Abstract
The renin angiotensin system (RAS) plays a central role in the brain to regulate blood pressure (BP). This role includes the modulation of sympathetic nerve activity (SNA) that regulates vascular tone, the regulation of secretion of neurohormones that have a critical role in electrolyte and fluid homeostasis, and by influencing behavioral processes to increase salt and water intake. Based on decades of research it is clear that angiotensin II (Ang II), the major bioactive product of the RAS, mediates these actions largely via its Ang II type 1 receptor (AT1R), located within hypothalamic and brainstem control centers. However, the mechanisms of brain RAS function have been questioned, due in large part to low expression levels of the rate limiting enzyme renin within the central nervous system. Tissue localized RAS has been observed in heart, kidney tubules and vascular cells. Studies have also given rise to the hypothesis for localized RAS function within the brain, so that Ang II can act in a paracrine manner to influence neuronal activity. The recently discovered (pro)renin receptor (PRR) may be key in this mechanism as it serves to sequester renin and prorenin for localized RAS activity. Thus, the PRR can potentially mitigate the low levels of renin expression in the brain to propagate Ang II action. In this review we examine the regulation, expression and functional properties of the various RAS components in the brain with particular focus on the different roles that PRR may have in BP regulation and hypertension.
Keywords: Hypertension, Tissue localized renin Angiotensin System, Blood pressure, Signaling, Angiotensin II type-1-Receptor, Sympathetic
I. Introduction
The brain plays a critical role in the regulation of blood pressure (BP) through the incorporation of a complex signaling network that processes information from the circulation regarding changes in vascular tone. In turn, this network responds by modulating sympathetic nerve activity (SNA) and by secreting hormones into the circulation to adjust the degree of vascular resistance in order to maintain a homeostatic BP set point that is known to be governed by renal pressure-natriuresis mechanisms (Dampney, 1994; Guyenet, 2006; Guyton, 1991). These compensatory mechanisms are temporary in nature, lasting up to several days. This period allows for adjustment of the pressure-natriuresis mechanism to take over at the kidney. However, an accumulating body of work has suggested that dysfunctions in the central nervous system (CNS) regulatory mechanisms of BP control can lead to sustained increase in SNA, which can lead to chronic hypertension (Colombari et al., 2001; de Wardener, 2001; Guyenet, 2006). In support of this idea, it is established that various animal models of hypertension, including the spontaneously hypertensive rat (SHR), the deoxycorticosterone acetate salt rat and the Dahl salt-sensitive rat each exhibit increased sympathetic activity (Cabassi et al., 2002; Leenen et al., 2002; Takeda and Bunag, 1980). Furthermore, studies in humans have revealed increased sympathetic activity in many cases of hypertension (Schlaich et al., 2004). This growing body of research lends credence to the theory that increased sympathetic activity is the most likely cause of neurogenic hypertension (Esler, 2000). Thus, factors that regulate sympathetic outflow from the brain are prime targets in understanding the mechanisms of neurogenic hypertension. The peptide angiotensin (Ang) II is of major importance in this regard. Ang II is a potent pressor hormone that is synthesized by the renin-Ang system (RAS). The pressor action of Ang II, mediated by the Ang II type-1 receptor (AT1R), involves direct vasoconstrictor actions as well as release of other pressor hormones (e.g. norepinephrine) in addition to other processes that result to increase cardiovascular tone (de Gasparo et al., 2000; Ferrario and Strawn, 2006; Lifton et al., 2001; Smith et al., 1995). However, Ang II is also a neuropeptide that is well known to act via the AT1R to increase the excitability of neurons in the cardiovascular regulatory centers of the hypothalamus and brain stem (Bader et al., 2001; Barnes et al., 2003; Kasparov and Teschemacher, 2008; Li et al., 2003; Li and Ferguson, 1993; Pan, 2004; Veerasingham and Raizada, 2003). In doing so, Ang II is able to modulate sympathetic outflow and blood pressure in rats, and overactivity of these Ang II/AT1R actions in the CNS contribute to neurogenic hypertension (Pan, 2004; Veerasingham and Raizada, 2003). While it is generally accepted that the brain has an intrinsic RAS and Ang II is known to have powerful actions mediated via AT1R in the brain, long-standing questions have arisen on the origin of the Ang II within the brain and on the CNS RAS in general. In fact the relevance of intrinsic RAS function in the CNS has been questioned since renin, the rate limiting enzyme for the RAS, is poorly expressed in the brain (Bader and Ganten, 2002; Lippoldt et al., 2001). However, there is strong evidence for a competent brain RAS mechanism. Research has shown that various tissues and organs can achieve significant levels of Ang II production within localized regions, even with limited renin availability. This localized RAS activity has been reported in the heart, kidney, blood vessels and the brain (Bader and Ganten, 2002; Bader et al., 2001; Kobori et al., 2007). The recently discovered (pro)renin receptor (PRR) is rapidly being acknowledged as an important player in this process. The PRR is expressed in various tissues and has been found to mediate localized RAS function at the kidney and heart (Ichihara et al., 2006a; Kobori et al., 2007; Nguyen et al., 2002). Since the PRR is highly expressed in the brain (Nguyen et al., 2002) it is conceivable that it may mitigate the low level of renin expression and thus support a competent, centrally mediated, RAS function.
In this review we will re-examine the scientific evidence for the various components of the RAS in the brain in order to discuss their potential role in BP control, with a special focus on the PRR and its potential contribution to cardiovascular regulation.
II. Overview of the brain control of Blood pressure
The BP regulatory brain centers are situated, for the most part, in the hypothalamus of the forebrain and in the medulla of the brainstem (Colombari et al., 2001; Dampney, 1994; de Wardener, 2001). These nuclei are highly networked and their major function in cardiovascular control involves the regulation of SNA to modulate BP tone (Dampney, 1994; Guyenet, 2006). In addition, these central cardiovascular pathways mediate neurohormone secretion, including arginine-vasopressin (AVP) that stimulates vasoconstriction and modulates the renal pressure-natriuresis mechanism (Guyenet, 2006; McKinley et al., 2001; Treschan and Peters, 2006). Hypothalamic control centers are also important in the regulation of electrolyte and plasma fluid volume via stimulation of thirst and increasing salt appetite.
Animal models of hypertension have been vital in detailing the contribution of various cardiovascular regulatory brain nuclei to neurogenic hypertension. Key nuclei have been involved in this disorder include; the paraventricular nucleus (PVN) of the hypothalamus, the rostral ventrolateral medulla (RVLM) and the nucleus of the solitary tract (NTS) of the brainstem (Colombari et al., 2001; Dampney, 1994; de Wardener, 2001; Guyenet, 2006). The PVN plays a pivotal role in regulating blood pressure in both the normotensive and hypertensive states. This nucleus receives neural input concerning cardiovascular status from regions such as the NTS, integrates the input and controls sympathetic outflow by sending projections directly to the intermediolateral cell column (IML) of the spinal cord, and by sending projections to other sympathetic regulatory regions including the RVLM (Swanson and Sawchenko, 1983). The PVN also regulates the production of hormones that influence cardiovascular function including glucocorticoids and vasopressin (Benarroch, 2005). The RVLM is also a key integration point for the central control of BP (Dampney, 1994; Guyenet, 2006). Excitatory and inhibitory signals from various BP regulatory brain regions converge at the RVLM. These signals are integrated and transmitted to the sympathetic preganglionic nucleus of the IML of the spinal cord, which then innervate the sympathetic ganglionic neurons to modulate SNA and subsequently BP at the target vascular beds (Colombari et al., 2001; Dampney, 1994; Guyenet, 2006). The NTS is important for central feedback regulation of BP control. One of the most potent regulatory feedback inputs into the NTS is the baroreflex (Chapleau and Abboud, 2004; Guyton, 1991). Changes in BP are immediately detected by aortic arch and carotid sinus baroreceptors, which then signal the information to the NTS, which processes these signals and modulates the synaptic output from RVLM through a relay involving the caudal ventrolateral medulla (Colombari et al., 2001; Dampney, 1994; Guyenet, 2006). It is hypothesized that neurogenic hypertension, mediated by a chronic increase in SNA, results from increased output from the brain (Esler, 2000; Schlaich et al., 2004). Mechanisms that have been proposed to alter sympathetic output include an increased neuronal activity in the RVLM or decreased sensitivity of the baroreflex at the NTS (Esler, 2000).
In addition to modulating SNA the brain stimulates behavioral changes that ultimately influence BP by stimulating salt intake and thirst. Ang II is a major factor in these responses, and circulating Ang II can stimulate both water and sodium intake by acting at AT1R within circumventricular organs (CVO) such as the subfornical organ (SFO) and the organum vasculosum of the lamina terminalis (OVLT). These salt and thirst responses also involve other brain nuclei, including the PVN and the median preoptic nucleus (MNPO) (McKinley et al., 2001). Fluid and electrolyte balance is also regulated by neurohormone section. AVP is both a pressor and an antidiuretic agent that is synthesized by the magnocellular neurons of the paraventricular nucleus (PVN) and the supraoptic nucleus (SON). AVP is secreted into the circulation via projection from the PVN and the SON to the neurohypophesis (posterior pituitary) in response to changes in osmolarity or neuronal stimulation (Bourque and Renaud, 1984; Mason, 1980; Pan, 2004; Treschan and Peters, 2006). The pressor role of AVP is mediated by V1 receptors in smooth muscle cells, although this action is mitigated by the baroreflex (Goldsmith, 1988). AVP acts as an antidiuretic with V2 receptors in the kidney to reverse natriuresis or the process of Na+ excretion. Reabsorption of Na+ results to elicit water retention, mediated by upregulation and targeting of epithelial Na+ -channels and Aquaporin-2-channels (Bankir et al., 2005; Treschan and Peters, 2006). As a result, urine becomes concentrated and reduced in volume (Bakris et al., 1997; Bankir et al., 2005; Bankir et al., 2007; Bursztyn et al., 1990).
Thus, by using a complex networking architecture, the brain processes feedback from the circulation to respond to changes in mean arterial blood pressure through multiple mechanisms. These mechanisms include modulation of the sympathetic pathway and secretion of various hormones to complement with circulatory mechanisms to maintain blood pressure tone. While the exact mechanism for brain processes to establish hypertension is still a matter of study, it is becoming evident that a neurogenic component is involved in some cases of chronic high blood pressure.
III. The current status of the Brain Renin Angiotensin System
The RAS is one of the most important mechanisms for regulating blood pressure, and the major bioactive hormone of this system is Ang II. Historically, the RAS took over 60 years to define (Cleland and Reid, 1996). This began in 1896 with the discovery of renin and its effects on blood pressure by Tigerstedt and Bergmen. Renin catalyzes the rate limiting step of converting the substrate angiogensinogen (Agt) to Ang I, which is then converted to Ang II by Angiotensin converting enzyme (ACE). Circulating Ang II stimulates vasoconstriction, and in addition, Ang II mediates the secretion of the steroid hormone aldosterone that mediates Na+ reabsorbtion and water retention (Cleland and Reid, 1996). Bickerton and Buckley (1961) were the first to demonstrate that administration of Ang II into to the brain of dogs mediated an increase in BP, which was propagated by the sympathetic nervous system. This and similar discoveries led to the proposal of an intrinsic RAS in the brain (Bader and Ganten, 2002), an idea which was strengthened by reports of renin-like activity in brain extracts (Fischer-Ferraro et al., 1971; Ganten et al., 1971). The pressor actions of Ang II are mediated by its binding to AT1R (de Gasparo et al., 2000). Since then, AT1R and all the components for the RAS have been found in various brain regions that are involved in the regulation of SNA as well as the regulation of fluid and electrolyte balance (Dampney, 1994; de Gasparo et al., 2000). Furthermore, the physiological significance of a brain RAS is underscored by observations of increased sensitivity to Ang II, and of increased AT1R expression in the cardiovascular regulatory brain areas of animal models of neurogenic hypertension (Guyenet, 2006; Veerasingham and Raizada, 2003). In addition, genetic or pharmacological intervention of brain RAS components have been shown to attenuate neurogenic hypertension (Morimoto et al., 2002; Phillips et al., 1977; Veerasingham and Raizada, 2003; Yamazato et al., 2007).
In addition, increased RAS activity in the brain increases AVP secretion, which is found to exacerbate hypertension in rats (Luft, 2007). Similar findings have been been reported in humans. Clinical studies indicate that the African American male population have an increased incidence of hypertension. Furthermore, recent re-examination of this clinical data show that hypertensive African American males exhibited symptoms that are associated with increased secretion of the antidiuretic hormone, AVP (Bakris et al., 1997; Bankir et al., 2005; Bankir et al., 2007). At the other extreme, a deficit of RAS function in key regions in the brain, such as the magnocellular region of the PVN or the SON, results in impaired production and secretion of AVP, the main cause of centrally mediated diabetes insipidus (Treschan and Peters, 2006). Consistent with this are reports showing that reduced Ang II production is observed in the genetic rat model of diabetes insipidus, the Brattleboro rat (Bundzikova et al., 2008).
Despite the above evidence for powerful cardiovascular actions of Ang II mediated by the CNS, the fact that renin is poorly expressed in the brain (Bader and Ganten, 2002; Lippoldt et al., 2001; Saavedra, 2005) has raised a doubt as to whether centrally generated Ang II has meaningful functional actions. As a result, this has given way to alternative proposals for the source of Ang I or Ang II in the CNS, such as their uptake and transport from the circumventricular organs, which are devoid of a blood brain barrier (Saavedra, 2005). However, several key pieces of evidence do support a competent RAS function in the brain. For example, ACE and Ang II-like immunoreactivity have been reported in neurons in various regions of the brain, including the SFO and the NTS (Lind et al., 1984; Pickel and Chan, 1995; Pickel et al., 1986). Expression of GFP, driven by the renin promoter, in transgenic mice has been used as a sensitive marker for renin expression. Examination of the brains of transgenic rats has defined renin expression within various brain cardiovascular regulatory centers (Lavoie et al., 2004a; Lavoie et al., 2004b). Other studies show that suppression of angiotensinogen (Agt) expression in the brain results in a significant reduction in BP in SHR (Gyurko et al., 1993). Of major significance is the demonstration that intracerebroventricular (ICV) injection of an angiotensin II receptor antagonist results in reduced BP in bilaterally nephrectomized SHR, which removes the source of renin in the periphery (Phillips et al., 1977). Work in other tissue preparations such as the heart, blood vessels and kidney tubules have demonstrated persistent renin activity even after extensive washout (Bader et al., 2001). In these examples RAS function is compartmentalized and the synthesized Ang II acts locally. Together, these results provide support for a functional and capable RAS function in the brain (Bader et al., 2001; Kobori et al., 2007). Furthermore, localized RAS function suggest the existence of a renin binding protein or receptor that would facilitate this action.
Recent work by a number of investigators has demonstrated that the recently discovered PRR is an integral component of the tissue localized RAS that serves to bind renin and compartmentalize Ang II biosynthesis and function. In addition, PRR also stimulates intracellular signaling. In many respects this signaling mechanism mimics the effects mediated by the AT1R, independently of Ang II action (Batenburg and Jan Danser, 2008; Nguyen and Contrepas, 2008a; Nguyen and Contrepas, 2008b). Taken together, these and other studies lend support to the hypothesis that the brain can sustain a functionally sufficient RAS.
An increase in Ang II production and increased AT1R activation have already been found to reduce baroreflex sensitivity at the NTS (Averill and Diz, 2000; Boscan et al., 2001; Merrill et al., 1996) and increase neuronal activity in the RVLM (Li and Guyenet, 1996; Tagawa et al., 2000), which are proposed actions that are hypothesized to mediate neurogenic hypertension. Therefore establishment of the mechanism for increased Ang II production in the brain and demonstration that this mechanism is upregulated in neurogenic hypertension would open a new avenue for the treatment.
IV. Central components of the brain RAS and their properties
Each component of the RAS has been identified in the brain. Evidence of their role in central BP control and function in hypertension has been determined, both pharmacologically and by genetic manipulation. Data from multiple laboratories show that brain RAS components are compartmentalized within specific brain nuclei and even within specific cells, which would result in increased efficiency of RAS function. These findings help assert that a functional and competent RAS is active in the brain. Furthermore, as will be discussed further below, pathological insults in the organs of the systemic circulation or in the brain alter central RAS function that exacerbates hypertension (Francis et al., 2004; Tan et al., 2004). In this section we will re-examine the various RAS components in the brain and discuss their individual role in BP control and hypertension.
IV.i. Angiotensinogen
Angiotensinogen (Agt) is an alpha 2 globulin and is the substrate for renin. It is a member of the serine protease inhibitor or serpin family of enzyme inhibitors, although Agt is not known to interact with any other enzyme besides renin (Doolittle, 1983; Hunt and Dayhoff, 1980). The liver is the chief source of Agt for the systemic circulation, whereas the astroglia is the main site of Agt synthesis in the brain (Milsted et al., 1990; Stornetta et al., 1988). However, studies have also demonstrated that Agt is produced by neurons in primary culture (Kumar et al., 1988; Richoux et al., 1988; Thomas et al., 1992).
Various studies imply a role for brain Agt in hypertension. For example, four week old SHR, prior to the onset of hypertension, exhibit elevated Agt expression in the hypothalamus region compared to age-matched WKY rats (Tamura et al., 1996). A further study indicated that Agt mRNA levels are significantly increased in the hypothalamus of SHR, compared to WKY rats; while, no differences in Agt transcript levels were observed between the liver of each strain (Yongue et al., 1991). In a separate study, transgenic rats that over express Agt exhibit hypertension. However, the high blood pressure was present only in rat lineages that over-express Agt in the liver and in specific areas of the brain that are involved in the regulation of BP (Kimura et al., 1992). Other work comparing gene promoter activity in rat brains demonstrated that cis-regulatory elements of the Agt promoter, AGF2 and AGF3, were more active in SHR, when compared to that in WKY rats (Nishii et al., 1999). These studies indicate that a breakdown in the regulation of Agt expression in the brain regions that mediate control of cardiovascular tone contributes to the onset of hypertension.
Inhibition or suppression of Agt expression in hypertensive animal models normalizes blood pressure. For example, intracerebroventricular (ICV) injection of Agt-antisense oligonucleotides reduced basal BP levels in SHR, with no comparable effect observed following injections into WKY rat brain (Gyurko et al., 1993). Other reports show that transgenic suppression of Agt in rats demonstrate polyurea and polydipsia and their urine show decreased osmolality, which are all symptoms associated with central diabetes insipidus or type II diabetes (Schinke et al., 1999). Therefore, while reduction of Agt levels in the brain has some protective effects on blood pressure, inhibition of Agt expression can lead to other pathological complications.
IV.ii. Renin
Renin is a critical member of the RAS, and catalyzes the rate limiting step of converting Agt to Ang I. Brain renin activity was first reported within dogs and rats (Fischer-Ferraro et al., 1971; Ganten et al., 1971) and the existence of renin within the brain has now been demonstrated by numerous investigators (Bader et al., 2001). However, isolation of meaningful levels of the enzyme from the brain has proven to be a challenge. This is due, in part, to very low expression levels of renin in the CNS (Lippoldt et al., 2001; Paul et al., 1988).
Active renin is formed by the proteolytic cleavage of the prosegment region from prorenin. The prosegment is 43 amino acids long, and is situated at the amino terminus of prorenin (Mercure et al., 1995). Included in the prosegment is a “gate” region that blocks the enzyme’s active site. Epitope specific antibodies raised against various regions of the prosegment were used to identify the handle region of the prosegment. Antibodies that bound the handle region resulted in non-proteolytic activation of the enzyme (Suzuki et al., 2003). These findings have led to the hypothesis that the PRR mediates non-proteolytic activation of prorenin by binding the handle region peptide.
RAS components from different species are found to be poorly compatible with each other (Bohlender et al., 1997; Ganten et al., 1992; Hatae et al., 1994). This knowledge has since been exploited in subsequent transgenic animal studies, which revealed specific aspects of RAS function in the brain as well as in the circulation (Bader and Ganten, 2002; Morimoto et al., 2002; Morimoto and Sigmund, 2002). In one study, human-renin was expressed in the brain using neuronal specific promoters in transgenic mice, which were bred with mice that express human-Agt driven by the mouse Agt promoter. Transgenic mice showed an AT1R dependent dipsogenic response, which was blocked by ablation of the human Agt gene, specifically at the SFO (Sakai et al., 2007; Sinnayah et al., 2006). Transgenic mice did not demonstrate changes in circulating levels of Ang II, showing that the response was limited to the brain. These findings suggest that functional RAS activity can occur in the brain, independent of peripherally derived components, to mediate a response that is characteristic of the SFO.
IV.iii. Angiotensin converting enzyme
Early work described that the product of Agt conversion by renin, Ang I, was rapidly converted to Ang II. Testing both substances on aortic strips demonstrated that Ang II is the true pressor agent (Helmer, 1957). Further effort resulted in the isolation and characterization of the enzyme now called ACE (Skeggs et al., 1956). ACE is expressed in various regions of the brain, including areas that regulate BP control. Electron microscopic studies using neurons show that ACE is found on neuronal plasma membrane and is also co-localized in vesicles with Ang II as well as with the inhibitory neurotransmitter gama-amino-butyric acid (Pickel and Chan, 1995; Pickel et al., 1986). Pharmacological interruption of ACE activity in the brain provides further evidence of brain RAS function. Intracarotid administration of the ACE inhibitor captopril revealed a tonic role of Ang II in the forebrain that mediates excitation of renal sympathetic nerve activity. This effect is normally obscured by the baroreflex, but the authors of this study suggest that this tonic activity may play a more significant role in the advent of impaired baroreflex and augmented RAS function (Wei and Felder, 2002). Moreover, altered ACE function in the brain is found to be induced by pathological insults such as myocardial infarcts that exacerbate cardiac hypertrophy in rats. Induction of myocardial infarct, by coronary arterial ligation, stimulates an increased AT1R and ACE activity in the heart, kidney and the brain. In the brain AT1R binding and ACE activity are increased at various CVO as well as the PVN (Tan et al., 2004). Finally, genetic transfer of human ACE into the hypothalamus of rats exhibited increased sympathetic activity as well as increased Na+ intake, reduced urine volume and increased concentration. Rats over-expressing ACE also demonstrated elevated mean arterial pressure as well as increased vasopressin levels in the circulation, which were blocked by ICV injection of ACE inhibitors (Nakamura et al., 1999).
IV.iv. AT1R
The AT1R mediates the peripheral pressor response of Ang II and is also found at all major cardiovascular control centers in the brain, as well as in regions that regulate electrolyte and fluid balance. Some of these areas include the PVN, the RVLM, the NTS, and the SFO, amongst others (Allen et al., 1999; Obermuller et al., 1991; Phillips et al., 1993; Tsutsumi and Saavedra, 1991).
The AT1R is a member of the 7 transmembrane superfamily of receptors and couples to the G-protein Gαq/11/12 that activates phospholipase C (PLC) - β (de Gasparo et al., 2000). The AT1R also stimulates PLC- γ1 by activation of tyrosine kinases (Ushio-Fukai et al., 1998b). PLC catalyzes the conversion of the membrane lipid phosphoinositol-bisphosphate to inositol-trisphosphate and diacylglycerol that lead to cytosolic Ca2+ mobilization and protein kinase C (PKC) activation (Berridge and Irvine, 1989; Ushio-Fukai et al., 1998b).
In addition, Ang II has been shown to increase reactive oxygen species (ROS) formation in endothelial cells, vascular smooth muscle cells as well as in neurons in a mechanism that involves the activation of xanthine oxidase (Chan et al., 2005; Griendling et al., 1994; Griendling and Ushio-Fukai, 1998; Sun et al., 2005). ROS, by themselves, mediate the stimulation of mitogen activated protein (MAP) kinases, including P38 and extracellular regulated kinase (ERK) 1/2 (Griendling et al., 1994; Rao and Berk, 1992; Ushio-Fukai et al., 1998a). ROS formation mediated by AT1R is also found to stimulate PI3 kinase and AKT/Protein kinase B (PKB) activity (Ushio-Fukai et al., 1999). There is also much evidence that Ang II elicits a PKC-dependent increase in NADPH oxidase activity, and that the reactive oxygen species that are generated serve to increase neuronal firing(Sun et al., 2005; Sun et al., 2002).
AT1R stimulation in neurons increases action potential-, or chronotropic-activity mediated by decreasing IA and IKV type potassium currents and by increasing Ca2+ channel current (Zhu et al., 1997). In addition, AT1R mediates increases in Ca2+ channel density at the membrane mediated by PI3-kinase (Macrez et al., 2001). Dissociated G-protein Gβγ subunit stimulates PI3-kinase and AKT/PKB to produce increased current (Macrez et al., 2001; Viard et al., 2004; Viard et al., 1999).
The AT1R can potentiate catecholaminergic signaling. This effect has an acute and a chronic component that is dependent on posttranscriptional and transcriptional mechanisms, respectively (Lu et al., 1996b). The chronic effects are mediated by increased transcription of tyrosine hydroxylase, dopamine β-hydroxylase and the norepinephrine transporter that involve MAP kinase signaling pathway (Lu et al., 1998a; Lu et al., 1996a; Lu et al., 1996b). This is mediated, in part, by a signaling cascade that involves activation of MAP kinase(Lu et al., 1998b; Lu et al., 1996b; Yang et al., 1996). In turn, MAP kinase activates transcription factors that bind to AP1 cis elements found in the promoter regions of the tyrosine hydroxylase, dopamine β-hydroxylase and the norepinephrine transporter genes (Goc and Stachowiak, 1994; Seo et al., 1996).
The AT1R is involved in control of SNA to modulate BP, to mediate control of fluid and electrolyte homeostasis and suppresses sensitivity to the baroreflex (Averill and Diz, 2000; Boscan et al., 2001; Hogarty et al., 1992; McKinley et al., 2001; Merrill et al., 1996; Veltmar et al., 1992). The RVLM of SHR rats show increased receptor density compared to WKY rat (Hu et al., 2002). Furthermore, bilateral injection of AT1R antagonists results in significant reduction in BP in hypertensive animal models, while no significant effect is seen in normotensive rats (Allen, 2001; Ito et al., 2003; Ito et al., 2002). SHR rats were found to have increased AT1R density in the RVLM compared to WKY rats. This same study showed that microinjection of Ang II into the RVLM of SHR mediated increased excitatory amino acid release in the IML compared to WKY rats, which would result in increased sympathetic activity (Hu et al., 2002).
Transgenic rats over-expressing AT1R, specifically in neurons, showed an exaggerated response to Ang II treatment administered ICV, which was curtailed by losartan pretreatment. However, basal BP levels were not significantly different compared to control rats (Lazartigues et al., 2002). Together, these results indicate that AT1R does have a role in mediating chronic hypertension, but that over-expression of the receptor itself is not sufficient to mediate this pathology, but requires concomitant activation.
In a more recent study, a constitutively active AT1R was over-expressed in the RVLM that resulted in a chronic increase in BP. Of interest is that histological examination showed that the exogenous receptor was expressed mostly in glial cells (Allen et al., 2006). Finally, these findings also suggest that glial cells may play more than a passive role in BP control in the brain.
In normotensive rats the role of AT1R in modulating basal BP in the brain is less defined. Peptide angiotensin receptor inhibitors, sarthran and sarile, reduced SNA and BP in normotensive rats (Ito and Sved, 1996; Tagawa et al., 1999). Subsequent studies questioned whether these inhibitory effects are in fact mediated by the AT1R. While microinjection of Ang II mediated an increase in BP that was blocked by losartan pretreatment, basal BP levels were unaffected (Hirooka et al., 1997). Treatment with an AT1R selective inhibitor in SHR resulted in a decrease in basal SNA and BP, which was not demonstrated in WKY rats (Allen, 2001). The work of Ito and colleagues found that while sarthran decreased BP in Sprague Dawley rats, this was not replicated by AT1R specific antagonists, but was inhibited by aminopeptidase A (APA) inhibitor, amastatin (Ito and Sved, 2000). This suggests that the modulator of basal BP in normotensive animal may be Ang III (more below) and the mediator of transient increased BP is mediated by the Ang II and the AT1R.
V. The (Pro)renin Receptor
V.i Discovery of prorenin receptor
In spite of the fact that there is strong evidence for the existence of all the components of an intrinsic RAS, or RAS-like mechanism in the brain, it has been difficult to resolve whether this system is functional. Some investigators have argued that a brain RAS mechanism is likely to operate on a microscopic level such as that seen in the heart, kidney, adrenal glands and vascular tissue (Bader et al., 2001). The recently discovered PRR has been found to have a key modulatory role in tissue localized RAS (Jan Danser, 2003; Nguyen and Contrepas, 2008a; Nguyen et al., 2002). PRR mRNA levels are highly expressed in the brain, comparable to levels seen in the heart and placenta and greater than the levels seen in the kidney, liver and pancreas (Nguyen et al., 2002). PRR is also a functional receptor, which triggers intracellular signaling cascades. Of interest is that the PRR, itself, stimulates signaling pathways that are hallmarks of AT1R activation (Burckle and Bader, 2006; Jan Danser, 2003; Nguyen and Contrepas, 2008a; Nguyen et al., 2002). In fact, prior to its discovery, evidence of PRR action had been reported over the past two decades through connections involving anomalous renin or prorenin expression and pathologies that include renal and cardiac hypertrophy, which could not be fully explained by altered RAS function. Some of these findings are discussed below.
The RAS has been known to increase tissue and organ hypertrophy in conditions that exacerbate hypertension, such as diabetes. For example, increased blood glucose levels increased Agt expression (Liu et al., 2008; Zhang et al., 2001). Of note is a report of a clinical finding that a high ratio of inactive renin to active renin is correlated with certain pathological conditions such as diabetes, retinopathy and albuminuria (Luetscher et al., 1985). Prorenin to renin ratios of 5 to 10 fold were seen in normal or asymptomatic conditions. Ratio levels reached from 20 fold to as much as 200 fold were observed in pathological conditions, with increasing ratios seen in more severe conditions (Luetscher et al., 1985). Furthermore, comparison of renin activity levels showed no significant differences in these groups regardless of the severity of the pathology. What role prorenin had in these pathological conditions could not be determined. However, they can be explained with what we now understand of PRR function. Current understanding of the PRR can explain the anomalous results from animal studies that over-expressed mouse renin in rats, described below.
Early attempts to link increased RAS function to hypertension was performed by transgenically over-expressing the mouse renin-2 isoform, TGR(mRen2)27 in rats. Plasma Ang I and Ang II levels were found to be unaffected and renin activity was actually reduced compared to the control rats in all areas except at the adrenal glands. However, prorenin levels were significantly increased as well as aldosterone levels. Complicating the interpretation was the finding that treatment with ACE-inhibitor, captopril, showed little effect on blood pressure (Mullins et al., 1990). In a similar study, human renin was over-expressed in the liver of rats driven by the alpha1-anti-trypsin promoter (Veniant et al., 1996). Male rats exhibited a 400 fold increase in renin levels, which was accompanied by chronic increase in BP. Interestingly, female rats only showed a 2-3 fold increase in renin expression and showed no difference in blood pressure compared to control rats. In-situ hybridization experiments showed increase prorenin mRNA levels only at the liver, with no change in the kidneys. Also, plasma renin activity was not different compared to control rats. Histological analysis showed increased lesions in cardiac, vascular and renal tissue. In the kidneys, male rats showed nephroangiosclerosis, glomerulosclerosis, tubulointerstitial atrophy, inflammation and arterial wall thickening. In the heart, the cardiomyocytes as well as the aortic wall were hypertrophic and there was increased coronary fibrosis (Veniant et al., 1996). Thus, it appears that the increased BP in mice was dependent on renin over-expression, but unrelated to RAS activity. The lack of RAS function could be explained by work showing poor interspecies compatibility of RAS components. Human renin has very poor reactivity for rat or mouse Agt and mouse or rat renin is inefficient to catalyze human Agt (Bohlender et al., 1997; Ganten et al., 1992; Hatae et al., 1994).
In an apparently separate direction of study, researchers have discovered that RAS function occurred locally at the tissue level. Concentrated Ang II had been reported in renal tissue at levels that could not be explained by formation in the circulation (Campbell et al., 1991). Studies have shown that Agt, ACE and discreet levels of renin are synthesized locally in the kidney tubules (Das and Soffer, 1976; Henrich et al., 1996; Ingelfinger et al., 1990; Moe et al., 1993). Further studies in vascular tissue preparations showed that renin administration maintained catalytic activity even after prolonged washout (Muller et al., 1995). These studies, coupled with the findings that ACE is expressed in the vessel wall during vascular stress, suggested the presence of a renin binding protein or receptor that could sequester renin for localized function (Jan Danser, 2003; Jan Danser and Deinum, 2005; Muller et al., 1997). The first candidate for a renin binding protein was the mannose-6-phosphate / insulin-like growth factor II. This receptor binds and internalizes mannosylated proteins with high affinity. This receptor also activated prohormones, including prorenin upon internalization. However, this receptor acts as a clearance receptor, which targets proteins for degradation (Jan Danser, 2003; Nguyen, 2006).
The PRR was first reported in mesangial cells. These cells showed renin binding affinity in the subnanomolar range. The (pro)renin receptor was later cloned and found to be a 350 amino acid protein and shown to migrate at 42 kD protein on western blot (Nguyen et al., 2002). The PRR binds and sequesters renin which results to increase catalytic activity by up to fivefold compared to soluble renin. In addition, PRR activates the precursor, prorenin, non-proteolytically. The second function stimulated by renin binding is receptor mediated signal transduction. (Nguyen et al., 2002; Schefe et al., 2006).
V.ii Properties of the Prorenin receptor
PRR mRNA levels in the brain are reported to be at very high levels, in humans. In spite of this, little information is available to even detail its distribution in this organ. In order to infer the possible roles of this receptor in the brain it is important to examine its function in organs and tissues such as the kidney, heart and smooth muscle cells. These studies provide evidence for PRR’s mediated facilitation of localized tissue RAS and show its role in tissue hypertrophy and organ failure, mediated by its intrinsic signaling properties. Furthermore, studies of receptor over-expression and diabetes show that PRR can mediate actions that are normally attributed to hyperactive AT1R stimulation. In some cases they show that PRR can mediate pathological actions in the absence of the AT1R.
The PRR gene, previously identified as ATP6AP2, is a 350 amino acid protein with one single transmembrane domain, with evidence to suggest that it functions as a dimerized receptor (Nguyen et al., 2002). Activation by renin caused phosphorylation of the receptor that is associated with MAP kinase activation. Western blotting shows that it is phosphorylated at two residues believed to be 337S and 355Y, which occur by 3 minutes and 10 minutes, respectively (Nguyen et al., 2002). In addition, the PRR is shown to have a number of regulatory motifs that regulate its function and localization. One of the regulatory motifs is an atypical endoplasmic reticulum-retention motif and the other is an endosomal/lysosomal sorting motif (Burckle and Bader, 2006). The ER retention motif K346IRMD, conforms to the dibasic KxRxx consensus sequence. The lysosomal sorting motif is Y335DSI that conforms to the consensus YxxØ (Ø = large hydrophobic residue) (Burckle and Bader, 2006). Scheffe and co-workers confirmed the function of these motifs on PRR localization. They tested three PRR constructs: a full length form, a PRR(K346 - R) substituted construct and an extracellular deleted - carboxyl-terminal construct. The full length GFP tagged form displayed predominantly perinuclear expression, with diffuse cytosolic expression. Expression of the PRR(K346 - R) construct lost the perinuclear localization and showed diffuse expression in the cell. The carboxyl-terminal construct exhibited strong co-localization at lysosomal vesicles (Schefe et al., 2006).
Prorenin binding to PRR equilibrates by 2 hours, and the half life for dissociation is approximately 4 hours in VSMC cells, similar to the half life of renin dissociation in mesangial cells (Batenburg and Danser, 2008; Batenburg et al., 2007; Nguyen et al., 1996). Several laboratories have examined binding affinity of renin and prorenin to the PRR (Nabi et al., 2006; Nguyen et al., 1996; Nguyen et al., 2002; Nurun et al., 2007). In vascular smooth muscle preparations the binding of the renin precursor, prorenin, was increased in PRR over-expressing cells compared to control cells; however, renin binding was not affected in cells that express exogenous PRR. The authors concluded that this is an indication that prorenin is the true agonist for PRR (Batenburg et al., 2007).
PRR mRNA is highly expressed in the brain (Nguyen et al., 2002). Our laboratory has reported on the presence of PRR mRNA expression in brain neurons determined by real time PCR and immunoreactivity (Shan et al., 2008b). In primary neuronal cultures we show high PRR mRNA levels in brain neurons and low expression levels in glial cells (Shan et al., 2008b).
V.iii Implication of the brain (pro)renin receptor and Ang II signaling
The role of Ang II action in the brain, in the modulation of cardiovascular tone, is not in dispute; and the existence of an intrinsic RAS in the brain is generally accepted (Guyenet, 2006; Veerasingham and Raizada, 2003). However, the mechanism of the biosynthesis of the RAS products has been questioned due to the poor expression of renin in the brain (Bader and Ganten, 2002; Lippoldt et al., 2001). Whether the effect on vascular tone and fluid and electrolyte balance are mediated by Ang II, Ang III, or subsequent metabolites, the processing of Agt to its final active product in the brain would require physiologically significant renin or renin like activity in the brain (Pan, 2004; Reaux et al., 2001; Veerasingham and Raizada, 2003). In this matter, the recent discovery of the PRR may help elucidate the mechanism for a brain localized RAS. PRR facilitated RAS function has been demonstrated in tissue preparations and in recombinant cell lines (Batenburg et al., 2007; Nguyen et al., 2002; Nurun et al., 2007); however, to our knowledge, no work has yet described PRR facilitated RAS in the brain. The presence of PRR in the brain would help tie together findings of anomalous expression and activity of RAS components in the brain that lead to hypertension.
Studies have shown that Agt expression in CV regulatory centers in the brain are increased in SHR, compared to WKY rat, which occur prior to the onset of hypertension (Tamura et al., 1996). This finding is highlighted in a study, which demonstrates that over-expression of Agt, in the brain as well as the liver, precedes the onset of chronic increase in BP in mice (Kimura et al., 1992). Moreover, this same study showed that hypertension was not observed in other transgenic lines when Agt was not over-expressed in key cardiovascular regulatory brain nuclei. Elevated brain renin-like activity has been reported in the anterior hypothalamus and NTS of SHR compared to WKY rats during the onset of hypertension (Ruiz et al., 1990). Furthermore, Ang II immunoreactivity and turnover are reported to be increased in the PVN and SON of adult SHR compared to WKY rat (Ganten et al., 1983; Hermann et al., 1984; Phillips and Kimura, 1988; Weyhenmeyer and Phillips, 1982). Furthermore, persistent Ang II levels as well as chronically elevated BP are reported in bilaterally nephrectomized SHR, which is the chief source of renin for the circulatory system. The increased BP was suppressed only after ICV administration with ACE inhibitors (Hermann et al., 1984; Phillips et al., 1977; Trolliet and Phillips, 1992). Finally, combined with studies that show that ICV injected Ang II and Ang III levels have very short half lives of 23 and 7.7 seconds, respectively (Harding et al., 1986), these findings suggest that the brain must have a capable RAS or RAS related mechanism in place in the brain.
In studies performed in recombinant cells as well as in tissue preparations PRR facilitated RAS function by binding and sequestering renin and prorenin (Figure 1). In doing so PRR activates prorenin, non-proteolytically and increases the catalytic activity of both enzymes (Batenburg et al., 2007; Nguyen et al., 2002; Nurun et al., 2007). PRR shows high expression levels in the brain and preliminary data show elevated expression levels in the hypothalamus (Nguyen et al., 2002). The co-localization of PRR with ACE would suggest an increase in the efficiency of localized RAS activity. Our laboratory has begun to examine PRR expression in the brain and our initial findings show increased immunoreactive density in the PVN, SON, NTS and the ventrolateral medulla of rats, which is corroborated by real time-PCR experiments examining PRR transcript levels (Shan et al., 2008a). It would be of importance to see if PRR is proximally localized to ACE as well as the AT1R in the cardiovascular regulatory brain centers. Furthermore, it would be of interest to compare PRR expression levels as well as function in various CV brain regions during development in various animal models of hypertension.
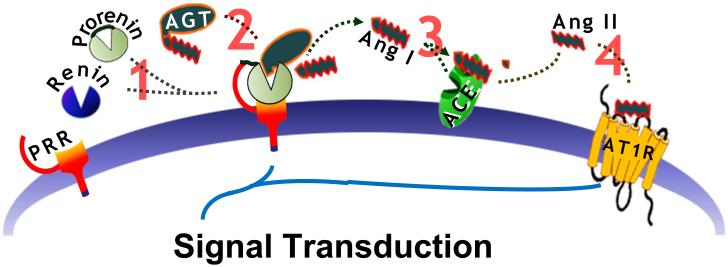
Figure1.
The roles that PRR is proposed to have, in the cardiovascular regulatory centers in the brain to mediate control of BP. PRR is hypothesized to facilitate localized tissue RAS function. 1) By binding and sequestering renin or prorenin in the brain, PRR increases the catalytic efficiency of 2) Agt conversion to Ang I. In this way the PRR is hypothesized to mitigate the limited expression of renin in the brain. 3) Membrane bound ACE, which is expressed in the brain, will then convert Ang I to Ang II in order to 4) stimulate the AT1R.
V.iv Implications on neuronal signaling by (pro)renin that is independent of AT1R
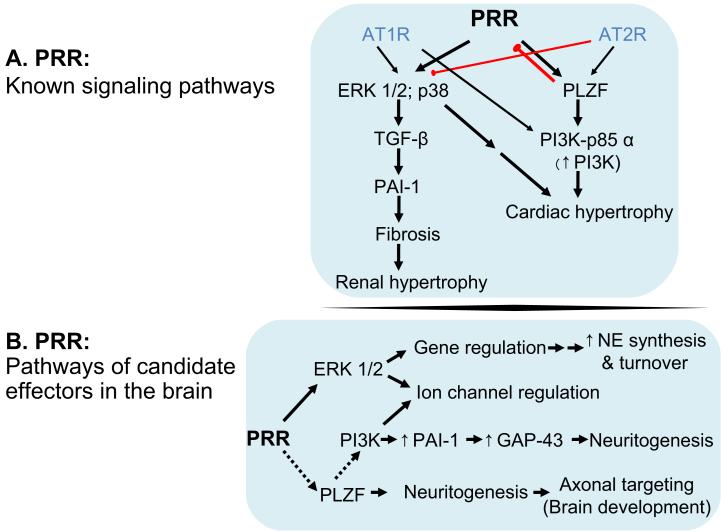
Most of PRR signaling cascade can be mapped to two known downstream signaling messengers, MAP kinase and the promyelocytic leukemia zinc finger (PLZF) transcription factor (Schefe et al., 2006; Schefe et al., 2008). Figure 2A illustrates that PRR’s downstream signaling events have been linked to profibrotic and proliferative activity that leads to hypertrophy and end organ damage in the heart, kidney and liver (Campbell, 2008; Jan Danser et al., 2007).
Figure 2.
Downstream signaling by the PRR and role of potential effectors in brain neurons. Top, Activation of PRR by renin or prorenin activates MAP kinases such as ERK 1/2 in the kidney, smooth muscle cells and in brain neurons as well as p38 in the heart. MAP kinase activation by PRR activates TGF-β to promote PAI-1secretion, which leads to fibrosis. In addition PRR stimulates the transcription factor PLZF, which mediates upregulation of the PI3 kinase adaptor protein p85α that promotes PI3-kinase activity. Bottom, Of the potential downstream effector of PRR, in the brain, only ERK 1/2 has been demonstrated, thus far. ERK 1/2, itself mediates gene regulation that include upregulation of enzymes and transporter proteins that promote norepinephrine turnover. ERK 1/2 is also found to modulate K+ channel function. Future studies may show that PRR is linked PLZF function to mediate neuronal maturation in the brain. PLZF is present in various regions of the brain where it is important for neuronal organization. PI3-kinase function, linked to PLZF action can further link PRR to channel regulation, in addition, promoting neurite growth. These actions are linked to tissue hypertrophy and end organ failure and also mediate neurogenic hypertension. Black Arrows, established signal transduction pathways; red arrow, inhibitory pathways; dotted lines, candidate pathways.
PRR transcript and protein expression have been reported in cultured brain neurons and mRNA transcripts are found to be highly expressed in the human brain (Nguyen et al., 2002; Shan et al., 2008b). Renin application to cultured brain neurons has been found to stimulate ERK 1/2 signaling in a concentration dependent manner (Shan et al., 2008b); while, PRR mediated PLZF signaling in neurons has not been demonstrated. In fact, to date, little has been reported on PRR’s signaling mechanism in the brain. However, as is the case with AT1R signaling, many of the downstream events that occur in the periphery also function in brain neurons and PRR may mediate similar events that are seen in the periphery.
Our laboratory has shown that ERK 1/2 activation, mediated by the AT1R, increase norepinephrine biosynthesis and turnover (Figure 2A), in part, by increasing the production of tyrosine hydroxylase, dopamine-β-hydroxylase and the norepinephrine transporter (Lu et al., 1998a; Lu et al., 1996a; Lu et al., 1996b). Our laboratory has detected PRR immunoreactivity in various regions of the hypothalamus, including the PVN, NTS and the ventrolateral medulla (unpublished observations). Thus, activation of PRR in these regions can conceivably upregulate enzymes that promote norepinephrine signaling (Figure 2B).
Similar to the AT1R, PRR is found to stimulate the production of the plasminogen activator-inhibitor (PAI)-1 via the sequential activation of ERK 1/2 production of transformation growth factor- β (TGF-β) (Huang et al., 2008; Huang et al., 2007; Huang et al., 2006). PAI-1 promotes fibrosis by acting as an inhibitor for plasminogen to decrease fibrinolysis (Figure 2A). AT1R mediated induction of PAI-1 also occurs in brain neurons (Yang et al., 1996; Yu et al., 1996). Stimulation of the AT1R in neurons activates phosphatidylinositol-3-kinase (PI3-kinase) which will sequentially activate protein kinase B (PKB)/AKT, p70s6-kinase to produce PAI-1. Secretion of PAI-1 results in increased expression of growth associated protein (GAP)-43, which mediates neurite extension (Yang et al., 1996; Yang and Raizada, 1999; Yang et al., 2002; Yang et al., 2001; Yu et al., 1996). PRR upregulates the p85α subunit of PI3-kinase mediated by the stimulation of PLZF transcription factor (Schefe et al., 2006; Schefe et al., 2008). In the brain, PLZF is reported to be expressed in a segmented pattern in the hypothalamus and hindbrain, where it is linked to the modulation of neuronal growth and targeting (Avantaggiato et al., 1995; Ivins et al., 2003). The p85α subunit of PI3-kinase, a splice variant of the p85 regulatory adaptor for PI3-kinase, is important for maintaining the p110 catalytic subunit of PI3-kinase (Steinberg, 2001; Vanhaesebroeck et al., 1997; Vanhaesebroeck and Waterfield, 1999). Thus, the involvement of PI3-kinase activation by PRR, in the brain may be found to play a role in neuronal development (Figure 2B).
The PRR may be important to brain development via its association with PLZF activation (Figure 2B). This may be suggested from the unsuccessful attempts to breed a PRR animal knockout (Burckle and Bader, 2006). In addition, recent investigation has found that a silent mutation (c.321C>T, p.D107D) resulted in the truncation of the open reading frame of the PRR mRNA. This truncation is associated with X-linked mental retardation and epilepsy. Recombinant expression of this PRR construct showed a modest, but significant deficit in ERK 1/2 phosphorylation (Ramser et al., 2005). However, it would be of interest to see if the PRR signals via PLZF in the brain and if this is affected by this mutation.
PRR mediated facilitation of Ang II formation, alone makes is a potentially powerful component for RAS in the brain. However, the coupling of prorenin to PRR can also mediate MAP kinase signaling pathways, in concert with activation by the AT1R. This would suggest that PRR may play a role in neurogenic hypertension. If this is found to be the case this would open a new avenue for therapeutic treatment strategies for neurogenic hypertension. Further demonstration that PRR is linked to PLZF signaling suggest that it may be further linked to BP and may be revealed to be essential to brain development.
VI.v Pharmacological profile of the Prorenin Receptor
The discovery of the prorenin receptor has given way to new answers in regards to formerly unexpected findings in the search for treatments of hypertension and its associated disorders. The detrimental effects that can potentially be caused by hyperactivation of the PRR, centrally and in the periphery, demands the need for an inhibitor for the receptor, both as a diagnostic tool as well as for treatment in the periphery as well as in the brain.
There are a few options for pharmacological characterization of PRR as a diagnostic tool. As stated, both renin and prorenin catalytic activity are potentiated by PRR. Conversely, renin and prorenin stimulate the PRR. However, the use of prorenin has advantages over renin as a measure for PRR function. Renin is enzymatically active in soluble form, while prorenin has poor catalytic activity until activated, non-proteolytically, upon binding to PRR (Nguyen et al., 2002; Suzuki et al., 2003). Moreover, binding studies suggest that prorenin is likely the true agonist for PRR, as it was found to be more sensitive in the quantitation of PRR over-expression in smooth muscle cell preparations, compared to renin in receptor binding experiments (Batenburg et al., 2008). Therefore, the difference in catalytic activity in the two enzyme forms can be a sensitive measure for qualitative assessment of PRR action.
The interspecies incompatibility of renin and Agt can be exploited to distinguish between the PRR facilitated Agt conversion component and the receptor mediated signaling. As described previously, renin of human origin will have poor catalytic activity for mouse or rat Agt or Agt-derived substrate (Bohlender et al., 1997; Ganten et al., 1992; Hatae et al., 1994). Rats and mice that transgenically over-express human PRR exhibit increased MAP kinase activity in the kidney and heart (Burckle et al., 2006; Kaneshiro et al., 2007) suggesting that the human form is activated by the endogenous mouse renin or prorenin. Thus, application of human renin to rat brain cultures mediated stimulation of ERK 1/2 phosphorylation. Thus prorenin or renin from one species can be used to stimulate PRR from another species with little contamination from AT1R mediated by endogenous Agt conversion. In addition renin inhibitors, such as aliskiren that blocks Agt conversion did not impair renin or prorenin coupling to PRR stimulated MAP kinase phosphorylation (Feldman et al., 2008; Feldt et al., 2008a; Feldt et al., 2008b). This shows that PRR mediated signaling can be isolated from PRR’s facilitation of renin or prorenin catalytic action.
One potential inhibitor of the PRR is the prorenin derived “handle region peptide” (HRP), which has also been referred to as the “PRR-blocker” (Ichihara et al., 2004; Kaneshiro et al., 2007). Several studies have shown the remarkable properties for inhibiting PRR mediated hypertrophy, both in the periphery as well as in brain neurons. HRP is derived from the 43 amino acid prosegment of prorenin that is proteolytically cleaved off for maturation of active renin. The HRP is a five amino acid segment, 11IFLKR15 of human prorenin, which is homologously conserved between mice and rats. It is situated contiguously to the carboxyl end of the gate region peptide 7TFKR10 (Suzuki et al., 2003).
HRP is described to have protective effects on PRR mediated hypertrophy in the streptozotocin induced diabetic model. Streptozotocin selectively destroys the beta cells in the pancreas (Szkudelski, 2001). Rats treated with this agent exhibit pathophysiology associated with diabetes, which includes elevations of blood glucose, urinary protein excretion and prorenin levels. Treated rats also showed reduction in body weight, plasma renin as well as reduced plasma Ang II levels (Ichihara et al., 2004). In the kidney, total renin, Ang I and Ang II levels were elevated, in contrast to plasma levels. In addition, these rats showed increased collagen expression and extensive glomeroschlerosis in the kidney. When streptozotocin was co-applied with HRP, the increased Ang I and Ang II levels were inhibited and glomeroschlerosis was suppressed (Ichihara et al., 2004). Application of mouse prorenin derived HRP containing HRP not only inhibited nephroschlerosis, but was also reported to reverse the scarring (Ichihara et al., 2006b; Takahashi et al., 2007).
The beneficial effects of HRP are not limited to diabetes. Studies show that stroke prone SHR (SHRsp) have elevated PRR mRNA levels in the heart and developed extensive fibrosis when examined in the left ventricle when compared to normotensive Wistar Kyoto (WKY) rat (Ichihara et al., 2006a). Rat-prorenin derived HRP treatment did reduce heart renin levels and suppressed collagen I/III levels. Our laboratory has found that renin application to neuronal brain cultures slows the frequency of spontaneous action potential formation, an effect that was blocked by HRP pretreatment (Shan et al., 2008b).
However, the HRP has also been met with significant controversy. The inhibitory effects of HRP on renin or prorenin binding to the PRR and coupled functions could not be confirmed in other studies (Batenburg et al., 2008; Batenburg et al., 2007; Feldman et al., 2008; Feldt et al., 2008a; Feldt et al., 2008b). Functional assays to measure renin and prorenin coupling to PRR are reported to be inhibited by HRP in recombinant PRR in COS-7 cells (Nurun et al., 2007). However, HRP failed to inhibit prorenin binding to PRR in vascular smooth muscle cells from either wild type rats or rats expressing human PRR (Batenburg et al., 2008). HRP treatment was ineffective in treating the 2 kidney-1 clipped Goldblatt rat hypertensive rat model that exhibited an increase in prorenin mRNA levels (Muller et al., 2008). While one study showed that HRP will stop the progression of renal hypertrophy in transgenic rats that over-express human PRR, another study failed to repeat these findings (Feldt et al., 2008b). Furthermore, in-vitro studies performed to measure PRR mediated ERK 1/2 phosphorylation were not inhibited by HRP treatment in either monocytes or in vascular smooth muscle cells, which express PRR (Feldt et al., 2008a; Feldt et al., 2008b).
What exactly is the cause of the discrepancy in the use of prorenin derived HRP to block prorenin effects mediated by PRR? One suggestion is that the dose or concentration range of HRP treatment must be fully characterized, with consideration of pharmacodynamics for in-vivo studies (Batenburg and Danser, 2008). Alternatively, it has been suggested that HRP’s beneficial effects may be due to action at an undiscovered target (Batenburg and Danser, 2008; Batenburg and Jan Danser, 2008). Hints that the latter may be the case has been provided by Ichihara and Kaneshiro (2006) who indicated that HRP may have weak agonistic function of its own. For now, until the controversy of HRP’s antagonistic function has been fully addressed the best method to pharmacologically inhibit PRR’s function is to suppress its expression by using siRNA (Huang et al., 2007; Huang et al., 2006; Schefe et al., 2006).
VII. Conclusion: Implications for PRR on autonomic regulation
Overexpression and increased activity of AT1R in the brain has been suggested to promote hypertension (Dampney, 1994; Veerasingham and Raizada, 2003). This has been demonstrated by direct infusion of Ang II into the brain at important BP centers, such as at the PVN and RVLM, which results in increased BP (Dampney, 1994; Pan, 2004). Similarly, treatment of AT1R antagonists at the RVLM of SHR resulted in marked reduction in BP with only modest to no effect observed in normotensive WKY rats (Veerasingham and Raizada, 2003). Furthermore, receptor binding studies have shown that AT1R levels are elevated in SHR compared to normotensive control WKY rats (Dampney, 1994; Veerasingham and Raizada, 2003). Thus, it is generally accepted that AT1R expression in the brain may at least contribute to hypertension and some argue that these receptors may play a causative role in neurogenic hypertension (Dampney, 1994; Ito et al., 2003; Ito et al., 2002; Veerasingham and Raizada, 2003). However, over-expression of the AT1R, alone, does not mediate increased blood pressure. Increased Ang II turnover is necessary to mediate increased SNA and increased BP (Allen et al., 2006; Lazartigues et al., 2002). The PRR may be key in this regard. The human brain is reported to show one of the highest levels of PRR mRNA levels when compared to other tissue (Nguyen et al., 2002). Any effect that would lead to increased expression of PRR in the brain may result in increased RAS activity. This phenomenon has been demonstrated to occur in both the heart and kidney (Ichihara et al., 2004; Ichihara et al., 2006a). The PRR can potentially mediate increased SNA by modulating norepinephrine signaling. This can be achieved in two ways: 1) by promoting AT1R activation and downstream activation of MAP kinase activity (Lu et al., 1998b; Lu et al., 1996b; Yang et al., 1996); or 2) by direct activation of MAP kinase signaling by the PRR itself, the latter of which has been demonstrated by our laboratory (Shan et al., 2008b).
One important consideration is that the current treatment of hypertension includes AT1R blockers and ACE inhibitors. In addition, the orally active renin inhibitor, Aliskiren, also shows promise for treatment. The latter of which, prolongs the half life of the PRR agonists, prorenin and renin and neither affect PRR signaling (Batenburg and Danser, 2008; Batenburg et al., 2008; Huang et al., 2007; Huang et al., 2006). Therefore, such treatments would give the PRR increased opportunity to promote hypertrophy in the tissues where it is expressed. Our initial data, from rat brains, show PRR immunoreactivity and mRNA levels in the cardiovascular control centers of rats and further show that some of these regions exhibit increased expression in SHR compared to WKY rats (Shan et al., 2008a). This suggests that expressional regulation of the PRR is an important factor in the regulation of BP tone. A significant change in basal PRR expression may have a detrimental impact on BP control. Further investigation of the actions of PRR in the CV regulatory centers in the brain may help to detail the mechanism of brain RAS function and the establishment of neurogenic hypertension.
Abbreviations
- Agt
Angiotensinogen
- Ang
angiotensin
- AT1R
Ang II-type 1 receptor
- ACE
Angiotensin converting enzyme
- AVP
arginine-vasopressin
- BP
blood pressure
- ERK
extracellular regulated kinase
- IML
intermediolateral cell column of the spinal cord
- ICV
intracerebral-ventricular
- MAP kinase
mitogen activated protein kinases
- NTS
nucleus of the solitary tract
- PVN
paraventricular nucleus
- PLC
phospholipase C
- PI3-kinase
phosphatidylinositol-3-kinase
- (PAI)-1
plasminogen activator-inhibitor
- PRR
(pro)renin receptor
- PKB
Protein kinase B
- PKC
protein kinase C
- ROS
reactive oxygen species
- RAS
renin-Angiotensin system
- RVLM
rostral ventrolateral medulla
- SHR
spontaneously hypertensive rat
- SNA
sympathetic nerve activity
- (WKY) rat
Wistar Kyoto
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
VII. References
- Allen AM. Blockade of angiotensin AT1-receptors in the rostral ventrolateral medulla of spontaneously hypertensive rats reduces blood pressure and sympathetic nerve discharge. J Renin Angiotensin Aldosterone Syst. 2001;2:s120–124. doi: 10.1177/14703203010020012101. [DOI] [PubMed] [Google Scholar]
- Allen AM, et al. Expression of constitutively active angiotensin receptors in the rostral ventrolateral medulla increases blood pressure. Hypertension. 2006;47:1054–1061. doi: 10.1161/01.HYP.0000218576.36574.54. [DOI] [PubMed] [Google Scholar]
- Allen AM, MacGregor DP, McKinley MJ, Mendelsohn FA. Angiotensin II receptors in the human brain. Regul Pept. 1999;79:1–7. doi: 10.1016/s0167-0115(98)00138-4. [DOI] [PubMed] [Google Scholar]
- Avantaggiato V, et al. Developmental analysis of murine Promyelocyte Leukemia Zinc Finger (PLZF) gene expression: implications for the neuromeric model of the forebrain organization. J Neurosci. 1995;15:4927–4942. doi: 10.1523/JNEUROSCI.15-07-04927.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averill DB, Diz DI. Angiotensin peptides and baroreflex control of sympathetic outflow: pathways and mechanisms of the medulla oblongata. Brain Res Bull. 2000;51:119–128. doi: 10.1016/s0361-9230(99)00237-3. [DOI] [PubMed] [Google Scholar]
- Bader M, Ganten D. It’s Renin in the Brain: Transgenic Animals Elucidate the Brain Renin-Angiotensin System. Circ Res. 2002;90:8–10. [PubMed] [Google Scholar]
- Bader M, et al. Tissue renin-angiotensin systems: new insights from experimental animal models in hypertension research. J Mol Med. 2001;79:76–102. doi: 10.1007/s001090100210. [DOI] [PubMed] [Google Scholar]
- Bakris G, Bursztyn M, Gavras I, Bresnahan M, Gavras H. Role of vasopressin in essential hypertension: racial differences. J Hypertens. 1997;15:545–550. doi: 10.1097/00004872-199715050-00011. [DOI] [PubMed] [Google Scholar]
- Bankir L, Fernandes S, Bardoux P, Bouby N, Bichet DG. Vasopressin-V2 receptor stimulation reduces sodium excretion in healthy humans. J Am Soc Nephrol. 2005;16:1920–1928. doi: 10.1681/ASN.2004121079. [DOI] [PubMed] [Google Scholar]
- Bankir L, Perucca J, Weinberger MH. Ethnic differences in urine concentration: possible relationship to blood pressure. Clin J Am Soc Nephrol. 2007;2:304–312. doi: 10.2215/CJN.03401006. [DOI] [PubMed] [Google Scholar]
- Barnes KL, DeWeese DM, Andresen MC. Angiotensin potentiates excitatory sensory synaptic transmission to medial solitary tract nucleus neurons. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1340–1353. doi: 10.1152/ajpregu.00505.2002. [DOI] [PubMed] [Google Scholar]
- Batenburg WW, Danser AJ. Prorenin and the (pro)renin receptor: binding kinetics, signalling and interaction with aliskiren. J Renin Angiotensin Aldosterone Syst. 2008;9:181–184. doi: 10.1177/1470320308097674. [DOI] [PubMed] [Google Scholar]
- Batenburg WW, et al. Aliskiren-binding increases the half life of renin and prorenin in rat aortic vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2008;28:1151–1157. doi: 10.1161/ATVBAHA.108.164210. [DOI] [PubMed] [Google Scholar]
- Batenburg WW, Jan Danser AH. The (pro)renin receptor: a new addition to the renin-angiotensin system? Eur J Pharmacol. 2008;585:320–324. doi: 10.1016/j.ejphar.2008.02.092. [DOI] [PubMed] [Google Scholar]
- Batenburg WW, et al. Prorenin is the endogenous agonist of the (pro)renin receptor. Binding kinetics of renin and prorenin in rat vascular smooth muscle cells overexpressing the human (pro)renin receptor. J Hypertens. 2007;25:2441–2453. doi: 10.1097/HJH.0b013e3282f05bae. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Paraventricular nucleus, stress response, and cardiovascular disease. Clin Auton Res. 2005;15:254–263. doi: 10.1007/s10286-005-0290-7. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Irvine RF. Inositol phosphates and cell signalling. Nature. 1989;341:197–204. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bickerton RK, Buckley JP. Evidence for a central mechanism in angiotensin induced hypertension. Proc Soc Exp Biol Med. 1961;106:834–837. [Google Scholar]
- Bohlender J, et al. High human renin hypertension in transgenic rats. Hypertension. 1997;29:428–434. doi: 10.1161/01.hyp.29.1.428. [DOI] [PubMed] [Google Scholar]
- Boscan P, Allen AM, Paton JF. Baroreflex inhibition of cardiac sympathetic outflow is attenuated by angiotensin II in the nucleus of the solitary tract. Neuroscience. 2001;103:153–160. doi: 10.1016/s0306-4522(00)00559-5. [DOI] [PubMed] [Google Scholar]
- Bourque CW, Renaud LP. Activity patterns and osmosensitivity of rat supraoptic neurones in perfused hypothalamic explants. J Physiol. 1984;349:631–642. doi: 10.1113/jphysiol.1984.sp015178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundzikova J, Pirnik Z, Zelena D, Mikkelsen JD, Kiss A. Response of substances co-expressed in hypothalamic magnocellular neurons to osmotic challenges in normal and Brattleboro rats. Cell Mol Neurobiol. 2008;28:1033–1047. doi: 10.1007/s10571-008-9306-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burckle C, Bader M. Prorenin and Its Ancient Receptor. Hypertension. 2006;48:549–551. doi: 10.1161/01.HYP.0000241132.48495.df. [DOI] [PubMed] [Google Scholar]
- Burckle CA, et al. Elevated Blood Pressure and Heart Rate in Human Renin Receptor Transgenic Rats. Hypertension. 2006;47:552–556. doi: 10.1161/01.HYP.0000199912.47657.04. [DOI] [PubMed] [Google Scholar]
- Bursztyn M, Bresnahan M, Gavras I, Gavras H. Pressor hormones in elderly hypertensive persons. Racial differences. Hypertension. 1990;15:I88–92. doi: 10.1161/01.hyp.15.2_suppl.i88. [DOI] [PubMed] [Google Scholar]
- Cabassi A, et al. Sympathetic activation in adipose tissue and skeletal muscle of hypertensive rats. Hypertension. 2002;39:656–661. doi: 10.1161/hy0202.103471. [DOI] [PubMed] [Google Scholar]
- Campbell DJ. Critical review of prorenin and (pro)renin receptor research. Hypertension. 2008;51:1259–1264. doi: 10.1161/HYPERTENSIONAHA.108.110924. [DOI] [PubMed] [Google Scholar]
- Campbell DJ, Lawrence AC, Towrie A, Kladis A, Valentijn AJ. Differential regulation of angiotensin peptide levels in plasma and kidney of the rat. Hypertension. 1991;18:763–773. doi: 10.1161/01.hyp.18.6.763. [DOI] [PubMed] [Google Scholar]
- Chan SH, et al. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced pressor effect via activation of p38 mitogen-activated protein kinase in the rostral ventrolateral medulla. Circ Res. 2005;97:772–780. doi: 10.1161/01.RES.0000185804.79157.C0. [DOI] [PubMed] [Google Scholar]
- Chapleau MW, Abboud FM. The baroreceptor reflex: Novel methods and mechanisms. In: Dun NJ, Benedito H, Pilowsky PM, editors. In Neural mechanisms of cardiovascular regulation. Kluwer Academic Publishers; Norwell, MA: 2004. pp. 1–29. [Google Scholar]
- Cleland SJ, Reid JL. The renin-angiotensin system and the heart: a historical review. Heart. 1996;76:7–12. doi: 10.1136/hrt.76.3_suppl_3.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombari E, et al. Role of the medulla oblongata in hypertension. Hypertension. 2001;38:549–554. doi: 10.1161/01.hyp.38.3.549. [DOI] [PubMed] [Google Scholar]
- Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- Das M, Soffer RL. Pulmonary angiotensin-converting enzyme antienzyme antibody. Biochemistry. 1976;15:5088–5094. doi: 10.1021/bi00668a022. [DOI] [PubMed] [Google Scholar]
- de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- de Wardener HE. The hypothalamus and hypertension. Physiol Rev. 2001;81:1599–1658. doi: 10.1152/physrev.2001.81.4.1599. [DOI] [PubMed] [Google Scholar]
- Doolittle RF. Angiotensinogen is related to the antitrypsin-antithrombin-ovalbumin family. Science. 1983;222:417–419. doi: 10.1126/science.6604942. [DOI] [PubMed] [Google Scholar]
- Esler M. The sympathetic system and hypertension. Am J Hypertens. 2000;13:99S–105S. doi: 10.1016/s0895-7061(00)00225-9. [DOI] [PubMed] [Google Scholar]
- Feldman DL, et al. Effects of Aliskiren on Blood Pressure, Albuminuria, and (Pro)Renin Receptor Expression in Diabetic TG(mRen-2)27 Rats. Hypertension. 2008;52:130–136. doi: 10.1161/HYPERTENSIONAHA.107.108845. [DOI] [PubMed] [Google Scholar]
- Feldt S, et al. Prorenin and renin-induced extracellular signal-regulated kinase 1/2 activation in monocytes is not blocked by aliskiren or the handle-region peptide. Hypertension. 2008a;51:682–688. doi: 10.1161/HYPERTENSIONAHA.107.101444. [DOI] [PubMed] [Google Scholar]
- Feldt S, Maschke U, Dechend R, Luft FC, Muller DN. The Putative (Pro)renin Receptor Blocker HRP Fails to Prevent (Pro)renin Signaling. J Am Soc Nephrol. 2008b;19:743–748. doi: 10.1681/ASN.2007091030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CM, Strawn WB. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol. 2006;98:121–128. doi: 10.1016/j.amjcard.2006.01.059. [DOI] [PubMed] [Google Scholar]
- Fischer-Ferraro C, Nahmod VE, Goldstein DJ, Finkielman S. Angiotensin and renin in rat and dog brain. J Exp Med. 1971;133:353–361. doi: 10.1084/jem.133.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis J, Wei SG, Weiss RM, Felder RB. Brain angiotensin-converting enzyme activity and autonomic regulation in heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H2138–2146. doi: 10.1152/ajpheart.00112.2004. [DOI] [PubMed] [Google Scholar]
- Ganten D, Hermann K, Bayer C, Unger T, Lang RE. Angiotensin synthesis in the brain and increased turnover in hypertensive rats. Science. 1983;221:869–871. doi: 10.1126/science.6879184. [DOI] [PubMed] [Google Scholar]
- Ganten D, et al. Angiotensin-forming enzyme in brain tissue. Science. 1971;173:64–65. doi: 10.1126/science.173.3991.64. [DOI] [PubMed] [Google Scholar]
- Ganten D, et al. Species specificity of renin kinetics in transgenic rats harboring the human renin and angiotensinogen genes. Proc Natl Acad Sci U S A. 1992;89:7806–7810. doi: 10.1073/pnas.89.16.7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goc A, Stachowiak MK. Bovine tyrosine hydroxylase gene-promoter regions involved in basal and angiotensin II-stimulated expression in nontransformed adrenal medullary cells. J Neurochem. 1994;62:834–843. doi: 10.1046/j.1471-4159.1994.62030834.x. [DOI] [PubMed] [Google Scholar]
- Goldsmith SR. Baroreceptor-mediated suppression of osmotically stimulated vasopressin in normal humans. J Appl Physiol. 1988;65:1226–1230. doi: 10.1152/jappl.1988.65.3.1226. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Ushio-Fukai M. Redox control of vascular smooth muscle proliferation. J Lab Clin Med. 1998;132:9–15. doi: 10.1016/s0022-2143(98)90019-1. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Guyton AC. Blood pressure control--special role of the kidneys and body fluids. Science. 1991;252:1813–1816. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- Gyurko R, Wielbo D, Phillips MI. Antisense inhibition of AT1 receptor mRNA and angiotensinogen mRNA in the brain of spontaneously hypertensive rats reduces hypertension of neurogenic origin. Regul Pept. 1993;49:167–174. doi: 10.1016/0167-0115(93)90438-e. [DOI] [PubMed] [Google Scholar]
- Harding JW, Yoshida MS, Dilts RP, Woods TM, Wright JW. Cerebroventricular and intravascular metabolism of [125I]angiotensins in rat. J Neurochem. 1986;46:1292–1297. doi: 10.1111/j.1471-4159.1986.tb00652.x. [DOI] [PubMed] [Google Scholar]
- Hatae T, Takimoto E, Murakami K, Fukamizu A. Comparative studies on species-specific reactivity between renin and angiotensinogen. Mol Cell Biochem. 1994;131:43–47. doi: 10.1007/BF01075723. [DOI] [PubMed] [Google Scholar]
- Helmer OM. Differentiation between two forms of angiotonin by means of spirally cut strips of rabbit aorta. Am J Physiol. 1957;188:571–577. doi: 10.1152/ajplegacy.1957.188.3.571. [DOI] [PubMed] [Google Scholar]
- Henrich WL, McAllister EA, Eskue A, Miller T, Moe OW. Renin regulation in cultured proximal tubular cells. Hypertension. 1996;27:1337–1340. doi: 10.1161/01.hyp.27.6.1337. [DOI] [PubMed] [Google Scholar]
- Hermann K, McDonald W, Unger T, Lang RE, Ganten D. Angiotensin biosynthesis and concentrations in brain of normotensive and hypertensive rats. J Physiol (Paris) 1984;79:471–480. [PubMed] [Google Scholar]
- Hirooka Y, Potts PD, Dampney RAL. Role of angiotensin II receptor subtypes in mediating the sympathoexcitatory effects of exogenous and endogenous angiotensin peptides in the rostral ventrolateral medulla of the rabbit. Brain Research. 1997;772:107. doi: 10.1016/s0006-8993(97)00861-5. [DOI] [PubMed] [Google Scholar]
- Hogarty DC, Speakman EA, Puig V, Phillips MI. The role of angiotensin, AT1 and AT2 receptors in the pressor, drinking and vasopressin responses to central angiotensin. Brain Res. 1992;586:289–294. doi: 10.1016/0006-8993(92)91638-u. [DOI] [PubMed] [Google Scholar]
- Hu L, et al. Expression of angiotensin II type 1 (AT1) receptor in the rostral ventrolateral medulla in rats. J Appl Physiol. 2002;92:2153–2161. doi: 10.1152/japplphysiol.00261.2001. [DOI] [PubMed] [Google Scholar]
- Huang W, et al. Aldosterone and TGF-beta1 synergistically increase PAI-1 and decrease matrix degradation in rat renal mesangial and fibroblast cells. Am J Physiol Renal Physiol. 2008;294:F1287–1295. doi: 10.1152/ajprenal.00017.2008. [DOI] [PubMed] [Google Scholar]
- Huang Y, Noble NA, Zhang J, Xu C, Border WA. Renin-stimulated TGF-beta1 expression is regulated by a mitogen-activated protein kinase in mesangial cells. Kidney Int. 2007;72:45–52. doi: 10.1038/sj.ki.5002243. [DOI] [PubMed] [Google Scholar]
- Huang Y, et al. Renin increases mesangial cell transforming growth factor-beta1 and matrix proteins through receptor-mediated, angiotensin II-independent mechanisms. Kidney Int. 2006;69:105–113. doi: 10.1038/sj.ki.5000011. [DOI] [PubMed] [Google Scholar]
- Hunt LT, Dayhoff MO. A surprising new protein superfamily containing ovalbumin, antithrombin-III, and alpha 1-proteinase inhibitor. Biochem Biophys Res Commun. 1980;95:864–871. doi: 10.1016/0006-291x(80)90867-0. [DOI] [PubMed] [Google Scholar]
- Ichihara A, et al. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J Clin Invest. 2004;114:1128–1135. doi: 10.1172/JCI21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara A, Kaneshiro Y. Response to Cardiovascular Effects of Nonproteolytic Activation of Prorenin. Hypertension. 2006 doi: 10.1161/01.HYP.0000247309.36509.7b. [DOI] [PubMed] [Google Scholar]
- Ichihara A, et al. Nonproteolytic activation of prorenin contributes to development of cardiac fibrosis in genetic hypertension. Hypertension. 2006a;47:894–900. doi: 10.1161/01.HYP.0000215838.48170.0b. [DOI] [PubMed] [Google Scholar]
- Ichihara A, et al. Prorenin receptor blockade inhibits development of glomerulosclerosis in diabetic angiotensin II type 1a receptor-deficient mice. J Am Soc Nephrol. 2006b;17:1950–1961. doi: 10.1681/ASN.2006010029. [DOI] [PubMed] [Google Scholar]
- Ingelfinger JR, Zuo WM, Fon EA, Ellison KE, Dzau VJ. In situ hybridization evidence for angiotensinogen messenger RNA in the rat proximal tubule. An hypothesis for the intrarenal renin angiotensin system. J Clin Invest. 1990;85:417–423. doi: 10.1172/JCI114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, et al. Ventrolateral medulla AT1 receptors support arterial pressure in Dahl salt-sensitive rats. Hypertension. 2003;41:744–750. doi: 10.1161/01.HYP.0000052944.54349.7B. [DOI] [PubMed] [Google Scholar]
- Ito S, Komatsu K, Tsukamoto K, Kanmatsuse K, Sved AF. Ventrolateral Medulla AT1 Receptors Support Blood Pressure in Hypertensive Rats. Hypertension. 2002;40:552–559. doi: 10.1161/01.hyp.0000033812.99089.92. [DOI] [PubMed] [Google Scholar]
- Ito S, Sved AF. Blockade of angiotensin receptors in rat rostral ventrolateral medulla removes excitatory vasomotor tone. Am J Physiol. 1996;270:R1317–1323. doi: 10.1152/ajpregu.1996.270.6.R1317. [DOI] [PubMed] [Google Scholar]
- Ito S, Sved AF. Pharmacological profile of depressor response elicited by sarthran in rat ventrolateral medulla. Am J Physiol Heart Circ Physiol. 2000;279:H2961–2966. doi: 10.1152/ajpheart.2000.279.6.H2961. [DOI] [PubMed] [Google Scholar]
- Ivins S, et al. Regulation of Hoxb2 by APL-associated PLZF protein. Oncogene. 2003;22:3685–3697. doi: 10.1038/sj.onc.1206328. [DOI] [PubMed] [Google Scholar]
- Jan Danser AH. Local renin-angiotensin systems: the unanswered questions. The International Journal of Biochemistry & Cell Biology. 2003;35:759. doi: 10.1016/s1357-2725(02)00178-4. [DOI] [PubMed] [Google Scholar]
- Jan Danser AH, Batenburg WW, van Esch JH. Prorenin and the (pro)renin receptor--an update. Nephrol Dial Transplant. 2007;22:1288–1292. doi: 10.1093/ndt/gfl846. [DOI] [PubMed] [Google Scholar]
- Jan Danser AH, Deinum J. Renin, prorenin and the putative (pro)renin receptor. Hypertension. 2005;46:1069–1076. doi: 10.1161/01.HYP.0000186329.92187.2e. [DOI] [PubMed] [Google Scholar]
- Kaneshiro Y, et al. Slowly progressive, angiotensin II-independent glomerulosclerosis in human (pro)renin receptor-transgenic rats. J Am Soc Nephrol. 2007;18:1789–1795. doi: 10.1681/ASN.2006091062. [DOI] [PubMed] [Google Scholar]
- Kasparov S, Teschemacher AG. Altered central catecholaminergic transmission and cardiovascular disease. Exp Physiol. 2008;93:725–740. doi: 10.1113/expphysiol.2007.041814. [DOI] [PubMed] [Google Scholar]
- Kimura S, et al. High blood pressure in transgenic mice carrying the rat angiotensinogen gene. Embo J. 1992;11:821–827. doi: 10.1002/j.1460-2075.1992.tb05119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- Kumar A, Rassoli A, Raizada MK. Angiotensinogen gene expression in neuronal and glial cells in primary cultures of rat brain. J Neurosci Res. 1988;19:287–290. doi: 10.1002/jnr.490190302. [DOI] [PubMed] [Google Scholar]
- Lavoie JL, Cassell MD, Gross KW, Sigmund CD. Adjacent Expression of Renin and Angiotensinogen in the Rostral Ventrolateral Medulla Using a Dual-Reporter Transgenic Model. Hypertension. 2004a;43:1116–1119. doi: 10.1161/01.HYP.0000125143.73301.94. [DOI] [PubMed] [Google Scholar]
- Lavoie JL, Cassell MD, Gross KW, Sigmund CD. Localization of renin expressing cells in the brain, by use of a REN-eGFP transgenic model. Physiol Genomics. 2004b;16:240–246. doi: 10.1152/physiolgenomics.00131.2003. [DOI] [PubMed] [Google Scholar]
- Lazartigues E, et al. Brain-selective overexpression of angiotensin (AT1) receptors causes enhanced cardiovascular sensitivity in transgenic mice. Circ Res. 2002;90:617–624. doi: 10.1161/01.res.0000012460.85923.f0. [DOI] [PubMed] [Google Scholar]
- Leenen FH, Ruzicka M, Huang BS. The brain and salt-sensitive hypertension. Curr Hypertens Rep. 2002;4:129–135. doi: 10.1007/s11906-002-0037-y. [DOI] [PubMed] [Google Scholar]
- Li DP, Chen SR, Pan HL. Angiotensin II stimulates spinally projecting paraventricular neurons through presynaptic disinhibition. J Neurosci. 2003;23:5041–5049. doi: 10.1523/JNEUROSCI.23-12-05041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YW, Guyenet PG. Angiotensin II decreases a resting K+ conductance in rat bulbospinal neurons of the C1 area. Circ Res. 1996;78:274–282. doi: 10.1161/01.res.78.2.274. [DOI] [PubMed] [Google Scholar]
- Li Z, Ferguson AV. Subfornical organ efferents to paraventricular nucleus utilize angiotensin as a neurotransmitter. Am J Physiol. 1993;265:R302–309. doi: 10.1152/ajpregu.1993.265.2.R302. [DOI] [PubMed] [Google Scholar]
- Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- Lind RW, Swanson LW, Ganten D. Angiotensin II immunoreactive pathways in the central nervous system of the rat: evidence for a projection from the subfornical organ to the paraventricular nucleus of the hypothalamus. Clin Exp Hypertens A. 1984;6:1915–1920. doi: 10.3109/10641968409046101. [DOI] [PubMed] [Google Scholar]
- Lippoldt A, Fuxe K, Luft FC. A view of renin in the brain. J Mol Med. 2001;79:71–73. doi: 10.1007/s001090100215. [DOI] [PubMed] [Google Scholar]
- Liu F, et al. Overexpression of angiotensinogen increases tubular apoptosis in diabetes. J Am Soc Nephrol. 2008;19:269–280. doi: 10.1681/ASN.2007010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Yang H, Lenox RH, Raizada MK. Regulation of angiotensin II-induced neuromodulation by MARCKS in brain neurons. J Cell Biol. 1998a;142:217–227. doi: 10.1083/jcb.142.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Yang H, Raizada MK. Angiotensin II regulation of neuromodulation: downstream signaling mechanism from activation of mitogen-activated protein kinase. J Cell Biol. 1996a;135:1609–1617. doi: 10.1083/jcb.135.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Yang H, Raizada MK. AT1 receptor-mediated nuclear translocation of Raf-1 in brain neurons. J Neurochem. 1998b;70:424–427. doi: 10.1046/j.1471-4159.1998.70010424.x. [DOI] [PubMed] [Google Scholar]
- Lu D, Yu K, Paddy MR, Rowland NE, Raizada MK. Regulation of norepinephrine transport system by angiotensin II in neuronal cultures of normotensive and spontaneously hypertensive rat brains. Endocrinology. 1996b;137:763–772. doi: 10.1210/endo.137.2.8593828. [DOI] [PubMed] [Google Scholar]
- Luetscher JA, Kraemer FB, Wilson DM, Schwartz HC, Bryer-Ash M. Increased plasma inactive renin in diabetes mellitus. A marker of microvascular complications. N Engl J Med. 1985;312:1412–1417. doi: 10.1056/NEJM198505303122202. [DOI] [PubMed] [Google Scholar]
- Luft FC. Vasopressin, urine concentration, and hypertension: a new perspective on an old story. Clin J Am Soc Nephrol. 2007;2:196–197. doi: 10.2215/CJN.04161206. [DOI] [PubMed] [Google Scholar]
- Macrez N, et al. Phosphoinositide 3-kinase isoforms selectively couple receptors to vascular L-type Ca(2+) channels. Circ Res. 2001;89:692–699. doi: 10.1161/hh2001.097864. [DOI] [PubMed] [Google Scholar]
- Mason WT. Supraoptic neurones of rat hypothalamus are osmosensitive. Nature. 1980;287:154–157. doi: 10.1038/287154a0. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, et al. Brain angiotensin and body fluid homeostasis. Jpn J Physiol. 2001;51:281–289. doi: 10.2170/jjphysiol.51.281. [DOI] [PubMed] [Google Scholar]
- Mercure C, et al. Molecular analysis of human prorenin prosegment variants in vitro and in vivo. J Biol Chem. 1995;270:16355–16359. doi: 10.1074/jbc.270.27.16355. [DOI] [PubMed] [Google Scholar]
- Merrill DC, et al. Chronic hypertension and altered baroreflex responses in transgenic mice containing the human renin and human angiotensinogen genes. J Clin Invest. 1996;97:1047–1055. doi: 10.1172/JCI118497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milsted A, Barna BP, Ransohoff RM, Brosnihan KB, Ferrario CM. Astrocyte cultures derived from human brain tissue express angiotensinogen mRNA. Proc Natl Acad Sci U S A. 1990;87:5720–5723. doi: 10.1073/pnas.87.15.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe OW, et al. Renin expression in renal proximal tubule. J Clin Invest. 1993;91:774–779. doi: 10.1172/JCI116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto S, Cassell MD, Sigmund CD. The brain renin-angiotensin system in transgenic mice carrying a highly regulated human renin transgene. Circ Res. 2002;90:80–86. doi: 10.1161/hh0102.102272. [DOI] [PubMed] [Google Scholar]
- Morimoto S, Sigmund CD. Angiotensin mutant mice: a focus on the brain renin-angiotensin system. Neuropeptides. 2002;36:194–200. doi: 10.1054/npep.2002.0894. [DOI] [PubMed] [Google Scholar]
- Muller DN, et al. Vascular angiotensin-converting enzyme expression regulates local angiotensin II. Hypertension. 1997;29:98–104. doi: 10.1161/01.hyp.29.1.98. [DOI] [PubMed] [Google Scholar]
- Muller DN, et al. Effects of human renin in the vasculature of rats transgenic for human angiotensinogen. Hypertension. 1995;26:272–278. doi: 10.1161/01.hyp.26.2.272. [DOI] [PubMed] [Google Scholar]
- Muller DN, et al. (Pro)renin receptor peptide inhibitor “handle-region” peptide does not affect hypertensive nephrosclerosis in Goldblatt rats. Hypertension. 2008;51:676–681. doi: 10.1161/HYPERTENSIONAHA.107.101493. [DOI] [PubMed] [Google Scholar]
- Mullins JJ, Peters J, Ganten D. Fulminant hypertension in transgenic rats harbouring the mouse Ren-2 gene. Nature. 1990;344:541–544. doi: 10.1038/344541a0. [DOI] [PubMed] [Google Scholar]
- Nabi AH, et al. Binding properties of rat prorenin and renin to the recombinant rat renin/prorenin receptor prepared by a baculovirus expression system. Int J Mol Med. 2006;18:483–488. [PubMed] [Google Scholar]
- Nakamura S, et al. Activation of the brain angiotensin system by in vivo human angiotensin-converting enzyme gene transfer in rats. Hypertension. 1999;34:302–308. doi: 10.1161/01.hyp.34.2.302. [DOI] [PubMed] [Google Scholar]
- Nguyen G. Renin//prorenin receptors. Kidney Int. 2006;69:1503. doi: 10.1038/sj.ki.5000265. [DOI] [PubMed] [Google Scholar]
- Nguyen G, Contrepas A. Physiology and pharmacology of the (pro)renin receptor. Current Opinion in Pharmacology. 2008a;8:127. doi: 10.1016/j.coph.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Nguyen G, Contrepas A. The (pro)renin receptors. J Mol Med. 2008b;86:643–646. doi: 10.1007/s00109-008-0319-1. [DOI] [PubMed] [Google Scholar]
- Nguyen G, Delarue F, Berrou J, Rondeau E, Sraer J-D. Specific receptor binding of renin on human mesangial cells in culture increases plasminogen activator inhibitor-1 antigen. Kidney Int. 1996;50:1897. doi: 10.1038/ki.1996.511. [DOI] [PubMed] [Google Scholar]
- Nguyen G, et al. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishii T, et al. Angiotensinogen gene-activating elements regulate blood pressure in the brain. Circ Res. 1999;85:257–263. doi: 10.1161/01.res.85.3.257. [DOI] [PubMed] [Google Scholar]
- Nurun NA, et al. Role of “handle” region of prorenin prosegment in the non-proteolytic activation of prorenin by binding to membrane anchored (pro)renin receptor. Front Biosci. 2007;12:4810–4817. doi: 10.2741/2429. [DOI] [PubMed] [Google Scholar]
- Obermuller N, et al. Distribution of angiotensin II receptor subtypes in rat brain nuclei. Neurosci Lett. 1991;132:11–15. doi: 10.1016/0304-3940(91)90420-x. [DOI] [PubMed] [Google Scholar]
- Pan HL. Brain angiotensin II and synaptic transmission. Neuroscientist. 2004;10:422–431. doi: 10.1177/1073858404264678. [DOI] [PubMed] [Google Scholar]
- Paul M, et al. Quantification of renin mRNA in various mouse tissues by a novel solution hybridization assay. J Hypertens. 1988;6:247–252. doi: 10.1097/00004872-198803000-00010. [DOI] [PubMed] [Google Scholar]
- Phillips MI, Kimura B. Brain angiotensin in the developing spontaneously hypertensive rat. J Hypertens. 1988;6:607–612. doi: 10.1097/00004872-198808000-00002. [DOI] [PubMed] [Google Scholar]
- Phillips MI, et al. Lowering of hypertension by central saralasin in the absence of plasma renin. Nature. 1977;270:445–447. doi: 10.1038/270445a0. [DOI] [PubMed] [Google Scholar]
- Phillips MI, Shen L, Richards EM, Raizada MK. Immunohistochemical mapping of angiotensin AT1 receptors in the brain. Regul Pept. 1993;44:95–107. doi: 10.1016/0167-0115(93)90233-x. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J. Co-localization of angiotensin II and gamma-aminobutyric acid in axon terminals in the rat subfornical organ. Neurosci Lett. 1995;193:89–92. doi: 10.1016/0304-3940(95)11673-k. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Ganten D. Dual peroxidase and colloidal gold-labeling study of angiotensin converting enzyme and angiotensin-like immunoreactivity in the rat subfornical organ. J Neurosci. 1986;6:2457–2469. doi: 10.1523/JNEUROSCI.06-08-02457.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramser J, et al. A unique exonic splice enhancer mutation in a family with X-linked mental retardation and epilepsy points to a novel role of the renin receptor. Hum Mol Genet. 2005;14:1019–1027. doi: 10.1093/hmg/ddi094. [DOI] [PubMed] [Google Scholar]
- Rao GN, Berk BC. Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ Res. 1992;70:593–599. doi: 10.1161/01.res.70.3.593. [DOI] [PubMed] [Google Scholar]
- Reaux A, Fournie-Zaluski MC, LlorensCortes C. Angiotensin III: a central regulator of vasopressin release and blood pressure. Trends Endocrinol Metab. 2001;12:157–162. doi: 10.1016/s1043-2760(01)00381-2. [DOI] [PubMed] [Google Scholar]
- Richoux JP, Bouhnik J, Clauser E, Corvol P. The renin-angiotensin system in the rat brain. Immunocytochemical localization of angiotensinogen in glial cells and neurons. Histochemistry. 1988;89:323–331. doi: 10.1007/BF00500633. [DOI] [PubMed] [Google Scholar]
- Ruiz P, Basso N, Cannata MA, Taquini AC. The renin-angiotensin system in different stages of spontaneous hypertension in the rat (S H R) Clin Exp Hypertens A. 1990;12:63–81. doi: 10.3109/10641969009074720. [DOI] [PubMed] [Google Scholar]
- Saavedra JM. Brain angiotensin II: new developments, unanswered questions and therapeutic opportunities. Cell Mol Neurobiol. 2005;25:485–512. doi: 10.1007/s10571-005-4011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, et al. Local production of angiotensin II in the subfornical organ causes elevated drinking. J Clin Invest. 2007;117:1088–1095. doi: 10.1172/JCI31242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schefe JH, et al. A novel signal transduction cascade involving direct physical interaction of the renin/prorenin receptor with the transcription factor promyelocytic zinc finger protein. Circ Res. 2006;99:1355–1366. doi: 10.1161/01.RES.0000251700.00994.0d. [DOI] [PubMed] [Google Scholar]
- Schefe JH, Unger T, Funke-Kaiser H. PLZF and the (pro)renin receptor. J Mol Med. 2008;86:623–627. doi: 10.1007/s00109-008-0320-8. [DOI] [PubMed] [Google Scholar]
- Schinke M, et al. Blood pressure reduction and diabetes insipidus in transgenic rats deficient in brain angiotensinogen. Proc Natl Acad Sci U S A. 1999;96:3975–3980. doi: 10.1073/pnas.96.7.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaich MP, et al. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and Angiotensin neuromodulation. Hypertension. 2004;43:169–175. doi: 10.1161/01.HYP.0000103160.35395.9E. [DOI] [PubMed] [Google Scholar]
- Seo H, Yang C, Kim HS, Kim KS. Multiple protein factors interact with the cis-regulatory elements of the proximal promoter in a cell-specific manner and regulate transcription of the dopamine beta-hydroxylase gene. J Neurosci. 1996;16:4102–4112. doi: 10.1523/JNEUROSCI.16-13-04102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Z, Cuadra AE, Lin F, Sumners C, Raizada MK. Increased expression of (pro)renin receptor in the brain from spontaneously hypertensive rats; Paper presented at: 62 High Blood Pressure Research Conference; Alanta, GA. 2008a. [Google Scholar]
- Shan Z, Cuadra AE, Sumners C, Raizada MK. Characterization of a functional (pro)renin receptor in rat brain neurons. Exp Physiol. 2008b;93:701–708. doi: 10.1113/expphysiol.2008.041988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnayah P, et al. Genetic ablation of angiotensinogen in the subfornical organ of the brain prevents the central angiotensinergic pressor response. Circ Res. 2006;99:1125–1131. doi: 10.1161/01.RES.0000250259.66683.f5. [DOI] [PubMed] [Google Scholar]
- Skeggs LT, Jr., Kahn JR, Shumway NP. The preparation and function of the hypertensin-converting enzyme. J Exp Med. 1956;103:295–299. doi: 10.1084/jem.103.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PR, Stoner LC, Viggiano SC, Angelides KJ, Benos DJ. Effects of vasopressin and aldosterone on the lateral mobility of epithelial Na+ channels in A6 renal epithelial cells. J Membr Biol. 1995;147:195–205. doi: 10.1007/BF00233547. [DOI] [PubMed] [Google Scholar]
- Steinberg SF. PI3King the L-type calcium channel activation mechanism. Circ Res. 2001;89:641–644. [PubMed] [Google Scholar]
- Stornetta RL, Hawelu-Johnson CL, Guyenet PG, Lynch KR. Astrocytes synthesize angiotensinogen in brain. Science. 1988;242:1444–1446. doi: 10.1126/science.3201232. [DOI] [PubMed] [Google Scholar]
- Sun C, Sellers KW, Sumners C, Raizada MK. NAD(P)H oxidase inhibition attenuates neuronal chronotropic actions of angiotensin II. Circ Res. 2005;96:659–666. doi: 10.1161/01.RES.0000161257.02571.4b. [DOI] [PubMed] [Google Scholar]
- Sun C, Sumners C, Raizada MK. Chronotropic action of angiotensin II in neurons via protein kinase C and CaMKII. Hypertension. 2002;39:562–566. doi: 10.1161/hy0202.103057. [DOI] [PubMed] [Google Scholar]
- Suzuki F, et al. Human prorenin has “gate and handle” regions for its non-proteolytic activation. J Biol Chem. 2003;278:22217–22222. doi: 10.1074/jbc.M302579200. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:537–546. [PubMed] [Google Scholar]
- Tagawa T, Fontes MA, Potts PD, Allen AM, Dampney RA. The physiological role of AT1 receptors in the ventrolateral medulla. Braz J Med Biol Res. 2000;33:643–652. doi: 10.1590/s0100-879x2000000600005. [DOI] [PubMed] [Google Scholar]
- Tagawa T, Horiuchi J, Potts PD, Dampney RA. Sympathoinhibition after angiotensin receptor blockade in the rostral ventrolateral medulla is independent of glutamate and gamma-aminobutyric acid receptors. J Auton Nerv Syst. 1999;77:21–30. doi: 10.1016/s0165-1838(99)00026-0. [DOI] [PubMed] [Google Scholar]
- Takahashi H, et al. Regression of nephropathy developed in diabetes by (Pro)renin receptor blockade. J Am Soc Nephrol. 2007;18:2054–2061. doi: 10.1681/ASN.2006080820. [DOI] [PubMed] [Google Scholar]
- Takeda K, Bunag RD. Augmented sympathetic nerve activity and pressor responsiveness in DOCA hypertensive rats. Hypertension. 1980;2:97–101. doi: 10.1161/01.hyp.2.1.97. [DOI] [PubMed] [Google Scholar]
- Tamura K, et al. Tissue-specific regulation of angiotensinogen gene expression in spontaneously hypertensive rats. Hypertension. 1996;27:1216–1223. doi: 10.1161/01.hyp.27.6.1216. [DOI] [PubMed] [Google Scholar]
- Tan J, Wang H, Leenen FH. Increases in brain and cardiac AT1 receptor and ACE densities after myocardial infarct in rats. Am J Physiol Heart Circ Physiol. 2004;286:H1665–1671. doi: 10.1152/ajpheart.00858.2003. [DOI] [PubMed] [Google Scholar]
- Thomas WG, Greenland KJ, Shinkel TA, Sernia C. Angiotensinogen is secreted by pure rat neuronal cell cultures. Brain Res. 1992;588:191–200. doi: 10.1016/0006-8993(92)91575-y. [DOI] [PubMed] [Google Scholar]
- Treschan TA, Peters J. The vasopressin system: physiology and clinical strategies. Anesthesiology. 2006;105:599–612. doi: 10.1097/00000542-200609000-00026. quiz 639-540. [DOI] [PubMed] [Google Scholar]
- Trolliet MR, Phillips MI. The effect of chronic bilateral nephrectomy on plasma and brain angiotensin. J Hypertens. 1992;10:29–36. doi: 10.1097/00004872-199201000-00006. [DOI] [PubMed] [Google Scholar]
- Tsutsumi K, Saavedra JM. Characterization and development of angiotensin II receptor subtypes (AT1 and AT2) in rat brain. Am J Physiol. 1991;261:R209–216. doi: 10.1152/ajpregu.1991.261.1.R209. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Alexander RW, Akers M, Griendling KK. p38 Mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II. Role in vascular smooth muscle cell hypertrophy. J Biol Chem. 1998a;273:15022–15029. doi: 10.1074/jbc.273.24.15022. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, et al. Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J Biol Chem. 1999;274:22699–22704. doi: 10.1074/jbc.274.32.22699. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Griendling KK, Akers M, Lyons PR, Alexander RW. Temporal dispersion of activation of phospholipase C-beta1 and - gamma isoforms by angiotensin II in vascular smooth muscle cells. Role of alphaq/11, alpha12, and beta gamma G protein subunits. J Biol Chem. 1998b;273:19772–19777. doi: 10.1074/jbc.273.31.19772. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Leevers SJ, Panayotou G, Waterfield MD. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem Sci. 1997;22:267–272. doi: 10.1016/s0968-0004(97)01061-x. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Waterfield MD. Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res. 1999;253:239–254. doi: 10.1006/excr.1999.4701. [DOI] [PubMed] [Google Scholar]
- Veerasingham SJ, Raizada MK. Brain renin-angiotensin system dysfunction in hypertension: recent advances and perspectives. Br J Pharmacol. 2003;139:191–202. doi: 10.1038/sj.bjp.0705262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltmar A, Culman J, Qadri F, Rascher W, Unger T. Involvement of adrenergic and angiotensinergic receptors in the paraventricular nucleus in the angiotensin II-induced vasopressin release. J Pharmacol Exp Ther. 1992;263:1253–1260. [PubMed] [Google Scholar]
- Veniant M, et al. Vascular damage without hypertension in transgenic rats expressing prorenin exclusively in the liver. J Clin Invest. 1996;98:1966–1970. doi: 10.1172/JCI119000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard P, et al. PI3K promotes voltage-dependent calcium channel trafficking to the plasma membrane. Nat Neurosci. 2004;7:939–946. doi: 10.1038/nn1300. [DOI] [PubMed] [Google Scholar]
- Viard P, et al. Gbetagamma dimers stimulate vascular L-type Ca2+ channels via phosphoinositide 3-kinase. Faseb J. 1999;13:685–694. doi: 10.1096/fasebj.13.6.685. [DOI] [PubMed] [Google Scholar]
- Wei SG, Felder RB. Forebrain renin-angiotensin system has a tonic excitatory influence on renal sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2002;282:H890–895. doi: 10.1152/ajpheart.2002.282.3.H890. [DOI] [PubMed] [Google Scholar]
- Weyhenmeyer JA, Phillips MI. Angiotensin-like immunoreactivity in the brain of the spontaneously hypertensive rat. Hypertension. 1982;4:514–523. doi: 10.1161/01.hyp.4.4.514. [DOI] [PubMed] [Google Scholar]
- Yamazato M, Yamazato Y, Sun C, Diez-Freire C, Raizada MK. Overexpression of Angiotensin-Converting Enzyme 2 in the Rostral Ventrolateral Medulla Causes Long-Term Decrease in Blood Pressure in the Spontaneously Hypertensive Rats. Hypertension. 2007;49:926–931. doi: 10.1161/01.HYP.0000259942.38108.20. [DOI] [PubMed] [Google Scholar]
- Yang H, Lu D, Yu K, Raizada MK. Regulation of neuromodulatory actions of angiotensin II in the brain neurons by the Ras-dependent mitogen-activated protein kinase pathway. J Neurosci. 1996;16:4047–4058. doi: 10.1523/JNEUROSCI.16-13-04047.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Raizada MK. Role of phosphatidylinositol 3-kinase in angiotensin II regulation of norepinephrine neuromodulation in brain neurons of the spontaneously hypertensive rat. J Neurosci. 1999;19:2413–2423. doi: 10.1523/JNEUROSCI.19-07-02413.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Shaw G, Raizada MK. ANG II stimulation of neuritogenesis involves protein kinase B in brain neurons. Am J Physiol Regul Integr Comp Physiol. 2002;283:R107–114. doi: 10.1152/ajpregu.00611.2001. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang X, Raizada MK. Characterization of signal transduction pathway in neurotropic action of angiotensin II in brain neurons. Endocrinology. 2001;142:3502–3511. doi: 10.1210/endo.142.8.8348. [DOI] [PubMed] [Google Scholar]
- Yongue BG, Angulo JA, McEwen BS, Myers MM. Brain and liver angiotensinogen messenger RNA in genetic hypertensive and normotensive rats. Hypertension. 1991;17:485–491. doi: 10.1161/01.hyp.17.4.485. [DOI] [PubMed] [Google Scholar]
- Yu K, Lu D, Paddy MR, Lenk SE, Raizada MK. Angiotensin II regulation of plasminogen activator inhibitor-1 gene expression in neurons of normotensive and spontaneously hypertensive rat brains. Endocrinology. 1996;137:2503–2513. doi: 10.1210/endo.137.6.8641204. [DOI] [PubMed] [Google Scholar]
- Zhang SL, et al. Effect of renin-angiotensin system blockade on the expression of the angiotensinogen gene and induction of hypertrophy in rat kidney proximal tubular cells. Exp Nephrol. 2001;9:109–117. doi: 10.1159/000052601. [DOI] [PubMed] [Google Scholar]
- Zhu M, et al. Modulation of K+ and Ca2+ currents in cultured neurons by an angiotensin II type 1a receptor peptide. Am J Physiol. 1997;273:C1040–1048. doi: 10.1152/ajpcell.1997.273.3.C1040. [DOI] [PubMed] [Google Scholar]