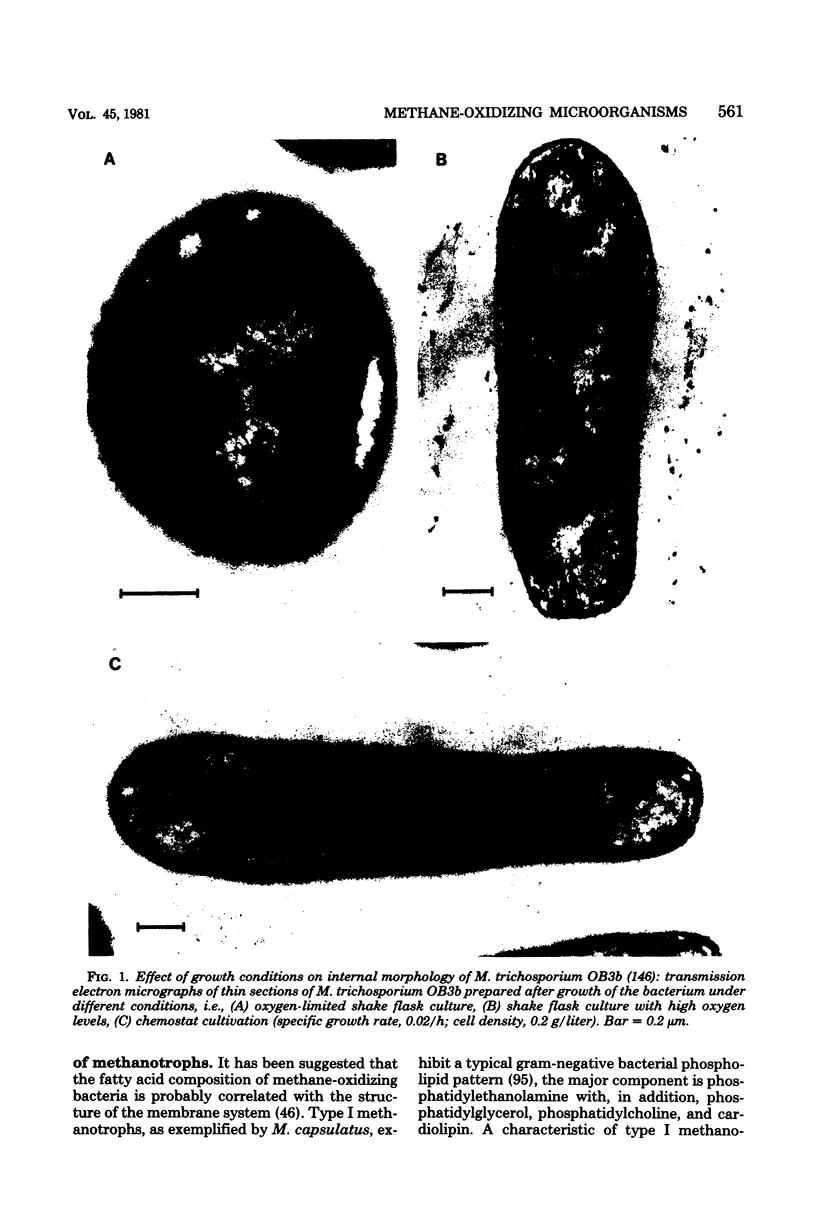
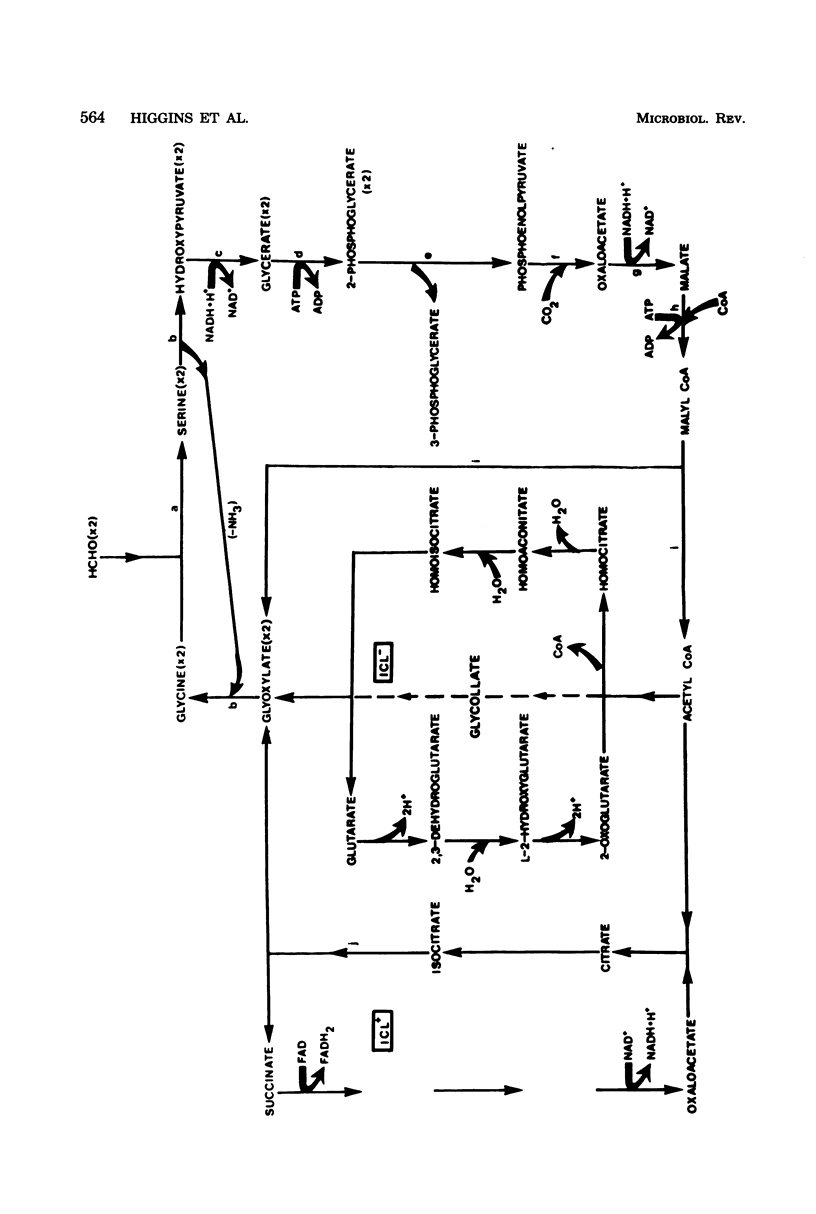
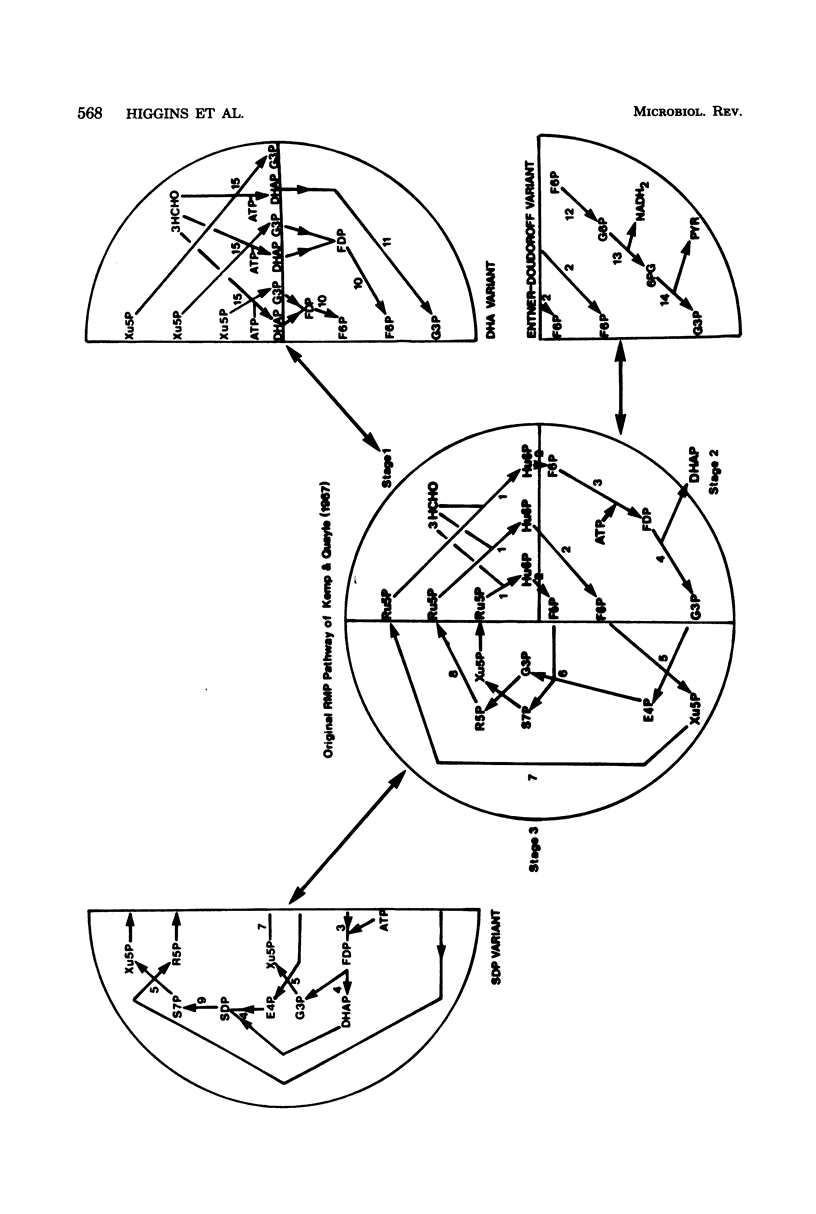
Full text
PDF














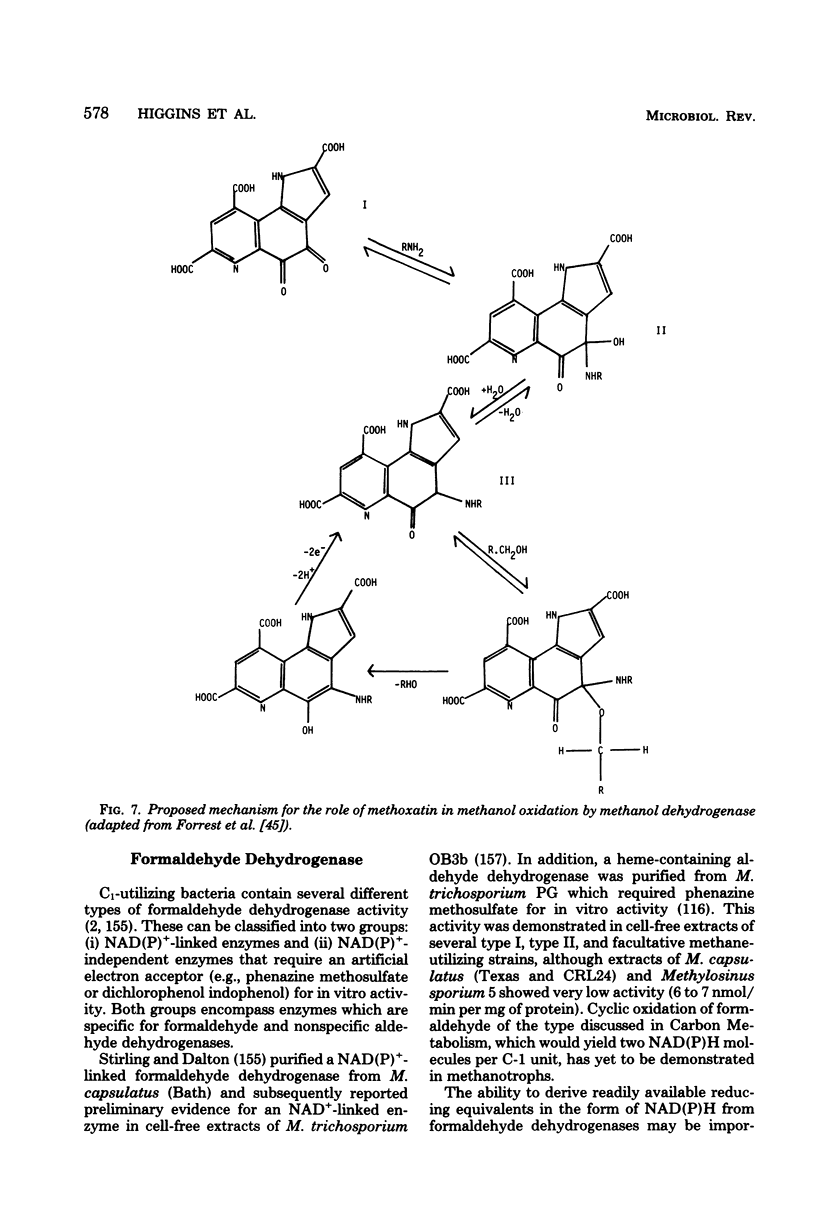












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony C. The biochemistry of methylotrophic micro-organisms. Sci Prog. 1975 Summer;62(246):167–206. [PubMed] [Google Scholar]
- Anthony C., Zatman L. J. The microbial oxidation of methanol. 1. Isolation and properties of Pseudomonas sp. M27. Biochem J. 1964 Sep;92(3):609–614. doi: 10.1042/bj0920609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellion E., Wu G. T. Alcohol dehydrogenases from a facultative methylotrophic bacterium. J Bacteriol. 1978 Jul;135(1):251–258. doi: 10.1128/jb.135.1.251-258.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird C. W., Lynch J. M., Pirt F. J., Reid W. W. Steroids and squalene in Methylococcus capsulatus grown on methane. Nature. 1971 Apr 16;230(5294):473–474. doi: 10.1038/230473a0. [DOI] [PubMed] [Google Scholar]
- Bont J. A. Hydrogenase activity in nitrogen-fixing methane-oxidizing bacteria. Antonie Van Leeuwenhoek. 1976;42(3):255–259. doi: 10.1007/BF00394122. [DOI] [PubMed] [Google Scholar]
- Colby J., Dalton H. Characterization of the second prosthetic group of the flavoenzyme NADH-acceptor reductase (component C) of the methane mono-oxygenase from Methylococcus capsulatus (Bath). Biochem J. 1979 Mar 1;177(3):903–908. doi: 10.1042/bj1770903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Dalton H. Resolution of the methane mono-oxygenase of Methylococcus capsulatus (Bath) into three components. Purification and properties of component C, a flavoprotein. Biochem J. 1978 May 1;171(2):461–468. doi: 10.1042/bj1710461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Dalton H. Some properties of a soluble methane mono-oxygenase from Methylococcus capsulatus strain Bath. Biochem J. 1976 Aug 1;157(2):495–497. doi: 10.1042/bj1570495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Dalton H., Whittenbury R. An improved assay for bacterial methane mono-oxygenase: some properties of the enzyme from Methylomonas methanica. Biochem J. 1975 Nov;151(2):459–462. doi: 10.1042/bj1510459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Dalton H., Whittenbury R. Biological and biochemical aspects of microbial growth on C1 compounds. Annu Rev Microbiol. 1979;33:481–517. doi: 10.1146/annurev.mi.33.100179.002405. [DOI] [PubMed] [Google Scholar]
- Colby J., Stirling D. I., Dalton H. The soluble methane mono-oxygenase of Methylococcus capsulatus (Bath). Its ability to oxygenate n-alkanes, n-alkenes, ethers, and alicyclic, aromatic and heterocyclic compounds. Biochem J. 1977 Aug 1;165(2):395–402. doi: 10.1042/bj1650395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Zatman L. J. Regulation of citrate synthase activity in methylotrophs by reduced nicotinamide-adenine dinucleotide, adenine nucleotides and 2-oxoglutarate. Biochem J. 1975 Jul;150(1):141–144. doi: 10.1042/bj1500141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DWORKIN M., FOSTER J. W. Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol. 1958 May;75(5):592–603. doi: 10.1128/jb.75.5.592-603.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DWORKIN M., FOSTER J. W. Studies on Pseudomonas methanica (Söhngen) nov. comb. J Bacteriol. 1956 Nov;72(5):646–659. doi: 10.1128/jb.72.5.646-659.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J. F., Whittenbury R., Wilkinson J. F. The distribution in the methylobacteria of some key enzymes concerned with intermediary metabolism. Arch Mikrobiol. 1972;87(4):359–366. doi: 10.1007/BF00409135. [DOI] [PubMed] [Google Scholar]
- Davies S. L., Whittenbury R. Fine structure of methane and other hydrocarbon-utilizing bacteria. J Gen Microbiol. 1970 May;61(2):227–232. doi: 10.1099/00221287-61-2-227. [DOI] [PubMed] [Google Scholar]
- De Boer W. E., Hazeu W. Observations on the fine structure of a methane-oxidizing bacterium. Antonie Van Leeuwenhoek. 1972;38(1):33–47. doi: 10.1007/BF02328075. [DOI] [PubMed] [Google Scholar]
- Duine J. A., Frank J., Jr The prosthetic group of methanol dehydrogenase. Purification and some of its properties. Biochem J. 1980 Apr 1;187(1):221–226. doi: 10.1042/bj1870221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duine J. A., Frank J., Verwiel P. E. Structure and activity of the prosthetic group of methanol dehydrogenase. Eur J Biochem. 1980;108(1):187–192. doi: 10.1111/j.1432-1033.1980.tb04711.x. [DOI] [PubMed] [Google Scholar]
- Duine J. A., Frank J., Westerling J. Purification and properties of methanol dehydrogenase from Hyphomicrobium x. Biochim Biophys Acta. 1978 Jun 9;524(2):277–287. doi: 10.1016/0005-2744(78)90164-x. [DOI] [PubMed] [Google Scholar]
- Eccleston M., Kelly D. P. Assimilation and toxicity of some exogenous C1 compounds, alcohols, sugars and acetate in the methane-oxidizing bacterium Methylococcus capsulatus. J Gen Microbiol. 1973 Mar;75(1):211–221. doi: 10.1099/00221287-75-1-211. [DOI] [PubMed] [Google Scholar]
- Ferenci T. Carbon monoxide-stimulated respiration in methane-utilizing bacteria. FEBS Lett. 1974 Apr 15;41(1):94–98. doi: 10.1016/0014-5793(74)80962-2. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Strom T., Quayle J. R. Oxidation of carbon monoxide and methane by Pseudomonas methanica. J Gen Microbiol. 1975 Nov;91(1):79–91. doi: 10.1099/00221287-91-1-79. [DOI] [PubMed] [Google Scholar]
- Forrest H. S., Salisbury S. A., Kilty C. G. A mechanism for the enzymic oxidation of methanol involving methoxatin. Biochem Biophys Res Commun. 1980 Nov 17;97(1):248–251. doi: 10.1016/s0006-291x(80)80161-6. [DOI] [PubMed] [Google Scholar]
- Gautier F., Bonewald R. The use of plasmid R1162 and derivatives for gene cloning in the methanol-utilizing Pseudomonas AM1. Mol Gen Genet. 1980;178(2):375–380. doi: 10.1007/BF00270487. [DOI] [PubMed] [Google Scholar]
- HARRINGTON A. A., KALLIO R. E. Oxidation of methanol and formaldehyde by pseudomonas methanica. Can J Microbiol. 1960 Feb;6:1–7. doi: 10.1139/m60-001. [DOI] [PubMed] [Google Scholar]
- HUTTON W. E., ZOBELL C. E. Production of nitrite from ammonia by methane oxidizing bacteria. J Bacteriol. 1953 Feb;65(2):216–219. doi: 10.1128/jb.65.2.216-219.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder W., Attwood M. M. Biology, physiology and biochemistry of hyphomicrobia. Adv Microb Physiol. 1978;17:303–359. doi: 10.1016/s0065-2911(08)60060-0. [DOI] [PubMed] [Google Scholar]
- Harder W., Matin A., Attwood M. M. Studies on the physiological significance of the lack of a pyruvate dehydrogenase complex in Hyphomicrobium sp. J Gen Microbiol. 1975 Feb;86(2):319–326. doi: 10.1099/00221287-86-2-319. [DOI] [PubMed] [Google Scholar]
- Harwood J. H., Williams E., Bainbridge B. W. Mutation of the methane oxidizing bacterium Methylococcus capsulatus. J Appl Bacteriol. 1972 Mar;35(1):99–108. doi: 10.1111/j.1365-2672.1972.tb03678.x. [DOI] [PubMed] [Google Scholar]
- Haubold R. Two different types of surface structures of methane utilizing bacteria. Z Allg Mikrobiol. 1978;18(7):511–515. doi: 10.1002/jobm.3630180707. [DOI] [PubMed] [Google Scholar]
- Hazeu W. Some cultural and physiological aspects of methane-utilizing bacteria. Antonie Van Leeuwenhoek. 1975;41(2):121–134. doi: 10.1007/BF02565044. [DOI] [PubMed] [Google Scholar]
- Hazeu W., Steennis P. J. Isolation and characterization of two vibrio-shaped methane-oxidizing bacteria. Antonie Van Leeuwenhoek. 1970;36(1):67–72. doi: 10.1007/BF02069009. [DOI] [PubMed] [Google Scholar]
- Higgins I. J., Best D. J., Hammond R. C. New findings in methane-utilizing bacteria highlight their importance in the biosphere and their commercial potential. Nature. 1980 Aug 7;286(5773):561–564. doi: 10.1038/286561a0. [DOI] [PubMed] [Google Scholar]
- Higgins I. J., Hammond R. C., Sariaslani F. S., Best D., Davies M. M., Tryhorn S. E., Taylor F. Biotransformation of hydrocarbons and related compounds by whole organism suspensions of methane-grown methylosinus trichosporium OB 3b. Biochem Biophys Res Commun. 1979 Jul 27;89(2):671–677. doi: 10.1016/0006-291x(79)90682-x. [DOI] [PubMed] [Google Scholar]
- Holloway B. W., Krishnapillai V., Morgan A. F. Chromosomal genetics of Pseudomonas. Microbiol Rev. 1979 Mar;43(1):73–102. doi: 10.1128/mr.43.1.73-102.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C. T., Laskin A. I., Patel R. N. Growth and Polysaccharide Production by Methylocystis parvus OBBP on Methanol. Appl Environ Microbiol. 1979 May;37(5):800–804. doi: 10.1128/aem.37.5.800-804.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C. T., Patel R. N., Laski A. I., Marczak I., Barnabe N. Microbial oxidation of gaseous hydrocarbons: production of alcohols and methyl ketones from their corresponding n-alkanes by methylotrophic bacteria. Can J Microbiol. 1981 Jan;27(1):107–115. doi: 10.1139/m81-017. [DOI] [PubMed] [Google Scholar]
- Hou C. T., Patel R. N., Laskin A. I., Barnabe N., Marczak I. Identification and purification of a nicotinamide adenine dinucleotide-dependent secondary alcohol dehydrogenase from C1-utilizing microbes. FEBS Lett. 1979 May 1;101(1):179–183. doi: 10.1016/0014-5793(79)81321-6. [DOI] [PubMed] [Google Scholar]
- Hou C. T., Patel R., Laskin A. I., Barnabe N., Marczak I. Microbial oxidation of gaseous hydrocarbons: production of methyl ketones from their corresponding secondary alcohols by methane- and methanol-grown microbes. Appl Environ Microbiol. 1979 Jul;38(1):135–142. doi: 10.1128/aem.38.1.135-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C. T., Patel R., Laskin A. I., Barnabe N. Microbial oxidation of gaseous hydrocarbons: epoxidation of C2 to C4 n-alkenes by methylotrophic bacteria. Appl Environ Microbiol. 1979 Jul;38(1):127–134. doi: 10.1128/aem.38.1.127-134.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson D. W., Whittenbury R., Dalton H. A possible role of free radicals in the oxidation of methane by Methylococcus capsulatus. J Theor Biol. 1976 May 21;58(2):325–335. doi: 10.1016/s0022-5193(76)80123-3. [DOI] [PubMed] [Google Scholar]
- Hyder S. L., Meyers A., Cayer M. L. Membrane modulation in a methylotrophic bacterium Methylococcus capsulatus (Texas) as a function of growth substrate. Tissue Cell. 1979;11(4):597–610. doi: 10.1016/0040-8166(79)90017-x. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W. Arene oxides: a new aspect of drug metabolism. Science. 1974 Aug 16;185(4151):573–582. doi: 10.1126/science.185.4151.573. [DOI] [PubMed] [Google Scholar]
- Johnson P. A., Quayle J. R. Microbial growth on C-1 compounds. 6. Oxidation of methanol, formaldehyde and formate by methanol-grown Pseudomonas AM-1. Biochem J. 1964 Nov;93(2):281–290. doi: 10.1042/bj0930281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALLIO R. E., HARRINGTON A. A. Sudanophilic granules and lipid of Pseudomonas methanica. J Bacteriol. 1960 Sep;80:321–324. doi: 10.1128/jb.80.3.321-324.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARGE P. J., PEEL D., QUAYLE J. R. Microbial growth on C1 compounds. II. Synthesis of cell constituents by methanol- and formate-grown Pseudomonas AM 1, and methanol-grown Hyphomicrobium vulgare. Biochem J. 1961 Dec;81:470–480. doi: 10.1042/bj0810470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEADBETTER E. R., FOSTER J. W. Bacterial oxidation of gaseous alkanes. Arch Mikrobiol. 1960;35:92–104. doi: 10.1007/BF00425597. [DOI] [PubMed] [Google Scholar]
- LUKINS H. B., FOSTER J. W. METHYL KETONE METABOLISM IN HYDROCARBON-UTILIZING MYCOBACTERIA. J Bacteriol. 1963 May;85:1074–1087. doi: 10.1128/jb.85.5.1074-1087.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large P. J., Peel D., Quayle J. R. Microbial growth on C(1) compounds. 3. Distribution of radioactivity in metabolites of methanol-grown Pseudomonas AM1 after incubation with [C]methanol and [C]bicarbonate. Biochem J. 1962 Mar;82(3):483–488. doi: 10.1042/bj0820483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large P. J., Quayle J. R. Microbial growth on C(1) compounds. 5. Enzyme activities in extracts of Pseudomonas AM1. Biochem J. 1963 May;87(2):386–396. doi: 10.1042/bj0870386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence A. J., Kemp M. B., Quayle J. R. Synthesis of cell constituents by methane-grown Methylococcus capsulatus and Methanomonas methanooxidans. Biochem J. 1970 Feb;116(4):631–639. doi: 10.1042/bj1160631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence A. J., Quayle J. R. Alternative carbon assimilation pathways in methane-utilizing bacteria. J Gen Microbiol. 1970 Nov;63(3):371–374. doi: 10.1099/00221287-63-3-371. [DOI] [PubMed] [Google Scholar]
- Linton J. D., Cripps R. E. The occurrence and identification of intracellular polyglucose storage granules in Methylococcus NCIB 11083 grown in chemostat culture on methane. Arch Microbiol. 1978 Apr 27;117(1):41–48. doi: 10.1007/BF00689349. [DOI] [PubMed] [Google Scholar]
- Loginova N. B., Trotsenko Iu A. Karboksilazy piruvata i fosfoenolpiruvata u metilotrofov. Mikrobiologiia. 1979 Mar-Apr;48(2):202–207. [PubMed] [Google Scholar]
- Lynch M. J., Wopat A. E., O'connor M. L. Characterization of two new facultative methanotrophs. Appl Environ Microbiol. 1980 Aug;40(2):400–407. doi: 10.1128/aem.40.2.400-407.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makula R. A. Phospholipid composition of methane-utilizing bacteria. J Bacteriol. 1978 Jun;134(3):771–777. doi: 10.1128/jb.134.3.771-777.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naguib M. Alternative carboxylation reactions in type II methylotrophs and the localization of carboxylase activities in the intra-cytoplasmic membranes. Z Allg Mikrobiol. 1979;19(5):333–342. doi: 10.1002/jobm.3630190505. [DOI] [PubMed] [Google Scholar]
- Newaz S. S., Hersh L. B. Reduced nicotinamide adenine dinucleotide-activated phosphoenolpyruvate carboxylase in Pseudomonas MA: potential regulation between carbon assimilation and energy production. J Bacteriol. 1975 Nov;124(2):825–833. doi: 10.1128/jb.124.2.825-833.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor M. L. Extension of the model concerning linkage of genes coding for C-1 related functions in Methylobacterium organophilum. Appl Environ Microbiol. 1981 Feb;41(2):437–441. doi: 10.1128/aem.41.2.437-441.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor M. L., Hanson R. S. Serine transhydroxymethylase isoenzymes from a facultative methylotroph. J Bacteriol. 1975 Nov;124(2):985–996. doi: 10.1128/jb.124.2.985-996.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor M., Wopat A., Hanson R. S. Genetic transformation in Methylobacterium organophilum. J Gen Microbiol. 1977 Jan;98(1):265–272. doi: 10.1099/00221287-98-1-265. [DOI] [PubMed] [Google Scholar]
- Panganiban A. T., Jr, Patt T. E., Hart W., Hanson R. S. Oxidation of methane in the absence of oxygen in lake water samples. Appl Environ Microbiol. 1979 Feb;37(2):303–309. doi: 10.1128/aem.37.2.303-309.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Bose H. R., Mandy W. J., Hoare D. S. Physiological studies of methane- and methanol-oxidizing bacteria: comparison of a primary alcohol dehydrogenase from Methylococcus capsulatus (Texas strain) and Pseudomonas species M27. J Bacteriol. 1972 May;110(2):570–577. doi: 10.1128/jb.110.2.570-577.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Felix A. Microbial oxidation of methane and methanol: crystallization and properties of methanol dehydrogenase from Methylosinus sporium. J Bacteriol. 1976 Oct;128(1):413–424. doi: 10.1128/jb.128.1.413-424.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Hoare D. S. Physiological studies of methane and methanol-oxidizing bacteria: oxidation of C-1 compounds by Methylococcus capsulatus. J Bacteriol. 1971 Jul;107(1):187–192. doi: 10.1128/jb.107.1.187-192.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Hoare S. L., Hoare D. S. (1-14C) acetate assimilation by obligate methylotrophs, Pseudomonas methanica and Methylosinus trichosporium. Antonie Van Leeuwenhoek. 1979;45(3):499–511. doi: 10.1007/BF00443287. [DOI] [PubMed] [Google Scholar]
- Patel R. N., Hou C. T., Derelanko P., Felix A. Purification and properties of a heme-containing aldehyde dehydrogenase from Methylosinus trichosporium. Arch Biochem Biophys. 1980 Sep;203(2):654–662. doi: 10.1016/0003-9861(80)90223-4. [DOI] [PubMed] [Google Scholar]
- Patel R. N., Hou C. T., Felix A. Microbial oxidation of methane and methanol: isolation of methane-utilizing bacteria and characterization of a facultative methane-utilizing isolate. J Bacteriol. 1978 Oct;136(1):352–358. doi: 10.1128/jb.136.1.352-358.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Hou C. T., Laskin A. I., Felix A., Derelanko P. Microbial Oxidation of Gaseous Hydrocarbons: Production of Methylketones from Corresponding n-Alkanes by Methane-Utilizing Bacteria. Appl Environ Microbiol. 1980 Apr;39(4):727–733. doi: 10.1128/aem.39.4.727-733.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Hou C. T., Laskin A. I., Felix A., Derelanko P. Microbial Oxidation of Gaseous Hydrocarbons: Production of Secondary Alcohols from Corresponding n-Alkanes by Methane-Utilizing Bacteria. Appl Environ Microbiol. 1980 Apr;39(4):720–726. doi: 10.1128/aem.39.4.720-726.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Hou C. T., Laskin A. I., Felix A., Derelanko P. Microbial oxidation of gaseous hydrocarbons. II. Hydroxylation of alkanes and epoxidation of alkenes by cell-free particulate fractions of methane-utilizing bacteria. J Bacteriol. 1979 Aug;139(2):675–679. doi: 10.1128/jb.139.2.675-679.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Mandy W. J., Hoare D. S. Physiological studies of methane- and methanol-oxidizing bacteria: immunological comparison of a primary alcohol dehydrogenase from Methylococcus capsulatus and Pseudomonas sp. M27. J Bacteriol. 1973 Feb;113(2):937–945. doi: 10.1128/jb.113.2.937-945.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R., Hoare L., Hoare D. S., Taylor B. F. Incomplete tricarboxylic acid cycle in a type I methylotroph, Methylococcus capsulatus. J Bacteriol. 1975 Jul;123(1):382–384. doi: 10.1128/jb.123.1.382-384.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patt T. E., Cole G. C., Bland J., Hanson R. S. Isolation and characterization of bacteria that grow on methane and organic compounds as sole sources of carbon and energy. J Bacteriol. 1974 Nov;120(2):955–964. doi: 10.1128/jb.120.2.955-964.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patt T. E., Hanson R. S. Intracytoplasmic membrane, phospholipid, and sterol content of Methylobacterium organophilum cells grown under different conditions. J Bacteriol. 1978 May;134(2):636–644. doi: 10.1128/jb.134.2.636-644.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce L. E., Meynell E. Specific chromosomal affinity of a resistant factor. J Gen Microbiol. 1968 Jan;50(1):159–172. doi: 10.1099/00221287-50-1-159. [DOI] [PubMed] [Google Scholar]
- Quayle J. R. Aspects of the regulation of methylotrophic metabolism. FEBS Lett. 1980 Aug 25;117 (Suppl):K16–K27. doi: 10.1016/0014-5793(80)80566-7. [DOI] [PubMed] [Google Scholar]
- Quayle J. R., Ferenci T. Evolutionary aspects of autotrophy. Microbiol Rev. 1978 Jun;42(2):251–273. doi: 10.1128/mr.42.2.251-273.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quayle J. R. Microbial assimilation of C1 compounds. The Thirteenth CIBA Medal Lecture. Biochem Soc Trans. 1980 Feb;8(1):1–10. doi: 10.1042/bst0080001. [DOI] [PubMed] [Google Scholar]
- Reed W. M., Dugan P. R. Distribution of Methylomonas methanica and Methylosinus trichosporium in Cleveland Harbor as Determined by an Indirect Fluorescent Antibody-Membrane Filter Technique. Appl Environ Microbiol. 1978 Feb;35(2):422–430. doi: 10.1128/aem.35.2.422-430.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed W. M., Dugan P. R. Study of Developmental Stages of Methylosinus trichosporium with the Aid of Fluorescent-Antibody Staining Techniques. Appl Environ Microbiol. 1979 Dec;38(6):1179–1183. doi: 10.1128/aem.38.6.1179-1183.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed W. M., Titus J. A., Dugan P. R., Pfister R. M. Structure of Methylosinus trichosporium exospores. J Bacteriol. 1980 Feb;141(2):908–913. doi: 10.1128/jb.141.2.908-913.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbons D. W., Michalover J. L. Methane oxidation by cell-free extracts of Methylococcus capsulatus. FEBS Lett. 1970 Nov 9;11(1):41–44. doi: 10.1016/0014-5793(70)80487-2. [DOI] [PubMed] [Google Scholar]
- Ribbons D. W. Oxidation of C1 Compounds by Particulate fractions from Methylococcus capsulatus: distribution and properties of methane-dependent reduced nicotinamide adenine dinucleotide oxidase (methane hydroxylase). J Bacteriol. 1975 Jun;122(3):1351–1363. doi: 10.1128/jb.122.3.1351-1363.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem A. R., Hacking A. J., Quayle J. R. Cleavage of malyl-Coenzyme A into acetyl-Coenzyme A and glyoxylate by Pseudomonas AM1 and other C1-unit-utilizing bacteria. Biochem J. 1973 Sep;136(1):89–96. doi: 10.1042/bj1360089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury S. A., Forrest H. S., Cruse W. B., Kennard O. A novel coenzyme from bacterial primary alcohol dehydrogenases. Nature. 1979 Aug 30;280(5725):843–844. doi: 10.1038/280843a0. [DOI] [PubMed] [Google Scholar]
- Smith A. J., Hoare D. S. Specialist phototrophs, lithotrophs, and methylotrophs: a unity among a diversity of procaryotes? Bacteriol Rev. 1977 Jun;41(2):419–448. doi: 10.1128/br.41.2.419-448.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling D. I., Colby J., Dalton H. A comparison of the substrate and electron-donor specificities of the methane mono-oxygenases from three strains of methane-oxidizing bacteria. Biochem J. 1979 Jan 1;177(1):361–364. doi: 10.1042/bj1770361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling D. I., Dalton H. Effect of metal-binding and other compounds on methane oxidation by two strains of Methylococcus capsulatus. Arch Microbiol. 1977 Jul 26;114(1):71–76. doi: 10.1007/BF00429633. [DOI] [PubMed] [Google Scholar]
- Stirling D. I., Dalton H. Fortuitous oxidations by methane-utilizing bacteria. Nature. 1981 May 14;291(5811):169–170. doi: 10.1038/291169a0. [DOI] [PubMed] [Google Scholar]
- Stirling D. I., Dalton H. Fortuitous oxidations by methane-utilizing bacteria. Nature. 1981 May 14;291(5811):169–170. doi: 10.1038/291169a0. [DOI] [PubMed] [Google Scholar]
- Stirling D. I., Dalton H. Properties of the methane mono-oxygenase from extracts of Methylosinus trichosporium OB3b and evidence for its similarity to the enzyme from Methylococcus capsulatus (Bath). Eur J Biochem. 1979 May 2;96(1):205–212. doi: 10.1111/j.1432-1033.1979.tb13030.x. [DOI] [PubMed] [Google Scholar]
- Stirling D. I., Dalton H. Purification and properties of an NAD(P)+-linked formaldehyde dehydrogenase from Methylococcus capsulatus (Bath). J Gen Microbiol. 1978 Jul;107(1):19–29. doi: 10.1099/00221287-107-1-19. [DOI] [PubMed] [Google Scholar]
- Strom T., Ferenci T., Quayle J. R. The carbon assimilation pathways of Methylococcus capsulatus, Pseudomonas methanica and Methylosinus trichosporium (OB3B) during growth on methane. Biochem J. 1974 Dec;144(3):465–476. doi: 10.1042/bj1440465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki I., Kwok S. C., Dular U. Competitive inhibition of ammonia oxidation in Nitrosomonas europaea by methane, carbon monoxide or methanol. FEBS Lett. 1976 Dec 15;72(1):117–120. doi: 10.1016/0014-5793(76)80825-3. [DOI] [PubMed] [Google Scholar]
- Takeda K., Tanaka K. Ultrastructure of intracytoplasmic membranes of Methanomonas margaritae cells grown under different conditions. Antonie Van Leeuwenhoek. 1980;46(1):15–25. doi: 10.1007/BF00422225. [DOI] [PubMed] [Google Scholar]
- Taylor I. J., Anthony C. A biochemical basis for obligate methylotrophy: properties of a mutant of Pseudomonas AM1 lacking 2-oxoglutarate dehydrogenase. J Gen Microbiol. 1976 Apr;93(2):259–265. doi: 10.1099/00221287-93-2-259. [DOI] [PubMed] [Google Scholar]
- Thomson A. W., O'Neill J. G., Wilkinson J. F. Acetone production by methylobacteria. Arch Microbiol. 1976 Sep 1;109(3):243–246. doi: 10.1007/BF00446635. [DOI] [PubMed] [Google Scholar]
- Tomaszewski J. E., Jerina D. M., Daly J. W. Deuterium isotope effects during formation of phenols by hepatic monoxygenases. Evidence for an alternative to arene oxide pathway. Biochemistry. 1975 May 6;14(9):2024–2031. doi: 10.1021/bi00680a033. [DOI] [PubMed] [Google Scholar]
- Tonge G. M., Harrison D. E., Higgins I. J. Purification and properties of the methane mono-oxygenase enzyme system from Methylosinus trichosporium OB3b. Biochem J. 1977 Feb 1;161(2):333–344. doi: 10.1042/bj1610333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonge G. M., Harrison D. E., Knowles C. J., Higgins I. J. Properties and partial purification of the methane-oxidising enzyme system from Methylosinus trichosporium. FEBS Lett. 1975 Oct 15;58(1):293–299. doi: 10.1016/0014-5793(75)80282-1. [DOI] [PubMed] [Google Scholar]
- Tyutikov F. M., Bespalova I. A., Rebentish B. A., Aleksandrushkina N. N., Krivisky A. S. Bacteriophages of methanotrophic bacteria. J Bacteriol. 1980 Oct;144(1):375–381. doi: 10.1128/jb.144.1.375-381.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadzinski A. M., Ribbons D. W. Oxidation of C1 compounds by particulate fractions from Methylococcus capsulatus: properties of methanol oxidase and methanol dehydrogenase. J Bacteriol. 1975 Jun;122(3):1364–1374. doi: 10.1128/jb.122.3.1364-1374.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadzinski A. M., Ribbons D. W. Utilization of acetate by Methanomonas emthanooxidans. J Bacteriol. 1975 Jul;123(1):380–381. doi: 10.1128/jb.123.1.380-381.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver T. L., Dugan P. R. Ultrastruct of Methylosinus trichosporium as revealed by freeze etching. J Bacteriol. 1975 Feb;121(2):704–710. doi: 10.1128/jb.121.2.704-710.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver T. L., Patrick M. A., Dugan P. R. Whole-cell and membrane lipids of the methylotrophic bacterium Methylosinus trichosporium. J Bacteriol. 1975 Nov;124(2):602–605. doi: 10.1128/jb.124.2.602-605.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerling J., Frank J., Duine J. A. The prosthetic group of methanol dehydrogenase from Hyphomicrobium X: electron spin resonance evidence for a quinone structure. Biochem Biophys Res Commun. 1979 Apr 13;87(3):719–724. doi: 10.1016/0006-291x(79)92018-7. [DOI] [PubMed] [Google Scholar]
- Whittenbury R., Davies S. L., Davey J. F. Exospores and cysts formed by methane-utilizing bacteria. J Gen Microbiol. 1970 May;61(2):219–226. doi: 10.1099/00221287-61-2-219. [DOI] [PubMed] [Google Scholar]
- Whittenbury R., Phillips K. C., Wilkinson J. F. Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol. 1970 May;61(2):205–218. doi: 10.1099/00221287-61-2-205. [DOI] [PubMed] [Google Scholar]
- Wilkinson P. C. Action of sphingomyelinase C and other lipid-specific agents as inhibitors of Fc binding and locomotion in human leucocytes. Immunology. 1977 Sep;33(3):407–412. [PMC free article] [PubMed] [Google Scholar]
- Wolf H. J., Christiansen M., Hanson R. S. Ultrastructure of methanotrophic yeasts. J Bacteriol. 1980 Mar;141(3):1340–1349. doi: 10.1128/jb.141.3.1340-1349.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf H. J., Hanson R. S. Alcohol dehydrogenase from Methylobacterium organophilum. Appl Environ Microbiol. 1978 Jul;36(1):105–114. doi: 10.1128/aem.36.1.105-114.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajic J. E., Volesky B., Wellman A. Growth of Graphium sp. on natural gas. Can J Microbiol. 1969 Oct;15(10):1231–1236. doi: 10.1139/m69-222. [DOI] [PubMed] [Google Scholar]
- Zehnder A. J., Brock T. D. Methane formation and methane oxidation by methanogenic bacteria. J Bacteriol. 1979 Jan;137(1):420–432. doi: 10.1128/jb.137.1.420-432.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]