1998;152:425–35. doi: 10.1001/archpedi.152.5.425. [DOI] [PubMed] [Google Scholar]
- 2.NIH Consensus development panel on the effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA. 1995;273:413–18. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 3.Leviton LC, Goldenberg RL, Baker CS, Schwartz RM, Freda MC, Fish LJ, et al. Methods to encourage the use of antenatal corticosteroid therapy for fetal maturation: a randomized controlled trial. JAMA. 1999;281:46–52. doi: 10.1001/jama.281.1.46. [DOI] [PubMed] [Google Scholar]
- 4.Tanswell AK, Byrne PJ, Han RN, Edelson JD, Han VK. Limited division of low-density adult rat type II pneumocytes in serum-free culture. Am J Physiol Lung Cell Mol Physiol. 1991;260:L395–L402. doi: 10.1152/ajplung.1991.260.6.L395. [DOI] [PubMed] [Google Scholar]
- 5.Willet KE, McMenamin P, Pinkerton KE, Ikegami M, Jobe AH, Gurrin L, et al. Lung Morphometry and Collagen and Elastin Content: Changes During Normal Development and After Prenatal Hormone Exposure in Sheep. Pediatr.Res. 1999;45(5):615–625. doi: 10.1203/00006450-199905010-00002. Part 1 of 2. [DOI] [PubMed] [Google Scholar]
- 6.Schwab M, Coksaygan T, Samtani MN, Jusko WJ, Nathanielsz PW. Kinetics of Betamethasone and Fetal Cardiovascular Adverse Effects in Pregnant Sheep After Different Doses. Obstet Gynecol. 2006;108:617–25. doi: 10.1097/01.AOG.0000232815.80648.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fowden AL, Forhead AJ. The role of hormones in intrauterine development. In: Barker DJP, editor. Fetal Origins of Cardiovascular and Like Disease. Marcel Decker; New York: 2000. pp. 199–228. [Google Scholar]
- 8.Koenen SV, Mecenas CA, Smith GS, Jenkins S, Nathanielsz PW. Effects of maternal betamethasone administration on fetal and maternal blood pressure and heart rate in the baboon at 0.7 of gestation. Am.J.Obstet.Gynecol. 2002;186:812–17. doi: 10.1067/mob.2002.121654. [DOI] [PubMed] [Google Scholar]
- 9.Antonow-Schlorke I, Schwab M, Li C, Nathanielsz PW. Glucocorticoid exposure at the dose used clinically alters cytoskeletal proteins and presynaptic terminals in the fetal baboon brain. J.Physiol. 2003;547(1):117–23. doi: 10.1113/jphysiol.2002.025700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liggins GC. Premature Delivery of Foetal Lambs Infused with Glucocorticoids. J.Endocrinol. 1969;45:515–23. doi: 10.1677/joe.0.0450515. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz T, Cox LA, Li C, Levine BA, Ford SP, McDonald TJ, et al. Prostaglandin E2 receptor expression in fetal baboon lung at 0.7 gestation after etamethasone exposure. Pediatr.Res. 2007;61(4):421–26. doi: 10.1203/pdr.0b013e318030d141. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert JS, Lang AL, Grant AR, Nijland MJ. Maternal nutrient restriction in sheep: hypertension and decreased nephron number in offspring at 9 months of age. J Physiol (Lond) 2005;565:137–47. doi: 10.1113/jphysiol.2005.084202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chao AC, Ziadeh BI, Diau GY, Wijendran V, Sarkadi-Nagy E, Hsieh AT, et al. Influence of dietary long-chain PUFA on premature baboon lung FA and dipalmitoyl PC composition. Lipids. 2003;38:425–29. doi: 10.1007/s11745-003-1079-8. [DOI] [PubMed] [Google Scholar]
- 14.Crowther CA, Haslam RR, Hiller JE, Doyle LW, Robinson JS. Neonatal respiratory distress syndrome after repeat exposure to antenatal corticosteroids: a randomised controlled trial. The Lancet. 367:1913–19. doi: 10.1016/S0140-6736(06)68846-6. [DOI] [PubMed] [Google Scholar]
- 15.Abbasi S, Hirsch D, Davis J, Tolosa J, Stouffer N, Debbs R, et al. Effect of single versus multiple courses of antenatal corticosteroids on maternal and neonatal outcome. Am.J.Obstet.Gynecol. 2000;182:1243–49. doi: 10.1067/mob.2000.104789. [DOI] [PubMed] [Google Scholar]
- 16.Newnham JP, Moss TJ. Antenatal glucocorticoids and growth: single versus multiple doses in animal and human studies. Seminars in Neonatology. 2001;6:285–92. doi: 10.1053/siny.2001.0064. [DOI] [PubMed] [Google Scholar]
- 17.Sloboda DM, Challis JR, Moss TJ, Newnham JP. Synthetic glucocorticoids: antenatal administration and long-term implications. Curr.Pharm.Des. 2005;11:1459–72. doi: 10.2174/1381612053507873. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q, Wu WX, Ma XH, Nathanielsz PW, Brenna JT. Pulmonary phospholipid saturation increases with glucocorticoid receptor mRNA in late gestation but not due to labor in the fetal rhesus monkey. Prost.Leuko.Ess.Fatty Acids. 1997;57:311–21. doi: 10.1016/s0952-3278(97)90550-0. [DOI] [PubMed] [Google Scholar]
- 19.Rider ED, Jobe AH, Ikegami M, Yamada T, Seidner S. Antenatal betamethasone dose effects in preterm rabbits studied at 27 days gestation. J.Appl.Physiol. 1990;68:1134–41. doi: 10.1152/jappl.1990.68.3.1134. [DOI] [PubMed] [Google Scholar]
- 20.Walfisch A, Hallak M, Mazor M. Multiple Courses of Antenatal Steroids: Risks and Benefits. Obstet Gynecol. 2001;98:491–97. doi: 10.1016/s0029-7844(01)01368-0. [DOI] [PubMed] [Google Scholar]
- 21.French NP, Hagan R, Evans SF, Godfrey M, Newnham JP. Repeated antenatal corticosteroids: Size at birth and subsequent development, . American Journal of Obstetrics and Gynecology. 1999;180:114–21. doi: 10.1016/s0002-9378(99)70160-2. [DOI] [PubMed] [Google Scholar]
- 22.Johnson JW, Mitzner W, Beck JC, LONDON WT, Sly DL, Lee PA, et al. Long-term effects of betamethasone on fetal development. Am.J.Obstet.Gynecol. 1981;141:1053–64. doi: 10.1016/s0002-9378(16)32697-7. [DOI] [PubMed] [Google Scholar]
- 23.Uno H, Eisele S, Sakai A, Shelton S, Baker E, DeJesus O, et al. Neurotoxicity of glucocorticoids in the primate brain. Horm.Behav. 1994;28:336–48. doi: 10.1006/hbeh.1994.1030. [DOI] [PubMed] [Google Scholar]
- 24.Modi N, Lewis H, Al-Naqeeb N, Ajayi-Obe M, Dore CJ, Rutherford M. The effects of repeated antenatal glucocorticoid therapy on the developing brain. Pediatr.Res. 2001;50:581–85. doi: 10.1203/00006450-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Davis EP, Townsend EL, Gunnar MR, Guiang SF, Lussky RC, Cifuentes RF, et al. Antenatal betamethasone treatment has a persisting influence on infant HPA axis regulation. J Perinatol. 2006;26:147–53. doi: 10.1038/sj.jp.7211447. [DOI] [PubMed] [Google Scholar]
- 26.Karemaker R, Kavelaars A, ter Wolbeek M, Tersteeg-Kamperman M, Baerts W, Veen S, et al. Neonatal Dexamethasone Treatment for Chronic Lung Disease of Prematurity Alters the Hypothalamus-Pituitary-Adrenal Axis and Immune System Activity at School Age. Pediatrics. 2008;121:e870–e878. doi: 10.1542/peds.2007-2454. [DOI] [PubMed] [Google Scholar]
- 27.Jobe AH, Moss TJM, Nitsos I, Ikegami M, Kallapur SG, Newnham JP. Betamethasone for lung maturation: testing dose and formulation in fetal sheep. American Journal of Obstetrics and Gynecology. 2007;197:523. doi: 10.1016/j.ajog.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]