Abstract
We describe a computer-controlled tactile stimulator for use in human psychophysical and monkey neurophysiological studies of 3-D shape perception. The stimulator is constructed primarily of commercially available parts, as well as a few custom-built pieces for which we will supply diagrams upon request. There are two components to the stimulator: a tactile component and a hand positioner component. The tactile component consists of multiple stimulating units that move about in a Cartesian plane above the restrained hand. Each stimulating unit contains a servo-controlled linear motor with an attached small rotary stepper motor, allowing arbitrary stimulus shapes to contact the skin through vibration, static indentation, or scanning. The hand positioner component modifies the conformation of the restrained hand through a set of mechanical linkages under motorized control. The present design controls the amount of spread between digits two and three, the spread between digits four and three, and the degree to which digit three is flexed or extended, thereby simulating different conformations of the hand in contact with objects. This design is easily modified to suit the needs of the experimenter. Because the two components of the stimulator are independently controlled, the stimulator allows for parametric study of the mechanoreceptive and proprioceptive contributions to 3-D tactile shape perception.
Keywords: somatosensory, tactile, haptic, orientation, stimulator, shape
Introduction
Our ability to study the neural mechanisms underlying tactile perception from the hand has been limited by the lack of stimulators that can accurately stimulate the hand while it is in natural positions. Previous psychophysical and neurophysiological studies have relied on two main approaches. The first approach has been to apply controlled stimuli to the hand while it is in a single, fixed conformation. In this approach the hand is either supinated or pronated, with the digits spread slightly apart, while stimuli are brought into contact with the skin using a controlled force or displacement. The advantage of this approach is that since the hand is immobilized, it is possible to repeatedly present the stimuli to the same locations on the skin. In neurophysiological studies this means that the receptive fields of peripheral and central neurons can be studied in a controlled manner. The stimulators that have used this approach have been mechanical vibrating probes that present stimuli that vary in frequency and amplitude (e.g., the Chubbuck stimulator; Chubbuck, 1966; Johnson, 1974), vibrating piezoelectric pins (e.g., the Optacon; Bliss et al., 1970; Craig, 1977), motors that statically indent spatial surfaces such as gratings, probes, or oriented bars (Phillips and Johnson, 1981; Burton and Sinclair, 1990; Fitzgerald et al., 2004), or rotating drums (Johnson and Lamb, 1981) that scan surfaces such as dots and letters across the finger pad.
The second approach has been to allow subjects (both human and nonhuman primates) to actively scan objects with their unrestrained hands (Iwamura and Tanaka, 1978; Lederman and Klatzky, 1987; Connor et al., 1990; Vega-Bermudez et al., 1991; Zhou and Fuster, 1996; Gardner et al., 2002). In this approach, subjects use whatever hand conformation, contact force, or scanning velocity they choose. The two approaches are complementary in the information they provide about hand function. The first approach allows the skin to be stimulated in a controlled fashion but ignores the effects of hand conformation and active touch. The second approach allows the subject to actively contact the stimuli in a natural manner but provides limited information necessary to study neural mechanisms since the detailed stimulus/skin interactions and hand conformations are not controlled. Thus, differences in the neural or behavioral responses obtained from these two approaches can be attributed to a variety of effects such as contact location, contact force, hand conformation, or motor and cognitive effects related to active touch.
Here we describe a different approach for studying hand function that combines the two approaches. The basic idea is to present controlled tactile stimuli to the hand while it is held in a moveable restraint that is under computerized control. The advantage of this approach is that it allows for controlled stimulus delivery while the hand is moved into different conformations, which simulates in a more natural way the conformations that are used in everyday tasks than when the hand is positioned in a single supinated conformation. Studying the effects of hand conformation may not be critical if one is simply interested in the cutaneous responses from a single finger pad, since all of the relevant information is probably contained in the mechanoreceptive responses of the afferents that innervate that region of skin. However, hand conformation becomes important if one is interested in studying how information about large shapes that contact multiple digits is processed in the nervous system. Understanding the interaction between cutaneous input and hand conformation is also important since we have reported that cutaneous responses are modulated by hand conformation (Hsiao et al., 2002). For large stimuli, information about the shape is potentially carried by both the mechanoreceptive afferents and the proprioceptive afferents, the latter of which convey information about hand conformation to the central nervous system. For example, the information about the shape of a ball is potentially carried by the interaction between the local curvature information of the ball on the skin and the position of the digits as they wrap around the ball. Understanding how shape is represented in the brain is complex because the hand has multiple degrees of freedom, resulting in a huge number of ways in which it can contact an object. Therefore, quantitative study of the interaction between mechanoreceptive and proprioceptive information in tactile shape processing requires that the degrees of freedom for movement of the hand be restricted, with systematic decoupling of these two types of information.
The stimulator we describe here can present controlled cutaneous stimulation to the hand while the hand is positioned in different conformations. Below we describe the stimulator in greater detail, how it is controlled, and show examples of how it has been used in psychophysical studies of humans and neurophysiological studies of non-human primates. Detailed descriptions of the computerized control algorithms and/or specifications for the parts of the stimulator that were built in-house are available upon request.
Materials and Methods
Description of the stimulator
As stated above, the stimulator comprises two components: tactile and hand positioner. The tactile component consists of independently controlled stimulating units (Fig. 1), where the heart of each stimulating unit is a linear motor that has a long stroke length. The stimulating units are mounted on a Cartesian planar system that allows them to be independently moved to different XY locations over the restrained hand. The servo-controlled linear motors allow for controlled motion in the Z axis and provide the motive force for indenting the stimulus surfaces into the skin. A force sensor is mounted on the bottom of each linear motor to detect skin contact, and can also be used as a feedback sensor if force control is desired rather than displacement control. A variety of stimulus configurations can potentially be mounted on the bottom of the force sensor. Below we provide more details on the tactile component of the stimulator.
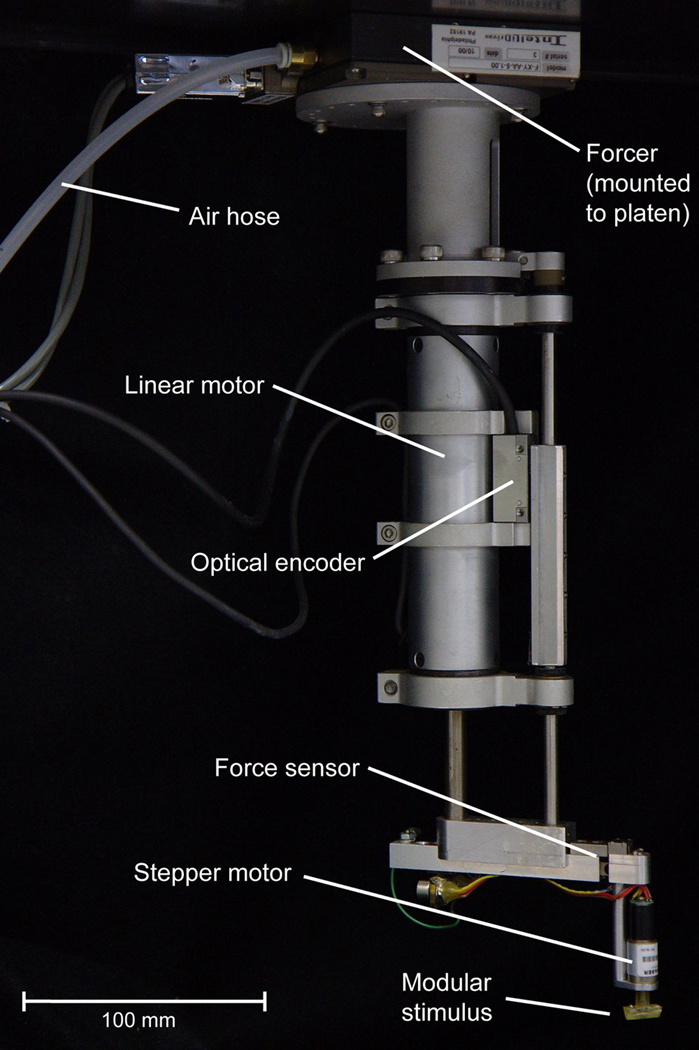
Figure 1.
Photograph of a stimulating unit. These units form the tactile component of the stimulator, where each unit consists of a forcer, linear motor, force sensor, and a small rotary stepper motor with an attached stimulus shape. The forcer allows the stimulating unit to move about in an XY plane just below the surface of a metal plate (platen), riding on frictionless air bearings above the restrained hand.
The tactile component consists of a single stimulating unit or multiple units, depending on the requirements for the experiment. Figure 1 shows a photograph of one stimulating unit. Each stimulating unit has a servo-controlled linear motor (Baldor Electric Company, Inc., Santa Clarita, CA), which is moved about using a planar system XY table (Intellidrives, Inc., Philadelphia, PA), which consists of a specially designed metal plate (platen) that is suspended over the hand, with linear motors attached to one or more forcers that move about on the plate. We use a platen that is about 102 × 61 cm and is attached, at its four corners, to a frame constructed of T-slotted modular aluminum framing material (80/20, Inc., Columbia City, IN) that has guides that allow the platen to be positioned manually in three dimensions over the hand. Below the platen are magnetic, planar forcers that glide on frictionless air bearings. Each forcer contains two stepper motor modules, oriented at 90 degrees to each other, that when activated function like stepper motors and lock the forcer in place on the platen. The forcer can then be moved incrementally in steps in either the X or Y dimension. The position and movement of the forcer are achieved using standard microstepping drives and indices with a 0.003 mm resolution and repeatability. One of the advantages of this planar system is that multiple stimulating units (see Figure 1 for one stimulating unit) can be mounted on the same platen. In our setup we typically use three forcers, with one tactile stimulator per forcer. To overcome the problem of the forcers having a relatively large footprint size of about 100 × 100 mm, the surfaces are mounted on the end of an extension arm (Figure 1) that is attached to the shaft of the linear motor. This allows us to place stimulus surfaces from the different forcers at arbitrary locations on the hand.
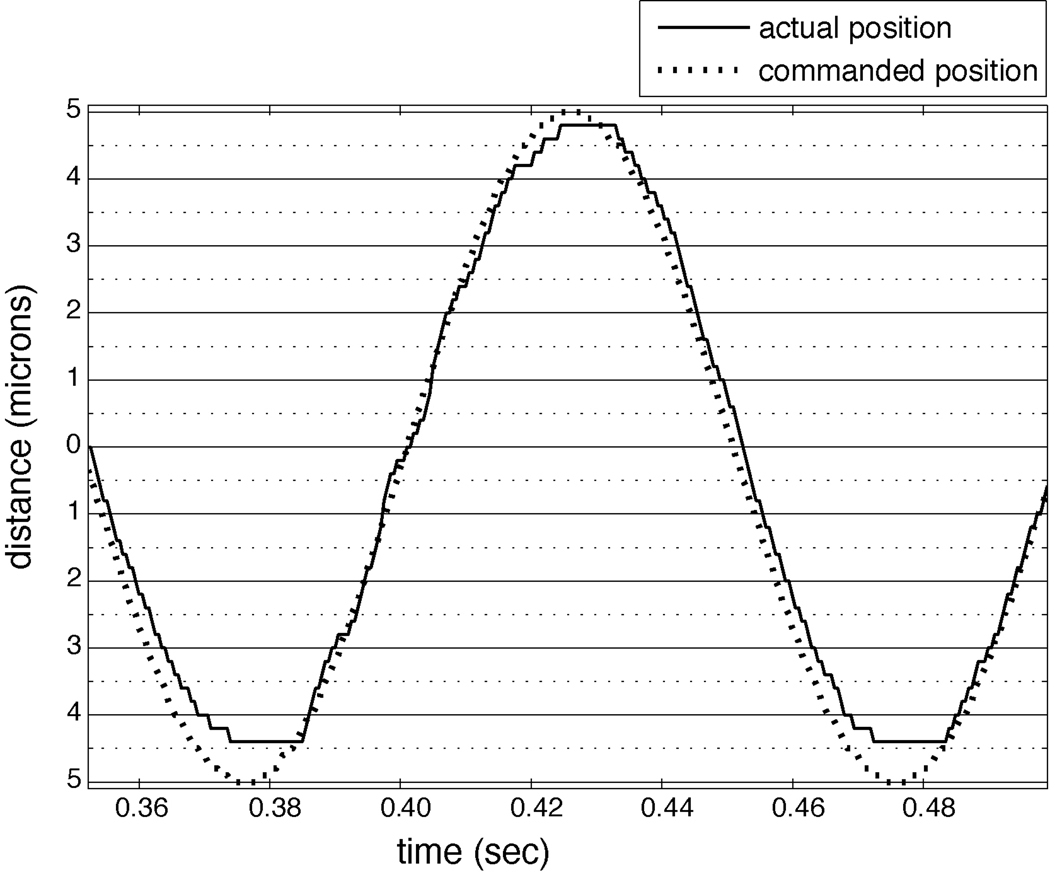
Servo-control of the Z position (of the shaft of the linear motor) is achieved by using a linear scale and optical encoder (Renishaw, Inc., Hoffman Estates, IL), which provides position feedback and gives the linear motor a travel of up to 40 mm with an accuracy of one micron. Figure 2 shows 1.5 cycles of a 10 Hz sinusoidal stimulus with a 10 micron amplitude, used to simulate vibration. As can be seen, the error between the commanded and actual position is less than one micron.
Figure 2.
Accuracy of the linear motor. Shown is the location of the linear motor’s shaft during part of a servo-controlled, 10 Hz sinusoidal stimulus with a 10 micron amplitude, used to simulate vibration. The dashed line indicates the commanded position of the shaft, and the solid line indicates the actual position. The error is less than one micron.
Attached to the shaft of the linear motor is an extension arm, a load cell (force sensor) (Strain Measurement Devices, Inc., Meriden, CT), and a small rotary stepper motor (Arsape, Inc., Switzerland) to which we attach the stimulus surfaces. The load cell has two purposes: to provide information about skin contact, and to provide a feedback signal that can be used for force control. The load cell has a resolution of 0.5 grams and a range of 500 grams. A wide variety of stimuli can be attached to the extension arm. One possibility, shown in Figure 1, is mounting a small rotary stepper motor vertically below the load cell and attaching a bar orthogonal to the shaft of the stepper motor. This configuration is well suited for studying the orientation tuning properties of neurons (Fig. 5) and also is useful for orienting the stimulus surface based on the positioning of the digits. The first version of this stimulator used a single linear motor and a rotary motor to study orientation tuning properties of second somatosensory cortex neurons (Fitzgerald et al. 2006a). An alternative configuration is to mount the stepper motor horizontally below the load cell and attach a small pivot arm orthogonal to the shaft of the stepper motor. In this configuration, rotating the shaft of the stepper motor changes the pitch of the bar. There are several requirements for the stepper motor. One is that the diameter must be small enough (<12 mm) that it does not interfere with stepper motors stimulating adjacent digits. Another is that it must be light enough to be moved quickly by the linear motor. Finally, it must have a sufficient number of steps per revolution to set the orientation or pitch precisely.
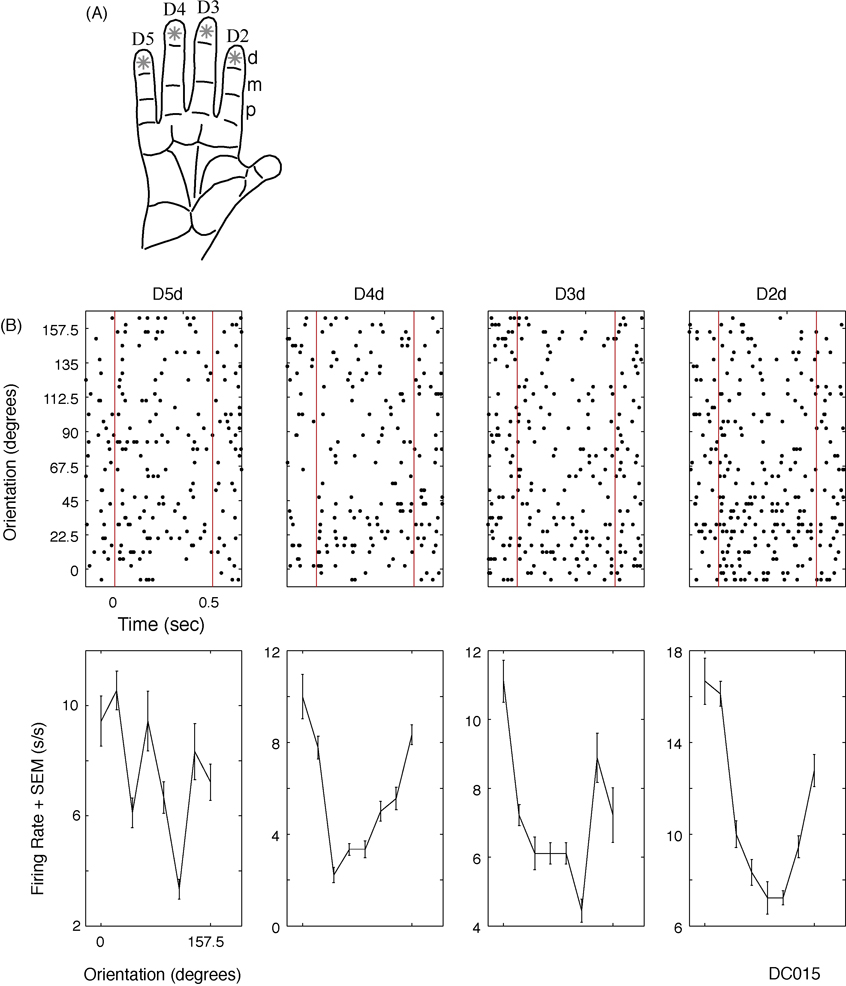
Figure 5.
Using the stimulator in single unit mode. A, Diagram showing how the four distal finger pads of digits 2–5 were stimulated with an oriented bar at eight, 22.5 degree separated orientations. B, An example SII neuron (DC015) raster (top) and orientation tuning diagram (bottom). Red lines indicate bar indentation into and withdrawal from the skin. Abbreviations: standard error of the mean (SEM), spikes per second (s/s).
A minor complication that occurs when using multiple stimulating units is the potential for collisions between the units. We addressed this problem by implementing code in the control computers that is capable of aborting motor commands and displaying a warning when a collision is predicted at the final commanded positions of the units. Since we know the size of the forcers, as well as the orientation and length of the extension arm of each unit, this is simply a test of the distance between the final positions of the units. However, the final positions of the units may not cause a collision but the paths they use to get there may result in one, so the burden is on the programmer to ensure that the units follow appropriate paths. Since the initial position of each unit is at a different corner of the platen, such caution is not difficult to implement.
The hand positioner component of the stimulator is designed to modify the conformation of the restrained hand by using a set of mechanical linkages under motorized control (Fig. 3). The hand positioner we describe here was designed specifically to investigate the representation of tactile curvature using the second (D2), third (D3), and fourth (D4) digits. There are two reasons why we have chosen to ignore the fifth digit (D5). The primary reason is to reduce the number of degrees of freedom, and the other is that the role of D5 in shape perception is probably less important than that of the other digits. For example, precision grasping is often done with the lateral digits (D1 and D2) (Glendinning et al. 1992), and the cortical representation of lateral digits may be larger than that of D5 (Fitzgerald et al. 2006b). If we consider just D2, D3, and D4, then only three degrees of freedom play primary roles in the perception of large, simple curved surfaces. A simple curved surface is one that can be defined by a single value (i.e., radius of curvature). One degree of freedom is the amount of spread between D2 and D3, the second is the spread between D4 and D3, and the third is the degree to which D3 is flexed or extended. The degree to which D2 and D4 are flexed/extended is the same for simple curved surfaces, and therefore the degree of flexion/extension for these two digits is kept equal and constant. Maintaining the same degree of flexion/extension for D2 and D4 is consistent with the natural covariation of digit movement (Kim et al. 2008).
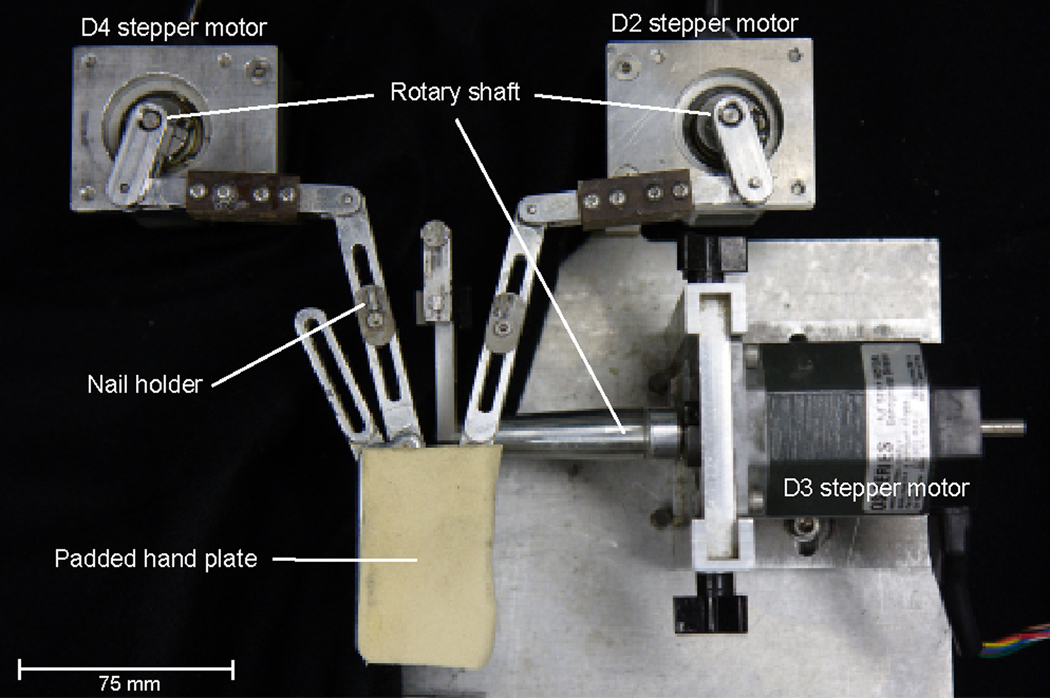
Figure 3.
Photograph of the hand positioner. Shown is a top down view of the positioner, configured for restraining the supinated right hand. Digits 2–5 are secured with glue to small nail holders that fasten to moveable aluminum supports. The digit 2 and digit 4 supports pivot mediolaterally, whereas the digit 3 support rotates upward (flexion) and downward (extension).
The hand positioner (Fig. 3) was designed specifically to study object curvature in monkeys. The animal’s arm is held in place with two aluminum half-tube restraints to which its upper arm and forearm are firmly secured with velcro straps. These restraints are positioned such that they form approximately a right angle, which aids in keeping the animal from pulling its arm free. The dorsum of the outstretched, supinated hand rests flat on an aluminum hand plate and is held in place with custom-molded thermoplastic (Smith & Nephew, Inc., Memphis, TN). Digits 2–5 are then secured by gluing them (with a small amount of quickly drying cyanoacrylate glue (MCM Electronics, Inc., Centerville, OH)) to small nail holders that fasten to moveable aluminum supports. The proximal end of the supports for D2, D4, and D5 is fastened to the hand plate by a swivel joint that permits only mediolateral pivoting, and D5 is spread away from the other digits. The distal end of the D2 and D4 supports is attached to a pivoting aluminum linkage that is in turn attached to the shaft of a stepper motor that controls the degree of spread between these two digits. The support for D3 is different. This support is attached to a rigid S-shaped aluminum linkage, with one end of the linkage attached to a rotary stepper motor’s shaft and the other end glued to the distal nail pad of D3. The center of rotation for the end of the S-shaped linkage that is attached to the motor is parallel to the proximal joint of D3. Thus, rotating the shaft of the stepper motor causes D3 to be either flexed or extended relative to the other digits.
Simulating object shape
The stimulator was designed, for one configuration, to simulate the hand and stimulus conditions that occur when contacting objects that vary in curvature, by positioning the digits such that they form a particular conformation, and by indenting up to three bars simultaneously, one into each of up to three digits, to produce contact that mimics the position and orientation of a single continuous curve. Thus to simulate a concave surface touching the right hand, D3 would be flexed, the surface would contact the lateral aspect of D2 and the medial aspect of D4, and the part of the surface contacting D3 would be oriented mediolaterally. Convex surfaces are simulated in a similar way, with D3 extended and the surface contacting the medial aspect of D2 and the lateral aspect of D4, while again contacting D3 in a mediolateral orientation. Because tactile stimulation and hand conformation are controlled separately, the stimulator allows for a systematic assessment of the relative contributions of the mechanoreceptive and proprioceptive inputs from each digit to the representation of surface curvature.
Computerized control
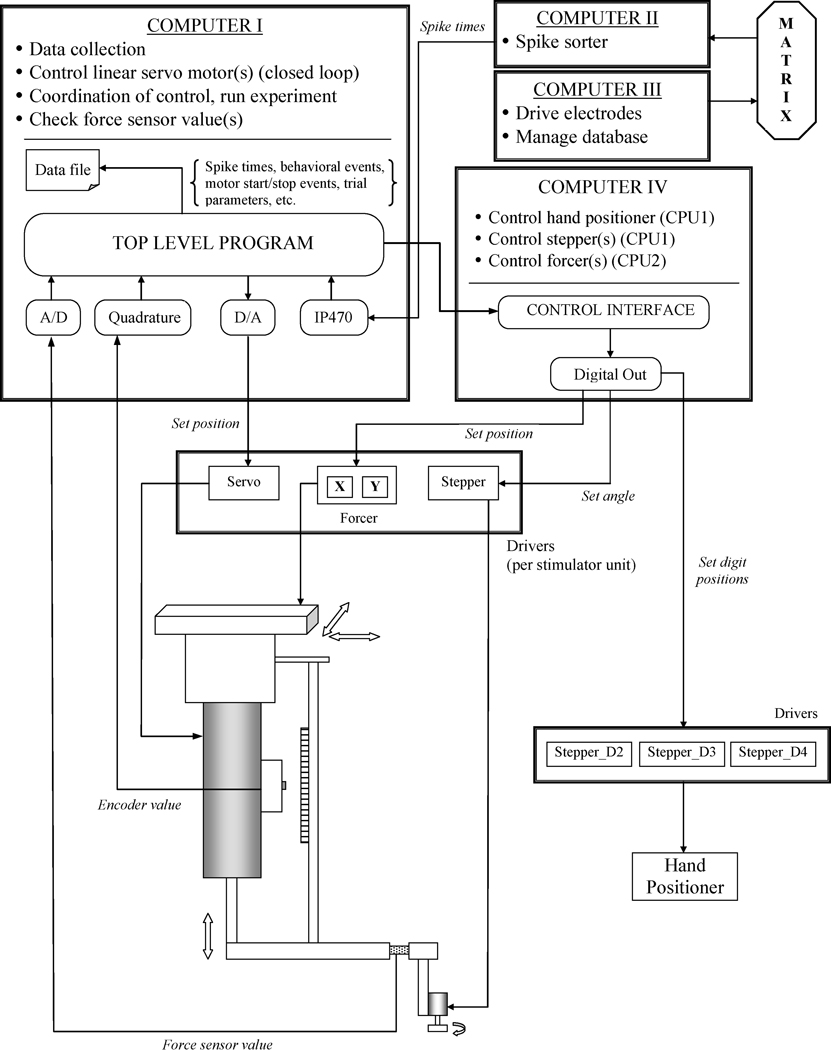
The entire stimulator is under computerized control. Figure 4 shows a block diagram of how the different parts of the stimulator are connected to our stimulation apparatus and neurophysiological data collection machines. One PC (Computer I) is devoted to data collection, running the linear servo motors, and coordinating the control signals for all the motors. The second PC (Computer IV) controls the three stepper motors that move the hand positioner, the XY position of the forcers, and the orientation or pitch of stimuli attached to the small rotary stepper motors. Both computers have interface cards that communicate with the linear drivers that provide current to the motors. These cards also have digital and analog inputs, and monitor the signals of the optical encoders and force sensors. The linear motors are controlled by a closed servo loop. In each computational cycle, a software PID controller reads the current position of the shaft of the motor from the optical encoder and then adjusts the speed and direction of the movement to match the commanded position.
Figure 4.
Computerized control of the stimulator. Shown is a block diagram of how the different parts of the stimulator are connected to two separate PCs. Computer I is devoted to data collection, running the linear servo motors, and coordinating the control signals for all the motors. Computer IV controls the three stepper motors that move the hand positioner, the orientation or pitch of stimuli attached to the small rotary stepper motors, and the XY position of the forcers. The matrix is our mechanical device for driving the microelectrodes into the brain.
In a typical experiment using one stimulating unit, during the interstimulus interval the linear motor moves the stimulus up and away from the hand, the forcer moves the stimulus to a new XY position (i.e., above another digit or pad), and the orientation of the stimulus and the hand conformation are also changed. To minimize the trial length, we control these motor movements using parallel processes running on different CPUs. A computational constraint for neurophysiological experiments is to make the computerized control “realtime” to ensure that the recorded action potential times are in synch with the recorded stimulation trial beginning and end times. To achieve this, we use the QNX realtime microkernel operating system (QNX Software Systems, Inc., Ottawa, Canada), which provides reliability, fault tolerance, and scalability for mission-critical applications. Since it is based on the POSIX standard, implementing UNIX, Linux, and other open source software is straightforward. One-way communication, in which the main computer (Computer I) sends the motor movement commands based on the experimental protocol to the second computer (Computer IV), which processes them and communicates with the motors, is implemented using pipes.
To determine where the forcers will move during an experiment, we use a MicroScribe (Immersion, Inc., San Jose, CA) that returns the 3-D coordinates of a point in space based on a frame of reference. The MicroScribe is used to specify locations on the hand that are to be stimulated. First, we use the MicroScribe to mark three points on the platen to use as an initial frame of reference that specifies the platen’s plane. The position of each forcer is then also marked. Using these measurements we can compute how much each forcer should move in the XY plane to reach any location on the hand. To ensure that identical points on the skin are stimulated when the hand is moved to different conformations, we visually mark the center of the finger pads with an ink spot. Note that if the MicroScribe remains in a fixed position, we only need to calibrate the platen and the forcer positions once, since the forcers are returned to their original positions at the end of the experiment.
Another calibration required at the beginning of an experiment is needed to determine the exact contact points of the stimulus with the hand. We use this additional calibration because the MicroScribe does not have sufficient resolution to ensure that contact is identical for each location on the hand. For each point of interest, first the forcer is moved to the marked position while the shaft of the linear motor is kept at its highest position. Then the shaft begins to move slowly downward. When contact is made with the hand, the load cell value increases and the shaft accordingly stops moving, and its position is used as the Z coordinate of the stimulating point. At this time, the forcer can also be moved a short distance to make fine adjustments in the stimulating point.
Single neuron recording
See Fitzgerald et al. 2004 for a description of our second somatosensory cortex (SII) recording techniques. Briefly, quartz coated microelectrodes were driven into the somatosensory cortex of a macaque monkey that was trained to sit quietly while receiving liquid rewards at random time intervals. After a single neuron with a receptive field located on the finger pads of the contralateral hand was isolated, the supinated hand was fastened to the hand positioner with the digits held flat and spread slightly apart. The centers of the finger pads and skin contact were determined manually as described above. Then the orientation of the stimulus bar was set, with zero degrees corresponding to a mediolateral orientation across the center of the distal pad. The neural recordings then began with the bar stimulating each of the four pads in a random sequence (Fig. 5A), indenting the skin 1300 microns from contact for 500 msec at one of eight orientations, separated by 22.5 degrees (similar to Fitzgerald et al. 2004—Fig. 2B). The maximum interstimulus interval was only 1.5 seconds, which was determined by the maximum distance traveled between finger pads, as well as a brief pause after the movement for the stimulator to stop shaking.
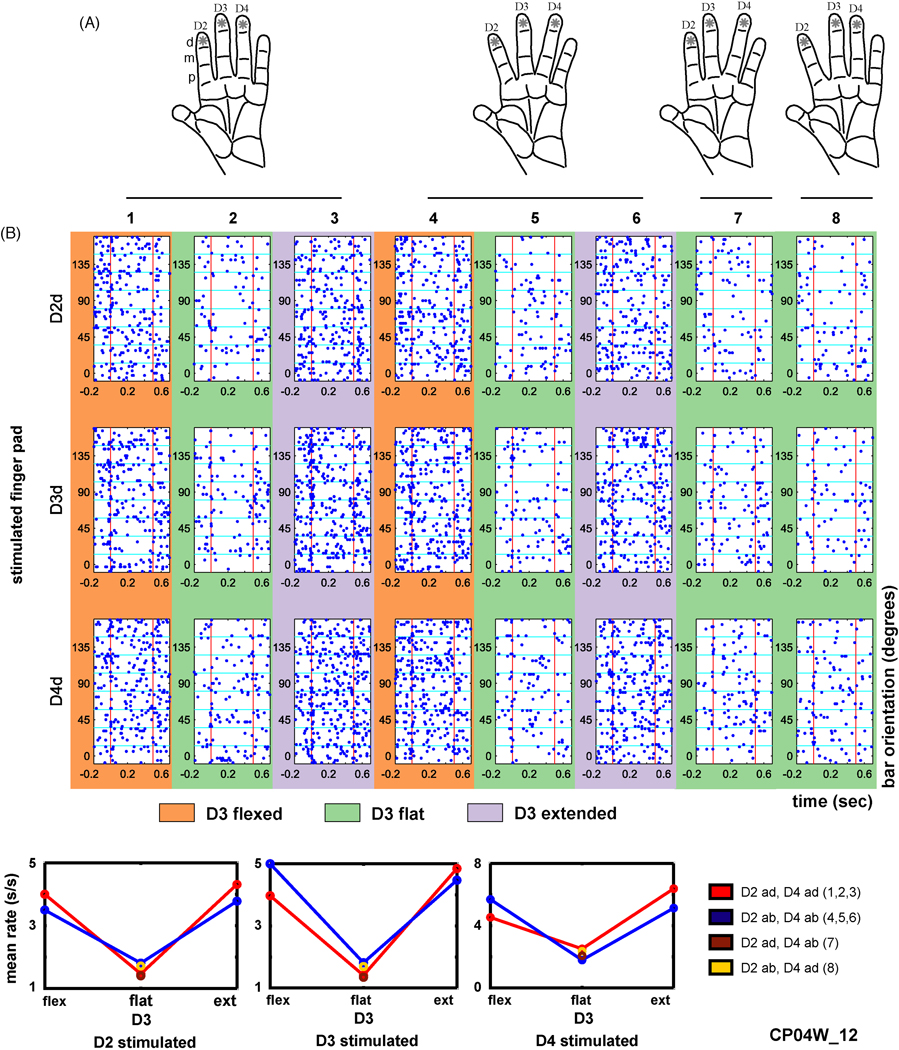
For the experiment shown in Figure 6, three hand conformations (1–3) simulated the positions of the digits when contacting small shapes (D2 and D4 adducted and D3 flexed, flat, or extended); three conformations (4–6) simulated contact with large shapes (D2 and D4 abducted and D3 flexed, flat, or extended); and we also used two intermediate asymmetrical conformations (7, 8) in which D3 was flat while D2 was adducted and D4 was abducted or vice versa.
Figure 6.
Combining mechanoreceptive and proprioceptive stimulation. A, Diagrams showing how the distal finger pads of digits 2–4 were stimulated with an oriented bar at eight, 22.5 degree separated orientations, while the hand was placed in one of eight conformations, where D2 and D4 were either adducted or abducted and D3 was flexed, flat, or extended (see Methods for more details). In conformations 1–3, D2 and D4 were adducted. In conformations 4–6, D2 and D4 were abducted. In conformation 7, D2 was adducted and D4 abducted. In conformation 8, D2 was abducted and D4 adducted. B, An example area 2 neuron (CP04W_12) raster (top), and mean firing rate diagram (bottom) averaged across the 500 msec indentation period. Abbreviations: spikes per second (s/s), flexed (flex), extended (ext), adducted (ad), abducted (ab).
Human psychophysics
In a human psychophysics study, subjects were presented with either a single long bar that touched D2d and D3d simultaneously, or were touched by two shorter co-linear bars, one on each of these two finger pads. The stimuli were hidden from view and auditory cues were eliminated. We tested four subjects in a two-alternative forced-choice task in which they were given two presentations per trial, one presentation with the single long bar and the other with the co-linear pair, and asked which presentation contained the single bar.
One modification to the stimulator which we used in a recent study (Yau et al. 2006) is replacement of the small rotary stepper motor with a custom-built, larger and more sturdy, vertically mounted rotary motor assembly, beneath which a pneumatic “gripper” motor (Pisco USA, Bensenville, IL) hangs vertically. This gripper motor is designed to clamp on to a stem that extends from the dorsum of each stimulus shape, using two movable wedge-shaped arms. In this configuration, the gripper is moved to a tray containing a large number of shapes. Changing stimuli is accomplished by releasing one shape in its proper slot in the tray, moving over to another slot, and picking up a new shape. Thus, arbitrary stimulus shapes can be presented to the hand.
Performance of the stimulator
We have used the stimulator in several psychophysical and neurophysiological experiments. Below we describe examples of uses that illustrate its versatility in presenting tactile surfaces to the hand of both humans and non-human primates. The stimulator has been used in three modes. In single unit mode, only one stimulating unit is used to present dynamic vibratory surfaces, statically indented surfaces, or moving surfaces to the hand. In multiple unit mode, two or more stimulating units are used to investigate aspects of tactile function that involve integration of information across the hand or both hands. In full object simulation mode, the stimulator is used to study the relative contributions of the mechanoreceptive and proprioceptive afferent systems to tactile shape processing. In this mode the conformation of the digits and positions of tactile stimuli are independently changed such that some cases are consistent with a particular shape and others are not.
Results
Single unit mode
Figure 5 shows an example of how the stimulator can be used in single unit mode. The aim of this neurophysiological study was to use a single stimulating unit to characterize the sizes and shapes of the receptive fields of neurons in SII cortex of an awake, behaving monkey. A receptive field of an orientation tuned SII neuron is shown in Figure 5B. The data are sorted according to finger pad and orientation of the bar. This neuron exhibited significant tuning (separate one-way ANOVA for each finger pad, p<0.01 in each case) on D2d, D3d, and D4d, and had a similar preferred orientation, centered at zero degrees, on these three finger pads. These results demonstrate that the stimulator is capable of stimulating different parts of the hand in an efficient manner.
Multiple unit mode
In multiple unit mode, two or more stimulating units that are controlled independently can be used to simultaneously stimulate the skin to study how large objects are represented in the nervous system. In a psychophysical study we tested whether subjects could tell if the stimulator was effectively simulating a single object. Subjects were presented, on two adjacent finger pads, with either one long bar across both pads or a co-linear pair of bars, one on each pad. In the first set of trials subjects were given no performance feedback, and in the second set of trials they were given feedback after each trial. Without feedback subjects performed near chance (mean: 45% correct), though performance improved to about 70% correct with feedback. These results show that there are some differences between the two types of stimulation that are detectible with feedback. However, subjects reported that the stimuli felt like a single continuous bar. These results demonstrate that the stimulator is capable of simulating contact with large objects.
Full object simulation mode
The third mode in which the stimulator can be used is to study the contributions of mechanoreceptive and proprioceptive inputs to object shape recognition. In this mode, these two inputs to the hand are independently presented, and the neural responses are then analyzed to determine their relative contributions. In a neurophysiological study (Hsiao et al. 2002), we used a single stimulating unit to determine whether the neural responses evoked by an indented oriented bar are affected by eight different hand conformations (Fig. 6). An example response from an area 2 neuron is shown in Figure 6B. For this neuron, the spontaneous firing rate and cutaneous responses to the bar on all three distal pads were clearly affected by the position of D3. When D3 was flexed or extended, the firing rate was higher, whereas when D3 was flat the firing rate was lower. These results suggest that this neuron is coding for contact of the hand with flat objects. More recently (Thakur et al. 2004), we extended these results and stimulated the digits while the hand was in 20 different conformations.
Discussion
We have demonstrated that this novel tactile stimulator is capable of stimulating the hand in a controlled fashion. Our studies of the human and monkey show that the stimulator can deliver a variety of stimuli that modulate behavioral and neural responses (Fig. 5, Fig. 6) in a systematic fashion. Below we elaborate further on its potential applications and limitations.
The stimulator can be used for a wide range of studies that require controlled stimulation of the skin while the hand is in specified conformations. In the simplest configuration, the stimulator uses a single stimulating unit that can deliver vibratory, statically indented, or scanned stimulus surfaces to any location on the hand. The stimulator is not limited to studying a single hand, as it can be used for bimanual stimulation as well. Although more than three stimulating units can be used at a time, there is a practical limit based on the footprint size of the platen. In addition, as the number of units increases, accurately controlling the stimulator also becomes increasingly difficult. The stimulator is very flexible; although we used oriented bars as stimuli, any light tactile stimulus surface can be used.
There are several limitations to the stimulator. One is that it can only indent stimuli vertically into the skin. This means that the tactile stimuli will not be identical when the degree to which a digit is flexed or extended is changed, since this produces different normal and tangential forces when the stimulus indents the skin. And since neurons in somatosensory cortex respond differently to variations in normal and tangential forces (Salimi et al. 1999a,b,c), this is potentially a serious limitation. One way to minimize these effects is to use only small degrees of digit flexion/extension. Another is to rotate the entire hand an amount equal to the degree of digit flexion/extension. Having the stimuli always presented in the vertical direction also poses a problem if one is studying responses from the thumb, which cannot be held flat relative to the other digits. It is also difficult to study complex aspects of hand function that involve grasping. However, grasping can be studied by changing the hand configuration component of the stimulator.
The hand positioner that we show here is only one of many possible hand restraints. In the experiments described above we limited the number of degrees of freedom in which the digits can move to three, which makes the study of tactile curvature more feasible. With some modifications, other configurations of the hand positioner could be implemented. For example the lateral spread between all three digits could be fixed and the degree to which D2 and D4 are flexed/extended could be changed. Such a configuration would more closely simulate the positioning of the digits that occurs when grasping objects.
Another limitation of the stimulator is that the simulation of curvature is only approximate since the local curvature on the skin is lost when only straight bars are used in the simulation. While this could be a problem when studying highly curved surfaces, it appears to have only minor effects when studying broadly curved surfaces (Pont et al. 1997). This limitation can also be minimized by using curved bars rather than straight ones.
Acknowledgements
This work was supported by National Institutes of Health Grant NS34086.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bliss JC, Katcher MH, Rogers CH, Shepard RP. Optical-to-tactile image conversion for the blind. IEEE Transactions on Man-Machine Systems. 1970;MMS-11:58–64. [Google Scholar]
- Burton H, Sinclair RJ. Second somatosensory cortical area in macaque monkeys. I. Neuronal responses to controlled, punctate indentations of glabrous skin on the hand. Brain Res. 1990;520:262–271. doi: 10.1016/0006-8993(90)91714-r. [DOI] [PubMed] [Google Scholar]
- Chubbuck JG. Small motion biological stimulator. Johns Hopkins APL Tech Digest. 1966:18–23. [Google Scholar]
- Connor CE, Hsiao SS, Phillips JR, Johnson KO. Tactile roughness: Neural codes that account for psychophysical magnitude estimates. J Neurosci. 1990;10:3823–3836. doi: 10.1523/JNEUROSCI.10-12-03823.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig JC. Vibrotactile pattern perception: Extraordinary observers. Science. 1977;196:450–452. doi: 10.1126/science.850791. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PJ, Lane JW, Thakur PH, Hsiao SS. Receptive field properties of the macaque second somatosensory cortex: Evidence for multiple functional representations. J Neurosci. 2004;24:11193–11204. doi: 10.1523/JNEUROSCI.3481-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PJ, Lane JW, Thakur PH, Hsiao SS. Receptive field (RF) properties of the macaque second somatosensory cortex: representation of orientation on different finger pads. J Neurosci. 2006a;26:6473–6484. doi: 10.1523/JNEUROSCI.5057-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PJ, Lane JW, Thakur PH, Hsiao SS. Receptive field (RF) properties of the macaque second somatosensory cortex: RF size, shape, and somatotopic organization. J Neurosci. 2006b;26:6485–6495. doi: 10.1523/JNEUROSCI.5061-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EP, Debowy DJ, Ro JY, Ghosh S, Babu KS. Sensory monitoring of prehension in the parietal lobe: A study using digital video. Behav Brain Res. 2002;135:213–224. doi: 10.1016/s0166-4328(02)00167-5. [DOI] [PubMed] [Google Scholar]
- Glendinning DS, Cooper BY, Vierck CJ, Jr, Leonard CM. Altered precision grasping in stumptail macaques after fasciculus cuneatus lesions. Somatosens Mot Res. 1992;9:61–73. doi: 10.3109/08990229209144763. [DOI] [PubMed] [Google Scholar]
- Hsiao SS, Pawluk D, Byrne A, Lane J. Effects of changes in hand conformation on neural responses in areas 3b, 1, and 2 of the awake behaving monkey. Soc Neurosci Abstr. 2002;650:14. [Google Scholar]
- Iwamura Y, Tanaka M. Postcentral neurons in hand region of area 2: Their possible role in the form discrimination of tactile objects. Brain Res. 1978;150:662–666. doi: 10.1016/0006-8993(78)90834-x. [DOI] [PubMed] [Google Scholar]
- Johnson KO. Reconstruction of population response to a vibratory stimulus in quickly adapting mechanoreceptive afferent fiber population innervating glabrous skin of the monkey. J Neurophysiol. 1974;37:48–72. doi: 10.1152/jn.1974.37.1.48. [DOI] [PubMed] [Google Scholar]
- Johnson KO, Lamb GD. Neural mechanisms of spatial tactile discrimination: Neural patterns evoked by braille-like dot patterns in the monkey. J Physiol. 1981;310:117–144. doi: 10.1113/jphysiol.1981.sp013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Shim JK, Zatiorsky VM, Latash ML. Finger inter-dependence: Linking the kinetic and kinematic variables. Hum Mov Sci. 2008;27:408–422. doi: 10.1016/j.humov.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman SJ, Klatzky RL. Hand movements: A window into haptic object recognition. Cognitive Psychology. 1987;19:342–368. doi: 10.1016/0010-0285(87)90008-9. [DOI] [PubMed] [Google Scholar]
- Phillips JR, Johnson KO. Tactile spatial resolution. II. Neural representation of bars, edges, and gratings in monkey primary afferents. J Neurophysiol. 1981;46:1192–1203. doi: 10.1152/jn.1981.46.6.1192. [DOI] [PubMed] [Google Scholar]
- Pont SC, Kappers AML, Koenderink JJ. Haptic curvature discrimination at several regions of the hand. Percept & Psychophys. 1997;59:1225–1240. doi: 10.3758/bf03214210. [DOI] [PubMed] [Google Scholar]
- Salimi I, Brochier T, Smith AM. Neuronal activity in somatosensory cortex of monkeys using a precision grip. I. Receptive fields and discharge patterns. J Neurophysiol. 1999a;81:825–834. doi: 10.1152/jn.1999.81.2.825. [DOI] [PubMed] [Google Scholar]
- Salimi I, Brochier T, Smith AM. Neuronal activity in somatosensory cortex of monkeys using a precision grip. II. Responses to object texture and weights. J Neurophysiol. 1999b;81:835–844. doi: 10.1152/jn.1999.81.2.835. [DOI] [PubMed] [Google Scholar]
- Salimi I, Brochier T, Smith AM. Neuronal activity in somatosensory cortex of monkeys using a precision grip. III. Responses to altered friction perturbations. J Neurophysiol. 1999c;81:845–857. doi: 10.1152/jn.1999.81.2.845. [DOI] [PubMed] [Google Scholar]
- Thakur PH, Fitzgerald PJ, Byrne AH, Pembeci I, Berryman LJ, Hsiao SS. Neural responses to changes in hand conformation in the second somatosensory cortex. Soc Neurosci Abstr. 2004 59.10. [Google Scholar]
- Vega-Bermudez F, Johnson KO, Hsiao SS. Human tactile pattern recognition: Active versus passive touch, velocity effects, and patterns of confusion. J Neurophysiol. 1991;65:531–546. doi: 10.1152/jn.1991.65.3.531. [DOI] [PubMed] [Google Scholar]
- Yau JM, Berryman LJ, Fitzgerald PJ, Connor CE, Hsiao SS. 2D shape representation in macaque second somatosensory cortex (SII) characterized with a genetic algorithm. Soc Neurosci Abstr. 2006 804.10. [Google Scholar]
- Zhou Y, Fuster JM. Mnemonic neuronal activity in somatosensory cortex. Proc Natl Acad Sci. 1996;93:10533–10537. doi: 10.1073/pnas.93.19.10533. [DOI] [PMC free article] [PubMed] [Google Scholar]