Abstract
Delayed corneal re-epithelialization is a complication of diabetes, and may lead to ulcers and erosions, which cause ocular morbidity and visual loss. This study examined the efficacy of naltrexone (NTX), a long-acting, potent opioid antagonist, applied topically, to facilitate the repair of standarized corneal abrasions in diabetic (alloxan-induced) New Zealand white rabbits (glucose levels). NTX at a concentration of 10−4 M, or sterile vehicle (SV), was administered topically 4 times per day for 7 days to the abraded eye of uncontrolled type 1 diabetic (DB), insulin-controlled type 1 diabetic (DB-IN), or nondiabetic (Normal) rabbits. Wound healing was monitored, and noninvasive (tonopen, pachymeter, hand-held slit lamp, and retinal camera) and invasive (histopathology) measurements evaluated. Corneal re-epithelialization in the uncontrolled DB rabbits was significantly enhanced (up to a 47% reduction in wound area) following treatment with NTX relative to both Normal SV and DB SV rabbits at 24, 48, and 56 hr following surgery. At 72 hr, DB NTX rabbits had residual defects that were 64%–82% smaller than Normal and DB SV animals. NTX treated DB-IN rabbits had residual defects that were 9–37% smaller than DB-IN rabbits receiving SV, and 6–40% smaller than Normal rabbits. No signs of toxicity from topical applications were noted. These data confirm and extend those documented in rats that demonstrated a lack of toxicity of NTX at a wide range of dosages, as well as efficacy for enhanced corneal epithelialization. These studies set the stage for clinical trials using NTX as a therapy for diabetic keratopathy.
Keywords: Naltrexone, Cornea, Epithelium, Diabetes, Wound Healing
1. Introduction
Corneal disease is the second leading cause of world blindness [8,9], and often results from a damaged ocular surface. The corneal epithelium contributes to maintaining the optical clarity of the cornea and provides a major protective barrier for the underlying tissues [2,23]. Wound repair is a basic and crucial property of all tissues including the cornea, and because of its exposed position at the surface of the eye it is at risk to a variety of injuries and diseases. The complete reepithelialization of the cornea after trauma or surgery, or as the result of disease, is essential for the restitution of normal visual function [23].
Corneal repair is often compromised in diabetic patients. Diabetic keratopathy has been estimated to eventually occur in 47% to 64% of diabetic patients [2,8,9]. With over 1 million individuals having type 1 diabetes alone [8], 500,000 or more patients may experience diabetic keratopathy at some time in the course of their disease. Moreover, corneal transplantation, removal of the corneal epithelium during vitrectomy in the course of treatment for diabetic retinopathy, and procedures such as laser photocoagulation, cataract surgery, and refractive surgery are risk factors for abnormal corneal epithelial healing in diabetic patients [7,20,28,31]. Corneal disorders associated with diabetic keratopathy include nonhealing epithelial defects, infectious corneal ulcers, and secondary scarring, which may result in a decreased or even permanent loss of vision [7,12,20,28,31]. Treatment of these diabetes-related epithelial defects may be resistant to conventional regimens (e.g., lubricants, antibiotics, bandage contact lens) [2,19]. Moreover, none of these therapies address the fundamental biological processes in corneal wound healing that are disturbed secondary to the pathophysiology of diabetes [19]. Topical administration of autologous serum has been investigated as therapy for corneal epithelial abrasions in diabetic patients following vitrectomy [32], however, this treatment is fraught with difficulties in application.
Corneal complications with respect to wound healing have been reported in both Type 1 and Type 2 diabetic animal models [6,19,35,39]. Altered cellular function is evidenced by decreased numbers of epithelial hemidesmosomes, increased expression of glycosyltransferases, and advanced glycation end (AGE) products, and altered epithelial basement membrane (BM) components such as laminin-10, nidogen-1, and the laminin-binding integrin α3β1 have been described in both human and animal diabetic corneas [1,20,24,25,27,28,37]. Several reports have documented an enhancement of MMP activity in healing corneal epithelium, both in vitro and in vivo suggesting involvement in diabetic keratopathy [33]. The changes observed in ex vivo corneas were reproduced in a corneal organ culture model [19]. Saghizadeh and colleagues [27] suggested that alterations of additional proteases, growth factors/cytokines, and BM components may occur in diabetic and DR corneas.
Naltrexone hydrochloride (NTX), an opioid antagonist, when applied topically in rats [21,22] and rabbits [46], systemically in normal and diabetic rats [40,45], or included in organ cultures of human [47] or rabbit [46] corneas, markedly accelerates DNA synthesis and corneal reepithelialization. The mechanism of action for NTX is the blockade of the opioid growth factor (OGF) interaction with the OGF receptor (OGFr) [41,44,46–49]. The OGF-OGFr axis serves as a tonically repressive pathway that regulates cell proliferation through cyclin-dependent inhibitory kinases and nucleocytoplasmic transport [4,5]. The repercussions of NTX application in the homeostatic (i.e., normal, untreated, unwounded) cornea include a decreased epithelial transit time from the basal layer to the suprabasal layers, and increases in linear thickness of the epithelium, basal cell proliferation, and packing density of suprabasal cells secondary to a decrease in cell diameter [48].
Although topical treatment with NTX accelerates corneal re-epithelialization in diabetic rats [21], it is unclear whether NTX restores corneal wound healing in other non-rodent animals with diabetes. The current study was designed to examine the toxicity and efficacy of NTX in facilitating corneal wound healing when applied topically to diabetic rabbits. Insulin-controlled (normoglycemic) and uncontrolled alloxan-induced diabetic rabbits were included in the study. Based on the data from the efficacy and toxicity studies of NTX in diabetic rats [21,41], a dosage of 10−4 M NTX was selected to administer topically 4 times each day. Outcome measures of the experiments included the size of the defect, as well as non-invasive parameters to assess toxicity such as intraocular pressure, corneal thickness, corneal topography, and retinal integrity. Additionally, histopathology was performed to evaluate the effects of NTX on peripheral corneal epithelium, limbus, and conjunctiva.
2. Methods
2.1. Animals and induction of diabetes
Male New Zealand White (RSI:NZW) rabbits (~1.5 kg) were purchased from RSI Farms (Mocksville, NC) and housed individually in stainless steel cages under standard laboratory conditions; water and food were continuously available. All investigations conformed to the regulations of the Association for Research in Vision and Ophthalmology, National Institutes of Health (NIH, Bethesda, MD), USDA, and the guidelines of the Department of Comparative Medicine of Pennsylvania State University (Hershey, PA). Fifty rabbits were used in these studies.
Diabetes was induced according to Herse [16–18] and Weekers [38] in fasting rabbits with a single intravenous injection (marginal ear vein) of 100 mg/kg alloxan monohydrate (Sigma, St. Louis, MO) dissolved in phosphate-buffered saline. Animals were anesthetized (intramuscularly) with a mixture of Domitor and Midazolam prior to the injection of alloxan. Sedation was reversed with Antisedan (0.1 mg/kg, subcutaneously). Immediately after alloxan induction, available drinking water had 10% dextrose (D-glucose; USB Corporation, Cleveland, OH) added; this water was provided as long as necessary to offset hypoglycemia [3,29].
2.2. Blood and urine glucose measurements, and body weights
Blood was collected from the marginal ear vein without anesthetic for assessment of glucose. The blood was analyzed using the True Track Smart System Glucometer (Home Diagnostic, Inc., Ft. Lauderdale, FL). Rabbits with a blood glucose level between 200 and 400 mg/dL were considered diabetic [3,29].
Baseline blood glucose measurements were taken before administration of alloxan (or sterile saline) and every 24 h post-alloxan injection until the rabbits were stabilized as either hyperglycemic or normoglycemic; blood glucose measurements were collected weekly thereafter. Body weights were monitored weekly throughout the study. Corneal wounds were created 8 weeks after injection with alloxan.
Urine samples of 6 rabbits in each group were tested after 9 weeks using Multistix 10 SG (Bayer Reagent Strips; Bayer Corporation, Elkhart, IN; USA). Bilirubin, ketone (acetoacetic acid), specific gravity, blood, pH, protein, urobilinogen, nitrite and leukocytes, as well as urine glucose were tested.
2.3. Establishment of normoglycemia
To establish normoglycemia (well-controlled diabetes), insulin pellets (Lin Shin, Scarborough, Ontario) were inserted into diabetic rabbits following published procedures [21,36,41]. Two implants were initially inserted into the shaved abdominal region (~2U/24h implant) of restrained, but not anesthetized, rabbits. Glucose levels were monitored weekly and, if glucose levels were >350 mg/dL, additional implants were inserted. After implantation, blood glucose levels were tested regularly to monitor normoglycemia and, if the animals became hypoglycemic, they were supplied with a 10% D-glucose water solution until their glucose levels returned to ~100 mg/dL.
2.4. Corneal abrasions
Procedures for the formation of circular corneal abrasions followed those in previous reports with rats [21,45,49,50]. In brief, rabbits were anesthetized with a ketamine-xylazine mixture (0.1 mg/100g; i.p.); Proparacine Hydrochloride Ophthalmic Solution 0.5% (Bausch & Lomb, Inc., Tampa, FL) was administered topically to the eye. Eyes were examined under a dissecting microscope (SZ-ET; Olympus, Tokyo, Japan) with a cold light source (Highlight 2000; Olympus) and a 12 mm diameter circle was demarcated using a disposable dermatology skin punch (Acu-Punch; Acuderm, Inc., Ft. Lauderdale, FL) on the surface epithelial layer between the inner limbal margins. The enclosed corneal epithelium was scraped off using a No. 15 Bard-Parker scalpel blade. Care was taken not to disturb the limbus or to injure the underlying corneal tissue. Wounds were created between 0730 and 0830 h, and only the right eye of each animal was abraded. Any rabbit that experienced bleeding, corneal opacities, ulcerations, inflammation, or infection was not included in the study.
2.5. Corneal photography
For the photography of corneal abrasions, rabbits were placed in restraining cages and the cornea was stained with topical fluorescein (Fluor-I-Strip, Ayerst Laboratories, Philadelphia, PA). Eyes were viewed using an Olympus dissecting microscope with a tungsten light source and gelatin Wratten No. 47 filter, and photographed with a SPOT RT3 CCD camera (Diagnostic Instruments, Sterling Heights, MI) at 1.5X magnification. Photographs of a particular rabbit were taken immediately after abrasion while the rabbit was anesthetized, and then at no less than 12 hr intervals in order to avoid interference with healing. The area of defect was determined using Optimas Software (Optimas Corporation, Bothell, WA) as the percentage of the original residual epithelial defect. Rabbit eyes were photographed at 24, 48, 56, 72, 80, and 96 h post-wounding.
2.6. Topical application of naltrexone
NTX (Sigma-Aldrich; Indianapolis, IN) was prepared at a concentration of 10−4 M in Vigamox (moxifloxacin hydrochloride ophthalmic solution, Alcon, Inc., Ft. Worth, TX); this concentration was selected based on data obtained earlier for rats [21,22,41,50]. Vigamox alone was administered as the control vehicle. Solutions were given as a single drop (~0.05 ml) to the central cornea of the abraded eye, with the lower eyelid held away from the eye to avoid overflow; the nictitating membrane and blink reflex were monitored so as to not interfere with drug administration. Eye drops were delivered to unanesthetized rabbits four times a day (0730, 1130, 1530 and 1830) for 7 consecutive days.
Six groups of animals were established: Normal rabbits receiving sterile vehicle (Normal SV) or 10−4 M NTX (Normal NTX), uncontrolled diabetic rabbits receiving sterile vehicle (DB SV) or 10−4 M NTX (DB NTX), or insulin controlled diabetic rabbits (DB-IN) receiving sterile vehicle (DB-IN SV) or 10−4 M NTX (DB-IN NTX).
2.7. Non-invasive procedures to monitor toxicity
Several measures of corneal integrity were assessed on all rabbits for both eyes 3–4 days prior to wounding, and 2 weeks post-wounding. No systemic anesthetic was utilized, however rabbits received topical anesthesia for some measurements.
2.7.1. General health of the cornea
To evaluate the general health of the cornea, animals were examined with a hand-held slit lamp (Zeiss HSO 10 Hand Slit Lamp, Dublin, CA) for general overall morphology and pathology (e.g., cataracts) that included edema, infiltrate, defects, scarring, and endothelial abnormalities such as thinning. The pupil was dilated with Phenylephrine Hydrochloride Ophthalmic Solution 2.5% (Bausch & Lomb Inc) and 1% Tropicamide Ophthalmic Solution (Falcon Pharmaceuticals Ltd., Forth Worth, TX). As the pupil was dilated the lens was inspected for cataracts.
2.7.2. Corneal thickness
Corneal thickness was determined by a pachymeter (DGH 550 Pachette 2, Pro Forma, Exton, PA). A topical anesthetic (Proparacaine Hydrochloride Ophthalmic Solution 0.5%) was used. For applanation, the pachymeter’s probe was positioned perpendicular to the center of the cornea allowing for 25 consecutive scans to be acquired for one measurement [17,30].
2.7.3. Intraocular pressure
Intraocular pressure was measured by a tonopen (Tono-Pen XL Tonometer, Medtronic, Jacksonville, FL). Topical anesthesia with Proparacaine was applied for measurements, and 4 readings/eye were recorded.
2.7.4. Corneal sensitivity
Corneal sensitivity was determined by the blinking reflex using the anesthesiometer with nylon threads (Cochet and Bonneau Aesthesiometer, Richmond Products, Boca Raton, FL) according to the instructions of the manufacturer; values (g/mm2) were determined directly from the protocol (and conversion table) supplied by the manufacturer.
2.7.5. Retinal examination
To examine the retina, optic nerve, and blood vessels in the eye, the fundus was photographed with a digital non-mydriatic retinal camera (NM-100 Type-D, Nidek Inc., Fremont, CA). To conduct these measurements, the pupils were dilated with topical 1% Tropicamide Ophthalmic Solution and 2.5% Phenylephrine Hydrochloride Ophthalmic Solution.
2.8. Plasma levels of OGF
Rabbits were euthanized 10 days after initial wounding by an i.p injection of pentobarbital (>100 mg/kg). Plasma was collected for radioimmunoassay (PerkinElmer, Waltham, MA) of [Met5]-enkephalin (OGF) levels.
2.9. Histology and morphometric analysis
Following euthanasia, both eyes were proptosed and enucleated. The eyes were fixed in 10% neutral buffered formalin for 24 h, embedded in paraffin, and sections (6 μm) of corneas were stained with hematoxylin and eosin. Eyes were evaluated by light microscopy for cell death and structure of the basement membrane, epithelium, and stroma. Using at least 2 sections/rabbit cornea, and 2–4 animals/group, assessment of the number of cell layers comprising the epithelium of the peripheral cornea, and the linear thickness of the corneal epithelium and stroma in the region of the peripheral cornea were ascertained in 5–8 independent fields/section.
2.10. Data analyses
All studies were conducted in a masked manner so that measurements were performed without knowing the treatment group. Body weights, glucose levels, non-invasive measurements, and morphometric data were evaluated using one-way analysis of variance with subsequent comparisons made with Newman-Keuls tests (GraphPad Prism 4.0 software); differences of p<0.05 were considered statistically significant.
3. Results
3.1. Body weights and general observations
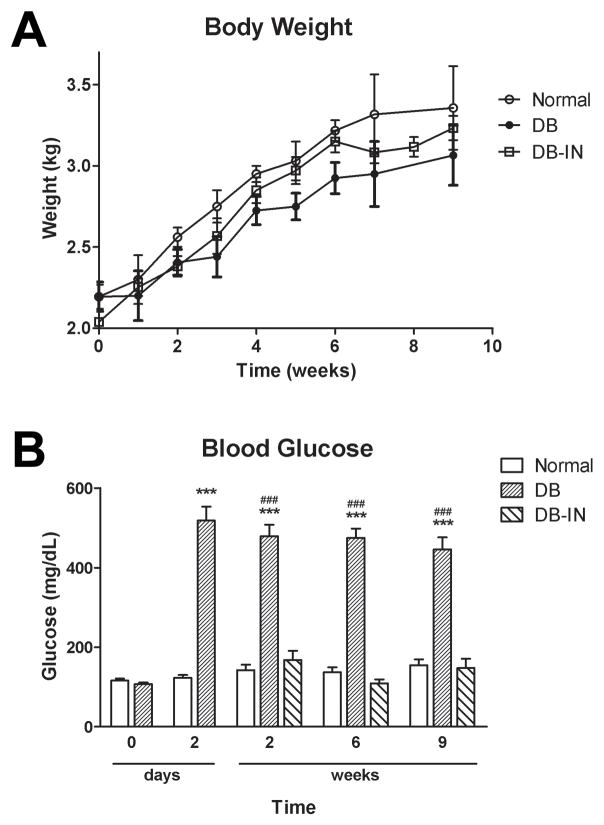
Rabbits were ~ 2.1 kg at the time of alloxan injection (Fig. 1A). No significant changes in body weights between hyperglycemic (DB), normoglycemic (DB-IN) and untreated (Normal) rabbits (Fig. 1A) were noted during the course of the following 9 weeks. At 9 weeks post-alloxan injection, the Normal, DB, and DB-IN rabbits weighed 3.4 ± 0.1, 2.8 ± 0.2, and 3.2 ± 0.1 kg, respectively. Topical NTX did not alter body weights in any group. No deaths were recorded in the Normal, DB, or DB-IN groups.
Figure 1.
Body weights (A) and blood glucose levels (B) of rabbits in the uncontrolled diabetic (DB), insulin-controlled diabetic (DB-IN) and normal (Normal) groups. Blood glucose levels were determined prior to alloxan injection (0 day), 2 days, and 2, 6, and 9 weeks after the administration of alloxan. Values are means ± SEM for 15 rabbits/group at each time point. Significantly different from Normal rabbits at p<0.001 (***) and from the DB-IN group at p<0.001 (###).
3.2. Glucose levels
Baseline blood glucose levels for 6 week-old rabbits were 100 mg/dL (Fig. 1B). Within 48 h of injection of alloxan, rabbits had blood glucose levels that were >450 mg/dL. At that time, insulin pumps were implanted into half of the DB rabbits, and glucose was controlled within 2 weeks; blood glucose for DB-IN rabbits was ~160 mg/dL and did not differ from mean values from Normal animals. At 6 and 9 weeks following injection with alloxan, glucose levels were comparable to values at 2 weeks. No rabbit experienced hyperglycemia (>600 mg/dL), and only one animal had hypoglycemia (<95 mg/dL). At 8 weeks after induction of diabetes, glucose levels for the poorly-controlled diabetic rabbits (DB) were 525 ± 42 mg/dL, whereas well-controlled diabetic (DB-IN) and Normal rabbits were 174 ± 27 mg/dL and 142 ± 4 mg/dL, respectively. NTX treatments did not change glucose levels.
Regardless of the treatment group, urine samples had a pH of 8.5 and tested negative for ketones, bilirubin, leukocytes, nitrites, protein, and blood. Urine glucose levels were >2000, 250–500, and negative in DB, DB-IN, and Normal rabbits, respectively.
3.3. Corneal re-epithelialization
The centrally positioned 12 mm diameter wound did not encroach on the limbus or conjunctiva. The initial area of abrasion used for analyses ranged from 104 – 125 mm2 and corresponded to corneal injuries of 11.4 – 12.6 mm in diameter. The size of initial abrasions was comparable among all groups.
Comparison of the repair of corneal abrasions in Normal animals receiving vehicle alone and DB rabbits receiving either SV or NTX is presented in Fig. 2. DB rabbits did not display significant delays in wound closure from Normal SV rabbits. In fact, at least at 72 h the DB rabbits had significantly smaller corneal defects than Normal animals. DB rabbits treated with NTX had significantly smaller wounds as early as 24 h following surgery relative to both Normal SV and DB SV animals; marked reductions in wound size were recorded at 48, 56, and 72 h relative to the size of residual epithelial defects in Normal SV animals. Corneal defects in DB NTX rabbits were significantly smaller than those in DB SV animals at 48 and 56 h. By 80 h, wound sizes were comparable among all groups and the DB NTX group had wounds that were over 92% healed; all animals in the DB NTX group were re-epithelialized by 96 h.
Figure 2.
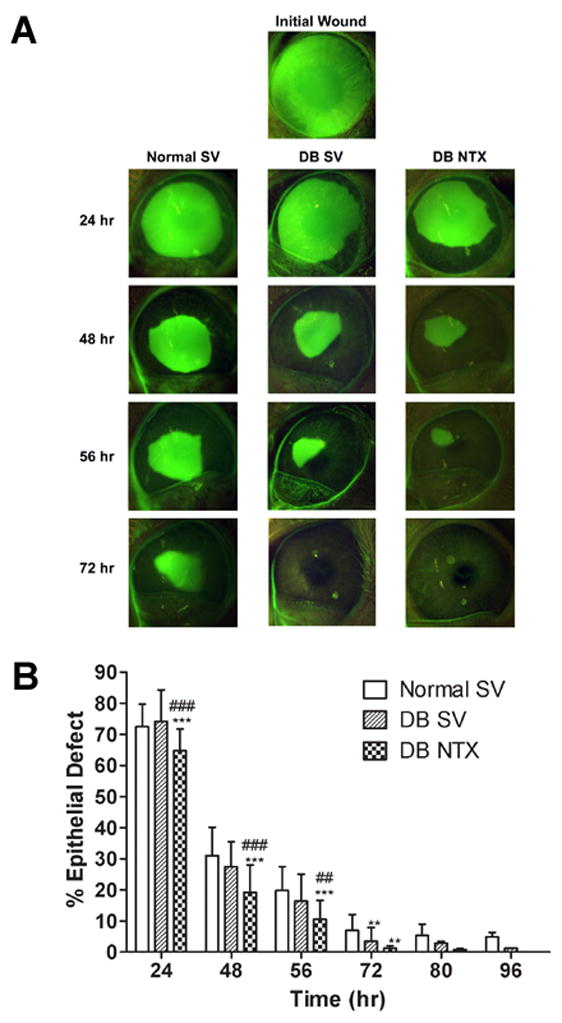
Photomicrographs (A) and areal measurements (B) of Normal and uncontrolled diabetic rabbits (DB) receiving either sterile vehicle (SV) or 10−4 M NTX. (A) Eyes were stained with fluorescein and photographs taken at 0 (Initial Wound), 24, 48, 56, and 72 h after abrasion. (B) Residual epithelial defect (means ± SEM; n = 10–15 animals/treatment group) as percentage of the original wound. Significantly different from Normal SV rabbits at p<0.01 (**) and p<0.001 (***), and from DB SV rabbits at p<0.01 (##) and p<0.001 (###).
Analyses of re-epithelialization in the insulin-controlled DB group revealed that NTX treated DB-IN rabbits had significantly smaller defects at 24, 48, and 56 h relative to Normal SV animals, and at 24 and 48 h relative to the DB-IN SV group (Fig. 3). NTX accelerated wound closure in the DB-IN NTX group by 9 – 37% at 24, 48, and 56 h. By 72 h, wound sizes were comparable between Normal SV, DB-IN SV, and DB-IN NTX groups. DB-IN SV rabbits did not display significant delays in wound closure from Normal SV rabbits.
Figure 3.
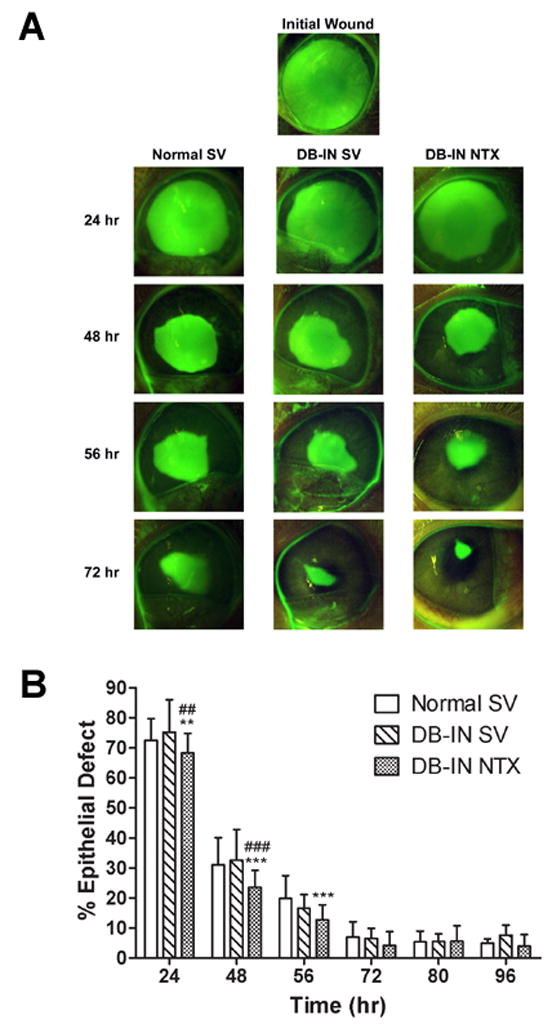
Photomicrographs (A) and areal measurements (B) of Normal and insulin controlled diabetic rabbits (DB-IN) receiving either sterile vehicle (SV) or 10−4 M NTX. (A) Eyes were stained with fluorescein and photographs taken at 0 (Initial Wound), 24, 48, 56, and 72 h after abrasion. (B) Residual epithelial defect (means ± SEM; n = 10–15 animals/treatment group) as percentage of the original wound. Significantly different from Normal SV rabbits at p<0.01 (**) and p<0.001 (***), and from DB-IN SV group at p<0.01 (##) and p<0.001 (###).
3.4. Non-invasive measurements
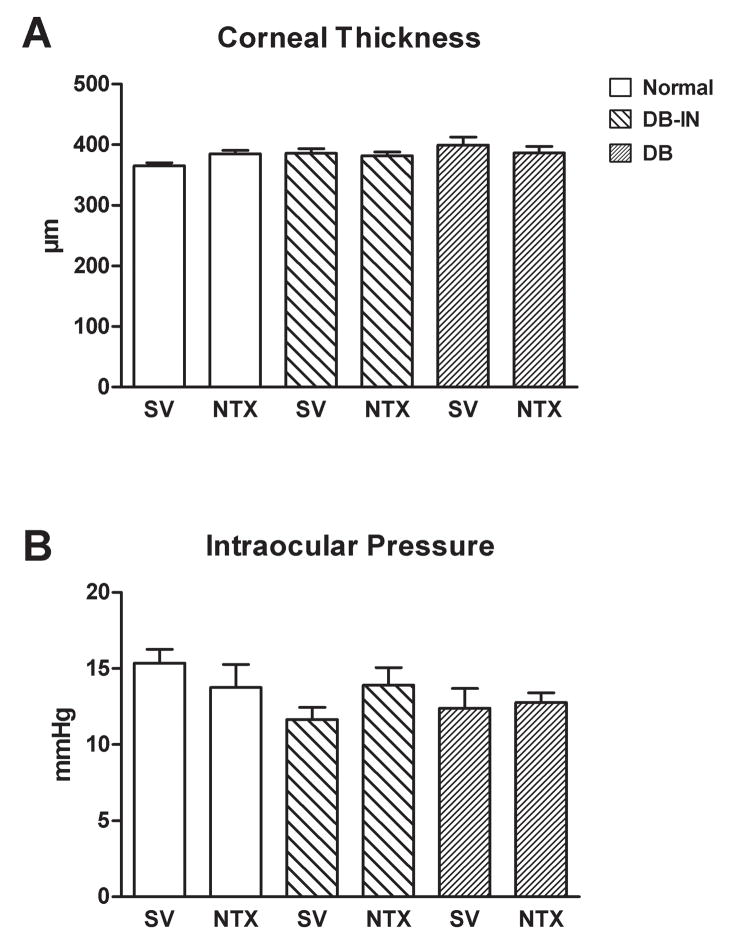
Corneal thickness (Fig. 4A) and intraocular pressure (Fig. 4B) were comparable among all vehicle and NTX treated groups at 3–4 days prior to (data not shown) and at 14 days after wounding. Ocular morphology as determined by slit lamp microscopy in the Normal, DB, and DB-IN groups was comparable at week 8 after the alloxan injection. However, cataracts were recorded in all DB rabbits, and this condition was independent of NTX administration. Analysis of retinal vessels, optic disk and retina revealed no differences based on SV or NTX treatment groups.
Figure 4.
Corneal thickness as measured with a pachymeter (A) and intraocular pressure monitored with a Tonopen (B) of rabbits in the uncontrolled diabetic (DB), insulin-controlled diabetic (DB-IN), and Normal groups 10 days after abrasion of the cornea, and treatment for 7 days with 10−4 M naltrexone (NTX) or sterile vehicle (SV). Values are means ± SEM for 10–15 rabbits in each treatment group.
Baseline values for corneal sensitivity did not differ between any group. At 10 days post-wounding, corneal sensation was decreased by 5-fold in the Normal SV and DB SV groups (2.2 ± 0.6 and 2.5 ± 0.8 g/mm2, respectively) compared to baseline values for the Normal SV group (0.51 ± 0.03 g/mm2) and DB SV group (0.53 ± 0.03 g/mm2). During the 10-day period following corneal injury, 10−4 M NTX did not alter the decreased sensitivity in the DB SV or Normal SV abraded groups.
3.5. Plasma levels of OGF
Evaluation of OGF levels revealed that Normal rabbits had 398 ± 20 pg/ml in comparison to DB rabbits with 482 ± 36 pg/ml; these values differed at p<0.05.
3.6. Histology and morphometric analysis
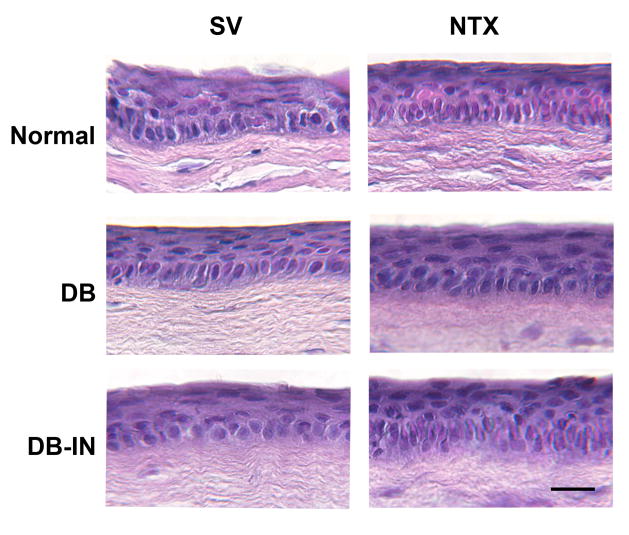
At 2 weeks after debridement, no differences in the morphology of the basal and suprabasal layers of the peripheral cornea, limbus, and conjunctiva, or stroma were observed in animals of the Normal SV and DB groups receiving NTX (Fig. 5). Moreover, the mean thickness and mean number of cell layers of the peripheral corneal epithelium (17.6 ± 1.2 μm and 3.8 ± 0.2, respectively), limbus (20.0 ± 0.4 μm and 4.0 ± 0.2, respectively), and conjunctiva (20.0 ± 1.2 μm and 4.0 ± 0.2, respectively) in the corneas of Normal animals did not differ from values of animals in the DB and DB-IN groups with or without treatment of NTX. The stroma underlying the peripheral cornea, limbus, and conjunctiva was 348 ± 16 μm, 276 ± 5 μm, and 436 ± 4 μm, respectively.
Figure 5.
Photomicrographs of histologic sections stained with hematoxylin/eosin of peripheral cornea from Normal, diabetic (DB), and insulin controlled diabetic (DB-IN) rabbits 2 weeks after corneal abrasion. Animals were treated with either 10−4- M naltrexone (NTX) or sterile vehicle (SV). No differences in morphology were detected between groups. Ep, epithelium; St, stroma. Bar = 10 μm.
4. Discussion
This study documented for the first time that topical application of NTX accelerated corneal reepithelialization in diabetic rabbits with uncontrolled hyperglycemia, equaling and even surpassing wound closure rates of those of Normal animals. Moreover, repair of corneal abrasions in rabbits with insulin-controlled diabetes (DB-IN) and receiving topical application of NTX also was accelerated above levels of Normal animals. The dosage of NTX used, 10−4 M, was not toxic, as determined using a variety of noninvasive and invasive measures. Moreover, topical administration of NTX accelerated wound healing without influencing blood glucose levels. Thus, the action of NTX was local rather than systemically driven. These observations support and extend earlier reports that topical NTX accelerates corneal reepithelialization without accompanying toxicity in rats that are diabetic with hyperglycemia, diabetic and euglycemic using insulin, and normal [21]. Thus, two Orders of animals in the Class Mammalia - Rodentia and Lagomorpha - are responsive to the stimulating effect of topical NTX insofar as corneal reepithelialization is concerned.
The model of alloxan-induced diabetes in rabbits used herein is based on extensive studies conducted earlier [11,12,16–18,36]. Within 2 days, both blood sugar and urine analysis indicated a hyperglycemic state, and remained so for the course of the 9 weeks of the experiment. Despite these extraordinary levels of hyperglycemia, and for an extended time, corneal reepithelialization was not altered from Normal rabbits. These results are consonant with those of Friend et al. [11] and Hatchell et al. [15] in which alloxan-injected rabbits were investigated for repair of mechanical and chemical, respectively, corneal abrasions. The duration of hyperglycemia did not play a role in the results as Friend et al. [11] examined animals at 2 weeks after induction of diabetes whereas Hatchell et al. [15] studied their cohort after 6 months of alloxan injection. However, these findings differ from those of Hatchell and colleagues [14] who used a transcorneal freezing model of abrasion and did record that corneal reepithelialization rates were more rapid than normal rabbits in animals that were diabetic for 6 months. Thus, there appears to be a difference in the healing rates of corneal epithelium in diabetic rabbits compared to diabetic rats such that the diabetic rabbit heals at a normal rate compared to the marked delay seen in diabetic rat [12,15,21,46]. Whether this difference in corneal healing rates reflects dissimilarities in the biology of the rat and rabbit and/or responses to the agents used to induce diabetes (i.e., alloxan in the rabbit, streptozotocin in the rat) is unclear. However, in view of this distinction between rats and rabbits, it is worthy to note that corneal wound healing is delayed in diabetic patients [2,8,9], suggesting the diabetic rat, rather than rabbit, model may have a greater similarity to the human condition.
This study shows that even in the face of marked hyperglycemia, the functional biologic system responsive to NTX is not impaired in rabbits. Moreover, as mentioned above, the effect of NTX at the local level was not reliant on systemic changes. These observations are in concert with in vivo and in vitro evidence that the endogenous opioid system (i.e., OGF-OGFr axis) is present [42–44,46] and functionally related to cell proliferation [44] and wound healing [46] in the normal rabbit, and now document safety and efficacy in the diabetic rabbit. Thus, in this study, the topical application of NTX, a pure opioid antagonist, blocks the interactions of the tonically active inhibitory pathway generated by the OGF-OGFr axis in the diabetic and normal cornea. OGF is targeted to the cyclin-dependent inhibitory kinase pathway that constitutes a regulatory system in these corneal epithelial cells. OGF has been found to be increased in patients with diabetes [10,26], genetically diabetic mice [13,34], and in the present study, diabetic rabbits, which would accentuate OGF-OGFr interaction, and thereby have an exaggerated response in inhibiting cell replication. Rabbit corneal epithelial cells do not appear as sensitive to the elevation in OGF levels as they are in the rat, at least as measured by changes in reepithelialization in diabetic rabbits, as evidenced by the fact that corneal repair in diabetic rabbits with no drug treatment is not altered. However, when OGF is prevented from interacting with OGFr in diabetic rabbits, this blockade of the pathway provides a mechanism of NTX’s action in accelerating repair of the ocular surface epithelium.
Given the vital role of the corneal epithelium in maintaining vision, the frequency of corneal complications related to diabetes (diabetic keratopathy) in humans, and the problems occurring in diabetic individuals postoperatively (e.g., vitrectomy, cataract extraction) in which the ocular surface epithelium is disturbed, an effective treatment to facilitate corneal epithelial wound healing in diabetic individuals is needed.
Unfortunately, conventional therapies such as artificial tears and bandage contact lenses fail frequently [2.19]. Schulze and colleagues [32] have reported that hourly exposure to autologous serum, but not hyaluronic acid (Vislube), accelerates closure of corneal epithelial abrasions by up to 40%. In comparison to the above therapies, the advantages of topical application of NTX for diabetic keratopathy is that this drug is readily available, of known chemical composition, easy to prepare, is required only 4 times/day, is not toxic to the cornea, and accelerates corneal wound closure by up to 60%. The safety and effectiveness of NTX in accelerating epithelial wound healing in both diabetic rats and rabbits support the need for clinical trials of NTX in treating human diabetic keratopathy.
Acknowledgments
This work was supported by NIH grant EY01666 (ISZ). The authors want to acknowledge the technical help of Ms. Cara Keiper.
Footnotes
Conflict of Interest Statement
None of the authors have any activity to disclose, and have no conflict of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Azar DT, Spurr-Michaud SJ, Tisdale AS. Altered epithelial-basement membrane interactions in diabetic corneas. Arch Ophthalmol. 1992;110:537–40. doi: 10.1001/archopht.1992.01080160115045. [DOI] [PubMed] [Google Scholar]
- 2.Cavallerano J. Ocular manifestations of diabetes mellitus. Optom Clin. 1992;2:93–116. [PubMed] [Google Scholar]
- 3.Changolkar AK, Hypolite JA, Disanto M, Oates PJ, Alan J, Chacko S. Diabetes induced decrease in detrusor smooth muscle force is associated with oxidative stress and overactivity of aldose reductase. J Urol. 2005;173(1):309–13. doi: 10.1097/01.ju.0000141583.31183.7a. [DOI] [PubMed] [Google Scholar]
- 4.Cheng F, McLaughlin PJ, Verderame MF, Zagon IS. The OGF-OGFr axis utilizes the p16INK4a and p21Waf1/Cip1 pathways to restrict normal cell proliferation. Mol Biol Cell. 2009;20(1):319–327. doi: 10.1091/mbc.E08-07-0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng F, McLaughlin PJ, Verderame MF, Zagon IS. Dependence on nuclear localization signals of the opioid growth factor receptor in the regulation of cell proliferation. Exp Biol Med. 2009;234:532–541. doi: 10.3181/0901-RM-16. [DOI] [PubMed] [Google Scholar]
- 6.Chikama T, Wakuta M, Liu Y, Nishida T. Deviated mechanism of wound healing in diabetic corneas. Cornea. 2007;26(Suppl 1):S75–S81. doi: 10.1097/ICO.0b013e31812f6d8e. [DOI] [PubMed] [Google Scholar]
- 7.Cisarik-Fredenburg P. Discoveries in research on diabetic keratopathy. Optometry. 2001;72:691–704. [PubMed] [Google Scholar]
- 8.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New Eng J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 9.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. New Eng J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fallucca F, Tonnarini G, Di Biase N, D’Alessandro M, Negri M. Plasma met-enkephalin levels in diabetic patients: Influence of autonomic neuropathy. Metabolism. 1996;45:1065–1068. doi: 10.1016/s0026-0495(96)90004-9. [DOI] [PubMed] [Google Scholar]
- 11.Friend JC, Kiorpes TC, Thoft RA. Diabetes mellitus and the rabbit corneal epithelium. Invest Ophthalmol Vis Sci. 1981;21:317–321. [PubMed] [Google Scholar]
- 12.Friend J, Toft RA. The diabetic cornea. Int Ophthalmol Clinic. 1984;24:111–123. [PubMed] [Google Scholar]
- 13.Greenberg J, Ellyin F, Pullen G, Ehrenpreis S, Singh SP, Cheng J. Methionine-enkephalin and β-endorphin levels in brain, pancreas, and adrenals of db/db mice. Endocrinology. 1985;116:328–331. doi: 10.1210/endo-116-1-328. [DOI] [PubMed] [Google Scholar]
- 14.Hatchell DL, Magolan JJ, Besson MJ, Goldman AI, Perderson HJ, Schultz KJ. Damage to the epithelial basement membrane in the corneas of diabetic rabbits. Arch Ophthalmol. 1983;101(3):469–471. doi: 10.1001/archopht.1983.01040010469029. [DOI] [PubMed] [Google Scholar]
- 15.Hatchell DL, Ubels JL, Stekiel T, Hatchell MC. Corneal epithelial wound healing in normal and diabetic rabbits treated with tretinoin. Arch Ophthalmol. 1985;103:98–100. doi: 10.1001/archopht.1985.01050010104029. [DOI] [PubMed] [Google Scholar]
- 16.Herse PR. Recovery from contact lens-induced edema is prolonged in the diabetic rabbit cornea. Optometry Vis Sci. 1990;67:466–470. doi: 10.1097/00006324-199006000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Herse PR. Diurnal and long-term variations in corneal thickness in the normal and alloxan-induced diabetic rabbit. Current Eye Res. 1990;9:451–457. doi: 10.3109/02713689008999611. [DOI] [PubMed] [Google Scholar]
- 18.Herse PR. Corneal hydration control in normal and alloxan-induced diabetic rabbits. Invest Ophthalmol Vis Sci. 1990;31:2205–2213. [PubMed] [Google Scholar]
- 19.Kabosova A, Kramerov AA, Aoki AM, Murphy G, Zierske JD, Ljubimov AV. Human diabetic corneas preserve wound healing, basement membrane, integrin and MMP-10 differences from normal corneas in organ culture. Exp Eye Res. 2003;77:211–217. doi: 10.1016/s0014-4835(03)00111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaji Y. Prevention of diabetic keratopathy. British J Ophthalmol. 2005;89:254–255. doi: 10.1136/bjo.2004.055541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klocek MS, Sassani JW, McLaughlin PJ, Zagon IS. Topically applied naltrexone restores corneal reepithelialization in diabetic rats. J Ocular Pharmacol Ther. 2007;23:89–102. doi: 10.1089/jop.2006.0111. [DOI] [PubMed] [Google Scholar]
- 22.Klocek MS, Sassani JW, McLaughlin PJ, Zagon IS. Naltrexone and insulin are independently effective but not additive in accelerating corneal epithelial healing in type 1 diabetic rats. Exp Eye Res. 2009 doi: 10.1016/j.exer.2009.06.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liebowitz HM. Clinical Diagnosis and Management. Saunders; Philadelphia: 1984. Corneal Disorder. [Google Scholar]
- 24.Ljubimov AV, Burgeson RE, Butkowski RJ, Couchman JR, Zardi L, Ninomiya Y, Sado Y, Huang ZS, Nesburn AB, Kenney MC. Basement membrane abnormalities in human eyes with diabetic retinopathy. J Histochem Cytochem. 1996;44:1469–1479. doi: 10.1177/44.12.8985139. [DOI] [PubMed] [Google Scholar]
- 25.Ljubimov AV, Huang Z, Huang GH, Burgeson RE, Gullberg D, Miner JH, Ninomiya Y, Sado Y, Kenney MC. Human corneal epithelial basement membrane and integrin alterations in diabetes and diabetic retinopathy. J Histochem Cytochem. 1998;46:1033–1041. doi: 10.1177/002215549804600907. [DOI] [PubMed] [Google Scholar]
- 26.Negri M, Tonnarini G, D’Alessandro M, Fallucca F. Plasma met-enkephalin in type 1 diabetes. Metabolism. 1992;41:460–461. doi: 10.1016/0026-0495(92)90200-t. [DOI] [PubMed] [Google Scholar]
- 27.Saghizadeh M, Kramerov AA, Tajbakhsh J, Aoki AM, Wang C, Chai NN, Ljubimova JY, Sasaki T, Sosne G, Carlson MRJ, Nelson SF, Ljubimov AV. Proteinase and growth factor alterations revealed by gene microarray analysis of human diabetic corneas. Invest Ophthamol Vis Sci. 2005;46:3604–3615. doi: 10.1167/iovs.04-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanchez-Thorin JC. The cornea in diabetes mellitus. Int Ophthalmol Clin. 1998;38:19–36. [PubMed] [Google Scholar]
- 29.Schratzberger P, Walter DH, Rittig K, Bahlmann FH, Pola R, Curry C, Silver M, Krainin JG, Weinberg DH, Ropper AH, Isner JM. Reversal of experimental diabetic neuropathy by VEGF gene transfer. J Clin Invest. 2001;107(9):1083–1092. doi: 10.1172/JCI12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schultz RO, Iliev ME, Frueh BE, Goldblum D. In vivo pachymetry in normal eyes of rats, mice, and rabbits with the optical low coherence reflectometer. Vision Res. 2003;43(6):723–8. doi: 10.1016/s0042-6989(03)00005-1. [DOI] [PubMed] [Google Scholar]
- 31.Schultz RO, Van Horn DL, Peters MA, Klewin KM, Schutten WH. Diabetic keratopathy. Trans Amer Ophthalmol Soc. 1981;79:180–199. [PMC free article] [PubMed] [Google Scholar]
- 32.Schulze SD, Sekundo W, Kroll P. Autologous serum for the treatment of corneal epithelial abrasions in diabetic patients undergoing vitrectomy. Amer J Ophthalmol. 2006;142:207–211. doi: 10.1016/j.ajo.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi H, Akiba K, Noguchi T, Ohmura T, Takahashi R, Ezure Y, Ohara K, Zieske JD. Matrix Metalloproteinase activity is enhanced during corneal wound repair in high glucose condition. Curr Eye Res. 2000;21:608–615. [PubMed] [Google Scholar]
- 34.Timmers K, Voyles NR, Zalenski C, Wilkins S, Recant L. Altered β-enkpehlain, met- and leu-enkephalins, and enkephalin-containing peptides in pancreas and pituitary of genetically obese (db/db) mice during development of diabetic syndrome. Diabetes. 1986;35:1143–1151. doi: 10.2337/diab.35.10.1143. [DOI] [PubMed] [Google Scholar]
- 35.Wakuta M, Morishige N, Chikama T, Seki K, Nagano T, Nishida T. Delayed wound closure and phenotypic changes in corneal epithelium of the spontaneously diabetic Goto-Kakizaki rat. Invest Ophthalmol Vis Sci. 2007;48:590–596. doi: 10.1167/iovs.05-1168. [DOI] [PubMed] [Google Scholar]
- 36.Wang PY. Control of hyperglycaemia in diabetic rabbits by a combination of implants. J Biomed Eng. 1993;15:106–112. doi: 10.1016/0141-5425(93)90038-z. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe H, Katakami C, Miyata S, Negi A. Corneal disorders in KKAy mouse: a type 2 diabetes model. Jap J Ophthamol. 2002;46:130–139. doi: 10.1016/s0021-5155(01)00487-7. [DOI] [PubMed] [Google Scholar]
- 38.Weekers F, Giulietti A, Michalaki M, Coopmans W, Van Herch E, Mathieu C, Van de Berghe G. Metabolic, endocrine, and immune effects on stress hyperglycemia in a rabbit model of prolonged critical illness. Endocrinology. 2003;144:5329–5338. doi: 10.1210/en.2003-0697. [DOI] [PubMed] [Google Scholar]
- 39.Xu KP, Li Y, Ljubimov AV, Yu FSX. High glucose suppresses epidermal growth factor receptor/phosphatidylinositol 3-kinase/Akt signaling pathway and attenuates corneal epithelial wound healing. Diabetes. 2009;58:1077–1085. doi: 10.2337/db08-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zagon IS, Jenkins JB, Sassani JW, Wylie JD, Ruth TB, Frey JL, Lang CM, McLaughlin PJ. Naltrexone, an opioid antagonist, facilitates reepithelialization of the cornea in diabetic rat. Diabetes. 2002;51(10):3055–3062. doi: 10.2337/diabetes.51.10.3055. [DOI] [PubMed] [Google Scholar]
- 41.Zagon IS, Klocek MS, Sassani JW, Mauger DT, McLaughlin PJ. Corneal safety of topically applied naltrexone. J Ocular Pharmacol Therap. 2006;22(5):377–387. doi: 10.1089/jop.2006.22.377. [DOI] [PubMed] [Google Scholar]
- 42.Zagon IS, Sassani JW, Allison G, McLaughlin PJ. Conserved expression of the opioid growth factor, [Met5]-enkephalin, and the zeta (ζ) opioid receptor in vertebrate cornea. Brain Res. 1995;671:105–111. doi: 10.1016/0006-8993(94)01314-8. [DOI] [PubMed] [Google Scholar]
- 43.Zagon IS, Sassani JW, Kane ER, McLaughlin PJ. Homeostasis of ocular surface epithelium in the rat is regulated by opioid growth factor. Brain Res. 1997;759:92–102. doi: 10.1016/s0006-8993(97)00238-2. [DOI] [PubMed] [Google Scholar]
- 44.Zagon IS, Sassani JW, McLaughlin PJ. Opioid growth factor modulates corneal epithelial growth in tissue culture. Amer J Physiol. 1995;268:R942–R950. doi: 10.1152/ajpregu.1995.268.4.R942. [DOI] [PubMed] [Google Scholar]
- 45.Zagon IS, Sassani JW, McLaughlin PJ. Re-epithelialization of the rat cornea is accelerated by blockade of opioid receptors. Brain Res. 1998;798:254–260. doi: 10.1016/s0006-8993(98)00427-2. [DOI] [PubMed] [Google Scholar]
- 46.Zagon IS, Sassani JW, McLaughlin PJ. Re-epithelialization of the rabbit cornea is regulated by opioid growth factor. Brain Res. 1998;803:61–68. doi: 10.1016/s0006-8993(98)00610-6. [DOI] [PubMed] [Google Scholar]
- 47.Zagon IS, Sassani JW, McLaughlin PJ. Reepithelialization of the human cornea is regulated by endogenous opioids. Invest Ophthalmol Vis Sci. 2000;41(1):73–81. [PubMed] [Google Scholar]
- 48.Zagon IS, Sassani JW, McLaughlin PJ. Adaptation of homeostatic ocular surface epithelium to chronic treatment with the opioid antagonist naltrexone. Cornea. 2006;25(7):821–829. doi: 10.1097/01.ico.0000224646.66472.aa. [DOI] [PubMed] [Google Scholar]
- 49.Zagon IS, Sassani JW, McLaughlin PJ. Insulin treatment ameliorates impaired corneal re-epithelialization in diabetic rats. Diabetes. 2006;55(4):1141–1147. doi: 10.2337/diabetes.55.04.06.db05-1581. [DOI] [PubMed] [Google Scholar]
- 50.Zagon IS, Sassani JW, Myers RL, McLaughlin PJ. Naltrexone accelerates healing without compromise of adhesion complexes in normal and diabetic corneal epithelium. Brain Res Bull. 2007;72(1):18–24. doi: 10.1016/j.brainresbull.2006.12.007. [DOI] [PubMed] [Google Scholar]