Abstract
Our previous data suggested the involvement of matrix metalloproteinase-10 (MMP-10) and cathepsin F (CTSF) in the basement membrane and integrin changes occurring in diabetic corneas. These markers were now examined in normal human organ-cultured corneas upon recombinant adenovirus (rAV)-driven transduction of MMP-10 and CTSF genes.
Fifteen pairs of normal autopsy human corneas were used. One cornea of each pair was transduced with rAV expressing either CTSF or MMP-10 genes. 1–2 × 108 plaque forming units of rAV per cornea were added to cultures for 48 hr with or without sildenafil citrate. The fellow cornea of each pair received control rAV with vector alone. After 6–10 days incubation without rAV, corneas were analyzed by Western blot or immunohistochemistry, or tested for healing of 5-mm circular epithelial wounds caused by topical application of n-heptanol.
Sildenafil significantly increased epithelial transduction efficiency, apparently through stimulation of rAV endocytosis through caveolae. Corneas transduced with CTSF or MMP-10 genes or their combination had increased epithelial immunostaining of respective proteins compared to fellow control corneas. Staining for diabetic markers integrin α3β1, nidogen-1, nidogen-2, and laminin γ2 chain became weaker and irregular upon proteinase transduction. Expression of phosphorylated Akt was decreased in proteinase-transduced corneas. Joint overexpression of both proteinases led to significantly slower corneal wound healing that became similar to that observed in diabetic ones.
The data suggest that MMP-10 and CTSF may be responsible for abnormal marker patterns and impaired wound healing in diabetic corneas. Inhibition of these proteinases in diabetic corneas may alleviate diabetic keratopathy symptoms.
Keywords: diabetic cornea, organ culture, MMP-10, cathepsin F, Akt, sildenafil
Introduction
Diabetes mellitus type I (insulin-dependent, IDDM) and type II (non insulin-dependent, NIDDM) is a systemic disease characterized by hyperglycemia. According to the World Health Organization, in 2000 there were 171 million diabetics worldwide, which is likely to double by 2030 [50]. Diabetic eye complications referred to as retinopathy (DR) are generally considered as a disease of retinal microvasculature. DR affects at least 50% of type II diabetics and over 80% of type I diabetics after 15–20 years of disease [25]. Retinal changes contribute most significantly to vision loss, but other parts of the eye (cornea, lens, optic nerve, and iris) are also affected. According to the available data, up to 70% of diabetics suffer from corneal problems [13,49], although they are rather rarely diagnosed [23]. Clinically, delayed and abnormal epithelial wound healing, ulcers, edema, recurrent erosions, superficial punctate keratitis, abnormalities of the endothelium and corneal nerves are major manifestations of diabetic corneal disease [8,12,18,39,47,51].
It has been long recognized that diabetic corneal changes involved alterations of the epithelial basement membrane (BM) and of its interactions with corneal epithelium. These changes include increased BM fragility [2,16,18], decreased number of hemidesmosomes [2,54], altered epithelial adhesion, and BM reassembly after wounding [48]. Glycation of BM components [23], abnormalities of the opioid growth factor-receptor axis [33] and other growth factors and mediators [42,43,45,52] could contribute to diabetic corneal abnormalities. Our previous studies identified decreased immunostaining of specific BM components including laminins and entactin/nidogen-1 as well as of a laminin receptor, integrin α3β1 in corneal epithelial BM [29]. These alterations were likely due to increased expression of specific proteinases, MMP-10 and cathepsin F, identified using immunohistochemistry, RT-PCR and gene microarrays [42,45]. The described diabetic changes could be readily reproduced in corneal organ cultures [22,45], apparently because of the diabetic memory phenomenon [57].
In the present paper the goal was to obtain a functional proof of principle for the involvement of these proteinases in diabetic corneal changes. To this end, normal corneas were transduced with recombinant adenoviruses (rAV) harboring full-length genes coding for MMP-10 and cathepsin F, and the expression of diabetes-associated markers and wound healing rates were examined. It is shown that overexpression of these two proteinases in normal corneas brings the patterns of diabetic markers and wound healing rates close to the diabetic ones. It may be thus suggested that MMP-10 and cathepsin F contribute to the abnormalities seen clinically in corneas of diabetic patients.
Material and Methods
Cell and organ culture
Human glioma U87MG, human embryonic kidney (HEK293), and Chinese hamster ovary (CHO) cell lines used for testing and optimizing rAV transduction were from the American Type Culture Collection (Rockville, MD). 293A cell line used for rAV production was from Invitrogen (Carlsbad, CA). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen) or a mixture of DMEM-Ham’s F-12 (1:1) with 10% fetal calf serum, L-glutamine, antibiotics, and sodium pyruvate, in a humidified atmosphere with 5% CO2 at 37°C.
Non-diabetic human autopsy corneas or whole globes were purchased from the National Disease Research Interchange (NDRI, Philadelphia, PA). NDRI human tissue collection protocol is approved by a managerial committee and is under National Institutes of Health oversight. A total of 31 corneas (Table 1) from 16 donors (4 females, 12 males, mean age 63.3 ± 15.5 years; one donor with insulin-dependent diabetes) were used. The whole corneas were cultured over agar-collagen gel [22] in 60-mm Petri dishes (BD Biosciences, Franklin Lakes, NJ), at a liquid-air interface (with medium covering the limbus), in serum-free medium with insulin-transferrin-selenite, antibiotics and antimycotic agent (Invitrogen, Carlsbad, CA). Two-three drops of medium were added one-two times a day to the top of the corneas for moistening the epithelium.
Table 1.
Donor characteristics
| Case number | Age | Gender | Cause of death | Culture treatment |
|---|---|---|---|---|
| 07-25 | 47 | F | natural causes | CF |
| 07-29 | 66 | M | sub-arachnoid bleed | M10 |
| 07-31, IDDM | 52 | M | sudden death during surgery | CF |
| 07-28 | 49 | M | heart disease | M10 |
| 08-45 | 64 | M | multi-organ failure | M10+CF |
| 08-58 (1) | 61 | F | congestive heart failure | GFP |
| 08-60 | 94 | M | cardiac arrest | M10+CF |
| 08-62 | 79 | M | myocardial infarction | GFP |
| 09-2 | 41 | F | brain tumor | M10+CF |
| 09-4 | 69 | M | metastatic cancer | M10+CF |
| 09-9 | 61 | M | congestive heart failure | M10+CF |
| 09-10 | 80 | M | heart disease | GFP |
| 09-15 | 76 | M | cardiac arrest | M10+CF |
| 09-18 | 75 | M | respiratory failure | M10+CF |
| 09-19 | 62 | F | heart disease | M10+CF |
| 09-20 | 37 | M | electrocution | M10+CF |
M10, MMP-10; CF, cathepsin F; M, male; F, female; IDDM, type I diabetes. In case 08-58 one cornea was analyzed.
Culture treatments
25-mg sildenafil citrate tablets (Viagra®; Pfizer Corp., New York, NY) were crushed into powder and dissolved in culture medium to a concentration of 3.5 mg/ml. The stock solution was centrifuged and sterile-filtered through 0.2 µm syringe filters (Pall Corp., Ann Arbor, MI). Fresh sildenafil (half-life 3.5 hrs [59]) was added twice to the corneas at 75 µg/ml during the first six hours of adenoviral transduction that lasted 48 hr. Stock solution (10 mg/ml) of filipin III (Sigma-Aldrich, St. Louis, MO), a specific inhibitor of caveolae endocytosis pathway, was prepared in dimethylsulfoxide (DMSO) and was stored in −20°C. It was added to organ-cultured corneas in fresh medium (1 µl/2 ml; final concentration 5 µg/ml) together with sildenafil citrate during viral transduction. Control organ-cultured corneas received sildenafil citrate and DMSO (1 µl/2 ml).
Epithelial wound healing
Epithelial debridement was performed by placing a 5-mm paper disc soaked in n-heptanol on the central corneal surface for one min [10,22].Contrary to mechanical debridement, n-heptanol does not disrupt fragile diabetic epithelial BM [16]. Both healing corneas, target gene and control treated, were photographed every 24 hr until the epithelial defect was completely healed and healing time recorded. After healing, corneas were cut in half, one half embedded in OCT compound (Ted Pella, Inc., Redding, CA) and processed for indirect immunofluorescence on cryostat sections, and the other half or the epithelium was frozen in liquid nitrogen for Western blot analysis.
Adenoviruses
The used rAV were E1/E3-deleted type 5 with genes of interest under the control of major immediate early cytomegalovirus promoter [28]. They expressed full-length open reading frames (ORF) of genes coding for matrix metalloproteinase-10 (rAV-MMP10) or cathepsin F (rAV-CTSF), or green fluorescent protein (rAV-GFP; positive control), or an empty vector as a negative control. All viruses were generated based on the Gateway recombination technology as per manufacturer’s instructions.
Briefly, the human MMP-10 and CTSF Ultimate™ full-length ORF clones in Gateway® entry vector (pENTR™221, Invitrogen) were transferred into rAV vectors (pAd/CMV/V5-DEST) using the ViraPower™ Adenoviral Gateway® Expression Kit and LR Clonase™ II enzyme mix (Invitrogen). pAd/CMV/V5/lacZ is included with each kit for use as a positive control in the ViraPower™ adenoviral expression system (rAV-gal). The reaction mixtures were transformed into DH5™ chemically competent E. coli to select for expression clones. The selected expression clones were sequenced at the UCLA core facility to confirm the correct orientation of the ORFs. The Pac I-digested vectors were used to transfect 293A cells to produce rAV stocks. The rAV were amplified by infecting the 293A producer cells with the crude viral lysates and purified using Vivapure® AdenoPACK™ kit (Sartorius Stedim SUS, Concord, CA). The titers of rAV stocks were twice determined using HEK-293 cells as described [53].
Viral transduction
Cell lines U87MG and CHO were first transduced with the rAV vectors expressing target genes to confirm their overexpression. Non-diabetic organ-cultured corneas were transduced with rAV-CTSF or rAV-MMP10 constructs; in some experiments both constructs were used simultaneously. The other cornea of each pair was treated with control rAV-gal or rAV-vector. The rAV particles were given at 1–2 × 108 plaque forming units (pfu) per cornea along with 75 µg/ml sildenafil citrate in culture medium for 48 hr. rAV-GFP was added to the corneas at five or 15 µl of a stock solution containing 5–15 × 107 pfu/µl. To ensure better transduction, corneas were kept under the medium in 24-well plates (BD Biosciences) for the whole period of incubation with rAV. Corneas were then transferred to new dishes with a Corning round end spatula (Fisher Scientific, Pittsburgh, PA) and cultured in medium without rAV. After the additional four to eight days at the liquid-air interface, rAV-treated corneas were processed or tested for healing (see below). Marker expression was analyzed by immunohistochemistry and Western blot.
Immunohistochemistry and Western blot analysis
These were performed as described before [26,45]. The antibodies used and their reactivities in different assays are presented in Table 1. For each marker of fellow corneas, the same exposure was used when photographing stained sections of a vector-transduced one and a proteinase gene-transduced one. For Western blots, 8–16% gradient Tris-glycine SDS polyacrylamide gels were used (Invitrogen). Gel loading was normalized by β-actin content [26] (Table 2).
Table 2.
Antibodies used in this study
| Antigen | Antibody & Cat. No. | Works in | Source/reference |
|---|---|---|---|
| Cathepsin F | goat pAb sc-9633 | IHC# | Santa Cruz Biotechnology |
| Cathepsin F | goat pAb AF2075 | IHC | R&D Systems |
| Cathepsin F | rabbit pAb sc-13987 | WB | Santa Cruz Biotechnology |
| Cathepsin F | rabbit pAb 11055-1-AP | IHC# | Proteintech Group |
| Cathepsin F | mouse mAb sc-73774 | Santa Cruz Biotechnology | |
| MMP-10 | goat pAb sc-9941 | IHC | Santa Cruz Biotechnology |
| MMP-10 | mouse mAb MAB9101 | R&D Systems | |
| MMP-10 | mouse mAb MAB9102 | R&D Systems | |
| MMP-10 | goat pAb sc-26694 | IHC# | Santa Cruz Biotechnology |
| MMP-10 | rabbit pAb 29580 | IHC | Anaspec |
| MMP-10 | mouse mAb NCL-MMP10 | WB | Novocastra |
| Nidogen-1 | mouse mAb A9 | IHC | [21] |
| Nidogen-1 | mouse mAb MAB2570 | IHC | R&D Systems |
| Nidogen-2 | rabbit pAb 1080 | IHC | [21] |
| Laminin γ1 chain | rat mAb A5 | IHC | [21] |
| Laminin γ2 chain | mouse mAb D4B5 | IHC | Millipore |
| Integrin α3β1 | mouse mAb MAB1992 | IHC | Millipore |
| Akt | mouse mAb 610860 | WB | BD Transduction Labs |
| p-Akt (Ser473) | rabbit pAb sc-7985-R | IHC, WB | Santa Cruz Biotechnology |
| p-Akt (Ser473) | rabbit pAb 9271 | IHC, WB | Cell Signaling |
| ERK1/2 | rabbit mAb 4695 | IHC, WB | Cell Signaling |
| p-ERK1/2 (Thr202/Tyr204) | rabbit mAb 4370 | WB | Cell Signaling |
| p-ERK1/2 (Thr185/Thr202) | rabbit pAb ab4819 | IHC | Abcam |
| p38 MAPK | rabbit pAb 9212 | WB | Cell Signaling |
| p-p38 (Thr180/Tyr182) | rabbit pAb AB3828 | WB# | Millipore |
| p-p38 (Thr180/Tyr182) | rabbit mAb 9215 | WB# | Cell Signaling |
| p-p38 (Thr180/Tyr182) | mouse mAb ab50012 | IHC | Abcam |
| p-EGFR (Tyr845) | rabbit pAb 44-784G | IHC | Invitrogen |
| p-EGFR (Tyr845) | rabbit mAb 2342-1 | Epitomics | |
| ZO-1 | rabbit pAb 40-2300 | IHC^ | Invitrogen |
| Claudin-1 | rabbit pAb 51-9000 | IHC^ | Invitrogen |
| Clathrin heavy chain | mouse mAb 610499 | IHC* | BD Transduction Labs |
| Caveolin-1 | rabbit pAb 610059 | IHC | BD Transduction Labs |
| Ki-67 | mouse mAb sc-101861 | IHC* | Santa Cruz Biotechnology |
| Activated caspase-3 | rabbit pAb G7481 | IHC | Promega |
| β-actin | mouse mAb A5316 | WB | Sigma |
mAb, monoclonal antibody; pAb, polyclonal antibody; IHC, immunohistochemistry; WB, Western blot; p-, phosphorylated;
weak reactivity;
acetone fixation needed;
95% ethanol followed by acetone fixation needed
Statistical analysis
Data were analyzed using InStat 3 software program (GraphPad Software, San Diego, CA). Wound healing rates in pairs of control and proteinase-transduced fellow corneas were analyzed by paired two-tailed t test. Comparison with previous data obtained on organ-cultured normal and DR corneas [22] was done using unpaired two-tailed t test, Welch corrected. P<0.05 was considered significant.
Results
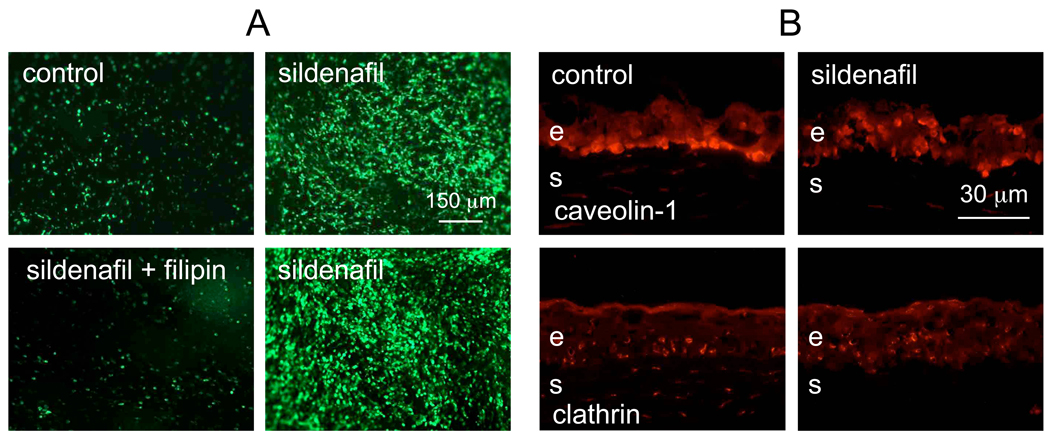
It was previously shown that vascular endothelial growth factor and inhibitors of phosphodiesterase 5 (PDE5) increased rAV delivery to the myocardium, presumably by enhancing vascular permeability via increased nitric oxide and cGMP [36]. A recent study using brain tumors showed that PDE5 inhibitors increased vascular permeability and adriamycin delivery through brain blood barrier [4]. We hypothesized that PDE5 inhibitors could also increase epithelial permeability to the rAV and tested this in organ-cultured corneas. As shown in Fig. 1A (left top row), addition of sildenafil during rAV-GFP infection of corneas did indeed significantly increase the number of GFP-positive epithelial cells. Because of the previous suggestion that PDE5 inhibitors may modulate endocytosis, the corneas treated with rAV-GFP with or without sildenafil were stained for the components of major endocytotic pathways. Distribution of clathrin (major protein of coated pits) did not appear to change in the epithelium upon sildenafil treatment (Fig. 1B, bottom row). Some decrease of stromal staining was variable and may not have contributed to the sildenafil effect because rAV in organ-cultured corneas do not transduce stromal cells [28]. However, caveolin-1 (major component of caveolae), which was predominantly seen in the basal epithelial cells in control corneas in accordance with previous data [1], changed its distribution upon sildenafil treatment with many suprabasal cells also becoming positive (Fig. 1B, top row). To test caveolae involvement more rigorously, corneas transduced with rAV-GFP were treated either with sildenafil or sildenafil with filipin III, an inhibitor of caveolae-mediated endocytosis. Filipin completely abrogated the effect of sildenafil on viral transduction (Fig. 1A, bottom row). These data suggested that sildenafil acted by predominantly stimulating caveolae-dependent rAV endocytosis.
Figure 1.
Effect of sildenafil on rAV transduction of organ-cultured corneas. A, live corneas. Top row: left panel, cornea transduced with rAV-GFP, 6 days after the addition of the virus; right panel, a fellow cornea transduced with the same virus in the presence of 75 µg/ml sildenafil. Note significant increase in transduction efficiency. Bottom row: left panel, addition of caveolae-dependent endocytosis inhibitor, filipin III (right panel), completely abolished the effect of sildenafil (left panel) on rAV-GFP transduction. The data suggest the importance of caveolae endocytosis in sildenafil-mediated increase in rAV transduction efficiency. Pictures of live corneas are shown. Scale bar = 150 µm.
B, immunofluorescence for markers of major endocytotic pathways. Top row: left panel, staining for caveolin-1 is most pronounced in the basal epithelial layer of a control cornea; right panel, after sildenafil incubation of a fellow cornea, caveolin-1 becomes also prominent in suprabasal layers. Bottom row: left panel, clathrin immunoreactivity is seen in basal and suprabasal cells in a control cornea; right panel, addition of sildenafil does not alter clathrin pattern in the epithelium of a fellow cornea. Pictures of corneal sections are shown. E, epithelium; s, stroma. Scale bar = 30 µm.
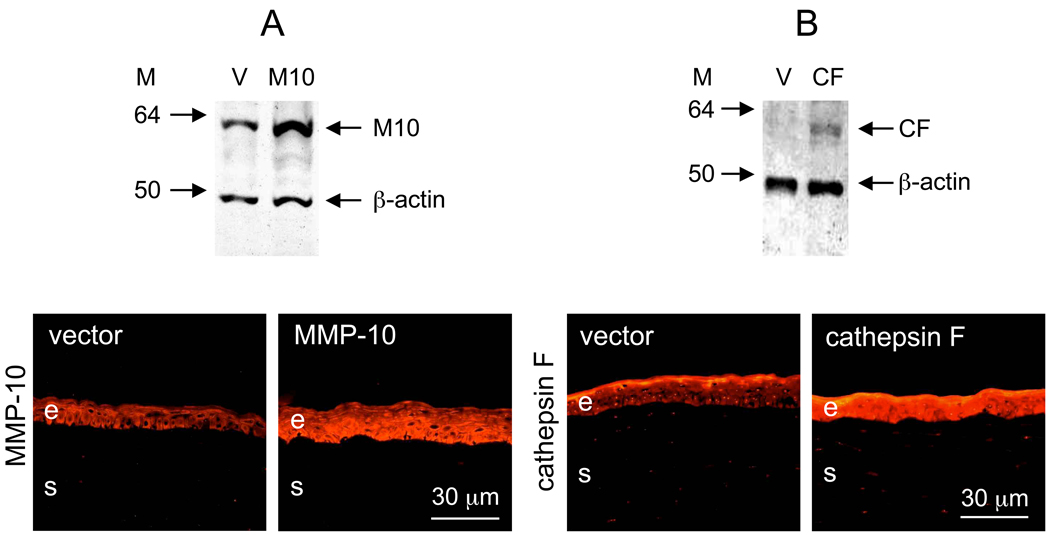
Before studying the effects of MMP-10 and cathepsin F transduction in human corneas, a confirmation of their overexpression was needed. Preliminary experiments with cells lines established this overexpression for both genes (data not shown here), and then the viruses were used in corneal organ cultures. Fairly efficient transduction of organ cultures could be achieved already within 24 hr when sildenafil was present (data not shown here) but without it, 48 hr ensured higher efficiency. Therefore, since some earlier experiments were done without sildenafil, 48 hr incubation time with the viruses was kept for all corneas. No deleterious effects were observed following this prolonged incubation with rAV and corneas always looked healthy and transparent. As shown in Fig. 2, both rAV-MMP10 (Fig. 2A) and rAV-CTSF (Fig. 2B) increased the expression of the transduced proteins as revealed by Western blot analysis of whole corneal lysates (top panels) and immunostaining of corneal sections (bottom panels). Staining for both proteins primarily increased in the epithelial cells. MMP-10 showed mostly cytoplasmic staining. Cathepsin F displayed cytoplasmic but also nuclear staining with several antibodies, in accordance with previous data [32].
Figure 2.
Overexpression of MMP-10 and cathepsin F after rAV transduction of respective genes. A, MMP-10 (M10); B, cathepsin F (CF). Top panel: Western blots; bottom panel: immunofluorescence on corneal sections (A, stained for M10; B, stained for CF). V, transduction with vector alone (rAV-vector). Note increased expression of M10 and CF after rAV transduction compared to vector alone by both methods. M, markers in kDa. The gel loading was normalized by β-actin content [26]. Bar = 30 µm.
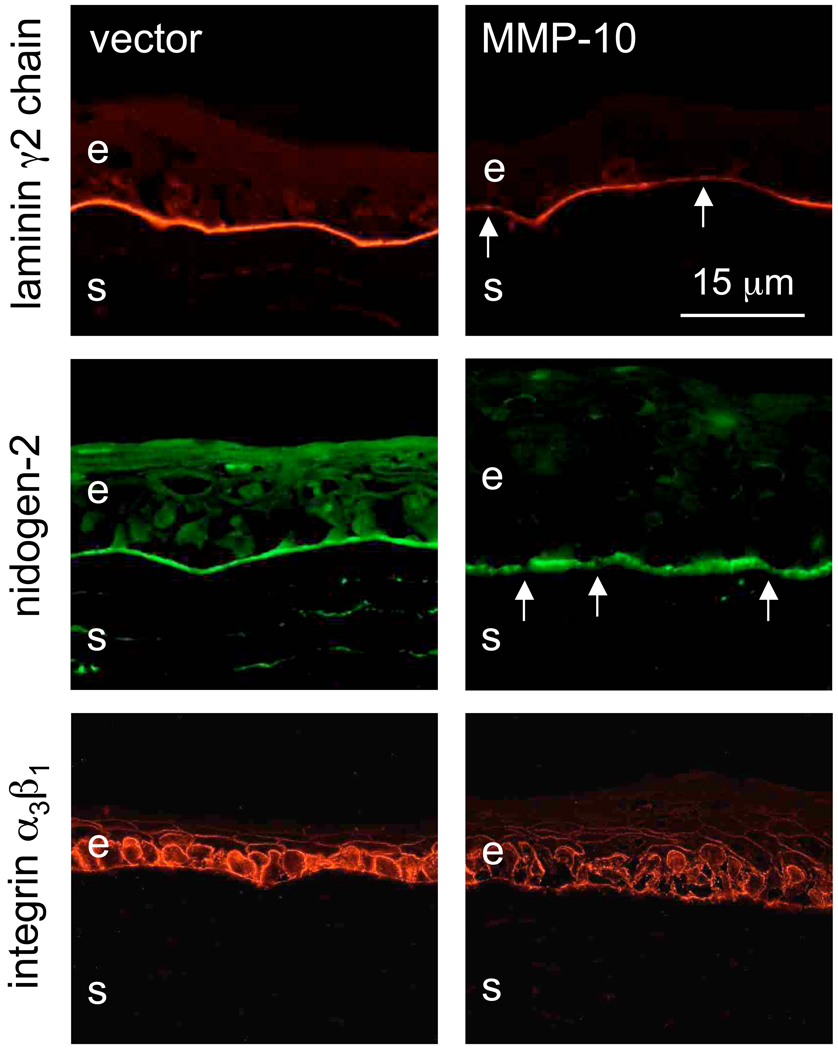
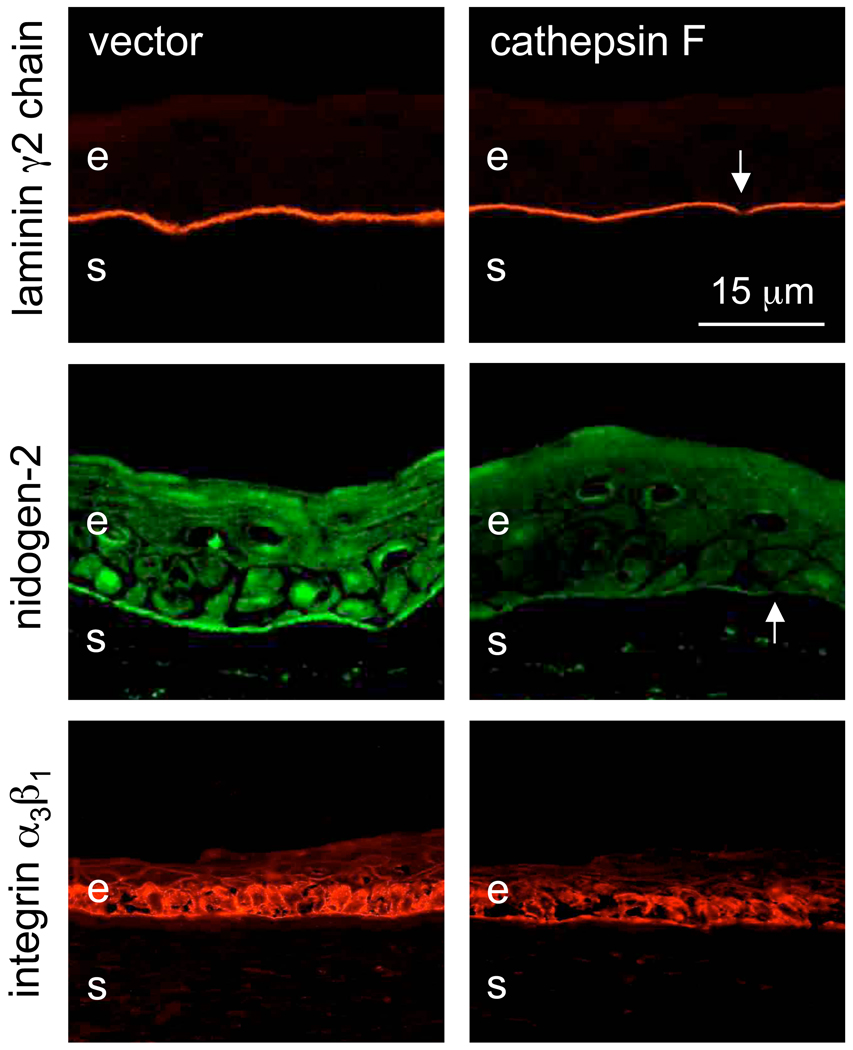
Upon rAV-MMP10 transduction, there were changes in the patterns of diabetic corneal markers compared to rAV-vector. Staining for basement membrane laminin-332 γ2 chain, nidogen-2 (Fig. 3) and nidogen-1 (data not shown here) became weak and/or discontinuous, which was similar to diabetic corneas [22,29]. Similarly, the staining for laminin receptor, integrin α3β1, became weaker and discontinuous. Essentially the same results were obtained following cathepsin F overexpression (Fig. 4), and when both proteinase genes were transduced together (data not shown here).
Figure 3.
Altered expression of diabetic markers after rAV-MMP10 transduction. Top row: laminin γ2 chain, middle row: nidogen-2. Note discontinuous (arrows) and markedly weaker (γ2 chain) staining of the epithelial basement membrane for both markers upon MMP-10 transduction. Bottom row: integrin α3β1. Note decreased and discontinuous staining upon MMP-10 transduction. E, epithelium; s, stroma. Immunofluorescence. Bar = 15 µm.
Figure 4.
Altered expression of diabetic markers after rAV-CTSF transduction. Top row: laminin γ2 chain, middle row: nidogen-2. Note markedly decreased (nidogen-2) and discontinuous (arrows) staining of the epithelial basement membrane for both markers after cathepsin F transduction. Bottom row: integrin α3β1. Note disorganized and weaker staining upon cathepsin F transduction. E, epithelium; s, stroma. Immunofluorescence. Bar = 15 µm.
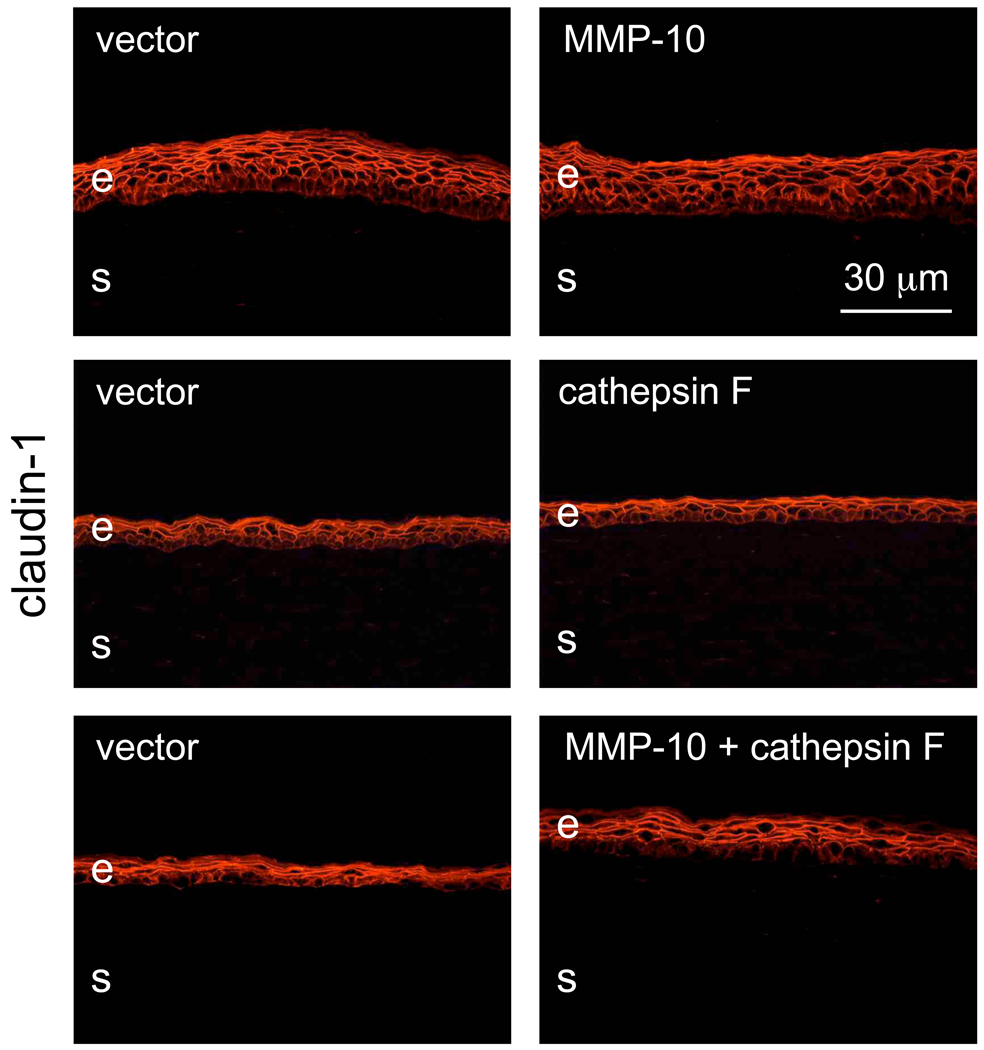
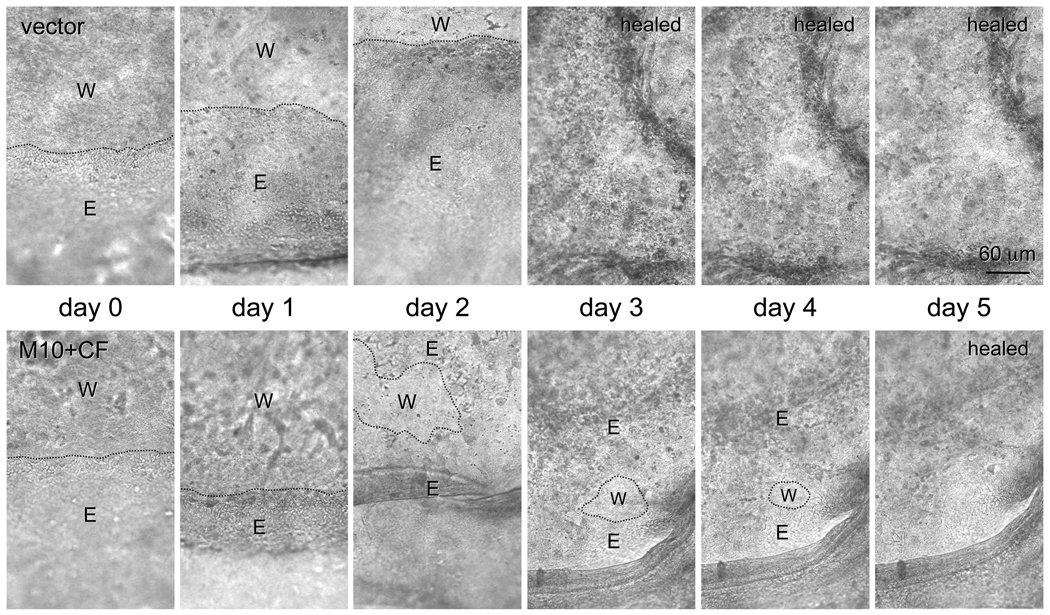
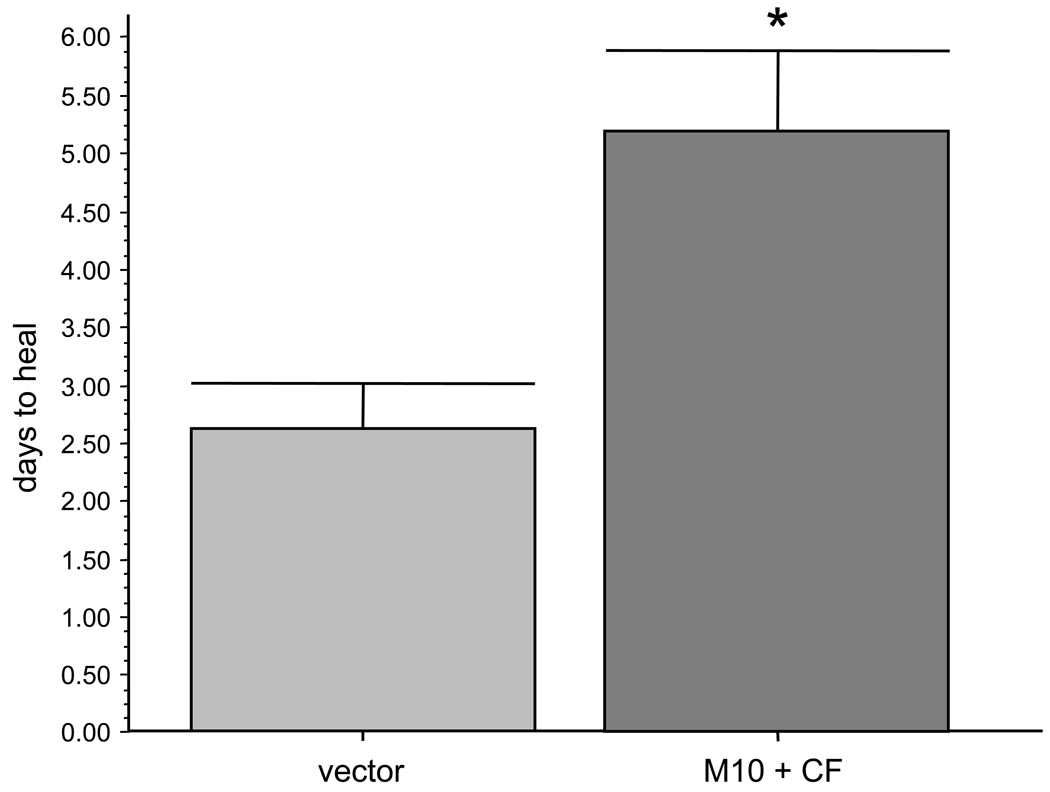
Major tight junction proteins, claudin-1 (Fig. 5), the main claudin type expressed in the corneal epithelium [61], and ZO-1 [3,19,58], did not reveal any staining pattern changes upon proteinase gene transduction. These data suggested that no significant compromise of epithelial barrier function occurred following proteinase transduction. As a physiological test for proteinase effects in organ-cultured corneas, wound healing dynamics was examined. Overexpression of either MMP-10 or cathepsin F did not produce a significant delay in wound healing compared to control. However, when viruses harboring genes for both proteinases were combined, transduced corneas healed significantly slower compared to vector controls. A typical time course picturing live corneas is shown on Fig. 6. On average, corneas transduced with rAV-MMP10 + rAV-CTSF healed twice as slowly as the fellow corneas transduced with rAV-vector (n=5, Fig. 7). Proteinase gene-transduced corneas healed on average in 5.2 ± 0.7 days (mean ± standard error) compared to 2.6 ± 0.4 days for vector controls (p<0.003). Comparison with our previous data on normal and DR corneas [22] showed that vector-transduced corneas healed similarly to normal corneas (2.6 days on average vs. 2.3 days, p>0.05, non-significant) and significantly faster than DR corneas (2.6 days vs. 4.5 days, p<0.04). Combined proteinase transduction made healing slower than in normal corneas (5.2 days vs. 2.3 days, p<0.02) effectively decreasing it to DR rates (5.0 days vs. 4.5 days, p>0.05, non-significant). It should be noted that time of wounding was important to show an effect. If wounds were made 3–6 days after transduction, the healing rates were not significantly changed in some experiments. More consistent results were obtained with wounds made 10 days after transduction or when the first wounds had been healed (within 4–5 days) and wounding was repeated. This could be due to a possible need for a certain time of proteinase action before functionally significant degradative changes occurred in the basement membrane and epithelial integrins. Wound healing delay upon proteinase transduction was more evident when the wounds became smaller, usually after the first two days (Fig. 6). During wound healing, the number of proliferating cells revealed by Ki-67 staining did not change upon proteinase transduction. Cells expressing activated caspase 3 were extremely rare in control or proteinase-transduced corneas (data not shown here).
Figure 5.
Staining of transduced corneas for a tight junction component claudin-1. Transduction with rAV-MMP10, rAV-CTSF or their combination does not cause any changes in claudin-1 patterns compared to rAV-vector (vector). E, epithelium; s, stroma. Immunofluorescence. Bar = 30 µm.
Figure 6.
Representative pictures of live healing corneas. One cornea was transduced with rAV-vector (vector), whereas the fellow corneas was transduced by rAV-MMP10 + rAV-CTSF (M10+CF). Note slower healing upon proteinase gene transduction (5 days) compared to rAV-vector (3 days), especially when the wound became smaller. E, epithelium; W, wound. Dotted lines denote wound margins. Day 0, the day when the wounds are made. Bar = 60 µm.
Figure 7.
Quantitation of healing in transduced corneas. Transduction with rAV-MMP10 + rAV-CTSF (M10+CF) slows down wound healing twofold compared to rAV-vector (vector). * p<0.003 by paired two-tailed t test.
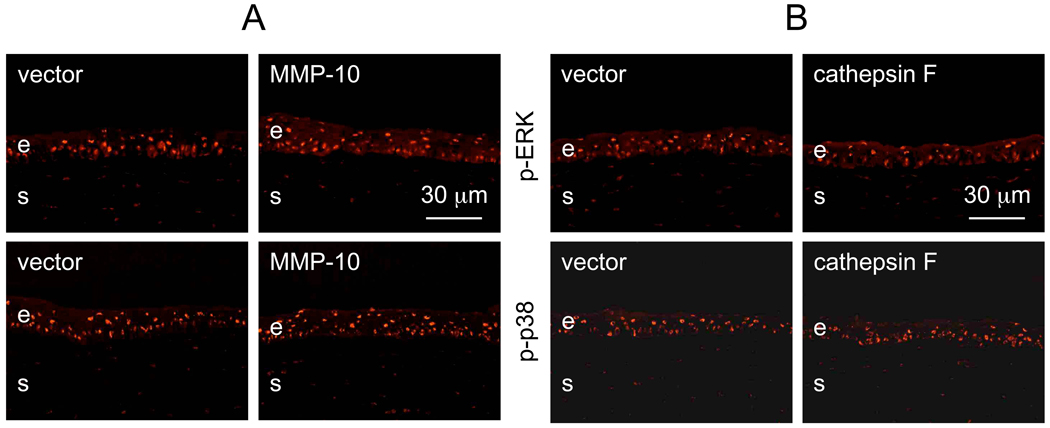
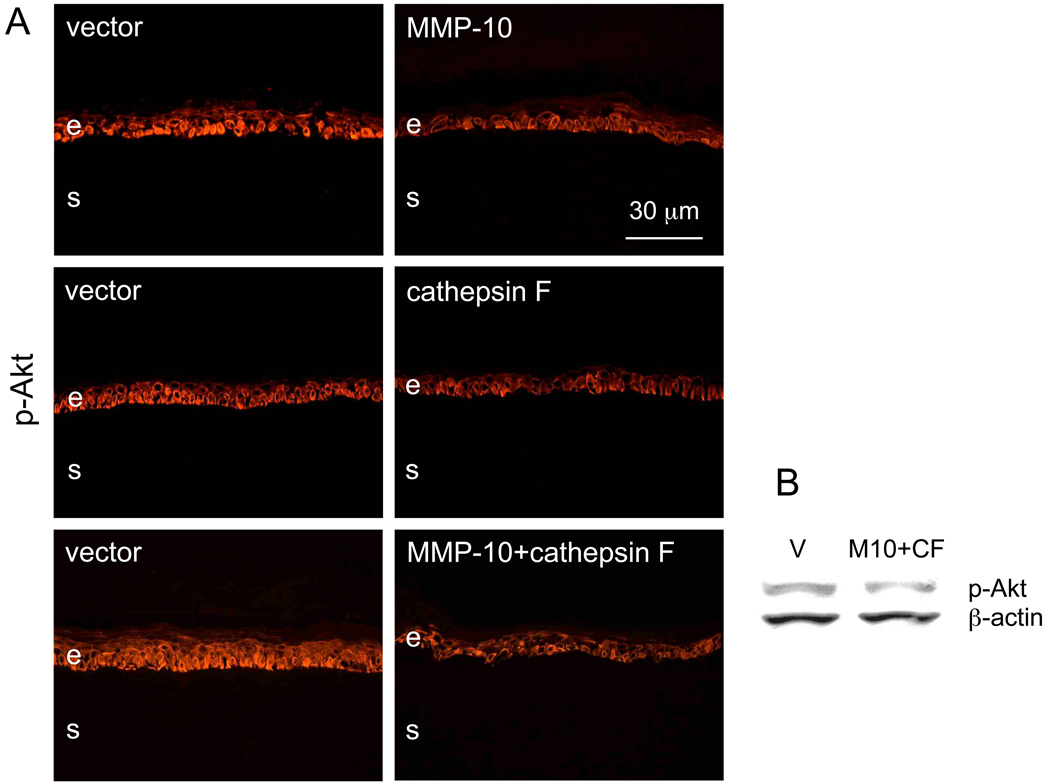
In order to gain insight into the possible mechanisms of the observed changes, patterns of major signaling intermediates in corneal wound healing were examined following proteinase gene transduction. No changes could be seen in the patterns of phosphorylated extracellular regulated kinase (p-ERK) or p38 mitogen-activated protein (MAP) kinase (p-p38) upon transduction of MMP-10, or cathepsin F (Fig. 8), or both together (data not shown here). However, overexpression of each proteinase alone or their combination led to decreased expression of phosphorylated Akt protein kinase (p-Akt) in the epithelial cells. Results were obtained by immunostaining (Fig. 9A) or Western blotting (Fig. 9B). It should be noted that immunostaining demonstrated the diabetic-like decrease in p-Akt [60] in non-wounded corneas or in those that had been wounded and then completely healed (6/7 cases). However, Western blot analysis was able to reveal decreased p-Akt expression only when corneas were actively healing epithelial wounds.
Figure 8.
Patterns of phosphorylated ERK (p-ERK) and p38 (p-p38) kinases in transduced corneas. A, MMP-10 transduction; B, CTSF transduction. There is no significant difference between corneas transduced with MMP-10 or CTSF compared to vector alone. E, epithelium; s, stroma. Immunofluorescence. Bar = 30 µm.
Figure 9.
Phosphorylated Akt (p-Akt) kinase in transduced corneas. A: immunofluorescent staining of corneal sections. Upon transduction with rAV-MMP10, rAV-CTSF or their combination, the epithelial staining for p-Akt (using pAb 9271) is noticeably decreased and becomes less regular in the basal cell layer. B: Western blot analysis. Upon transduction with rAV-MMP10 + rAV-CTSF, p-Akt is decreased as revealed by pAb 9271; pAb sc-7985-R gave the same result (data not shown here). The gel loading was normalized by β-actin content [26]. E, epithelium; s, stroma; V, vector; M10, MMP-10; CF, cathepsin F. Bar = 30 µm.
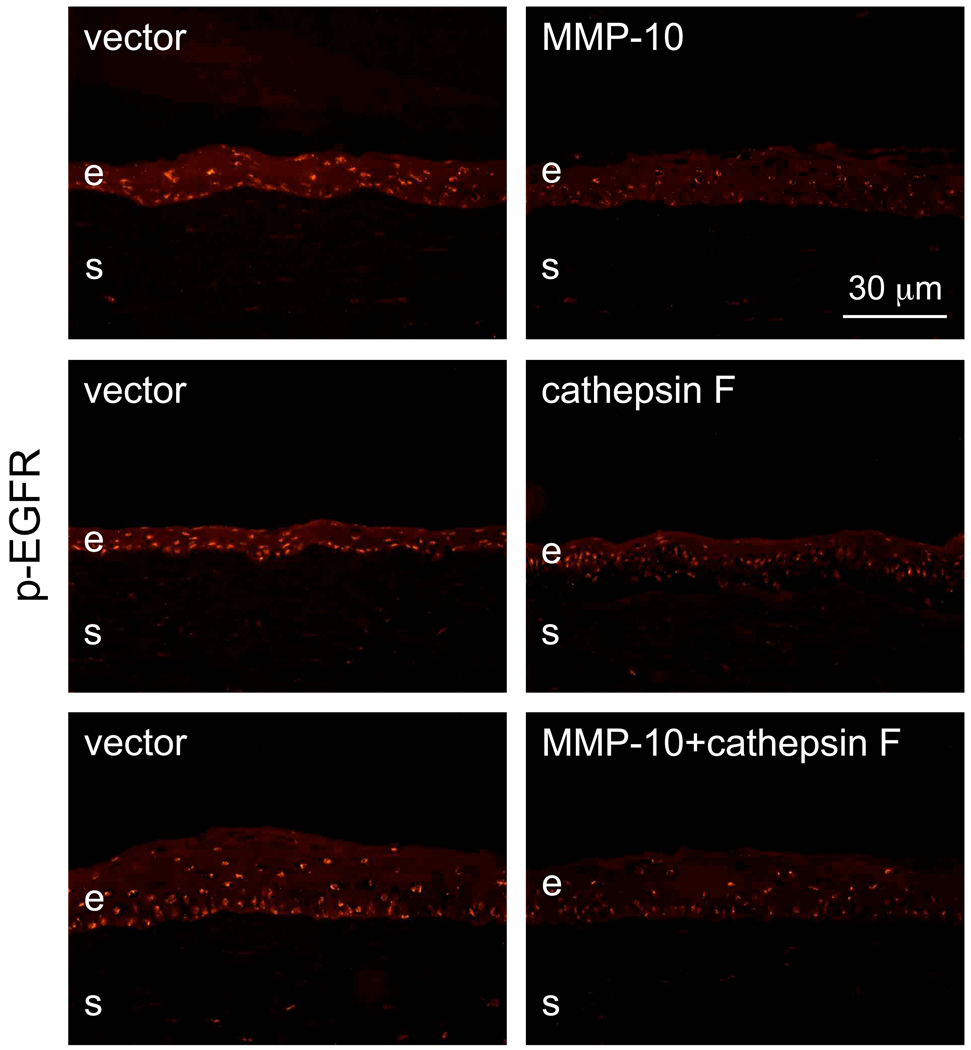
Because Akt is important for wound healing induced by epidermal growth factor (EGF) signaling [60] through its receptor tyrosine kinase (EGFR), we hypothesized that p-Akt decrease might be due to reduced activity of EGFR. Therefore, the expression of phosphorylated (activated) EGFR (p-EGFR) was also examined. As shown in Fig. 10, p-EGFR staining was indeed decreased in corneas overexpressing MMP-10, cathepsin F or both proteinases (6/7 cases) compared to vector-transduced fellow corneas.
Figure 10.
Staining patterns of transduced corneas for phosphorylated EGFR (p-EGFR). Note decreased epithelial nuclear staining (both by intensity and number of positive cells) upon transduction with rAV-MMP10, rAV-CTSF or their combination. E, epithelium; s, stroma. Immunofluorescence. Bar = 30 µm.
Discussion
Major diabetic corneal complications including keratopathy and neuropathy are a recognized clinical problem. However, patients with clinically manifested corneal diabetes only receive topical symptomatic treatment including artificial tears, autologous serum possibly supplying adhesive and growth factors for healing, contact lenses as a corneal bandage, punctal occlusion to increase tear production, topical antibiotics to control infection in case of persistent epithelial defects [7,23,46]. Naltrexone (opioid growth factor antagonist), insulin, aldose reductase inhibitors, and substance P have been tested with varying degree of success for alleviating diabetic keratopathy, but they are still in various phases of preclinical and clinical testing [33,35,37].
Overall, there is a clear need for a more specific treatment approach for diabetic keratopathy, such as gene therapy targeting abnormally expressed corneal proteins. Corneal tissue is easily accessible to various vectors, is immune privileged, and in case of diabetes may need only a rather transient gene expression change, e.g., for accelerating of epithelial wound healing. All these qualities make cornea a very promising target for gene therapy [24,35].
We have exploited this approach using adenoviral vectors. Our previous studies have shown that diabetic corneas abnormally express several epithelial markers including specific proteinases [42,45]. Contrary to adeno-associated viruses, rAV has an advantage of transducing epithelium but not keratocytes in corneal organ culture [28] and was chosen as a gene therapy vector because of this property and its high transduction efficiency. In the present study genes coding for proteinases altered in diabetic corneas, MMP-10 and cathepsin F, were transduced into normal human organ-cultured corneas using rAV vectors. We then tested whether their overexpression would bring about diabetic-like changes in marker protein expression and wound healing. The experiments aimed at establishing functional importance of MMP-10 and cathepsin F in diabetic corneal changes.
Corneal epithelial cell transduction with rAV has a rather low efficiency at clinically relevant incubation times necessitating long exposure; in this work 48 hrs period was used to obtain a functional effect. Therefore, there was a need to optimize rAV transduction, with increased efficacy at shorter incubation times. Previous work suggested that rAV-mediated gene transfer into myocardium was facilitated by PDE5 inhibitors [36], which also enhanced drug transport across tight-junction-controlled blood brain barrier [4]. Since we used whole corneas with epithelium containing tight junctions and rAV as a delivery vector, we tested sildenafil as the most water-soluble PDE5 inhibitor for possible increase in gene delivery to corneal cells. As expected, this reagent dramatically increased rAV-mediated gene transduction into organ-cultured corneas monitored by GFP expression. Using sildenafil it was also possible to reduce transduction time to 24 hrs still maintaining fairly high transduction efficiency.
Adenovirus usually uses clathrin-dependent coated pit pathway for cell entry [9]. However, in some cells it can also use caveolin-dependent lipid raft mechanism [9,38]. Clathrin staining of sections of corneas incubated with sildenafil did not reveal marked changes in expression or distribution. At the same time, in control corneas caveolin-1 staining was mainly observed in basal epithelial cells in accordance with previous data [1], but it was also seen in suprabasal cells after sildenafil reatment suggesting activation of this endocytotic pathway. This assumption was corroborated by incubating corneas with sildenafil in the presence of a specific caveolae-dependent pathway inhibitor, filipin. It dramatically reduced sildenafil-enhanced rAV-driven GFP transduction into cornea to levels below control (Fig. 1). These data strongly suggested that stimulation of caveolae-dependent endocytosis was the likely mechanism of sildenafil effect on rAV-mediated gene transfer to cornea. Our data, however, cannot completely exclude the involvement of clathrin-dependent pathway in adenoviral gene transfer and sildenafil effect on it.
Proteinase transduction apparently did not compromise epithelial barrier function as suggested from lack of changes in tight junction protein patterns. At the same time, overexpression of both proteinases in normal corneas (confirmed by Western blot and immunofluorescent staining) led to a change in the patterns of several markers [22] towards those observed in diabetic corneas. In particular, integrin α3β1 staining became weak and disorganized. Basement membrane components, nidogens (Fig. 3, Fig. 4) and laminin β1 chain (data not shown here) also showed discontinuous patterns compared to rAV-vector transduced fellow corneas. For a secreted enzyme MMP-10 that can degrade basement membrane and extracellular matrix proteins [5,56], these data agree well with previous results on skin wound healing [27]. Overexpression of constitutively active MMP-10 mutant in skin also led to the disruption of laminin-5 (now called laminin-332) apparently by direct proteolysis of γ2 chain, and disorganized staining for β1 chain-containing integrins. Although cathepsins are generally considered as lysosomal proteinases, accumulating evidence suggests that they may also exert their action outside of the cell and digest extracellular matrix [40]. Cathepsins including cathepsin F were found at sites of Bowman’s layer rupture in keratoconus corneas [6], in corneas with keratectasia after failed LASIK procedure [30], and in the corneal stroma around implanted intracorneal rings [31]. Moreover, cathepsin F secretion was observed in macrophages stimulated by angiotensin II [20]. Therefore, one may suggest that alterations of basement membrane and integrin observed in rAV-CTSF transduced corneas could also be due to a direct action of extracellular cathepsin F.
An important functional consequence of proteinase transduction was a significant 2-fold delay in epithelial wound healing compared to vector-transduced corneas. This result may be puzzling because proteinases, especially MMPs are generally considered as facilitators of wound healing [11,17,55]. However, neither overexpression of MMP-10 in skin nor its knockout changed the skin or corneal wound healing rate, respectively [15,27]. Indirect data suggest that MMP-10 may even delay wound healing. Mice immunosuppressed by dexamethasone treatment demonstrated delayed cutaneous wound healing but MMP-10 was overexpressed. Reduction of MMP-10 expression by recombinant interleukin-6 was accompanied by accelerated wound healing [14]. These data generally agree with our results showing that overexpression of either MMP-10 or cathepsin F alone did not change corneal epithelial wound healing rate, although degradative changes of the basement membrane and a laminin-binding integrin were induced. Apparently, the magnitude of the effect was not enough to delay wound healing in this system. Only when two proteinases were overexpressed together, there was a significant slowdown of wound healing. Joint overexpression appears to better mimic the situation in diabetic corneas that also show increased levels of both MMP-10 and cathepsin F [42,45] and slower than normal wound healing [22]. In this respect, proteinase-transduced corneas became similar to the diabetic ones suggesting that specific proteinase overexpression does contribute to diabetic corneal wound healing abnormalities.
Abnormal intracellular signaling would be a possible molecular mechanism of delayed wound healing upon proteinase gene transduction. Although phosphorylation/activation of major mediators, ERK and p38 MAPK, was unchanged, p-Akt showed a marked decrease compared to vector-transduced corneas. The p-Akt change was reproducibly observed by immunohistochemistry in healing or healed corneas, and by Western blot during active healing. These data are in agreement with previous evidence. In skin wounds of mice overexpressing MMP-10 in skin cells p-Akt was also depressed during healing [27]. Although short exposure of neutrophils to cathepsin G led to Akt stimulation, prolonged exposure (as in our experiments) led to Akt degradation and apoptosis activation [41]. In the cornea, where Akt mediated EGFR-dependent epithelial wound healing, a similar reduction of p-Akt was observed after high-glucose treatment mimicking diabetic conditions [60]. It may be suggested that decreased Akt activation upon proteinase gene transduction could lead to decreased cell migration translated in slower wound healing. Because Akt is downstream of several motogenic growth factors, especially EGF [60], the activation of EGFR was also examined. Interestingly, staining for p-EGFR also decreased upon MMP-10 and cathepsin F overexpression. These results suggest that MMP-10 and cathepsin F overexpression in normal corneas may interfere with EGFR signaling thereby affecting wound healing.
Overall, the data reported herein provide a proof of principle for a functional involvement of MMP-10 and cathepsin F overexpression in certain abnormalities associated with corneal diabetes including basement membrane alterations and wound healing slowdown. This makes them promising candidates for gene silencing therapy aimed at alleviating these signs of diabetic keratopathy. RNA interference and/or antisense treatment would be strategies of choice to inhibit MMP-10 and cathepsin F activity in order to restore normal structure and function to diabetic corneas. These treatments may be combined with overexpressing specific mediators decreased in diabetic corneas, such as c-met proto-oncogene, which can positively influence corneal markers and wound healing [44,45].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amino K, Honda Y, Ide C, Fujimoto T. Distribution of the plasmalemmal Ca2+-pump and caveolin in the corneal epithelium during the wound healing process. Curr. Eye Res. 1997;16:1088–1095. doi: 10.1076/ceyr.16.11.1088.5098. [DOI] [PubMed] [Google Scholar]
- 2.Azar DT, Spurr-Michaud SJ, Tisdale AS, Gipson IK. Altered epithelial-basement membrane interactions in diabetic corneas. Arch. Ophthalmol. 1992;110:537–540. doi: 10.1001/archopht.1992.01080160115045. [DOI] [PubMed] [Google Scholar]
- 3.Ban Y, Dota A, Cooper LJ, Fullwood NJ, Nakamura T, Tsuzuki M, Mochida C, Kinoshita S. Tight junction-related protein expression and distribution in human corneal epithelium. Exp. Eye Res. 2003;76:663–669. doi: 10.1016/s0014-4835(03)00054-x. [DOI] [PubMed] [Google Scholar]
- 4.Black KL, Yin D, Ong JM, Hu J, Konda BM, Wang X, Ko MK, Bayan JA, Sacapano MR, Espinoza A, Irwin DK, Shu Y. PDE5 inhibitors enhance tumor permeability and efficacy of chemotherapy in a rat brain tumor model. Brain Res. 2008;1230:290–302. doi: 10.1016/j.brainres.2008.06.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bister V, Mäkitalo L, Jeskanen L, Saarialho-Kere U. Expression of MMP-9, MMP-10 and TNF-α and lack of epithelial MMP-1 and MMP-26 characterize pyoderma gangrenosum. J. Cutan. Pathol. 2007;34:889–898. doi: 10.1111/j.1600-0560.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- 6.Brookes NH, Loh IP, Clover GM, Poole CA, Sherwin T. Involvement of corneal nerves in the progression of keratoconus. Exp. Eye Res. 2003;77:515–524. doi: 10.1016/s0014-4835(03)00148-9. [DOI] [PubMed] [Google Scholar]
- 7.Cavallerano J. Ocular manifestations of diabetes mellitus. Optom. Clin. 1992;2:93–116. [PubMed] [Google Scholar]
- 8.Chen WL, Lin CT, Ko PS, Yeh PT, Kuan YH, Hu FR, Yang CM. In vivo confocal microscopic findings of corneal wound healing after corneal epithelial debridement in diabetic vitrectomy. Ophthalmology. 2009;116:1038–1047. doi: 10.1016/j.ophtha.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Colin M, Mailly L, Rogée S, D’Halluin JC. Efficient species C HAdV infectivity in plasmocytic cell lines using a clathrin-independent lipid raft/caveola endocytic route. Mol. Ther. 2005;11:224–236. doi: 10.1016/j.ymthe.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Chung JH, Kim WK, Lee JS, Pae YS, Kim HJ. Effect of topical Na-hyaluronan on hemidesmosome formation in n-heptanol-induced corneal injury. Ophthalmic Res. 1998;30:96–100. doi: 10.1159/000055460. [DOI] [PubMed] [Google Scholar]
- 11.Daniels JT, Geerling G, Alexander RA, Murphy G, Khaw PT, Saarialho-Kere U. Temporal and spatial expression of matrix metalloproteinases during wound healing of human corneal tissue. Exp. Eye Res. 2003;77:653–664. doi: 10.1016/j.exer.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 12.De Cillà S, Ranno S, Carini E, Fogagnolo P, Ceresara G, Orzalesi N, Rossetti L. Corneal subbasal nerves changes in patients with diabetic retinopathy: an in vivo confocal study. Invest. Ophthalmol. Vis. Sci. 2009 Jun 24; doi: 10.1167/iovs.09-3384. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Didenko TN, Smoliakova GP, Sorokin EL, Egorov VV. [Clinical and pathogenetic features of neurotrophic corneal disorders in diabetes] (in Russian) Vestn. Oftalmol. 1999;115:7–11. [PubMed] [Google Scholar]
- 14.Gallucci RM, Sugawara T, Yucesoy B, Berryann K, Simeonova PP, Matheson JM, Luster MI. Interleukin-6 treatment augments cutaneous wound healing in immunosuppressed mice. J. Interferon Cytokine Res. 2001;21:603–609. doi: 10.1089/10799900152547867. [DOI] [PubMed] [Google Scholar]
- 15.Gordon GM, Fini ME. Roles for MMP-10 in corneal resurfacing, Annual Meeting Abstract and Program Planner. Association for Research in Vision and Ophthalmology. 2009 Abstract 6301. [Google Scholar]
- 16.Hatchell DL, Magolan JJ, Jr, Besson MJ, Goldman AI, Pederson HJ, Schultz KJ. Damage to the epithelial basement membrane in the corneas of diabetic rabbits. Arch. Ophthalmol. 1983;101:469–471. doi: 10.1001/archopht.1983.01040010469029. [DOI] [PubMed] [Google Scholar]
- 17.Hattori N, Mochizuki S, Kishi K, Nakajima T, Takaishi H, D'Armiento J, Okada Y. MMP-13 plays a role in keratinocyte migration, angiogenesis, and contraction in mouse skin wound healing. Am. J. Pathol. 2009;175:533–546. doi: 10.2353/ajpath.2009.081080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herse PR. A review of manifestations of diabetes mellitus in the anterior eye and cornea. Am. J. Optom. Physiol. Opt. 1988;65:224–230. doi: 10.1097/00006324-198803000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Hutcheon AE, Sippel KC, Zieske JD. Examination of the restoration of epithelial barrier function following superficial keratectomy. Exp. Eye Res. 2007;84:32–38. doi: 10.1016/j.exer.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaakinen R, Lindstedt KA, Sneck M, Kovanen PT, Oörni K. Angiotensin II increases expression and secretion of cathepsin F in cultured human monocyte-derived macrophages: an angiotensin II type 2 receptor-mediated effect. Atherosclerosis. 2007;192:323–327. doi: 10.1016/j.atherosclerosis.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Kabosova A, Azar DT, Bannikov GA, Campbell KP, Durbeej M, Ghohestani RF, Jones JCR, Kenney MC, Koch M, Ninomiya Y, Patton BL, Paulsson M, Sado Y, Sage EH, Sasaki T, Sorokin LM, Steiner-Champliaud MF, Sun TT, SundarRaj N, Timpl R, Virtanen I, Ljubimov AV. Compositional differences between infant and adult human corneal basement membranes. Invest. Ophthalmol. Vis. Sci. 2007;48:4989–4999. doi: 10.1167/iovs.07-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kabosova A, Kramerov AA, Aoki AM, Murphy G, Zieske JD, Ljubimov AV. Human diabetic corneas preserve wound healing, basement membrane, integrin and MMP-10 differences from normal corneas in organ culture. Exp. Eye Res. 2003;77:211–217. doi: 10.1016/s0014-4835(03)00111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaji Y. Prevention of diabetic keratopathy. Br. J. Ophthalmol. 2005;89:254–255. doi: 10.1136/bjo.2004.055541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klausner EA, Peer D, Chapman RL, Multack RF, Andurkar SV. Corneal gene therapy. J. Control. Release. 2007;124:107–133. doi: 10.1016/j.jconrel.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 25.Klein R. The epidemiology of diabetic retinopathy. In: Duh E, editor. Contemporary Diabetes: Diabetic Retinopathy. Totowa, NJ: Humana Press; 2008. pp. 67–107. [Google Scholar]
- 26.Kramerov AA, Saghizadeh M, Pan H, Kabosova A, Montenarh M, Ahmed K, Penn JS, Chan CK, Hinton DR, Grant MB, Ljubimov AV. Expression of protein kinase CK2 in astroglial cells of normal and neovascularized retina. Am. J. Pathol. 2006;168:1722–1736. doi: 10.2353/ajpath.2006.050533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krampert M, Bloch W, Sasaki T, Bugnon P, Rülicke T, Wolf E, Aumaiiley M, Parks WC, Werner S. Activities of the matrix metalloproteinase stromelysin-2 (MMP-10) in matrix degradation and keratinocyte organization in wounded skin. Mol. Biol. Cell. 2004;15:5242–5254. doi: 10.1091/mbc.E04-02-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Saghizadeh M, Tuli SS, Kramerov AA, Lewin AS, Bloom DC, Hauswirth WW, Castro MG, Schultz GS, Ljubimov AV. Different tropism of adenoviruses and adeno-associated viruses to corneal cells: implications for corneal gene therapy. Mol. Vis. 2008;14:2087–2096. [PMC free article] [PubMed] [Google Scholar]
- 29.Ljubimov AV, Huang ZS, Huang GH, Burgeson RE, Gullberg D, Miner JH, Ninomiya Y, Sado Y, Kenney MC. Human corneal epithelial basement membrane and integrin alterations in diabetes and diabetic retinopathy. J. Histochem. Cytochem. 1998;46:1033–1041. doi: 10.1177/002215549804600907. [DOI] [PubMed] [Google Scholar]
- 30.Maguen E, Maguen B, Regev L, Ljubimov AV. Immunohistochemical evaluation of two corneal buttons with post-LASIK keratectasia. Cornea. 2007;26:983–991. doi: 10.1097/ICO.0b013e3180de1d91. [DOI] [PubMed] [Google Scholar]
- 31.Maguen E, Rabinowitz YS, Regev L, Saghizadeh M, Sasaki T, Ljubimov AV. Alterations of extracellular matrix components and proteinases in human corneal buttons with INTACS for post-laser in situ keratomileusis keratectasia and keratoconus. Cornea. 2008;27:565–573. doi: 10.1097/ICO.0b013e318165b1cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maubach G, Lim MC, Zhuo L. Nuclear cathepsin F regulates activation markers in rat hepatic stellate cells. Mol. Biol. Cell. 2008;19:4238–4248. doi: 10.1091/mbc.E08-03-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLaughlin PJ, Sassani JW, Klocek MS, Zagon IS. Diabetic keratopathy and treatment by modulation of the opioid growth factor (OGF) - OGF receptor (OGFr) axis with naltrexone: A review. Brain Res. Bull. 2009 doi: 10.1016/j.brainresbull.2009.08.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohan RR, Sharma A, Netto MV, Sinha S, Wilson SE. Gene therapy in the cornea. Prog. Retin. Eye Res. 2005;24:537–559. doi: 10.1016/j.preteyeres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Nakahara M, Miyata K, Otani S, Miyai T, Nejima R, Yamagami S, Amano S. A randomised, placebo controlled clinical trial of the aldose reductase inhibitor CT-112 as management of corneal epithelial disorders in diabetic patients. Br. J. Ophthalmol. 2005;89:266–268. doi: 10.1136/bjo.2004.049841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagata K, Marbán E, Lawrence JH, Donahue JK. Phosphodiesterase inhibitor-mediated potentiation of adenovirus delivery to myocardium. J. Mol. Cell. Cardiol. 2001;33:575–580. doi: 10.1006/jmcc.2000.1322. [DOI] [PubMed] [Google Scholar]
- 37.Nishida T, Yanai R. Advances in treatment for neurotrophic keratopathy. Curr. Opin. Ophthalmol. 2009;20:276–281. doi: 10.1097/icu.0b013e32832b758f. [DOI] [PubMed] [Google Scholar]
- 38.Rogée S, Grellier E, Bernard C, Loyens A, Beauvillain JC, D’Halluin JC, Colin M. Intracellular trafficking of a fiber-modified adenovirus using lipid raft/caveolae endocytosis. Mol. Ther. 2007;15:1963–1972. doi: 10.1038/sj.mt.6300283. [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg ME, Tervo TM, Immonen IJ, Müller LJ, Grönhagen-Riska C, Vesaluoma MH. Corneal structure and sensitivity in type 1 diabetes mellitus. Invest. Ophthalmol. Vis. Sci. 2000;41:2915–2921. [PubMed] [Google Scholar]
- 40.Roycik MD, Fang X, Sang QX. A fresh prospect of extracellular matrix hydrolytic enzymes and their substrates. Curr. Pharm. Des. 2009;15:1295–1308. doi: 10.2174/138161209787846676. [DOI] [PubMed] [Google Scholar]
- 41.Sabri A, Alcott SG, Elouardighi H, Pak E, Derian C, Andrade-Gordon P, Kinnally K, Steinberg SF. Neutrophil cathepsin G promotes detachment-induced cardiomyocyte apoptosis via a protease-activated receptor-independent mechanism. J. Biol. Chem. 2003;278:23944–23954. doi: 10.1074/jbc.M302718200. [DOI] [PubMed] [Google Scholar]
- 42.Saghizadeh M, Brown DJ, Castellon R, Chwa M, Huang GH, Ljubimova JY, Rosenberg S, Spirin KS, Stolitenko RB, Adachi W, Kinoshita S, Murphy G, Windsor LJ, Kenney MC, Ljubimov AV. Overexpression of matrix metalloproteinase-10 and matrix metalloproteinase-3 in human diabetic corneas: A possible mechanism of basement membrane and integrin alterations. Am. J. Pathol. 2001;158:723–734. doi: 10.1016/S0002-9440(10)64015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saghizadeh M, Chwa M, Aoki A, Lin B, Pirouzmanesh A, Brown DJ, Ljubimov AV, Kenney MC. Altered expression of growth factors and cytokines in keratoconus, bullous keratopathy and diabetic human corneas. Exp. Eye Res. 2001;73:179–189. doi: 10.1006/exer.2001.1028. [DOI] [PubMed] [Google Scholar]
- 44.Saghizadeh M, Kramerov AA, Castro MG, Ljubimov AV. C-met overexpression restores normal wound healing and marker patterns in organ-cultured human diabetic corneas. Annual Meeting Abstract and Program Planner, Association for Research in Vision and Ophthalmology. 2009 Abstract 3494. [Google Scholar]
- 45.Saghizadeh M, Kramerov AA, Tajbakhsh J, Aoki AM, Wang C, Chai NN, Ljubimova JY, Sasaki T, Sosne G, Carlson MR, Nelson SF, Ljubimov AV. Proteinase and growth factor alterations revealed by gene microarray analysis of human diabetic corneas. Invest. Ophthalmol. Vis. Sci. 2005;46:3604–3615. doi: 10.1167/iovs.04-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakamoto A, Sasaki H, Kitagawa K. Successful treatment of diabetic keratopathy with punctal occlusion. Acta Ophthalmol. Scand. 2004;82:115–117. doi: 10.1111/j.1395-3907.2004.0189h.x. [DOI] [PubMed] [Google Scholar]
- 47.Sánchez-Thorin JC. The cornea in diabetes mellitus. Int. Ophthalmol. Clin. 1998;38:19–36. [PubMed] [Google Scholar]
- 48.Sato N, Nakamura M, Chikama T, Nishida T. Abnormal deposition of laminin and type IV collagen at corneal epithelial basement membrane during wound healing in diabetic rats. Jpn. J. Ophthalmol. 1999;43:343–347. doi: 10.1016/s0021-5155(99)00095-7. [DOI] [PubMed] [Google Scholar]
- 49.Schultz RO, Van Horn DL, Peters MA, Klewin KM, Schutten WH. Diabetic keratopathy. Trans. Am. Ophthalmol. Soc. 1981;79:180–199. [PMC free article] [PubMed] [Google Scholar]
- 50.Setacci C, de Donato G, Setacci F, Chisci E. Diabetic patients: epidemiology and global impact. J. Cardiovasc. Surg. 2009;50:263–273. [PubMed] [Google Scholar]
- 51.Shenoy R, Khandekar R, Bialasiewicz A, Al Muniri A. Corneal endothelium in patients with diabetes mellitus: a historical cohort study. Eur. J. Ophthalmol. 2009;19:369–375. doi: 10.1177/112067210901900307. [DOI] [PubMed] [Google Scholar]
- 52.Sosne G, Qiu P, Kurpakus-Wheater M. Thymosin β-4 and the eye: I can see clearly now the pain is gone. Ann. N. Y. Acad Sci. 2007;1112:114–122. doi: 10.1196/annals.1415.004. [DOI] [PubMed] [Google Scholar]
- 53.Southgate T, Kroeger KM, Liu C, Lowenstein PR, Castro MG. Gene transfer into neural cells in vitro using adenoviral vectors, Chapter 4. Curr. Protoc. Neurosci. 2008 doi: 10.1002/0471142301.ns0423s45. Unit 4.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tabatabay CA, Bumbacher M, Baumgartner B, Leuenberger PM. Reduced number of hemidesmosomes in the corneal epithelium of diabetics with proliferative vitreoretinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 1988;226:389–392. doi: 10.1007/BF02172973. [DOI] [PubMed] [Google Scholar]
- 55.Tripathi BK, Stepp MA, Gao CY, Zelenka PS. The Cdk5 inhibitor olomoucine promotes corneal debridement wound closure in vivo. Mol. Vis. 2008;14:542–549. [PMC free article] [PubMed] [Google Scholar]
- 56.Vaalamo M, Karjalainen-Lindsberg ML, Puolakkainen P, Kere J, Saarialho-Kere U. Distinct expression profiles of stromelysin-2 (MMP-10), collagenase-3 (MMP-13), macrophage metalloelastase (MMP-12), and tissue inhibitor of metalloproteinases-3 (TIMP-3) in intestinal ulcerations. Am. J. Pathol. 1998;152:1005–1014. [PMC free article] [PubMed] [Google Scholar]
- 57.Villeneuve LM, Reddy MA, Lanting LL, Wang M, Meng L, Natarajan R. Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc. Natl. Acad. Sci. USA. 2008;105:9047–9052. doi: 10.1073/pnas.0803623105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y, Chen M, Wolosin JM. Wolosin, ZO-1 in corneal epithelium; stratal distribution and synthesis induction by outer cell removal. Exp. Eye Res. 1993;57:283–292. doi: 10.1006/exer.1993.1126. [DOI] [PubMed] [Google Scholar]
- 59.Wright PJ. Comparison of phosphodiesterase type 5 (PDE5) inhibitors. Int. J. Clin. Pract. 2006;60:967–975. doi: 10.1111/j.1742-1241.2006.01049.x. [DOI] [PubMed] [Google Scholar]
- 60.Xu KP, Li Y, Ljubimov AV, Yu FS. High glucose suppresses epidermal growth factor receptor/phosphatidylinositol 3-kinase/Akt signaling pathway and attenuates corneal epithelial wound healing. Diabetes. 2009;58:1077–1085. doi: 10.2337/db08-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshida Y, Ban Y, Kinoshita S. Tight junction transmembrane protein claudin subtype expression and distribution in human corneal and conjunctival epithelium. Invest. Ophthalmol. Vis. Sci. 2009;50:2103–2108. doi: 10.1167/iovs.08-3046. [DOI] [PubMed] [Google Scholar]